Abstract
The Fontan operation remains the final palliation for thousands of patients with complex congenital heart disease. By creating a Fontan circuit, control of cardiac output and congestion is wrested away from the ventricle and new haemodynamic forces take control. Understanding how to control the flow in the Fontan circuit will enable clinicians to improve patient management and possibly prevent future complications.
Conclusion
This review proposes a framework of principles to conceptualise the functionality and limitations of a Fontan circulation.
Keywords: circulation failure, diastolic function, Fontan, pulmonary vasculature, pulmonary vascular resistance
Abbreviations
- BSA
body surface area
- FPC
Fontan portal complex: refers to the flow restriction determined by the upstream push, the Fontan portal system, and the downstream pull
- FPS
Fontan portal system: refers to all structures‐resistances between the caval veins and left atrium: surgical venous connection, the pulmonary arteries, the pulmonary capillary vasculature, the pulmonary veins and the veno‐atrial connection
- LVEDP
left ventricular end‐diastolic pressure
- VEDP
ventricular end‐diastolic pressure
Key Notes.
This review proposes a framework of principles to conceptualise the functionality and limitations of Fontan circulation for patients with complex congenital heart disease.
By creating a Fontan circuit, control of cardiac output and congestion is wrested away from the ventricle and new haemodynamic forces take control.
Understanding how to control the flow in the Fontan circuit will enable clinicians to target therapies to improve patient management and possibly prevent future complications.
1. INTRODUCTION
After five decades, the Fontan operation remains the final palliation for several thousands of patients with complex congenital heart disease. However, mortality and morbidity in these patients remain high.1 Standard paradigms of management have religiously been pursued with minor effect on outcome. Even though surgical mortality has improved remarkably and mechanical design and construction of the circuit have vastly improved, late failure of the Fontan circuit occurs and remains difficult to treat.2, 3 Exercise intolerance, acute and chronic circulation failure with congestion and ascites, cyanosis, arrhythmias, thromboembolism, protein‐losing enteropathy, plastic bronchitis, liver disease, renal dysfunction and premature death have become major determinants of outcome.4, 5
The aim of this review is to propose a framework of principles in which to best conceptualise a Fontan circulation, its functionality and limitations. By creating a Fontan circuit, control of cardiac output and congestion is wrested away from the ventricle and new haemodynamic forces take control. Understanding the control of flow in the Fontan circuit will enable clinicians dealing with these patients to improve their management and possibly prevent future complications.
1.1. What is a Fontan circuit?
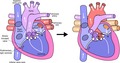
A Fontan circulation is offered to patients with severe congenital heart disease who have only one functional ventricle or the inability to split the ventricular cavity into two adequate pumps. The future candidate for a Fontan operation is prepared stepwise for such circulation from birth. In the neonatal period or early infancy, adequate flow to the body must be guaranteed by interventions (if obstruction: coarctectomy, Damus‐Kaye‐Stansel or Norwood arch repair) and pulmonary flow must appropriately be balanced (banding or shunt). The desaturated blood from the caval veins is then diverted directly to the pulmonary arteries step by step: the superior caval vein is connected to the pulmonary artery at the age of 3‐9 months (bidirectional Glenn shunt or partial cavopulmonary shunt), followed at the age of 2‐4 years by completion of the Fontan circuit by also connecting the inferior caval vein to the pulmonary arteries. The patient then has the modern version of a Fontan circuit: a total cavopulmonary circulation TCPC6 (Figure 1). Thus, by creating such Fontan circuit, the pulmonary circulation is positioned, like a portal system, between the venous return of the body and the systemic ventricle. A portal system is defined as a capillary network that is interposed between another capillary network and the heart. Humans have two portal systems: the hypophyseal and the hepatic portal system. The latter consists of drainage from gastrointestinal capillaries that join together into portal veins that then branch again into the liver parenchymal capillary system before routing to the heart. By creating a Fontan circuit, one is establishing a new pulmonary portal system as blood must now after having passed through the systemic capillary bed, traverse the pulmonary capillary bed before returning to the heart.
Figure 1.
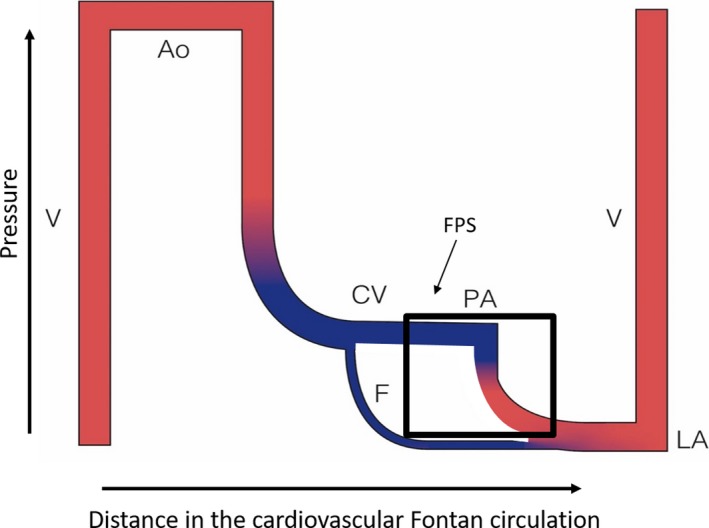
Flow/pressure/saturation diagram of Fontan circulation: critical bottleneck. Schematic representation of a modern Fontan circuit: the caval veins are directly connected to the pulmonary arteries; systemic venous pressures are markedly elevated. The Fontan portal complex (see text) demonstrated by black rectangle. A fenestration is an efficient bypass as it relieves venous congestion and low flow. The critical bottleneck is therefore pinpointed: it has to be between the beginning and the end of the bypass. Ao, aorta; CV, caval veins; F, fenestration; LA, left atrium; PA, pulmonary artery; V, single ventricle. Line thickness reflects output, height reflects pressure and colour reflects oxygen saturation
This pulmonary portal system functions like a dam in the cardiovascular circuit, with all of its characteristics: upstream congestion and downstream decreased flow; the energy from the dam is used to get the blood through the pulmonary circulation which allows oxygenation. The dam takes over control of the circulation as it has become the new critical bottleneck. The unwanted effects of that dam like pooling the systemic venous blood and reducing the overall output are the root cause of all the early and late problems of the Fontan circuit.3
A Fontan operation in essence is an extra‐cardiac or vascular operation: the surgeon can create a Fontan circuit on a beating heart without touching the ventricle! Cardiopulmonary bypass is frequently used to avoid perioperative congestion, but is not essential for the Fontan operation. The ventricle which is supposed to come untouched out of the operation is well downstream from the new obstruction: this implies that at least the early ventricular changes are not primary effects, but secondary effects to the new upstream flow restrictor.
In the preceding paragraphs, we used some important hydrodynamic principles that require some explanation: critical bottleneck, primary vs secondary effects and upstream flow restrictor.
1.2. Basic principles that apply to a Fontan circuit
1.2.1. Flow restriction, dam, critical bottleneck
When engineers construct a dam wall in a river, the following basic principles apply: the dam is a flow restrictor which causes upstream congestion and downstream controlled diminished flow. The potential energy created by the dam is reflected by the height of the dammed lake; the energy can be used for creation of electricity or in the case of a Fontan circuit: oxygenation. Building secondary dams at a distance lower down in the river system will have negligible effects on the downstream flow through the primary dam except in the unlikely event of flooding.
Every dam in the river is a flow restrictor and therefore a potential bottleneck. Every such dam will independently determine the height of the water immediately upstream only. However, on the path of the river, the dam with the highest flow restriction will become the critical bottleneck determining the maximal flow downstream. It is called critical because only changes that will affect flow through this dam will affect flow through the downstream system; changes at any other flow restrictor will not affect the overall flow and are therefore even irrelevant for overall output. If unclear where the critical bottleneck is, a bypass will elegantly identify the critical bottleneck: if a bypass increases the flow, the critical bottleneck will be between the two points of the bypass (Figure 2).
Figure 2.
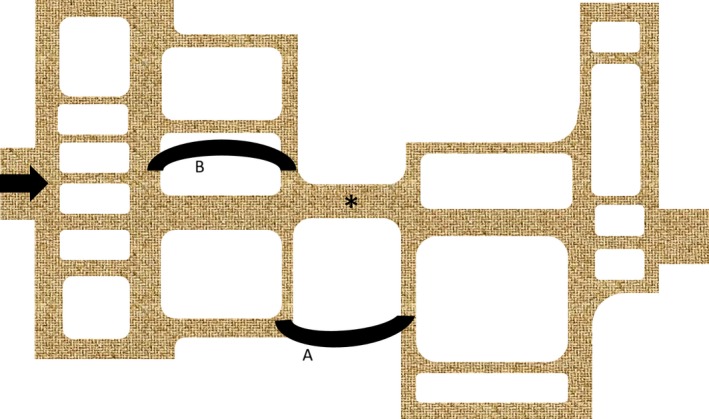
Critical Bottleneck in circuit. Pathway where the flow has to go from left to right (black arrow). * Indicates the critical bottleneck. Bypass A will relieve flow obstruction, while B will have no effect on flow distal to the bottleneck. See the text for details
If you apply this concept in a closed circuit, there will be at any time only one critical bottleneck that determines the flow through the system; that bottleneck will also determine the height of its adjacent upstream lake, while other dams in the circuit will determine the height of their own impoundment lake. Shift of bottleneck may occur once the primary critical bottleneck is relieved and other new bottlenecks may be unmasked; however, the next severe bottleneck will once again become the critical bottleneck and main determinant of flow.
The flow through a bottleneck is always determined by three factors: the resistance of the bottleneck, the pressure above and the runoff or suction below. When faced with an unwanted flow restriction across the critical bottleneck, three options are available to increase flow. Decreasing the resistance of the bottleneck is intuitive and is usually the most efficient approach. Increasing upstream pressure is the easiest approach, but is typically less efficient. Decreasing pressure downstream using suction is typically the least efficient.
These very general concepts apply in many fields such as hydrodynamics, factory production lines, business management and also the cardiovascular system.3, 7 Cardiologists have intuitively been thinking like this in many simple cardiac conditions and used it to efficiently treat cardiac conditions. These are illustrated in Figure 3. This distance‐pressure graph shows flow throughout the cardiovascular system and several problems can be superimposed on the graph. The first is congestive cardiomyopathy. The main problem or critical bottleneck is impaired muscle function. Flow can be increased by enhancing contractility or by increasing filling pressure, which is limited by retrograde pulmonary hypertension and over‐distention of the actin‐myosin complex, or by decreasing afterload.
Figure 3.
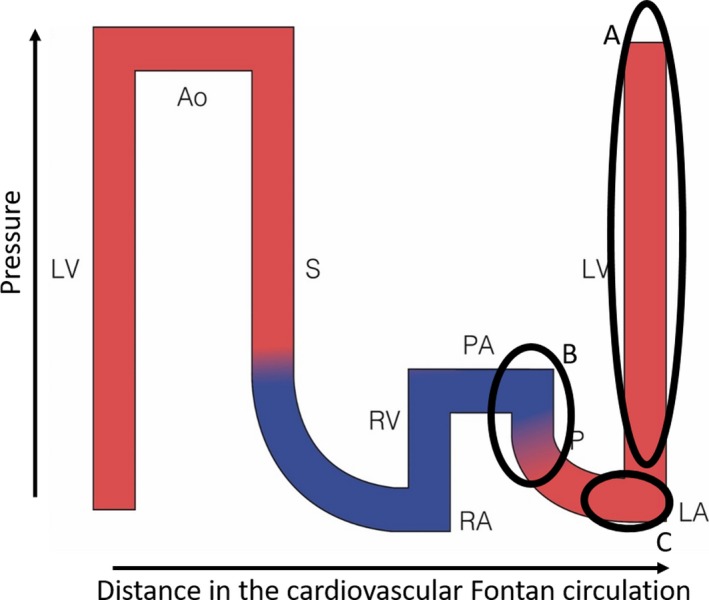
Flow/pressure/saturation diagram of a normal circulation: critical bottleneck in various disease conditions. Schematic representation of normal circulation: localisation of critical bottleneck in various diseases. A: Cardiomyopathy (CMP); B: Primary pulmonary hypertension PPHT; and C: Mitral stenosis (MS). See text for details. Ao, aorta; CV, caval veins; LA, left atrium; PA, pulmonary artery; LV, left ventricle; RA, right atrium; RV, right ventricle
A second example is primary pulmonary hypertension: the critical bottleneck is the pulmonary vasculature. Flow can be increased with pulmonary vasodilators, by increasing upstream pressure (right ventricular hypertension) or by decreasing left atrial pressure (not possible due to lack of adequate lusitropic drugs).
A third example is mitral valve stenosis: the critical bottleneck is the mitral valve. Flow can best be increased by dealing with the stenosis, less by increasing pressure upstream (left atrial pressure, limited by retrograde pulmonary hypertension) or least efficient by enhancing suction (no lusitropic drug available, bradycardia to prolong diastolic filling time).
1.2.2. Primary versus secondary effects
An additional important principle is the difference between primary and secondary effect. An intervention may have immediate and primary effects; these primary effects in turn may have distant secondary or tertiary effects. Altering the primary effect may enhance or neutralise the initial intervention, but in contrast, altering the secondary effects rarely will do so. For example, an aortic rupture will cause hypotension and shock as primary effect; secondary effects are hemothorax and cardiac arrest. Dealing with the secondary effects (thoracic drain and heart massage) may defer final outcome, but dealing with the primary effect such as aggressive volume repletion may be more effective; most effective will be to seal off the rupture. A secondary effect may occasionally become dominant as in an avalanche or a nuclear explosion, but this is exceptional and its relevance should be proven, certainly not taken for granted. This is a frequent problem when subspecialists look with tunnel vision at the secondary effects, neglecting the importance of the root problem and its primary effects.
1.3. Principles applied to the Fontan circuit
When creating the Fontan circuit, the surgeon establishes a pulmonary portal system, building a dam upstream of the ventricle. By doing so, this shifts the critical bottleneck outside and upstream of the heart itself: the dam will control overall flow and the degree of congestion upstream. Technically, the Fontan operation can be created without touching the ventricle; therefore, all changes observed at ventricular level such as hypocontractility, diastolic dysfunction and blunted heart rate response are downstream secondary phenomena and as such of little relevance when aiming to improve venous congestion and low output. The ventricle, while still the engine of the circuit, no longer controls the flow but it will pump the (reduced) blood volume that is offered: this explains why treatments that target heart rate, contractility and afterload have minimal impact on Fontan haemodynamics when within physiological range.3, 4, 7, 8 Decreasing the ventricular filling pressure (which is at the end of the new bottleneck) can increase overall flow; however, the ventricle cannot generate sufficient suction to compensate for the upstream damming, and there are no effective lusitropic type drugs at our disposal.
A very efficient and reliable way to decrease the venous congestion and increase overall flow in the Fontan circuit is to create a fenestration: such fenestration will bypass the Fontan portal system, thereby illustrating or even proving that this system has become the new critical bottleneck (Figure 1). Lowering the pulmonary vascular resistance (vasodilators and negative pressure ventilation) can also help.
1.4. Components of the flow resistor
The flow through the circuit is determined by the overall resistance from caval vein to pulmonary atrium—the pulmonary portal system—and the pressure gradient across this resistance.
1.4.1. The Fontan circuit and neo‐pulmonary portal system
By creating a Fontan connection, the pulmonary circulation is positioned like a portal system between the venous return of the body and the systemic ventricle. The composite of the Fontan pulmonary portal system therefore consists of the surgical venous connection and the graft (if extra‐cardiac), the pulmonary arteries, the pulmonary capillary vasculature including the pre‐capillary sphincters, the pulmonary veins and the veno‐atrial connection. Impediments in any region of the new portal system will markedly alter output of the Fontan circuit to a much greater extent than in a normal biventricular circulation. Such impairments may include pulmonary hypoplasia, stenosis, distortion, loss or exclusion of large vessels or microvessels, pulmonary vasoconstriction, pulmonary vascular disease, turbulence and flow collision, collateral flow, flow mismatch or obstruction by external extra‐vascular compression.
1.4.2. Flow through the Fontan pulmonary portal system
The flow through the Fontan pulmonary portal system (and thus the entirety of cardiac output) is determined by the overall resistance and the pressure gradient across this resistor. The resistance is predominantly determined by the pulmonary vascular resistance9, 10, 11; all other factors are typically reduced to a minimum (unless there is anatomical vessel or graft obstruction). The upstream central venous pressure will always be elevated in a Fontan patient with little variability at rest; out of experience we know that pressures chronically above 18‐20 mm Hg are poorly tolerated.11, 12 The downstream atrial pressure is in the absence of severe mitral valve dysfunction nearly completely determined by the ventricular filling pressure.13, 14 These three variables which control output through the FPS and the whole circuit, alter in time after creation of the Fontan circuit, resulting in a change in output and in congestion during Fontan life. Figure 4 reflects what happens in most Fontan over time: there is a steady increase of central venous pressure, pulmonary vascular resistance and ventricular filling pressure, resulting in a progressive decrease in flow.
Figure 4.
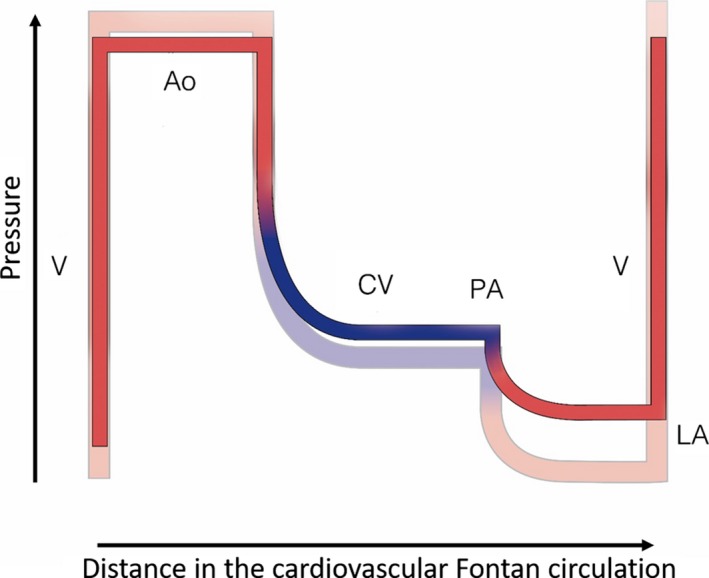
Flow/pressure/saturation diagram of the Fontan circulation: changes over time. Fontan haemodynamics late (full colour) superimposed on Fontan early (transparent): with time the ventricular end‐diastolic pressure and pulmonary vascular resistance increase, resulting in overall decreased flow and increased caval vein pressure/congestion. A downward progressive spiral ensues. Ao, aorta; CV, caval veins; LA, left atrium; PA, pulmonary artery; V, single ventricle
Pulmonary vascular resistance is known to increase with time, but likely more so in a Fontan circulation. The overall health of the pulmonary vasculature is impaired because of chronically decreased flow, minimal to mild desaturation, increased collateral flow, suboptimal mixing of inferior and superior caval flow streams, absence of pulsatility, endothelial dysfunction, and absence of flushing/maximal recruitment by episodes of high pulmonary flow and high pulmonary pressure as are normally seen during exercise in subjects with a normal right ventricle.
Ventricular filling pressure is also known to increase with age, partly due to the natural degenerative change of impaired ventricular relaxation related to fibrosis, partly due to deconditioning. This likely occurs more so in a Fontan ventricle with chronic volume deprivation resulting in reduced to absent exercise‐induced ventricular stretch.14 Any muscle that is underused and poorly or never stretched above its operating length will become more‐stiff; in the ventricle this will result in increasing filling pressures.
It should therefore be of no surprise that due to increasing pulmonary vascular resistance and ventricular filling pressure, systemic venous pressure and congestion also increase.3, 11
1.5. Control of flow in the Fontan circuit over time
Clinicians can differentiate several scenarios when observing congestion and cardiac output after creation of the Fontan circuit. Much can be predicted and explained by determining the critical bottleneck and its changes over time. In order to facilitate conceptualisation, one can pool the three determinants (resistance of the pulmonary portal system, central venous pressure as driving force and ventricular filling pressure as pulling force) and refer to this as the Fontan portal complex (FPC). In parallel are the characteristics of the single ventricle/univentricular heart: the mechanics of relaxation and or contraction as well as atrioventricular valve function that is rarely normal, and with a natural course of degeneration anticipated. Let us go through a series of thought experiments and discuss a number of scenarios, using our framework model. These are also outlined in a series of schematic diagrams (Figures 5, 6, 7). The restriction when the Fontan circuit is created (in diagram: “Fontan creation”) and the restriction at the end of Fontan life (“demise”) are nearly equal for all Fontan patients; some variability exists between these two points:
Figure 5.
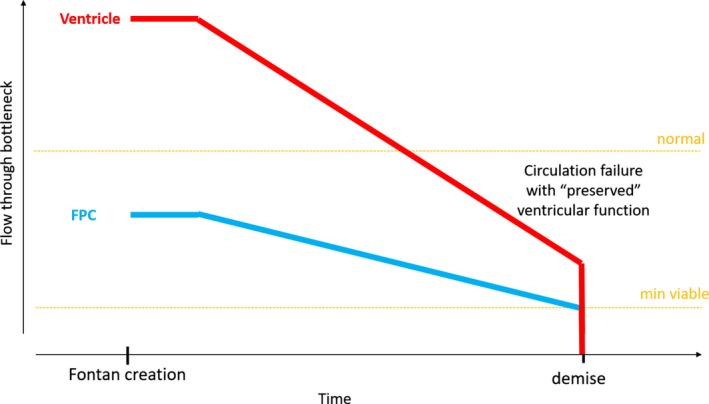
Change of restriction by the bottlenecks in the Fontan circuit: circulatory failure with ‘preserved’ ventricular function. Normal flow at rest in normal human and minimal output to remain functional is indicated by the orange lines. The restriction of the two dominant bottlenecks in the Fontan circuit is depicted: (a) the Fontan portal complex FPC (blue) and (b) the ventricle (red). Height of the bottleneck reflects its restriction: the lower the more restrictive. Flow through the circuit will be determined by the most restrictive bottleneck. Early on, the ventricle is able to pump more flow than provided by venous return. Immediately after establishment of a Fontan circuit, the FPC is the most restrictive and thereby becomes the main controller of flow and congestion. Ventricular function is not restrictive (good cardiac reserve and extra‐cardiac operation) at the start, but may gradually decrease for various reasons. Over time, restriction related to FPC increases resulting in decreasing cardiac output; the FPC remains the most important determinant of cardiac output. Once flow reaches below a critical minimal, the circulation fails and demise ensues
Figure 6.
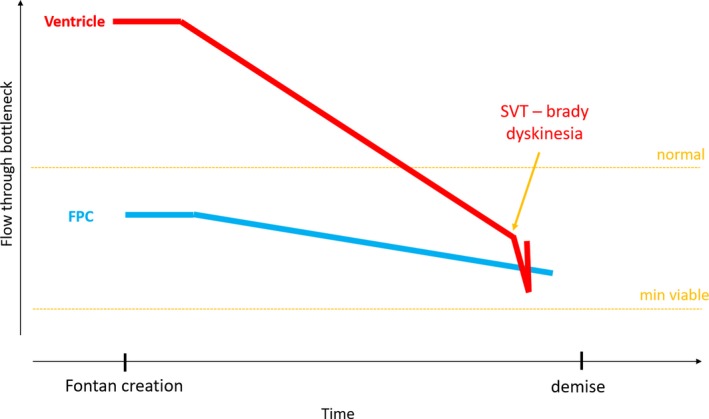
Change of restriction by the bottlenecks in the Fontan circuit: acute ventricular dysfunction. Acute changes of the ventricle such as severe bradycardia or tachycardia, ventricular dysfunction or acute excessive afterload may shift the critical bottleneck towards the ventricle. Such an episode will severely compromise the patient and may kill him if the ensuing rise of ventricular filling pressure reaches the level of systemic venous pressure. Treating the underlying ventricular problem may re‐shift the bottleneck and restore the prior output
Figure 7.
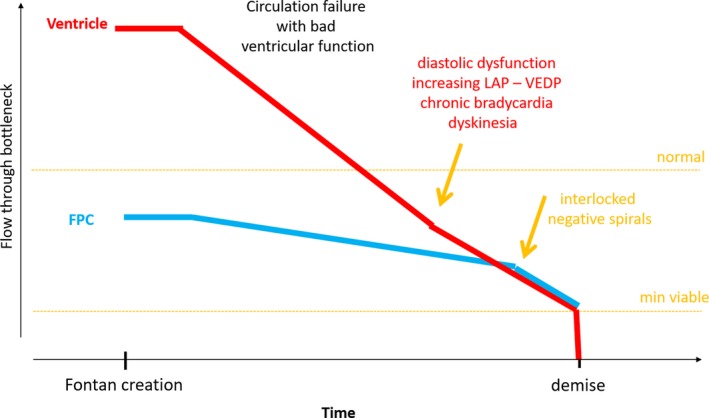
Change of restriction by the bottlenecks in the Fontan circuit: accelerated progressive ventricular dysfunction with interlocked bottlenecks (circulatory failure with poor ventricular function). Accelerated ventricular dysfunction may result in convergence of the two lines; the bottlenecks may become interlocked. Treatment of one and neglecting the other will inevitably lead to treatment failure. Once the two lines become interlocked, a vicious negative spiral ensues
1.5.1. Flow restriction immediately after the Fontan operation
At initial creation of the Fontan circuit, the FPC is the primary controller of output and congestion (point “Fontan creation” in Figures 5, 6, 7). The ventricle at this point likely has plenty of reserve but has become an “innocent bystander” to the new haemodynamics and will play no role, unless in the exceptional event of being markedly unwell to start with, critically unloaded at its closing volume or severely damaged by the operation.
1.5.2. Flow restriction at end of the Fontan life
In order to maintain survival and keep all organs functional, a minimal cardiac output is required (point “demise” in Figures 5, 6, 7). If for any reason this minimal output is not provided by the Fontan circuit, the heart will cease to function resulting in demise.
1.5.3. Flow restriction between beginning and end of Fontan life
Several scenario's can be postulated and observed.
As mentioned previously, flow restriction by the FPC will increase over time, thereby progressively limiting output. Ventricular performance may remain satisfactory of even quite “good” and never become the limiting factor. In these patients, FPC dysfunction may be the main reason for cardiac output to reach a minimum threshold with ensuing death. This scenario can be described as “Fontan circulatory failure with preserved ventricular function” (Figure 5).
Another scenario (Figure 6) is one in which there are acute changes of the ventricle such as severe bradycardia or tachycardia, ventricular dysfunction or acute excessive afterload, which may shift the critical bottleneck towards the ventricle. Such an episode may severely compromise the patient and may result in death if the ensuing rise of ventricular filling pressure reaches the level of systemic venous pressure, which will stop flow across the Fontan portal system. Treating the underlying ventricular problem may re‐shift the bottleneck and restore cardiac output.
A third and not uncommon scenario (Figure 7) is that the two lines of upper flow limits converge and the two bottlenecks may become interlocked as they share diastolic dysfunction. This results in a vicious spiral where improvement of one bottleneck without simultaneous improvement of the other will have no impact. The patient may survive as long as sufficient driving force across the Fontan pulmonary system exists, until the flow through the critical bottleneck reaches the minimal output line.10, 14 This scenario can be described as “circulation failure with severe ventricular dysfunction”.
1.6. Present and future
In essence, after completion of the Fontan operation the system effectively runs on autopilot with little margin for manipulation. The patient can increase his circulatory output by using peripheral muscles to pump blood into his caval veins to increase systemic venous pressure to drive blood through the critical bottleneck; respiratory pattern and force may also be helpful.15, 16 The clinician may improve output by lowering pulmonary vascular resistance but with limited result as many pulmonary lesions such as hypoplasia, stenosis, exclusion and others are not amenable to dilation; a great desire exists for improving the “suction of the ventricle”, but no lusitropic drugs nor efficient manoeuvres to lower VEDP are available.4, 7, 8 Increasing lower extremity muscle mass may impact active systemic venous return; however, this is a concept not yet well studied. Using our framework principles, a rational therapeutic approach can be conceived.
1.6.1. Prevention
One should ensure that, during pre‐staging for Fontan, the future FPC is as well developed as possible with a low PVR and high capacitance, combined with a ventricle that will function at low filling pressures for a long time.17 The clinician may need to choose between priorities: should one target the best possible ventricle, or aim for the best possible FPC?
1.6.2. Creating the best possible Fontan circuit
Create the best Fontan Portal Complex with low pulmonary vascular resistance
The most important period for (catch‐up) growth of the pulmonary arteries is between birth and the Glenn shunt. The initial palliation with “high‐pressure” pulmonary flow is therefore of the upmost importance: the goal should be to create adequate flow to promote optimal symmetric growth, avoiding over‐shunting that might cause pulmonary vascular disease. As no significant catch‐up growth can be expected with the limited flow of a partial cavopulmonary connection, pulmonary vasculature must be proven to be adequate before proceeding to the Glenn shunt; else the Glenn must be delayed and additional/different high‐pressure flow may be indicated. Such flow may indeed overload the ventricle, but the highest priority at this stage is to promote the best possible pulmonary vasculature, even at the price of mild late ventricular dysfunction.
Encourage development of an adequate ventricle with low filling pressure
The size of a ventricle is determined by its chronic volume load: the normal relationship between output and BSA is lost in single‐ventricle patients at every stage of their life (foetal, shunted, after partial or complete cavopulmonary connection).18 At birth, the single ventricle is already larger for BSA than a normal left ventricle in a biventricular heart. The initial pulmonary overflow will induce further dilation and overgrowth (for BSA) of that ventricle; acute and severe over‐shunting will damage any ventricle. When creating the partial cavopulmonary connection, the previous high‐pressure flow is usually interrupted, thereby reducing the ventricular preload to levels below normal for BSA. The preload of the ventricle will further be reduced when completing the Fontan circuit. The ventricle evolves thus from large and overloaded, to overgrown (for both BSA and certainly for its post‐Fontan preload) and severely deprived. The degree of deprivation (determined by the restriction of the FPC) is enhanced by any degree of ventricular overgrowth‐dilation (determined by the size of the previous shunt). Avoidance of acute large over‐shunting and severe overgrowth‐dilation is important to avoid later high and increasing ventricular filling pressures.
The volume requirements for optimal growth and development of the ventricle and the lungs are thus different and divergent. Avoiding severe overload of the ventricle is important, but excessive protection from volume overload is not necessary and may result in pulmonary artery hypoplasia, which in turn will severely affect functionality of the eventual Fontan circuit.17
1.6.3. Maintain a good Fontan circulation
Once created, many Fontan circuits may show progressive decrease in functionality. Overall surveillance as a means for maintaining good health in the face of this unique physiology becomes very important.19
Keep pulmonary vascular resistance low
Lifestyle adjustments such as maintaining good body weight, smoking avoidance and protection of the pulmonary vasculature (vaccinations, altitude avoidance, etc) may be more important than previously recognised.20 The most appropriate degree of anti‐aggregation or anticoagulation for various clinical and haemodynamic conditions to avoid micro‐emboli is still being explored.21 Furthermore, current evidence suggests that exercise is beneficial for the Fontan circulation: the muscle pump will increase pulmonary pressure and output, resulting in “flushing” the vasculature and contributing to intermittent recruitment and vasodilation of pulmonary vessels.22 The effect of vasodilators is currently being investigated, as well as the effect of a temporary or intermittent right ventricular assist device.
Keep LVEDP low
The debate is still ongoing as to why ventricular compliance diminishes over time, but it seems logical that by slowing the effects of ageing, some of the sequelae may be at least delayed. As the chronic low flow with absence of stretch might be a major contributor for progressive stiffness, studies of exercise to augment flow while avoiding tachycardia to promote stretch are of great interest.15
1.7. Future directions
The most logical solution would be to insert a pump into the circuit to propel blood forward: this is the solution that evolution has developed for all mammals. Cardiologist is very unlikely to beat this solution provided by millions of years of evolution. Some experiments with a subpulmonary pump have been conducted. Subsystems like the lymphatic system may be helped by specific strategies.
2. CONCLUSION
After 50 years of the Fontan operation, cardiologists have had little impact in managing the failing Fontan circulation. Conceptualising this unique circulation using a particular framework can help improve thinking and provide a platform for solutions. Recognition of the importance of the building blocks—pulmonary vasculature and ventricle—is essential. In order to treat the patient with a failing single ventricle, time has come to shift our focus beyond the notion of diastolic function of the heart, and more to the framework principles we outline with the principal being the Fontan portal complex.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Gewillig M, Brown SC, van de Bruaene A, Rychik J. Providing a framework of principles for conceptualising the Fontan circulation. Acta Paediatr. 2020;109:651–658. 10.1111/apa.15098
This paper was presented at The cardiac patient from birth to adulthood in Stockholm in February 2019
Funding information
This work was supported by grants from the Eddy Merckx Research Foundation and the parent organisation “de Kleine Hartjes”.
REFERENCES
- 1. Poh CL, D'Udekem Y. Life after surviving Fontan surgery: a meta‐analysis of the incidence and predictors of late death. Hear Lung Circ. 2018;27(5):552‐559. [DOI] [PubMed] [Google Scholar]
- 2. Caneo LF, Turquetto ALR, Neirotti RA, et al. Lessons learned from a critical analysis of the Fontan operation over three decades in a single institution. World J Pediatr Congenit Heart Surg. 2017;8(3):376‐384. [DOI] [PubMed] [Google Scholar]
- 3. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102(14):1081‐1086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Res. 2018;7:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lüscher TF, Camm JA, Maurer G, Serruys P. ESC cardiomed. Cardiol J. 2018;25(5):565‐568. [DOI] [PubMed] [Google Scholar]
- 6. de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96(5):682‐695. [PubMed] [Google Scholar]
- 7. Gewillig M, Brown SC, Eyskens B, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg. 2010;10(3):428‐433. [DOI] [PubMed] [Google Scholar]
- 8. Ohuchi H. Where is the “optimal” fontan hemodynamics? Korean Circ J. 2017;47(6):842‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snarr BS, Paridon SM, Rychik J, Goldberg DJ. Pulmonary vasodilator therapy in the failing Fontan circulation: rationale and efficacy. Cardiol Young. 2015;25(8):1489‐1492. [DOI] [PubMed] [Google Scholar]
- 10. Egbe AC, Connolly HM, Miranda WR, et al. Hemodynamics of Fontan failure: the role of pulmonary vascular disease. Circ Hear Fail. 2017;10(12):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egbe AC, Reddy YNV, Khan AR, et al. Venous congestion and pulmonary vascular function in Fontan circulation: implications for prognosis and treatment. Int J Cardiol. 2018;271:312‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Myers CD, Ballman K, Riegle LE, Mattix KD, Litwak K, Rodefeld MD. Mechanisms of systemic adaptation to univentricular Fontan conversion. J Thorac Cardiovasc Surg. 2010;140(4):850‐856.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Margossian R, Sleeper LA, Pearson GD, et al. Assessment of diastolic function in single‐ventricle patients after the Fontan procedure. J Am Soc Echocardiogr. 2016;29(11):1066‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura Y, Yagihara T, Kagisaki K, Hagino I, Kobayashi J. Ventricular performance in long‐term survivors after Fontan operation. Ann Thorac Surg. 2011;91(1):172‐180. [DOI] [PubMed] [Google Scholar]
- 15. Cordina R, Celermajer DS, D'Udekem Y. Lower limb exercise generates pulsatile flow into the pulmonary vascular bed in the setting of the Fontan circulation. Cardiol Young. 2018;28(5):732‐733. [DOI] [PubMed] [Google Scholar]
- 16. Laohachai K, Winlaw D, Selvadurai H, et al. Inspiratory muscle training is associated with improved inspiratory muscle strength, resting cardiac output, and the ventilatory efficiency of exercise in patients with a fontan circulation. J Am Heart Assoc. 2017;6(8):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gewillig M, Brown SC, Heying R, et al. Volume load paradox while preparing for the Fontan: not too much for the ventricle, not too little for the lungs. Interact Cardiovasc Thorac Surg. 2010;10(2):262‐265. [DOI] [PubMed] [Google Scholar]
- 18. Gewillig M, Kalis N. Pathophysiological aspects after cavopulmorary anastomosis Gewillig.pdf. Thorac Cardiovasc Surg. 2000;48:336‐341. [DOI] [PubMed] [Google Scholar]
- 19. Rychik J, Atz AM, Celermajer DS, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019. 10.1161/CIR.0000000000000696 [DOI] [PubMed] [Google Scholar]
- 20. Bradley EA, Jassal A, Moore‐Clingenpeel M, Abraham WT, Berman D, Daniels CJ. Ambulatory Fontan pressure monitoring: results from the implantable hemodynamic monitor Fontan feasibility cohort (IHM‐FFC). Int J Cardiol. 2019;284:22‐27. [DOI] [PubMed] [Google Scholar]
- 21. Balling G. Fontan anticoagulation: a never‐ending debate? J Am Coll Cardiol. 2016;68(12):1320‐1322. [DOI] [PubMed] [Google Scholar]
- 22. Cordina R, d'Udekem Y. Long‐lasting benefits of exercise for those living with a Fontan circulation. Curr Opin Cardiol. 2019;34(1):79‐86. [DOI] [PubMed] [Google Scholar]


