Summary
Proper immune cell development at early ontogenic stages is critical for life-long health. How resident immune cells are established in barrier tissues at neonatal stages to provide early protection is an important but still poorly understood question. We herein report that a developmentally programmed preferential generation of skin-homing group 1 innate lymphoid cells (ILC1s) at perinatal stages helps regulate early skin microbiota colonization. We found that a population of skin-homing NK1.1+ ILC1s was preferentially generated in the perinatal thymi of mice. Unique thymic environments and progenitor cells are responsible for the preferential generation of skin-homing NK1.1+ ILC1s at perinatal stages. In the skin, NK1.1+ ILC1s regulate proper microbiota colonization and control the opportunistic pathogen Pseudomonas aeruginosa in neonatal mice. These findings provide insight into the development and function of tissue-specific immune cells at neonatal stages, a critical temporal window for establishment of local tissue immune homeostasis.
Subject Areas: Immunology, Microbiology
Graphical Abstract
Highlights
-
•
Perinatal thymi support preferential generation of skin-homing CCR10+ NK1.1+ ILC1s
-
•
PLZF is crucial for the perinatal thymic development of CCR10+ NK1.1+ ILC1s
-
•
Perinatally derived NK1.1+ ILC1s contribute to skin immune system until adulthood
-
•
NK1.1+ ILC1s control skin commensal bacterial colonization during neonatal stages
Immunology; Microbiology
Introduction
During ontogeny, innate and innate-like lymphocytes develop earlier than conventional αβT cells of the adaptive immune system. In mice, innate-like Vγ3+ γδT cells are the first T cell population generated in the fetal thymi (Havran and Allison, 1990). Subsequently, innate-like Vγ4+ and Vγ2+ γδT cells that possess interleukin (IL)-17-producing capacities (γδT17) are preferentially generated in later fetal and neonatal thymi (Cai et al., 2011, Cai et al., 2014, Gray et al., 2011, Haas et al., 2012, Sandrock et al., 2018). In addition, innate lymphoid cells (ILCs) are also generated more preferentially in fetal thymi than in adult thymi (Carlyle et al., 1998, Cherrier et al., 2012, Vosshenrich et al., 2006), which suggests a vital, evolutionarily conserved function of innate or innate-like lymphocytes during the early ontogenic stage.
The fetal/neonatal thymus-derived innate and innate-like lymphocytes have particularly important contribution to establishment of the local immune system in barrier tissues such as the skin. In mice, fetal thymus-derived Vγ3+ γδT cells are developmentally programmed with a skin-homing property such as high expression of the skin-homing chemokine receptor CCR10 for their preferential localization into the skin, where they reside mostly in the epidermis and account for the vast majority of dendritic epidermal T cells (DETCs) (Havran and Allison, 1990, Jin et al., 2010a, Jin et al., 2010b, Xiong et al., 2004, Xiong and Raulet, 2007). DETCs play important roles in immune defense and homeostatic regulation of the skin (Girardi et al., 2001, Girardi et al., 2003, Hayday, 2000, Jameson et al., 2002). The perinatal thymus-originated γδT17 cells are found abundantly in the dermis of mice where they are involved in anti-microbial immunity, but during dysregulation they may also contribute to skin inflammation (Cai et al., 2011, Cai et al., 2014, Gray et al., 2011, Sandrock et al., 2018, Sumaria et al., 2011). ILCs are found abundantly in the skin of adult mice and humans where they play important roles in local immune homeostasis and inflammation (Ebert et al., 2006, Imai et al., 2013, Kim et al., 2013, Kobayashi et al., 2019, Luci et al., 2009, Pantelyushin et al., 2012, Roediger et al., 2013, Salimi et al., 2013, Sojka et al., 2014, Yang et al., 2016). However, their developmental origin and function in early immune protection of the skin are less understood.
ILCs are a family of innate lymphocytes, which are divided into three groups (1–3) in addition to the conventional natural killer (cNK) cells and lymphoid tissue-inducing (LTi) cells, based on their functional potentials and developmental pathways (Spits et al., 2013, Vivier et al., 2018). Upon activation, group 1 ILCs (ILC1s) predominantly produce TH1-type cytokines such as interferon (IFN)-γ; ILC2s produce TH2-type cytokines such as IL-5 and IL-13; and ILC3s produce TH17-type cytokines such as IL-17 and IL-22 (Spits et al., 2013, Vivier et al., 2018). There are overlapping functions between ILC1s and cNK cells in promoting the type 1 immunity (Vivier et al., 2018). However, ILC1s and cNK cells are developmentally programmed by different transcription factors (Constantinides et al., 2014, Constantinides et al., 2015, Seillet et al., 2016, Vivier et al., 2018). Functionally, ILC1s produce the cytokines IFN-γ, tumor necrosis factor (TNF)-α, and IL-2 more potently than cNK cells, whereas cNK cells more preferentially express high levels of cell-killing effector molecules such as granzyme B (grzmB) and thus possess more potent cytotoxic capacities than ILC1s (Fuchs et al., 2013, Klose et al., 2014, Robinette et al., 2015, Seillet et al., 2016, Sojka et al., 2014). However, ILC1s could also express certain cell-killing molecules such as granzyme C (grzmC) (Daussy et al., 2014, Gury-BenAri et al., 2016, Robinette et al., 2015), consistent with their overlapping functions with cNK cells.
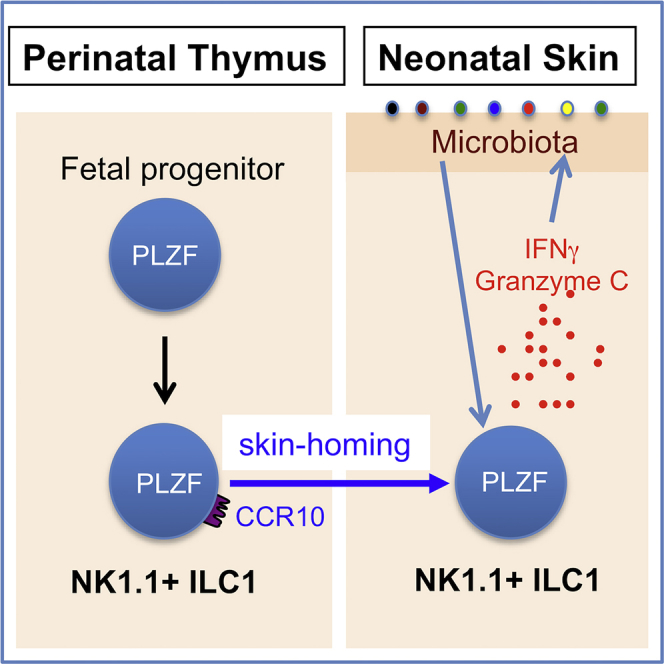
The skin of adult mice contains predominantly ILC2s and ILC3s, but few ILC1s (Kobayashi et al., 2019, Roediger et al., 2013, Yang et al., 2016, Yang et al., 2017). We previously reported that skin ILC2/3s in adult mice originate in skin-draining lymph nodes (sLNs), where their precursors are imprinted by skin-derived dendritic cells to express the skin-homing chemokine receptor CCR10 and associated homing molecules for their skin localization and function in maintenance of skin-resident T cell homeostasis (Yang et al., 2016). However, the development and function of ILCs at fetal/neonatal stages are not regulated in the same fashion as at the adult stage. In this study, we discovered a distinct mechanism of predominant establishment of ILC1s in the skin of fetal and early postnatal stages for regulation of commensal bacterial colonization. We found that like fetal thymus-derived skin-homing innate-like γδT cells, NK1.1+ ILC1s generated in fetal thymi are also programmed with preferential acquisition of the CCR10+ skin-homing property. Correspondingly, most skin ILCs in neonatal mice are NK1.1+ ILC1s, which play an important role in regulation of the skin colonization of commensal bacteria. This finding provides insight into the development of skin-resident ILCs and their function in protection of the barrier tissue at early postnatal stages, a critical temporal window for establishment of tissue homeostasis that profoundly impacts the life-long health of individuals.
Results
Preferential Generation of Skin-Homing NK1.1+ ILC1s in Fetal and Neonatal Thymi
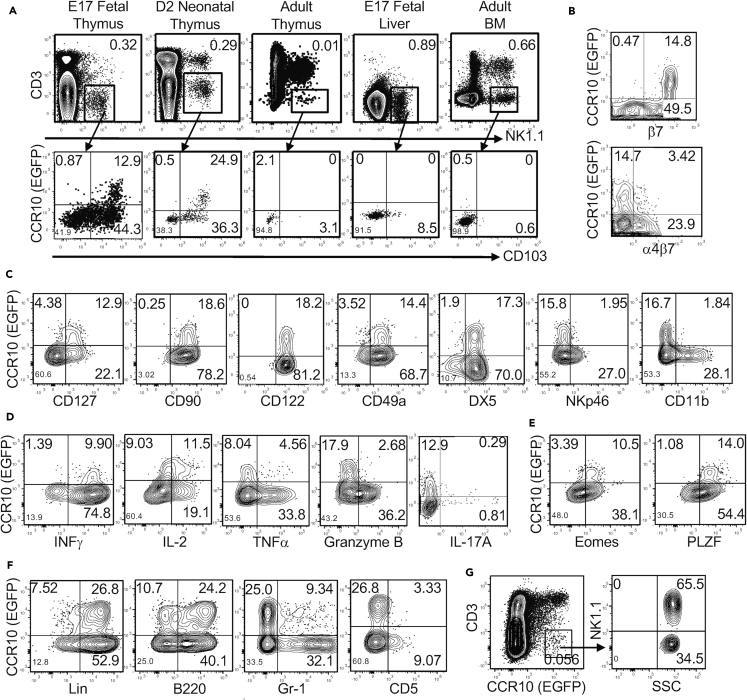
We previously found that innate-like γδT cells were programmed with a CCR10+ skin-homing property during their fetal thymic developmental stages (Jin et al., 2010a, Jin et al., 2010b). To determine whether fetal thymic environments also supported the generation of skin-homing ILCs, we analyzed the expression of CCR10 and related skin-homing molecules on fetal thymic CD3−NK1.1+ cells, a major subset of innate lymphocytes generated in the fetal thymus (Carlyle et al., 1998). Indeed, a significant fraction of embryonic day 17 (E17) fetal thymic CD3−NK1.1+ cells expressed CCR10(EGFP+) in CCR10+/EGFP (or CCR10+/− for simplicity) reporter mice in which the coding sequence of one CCR10 allele is replaced by a DNA sequence coding for enhanced green fluorescent protein (EGFP) to report endogenous CCR10 expression (Figure 1A) (Hu et al., 2011, Jin et al., 2010b). Like E17 fetal thymi, neonatal (2 days old) thymi also contained a significant percentage of CCR10+ CD3−NK1.1+ cells (Figure 1A). The percentage of CD3−NK1.1+ cells drastically decreased in adult thyme, and few expressed CCR10 (Figure 1A). In addition to the thymus, the primary lymphoid organs fetal liver and adult bone marrow (BM) also support generation of NK1.1+ ILCs and/or NK cells (Constantinides et al., 2015), which, however, did not express CCR10 (Figure 1A). All CCR10+ fetal/neonatal thymic CD3−NK1.1+ cells expressed CD103 (αE) and β7, two integrins involved in the establishment and retention of lymphocytes in the skin, but not the gut-homing molecule α4β7 integrin (Figures 1A and 1B). The homing molecule expression pattern of thymic CD3−NK1.1+ cells is identical to that of fetal thymic skin-homing Vγ3+ γδT cells (Jin et al., 2010b). These results suggest that fetal/neonatal thymi preferentially support generation of skin-homing NK1.1+ innate lymphocytes.
Figure 1.
Preferential Generation of NK1.1+ ILC1s with a CCR10+ Skin-Homing Property in Fetal and Neonatal Thymi
(A) Flow cytometric analysis of CD3−NK1.1+ cells of indicated lymphoid organs of CCR10+/EGFP mice of different ages (top row) and their expression of CCR10(EGFP) and CD103 (bottom). Data are representative of at least three experiments for each organ.
(B) Flow cytometric analysis of CCR10 and β7 or α4β7 on gated CD3−NK1.1+ thymocytes of E17 fetal CCR10+/EGFP mice.
(C) Flow cytometric analysis of CCR10 and indicated surface molecules on gated CD3−NK1.1+ thymocytes of fetal/neonatal CCR10+/EGFP mice.
(D) Flow cytometric analysis of CCR10 and IL-2, IFN-γ, TNF-α, granzyme B, or IL-17A in gated CD3−NK1.1+ thymocytes of fetal/neonatal CCR10+/EGFP mice. Data are representative of at least four experiments.
(E) Flow cytometric analysis of CCR10 and transcription factors Eomes and PLZF in gated CD3−NK1.1+ thymocytes of fetal/neonatal CCR10+/EGFP mice. Data are representative of at least four independent experiments.
(F) Flow cytometric analysis of CCR10 and indicated lineage markers on gated CD3−NK1.1+ thymocytes of fetal/neonatal CCR10+/EGFP mice. Data are representative of at least four experiments.
(G) Flow cytometric analysis of gated CCR10(EGFP)+CD3- thymocytes (left) of fetal/neonatal CCR10+/EGFP mice for their expression of NK1.1 (right). Data are representative of at least four experiments.
We further characterized the CCR10+ fetal/neonatal thymic NK1.1+ innate lymphocytes. All or most of CCR10+ thymic CD3−NK1.1+ cells expressed the cell surface molecules CD127, CD122, CD90, CD49a, and DX5, but not CD11b or NKp46 (Figure 1C). A large fraction co-expressed CD127, a marker for helper ILCs, and DX5, a marker of cNK cells (Figure S1A). They could produce high levels of IL-2, IFN-γ, and TNF-α, but not grzmB or IL-17, when stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Figure 1D). CCR10+ thymic CD3−NK1.1+ cells expressed PLZF and Eomes, the transcription factors commonly associated, respectively, with ILCs and NK cells (Figure 1E) (Constantinides et al., 2014, Constantinides et al., 2015, Fuchs et al., 2013, Klose et al., 2014, Robinette et al., 2015, Sojka et al., 2014). All these cells also expressed T-bet, a transcription factor associated with ILC1s and cNK cells, and many expressed GATA3, a transcription factor important for thymic NK development (Figure S1B) (Vosshenrich et al., 2006). These results indicate that CCR10+ fetal/neonatal thymic NK1.1+ ILCs are a unique population of ILC1s with overlapping features of NK cells. Notably, unlike other ILC subsets, the CCR10+ NK1.1+ ILC1s were Lin+ and mostly expressed the “B cell lineage marker” B220, but not Gr1 or CD5 (Figure 1F). They did not express IgM or CD19, confirming that they were not contaminated B cells (Figure S1C). CCR10+ NK1.1+ ILC1s accounted for most CCR10+ CD3- fetal/neonatal thymocytes, suggesting that they are the major skin-homing ILC subset generated in fetal/neonatal thymi (Figure 1G).
NK1.1+ ILC1s Are Highly Enriched in the Skin of Newborn Mice
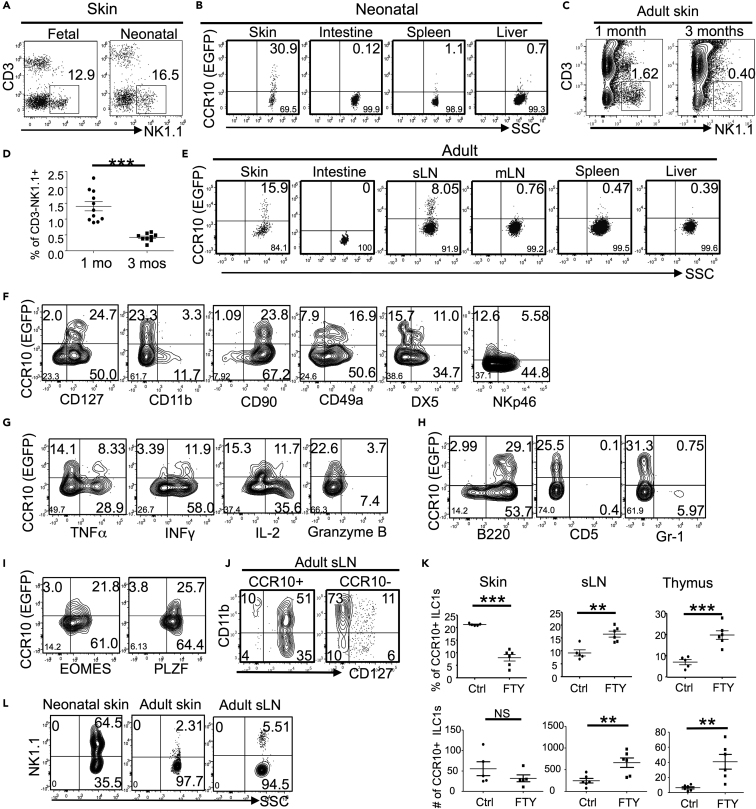
We then determined whether there was preferential enrichment of NK1.1+ ILC1s in the skin during early ontogenic stages. Indeed, in late fetal (E18) and newborn (day 1–4) mice, CD3−NK1.1+ cells accounted for more than 10% of skin CD45+ immune cells and a significant percentage (20%–30%) expressed CCR10 (Figures 2A and 2B). The percentage of CD3−NK1.1+ cells of total skin CD45+ cells gradually decreased in adult mice (Figures 2C and 2D). In addition, a smaller fraction of adult skin CD3−NK1.1+ CD45+ cells expressed CCR10 than neonatal skin CD3−NK1.1+ CD45+ cells (Figure 2E). In contrast to the skin, CD3−NK1.1+ cells of intestines did not express CCR10 (Figures 2B and 2E). Associated with these, CD3−NK1.1+ cells of intestine-draining mesenteric lymph nodes did not express CCR10, whereas a small fraction of CD3−NK1.1+ cells of sLNs expressed CCR10 (Figure 2E). No CD3−NK1.1+ cells of spleens or livers of any ontogenic stage expressed CCR10 (Figures 2B and 2E). These results demonstrate that CCR10+ CD3+NK1.1+ cells are skin-specific ILCs preferentially enriched in the skin of fetal/neonatal mice. Further analysis confirmed that neonatal skin CCR10+ NK1.1+ ILCs had the same molecular expression pattern of cell surface markers, ability of cytokine production, and expression of the transcription factors PLZF and Eomes as the thymic CCR10+ NK1.1+ ILC1s (Figures 2F–2I, summarized in Table S1), also supporting the notion that preferential generation of skin-homing NK1.1+ ILC1s in fetal/neonatal thymi contributes to specific enrichment of NK1.1+ ILC1s in the neonatal skin.
Figure 2.
NK1.1+ ILC1s Accounted for a Higher Percentage of Lymphocytes in the Skin of Fetal/Neonatal Mice than of Adult Mice
(A) Flow cytometric detection of CD3−NK1.1+ cells in gated CD45+ lymphocytes of the skin of late fetal (E18) and neonatal (day1–4) CCR10+/EGFP mice. Data are representative of three experiments with at least five mice for each experiment.
(B) Flow cytometric analysis of CCR10(EGFP) expression on gated CD3−NK1.1+CD45.2+ cells of the skin, intestine, spleen, and liver of fetal/neonatal CCR10+/EGFP mice. Data are representative of 3–5 experiments.
(C) Flow cytometric detection of CD3−NK1.1+ cells in gated CD45.2+ lymphocytes of the skin of 1- and 3-month-old mice.
(D) Average percentages of CD3−NK1.1+ cells of skin CD45+ lymphocytes based on the analysis in (C). One dot represents one mouse.
(E) Flow cytometric analysis of CCR10(EGFP) expression on gated CD3−NK1.1+CD45.2+ cells of the skin, intestine, spleen, sLN, mesenteric LN (mLN), and liver of adult CCR10+/EGFP mice. Representative of at least 10 experiments for skin, sLN, mesenteric LN (mLN), spleen, and liver.
(F–I) Flow cytometric analysis of the expression of CCR10 and indicated molecules in gated CD3−NK1.1+CD45.2+ cells of the skin of newborn CCR10+/EGFP mice. Data are representative of at least two experiments.
(J) Flow cytometric analysis of the CD127 and CD11b expression on gated CCR10+ and CCR10- CD3−NK1.1+CD45.2+ cells of sLNs of adult CCR10+/EGFP mice. Data are representative of at least 10 experiments.
(K) Comparison of percentages and numbers of the CCR10+ and CD127+CD11b− CD3−NK1.1+ cells in the skin, sLN, and thymus of adult CCR10+/EGFP mice fed with FTY720 (FTY) or not (Ctrl). One dot represents one mouse.
(L) Flow cytometric analysis of gated EGFP+ CD3- CD45+ cells of newborn and adult skin and adult sLNs of CCR10+/EGFP mice for their expression of NK1.1.
Data are representative of at least three experiments. NS, not significant, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by two-tailed Student's t test.
CCR10+ CD3−NK1.1+ cells in the skin of adult mice were more heterogeneous than CCR10+ NK1.1+ ILC1s of the fetal/neonatal skin and thymus (Figure S2A, Table S1), suggesting that they had more diverse sources of origins and/or activation statuses. As we previously found that the sLN is a site supporting generation of CCR10+ skin-homing ILC2/3s in adult mice (Yang et al., 2016), we tested whether CCR10+ CD3−NK1.1+ cells of sLNs also contributed to skin NK1.1+ ILC1s in adult mice. Consistent with this notion, the majority of CCR10+ CD3−NK1.1+ cells of adult sLNs were CD127+ ILC1s, whereas CCR10−CD3−NK1.1+ cells were mostly CD127−CD11b+ cNK cells (Figure 2J and Table S1). Furthermore, after treatment with FTY720, a drug that blocks the migration of ILCs of the sLNs and thymus (Matloubian et al., 2004, Yang et al., 2016), percentages of CCR10+ NK1.1+ ILC1s increased in the sLNs but decreased in the skin compared with untreated controls (Figures 2K and S2B), confirming that CCR10+ ILC1s of the sLNs migrate into the skin to contribute to the skin NK1.1+ ILC1 pool. The FTY720 treatment also increased the percentage of CCR10+ NK1.1+ ILCs in adult thymi compared with untreated controls (Figures 2K and S2B), indicating that although generation of skin-homing CCR10+ NK1.1+ ILC1s is drastically lower in adult than fetal thymi, inhibition of their egress results in accumulation of these cells in adult thymi. These results suggest that skin-homing NK1.1+ ILC1s of thymi could still contribute to the skin ILC repertoire in adult mice, although the relative contribution is much less than during perineonatal stages. In agreement, CCR10+ NK1.1+ ILC1s accounted for only a very small fraction (2%–5%) of total CCR10+ ILCs in the skin or sLNs of adult mice, whereas they accounted for the majority (>50%) of CCR10+ ILCs in the neonatal skin (Figure 2L). Together, these results revealed distinct mechanisms for establishment of different ILC populations in the skin of neonatal versus adult stages.
Unique Thymic Environments and Progenitor Cells Determine Preferential Generation of Skin-Homing NK1.1+ ILC1s in Fetal/Neonatal Mice
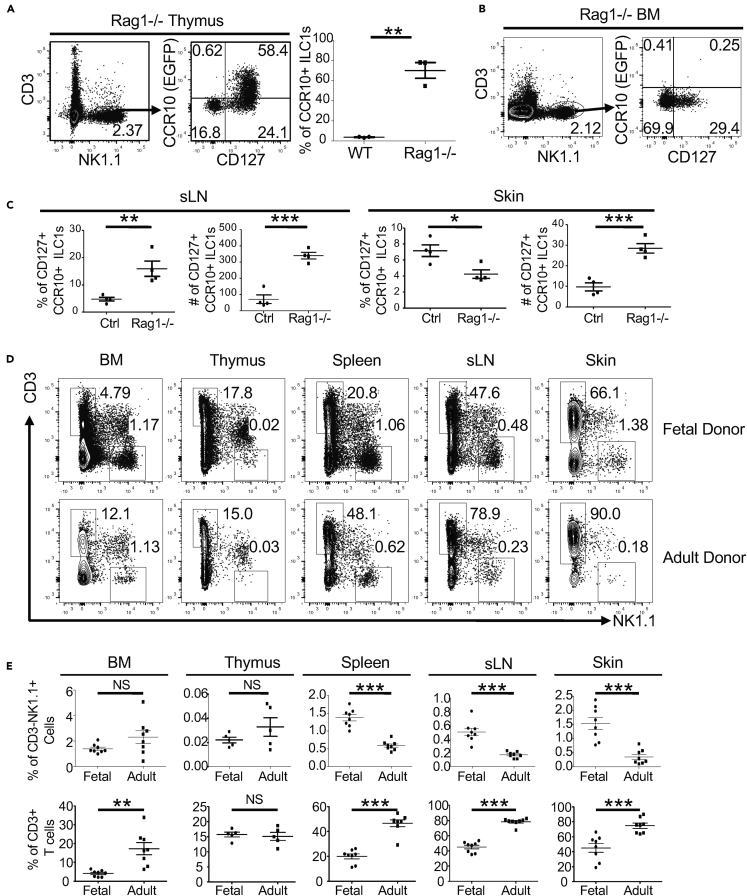
Both unique thymic environments and intrinsic properties of progenitor cells could play a role in the preferential generation of skin-homing NK1.1+ ILC1s in fetal/neonatal thymi compared with adult thymi or other primary lymphoid organs. One major difference between fetal and adult thymic environments is that the adult thymus is dominated by a large number of developing T cells, which are much less abundant in fetal thymi. We therefore tested whether absence of T cells in thymi of adult Rag1−/− mice would increase generation of CCR10+ NK1.1+ ILC1s. Strikingly, most thymic CD3−NK1.1+ cells of adult Rag1−/− mice expressed CCR10(EGFP+), which markedly contrasted to those of adult wild-type (WT) mice (Figures 1A and 3A). CCR10+ thymic CD3−NK1.1+ cells of adult Rag1−/− mice expressed CD127, CD103, integrin β7, and other markers associated with skin-homing ILC1s (Figures 3A and S3A). On the contrary, CD3−NK1.1+ cells of the BM of adult Rag1−/− mice remained CCR10(EGFP) negative (Figure 3B). These results indicate that thymic, but not BM, environments specifically support the generation of skin-homing NK1.1+ ILCs, which, however, is suppressed by a large number of developing T cells in the adult thymi. Associated with the increased generation of skin-homing NK1.1+ ILC1s in the thymi of adult Rag1−/− mice, percentages and numbers of CCR10+ NK1.1+ ILCs also increased in the sLNs compared with those of WT mice (Figure 3C). A large fraction of CCR10+ NK1.1+ ILCs in the sLNs of adult Rag1−/− mice expressed B220, whereas those in the thymi of adult Rag1−/− mice expressed only low levels of B220 (Figure S3B). Total numbers of CCR10+ NK1.1+ ILCs in the skin of adult Rag1−/− mice increased compared with WT controls, although percentages of the CCR10+ subset of total CD3−NK1.1+ cells decreased compared with WT controls (Figure 3C), which is consistent with our previous finding that skin ILC2/3s downregulate CCR10 upon activation in response to local environmental changes in the absence of T cells in Rag1−/− mice (Yang et al., 2016).
Figure 3.
Different Thymic Environments and Progenitor Cells Determine Preferential Generation of CCR10+ NK1.1+ ILC1s in Fetal/Neonatal Versus Adult Thymi
(A) Flow cytometric analysis of CD3−NK1.1+ thymocytes of adult Rag1−/−CCR10+/EGFP mice for CCR10(EGFP) and CD127 expression. The dot graph compares the percentages of CCR10+ subset of CD3−NK1.1+ thymocytes in Rag1−/−CCR10+/EGFP and CCR10+/EGFP controls. One dot represents one mouse.
(B) Flow cytometric analysis of CD3−NK1.1+ cells from the BM of adult Rag1−/−CCR10+/EGFP mice for their CCR10(EGFP) and CD127 expression.
(C) Comparison of percentages and numbers of the CCR10+CD127+ subset of CD3−NK1.1+ cells in the sLNs and skin of Rag1−/−CCR10+/EGFP mice and corresponding CCR10+/EGFP controls. One dot represents one mouse.
(D) Flow cytometric detection of CD3−NK1.1+ cells of gated CD45+ cells of fetal liver and adult BM donor progenitors in indicated tissues of the same recipient mice analyzed 2 months after transfer.
(E) Comparison of percentages of CD3−NK1.1+ cells and CD3+ T cells of fetal and adult donor-derived CD45+ cells in indicated tissues.
One dot represents one mouse. NS, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by two-tailed Student's t test.
To test whether fetal progenitor cells gave rise to skin-homing NK1.1+ ILC1s more efficiently than adult progenitor cells, we reconstituted irradiated adult WT mice with co-transfer of fetal liver and adult BM progenitor cells. Similar percentages of CD3−NK1.1+ cells of fetal and adult donor progenitors were found in the BM of recipients 2 months after reconstitution (Figures 3D and 3E), consistent with the previous findings that adult progenitor cells replace the fetal progenitor cells within 2–4 weeks after birth to become the dominant source of lymphocyte development in adults (Jotereau et al., 1987) (Yuan et al., 2012). As in adult WT mice, few CD3−NK1.1+ cells of the fetal or adult donor origin were found in the thymi of recipients (Figures 3D and 3E). However, in peripheral sites, CD3−NK1.1+ cells of fetal donor progenitors were found at much higher percentages in the skin, sLNs, and spleens of recipients than those of adult donor progenitors, whereas percentages of fetal donor-derived T cells were lower than those of adult donor-derived T cells (Figures 3D and 3E). Notably, the difference between percentages of fetal versus adult donor-derived CD3−NK1.1+ cells in the skin (∼5-fold) was significantly greater than that in sLNs (∼2.5-fold) or spleens (∼2-fold) (Figures 3D and 3E), suggesting that fetal progenitors were more effective in the generation of skin NK1.1+ ILCs than adult progenitors. Together, these results demonstrate that unique properties of thymic environments and progenitor cells both contribute to preferential generation of skin-homing NK1.1+ ILC1s at fetal/neonatal stages compared with adult stages.
PLZF Is Crucial for Thymic Generation of CCR10+ NK1.1+ ILC1s and Establishment of Skin NK1.1+ ILC1s at Early Postnatal Stages
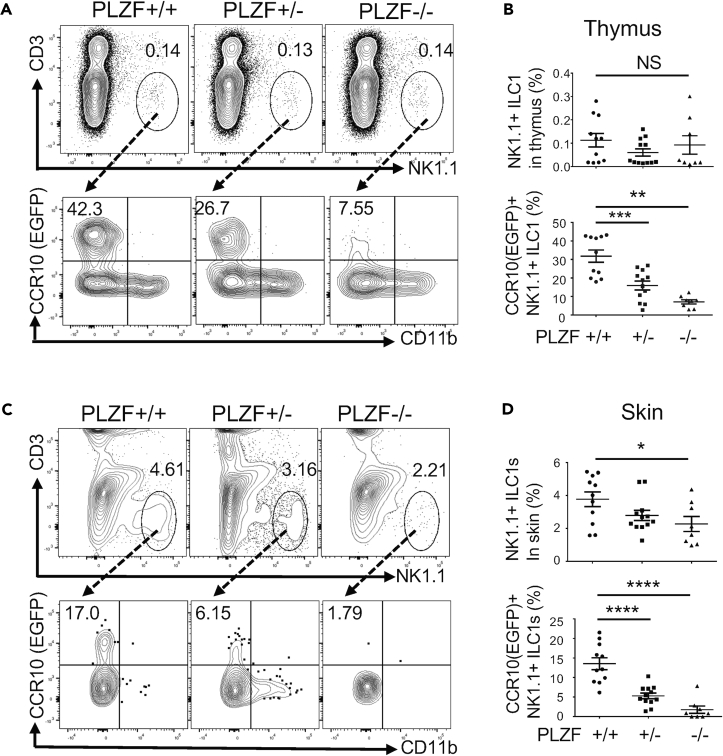
PLZF is a transcription factor highly expressed in CCR10+ thymic NK1.1+ ILCs (Figure 1E) and skin NK1.1+ ILCs at fetal/neonatal stages (Figure 2I). Previous studies found that the transient expression of PLZF distinguishes ILCs from NK and LTi cells (Constantinides et al., 2014, Constantinides et al., 2015). However, PLZF itself is not required for development of most ILCs (Constantinides et al., 2014, Constantinides et al., 2015). Similarly, PLZF is expressed in the fetal thymic precursors of skin-resident γδT cells, but is not critical for their development (Lu et al., 2015). On the other hand, PLZF is crucial for development of innate-like invariant NK αβT (iNKT) cells, mucosal-associated invariant αβT (MAIT) cells, and a subset of NKT-like γδT cells (Kovalovsky et al., 2008, Rahimpour et al., 2015, Savage et al., 2008). Continuous expression of PLZF in CCR10+ NK1.1+ thymic and skin ILC1s suggests that it might play a role in their development. To test this, we analyzed PZLF-knockout (KO) mice. The mice also carried a CCR10-KO/EGFP-knockin allele (CCR10+/EGFP) to report CCR10 expression with EGFP (Hu et al., 2011, Jin et al., 2010b). There were similar percentages of total CD3−NK1.1+ cells in the thymocytes of neonatal wild-type PLZF+/+, heterozygous PLZF+/−, and homozygous PLZF−/− KO mice (Figures 4A and 4B). However, within the thymic CD3−NK1.1+ population, there were greatly reduced percentages of CCR10(EGFP)+ NK1.1+ ILCs in PLZF−/− mice compared with PLZF+/+ controls. PLZF+/− mice also had lower percentages of CCR10(EGFP)+ NK1.1+ ILCs than PLZF+/+ controls (Figures 4A and 4B). These results demonstrate that PLZF is important for the thymic generation of CCR10+ NK1.1+ ILCs. Associated with this, there was significant reduction of total as well as CCR10+ NK1.1+ ILC1s in the skin of PLZF−/− mice compared with PLZF+/+ mice, whereas PLZF+/− mice also had modest reduction of the skin NK1.1+ ILC1s compared with PLZF+/+ mice (Figures 4C and 4D). These results demonstrate that PLZF is crucial for thymic generation of skin-homing CCR10+ NK1.1+ ILC1s and establishment of NK1.1+ ILC1s in the skin at early postnatal stages.
Figure 4.
PLZF Is Critical for Thymic Generation of Skin-Homing CCR10+ NK1.1+ ILCs and Establishment of Skin NK1.1+ ILCs in Neonatal Mice
(A) Flow cytometric detection of CD3−NK1.1+ thymocytes (top row) and their expression of CCR10(EGFP) and CD11b (bottom row) in neonatal PLZF+/+, PLZF+/−, and PLZF−/− mice. All mice carry a CCR10-KO/EGFP-knockin allele (CCR10+/EGFP) for purpose of reporting CCR10 expression with EGFP.
(B) Comparison of the percentages of CD3−NK1.1+ thymocytes (top) and percentages that express CCR10(EGFP) (bottom) in neonatal PLZF+/+, PLZF+/−, and PLZF−/− mice. One dot represents one mouse.
(C) Flow cytometric detection of CD3−NK1.1+ cells of gated CD45+ skin immune cells (top row) and their expression of CCR10(EGFP) and CD11b (bottom) in neonatal PLZF+/+, PLZF+/−, and PLZF−/− mice.
(D) Comparison of percentages of NK1.1+ ILCs of skin CD45+ cells and percentages of skin NK1.1+ ILCs that express CCR10(EGFP) in neonatal PLZF+/+, PLZF+/−, and PLZF−/− mice.
One dot represents one mouse. NS, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.001 as determined by two-tailed Student's t test.
Consistent with the finding that PLZF is not crucial for development of skin-resident γδT cells (Lu et al., 2015), there were similar percentages of CCR10(EGFP)+ thymic γδT cells in wild-type and PLZF-KO neonatal mice (Figure S4). Therefore, although skin-homing innate-like γδT cells and NK1.1+ ILCs are both generated in the fetal/neonatal thymus, they have different dependency on PLZF for their development.
CCR10 in Regulating Migration and Skin Establishment of NK1.1+ ILC1s
We previously found that although most skin lymphocytes (αβT, γδT, and ILCs) express CCR10, different subsets have different degrees of dependency on CCR10 for their establishment in the skin, with Treg and CD8+ T cells significantly affected and γδT17 cells unaffected by CCR10-KO (Cai et al., 2014, Jin et al., 2010b, Xia et al., 2014, Yang et al., 2016). We therefore also tested whether CCR10 was required for skin localization of NK1.1+ ILCs. In an in vitro migration assay, EGFP+ CD3−NK1.1+ cells of heterozygous CCR10+/EGFP (CCR10+/−) mice migrated efficiently toward CCL27, whereas EGFP+ CD3−NK1.1+ cells of homozygous CCR10EGFP/EGFP (CCR10−/−) KO mice did not (Figure S5A), indicating that CCR10 is the critical receptor for migration of CCR10+ NK1.1+ ILCs to the skin-specific ligand CCL27. However, there was no significant difference in percentages of EGFP+ NK1.1+ ILC1s in the skin of CCR10+/EGFP and CCR10EGFP/EGFP mice at either neonatal or adult stage (Figures S5B and S5C), suggesting that other homing molecules might compensate for loss of CCR10 in directing these cells to the skin in CCR10-KO mice.
Important Contribution of Fetal/Neonatal-Derived NK1.1+ ILC1s to the Skin Immune System
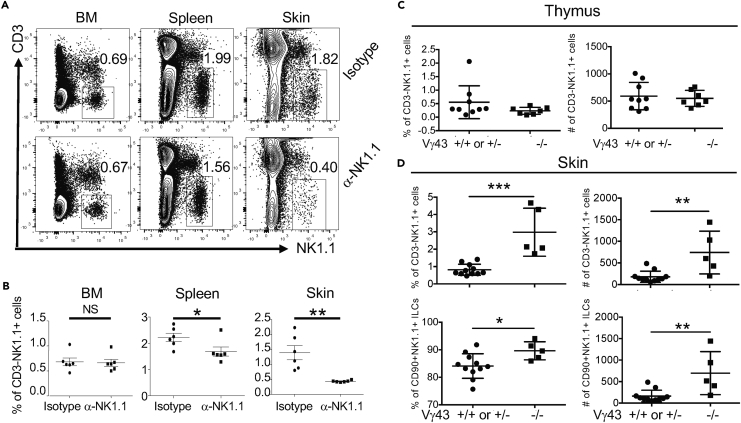
Fetal/neonatal thymus-derived innate-like γδT cells contribute to a significant fraction of the skin immune cell compartment of mice until adulthood (Havran and Allison, 1990) (Sumaria et al., 2011) (Gray et al., 2011) (Cai et al., 2011, Cai et al., 2014, Sandrock et al., 2018). As NK1.1+ ILCs were generated at the same fetal/neonatal ontogenic stages as innate-like γδT cells, we assessed whether fetal/neonatal-derived NK1.1+ ILC1s also had a long-lasting contribution to the establishment of the skin immune system. For this purpose, we injected pregnant mice with anti-NK1.1 antibodies to deplete NK1.1+ ILC1s in the developing fetuses (Figure S6A). Although the anti-NK1.1 antibody injection could also deplete other NK1.1-expressing cells, the depletion is mostly specific for NK1.1+ ILCs because very few other NK1.1-expressing lymphocytes exist in the skin of neonatal mice (Figure 1A). Six weeks after birth, the in utero antibody-treated mice were analyzed for NK1.1+ ILC1s in the skin. Markedly, the in utero anti-NK1.1 antibody-treated mice had significantly (2- to 3-fold) reduced percentages and numbers of NK1.1+ ILCs in the skin compared with isotype control antibody-treated mice (Figures 5A, 5B, and S6B). In contrast, the in utero NK1.1 antibody-treated mice had similar or only slightly reduced percentages of CD3−NK1.1+ cells in the BM and spleens compared with the controls (Figures 5A and 5B). The in utero NK1.1 antibody-treated mice also had similar percentages of NK1.1+ T cells in the BM, spleen, and skin as isotype control antibody-treated mice (Figures S6C and S6D), indicating that the in utero anti-NK1.1 antibody treatment did not significantly affect establishment of NK1.1-expressing T cells in the skin. These results demonstrate that like skin-resident innate-like γδT cells, skin NK1.1+ ILC1s are also predominantly derived from fetal/neonatal stages and their in utero depletion resulted in defective skin NK1.1+ ILCs until adulthood.
Figure 5.
Fetal/Neonatal NK1.1+ ILCs Significantly Contribute to the Establishment of the Skin Immune System
(A) Flow cytometric detection of CD3−NK1.1+ cells in gated CD45+ lymphocytes of the BM, spleen, and skin of CCR10+/EGFP mice treated in utero with anti-NK1.1 or isotype-matched control antibodies. Mice were analyzed 6 weeks after birth.
(B) Comparison of the average percentages of CD3−NK1.1+ cells in BM, spleen, and skin of in utero anti-NK1.1 and control antibody-treated mice. One dot represents one mouse. Data are pooled from two independent experiments as performed in (A).
(C) Comparison of the percentages (left) and numbers (right) of total CD3−NK1.1+ cells in thymi of 2- to 3-day-old WT (+/+) or Vγ43+/− versus Vγ43−/− mice. One dot represents one mouse. Data are combined from two litters.
(D) Comparison of the percentages and numbers of total and CD90.2+ CD3−NK1.1+CD45+CD11b− ILCs in the skin of 10-day-old WT (+/+) or Vγ43+/− versus Vγ43−/− mice.
One dot represents one mouse. NS, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by unpaired two-tailed Student's t test. Error bars denote mean ± SD, and data are combined from five separate litters.
Perinatally derived innate-like γδT cells migrate to the skin to help promote local homeostasis (Jameson and Havran, 2007, Nielsen et al., 2017). The preferential establishment of NK1.1+ ILCs in the skin at perinatal stages suggests that they might coordinate with innate-like γδT cells to provide early skin immune protection. To further understand this notion, we analyzed NK1.1+ ILCs in the skin of Vγ43-KO (Vγ43−/−) mice that are devoid of Vγ4+ and Vγ3+ γδT cells, the two major populations of skin innate-like γδT cells that are generated at fetal stages (Xiong et al., 2008). Newborn Vγ43−/− mice had similar percentages of NK1.1+ ILCs as WT or heterozygous Vγ43+/− littermates in thymi, suggesting that lack of Vγ4/3+ γδT cells did not alter thymic generation of NK1.1+ ILCs (Figure 5C). In striking contrast, there was a significantly increased frequency and number of NK1.1+ ILCs in the skin of neonatal Vγ43−/− mice compared with corresponding WT or heterozygous controls (Figure 5D), suggesting a compensatory expansion of NK1.1+ ILCs in the skin in the absence of Vγ4/3+ γδT cells, likely in response to local environmental stimulation to help local homeostasis.
NK1.1+ ILC1s Actively Respond to the Skin Commensal Bacterial Colonization at the Neonatal Period
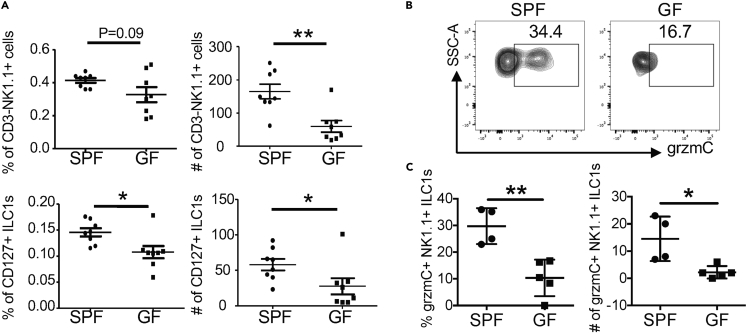
The skin is immediately colonized by commensal bacteria after birth (Capone et al., 2011). The preferential enrichment and increased expansion of NK1.1+ ILC1s in the skin of neonatal WT and Vγ43−/− mice raised the possibility that they might respond to the early commensal bacterial colonization to help local homeostasis. To test this, we compared NK1.1+ ILCs in the skin of neonatal mice raised in specific pathogen-free (SPF) versus germ-free (GF) conditions. Notably, neonatal mice raised in SPF conditions had a significantly higher percentage and number of NK1.1+ ILCs in the skin than age-matched mice raised in GF conditions (Figure 6A), suggesting that skin NK1.1+ ILCs increased in response to commensal bacterial colonization. Furthermore, compared with skin NK1.1+ ILCs of neonatal mice raised in GF conditions, a significantly higher percentage and number of skin NK1.1+ ILCs of littermate mice cross-fostered in SPF conditions expressed high levels of grzmC (Figures 6B and 6C), an effector molecule associated with activated ILC1s (Daussy et al., 2014, Robinette et al., 2015) (Gury-BenAri et al., 2016). These results demonstrate that skin NK1.1+ ILCs actively respond to early commensal bacterial colonization in neonatal mice.
Figure 6.
Skin NK1.1+ ILC1s Actively Respond to Bacterial Colonization at Early Postnatal Stages
(A) Comparison of percentages and numbers of total and CD127+ CD3−NK1.1+CD45+ lymphocytes of the skin of 10-day-old WT mice raised in SPF versus GF conditions.
(B) Flow cytometric analysis of skin NK1.1+ ILCs of 9-day-old WT neonatal mice raised in SPF versus GF conditions for granzyme C (grzmC) expression. NK1.1+ ILCs are gated on CD45+CD3−NK1.1+CD90+ lymphocytes.
(C) Comparison of average percentages and numbers of grzmC-expressing NK1.1+ ILCs in the skin of neonatal mice raised in the SPF versus GF conditions.
One dot represents one mouse. Data are combined from two experiments. ∗p < 0.05, ∗∗p < 0.01 as determined by Student's unpaired two-tailed t test.
NK1.1+ ILC1s Play an Important Role in Regulation of Early Commensal Bacterial Colonization of the Skin at the Neonatal Stage
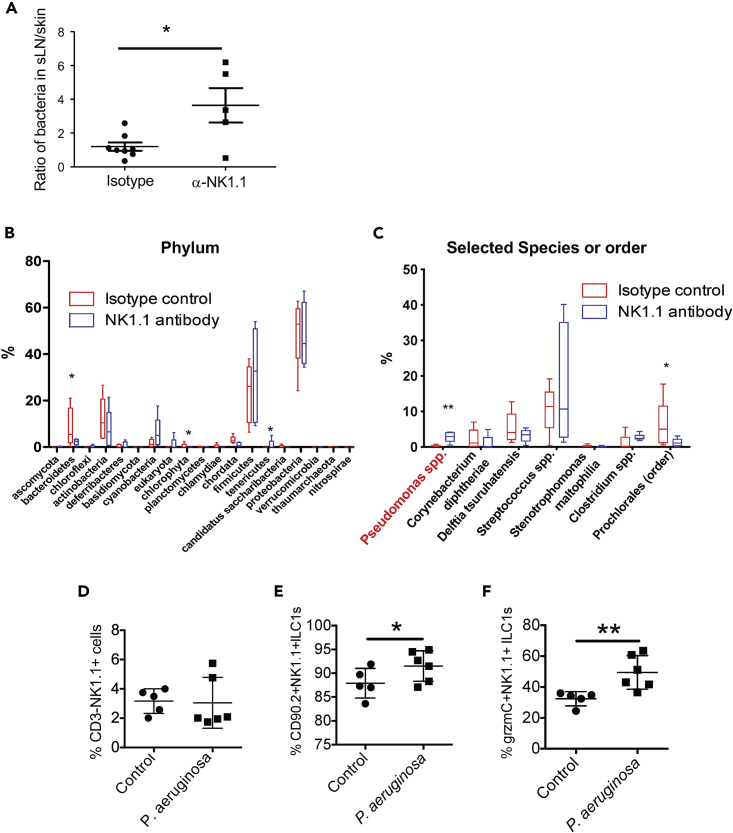
To demonstrate a direct role of NK1.1+ ILCs in the regulation of commensal bacteria, we tested whether in utero depletion of NK1.1+ cells would result in increased infiltration of commensal bacteria into the deeper tissue such as sLNs (Shen et al., 2014). Pregnant mice were treated with anti-NK1.1 or isotype control antibodies. Ten days after birth, bacteria in the skin and sLNs of the treated mice were assessed by the quantitative PCR analysis of bacterial 16S rRNA gene levels in genomic DNA isolated from the skin and sLNs. Although there was no statistical difference in the levels of bacteria in the skin or sLNs of anti-NK1.1 versus control antibody-treated mice (Figure S7A), anti-NK1.1 antibody-treated mice had a significantly increased ratio of bacteria in the sLNs relative to the skin compared with control-treated mice (Figure 7A), suggesting that skin NK1.1+ ILC1s might be important for barrier defense.
Figure 7.
NK1.1+ ILC1s Play an Important Role in Regulating Proper Bacterial Colonization in the Skin at Early Postnatal Stages
(A) Comparison of relative levels of commensal bacteria in sLNs versus skin of in utero anti-NK1.1 and isotype control antibody-treated WT 9-day-old mice. ∗p < 0.05 as determined by unpaired two-tailed Student's t test.
(B) Comparison of bacterial compositions at the phylum level in the skin of in utero anti-NK1.1 antibody versus isotype control antibody-treated 10-day-old mice. N = 5 each of anti-NK1.1 and control antibody-treated mice.
(C) Comparison of selected bacterial compositions in the skin of in utero anti-NK1.1 antibody versus isotype control antibody-treated mice. ∗p < 0.05, ∗∗p < 0.01 in the (B and C) as determined by Kruskal-Wallis test.
(D–F) Comparison of the total number (D) and frequency (E) of skin NK1.1+ ILC1s and their expression of grzmC (F) in neonatal mice topically applied with P. aeruginosa or PBS (control) on the skin.
One dot represents one mouse. Error bars denote mean ± SD, and data are combined from two experiments. ∗p < 0.05, ∗∗p < 0.01 as determined by Student's unpaired, two-tailed t test.
We then performed metagenomic analysis to determine how in utero depletion of NK1.1+ ILCs affected the bacterial composition in the skin of newborn mice. For this, total genomic DNA isolated from the skin of the in utero antibody-treated 10-day-old neonates was analyzed by deep sequencing of the PCR products of the bacterial 16S rRNA genes. The composition of commensal bacteria in the skin of anti-NK1.1 and control antibody-treated mice was compared based on the 16S rRNA gene sequencing analysis. Bacteria of the Proteobacteria, Fermicutes, Actinobacteria, and Bacteroidetes phyla dominated the skin of control newborn mice (Figure S7B), similar to that reported in the skin of adult mice (Naik et al., 2015). Compared with the control, the skin of in utero anti-NK1.1 antibody-treated mice had significantly decreased bacteria belonging to the phylum Bacteroidetes, whereas the phylum Tenericutes significantly increased (Figure 7B). Further analysis found that bacteria of the Pseudomonas genus and the Mollicutes class were most significantly increased in the skin of anti-NK1.1 antibody-treated mice compared with the controls (Figures 7C and S7C). These results demonstrate that NK1.1+ ILCs regulate homeostatic colonization of commensal bacteria, such as Pseudomonas, in the skin at the neonatal stage.
Pseudomonas aeruginosa Is a Major Species of Bacteria that Activates Skin NK1.1+ ILC1s
Among different species of Pseudomonas, P. aeruginosa is most clinically relevant. P. aeruginosa is commonly present on the skin and mucosal sites such as the nostril and gut of immunocompetent individuals as commensals (Cogen et al., 2008). The newborn may acquire P. aeruginosa from the mother through birth, but is capable of controlling their homeostatic colonization. As commensals, P. aeruginosa may have beneficial effects on hosts by inhibiting pathogenic microorganisms. However, P. aeruginosa could become a pathogen in immunocompromised hosts (Cogen et al., 2008, Lyczak et al., 2000), suggesting that the immune system is crucial to maintain homeostasis of the bacteria and hosts. Considering the clinical importance of P. aeruginosa, we directly tested whether NK1.1+ ILC1s responded to P. aeruginosa inoculation on the skin of newborn mice. Neonatal littermates were topically applied with P. aeruginosa or PBS (control) on the skin for 2 consecutive days and were analyzed 5 days later. Notably, although the percentage and number of NK1.1+ ILC1s were unaltered or only slightly increased in the skin that was topically applied with P. aeruginosa compared with the PBS-applied control (Figures 7D and 7E), the percentage of grzmC-expressing NK1.1+ ILC1s in the P. aeruginosa-inoculated skin was significantly higher than that in the PBS-treated controls (Figure 7F). These results indicate that NK1.1+ ILCs actively respond to the P. aeruginosa colonization and could be potentially involved in regulation of the bacteria in the skin at neonatal stages.
Discussion
The skin is exposed to commensal bacteria and other environment insults immediately after birth. How skin-resident immune cells are established at early postnatal stages to protect the skin and maintain the local homeostasis is not well understood. Here, we found that perinatal thymus-derived NK1.1+ ILC1s preferentially acquire skin-homing properties and contribute to regulation of commensal bacterial colonization in the skin of newborn mice, revealing differing mechanisms of development and function of skin-resident ILCs at early postnatal stages than at adult stages when sLN-generated skin-homing NK1.1- ILC2/3s contribute to their dominance in the skin (Yang et al., 2016).
CD3−NK1.1+ cells were found in the thymi of both fetal and adult mice long ago and were considered as a population of NK cells (Carlyle et al., 1998, Vosshenrich et al., 2006). The thymic NK cells can be found as early as day 13 of gestation in mice (Carlyle et al., 1998). Until recently, it was not clear whether fetal thymic NK cells are a distinct population of innate lymphocytes. Studies of adult thymic NK cells found that they are different from cNK cells because thymic NK cells depend on GATA3 and IL-7 for their development (Vosshenrich et al., 2006). Thymic NK cells also express Eomes and develop independent of T-bet (Gabrielli et al., 2017). Unlike cNK cells, thymic NK cells do not express CD11b, whereas they both express DX5 and NKG2D. When stimulated with PMA/ionomycin, thymic NK cells express higher IFN-γ and TNF, but lower grzmB than cNK cells (Vosshenrich et al., 2006). Based on these features, adult thymic NK cells are closely related to the CCR10+ fetal/neonatal thymic NK1.1+ ILCs we describe herein. However, because CCR10+ fetal/neonatal thymic NK1.1+ ILCs depend on PLZF for their development, we conclude that they are ILC1s, but not NK cells (Constantinides et al., 2014, Constantinides et al., 2015). Additional studies are needed to establish their relationship with adult thymic NK cells. Interestingly, whereas few thymic NK cells of adult mice express CCR10, the majority of thymic NK1.1+ cells of adult Rag1−/− mice expressed CCR10, indicating that they may be more related to fetal/neonatal CCR10+ ILC1s. However, there are some differences between CCR10+ NK1.1+ thymocytes of adult Rag1−/− mice and fetal/neonatal NK1.1+ ILC1s. Most CCR10+ fetal/neonatal NK1.1+ ILC1s express B220, but not NKp46, whereas the adult CCR10+ NK1.1+ thymocytes express NKp46, but low B220. Interestingly, CCR10+ NK1.1+ cells in sLNs of adult Rag1−/− mice highly express B220. As thymic NK cells are suggested to preferentially export into peripheral LNs, including sLNs (Vosshenrich et al., 2006), it is possible that the CCR10+ adult thymic NK cells could differentiate further in the sLN environments before their localization into the skin.
It is notable that fetal/neonatal thymus preferentially supports the generation of skin-homing ILCs and innate-like γδT cells. However, molecular mechanisms involved in their generation are not identical. We previously reported that T cell receptor-derived signals are required for the upregulation of CCR10 on skin-homing innate-like γδT cells in the thymi (Jin et al., 2010a, Jin et al., 2010b, Xiong et al., 2004). However, the molecular mechanisms involved in the thymus-specific generation of CCR10+ NK1.1+ ILC1s have yet to be defined. Considering that thymic, but not BM, environments support the generation of skin-homing NK1.1+ ILCs, it is plausible to hypothesize that thymic stroma-specific molecular signals are required for the generation of skin-homing NK1.1+ ILCs. Possible candidates include Notch ligands that are known to be highly expressed in thymic, but not BM, stromal cells (Bajoghli et al., 2009, Itoi et al., 2007, Nowell et al., 2011, Tsukamoto et al., 2005, Xiao et al., 2008). Our finding that developing T cells suppress the generation of CCR10+ NK1.1+ ILC1s in adult thymi is consistent with the notion that a Notch/ligand signal may be involved in the preferential generation of skin-homing CCR10+ NK1.1+ ILC1s in fetal and neonatal thymi where fewer developing T cells compete for interaction with Notch ligands expressed on thymic stromal cells. Whether and how different Notch ligands and receptors are involved in the thymic generation of skin-homing NK1.1+ ILC1s remains to be determined. In addition, PLZF is critical for the thymic generation of CCR10+ NK1.1+ ILCs, but not CCR10+ γδT cells, also suggesting different mechanisms for their development.
While the unique thymic environment supports generation of skin-homing NK1.1+ ILC1s at perinatal stages, our previous studies found that sLNs also support generation of skin-homing ILCs in adult mice (Yang et al., 2016). However, sLNs in adult mice preferentially support generation of skin-homing ILC2/3s and may not be a major site for the generation of skin-resident NK1.1+ ILCs. Consistent with this notion, depletion of NK1.1+ ILCs in utero, when the sLNs are not well developed or functional, severely impaired the establishment of skin NK1.1+ ILCs until adulthood, demonstrating that most NK1.1+ ILCs in the skin originate at perinatal stages. However, the exact contribution of fetal/neonatal thymus-derived skin-homing NK1.1+ ILCs to the total skin NK1.1+ ILC repertoire is not clear. Many NK1.1+ ILCs of the neonatal skin do not express CCR10. Although it is possible that CCR10+ ILCs differentiate into CCR10- ILCs upon activation in the skin (Yang et al., 2016), it could not be ruled out that CCR10- NK1.1+ ILCs originate from different developmental processes and/or origins. The relationship of CCR10+ and CCR10- NK1.1+ skin ILCs needs further investigation.
The enrichment of NK1.1+ ILC1s in the skin at early postnatal stages is important in the regulation of commensal bacterial colonization. Compared with skin NK1.1+ ILC1s of neonatal mice raised in GF conditions, skin NK1.1+ ILC1s of neonatal mice raised in SPF conditions are more active, indicated by increased grzmC expression. Skin NK1.1+ ILC1s also had increased expression of grzmC in response to over-population with Pseudomonas aeruginosa. The increased grzmC expression by activated ILC1s in regulation of bacterial colonization has yet to be determined. Potentially, the molecule could help regulate proper bacterial colonization by killing bacterium-infected cells or targeting bacteria directly (Cai et al., 2009, Dotiwala et al., 2017, Getachew et al., 2008). In addition, cytokines produced by NK1.1+ ILC1s, such as IFN-γ, could stimulate other immune and non-immune cells for the production of antimicrobial molecules. Interestingly, activated NK1.1+ ILC1s express grzmC but not grzmB, indicating that they use different effector mechanisms to protect the host than NK cells, which preferentially express grzmB over grzmC (Cai et al., 2009). The differential expression of granzymes in ILC1s and NK cells might be relevant to their different, but compensatory, functions. Functional compensation of NK1.1+ ILC1s and innate-like γδT cells was also identified in our current study, as NK1.1+ ILC1s expanded in the skin of neonatal mice deficient in Vγ3/Vγ4+ γδT cells. Mechanisms of functional compensation and coordination of different innate or innate-like lymphocytes in the skin homeostatic regulation require further investigation.
Early immune cell development plays a critical role in the establishment of long-term immune homeostasis. Disturbance of perinatal immune cell development has lasting effects on the health of individuals and could be a causative factor in the development of autoimmune diseases in adults (Bjorksten, 2009). We found that perinatally derived NK1.1+ ILCs have long-lasting contribution to the skin immune cell repertoire until adulthood. However, this population steadily decreases with increase in age. By age 3 months, NK1.1+ ILCs account for a very minor population (<1%) of total lymphocytes in the skin. These suggest that the perinatal generation of NK1.1+ ILC1s represents a temporally regulated developmental mechanism to provide rapid innate immune protection of the skin at the early postnatal stage, when adaptive immune cells are not well developed. In the adult skin, NK1.1+ ILC1s may be less required likely because adaptive T cells could functionally substitute for NK1.1+ ILC1s. Consistent with this notion, there are increased NK1.1+ ILC1s in the skin of adult Rag1−/− mice, which lack adaptive immune T/B cells.
Limitations of the Study
The functional importance and mechanisms of skin-resident NK1.1+ ILC1s in helping establishment of the skin homeostasis throughout early postnatal stages require further investigation. In our experiments of using anti-NK1.1 antibodies to deplete NK1.1+ ILC1s to study their roles in regulating the local tissue homeostasis and commensal bacterial colonization in the skin of neonatal mice, the antibody treatment is not completely specific to NK1.1+ ILC1s because it could target the other NK1.1-expressing cells such as cNK and iNKT cells. Therefore, although NK1.1+ ILC1s account for the majority of NK1.1-expressing immune cells in the neonatal skin, it could not be ruled out that the other NK1.1-expressing immune cells also contributed to the increased Pseudomonas species observed in the anti-NK1.1 antibody-treated wild-type mice. Additional studies are needed to confirm that NK1.1+ ILC1s play a crucial role in regulating the skin colonization or infection of P. aeruginosa. Potentially, these conclusions could be strengthened if specific ILC1-deficient mice have increased P. aeruginosa colonization. However, currently there is no specific method to deplete only ILC1s from mice for the study. Molecular mechanisms underlying activation and function in response to the commensal colonization are also yet to be determined.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Research reported in this publication was partly supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis, Musculoskeletal and Skin Diseases and the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Numbers U01AI131393 and R01AR064831 (to N.X.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Stefan Muljo and Joan Yuan for comments and suggestions on the manuscript and the staff of Microscopy and Cytometry Facilities of the Penn State University and University of Texas Health Science Center at San Antonio for excellent technical support.
Author Contributions
J.Y., K.H.R., M.X., E.H.S., L.Z., and S.H., P.L., and W.-B.W. performed experiments. N.X., J.Y., K.H.R., and M.X. designed the study. J.Y., K.H.R., M.X., E.H.S., and N.X analyzed the data. N.X., J.Y., K.H.R., and M.X. wrote the manuscript. All approved the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101014.
Data and Code Availability
The data will be deposited into the ImmPort (www.Immport.org) for public access.
Supplemental Information
References
- Bajoghli B., Aghaallaei N., Hess I., Rode I., Netuschil N., Tay B.H., Venkatesh B., Yu J.K., Kaltenbach S.L., Holland N.D. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Bjorksten B. Disease outcomes as a consequence of environmental influences on the development of the immune system. Curr. Opin. Allergy Clin. Immunol. 2009;9:185–189. doi: 10.1097/ACI.0b013e32832abfc2. [DOI] [PubMed] [Google Scholar]
- Cai S.F., Fehniger T.A., Cao X., Mayer J.C., Brune J.D., French A.R., Ley T.J. Differential expression of granzyme B and C in murine cytotoxic lymphocytes. J. Immunol. 2009;182:6287–6297. doi: 10.4049/jimmunol.0804333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V.R., Zhang H.G., Wang T., Zheng J. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Xue F., Fleming C., Yang J., Ding C., Ma Y., Liu M., Zhang H.G., Zheng J., Xiong N. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat. Commun. 2014;5:3986. doi: 10.1038/ncomms4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone K.A., Dowd S.E., Stamatas G.N., Nikolovski J. Diversity of the human skin microbiome early in life. J. Invest. Dermatol. 2011;131:2026–2032. doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle J.R., Michie A.M., Cho S.K., Zuniga-Pflucker J.C. Natural killer cell development and function precede alpha beta T cell differentiation in mouse fetal thymic ontogeny. J. Immunol. 1998;160:744–753. [PubMed] [Google Scholar]
- Cherrier M., Sawa S., Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J. Exp. Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen A.L., Nizet V., Gallo R.L. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., Gudjonson H., McDonald B.D., Ishizuka I.E., Verhoef P.A., Dinner A.R., Bendelac A. PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl. Acad. Sci. U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., McDonald B.D., Verhoef P.A., Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daussy C., Faure F., Mayol K., Viel S., Gasteiger G., Charrier E., Bienvenu J., Henry T., Debien E., Hasan U.A. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotiwala F., Sen Santara S., Binker-Cosen A.A., Li B., Chandrasekaran S., Lieberman J. Granzyme B disrupts central metabolism and protein synthesis in bacteria to promote an immune cell death program. Cell. 2017;171:1125–1137.e11. doi: 10.1016/j.cell.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert L.M., Meuter S., Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J. Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- Fuchs A., Vermi W., Lee J.S., Lonardi S., Gilfillan S., Newberry R.D., Cella M., Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli S., Sun M., Bell A., Zook E.C., de Pooter R.F., Zamai L., Kee B.L. Murine thymic NK cells are distinct from ILC1s and have unique transcription factor requirements. Eur. J. Immunol. 2017;47:800–805. doi: 10.1002/eji.201646871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew Y., Stout-Delgado H., Miller B.C., Thiele D.L. Granzyme C supports efficient CTL-mediated killing late in primary alloimmune responses. J. Immunol. 2008;181:7810–7817. doi: 10.4049/jimmunol.181.11.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M., Glusac E., Filler R.B., Roberts S.J., Propperova I., Lewis J., Tigelaar R.E., Hayday A.C. The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. J. Exp. Med. 2003;198:747–755. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M., Oppenheim D.E., Steele C.R., Lewis J.M., Glusac E., Filler R., Hobby P., Sutton B., Tigelaar R.E., Hayday A.C. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Gray E.E., Suzuki K., Cyster J.G. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gury-BenAri M., Thaiss C.A., Serafini N., Winter D.R., Giladi A., Lara-Astiaso D., Levy M., Salame T.M., Weiner A., David E. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166:1231–1246.e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Haas J.D., Ravens S., Duber S., Sandrock I., Oberdorfer L., Kashani E., Chennupati V., Fohse L., Naumann R., Weiss S. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Havran W.L., Allison J.P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- Hayday A.C. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Hu S., Yang K., Yang J., Li M., Xiong N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc. Natl. Acad. Sci. U S A. 2011;108:E1035–E1044. doi: 10.1073/pnas.1100156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Yasuda K., Sakaguchi Y., Haneda T., Mizutani H., Yoshimoto T., Nakanishi K., Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi M., Tsukamoto N., Yoshida H., Amagai T. Mesenchymal cells are required for functional development of thymic epithelial cells. Int. Immunol. 2007;19:953–964. doi: 10.1093/intimm/dxm060. [DOI] [PubMed] [Google Scholar]
- Jameson J., Havran W.L. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol. Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W.L. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Jin Y., Xia M., Saylor C.M., Narayan K., Kang J., Wiest D.L., Wang Y., Xiong N. Cutting edge: intrinsic programming of thymic gammadeltaT cells for specific peripheral tissue localization. J. Immunol. 2010;185:7156–7160. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Xia M., Sun A., Saylor C.M., Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J. Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J. Immunol. 1987;138:1026–1030. [PubMed] [Google Scholar]
- Kim B.S., Siracusa M.C., Saenz S.A., Noti M., Monticelli L.A., Sonnenberg G.F., Hepworth M.R., Van Voorhees A.S., Comeau M.R., Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C.S.N., Flach M., Mohle L., Rogell L., Hoyler T., Ebert K., Fabiunke C., Pfeifer D., Sexl V., Fonseca-Pereira D. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Voisin B., Kim D.Y., Kennedy E.A., Jo J.H., Shih H.Y., Truong A., Doebel T., Sakamoto K., Cui C.Y. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–997.e16. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D., Uche O.U., Eladad S., Hobbs R.M., Yi W., Alonzo E., Chua K., Eidson M., Kim H.J., Im J.S. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Cao X., Zhang X., Kovalovsky D. PLZF controls the development of fetal-derived IL-17+Vgamma6+ gammadelta T cells. J. Immunol. 2015;195:4273–4281. doi: 10.4049/jimmunol.1500939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C., Reynders A., Ivanov I.I., Cognet C., Chiche L., Chasson L., Hardwigsen J., Anguiano E., Banchereau J., Chaussabel D. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Lyczak J.B., Cannon C.L., Pier G.B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Naik S., Bouladoux N., Linehan J.L., Han S.J., Harrison O.J., Wilhelm C., Conlan S., Himmelfarb S., Byrd A.L., Deming C. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.M., Witherden D.A., Havran W.L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell C.S., Bredenkamp N., Tetelin S., Jin X., Tischner C., Vaidya H., Sheridan J.M., Stenhouse F.H., Heussen R., Smith A.J. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi: 10.1371/journal.pgen.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S., Haak S., Ingold B., Kulig P., Heppner F.L., Navarini A.A., Becher B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B., Meehan B., Chen Z., Whittle B., Liu L., Fairlie D.P. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette M.L., Fuchs A., Cortez V.S., Lee J.S., Wang Y., Durum S.K., Gilfillan S., Colonna M., Immunological Genome C. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B., Kyle R., Yip K.H., Sumaria N., Guy T.V., Kim B.S., Mitchell A.J., Tay S.S., Jain R., Forbes-Blom E. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X., Huang L.C., Johnson D., Scanlon S.T., McKenzie A.N. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock I., Reinhardt A., Ravens S., Binz C., Wilharm A., Martins J., Oberdorfer L., Tan L., Lienenklaus S., Zhang B. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing gammadelta T cells. J. Exp. Med. 2018;215:3006–3018. doi: 10.1084/jem.20181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C., Belz G.T., Huntington N.D. Development, homeostasis, and heterogeneity of NK cells and ILC1. Curr. Top Microbiol. Immunol. 2016;395:37–61. doi: 10.1007/82_2015_474. [DOI] [PubMed] [Google Scholar]
- Shen W., Li W., Hixon J.A., Bouladoux N., Belkaid Y., Dzutzev A., Durum S.K. Adaptive immunity to murine skin commensals. Proc. Natl. Acad. Sci. U S A. 2014;111:E2977–E2986. doi: 10.1073/pnas.1401820111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka D.K., Plougastel-Douglas B., Yang L., Pak-Wittel M.A., Artyomov M.N., Ivanova Y., Zhong C., Chase J.M., Rothman P.B., Yu J. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Sumaria N., Roediger B., Ng L.G., Qin J., Pinto R., Cavanagh L.L., Shklovskaya E., Fazekas de St Groth B., Triccas J.A., Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N., Itoi M., Nishikawa M., Amagai T. Lack of Delta like 1 and 4 expressions in nude thymus anlages. Cell Immunol. 2005;234:77–80. doi: 10.1016/j.cellimm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E. Innate lymphoid cells: 10 Years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Vosshenrich C.A., Garcia-Ojeda M.E., Samson-Villeger S.I., Pasqualetto V., Enault L., Richard-Le Goff O., Corcuff E., Guy-Grand D., Rocha B., Cumano A. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Xia M., Hu S., Fu Y., Jin W., Yi Q., Matsui Y., Yang J., McDowell M.A., Sarkar S., Kalia V. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J. Allergy Clin. Immunol. 2014;134:634–644 e610. doi: 10.1016/j.jaci.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Su D.M., Manley N.R. T cell development from kit-negative progenitors in the Foxn1Delta/Delta mutant thymus. J. Immunol. 2008;180:914–921. doi: 10.4049/jimmunol.180.2.914. [DOI] [PubMed] [Google Scholar]
- Xiong N., Kang C., Raulet D.H. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Xiong N., Raulet D.H. Development and selection of gammadelta T cells. Immunol. Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- Xiong N., Zhang L., Kang C., Raulet D.H. Gene placement and competition control T cell receptor gamma variable region gene rearrangement. J. Exp. Med. 2008;205:929–938. doi: 10.1084/jem.20071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hu S., Zhao L., Kaplan D.H., Perdew G.H., Xiong N. Selective programming of CCR10(+) innate lymphoid cells in skin-draining lymph nodes for cutaneous homeostatic regulation. Nat. Immunol. 2016;17:48–56. doi: 10.1038/ni.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhao L., Xu M., Xiong N. Establishment and function of tissue-resident innate lymphoid cells in the skin. Protein Cell. 2017;8:489–500. doi: 10.1007/s13238-017-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Nguyen C.K., Liu X., Kanellopoulou C., Muljo S.A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be deposited into the ImmPort (www.Immport.org) for public access.