SUMMARY
Longitudinal studies that have examined the association of insomnia with incident depression using objective sleep measures are very limited. The aim of this study was to examine the predictive role of the severity of insomnia for incident depression in a general population sample using psychometric and polysomnographic data. From a random, general population sample of 1741 individuals of the Penn State Adult Cohort, 1137 adults without depression were followed up with a structured telephone interview after 7.5 years. All subjects completed a full medical evaluation, 1-night polysomnogram and Multiphasic Minnesota Personality Inventory at baseline. The incidence of depression was 15%. Poor sleep (odds ratio = 1.5, P = 0.001) and insomnia (odds ratio = 1.9, P = 0.031) were significantly associated with incident depression. The odds of incident depression were highest (odds ratio = 2.2, P = 0.019) in insomnia with objective short sleep duration and independent of Multiphasic Minnesota Personality Inventory Ego Strength scores, an index of poor coping resources. The persistence of insomnia and worsening of poor sleep into insomnia significantly increased the odds of incident depression (odds ratios ranged from 1.8 to 6.3), whereas their full remission did not (odds ratio ranged from 1.2 to 1.8). Insomnia with short sleep duration is associated with incident depression independent of poor coping resources, whereas the association of insomnia with normal sleep duration with incident depression was mediated by poor coping resources. Persistence and worsening of poor sleep or insomnia, but n0ot their full remission, are significant predictors of incident depression. These data suggest that there is a significant relationship between the severity of insomnia and incident depression.
Keywords: depression, longitudinal, insomnia, severity, polysomnography
INTRODUCTION
Depression is a mental disorder associated with significant morbidity and mortality (Gadermann et al., 2012). The lifetime prevalence of depression in the general population is about 16–19% (Kessler et al., 2003), and longitudinal population-based studies have estimated that its incidence is about 10–15% (Murphy et al., 2002), which makes it a growing major public health concern in need of better prevention. It is, thus, essential to better define potential factors preceding the development of depression. Sleep disorders, and particularly insomnia, have been associated with depression in clinical and population studies (Baglioni et al., 2011; Harris et al., 2009). However, longitudinal population-based studies on incident depression that have included objective sleep data are very limited.
A recent meta-analysis of longitudinal studies reported that individuals with insomnia have twofold odds of incident depression, but pointed out a significant degree of heterogeneity and limitations across studies (Baglioni et al., 2011). For example, based on the available studies the authors could not evaluate whether chronic insomnia, which affects about 8–10% of the population (Bixler et al., 2002; Ohayon, 2002), had a greater association with incident depression as compared with the experience of ‘poor sleep’, which affects about 20–30% of the population (Bixler et al., 2002; Ohayon, 2002). Furthermore, Baglioni et al. (2011) noted that few studies considered the role of potential confounders, such as psychiatric (e.g. alcohol and drug abuse; Kessler et al., 2003), or physical health problems (e.g. obesity and sleep apnoea; Harris et al., 2009; Luppino et al., 2010), an important limitation when estimating the independent association of insomnia with incident depression. Moreover, none of the previous studies examined possible mediating factors, for example stress response or coping resources (Healey et al., 1981; Kessler et al., 1985; Morin et al., 2003; Stetler and Miller, 2011), in the association of insomnia with incident depression (Baglioni et al., 2011). Finally, no study to date has examined the association of insomnia with objective short sleep duration with incident depression.
We and others have recently reported that insomnia with objective short sleep duration is associated with hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, increased metabolic rate, impaired heart rate variability, glucose and insulin alterations (Bonnet and Arand, 2010; Knutson et al., 2011;Spiegelhalder et al., 2011;Vasishtet al.,2013;Vgontzas et al., 2013), neurocognitive deficits (Fernandez-Mendoza et al., 2010), increased risk of hypertension (Fernandez-Mendozaet al. ,2012a;Vgontzaset al.,2009a),diabetes(Vgontzas et al., 2009b) and mortality (Vgontzas et al., 2010), as well as a persistent and unremitting course (Fernandez-Mendoza et al., 2012b;Vgontzas et al., 2012). Incontrast, insomnia with normal sleep duration is associated with sleep misperception, dysfunctional beliefs, depressive and anxious-ruminative traits, and poor coping resources (Edinger et al., 2000; Fernandez-Mendoza et al., 2011), but not with the physiological changes mentioned above. Based on these findings, we have hypothesized that insomnia with objective short sleep duration may be linked to depression through HPA axis activation, whereas insomnia with normal sleep duration may be associated with depression through psychological mechanisms, such as poor coping resources (Vgontzas et al., 2013).
Thus, the aims of this study were to examine the following hypotheses: (1) there is a significant relationship between the severity of insomnia and incident depression, where insomnia with objective short sleep duration, as measured by polysomnography (PSG), is associated with the highest odds of incident depression; (2) poor coping resources play a significant role in the association of insomnia with objective normal sleep duration with incident depression; and (3) persistent insomnia and worsening of ‘poor sleep’ into insomnia are significantly associated with incident depression, while their full remission is not.
MATERIALS AND METHODS
Participants
The data presented here were collected as part of a population-based study of sleep disorders, which used a two-phase protocol to recruit participants from various age groups (Bixler et al., 2001, 2002). In the first phase of the study, telephone interviews were conducted with 4364 age-eligible men and 12 219 age-eligible women residing in the sample households (Kish, 1965; Waksberg, 1978), with response rates of 73.5 and 74.1%, respectively. In the second phase of this study, a subsample of 741 men and 1000 women, selected randomly from the first phase, were studied in the sleep laboratory, with response rates of 67.8 and 65.8%, respectively. After giving a complete description of the study to the subjects, written informed consent was obtained. Of the 1741 subjects who completed the sleep laboratory evaluation (Phase II), 1395 subjects were followed-up after an average duration of 7.5 years via telephone interview with a standardized questionnaire (Fernandez-Mendoza et al., 2012a,b; Singareddy et al., 2012; Vgontzas et al., 2012). The response rate of the follow-up study was 79.7%. After complete description of the follow-up study to the subjects, verbal informed consent was obtained. The study was approved by the university’s institutional review board. Fig. 1 shows the participant flow in the present study.
Figure 1.
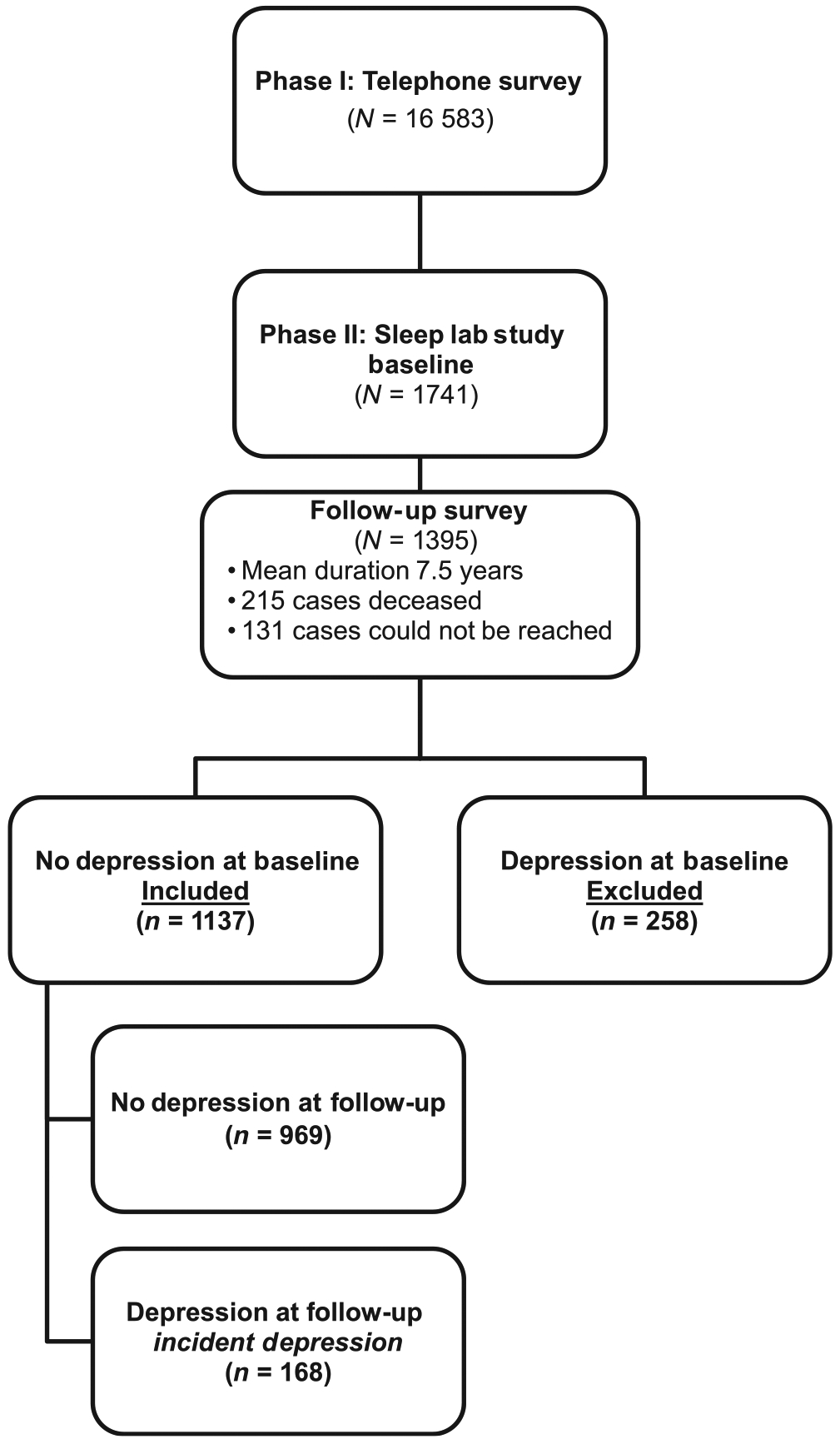
Participants’ flow in the study.
Definition of incident depression
The presence of depression at baseline was defined by a self-report of a past or current (i.e. lifetime) history of physician diagnosis or treatment of depression, based on a standardized questionnaire completed by the subjects on the evening of their sleep laboratory visit (Bixler et al., 2001, 2002). Of the 1395 subjects who were followed up, 1137 did not have depression at baseline and were selected for the present study. The definition of depression at follow-up was commensurate with the baseline definition. A total of 168 subjects were incident cases of depression, whereas 969 did not have depression at follow-up (Fig. 1).
Sleep laboratory evaluation
All subjects were evaluated for 1 night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. Each subject was continuously monitored for 8 h (fixed-time period) using 16-channel PSG including electroencephalogram, electrooculogram and electromyogram. The sleep recordings were subsequently scored independently, according to Rechtschaffen and Kales (1968) criteria. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges (Bixler et al., 2001). The presence of sleep apnoea was defined as an obstructive apnoea/hypopnoea index ≥5 events per hour of sleep (Bixler et al., 2001). From the objectively recorded total sleep time data with a population median of 6 h (Fernandez-Mendoza et al., 2010, 2011, 2012a,b; Vgontzas et al., 2009a,b, 2010, 2012), we regrouped individuals in each age decade into two ordinal groups: the top 50% of persons above the median total sleep time (‘normal sleep duration group’) and the 50% of persons in the bottom half (‘short sleep duration group’). Thus, we created the following two sleep duration groups: the ‘normal sleep duration group’ who slept on average 6.6 ± 0.6 h and the ‘short sleep duration group’ who slept on average 5.0 ± 1.1 h. Body mass index was based on measured height (cm) and weight (kg) during the subjects’ sleep laboratory visit.
Insomnia and other measurements
The presence of sleep difficulty at baseline was established on three mutually exclusive levels of severity based on the standardized questionnaire, which commensurate with our previous studies (Bixler et al., 2002; Fernandez-Mendoza et al., 2010, 2011, 2012a,b; Singareddy et al., 2012; Vgontzas et al., 2009a,b, 2010, 2012). First, ‘insomnia’ was defined by a complaint of insomnia with a duration of at least 1 year. Second, ‘poor sleep’ was defined as a moderate to severe complaint of difficulty falling asleep, difficulty staying asleep, early final awakening, or non-restorative sleep without any duration criterion. Finally, ‘normal sleep’ was defined as the absence of either of these two categories.
The presence of hypertension at baseline was defined by a self-report of use of antihypertensive medication or by a diastolic blood pressure ≥90 mmHg and/or a systolic blood pressure ≥140 mmHg taken during the evening of the sleep lab evaluation (Vgontzas et al., 2009a). The presence of diabetes was defined by a self-report of being treated for diabetes or by having fasting blood glucose levels ≥126 mg dL−1 from blood drawn the morning after the subject’s sleep lab evaluation (Vgontzas et al., 2009b). We also ascertained at baseline participants’ daily caffeine (number of cups per day), tobacco (number of cigarettes per day) and alcohol (number of drinks per day) consumption, as well as lifetime history of alcohol or drug use disorder, suicide thoughts or attempts and feelings of loneliness. Participants also completed at baseline the Minnesota Multiphasic Personality Inventory-2 following the standardized rules and scored accordingly (Butcher et al., 2001). The Ego Strength scale was used as a measure of poor coping resources given its previously reported association with insomnia and depression (Butcher et al., 2001; Fernandez-Mendoza et al., 2011, 2012b; Singareddy et al., 2012; Vgontzas et al., 2012).
Statistical analyses
Categorical variables are expressed as proportions, while continuous variables are expressed as mean and standard deviation. Chi-square test for categorical variables and Student’s t-test for continuous variables were used to examine the association of demographic, clinical and behavioural factors with incident depression. Multivariable logistic regression models were used to examine the association of sleep difficulty with incident depression after progressively adjusting for potential major confounding factors associated with either sleep difficulties or depression [i.e. gender, race, age, body mass index (BMI), obstructive sleep apnoea (OSA), hypertension, diabetes, caffeine, tobacco, alcohol consumption, and alcohol use disorder, as well as drug use disorder, suicide thoughts or attempts, and feelings of loneliness]. Based on the findings of previous studies on the role of objective sleep duration in insomnia reviewed above, we created six subgroups based on the combination of the three-level sleep difficulty and the two-level objective sleep duration variables, i.e. normal sleep with normal (n = 327) or short (n = 431) sleep duration, poor sleep with normal (n = 111) or short (n = 174) sleep duration, and insomnia with normal (n = 32) or short (n = 62) sleep duration. To test the hypothesis that poor coping resources, as measured by Ego Strength scores, may play a role in the development of depression in insomnia with normal sleep duration (Fernandez-Mendoza et al., 2011; Vgontzas et al., 2013), we examined the unadjusted and adjusted incidence of depression across these subgroups as well as their independent predictive role using fully adjusted multivariable logistic regression. Finally, based on our previous findings that objective short sleep duration predicts the persistence of insomnia and the worsening of poor sleep into insomnia (Fernandez-Mendoza et al., 2012b; Vgontzas et al., 2012), we performed a set of multivariable logistic regression models that included nine dummy groups representing the natural history of sleep difficulty: ‘normal sleep’ (i.e. normal sleep both at baseline and at follow-up; n = 540); ‘fully remitted poor sleep’ (i.e. poor sleep at baseline and normal sleep at follow-up; n = 134); ‘fully remitted insomnia’ (i.e. insomnia at baseline and normal sleep at follow-up; n = 26); ‘persistent poor sleep’ (i.e. poor sleep both at baseline and at follow-up; n = 107); ‘partially remitted insomnia’ (i.e. insomnia at baseline and poor sleep at follow-up; n = 29); ‘persistent insomnia’ (i.e. insomnia both at baseline and at follow-up; n = 39); ‘normal sleep evolving into poor sleep’ (i.e. normal sleep at baseline and poor sleep at follow-up; n = 162); ‘normal sleep evolving into insomnia’ (i.e. normal sleep at baseline and insomnia at follow-up; n = 56); and ‘poor sleep evolving into insomnia’ (i.e. poor sleep at baseline and insomnia at follow-up; n = 44). All analyses were conducted with SPSS version 21.0 (Armonk, NY, USA).
RESULTS
The final study sample of individuals without depression at baseline was comprised of 1137 adults (49.3 ± 13.3 years), of whom 48% were females and 94% Caucasian. The incidence of depression was 15% (SE = 1.1%, 95% CI = 13–17%), and the demographic, clinical and behavioural characteristics associated with incident depression are presented in Table 1.
Table 1.
Demographic, clinical and behavioural characteristics and incident depression
| Incident depression | |||
|---|---|---|---|
| No (n = 969) | Yes(n = 168) | P | |
| Sex | |||
| Male, % | 93.2 | 6.8 | 0.00001** |
| Female, % | 78.2 | 21.8 | |
| Race | |||
| Caucasian, % | 85.2 | 14.8 | 0.886 |
| Non-Caucasian, % | 85.7 | 14.3 | |
| Age (years) | 53.4 ± 13.1 | 52.2 ± 12.2 | 0.267 |
| 20–29, % | 95.9 | 4.1 | 0.040* |
| 30–39, % | 80.0 | 20.0 | |
| 40–49, % | 81.8 | 18.2 | |
| 50–59, % | 87.7 | 12.3 | |
| 60–69, % | 85.2 | 14.8 | |
| >70, % | 87.6 | 12.4 | |
| BMI, kg m−2 | 30.6 ± 6.0 | 32.9 ± 6.7 | 0.00001** |
| <25, % | 91.7 | 8.3 | 0.003** |
| >25, % | 87.2 | 12.8 | |
| >30, % | 81.9 | 18.1 | |
| OSA | |||
| No, % | 85.5 | 15.5 | 0.165 |
| Yes, % | 88.0 | 12.0 | |
| Hypertension | |||
| No, % | 84.6 | 15.4 | 0.617 |
| Yes, % | 85.7 | 14.3 | |
| Diabetes | |||
| No, % | 85.4 | 14.6 | 0.790 |
| Yes, % | 84.7 | 15.3 | |
| Caffeine, cups per day | 2.3 ± 2.6 | 1.8 ± 2.8 | 0.079 |
| No, % | 81.5 | 18.5 | 0.008** |
| >1 cup/day, % | 87.3 | 12.7 | |
| Tobacco, cigarettes per day | 2.9 ± 8.6 | 2.4 ± 6.3 | 0.338 |
| No, % | 85.4 | 14.6 | 0.696 |
| >1/day, % | 84.3 | 15.7 | |
| Alcohol, drinks per day | 1.0 ± 4.8 | 0.4 ± 1.7 | 0.002* |
| No, % | 83.3 | 16.7 | 0.001** |
| >1 drink/day, % | 91.8 | 8.2 | |
| Alcohol use disorder | |||
| No, % | 86.0 | 14.0 | 0.082 |
| Yes, % | 70.6 | 29.4 | |
| Ego Strength, f-score | 48.6 ±9.1 | 44.5 ± 10.0 | 0.00002** |
Descriptive data are mean ± standard deviation (SD) for continuous variables and proportions for categorical variables.
BMI, body mass index; OSA, obstructive sleep apnoea.
P ≤ 0.05.
P ≤ 0.01.
Table 2 shows that poor sleep (P = 0.001) and insomnia (P = 0.002) were significantly associated with incident depression (P-linear = 0.003), and that this association remained significant after progressively adjusting for sociodemographic, clinical and behavioural factors (see Model 3 in Table 2). Fig. 2 depicts the role of objective sleep duration and poor coping resources in the association of insomnia with incident depression. As shown in Fig. 2a, the incidence of depression was highest (27.0%) in insomnia with short sleep duration (OR = 2.81, 95% CI = 1.52–5.20, P = 0.001), followed by poor sleep with short sleep duration (19.4%, OR = 1.82, 95% CI = 1.16–2.84, P = 0.009) and poor sleep (19.2%) or insomnia (19.4%) with normal sleep duration (OR = 1.80, 95% CI = 1.11–2.92, P = 0.017); P-linear within short sleepers = 0.004, P-linear within normal sleepers = 0.292. As shown in Fig. 2b, Ego Strength scores were lowest in insomnia with normal sleep duration (P = 0.019), followed by poor sleep and insomnia with short sleep duration (P = 0.001 and P = 0.066, respectively) and poor sleep with normal sleep duration (P = 0.798) compared with normal sleepers. Consistently, Fig. 2c shows that the incidence of depression in insomnia or poor sleep with short sleep duration remained elevated (24.1%, P = 0.015 and 19.0%, P = 0.058, respectively) after adjusting for Ego Strength, whereas that in poor sleep or insomnia with normal sleep duration decreased to a non-significant degree (16.0%, P = 0.293 and 13.2%, P = 0.898, respectively). In fact, fully adjusted multivariable analyses (i.e. gender, race, BMI, OSA, hypertension, diabetes, caffeine, tobacco, alcohol consumption, alcohol use disorder, drug use disorder, suicide thoughts or attempts, feeling of loneliness, and Ego Strength) showed that insomnia with short sleep duration was associated with a significant twofold odds of incident depression (OR = 2.20, 95% CI = 1.14–4.26, P = 0.019), while poor sleep with short sleep duration was associated with a marginally significant 1.6-fold odds of incident depression (OR = 1.59, 95% CI = 0.98–2.57, P = 0.060). Consistent with the data presented in Fig. 2, objective short sleep duration alone was not significantly associated with incident depression (OR = 1.16, 95% CI = 0.81–1.66, P = 0.417), and the interaction effect with sleep difficulty was twofold without reaching statistical significance (OR = 2.04, 95% CI = 0.61–6.77, P = 0.245).
Table 2.
Multivariable odds ratio (95% CI) of incident depression for sleep difficulty
| Depression Incidence | Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% Cl | OR | 95% Cl | OR | 95% Cl | OR | 95% Cl | ||
| Sleep difficulty | - | ||||||||
| Normal sleep | 11.9 | - | - | - | - | - | - | - | - |
| Poor sleep | 19.6 | 1.82 | 1.26–2.62** | 1.56 | 1.05–2.31* | 1.54 | 1.04–2.29* | 1.52 | 1.02–2.27* |
| Insomnia | 23.4 | 2.27 | 1.34–3.84** | 1.84 | 1.06–3.20* | 1.80 | 1.03–3.15* | 1.85 | 1.06–3.23* |
Model 1 = adjusted for gender, race, age and BMI.
Model 2 = adjusted for gender, race, age, BMI, OSA, hypertension, diabetes, caffeine, tobacco, alcohol consumption and alcohol use disorder.
Model 3 = adjusted for gender, race, age, BMI, OSA, hypertension, diabetes, caffeine, tobacco, alcohol consumption, alcohol use disorder, drug use disorder, suicide thoughts or attempts, and feelings of loneliness.
P ≤ 0.05.
P ≤ 0.01.
Figure 2.
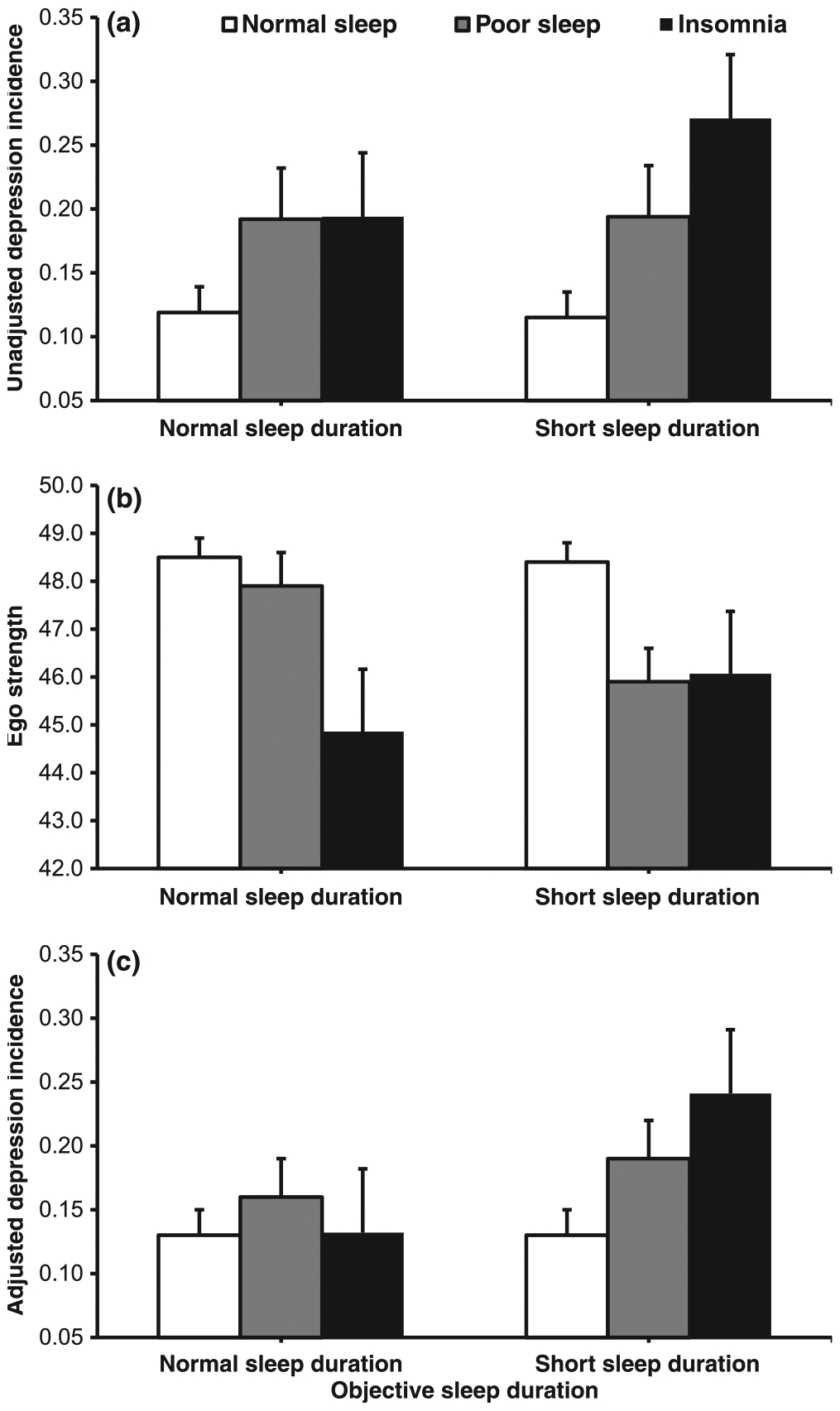
Insomnia and incidence of depression: role of objective sleep duration and coping resources. (a) The incidence of depression was highest in insomnia with short sleep duration (P = 0.001), followed by poor sleep with short sleep duration (P = 0.009) and poor sleep or insomnia with normal sleep duration (P = 0.017). (b) Ego Strength scores were lowest in insomnia with normal sleep duration (P = 0.019), followed by poor sleep and insomnia with short sleep duration (P = 0.001 and P = 0.066, respectively) and poor sleep with normal sleep duration (P = 0.798). (c) The incidence of depression in insomnia or poor sleep with short sleep duration remained elevated (P = 0.015 and P = 0.058, respectively) after adjusting for Ego Strength, whereas that in poor sleep or insomnia with normal sleep duration decreased to a non-significant degree (P = 0.293 and P = 0.898, respectively). Data are mean ± standard error (SE).
Furthermore, we examined the relationship between the natural history of sleep difficulty and incident depression. As shown in Table 3, persistent insomnia (P = 0.049), persistent poor sleep (P = 0.028), partially remitted insomnia (P = 0.032), normal sleep evolving into poor sleep (P = 0.003) and poor sleep evolving into insomnia (P = 0.0001) were all associated with incident depression. In contrast, fully remitted poor sleep or insomnia were not associated with significantly increased odds of incident depression (P = 0.660 and P = 0.259, respectively).
Table 3.
Multivariable odds ratio (95% CI) of incident depression for the natural history of sleep difficulty
| Depression incidence | Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% Cl | OR | 95% Cl | OR | 95% Cl | OR | 95% Cl | ||
| Natural history of sleep difficulty | |||||||||
| Normal sleep | 9.6 | - | - | - | - | - | - | - | - |
| Fully remitted poor sleep | 12.7 | 1.36 | 0.76–2.44 | 1.19 | 0.64–2.21 | 1.18 | 0.63–2.18 | 1.15 | 0.62–2.14 |
| Fully remitted insomnia | 19.2 | 2.23 | 0.81–6.17 | 1.78 | 0.63–5.07 | 1.80 | 0.63–5.14 | 1.83 | 0.64–5.22 |
| Persistent poor sleep | 17.8 | 2.03 | 1.14–3.59* | 1.82 | 1.03–3.36* | 1.82 | 1.02–3.37* | 1.81 | 1.01–3.36* |
| Partially remitted insomnia | 27.6 | 3.58 | 1.51–8.47** | 2.61 | 1.07–6.40* | 2.61 | 1.07–6.40* | 2.60 | 1.06–6.39* |
| Persistent insomnia | 23.1 | 2.82 | 1.27–6.25** | 2.60 | 1.13–6.02* | 2.44 | 1.05–5.70* | 2.53 | 1.09–5.89* |
| Normal sleep developing | 18.5 | 2.13 | 1.31–3.48** | 2.24 | 1.34–3.76** | 2.29 | 1.36–3.83** | 2.27 | 1.35–3.81** |
| poor sleep | |||||||||
| Normal sleep developing | 14.3 | 1.56 | 0.70–3.49 | 1.54 | 0.67–3.57 | 1.52 | 0.66–3.54 | 1.43 | 0.61–3.34 |
| insomnia | |||||||||
| Poor sleep developing | 45.5 | 7.82 | 4.05–15.1** | 6.35 | 3.10–13.0** | 6.09 | 2.96–12.5** | 6.03 | 2.92–12.4** |
| insomnia | |||||||||
Model 1 = adjusted for gender, race, age and BMI.
Model 2 = adjusted for gender, race, age, BMI, OSA, hypertension, diabetes, caffeine, tobacco, alcohol consumption and alcohol disorder.
Model 3 = adjusted for gender, race, age, BMI, OSA, hypertension, diabetes, caffeine, tobacco, alcohol consumption, alcohol use disorder, drug use disorder, suicide thoughts or attempts, and feelings of loneliness.
P ≤ 0.05.
P ≤ 0.01.
Finally, we examined whether specific PSG parameters, particularly increased rapid eye movement (REM) sleep or reduced REM latency, were pivotal in the association of sleep difficulty with incident depression. As shown in Table S1, the groups with significant odds of incident depression, i.e. poor sleepers and insomniacs, were not significantly different in terms of PSG parameters from their respective normal sleeping controls. Specifically, PSG parameters were not significantly different between normal sleepers with short sleep duration versus poor sleepers or insomniacs with short sleep duration, or between normal sleepers with normal sleep duration versus poor sleepers or insomniacs with normal sleep duration. In this general population sample, the amount of REM sleep was not significantly different between individuals with and without incident depression (15.6 ± 7.4% versus 15.5 ± 7.0%, respectively, P = 0.766). Similarly, REM sleep latency was not significantly different between individuals with and without incident depression (133.2 ± 83.8 min versus 137.9 ± 85.4 min, respectively, P = 0.505).
DISCUSSION
This study shows a significant relationship between the severity of insomnia and incident depression. First, we found that poor sleep and insomnia were associated with incident depression in a dose-response manner. Second, insomnia with objective short sleep duration was associated with incident depression independent of poor coping resources. In contrast, the association of insomnia with objective normal sleep duration with incident depression was mediated by poor coping resources. Finally, persistent insomnia and worsening of poor sleep into insomnia were associated with significant odds of incident depression, whereas their full remission was not.
This is the first study to demonstrate a significant relationship between the incidence of depression and the severity of insomnia, using subjective definitions commonly used in epidemiological studies (Bixler et al., 2002; Ohayon, 2002) and a wide array of potential confounders. In fact, poor sleep and insomnia were associated with incident depression not only independent of sociodemographic, physical health and behavioural factors, but also independent of a history of suicide thoughts or attempts and feelings of loneliness, which indicates that insomnia may be a premorbid risk or mechanistic factor for depression rather than simply a prodromal symptom.
The novel finding of this study is that insomnia with objective short sleep duration is associated with the highest odds of incident depression independent of psychological factors predisposing to depression, such as poor coping resources (Kessler et al., 1985). This finding is to some extent consistent with those of Szklo-Coxe et al. (2010) who found objectively-defined markers of insomnia (e.g. increased sleep latency, increased wake after sleep onset and short sleep duration) to be strongly associated with incident depression, and expand the findings of previous studies suggesting that insomnia with objective short sleep duration is the most biologically severe phenotype of the disorder (Vgontzas et al., 2013). Insomnia with objective short sleep duration has been associated with hyperactivity of the HPA axis as well as cardiometabolic (Bonnet and Arand, 2010; Fernandez-Mendoza et al., 2012a; Knutson et al., 2011; Spiegelhalder et al., 2011; Vasisht et al., 2013; Vgontzas et al., 2009a,b) and neurocognitive (Fernandez-Mendoza et al., 2010) morbidity and mortality (Vgontzas et al., 2010). Given the association of depression with the HPA axis (Stetler and Miller, 2011), it is possible to speculate that hyperactivity of the stress system, present in insomnia with objective short sleep duration, may be a biological mechanism implicated in the independent association of this insomnia subtype with incident depression. In contrast, insomnia with normal sleep duration was not independently associated with incident depression after accounting for poor coping resources; in fact, this insomnia subtype showed the poorest scores in coping resources, a finding that is consistent with our previous study (Fernandez-Mendoza et al., 2011). Together, these findings suggest that cognitive-emotional factors may play a role in the development of depression in the less biologically severe subtype of the disorder (Vgontzas et al., 2013). Among many other potential underlying mechanisms, we did not find evidence that REM sleep parameters played a pivotal role in the association of insomnia with incident depression in this general population sample. Future longitudinal studies combining neurobiological and psychological measures should examine the specific mechanisms involved in the development of depression in these two insomnia subtypes.
In the present study, we also showed that the odds of incident depression increase as poor sleep or insomnia persist or worsen over time, which is consistent with the findings of previous studies (Buysse et al., 2008; Okajima et al., 2012; Suh et al., 2013; Zhang et al., 2012). We have previously shown that objective short sleep duration and mental health problems predict the persistence of insomnia as well as who amongst poor sleepers worsen into insomnia, whereas physical health problems predict who amongst normal sleepers evolve into insomnia (Fernandez-Mendoza et al., 2012b; Vgontzas et al., 2012). Our current study expands these findings and provides further support for the concept that objective short sleep duration is a marker of the biological severity of insomnia, including its natural course and associated risk of mental health problems, i.e. depression. Interestingly, fully remitted poor sleep was not associated with significant increased odds of incident depression. In contrast, the odds of incident depression in fully remitted insomnia, although not statistically significant, were 1.8-fold. This finding suggests an aetiological link independent of current symptomatology (e.g. genetic, biological) between the two disorders. Future studies should examine what mechanisms continue to put some individuals at risk of depression after full remission of insomnia.
From a clinical standpoint, the accumulating evidence from clinical, experimental and epidemiological studies demonstrating that objective measures are useful in subtyping and predicting the biological severity of insomnia, suggest that these two insomnia subtypes may respond differently to treatment (Vgontzas et al., 2013). Future clinical trials should test whether these two insomnia subtypes respond differentially to cognitive-behavioural therapy for insomnia, hypnotic medication, or a combination of the two, and whether targeting potential underlying mechanisms (e.g. HPA axis activation or poor coping skills) reduces the risk of new onset depression.
Some limitations should be taken into account when interpreting our results. First, our definition of depression was based on a self-report of a physician diagnosis or treatment for depression, which may not capture untreated cases, specific diagnoses (e.g. major depressive disorder) or severity of depressive symptoms. Nevertheless, the prevalence (17%), incidence (15%), gender-related and age-related rates of depression in the Penn State Adult Cohort (Fernandez-Mendoza et al., 2010, 2012a; Vgontzas et al., 2009a,b, 2010) are consistent with those of previous population-based studies (Eaton et al., 1997; Kessler et al., 2003; Murphy et al., 2002), which increases our confidence about the replicability and generalizability of our findings. Second, the objective sleep duration in this study was based on 1 night of PSG, which may not be representative of the participants’ habitual sleep duration and may be affected by the first night effect. Nevertheless, large epidemiological studies have shown an average sleep duration of 6 h independent of whether sleep is recorded at home with PSG (Silva et al., 2007), for three consecutive nights with actigraphy (Lauderdale et al., 2006) or in the sleep laboratory with PSG (Vgontzas et al., 2009a); the consistency among these three large epidemiological studies increases our confidence about the replicability and generalizability of the present findings. Furthermore, our sleep duration measurement precluded examining ‘long’ sleep duration as defined in previous studies (i.e. >8 h); however, we did not find a U-shaped relationship between PSG-measured sleep duration, modelled as a continuous variable, and incident depression (P = 0.259). There is a need to better understand what self-reported ‘long’ sleep duration means, particularly in individuals who have already developed depression. Third, we did not have available self-reported data of physician diagnosis or treatment for anxiety disorders at baseline. The possibility exists that in some individuals with insomnia the odds of incident depression may be associated with the presence of comorbid anxiety disorders. Future prospective studies are needed to address this issue. Fourth, we did not have available data on how long after the initial assessment depression was developed, which precluded performing Cox proportional hazards analyses. Finally, we did not assess daytime consequences associated with insomnia in the present study; however, our previous studies have supported the face, construct and predictive validity of the poor sleep and insomnia definitions used (Bixler et al., 2002; Fernandez-Mendoza et al., 2012b).
In summary, this study shows that there is a significant relationship between the severity of insomnia and the incidence of depression. Importantly, insomnia with objective short sleep duration is associated with incident depression independent of psychological factors such as coping resources, while the latter played a mediating role in the association of insomnia with objective normal sleep duration and incident depression. This study suggests that the persistence and worsening of sleep difficulties are significantly associated with incident depression. Future prospective studies should investigate the potential effect of specific insomnia treatments in preventing the onset of depression.
Supplementary Material
Table S1. Polysomnographic parameters of sleep difficulty and objective sleep duration at baseline (N = 1137).
ACKNOWLEDGEMENTS
This work was performed at the Sleep Research and Treatment Center at the Pennsylvania State University College of Medicine/Milton S. Hershey Medical Center, and the staff is especially commended for their efforts. This research was funded in part by the National Institutes of Health grants RO1 HL51931, RO1 HL40916 and RO1 HL64415.
Footnotes
CONFLICT OF INTEREST
The authors indicate no financial conflicts of interest.
REFERENCES
- Baglioni C, Battagliese G, Feige B et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemicological studies. J. Affect. Disord, 2011, 135: 10–19. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am. J. Respir. Crit. Care Med, 2001, 163: 608–613. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A and Kales A Insomnia in central Pennsylvania. J. Psychosom. Res, 2002, 53: 589–592. [DOI] [PubMed] [Google Scholar]
- Bonnet MH and Arand DL Hyperarousal and insomnia: state of the science. Sleep Med. Rev, 2010, 14: 9–15. [DOI] [PubMed] [Google Scholar]
- Butcher JN, Graham JR, Ben-Porath YS et al. MMPI-2: Manual for Administration, Scoring and Interpretation Revised edn. University of Minnesota Press, Minneapolis, MN, 2001. [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D and Rössler W Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 2008, 31: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Anthony JC and Gallo J Natural history of Diagnostic Interview Schedule/DSM-IV major depression. The Baltimore Epidemiologic Catchment Area follow-up. Arch. Gen. Psychiatry, 1997, 54: 993–999. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Glenn DM et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J. Consult. Clin. Psychol, 2000, 68: 586–593. [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep, 2010, 33: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun SL, Bixler EO et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom. Med, 2011, 73: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension, 2012a, 60: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Bixler EO et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep, 2012b, 35: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM and Kessler RC Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R). Depress. Anxiety, 2012, 29: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Glozier N, Ratnavadivel R and Grunstein RR Obstructive sleep apnea and depression. Sleep Med. Rev, 2009, 3: 437–444. [DOI] [PubMed] [Google Scholar]
- Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K and Soldatos CR Onset of insomnia: role of life-stress events. Psychosom. Med, 1981, 43: 439–451. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Price RH and Wortman CB Social factors in psychopathology: stress, social support, and coping processes. Annu. Rev. Psychol, 1985, 36: 531–572. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA, 2003, 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- Kish L Survey Sampling. John Wiley, New York, NY, 1965. [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K and Lauderdale DS Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care, 2011, 34: 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am. J. Epidemiol, 2006, 164: 5–16. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry, 2010, 67: 220–229. [DOI] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S and Ivers H Role of stress, arousal, and coping skills in primary insomnia. Psychosom. Med, 2003, 65: 259–267. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Nierenberg AA, Laird NM, Monson RR, Sobol AM and Leighton AH Incidence of major depression: prediction from subthreshold categories in the Stirling County Study. J. Affect. Disord, 2002, 68: 251–259. [DOI] [PubMed] [Google Scholar]
- Ohayon MM Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev, 2002, 6: 97–111. [DOI] [PubMed] [Google Scholar]
- Okajima I, Komada Y, Nomura T, Nakashima K and Inoue Y Insomnia as a risk for depression: a longitudinal epidemiologic study on a Japanese rural cohort. J. Clin. Psychiatry, 2012, 73: 377–383. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A and Kales A A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. National Institutes of Health, Bethesda, MD, 1968. [Google Scholar]
- Silva GE, Goodwin JL, Sherrill DL et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J. Clin. Sleep Med, 2007, 3: 622–630. [PMC free article] [PubMed] [Google Scholar]
- Singareddy R, Vgontzas AN, Fernandez-Mendoza J et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med, 2012, 13: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Fuchs L, Ladwig J et al. Heart rate and heart rate variability in subjectively reported insomnia. J. Sleep Res, 2011, 20: 137–145. [DOI] [PubMed] [Google Scholar]
- Stetler C and Miller GE Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med, 2011, 73: 114–126. [DOI] [PubMed] [Google Scholar]
- Suh S, Kim H, Yang HC, Cho ER, Lee SK and Shin C Longitudinal course of depression scores with and without insomnia in non-depressed individuals: a 6-year follow-up longitudinal study in a Korean cohort. Sleep, 2013, 36: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklo-Coxe M, Young T, Peppard PE, Finn LA and Benca RM Prospective associations of insomnia markers and symptoms with depression. Am. J. Epidemiol, 2010, 171: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasisht KP, Kessler LE, Booth JN 3rd, Imperial JG and Penev PD Differences in insulin secretion and sensitivity in short-sleep insomnia. Sleep, 2013, 36: 955–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP and Vela-Bueno A Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep, 2009a, 32: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M and Bixler EO Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care, 2009b, 32: 1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep, 2010, 33: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Bixler EO et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep, 2012, 35: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D and Bixler EO Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med. Rev, 2013,17: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksberg J Sampling methods for random digit dialing. J. Am. Stat. Assoc, 1978, 73: 40–46. [Google Scholar]
- Zhang J, Lam SP, Li SX et al. Long-term outcomes and predictors of chronic insomnia: a prospective study in Hong Kong Chinese adults. Sleep Med., 2012, 13: 455–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Polysomnographic parameters of sleep difficulty and objective sleep duration at baseline (N = 1137).


