Abstract
Cells are sensitive to chemical stimulation which is converted into intracellular biochemical signals by the activation of specific receptors. Mechanical stimulations can also induce biochemical responses via the activation of various mechano-sensors. Although principally appreciated for their chemosensory function, G-protein-coupled receptors (GPCRs) may participate in mechano-transduction. They are indirectly activated by the paracrine release of chemical compounds secreted in response to mechanical stimuli, but they might additionally behave as mechano-sensors that are directly stimulated by mechanical forces. Although several studies are consistent with this latter hypothesis, the molecular mechanisms of a potential direct mechanical activation of GPCRs have remained elusive until recently. In particular, investigating the activation of the catecholamine β2-adrenergic receptor by a pathogen revealed that traction forces directly exerted on the N-terminus of the receptor via N-glycan chains activate specific signaling pathways. These findings open new perspectives in GPCR biology and pharmacology since most GPCRs express N-glycan chains in their N-terminus, which might similarly be involved in the interaction with cell-surface glycan-specific lectins in the context of cell-to-cell mechanical signaling.
Keywords: mechanosensors, mechano-transduction, shear stress, pilins, lectins, sialic acid, host−pathogen
Mechanical stimuli acting at the level of organs, tissues, individual cells, or molecular complexes regulate multiple physiological and developmental processes. Mechano-transduction, the molecular process converting mechanical stimuli into biochemical signals, is elicited by the direct activation of mechano-sensors, which are the molecules that directly respond to changes in mechanical cues. Different types of plasma membrane-associated mechano-sensors have been identified, which respond to various types of mechanical stimuli and activate distinct signaling pathways.
“Typical” Mechano-Sensors
The most extensively investigated mechano-sensors so far do not belong to the G-protein-coupled receptors (GPCRs) family. Extracellular matrix (ECM) stiffness provides mechanical signals regulating cell differentiation, proliferation, migration, and apoptosis.1−4 Rigidity sensing involves transmembrane integrin molecules that bind the ECM at their extracellular side, and the actin cytoskeleton, via adapter proteins, at their intracellular side (see ref (5) for a recent review). Platelet endothelial cell adhesion molecule (PECAM)-1 present on adherent junctions forms a mechano-sensory complex6 with VE-cadherin and vascular endothelial growth factor receptor (VEGFR)-2/VEGFR3. In endothelial cells, this complex is activated by shear stress resulting in the phosphorylation of PECAM-1 and Src-dependent activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K). This process in turn activates integrins to bind to ECM by an inside-out mechanism. Piezo family mechanically activated ion channels7,8 play a major role in vascular development and function, pulmonary function, and sensory transduction (reviewed in ref (9)). In mammals, two distinct genes code for PIEZOs: PIEZO1, principally expressed in non-neuronal cells, and PIEZO2, restricted to sensory neurons and mechano-sensory structures. These proteins are arranged as trimers. Their activation results in an influx of Na+ and Ca2+. Membrane stretching and shear stress activate PIEZOs by generating a membrane tension, which causes the reorganization of lipids around the protein and channel opening.10 Additional types of mechanically activated ions channels have been discovered in lower organisms and in mammals.11 Among these, two-pore-domain potassium channels (K2P) constitute a family of 15 members sharing a 4 transmembrane (TM) domain topology. Some of them directly sense mechanical forces imparted through the lipid membrane.12 Mechano-transduction also plays an important role in adaptive immune response. Properly applied mechanical forces provide a direct synergistic activation of αβ T-cell receptors (TCR), which have established a chemical bond with an antigenic peptide ligand bound to major histocompatibility complex (MHC) molecules. These forces reduce by orders of magnitude the number of peptide-bound MHC molecules sufficient to trigger a Ca2+ flux in T cells.13
GPCR Participation in Mechano-Transduction
Although principally known for their chemo-sensory function which converts the signals conveyed by chemical ligands into intracellular biochemical signals, GPCRs can be involved in mechano-transduction (Table 1). However, although the participation of GPCRs in the transmission of external mechanical stimuli has been known for more than 25 years,14 experimental evidence that mechanical forces can directly activate these receptors has only recently emerged.
Table 1. GPCRs Involved in Mechano-Transduction.
| mechano-sensory receptor/natural ligand | cell type | type of mechanical activation | outputs, mechanisms of activation | ref |
|---|---|---|---|---|
| adhesion GPGRs (aGPCRs): dCIRL/tether | sensory neurons (Drosophila) | mechanical vibrations; activation of tethered large extracellular domain (ECL), not necessitating ECL proteolysis (which is typically involved in the activation of aGPCRs) | cAMP decrease in the cell, possibly due to the activation of Gαi; downstream alteration of the NOMPC anion channel activity; fine-tuning of neuronal response | (24) |
| APJ/apelin | cardiomyocytes | stretch | stretch signals in an APJ- and β-arrestin-dependent, G-protein-independent fashion to induce hypertrophy | (49) |
| endothelial cells | blood flow (shear stress) | Hypothesis: upregulation of APJ in vascular cells generated by the mechanosensitive properties of the receptor itself | (50) | |
| AT1R/angiotensin II | cardiomyocytes, HEK293 and COS7 cells | mechanical stretching | stretch-induced hypertrophy of cardiac myocytes caused by autocrine release of Ang II | (14) |
| rat aortic A7r5 cells, HEK293 cells | hypo-osmotic stretch, application of positive pressures in patch-clamp experiments | agonist-independent receptor activation (unidentified mechanism), activation of Gq/11 and Jak2 | (18) | |
| ventricular endothelial cells | hypotonicity, membrane stretch, increased intravascular pressure stretch (left ventricle balloon stretch) | stretch-dependent, agonist-independent Gq/11 activation by receptors upon antagonist-blocked conformational change (H1R > AT1R > M5R > V1AR); secondary activation of transient receptor potential (TRP)-6 cation channel; increase of myogenic tone of vascular smooth muscle cells | (19) | |
| coupling to Gαi; Gαi-dependent recruitment of β-arrestins, EGFR transactivation and ERK phosphorylation | (51) | |||
| β2AR/catecholamines | human brain microvasculature endothelial cells (D3 cell line); transfected cells expressing β2AR and β-arrestins | shear stress (indirect) and traction of baterial (meningococcus) pili | traction forces directly applied on the N-terminus of the β2AR via a specific glycan (Neu5Ac). β-arrestin-biased activation of Src-cortactin pathways and recruitment of junctional proteins under bacterial colonies causing the opening of the intercellular spaces of endothelial cells | (38) |
| B2R/bradykinin | bovine aortic endothelial cells | fluid shear stress, hypo-osmotic stimulation, increase in plasma membrane fluidity | changes in cell membrane tension and membrane fluidity affect conformational dynamics of GPCRs (agonist independent but blocked by selective antagonists) | (19,20) |
| ET1AR/endothelin | HEK293 cells | stretch | stretch-dependent, agonist-independent Gq/11 activation (H1R > AT1R > M5R > V1AR); secondary activation of transient receptor potential (TRP)-6 cation channel R | (19) |
| GPR68 | MDA-MB-231 cells (breast cancer cells used for screening); murine primary microvascular endothelial cells. GRPR is naturally expressed in endothelial cells of small diameter arteries and in immune cells. | shear stress imposed by disturbed and laminar flow; probable role in flow mediated dilation | direct mechanical stimulation inducing receptor conformational changes or indirectly downstream a still unknown mechanosensor; Gq/11-coupled, PLC activation, Ca++ release from ER stores | (21) |
| H1R/histamine | HEK293 cells | stretch, shear stress | stretch-dependent, agonist-independent Gq/11 activation (H1R > AT1R > M5R > V1AR); secondary activation of transient receptor potential (TRP)-6 cation channel | (19) |
| endothelial cells | traction over helix 8 | (25) | ||
| M5R/acetylcholine | HEK293 cells | stretch | stretch-dependent, agonist-independent Gq/11 activation (H1R > AT1R > M5R > V1AR); secondary activation of transient receptor potential (TRP)-6 cation channel | (19) |
| PTH1R/parathormone | bone cells, murine preosteoblastic cells (MC3T3-E1) | fluid shear stress | changes in conformational equilibrium of the PTH1R, inhibited by the agonist; mimicked by membrane fluidization using benzyl alcohol or cholesterol extraction | (52) |
| receptor-independent, S1P3-coupled G protein | human coronary artery endothelial cells (HCECs) | shear stress | association between G q/11 and PECAM-1 under basal conditions; dissociation from one another in response to shear stress; direct activation of G q/11, independent of GPCR activation (the possible role of another GPCR in heteromeric complex with S1P3 cannot be excluded) | (53) |
| SIP1/sphingosine 1-phosphate | endothelial cells during angiogenesis | laminar shear stress | direct SIP1 ligand-independent activation by shear stress stabilizes blood vessels in development and homeostasis | (54) |
| V1R/vasopressin | HEK293 cells | stretch, shear stress | stretch-dependent, agonist-independent Gq/11 activation (H1R > AT1R > M5R > V1AR); secondary activation of transient receptor potential (TRP)-6 cation channel | (19) |
| traction over helix 8 | (25) | |||
| indirect GPCR (ETRs, TXA2R, PGH2R, S1PR, P2YR), activation by locally released ligands | endothelial cells acting on smooth muscle cells (paracrine activation) | vascular stretch | myogenic tone generated by the indirect activation of GPCRs coupled to Gq-11 or G12–13; stimulation occurs in reponse to peptides, lipids, nucleotides, and amines released locally by endothelial cells; activation of actin–myosin interaction | (15,16) |
| indirect activation of adrenomedullin R | endothelial cells | shear stress | PIEZO1 mediated fluid shear stress-induced release of adrenomedullin, receptor activation, Gs-dependent cAMP production, and eNOS activation | (17) |
Since the discovery that stretch-induced hypertrophy of cardiac myocytes was caused by autocrine release of angiotensin II (Ang II) and subsequent activation of Ang II receptors,14 several GPCRs of smooth muscle cells were reported to be activated by the paracrine release of peptides, lipids, nucleotides, and amines by endothelial cells in response to vascular stretch. In turn, the activated receptors stimulated their cognate G proteins such as Gs, Gq/11, or G12/13 to generate a myogenic tone.15−17 Other investigations revealed that mechanical stimuli could also activate GPCRs without the involvement of their cognate agonists,18−20 but the molecular mechanism of receptor activation has remained elusive in most cases. The hypothesis that GPCRs might themselves be mechano-sensors has been raised by several studies, in which GPCR conformational changes consistent with receptor activation, productive G-protein coupling and β-arrestin recruitment, were documented in response to mechanical stimuli.19 Supporting this hypothesis further, it was observed that mechanical perturbation of the plasma membrane (caused by shear stress, hypotonic stress, or membrane-fluidizing agents) elicited real-time ligand-independent conformational transitions of GPCRs.20 A large-scale automated screening assay was conducted with a mechano-sensitive cell line that exhibits shear-stress-activated calcium transients to identify shear-stress-sensitive mechano-transducers.21 With this assay and complementary gain- or loss-of-function experiments, the authors found that GPR68, a Gq/11-coupled receptor expressed in endothelial cells of small diameter vessels, was necessary and sufficient for shear stress responses in these cells. Although satisfying all criteria for a mechano-sensor (i.e., essential for immediate response in mechano-sensitive cells, which naturally express the receptor and responsive to the relevant mechanical stimuli when expressed in heterologous cells), it could not be formally established whether GPR68 is directly activated by mechanical forces, by a putative upstream mechano-sensor, or by the local release of protons, which were also reported to activate this GPCR.
Evidence that a mechanical stimulus can directly activate a GPCR also comes from studies on receptors belonging to a special subfamily of GPCRs, namely adhesion GPCRs (aGPCRs, see refs (22) and (23) for recent reviews). aGPCRs possess a long extracellular N-terminus with adhesive properties that anchors the receptor to the extracellular matrix or to opposed cell surfaces via cognate ligands. They also contain a juxta-membrane GPCR autoproteolysis-inducing (GAIN) domain, which during protein maturation catalyzes receptor cleavage in N- and C-terminal fragments (NTF and CTF) that remain noncovalently associated. aGPCRs function as adhesion molecules due to the NTF and as classical GPCRs through the CTF. Receptor activation exposes a cryptic tethered agonist ligand that activates the receptor. The latrophilin/dCIRL aGPCR, for example, is found in mechano-sensory neurons of Drosophila chordotonal organs and modulates ionotropic receptor currents. Whereas autoproteolysis of the GAIN domain is not essential for dCIRL activity, the authors found that progressive lengthening of dCIRL’s N-terminal fragment gradually reduces mechano-sensory neuronal responses. These findings are consistent with a model in which mechanical tension applied to the receptor determines the extent of its activity.24
A different mechanism of mechanical GPCR activation has been reported in a very recent article investigating the activation of endothelial histamine H1 receptors (H1Rs) by shear stress and membrane stretch.25 Mechanical activation of H1Rs was found to be agonist-independent, insensitive to inverse agonists, and G11/q-protein-dependent, resulting in NO production. Conformational changes promoted by shear stress and stretch were distinct from those elicited by receptor agonists. Interestingly, the mechanically activated pathway was strictly dependent on the presence of an eighth helix (H8) in the C-terminal tail of the receptor: transfer of the H8 to nonresponsive GPCRs conferred mechano-sensitivity to nonresponsive GPCRs, whereas H8 removal in H1Rs abolished it. These observations suggest that the mechanical elongation of H8 would activate the G protein.
GPCR Activation by Pathogen Pulling on N-Terminal Glycan Chains
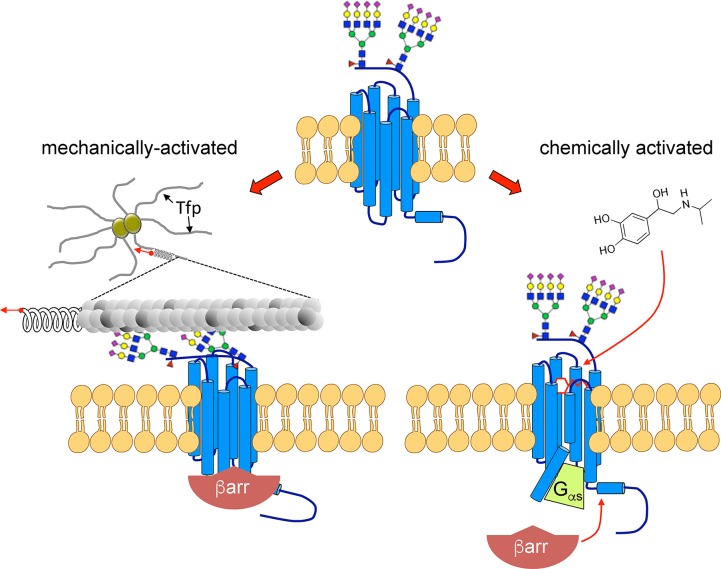
Meningococcus (Neisseria meningitidis) is a human-specific Gram-negative bacterium, which colonizes the mucosa of nose and pharynx. However, when meningococci enter into the bloodstream, they rapidly spread and become pathogenic. After blood brain barrier crossing they can invade meninges, causing cerebrospinal meningitis.26 When bacterial load is high, infection spreads to peripheral vessels causing septic shock, the most severe life-threatening form being purpura fulminans.27 The interaction of the pathogen with endothelial cells initiates peripheral vascular lesions28 and is responsible for the opening of the blood brain barrier.29 This interaction involves long bacterial filamentous organelles known as Type IV pili (Tfp), which mediate the adhesion of virulent capsulated meningococci to endothelial cells in vitro and in vivo.29,30 Tfp interact with two host cell receptors. The first is CD147, an IgG-family single TM receptor, which does not transduce any signal but is essential for early adhesion of bacteria to the endothelial cells.31 The second is a GPCR, the catecholamine β2-adrenergic receptor (β2AR). Interaction of Tfp with the N-terminus of the β2AR induces an allosteric β-arrestin (βarr)-biased signaling cascade in host endothelial cells, which is not blocked by the antagonist propranolol.32 Although the Gs/cAMP pathway is not affected upon bacterial binding to endothelial cells, bacteria induce a GRK-dependent recruitment of βarrs, leading to the activation of ERK and Src, and the subsequent recruitment under bacterial colonies of various cytoskeletal (actin and ezrin) and junctional proteins. Interestingly, all these signaling events are necessary for two essential processes of meningococcus pathophysiology, namely, the stabilization of growing bacterial colonies under blood flow33 and the opening of endothelial junctional spaces, which become loose and ultimately allow bacteria to penetrate into tissues34 such as meninges. The interaction of the pathogen with these two distinct receptors is facilitated by the fact that the β2AR and CD147 are preassembled in endothelial cells forming heteromeric structures stabilized by the cytoskeletal protein alpha-actinin-4.35 Although these studies outlined well the principal pathophysiological events permitting meningococcus to penetrate into meninges, a major issue remained unresolved for several years, namely, how a GPCR activated by nonsoluble bacterial proteins is able to transduce a signaling cascade which is usually induced by cognate receptor ligands.
Besides their passive “hooking” role, Tfp also generate mechanical forces. Studies in Neisseria species demonstrated that pilus retraction driven by the bacterial ATPase PilT produces forces in vivo that allow bacteria to move over surfaces.36,37 Moreover, bacteria growing on the endothelial cell surface are constantly submitted to forces produced by blood flow, which can be propagated to endothelial cell plasma membranes via Tfp attachment. Although Tfp binding to host endothelial cells is necessary to engender pulling forces, the molecular plasma membrane tether involved in this process remained elusive. Studies recapitulating the hemodynamic conditions existing in capillaries showed that the β2AR is involved in the signaling cascade that allows small meningococcal colonies to remain stable under flow.32 A possible mechanistic explanation of the above findings would be that the β2AR is simultaneously a mechano-sensor and a transducing receptor for meningococcal Tfp. The hypothesis that β2AR is a mechano-sensor for Tfp-mediated traction was confirmed recently and represents the first demonstration that a “classical GPCR” can directly be activated by mechanical forces.38
In an attempt to characterize the binding site of pilins on β2AR, a chimeric receptor approach with the angiotensin II receptor AT1R was used in a fully controlled reconstitution cell system. AT1R, also present in endothelial cells, is incompetent for N. meningitidis signaling. Substituting its N-terminal region with that of human β2AR generated a chimera, which could be activated by N. meningitidis in vitro, suggesting that pilin binding to the β2AR N-terminus is sufficient to mediate βarr-biased activation.32 Direct binding of purified pilins to this receptor region, consisting of 27 amino acid residues, was subsequently demonstrated by homogeneous time-resolved FRET. Although only humans are infected by N. meningitidis,26 this region is conserved in mammals and is almost identical between human and monkeys. Moreover, whereas human HEK-293 cells reconstituted with mouse β2AR could be activated by meningococcus, mouse 3T3 cells and mouse C166 endothelial cells expressing exogenous human β2AR were not activated by the bacterium. These data indicated that the capacity of N. meningitidis to bind and activate β2AR in human cells involves human host-cell-specific post-translational modification of the receptor. The β2AR displays two asparagine-branched glycan chains in its N-terminus.39 It was then investigated whether these glycans might be involved in receptor activation by meningococcus. Alanine substitution of either Asn residue reduced signaling under meningococcal colonies to control levels. In a gain-of-function strategy, two glycosylation sites, nine residues distant as in β2AR, were produced by mutagenesis in the AT1R: This mutant reconstituted meningococcus-promoted signaling in vitro, whereas mutants expressing only one of the glycan chains failed (after substitution with Ala). These data supported a model where both glycan chains of the β2AR are necessary and sufficient to permit receptor activation by N. meningitidis. Using a panel of lectins to inhibit meningococcus-promoted signaling in vitro, it could be determined that terminally exposed α2–3 branched sialic acids play an important role in the activation of the β2AR by the pathogen.
This finding sheds some light on the mechanism of meningococcus species selectivity. Two forms of sialic acids are predominant in mammals, Neu5Ac and Neu5Gc, which differ by a single oxygen atom.40 Only Neu5Ac is produced in humans, contrasting with the predominant presence of Neu5Gc in other mammals.41 Indeed, Neu5Gc is synthesized from Neu5Ac by the cytidine monophosphate-N-acetylneuraminic acid hydrolase (CMAH),42 which is absent in humans, due to a mutation in the cmah gene. The observations above raised the hypothesis that species selectivity of N. meningitidis for humans might be explained by the requirement for two terminally exposed Neu5Ac at a particular distance.38 Consistently, it was found that deletion of cmah in mouse cells restored meningococcal signaling via β2AR, while the mouse β2AR receptor was activated by meningococci when expressed in human cells.
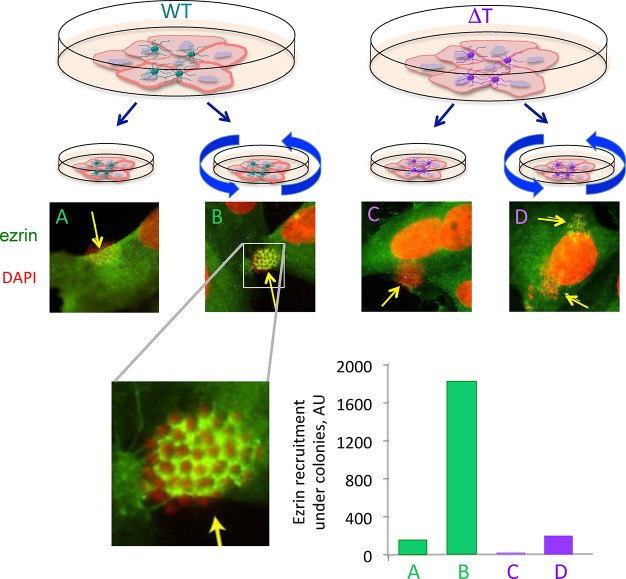
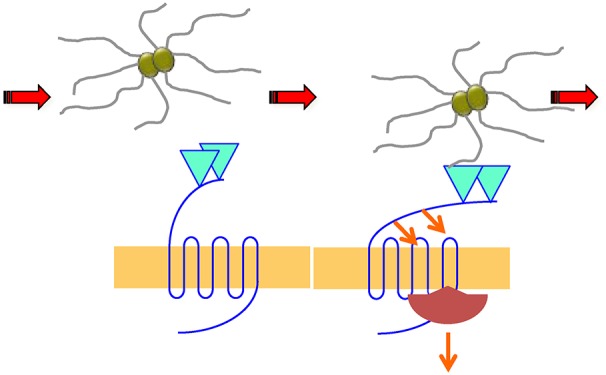
From the above data, a plausible scenario emerged postulating that traction forces applied by bacterial Tfp bound to sialic acid residues would activate the β2AR (Figure 1). To test this hypothesis, wild-type and mutant meningococci deficient in PilT activity (referred to as ΔT) were compared for their ability to induce signaling in endothelial cells (Figure 2). Signaling of ΔT meningococci was significantly impaired compared to wild type bacteria under basal, static conditions. Next, adherent meningococci were submitted to orbital rotation to apply a centrifugal force on bacteria. Rotation promoted signaling under meningococcus colonies both in wild-type and ΔT meningococci, but the effect was superior for wild-type colonies, indicating that maximal signaling depends on added effects of PilT-induced pilus retraction and exogenous forces applied to bacteria. Interestingly, sialic acid selective lectin-coated beads submitted to the same orbital rotation recapitulated the signaling features observed with meningococci. Traction forces specifically applied on β2AR via its N-glycan chains can therefore induce βarr-biased activation and downstream ezrin recruitment independent of bacteria. Moreover, whereas meningococcal activation of the β2AR was strictly dependent on the presence of two glycan chains at a particular distance, probably reflecting structural constraints, such as pilin orientation or spacing within the bacterial pilus, β2AR mutants lacking either N-glycosylation site retained their capacity for being activated by lectin-coated beads.38 Thus, a force applied via a single N-glycan chain is potentially sufficient to promote the mechanical activation of a GPCR.
Figure 1.
Chemical and mechanical activation of β2AR. β2AR is principally known for its chemosensory functions (right). Agonist binding (isoproterenol in the figure) to the receptor orthosteric binding pocket induces conformational changes and coupling with its cognate GS heterotrimeric G protein. Subsequent phosphorylation by G protein receptor kinases (GRKs) and βarr recruitment extend receptor signaling to additional pathways (not shown). βarrs are also involved in the uncoupling of the β2AR with the Gs protein and in receptor endocytosis. Meningococcal pili (Tfp) mechanically activate the β2AR (left). Pilus components PilE and PilV bind to β2AR N-terminal glycans by interacting with terminal Neu5Ac (purple diamonds) branched on galactose residues (yellow circles). Bacteria “pull” the receptor N-terminus and induce a conformational change of the β2AR, which recruits βarrs without coupling with the Gs G protein. This mechanical activation can also be reproduced by beads coated with lectins that recognize Neu5Ac if beads are submitted to centrifugal forces.
Figure 2.
Extrinsic and intrinsic forces contribute to β2AR activation. Once in the blood, meningococci adhere to endothelial cells via their type-IV pili. Growing colonies at the apical surface of endothelial cells are then submitted to forces exerted by blood flow. In addition, meningococcal PilT ATPase generates traction forces via pili in vivo allowing bacteria to crawl over surfaces. To evaluate the respective contribution of intrinsic and extrinsic forces to the mechanical activation of the β2AR by meningococci, the activation of receptor signaling was compared in wild-type and PilT defective bacteria (ΔT). Human endothelial cells (hCMEC/D3 cell line) were infected with wild-type or mutant bacteria and ezrin recruitment under colonies (a downstream signaling event elicited by meningococcus in host cells) was analyzed under static conditions (2 h of incubation) or under orbital rotation (15 min after initial static incubation) aimed at applying a centrifugal force on bacteria. After cell fixation, for each experimental condition the percent of colonies which recruit ezrin was counted under a fluorescent microscope. The amount of ezrin recruitment under each colony was quantified from Apotome acquired images using ImageJ software (expressed as arbitrary fluorescence value per pixel). The histogram values were obtained by multiplying the percent values with the median values of ezrin fluorescence.
Recent structural and molecular studies have identified the events, which control GPCR function at the molecular level.43 For small-molecule-activated class A GPCRs, the class to which β2AR belongs, the orthosteric binding site used by endogenous agonists to activate the receptor is situated in the middle of the seven-transmembrane helical bundle, between the extracellular loops and the middle plane of the plasma membrane. Allosteric agonists bind to the receptor in varying positions outside the orthosteric site. For example, a small allosteric modulator of the β2AR was found to bind to a pocket formed by the cytoplasmic ends of TM domains 1, 2, 6, and 7, the intracellular loop 1, and helix 8.44 An allosteric modulator of the M2 muscarinic receptor instead binds above the orthosteric site and establishes molecular interactions with the extracellular vestibule, which includes the second extracellular loop.45 The exosite for the aryloxyalkyl tail of salmeterol, a selective β2AR long-acting agonist was investigated in a recent study. The salmeterol tail extends toward the extracellular surface of the receptor, filling a cleft formed by amino acid residues belonging to extracellular loops 2 and 3 and to the extracellular ends of TM domains 6 and 7.46 These findings, and the observation that the orthosteric site for peptide-activated GPCRs can involve the N-terminal region and is stabilized by extracellular loops,47 suggests a mechanism in which the traction applied via meningococcal pili bound to β2AR glycan chains engages the helical bundle region through the recruitment of the N-terminal region, which creates interactions with the transmembrane core. This model is supported by the fact that applying traction forces on the β2AR, via beads that are coated with lectins specific for the exposed sialic acid of receptor glycan chains, reproduces a pili-like activation.38
Conclusion and Perspectives
Over the past 20 years, an increasing number of observations supported the role of GPCRs in the transduction of mechanical signals. Although in most cases GPCR activation appears as a downstream event caused by the release of receptor agonists in response to the mechanical stimulation, recent studies support that GPCRs may function as direct mechano-sensors and that mechanical forces can activate these receptors in different ways. In this context, the previously unreported mechanism of mechanical activation of a GPCR, involving traction forces applied on glycan chains terminated with sialic acid, raises the question of its physiological relevance. This may suggest that there are supplementary functions for the β2AR in the vascular system. For instance, macrophages which adhere to and roll over endothelial cells before entering into tissues express the sialic acid specific sialoadhesin Siglec-1,48 which displays the same glycan specificity as Mal-I. Siglec-1 binding to β2AR might induce some signaling in the context of cell-to-cell interactions and/or diapedesis. More generally, most (80%) GPCRs express at least one N-glycan chain in their N-terminus. These glycan chains might similarly be involved in the interaction with cell surface lectins and allosterically activated in the context of cell-to-cell mechanical signaling. However, it is currently difficult to evaluate how many GPCRs could actually be activated via a traction applied on their N-glycans. For example, lectin-coated beads failed to activate the AT1R, indicating that each receptor should be tested individually for its aptitude of being directly activated by mechanical forces. Also, additional studies will be necessary to establish how a traction force exerted on its N-terminus can structurally activate a GPCR.
Acknowledgments
This article was supported by grants ANR-14-IFEC-0006-01, ANR-15-CE15-0002, and ANR-19-CE14-0045. S.M. and M.C. are also supported by the INSERM, the CNRS, and the Université de Paris.
The authors declare no competing financial interest.
References
- Folkman J.; Moscona A. (1978) Role of cell shape in growth control. Nature 273, 345–9. 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Watt F. M.; Jordan P. W.; O’Neill C. H. (1988) Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl. Acad. Sci. U. S. A. 85, 5576–80. 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S.; Mrksich M.; Huang S.; Whitesides G. M.; Ingber D. E. (1997) Geometric control of cell life and death. Science 276, 1425–8. 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Engler A. J.; Sen S.; Sweeney H. L.; Discher D. E. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–89. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Wolfenson H.; Yang B.; Sheetz M. P. (2019) Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 81, 585–605. 10.1146/annurev-physiol-021317-121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E.; Irani-Tehrani M.; Kiosses W. B.; Dejana E.; Schultz D. A.; Engelhardt B.; Cao G.; DeLisser H.; Schwartz M. A. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–31. 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Syeda R.; Florendo M. N.; Cox C. D.; Kefauver J. M.; Santos J. S.; Martinac B.; Patapoutian A. (2016) Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 17, 1739–46. 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D.; Bae C.; Ziegler L.; Hartley S.; Nikolova-Krstevski V.; Rohde P. R.; Ng C. A.; Sachs F.; Gottlieb P. A.; Martinac B. (2016) Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 7, 10366. 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. E.; Dubin A. E.; Patapoutian A. (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–83. 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- Lewis A. H.; Grandl J. (2015) Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4, e12088. 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade S. S.; Syeda R.; Patapoutian A. (2015) Mechanically Activated Ion Channels. Neuron 87, 1162–79. 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S. G.; Su Z.; MacKinnon R. (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. U. S. A. 111, 3614–9. 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Brazin K. N.; Kobayashi E.; Mallis R. J.; Reinherz E. L.; Lang M. J. (2017) Mechanosensing drives acuity of alphabeta T-cell recognition. Proc. Natl. Acad. Sci. U. S. A. 114, E8204–E13. 10.1073/pnas.1703559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J.; Xu Y.; Slayter H. S.; Izumo S. (1993) Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75, 977–84. 10.1016/0092-8674(93)90541-W. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G.; Laher I.; Matrougui K.; Guerineau N. C.; Henrion D. (2012) Emerging role of G protein-coupled receptors in microvascular myogenic tone. Cardiovasc. Res. 95, 223–32. 10.1093/cvr/cvs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Iring A.; Strilic B.; Albarran Juarez J.; Kaur H.; Troidl K.; Tonack S.; Burbiel J. C.; Muller C. E.; Fleming I.; Lundberg J. O.; Wettschureck N.; Offermanns S. (2015) P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125, 3077–86. 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iring A.; Jin Y. J.; Albarran-Juarez J.; Siragusa M.; Wang S.; Dancs P. T.; Nakayama A.; Tonack S.; Chen M.; Kunne C.; Sokol A. M.; Gunther S.; Martinez A.; Fleming I.; Wettschureck N.; Graumann J.; Weinstein L. S.; Offermanns S. (2019) Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 129, 2775–91. 10.1172/JCI123825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.; Akazawa H.; Qin Y.; Sano M.; Takano H.; Minamino T.; Makita N.; Iwanaga K.; Zhu W.; Kudoh S.; Toko H.; Tamura K.; Kihara M.; Nagai T.; Fukamizu A.; Umemura S.; Iiri T.; Fujita T.; Komuro I. (2004) Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 6, 499–506. 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M.; Storch U.; Meibers S.; Nurwakagari P.; Breit A.; Essin K.; Gollasch M.; Gudermann T. (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 27, 3092–103. 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachisvilis M.; Zhang Y. L.; Frangos J. A. (2006) G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 103, 15463–8. 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Mathur J.; Vessieres E.; Hammack S.; Nonomura K.; Favre J.; Grimaud L.; Petrus M.; Francisco A.; Li J.; et al. (2018) GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173, 762–775.e16. 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T.; Piao X.; Monk K. R. (2016) Adhesion G protein-coupled receptors in nervous system development and disease. Nat. Rev. Neurosci. 17, 550–61. 10.1038/nrn.2016.86. [DOI] [PubMed] [Google Scholar]
- Folts C. J.; Giera S.; Li T.; Piao X. (2019) Adhesion G Protein-Coupled Receptors as Drug Targets for Neurological Diseases. Trends Pharmacol. Sci. 40, 278–93. 10.1016/j.tips.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz N.; Guan C.; Nieberler M.; Grotemeyer A.; Maiellaro I.; Gao S.; Beck S.; Pawlak M.; Sauer M.; Asan E. (2017) Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. eLife 6, 28360. 10.7554/eLife.28360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogmus S.; Storch U.; Danner L.; Becker J.; Winter M.; Ziegler N.; Wirth A.; Offermanns S.; Hoffmann C.; Gudermann T.; Mederos Y. S. M. (2019) Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat. Commun. 10, 5784. 10.1038/s41467-019-13722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M.; Bourdoulous S.; Marullo S.; Nassif X. (2014) Invasive meningococcal disease: a disease of the endothelial cells. Trends Mol. Med. 20, 571–8. 10.1016/j.molmed.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Stephens D. S.; Greenwood B.; Brandtzaeg P. (2007) Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369, 2196–210. 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- Lemichez E.; Lecuit M.; Nassif X.; Bourdoulous S. (2010) Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 8, 93–104. 10.1038/nrmicro2269. [DOI] [PubMed] [Google Scholar]
- Virji M. (2009) Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7, 274–86. 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- Join-Lambert O.; Lecuyer H.; Miller F.; Lelievre L.; Jamet A.; Furio L.; Schmitt A.; Pelissier P.; Fraitag S.; Coureuil M.; Nassif X. (2013) Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. J. Infect. Dis. 208, 1590–7. 10.1093/infdis/jit301. [DOI] [PubMed] [Google Scholar]
- Bernard S. C.; Simpson N.; Join-Lambert O.; Federici C.; Laran-Chich M. P.; Maissa N.; Bouzinba-Segard H.; Morand P. C.; Chretien F.; Taouji S.; Chevet E.; Janel S.; Lafont F.; Coureuil M.; Segura A.; Niedergang F.; Marullo S.; Couraud P. O.; Nassif X.; Bourdoulous S. (2014) Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–31. 10.1038/nm.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M.; Lécuyer H.; Scott M. G. H.; Boularan C.; Enslen H.; Soyer M.; Mikaty G.; Bourdoulous S.; Nassif X.; Marullo S. (2010) Meningococcus hijack a ß2-adrenoceptor-ß-arrestin pathway to cross brain microvasculature endothelium. Cell 143, 1149–60. 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Mikaty G.; Soyer M.; Mairey E.; Henry N.; Dyer D.; Forest K. T.; Morand P.; Guadagnini S.; Prevost M. C.; Nassif X.; Dumenil G. (2009) Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 5, e1000314. 10.1371/journal.ppat.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M.; Mikaty G.; Miller F.; Lecuyer H.; Bernard C.; Bourdoulous S.; Dumenil G.; Mege R. M.; Weksler B. B.; Romero I. A.; Couraud P. O.; Nassif X. (2009) Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 325, 83–7. 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïssa N.; Covarelli V.; Janel S.; Durel B.; Simpson N.; Bernard S. C.; Pardo-Lopez L.; Bouzinba-Ségard H.; Faure C.; Scott M. G. H.; et al. (2017) Strength of Neisseria meningitidis binding to endothelial cells requires highly-ordered CD147/β2-adrenoceptor clusters assembled by alpha-actinin-4. Nat. Commun. 8, 15764. 10.1038/ncomms15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz A. J.; So M.; Sheetz M. P. (2000) Pilus retraction powers bacterial twitching motility. Nature 407, 98–102. 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- Biais N.; Ladoux B.; Higashi D.; So M.; Sheetz M. (2008) Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 6, e87. 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virion Z.; Doly S.; Saha K.; Lambert M.; Guillonneau F.; Bied C.; Duke R. M.; Rudd P. M.; Robbe-Masselot C.; Nassif X.; Coureuil M.; Marullo S. (2019) Sialic acid-mediated mechanical activation of β2 adrenergic receptors by bacterial pilins. Nat. Commun. 10, 4752. 10.1038/s41467-019-12685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E.; Candelore M. R.; Cheung A. H.; Hill W. S.; Strader C. D.; Dixon R. A. (1990) Mutational analysis of beta-adrenergic receptor glycosylation. J. Biol. Chem. 265, 10759–10764. [PubMed] [Google Scholar]
- Varki A. (2001) Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am. J. Phys. Anthropol. 116, 54–69. 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. (2007) Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446, 1023–9. 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- Kawano T.; Koyama S.; Takematsu H.; Kozutsumi Y.; Kawasaki H.; Kawashima S.; Kawasaki T.; Suzuki A. (1995) Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J. Biol. Chem. 270, 16458–63. 10.1074/jbc.270.27.16458. [DOI] [PubMed] [Google Scholar]
- Wacker D.; Stevens R. C.; Roth B. L. (2017) How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–27. 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Ahn S.; Kahsai A. W.; Meng K. C.; Latorraca N. R.; Pani B.; Venkatakrishnan A. J.; Masoudi A.; Weis W. I.; Dror R. O.; Chen X.; Lefkowitz R. J.; Kobilka B. K. (2017) Mechanism of intracellular allosteric beta2AR antagonist revealed by X-ray crystal structure. Nature 548, 480–4. 10.1038/nature23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A. C.; Ring A. M.; Manglik A.; Hu J.; Hu K.; Eitel K.; Hubner H.; Pardon E.; Valant C.; Sexton P. M.; Christopoulos A.; Felder C. C.; Gmeiner P.; Steyaert J.; Weis W. I.; Garcia K. C.; Wess J.; Kobilka B. K. (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–6. 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masureel M.; Zou Y.; Picard L.-P.; van der Westhuizen E.; Mahoney J. P.; Rodrigues J. P. G. L. M.; Mildorf T. J.; Dror R. O.; Shaw D. E.; Bouvier M.; Pardon E.; Steyaert J.; Sunahara R. K.; Weis W. I.; Zhang C.; Kobilka B. K. (2018) Structural insights into binding specificity, efficacy and bias of a β2AR partial agonist. Nat. Chem. Biol. 14, 1059–66. 10.1038/s41589-018-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Sun B.; Feng D.; Hu H.; Chu M.; Qu Q.; Tarrasch J. T.; Li S.; Sun Kobilka T.; Kobilka B. K.; Skiniotis G. (2017) Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–53. 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. (2017) Biological roles of glycans. Glycobiology 27, 3–49. 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimia M. C.; Hurtado C.; Ray S.; Metzler S.; Wei K.; Wang J.; Woods C. E.; Purcell N. H.; Catalucci D.; Akasaka T.; Bueno O. F.; Vlasuk G. P.; Kaliman P.; Bodmer R.; Smith L. H.; Ashley E.; Mercola M.; Brown J. H.; Ruiz-Lozano P. (2012) APJ acts as a dual receptor in cardiac hypertrophy. Nature 488, 394–8. 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R.; Strohbach A.; Pennewitz M.; Lorenz F.; Bahls M.; Busch M. C.; Felix S. B. (2015) Regulation of the endothelial apelin/APJ system by hemodynamic fluid flow. Cell. Signalling 27, 1286–96. 10.1016/j.cellsig.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Wang J.; Hanada K.; Gareri C.; Rockman H. A. (2018) Mechanoactivation of the angiotensin II type 1 receptor induces beta-arrestin-biased signaling through Galphai coupling. J. Cell. Biochem. 119, 3586–97. 10.1002/jcb.26552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. L.; Frangos J. A.; Chachisvilis M. (2009) Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am. J. Physiol Cell Physiol 296, C1391–9. 10.1152/ajpcell.00549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Paz N. G.; Melchior B.; Frangos J. A. (2017) Shear stress induces Galphaq/11 activation independently of G protein-coupled receptor activation in endothelial cells. Am. J. Physiol Cell Physiol 312, C428–C37. 10.1152/ajpcell.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B.; Obinata H.; Galvani S.; Mendelson K.; Ding B. S.; Skoura A.; Kinzel B.; Brinkmann V.; Rafii S.; Evans T.; Hla T. (2012) Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–10. 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]