Abstract
Peptide-liganded G protein-coupled receptors (GPCRs) are a growing fraction of GPCR drug targets, concentrated in two of the five major GPCR structural classes. The basic physiology and pharmacology of some within the rhodopsin class, for example, the enkephalin (μ opioid receptor, MOR) and angiotensin (ATR) receptors, and most in class B, all the members of which are peptide receptors, are well-known, whereas others are less so. Furthermore, with the notable exception of opioid peptide receptors, the ability to translate from peptide to “drug-like” (i.e., low-molecular-weight nonpeptide) molecules, with desirable oral absorption, brain penetrance, and serum stability, has met with limited success. Yet, peripheral peptide administration in patients with metabolic disorders is clinically effective, suggesting that “drug-like” molecules for peptide receptor targets may not always be required for disease intervention. Here, we consider recent developments in GPCR structure analysis, intracellular signaling, and genetic analysis of peptide and peptide receptor knockout phenotypes in animal models. These lines of research converge on a better understanding of how peptides facilitate adaptive behaviors in mammals. They suggest pathways to translate this burgeoning information into identified drug targets for neurological and psychiatric illnesses such as obesity, addiction, anxiety disorders, and neurodegenerative diseases. Advances centered on the peptide ligands oxytocin, vasopressin, GLP-1, ghrelin, PACAP, NPY, and their GPCRs are considered here. These represent the spectrum of progress across the “virtual pipeline”, of peptide receptors associated with many established drugs, those of long-standing interest for which clinical application is still under development, and those just coming into focus through basic research.
Keywords: GPCR, G-protein coupled receptor, regulatory peptide, neurotherapeutics
Overview
In March, 2019, the National Institute of Mental Health Intramural Research Program (NIMH-IRP) convened a workshop to examine emerging neurotranslational opportunities for peptide-liganded receptors as drug targets. The primary motivations were 2-fold: first, to gauge current progress in the field and inform NIMH and NIH at large about areas of promise for neuropsychiatric disease, and second, to attempt to create a “virtual pipeline” for regulatory peptide-related drug development focused on a “soup to nuts” approach to peptide-liganded receptors as studied by basic scientists, associated with specific neuropathologies, and translated to medical practice through drug development and clinical assessment. The NIMH Workshop addressed four broad questions. First, what is unique about the physiology of peptide modulation that recommends it as a target, per se, for behavioral intervention; second, does the drug pipeline require rethinking in terms of conversion of physiological ligands to drug-like congeners and other traditional milestones for drug development; third, are there lessons to be learned from existing peptide-liganded drug targets that can inform the creation of guidelines/best practices for regulatory peptide-centric drug development; and fourth, can “proof-of-concept” be adequately defined for resource deployment to support drug development throughout the virtual drug pipeline? The structure of this review reflects the structure of the workshop, culminating in a panel discussion of potential best candidates for further attention based on the concept of a virtual drug pipeline to which all sectors of biomedicine, from fundamental neuroscience through acceleration of translational research and culminating in human drug development, can contribute.
What Are the Unique Features of Peptides as Targets for Behavioral Intervention?
The session was chaired by Hugo Tejeda, NIMH-IRP, whose own work on peptide neuromodulation has focused on the action of kappa opioid receptor agonists in coordinating the actions of dopamine during reward-associated behavioral consolidation by biasing excitatory and inhibitory input signaling to dopaminoceptive neurons of the nucleus accumbens.1 Participants in the session included Eve Marder, Brandeis University, Ben White, NIMH-IRP, and Patrick Sexton of Monash University. A cohort of unique features of peptide neurotransmission was identified by speakers in this session. Marder illustrated, from computational analyses of the crustacean stomatogastric ganglion, the role of synaptic peptides in stabilizing neuronal circuits under stressful challenges, even though individuals varied in their susceptibility to stress-induced changes in circuit dynamics. The ability of peptide cotransmission to enhance flexibility of output responses in these circuits requires peptide-driven combinatorial changes in firing patterns that are reflective of environmental context as well as homeostatic status of the animal in toto.2 White and colleagues exemplify this in description of the highly integrative role of Drosophila peptides such as crustacean cardioactive peptide (CCAP) and bursicon to trigger life events, such as metamorphosis during development, that require the sequential actions of several motor systems. The timing of these events must be precise, and therefore requires “master regulation” by first messengers anatomically placed to achieve this sequence.3 The perspective offered by Marder and White strongly suggests that despite structural and functional differences in regulatory peptides across phyla as divergent as lobsters and primates, general principles of peptide action may be conserved. The case for acquiring insights into regulatory peptide control of behavior in humans, by investigations in other species, has been made in detail by White and colleagues.4
A particular challenge for consideration of peptide receptors as drug targets for behavioral disorders is that regulatory peptides invariably have parallel role(s) in integrating both homeostatic physiological responses, and behavior adaptation to other environmental cues and drives. How can the effects of oxytocin on parturition and affiliative behavior, those of vasopressin on hydromineral balance and social behavior, those of PACAP on pituitary hormone secretion and during stress and anxiety, for example, be physiologically dissected? Can this analysis be extended to pharmacological selectivity at the same receptor at different anatomical locations, usually the hypothalamus for homeostatic regulation, and more rostral brain structures for behavioral responses? The Sexton laboratory, and its collaborative network, has focused on “distinctions with a difference” both between different ligands acting at the same receptor, and individual ligands at related receptors. A prominent example is the GLP-1 receptor, which recognizes multiple endogenous peptides including GLP-1 and oxyntomodulin, another biologically active gut peptide (see Figure 1). Both of these peptides trigger pleiotropin signaling, but may preferentially engage either arrestin/ERK-regulated responses, or G-protein-coupled calcium and cyclic AMP signaling, as a function of differential binding to the GLP-1 receptor.5 The discovery of receptor activity-modulating proteins (RAMPs) provides an additional level of complexity, which can be exploited with peptide-based agonist and antagonist compounds. The calcitonin receptor (CTR) binds either calcitonin itself or amylin, depending on the presence of a RAMP, while the closely related calcitonin-like receptor (CLR) shows RAMP-dependent preference for either CGRP or adrenomedullin.6,7 As bioinformatic and molecular modeling workflows for dynamic interrogation of ligand–receptor interactions converges on improved structure elucidation, it can be expected that the understanding of protein structural dynamics and their regulation by allosteric factors will become an important avenue for peptide-centric drug development.8,9
Figure 1.
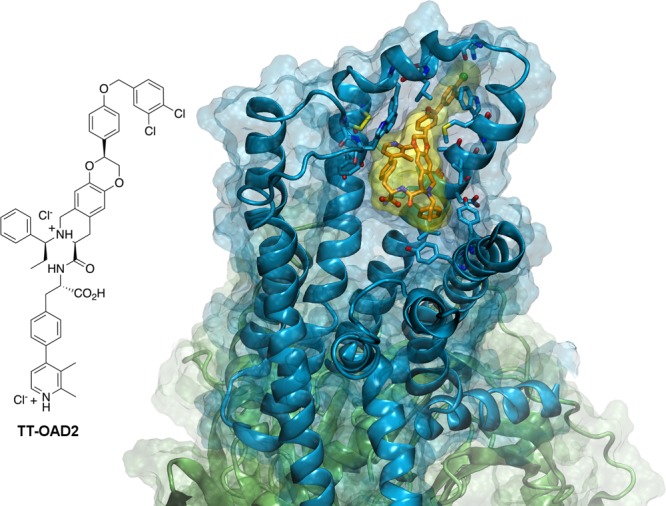
GLP-1 Receptor as a paradigm for peptide-liganded receptors. The cryo-electron microscopy structure of TT-OAD2, a nonpeptide agonist, bound to the active glucagon-like peptide-1 receptor (GLP-1R) in complex with its G protein.31 The ligand position is roughly similar to other Class B GPCRs, in that agonists (peptides or peptidomimetics) are located within the contact regions between upper parts of the TMs and the extracellular loops (ELs). However, the binding pose of this biased agonist has minimal overlap with the poses of full-length peptide agonists of the same receptor. The ligand, the 2D structure of which is reported on the left, is depicted by orange sticks and a yellow surface. The receptor is rendered by cyan ribbon and surface, and residues within 4 Å of the ligand are shown as cyan sticks. The heterotrimeric Gs protein is depicted by green ribbon and surface. The complex was subjected to the Protein Preparation Wizard Tool101 of the Schrödinger suite (Schrödinger Release 2019-3: Maestro, Schrödinger, LLC, New York, NY, 2019) and the figure was realized with VMD 1.9.31996.102 This figure is an original graphic realized from the atomic coordinates of the structure reported, and the chemical structure of the ligand was drawn using ChemDraw.
A further elaboration on specificity for peptide-liganded receptor targets on specific cell types has been championed by DiMarchi and colleagues, including the intriguing use of chimeric peptides for multiple ligands involved in metabolic regulation, such as GLP-1 and glucagon/GIP, as well as peptide-steroid hormone adducts that use the peptide ligand for homing to cell type, and the steroid hapten for modulation of activity of the particular cell targeted.10 Whether peptide receptors per se are uniquely suited to the chimeric and combinatorial drug design of this type remains to be established, but if so, this would be a powerful approach for limiting the impact of peptide-liganded receptor targeting to specific subpopulations of such targets in the nervous system.
Peptides as Drugs: Rethinking the Drug Pipeline?
The NIMH Workshop was keynoted by NIMH Scientific Director Susan Amara. As codiscoverer of calcitonin gene-related peptide (CGRP), a splice variant-generated neuropeptide from the calcitonin gene locus,11 Amara provided a unique perspective on the march of CGRP toward therapeutic application that may be paradigmatic for future exploitation of many neuropeptide/receptor systems, building from discovery to physiology, to pathophysiology, to drug target, and finally to therapy for migraine. Across this development, spanning some 30 years, there has been much interchange of knowledge between clinical observations, basic findings, and their translation into clinical practice. The CGRP saga eloquently illustrates the importance of “reverse translation”—learning from early attempts at bench to bedside translation that some hurdles to drug development cannot be overcome only by applying empirical solutions that rely on moving from “validated drug target” to “valid drug”—but rather require substantial rethinking about basic tenets of biology to overcome these hurdles efficiently.12
Some of the common barriers to peptide penetration to the brain are illustrated in Figure 2. While there is increasing evidence that many peptides can enter the brain from blood and nasal mucosa, this is a pathway forbidden to many other peptides, and a source of motivation to create more “drug-like” molecules. Another approach to overcome the bioavailability hurdle is the “post-translation” modification of peptides, i.e. the chemical synthesis of “peptoids”, that is, peptides modified by derivatization, d-amino acid substitution, substituent moiety addition, or cyclization. James Collins of the Fering Peptide Foundation, and colleagues, have concentrated on the production of a second generation of peptide ligands featuring glycosylation and macrocyclization as two modes of peptide modification that can improve oral absorption and other aspects of pharmacokinetics, including stability through metabolic resistance, and pharmacodynamic potency via conformational stabilization.13 These features can allow peptides to overcome substantial barriers to brain entry from a variety of routes of administration, including intranasal, oral, and intravenous. It is worth noting that various endogenous peptides require post-translational modification for activity, including cholecystokinin (sulfation) and ghrelin (acylation).14,15 Thus, some peptide binding sites may have evolved to accommodate allosteric receptor activation in conjunction with that provided by the primordial peptide sequence itself. If so, focusing on allosteric interactions between ligand and receptor, along with the entropic advantage of coupling with the peptide (orthosteric) ligand itself, may represent a human-engineered strategy that is already copied from nature. Closer examination of receptors accommodating post-translationally altered peptides may provide further clues for copying this strategy even more effectively.
Figure 2.
Barriers to peptide drug development. Illustration of the steps, involving the overcoming of sequential “barriers” to peptide-based drug design. (A) Validation of target. (B) Refining the target space and potential drug ligands for the validated receptor. (C) Optimization of drug delivery, potentially very different in approach depending on whether a “rule-of-five” receptor agonist/antagonist or a peptide or peptoid based on the endogenous ligand is chosen/obtained by screening. (D) Pharmacogenomics in pathogenic and therapeutic assessment. Figure produced using Biorender.
The idea of creating a “toolbox” of chemistry applicable to peptides in general, and tailored to specific peptide ligands, is in harmony with the understanding that the blood–brain barrier is not homogeneous with respect to access to all brain regions. Furthermore, peptides may differ dramatically in their rates of transport into and from the brain based on the heterogeneity of peptide transport systems existing at the blood–brain barrier.16−18
Another mode of peptide modification leading to enhanced biological activity occurs in “peptide chimerism”, an approach used extensively by Anish Konkar and colleagues, at Sanofi-Aventis, for the development of antidiabetic and antiobesity peptide therapeutics.19,20 Although chimeric peptides appear to be effective, compared to administration of combinations of individual peptides, the exact reason why this might be so is as yet unexplored. Thus, chimeric peptides are another example of the pressing need for “reverse translation”—finding out not just what works but why that which already works, does so, so that new general principles of peptide action can emerge. Such approaches hold promise for future drug development, building upon other ligand–receptor dyads and novel chimeric peptide combinations.
The considerations above also raise the issue of whether or not merely ascertaining free peptide concentrations in brain is the best way to guide peptide/nonpeptide drug modification. Quantifying “target engagement”—demonstrating that peptides/other drugs reach their receptors in brain—is important in drug development. This has usually necessitated the introduction of radiolabeled congeners for PET, or other imaging modalities, during late-stage drug development.21,22 However, Konkar and others have explored the additional possibility of developing indices of functional target engagement, such as measurement in animal models of brain immediate early gene activation following drug administration. This approach has some attractive features: while it does not answer directly whether a peptide enters the brain, it does indicate whether CNS responses to the drug occur. Ultimately determination of target engagement in human subjects requires noninvasive methods. Yet, functional target engagement in animal models can provide a powerful translational impetus to drug development efforts. Target engagement studies reveal critical basic information about how peptide-dependent information processing occurs in the brain, and they suggest parameters for optimization of drug design that both increase the probability of success, and increase the probability of leveraging failure into future progress. Increasingly, the description of peptidergic brain circuitry regulating behavior depicts a widespread pattern of brain activation from one or a few clusters of peptidergic cells.23−28 In this context, we can determine what might be called specific target engagement—the ability of a drug to affect behavior via action at a subset of functional consequent projections or synapses—through immediate early gene activation and other signals of receptor activation in laboratory animals. This knowledge will be extremely useful for focusing receptor subtype-specific, and perhaps signaling pathway-specific pharmacology on discrete behaviors comprising both symptomatic and underlying aspects of human brain disorders. In the final analysis, empirical evidence for peptide ligand entry into the brain is essential. This is especially true as several of the peptides currently administered for engagement of peripheral receptors, for example in management of diabetes, may have central actions that are related to therapeutic outcome; for example weight loss associated with administration of GLP-1 for primary treatment of type II diabetes. Understanding where a peptide acts, and by analogy the relative distribution of a mimetic drug in the body, critically links the mechanism to drug action for metabolic as well as behavioral disorders, especially for treatments that potentially address both domains.
In general, the accepted wisdom that the drug pipeline for peptide-liganded receptor targets requires first and foremost production of a nonpeptide equivalent ligand for the receptor is being increasingly questioned. The archetypal examples, morphine and naloxone as agonist and antagonist at the μ opioid (MOR) receptor, and their potencies relative to the endogenous ligand enkephalin, helped establish the principal that drug equivalents, rather than endogenous ligands are required for therapeutic purposes. However, this precedent was set mainly because the discovery of the nonpeptide ligand preceded the discovery of the actual endogenous peptide ligand. Even the realization that there might be an endogenous ligand for the “opiate receptor”, lagged behind, by hundreds of years, the medical use of morphine as well as naloxone, and in fact the nonpeptide agonist and antagonist were actually tools used in establishing that the peptide, “enkephalin”, did actually exist in the mammalian brain and gut.29 The number of potent, conveniently bioavailable nonpeptide agonists or antagonists at other peptide-liganded receptors is growing. Aprepinant, an orally active substance P receptor antagonist used to treat chemotherapy-induced nausea,30 is one of them, and analogues of bombesin, cholecystokinin, and recently announced nonpeptide GLP-1 agonists also exist.31,32 Several nonpeptide GLP-1 agonists are designated for clinical trials for type 2 diabetes (ClinicalTrials.gov Identifiers: NCT02653599;32), and receptor complexes with both peptide and nonpeptide agonists (see Figure 1) have been reported.31,33 However, overcoming the supposed pharmacological limitations of peptides per se, as potential therapeutics, would be useful to circumvent the difficulties of screening for and identifying nonpeptide ligands for peptide receptors. It would also likely represent an important accelerant to peptide-based neurotherapeutics development. In this context, two approaches are notable: the use of modified peptides, including substitution of isomeric d- for l-amino acid residues imparting greater peptidase stability in vivo, and the use of peptide antibodies, essentially to “immunize” against deleterious effects of an endogenous peptide, such as CGRP in migraineurs.34
Overall, a return to the discovery of orally active peptide congeners, or administration of actual endogenous peptides as drugs, is experiencing a resurgence,35 and we can expect that better structural information (see below) will accelerate this trend. In addition, the perceived wisdom that serum half-life predicts peptide action is also earning a reappraisal. While free drug concentration in serum at any given time after drug administration predicts future drug action at its receptor, it does not follow that the inability to detect drug (or peptide) in serum predicts that the drug is no longer acting at its receptor, and indeed may continue to act at its receptor for some considerable additional period of time. Thus, careful attention to whether or not prolongation of serum half-life actually lengthens the period of drug action will no doubt be a part of the regulatory peptide “virtual drug pipeline” in the future. For now, it is clear that there are actual peptides that can be administered, with extended efficacy, at intervals exceeding the plasma half-life of the administered peptides.36,37 Learning more about receptor binding and internalization by peptide ligands on a case-by-case basis, and establishing more rational ‘behavioral pharmacodynamic’ assays to assess their actual efficacy half-lives, will likely help to change the paradigms that currently guide drug discovery efforts in this arena.
Neuropeptide Y (NPY) has been considered a potential treatment for PTSD since at least 2000, when Charney and colleagues made the observation that plasma NPY levels were lower, and elevated less with yohimbine, in combat veterans with PTSD compared to those without.38 It was hypothesized that lower levels of NPY secretion in the sympathetic nervous system contributed to sympathetic hyperreactivity in PTSD. Other investigators, including the laboratory of Esther Sabban, at New York Medical College, focused on NPY expression using the single prolonged stress (SPS) rodent model for PTSD, and found that SPS induced central noradrenergic up-regulation and anxious and depressive behaviors, and that these were reversed by intranasal treatment with NPY peptide,39,40 in accord with findings (see ref (41)) that manipulation of NPY levels in rodent models correlates negatively with anxious behavior (that NPY is anxiolytic). Sabban, at this workshop, and Charney and colleagues,42 speculated on the future of NPY neurotherapeutics and how to accelerate the process. It has been emphasized that (1) serum NPY is a marker for PTSD, but requires further evaluation for trauma specificity due to uncertainty over where and through which receptor(s) endogenous NPY acts; (2) as NPY is expressed in both sympathetic nervous system and brain its actions at both sites needs systematic evaluation; and (3) given the number of NPY receptors (Y1–Y5), systematic pharmacological identification of the receptor subtype profile for PTSD needs better definition (Y1 and Y5 receptors are currently favored as major targets in anxiety/PTSD). Clearly there is a need for reverse translation to fill critical gaps in knowledge—including sex-dependent trauma variation and potential treatment effectiveness43—that persist even as NPY for treatment of PTSD enters phase II clinical trials (see ClinicalTrials.gov, identifiers NCT01533519 and NCT04071600). Schmeltzer and colleagues in their excellent review of the possibilities for NPY-based treatment of PTSD stated “···the NPY system is an attractive target in terms of understanding the physiological basis of PTSD as well as treatment of the disorder”.41 This is certainly true as long as impending clinical trials are designed to provide knowledge of why they were as effective as they were, regardless of the outcome. The status of NPY as a potential treatment for PTSD is highly illustrative of the critical need for a “virtual drug pipeline” that allows the constant evaluation and re-evaluation of the entire physiological profile for a given peptide, and the common potential for peptide action at multiple receptors, in the context of drug development for a specific human disorder or malady. For NPY, it remains unknown exactly where target engagement occurs after intranasal administration in SPS; which specific NPY receptor agonists can mimic the effects of NPY itself; and whether the distribution of putative target receptor(s) is similar across mammalian species. Filling these additional gaps in part defines what is meant by the “virtual drug pipeline”.
Marc Ferrer represented the National Center for Advancing Translational Sciences (NCATS), created by Congress in 2011 to ‘catalyze the generation of innovative methods and technologies that will enhance the development, testing, and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions.’ An example of innovation in this area is the development of analogues for the peptide relaxin, which acts at the RXP-1 receptor. NCATS and its collaborators were able to leverage structural information about relaxin and a high-throughput cell-based assay for relaxin action at RXP-1, in a cell line harboring the endogenous receptor, into development of a drug-like molecule with a much longer half-life than relaxin itself, and effective in treatment of preclinical models of liver fibrosis.44,45 Assays such as those reported by Ferrer and colleagues may also be useful in the development of more specific compounds for antagonizing or mimicking relaxin’s actions at RXP-3 receptors in the central nervous system (vide infra).
Ferrer also described a collaboration with NIMH scientists in which an assay for PACAP signaling at the PAC1 receptor was developed for both high-throughput screening of novel PACAP antagonists, and confirmation of bioactivity of previously reported PACAP antagonists. Their studies demonstrated that compounds previously documented as PAC1-specific based on receptor binding assays46 may in fact interact with other PACAP receptors, including the VIP-preferring VPAC1 and VPAC2, in whole-cell assays. The recent report of the cryo-EM structures of PAC1 by the Monash group47 and others48,49 may be useful in further development of PACAP antagonists with greater specificity than those currently reported.50−52
Another “wrinkle” in the drug pipeline is that evaluation of animal models is critical at all steps of evaluation: pharmacodynamics, pharmacokinetics, proof of concept, and specific drug development. This is true in general and for CNS neurotherapeutics in particular. For example, although the human and rodent 28-amino acid ghrelin peptides differ by only two amino acids, significant differences in ligand recognition at its receptor, as well as differences in activities of partial agonists and inverse agonists on constitute receptor activity in various organs and species, warrants careful “reverse translation” of the reported physiological effects of ghrelin-based compounds in rodent animal models.53,54 For another target of metabolic interest, the relatively mild GLP-1 knockout phenotype of mice belies the fact that, in human, GLP-1 is a major actor and entry point for therapeutics for metabolic disease.55 Animal models themselves may require some considerable rethinking if they are to be the “default pathway” to drug development for regulatory peptide-based therapeutics. It remains as true today as a decade ago that animal models for major neuropsychiatric disorders map poorly to disease treatment. This is, in large part, because psychiatric disorders are not conceptualized as symptom clusters, but rather considered globally, frustrating robust surrogacy in animal model screening.56 However, research domain criteria (RDoC), and other tools proposed for reconceptualizing various disorders as symptom-clusters centered on arousal, anxiety, fear, aggression, and other areas, may advance the field of neurotherapeutics and re-establish a viable pipeline from laboratory to clinic, in coming years.57,58
Samer Hattar from NIMH-IRP, who chaired this session, has studied the role of neuropeptides coreleased from innately photosensitive retinal ganglion cells with projections throughout the brain, including hypothalamus, habenula, and retina-associated stations in brain stem and thalamus. He has pioneered the use of conditional knockouts, knock-ins, and optogenetic analysis to explore the role of this unique peptide-containing projection system to convey light information to the brain not to be decoded as vision, as for secondary sensory (postphotosensitive) retinal ganglion cells, but for detection of day and night cycles in order to coordinate brain states with the tasks associated with light and dark in mammalian species.59,60 Humans continue to adapt to the increasingly “noncircadian” environment recently palimpsested (by electric lighting) upon the more ancient circadian one provided by the sun, and peptide cotransmission may play an important role in the coordination of behavior and mood with light, a complex process being slowly understood.
Peptide Paradigms: Ghrelin, GLP-1 and Vasopressin Receptors for CNS-Based Therapeutics
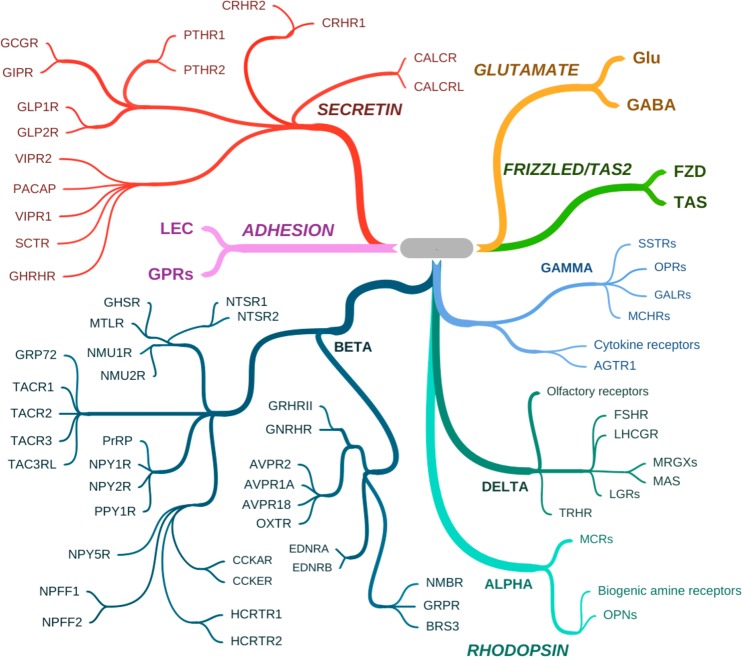
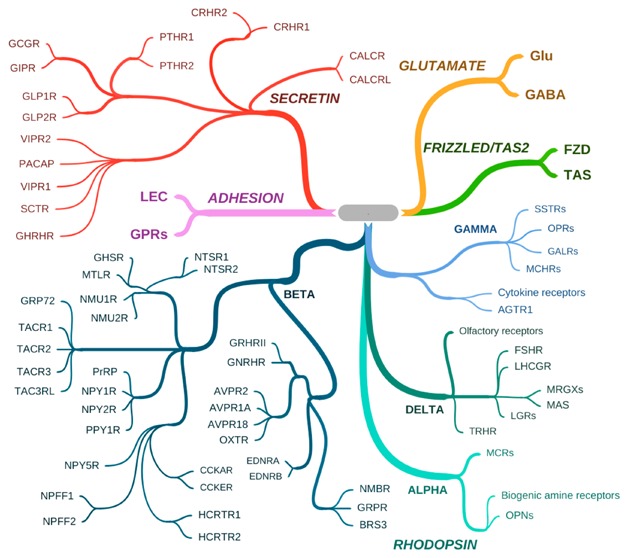
The peptide-liganded GPCRs, each potentially “druggable”, are illustrated in Figure 3, within the common GRAFS classification of the G-protein coupled receptor family. The session on peptide paradigms focused on three specific peptide receptors that are candidates for CNS-based therapeutics in diverse behavioral realms. The session was chaired by Mario Penzo, whose own work on peptidergic neurons has demonstrated the gating of stress responsivity by the paraventricular nucleus of the thalamus. This occurs via attention to arousal signals from locus coeruleus and elsewhere, and can be traced to regulation of the synaptic potentiation of somatostatinergic neurons of the lateral division of the central amygdala (CeL). Projections from these neurons in turn enables expression of fear learning (fear conditioning)61 thought to underlie psychiatric disorders including PTSD.
Figure 3.
Peptide-liganded GCPRs. Peptide-liganded receptors are found within two of the five major GPCR classes.103 The figure shows representative peptide receptors within the SECRETIN (also called family B) and RHODOPSIN (also called family A) classes. The SECRETIN class contains only peptide-liganded receptors, those for GHRH, secretin (SCT), PACAP, VIP, GLP-1, GIP, glucagon (GCG), parathyroid hormone (PTH), corticotropin releasing hormone (CRH), calcitonin (CALC), and calcitonin gene-related peptide (CGRP), which recognizes the calcitonin-related receptor (CALCRL). Depending on the presence of RAMPs, CALCR and CALCRL also recognize amylin and adrenomedullin, respectively. The RHODOPSIN class contains many other peptide-liganded GPCRs within the subclasses ALPHA, BETA, GAMMA, and DELTA. In the ALPHA subclass, the melanocortin receptors (MCRs) which recognize alpha-MSH and ACTH are shown, with the ALPHA class nonpeptide receptors for biogenic amines (e.g., dopamine, norepinephrine, serotonin), and the opsins (OPNs) which are the founding members of the RHODOPSIN class, also shown to provide context. The BETA subclass contains the largest number of peptide-liganded receptors including those for neurotensin (NTSR), neuromedins (NMURs), motilin (MTLR), tachykinins (TACRs), prolactin releasing peptide (PrRP), neuropeptide Y (NPY), pancreatic polypeptide (PPY), cholecystokinin (CCK), neuropeptide FF (NPFF), hypocretin/orexin (HCRTR), ghrelin, or growth hormone secretagogue (GHS), growth hormone-releasing hormone (GH-RH), gonadotropin-releasing hormone (GNRHR), vasopressin (AVPRs), oxytocin (OXTR), endothelin (EDNR), neuromedin B/mammalian bombesin analogue (NMBR), gastrin releasing peptide (GRPR), and bombesin (subtype 3) (BRS3). The GAMMA subclass contains the receptors for somatostatin (SSTRs), the opioid peptides dynorphin (OPRK1 or kappa receptor), endorphin (OPRD1 or delta receptor), enkephalin (OPRM1, or mu receptor) and nociception/peptide FQQ (OPRL1), galanin (GALRs), melanin-concentrating hormone (MCHRs), angiotensin II (AGTR1), and the polypeptide-liganded cytokine receptors, the largest by number in this subclass. The DELTA subclass contains the receptors for the follicle stimulating and luteinizing hormone receptors (FSHR and LHCGR), Mrg (MAS-related G protein-coupled) and MAS receptors, with ligands including opioid peptide BAM22P (for MRGPRX3) and potentially PACAP-38, leucine-rich repeat-containing G-protein coupled receptors (LGRs) which contain the deorphanized relaxin receptors RXFP1/LGR7 and RXFP3GPCR135, thyrotropin-releasing hormone (TRHR), and the nonpeptide-liganded founder members of this subclass, the large group of olfactory receptors, shown to provide context. Orphan receptors tentatively associated with peptide activation, for example, gpr160/GPCR1, associated with activation by cocaine amphetamine regulated transcript (CART) peptide), and GPCRs associated with neuropeptide S and others, are not shown here.
Ghrelin is a regulatory peptide produced in the gut, but with far-reaching effects in the periphery, in the pituitary, and in the brain, including a behavioral linkage between hunger and fear also thought to be mediated via amygdalar circuitry. Ghrelin was first identified as the ligand for the orphan growth hormone secretagogue receptor 1a (GHS-R1a).62,63 Surprisingly, the peptide hormone ghrelin acting on somatotrophs of the pituitary is produced in enteroendocrine cells of the gut, and perhaps more surprisingly, ghrelin (although likely produced locally) also acts at the arcuate nucleus of the hypothalamus to affect appetite, and at other brain regions including hippocampus and amygdala, to mediate stress-dependent fear behaviors.63 Studies in knockout mice have shown convincingly that ghrelin is primarily involved in mediating satiety.54,64 Ghrelin has been proposed as a mediator of anxiety and food reward in addition to its role(s) in signaling satiety and controlling growth hormone secretion.65,66 Ki Goosens, from the Icahn School of Medicine, has advanced ghrelin as a biomarker for chronic stress, and demonstrated in rodent stress models that GHS-R1a antagonism can prevent stress-enhanced fear, suggesting both a treatment for anxiety disorders such as PTSD, and a means of stratifying patients for such treatment.67 The complexity of ghrelinergic signaling offers many potential therapeutic points for intervention in stress-associated disorders (Figure 4), including several in the periphery. These represent especially attractive targets because blood-brain barrier penetrance does not need to be overcome.
Figure 4.
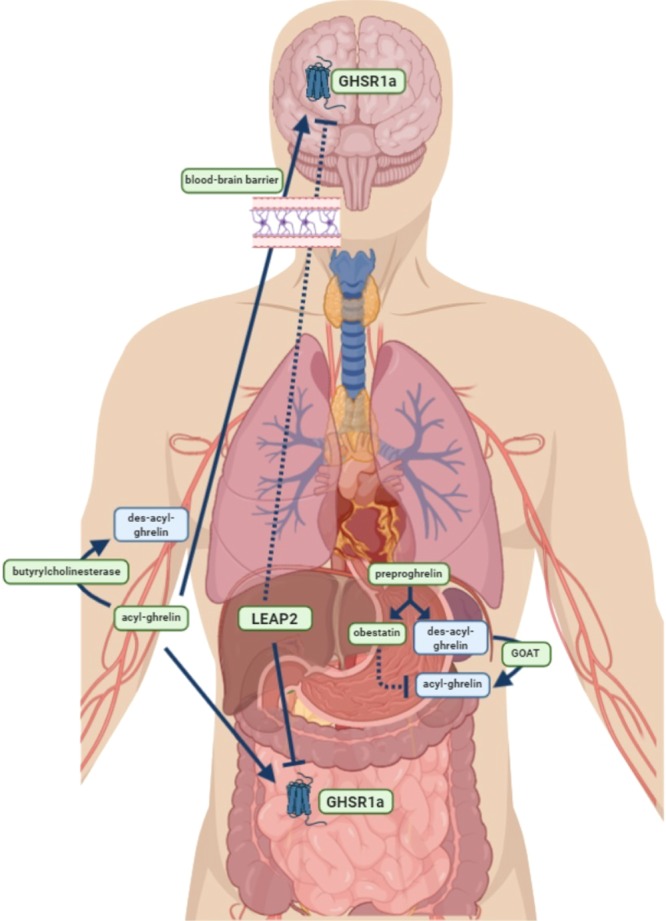
Multiple theoretical targets for altering ghrelinergic signaling. Ghrelinergic signaling involves a complex network of proteins and enzymes in the brain and periphery. Specialized endocrine cells in the fundus of the stomach produce the prohormone preproghrelin, which is post-translationally spliced into both obestatin and des-acyl-ghrelin. The GOAT (ghrelin-O-acyltransferase) enzyme, also present in the stomach, acylates des-acyl-ghrelin at a single residue to form acyl-ghrelin. This is a critical and necessary step for ghrelin to bind to its receptor (GHSR1a; for growth hormone secretagogue receptor 1a) and cross the blood-brain barrier. From the stomach, acyl-ghrelin is released into the bloodstream, where it can travel to bind to its receptor in the periphery (depicted here only in the gut, but present in many organs, circulating immune cells, and other tissues) or cross the blood–brain barrier to bind to its receptor in the brain. In the bloodstream and brain, acyl-ghrelin is inactivated by the enzyme butyrylcholinesterase and becomes des-acyl-ghrelin. Whether des-acyl-ghrelin exerts biological activity is not clear. LEAP2 (liver-expressed antimicrobial peptide 2) is an enzyme produced by the liver and gut and secreted into the bloodstream; LEAP2 antagonizes the effects of ghrelin by competitive binding at the GHSR1a. Obestatin is also known to antagonize the effects of ghrelin, but the mechanisms by which this is achieved are unknown. From a therapeutic standpoint, there are many potential points at which ghrelinergic signaling can be modulated. This includes the synthesis of acyl-ghrelin in the stomach, the breakdown of acyl-ghrelin in the blood, the passage of acyl-ghrelin through the blood-brain barrier, and the activity of GHSR1a. These targets are depicted in green boxes. Lines indicate the effects of the indicated hormone or enzyme on its target (stimulatory or inhibitory); dotted lines indicate uncertain effects. Figure produced using Biorender.
Like ghrelin, GLP-1 is produced in gut enteroendocrine cells and released into the blood to effect hormone secretion from endocrine pancreas (that is, it acts as an incretin). The GLP-1 analogues exenatide (a reptilian paralog of mammalian GLP-1) and liraglutide are employed in the treatment of type II diabetes, with the latter also approved for treatment of obesity, with the presumptive mechanism of action of mimicking the incretin action of GLP-1 in enhancing glucose-stimulated insulin release from pancreatic beta cells.68 GLP-1 also causes weight loss, through a behavioral mechanism involving reduced food intake, and this effect, even of peripherally administered GLP-1 or GLP-1 agonists, may be mediated through activation of GLP1r1 receptors within the central nervous system.69,70 Separation of the physiological and pharmacological effects of GLP-1 agonists to endocrine (presumably enteroendocrine) cells and neurons using cell-type deletion of the GLP-1 receptor suggests that different receptor populations mediate metabolic and feeding effects,71 offering an avenue to targeting these effects separately, using either biased agonism, differential drug pharmacokinetics, or combinatorial approaches.
The neuropeptide focus (on, e.g., ghrelin, GLP-1, leptin, oxytocin) of the NIDA/NIAAA-funded Clinical Psychoneuroendocrinology and Neuropsychopharmacology (CPN) Section, led by Lorenzo Leggio, reflects how ingestive behaviors can generalize from food to other rewarding/addictive comestibles such as alcohol and drugs of abuse. Also, a continuum of associative learning neuronal circuits, all intimately involving neuropeptides, governs decision-making leading to seeking behaviors. Quantifying drug-seeking behaviors in animal models of addiction, in transgenic rodent models, and in clinical populations studied via small Phase 1b/2a proof-of-concept human laboratory studies performed under rigorous and well-controlled conditions, has allowed Leggio and colleagues to gather data about the likelihood that addictive behaviors can be changed by physiological, transgenic, or pharmacological manipulations of a peptide system. An example of this approach is work conducted on the potential role of the ghrelin system in alcohol use disorder (for a recent review, see ref (72)). The ability to carry out such studies to accelerate further clinical development is balanced against the difficulties of establishing across multiple treatments a “false discovery rate” that accurately informs and justifies such efforts. This is a critical consideration for all stakeholders in CNS neurotherapeutics, peptide or otherwise.
Vasopressin perhaps shares pride of place only with insulin as a bioactive peptide with long-standing clinical utility. This remains, for vasopressin’s peripheral actions, but is only recently being developed for treatment of behavioral disorders. Limei Zhang, from the University of Mexico, discussed emerging understanding of vasopressin not as a hormone, but as a central neurotransmitter. This realization was spurred by classical observations by Buijs and others that vasopressinergic neurons existed in brain regions far from the hypothalamus, necessitating the consideration that vasopressin’s actions in the brain may not be hormonally mediated.73,74 Subsequently, neuroanatomical work of the Zhang laboratory demonstrated not only vasopressinergic projections to various limbic brain regions from magnocellular vasopressinergic neurons of the hypothalamus, but the establishment of bona fide synapses by these projections.75−79 Several themes have emerged from this work. One is that vasopressin likely acts as a cotransmitter, rather than alone, in mediating its effects at these invariably glutamatergic synapses. A second is that changes in vasopressin expression in the hypothalamus, driven by hydromineral balance, affect not only vasopressin secretion from the pituitary posterior lobe, but its release at dually projecting nerve terminals in hippocampus, amygdala, habenula, or locus coeruleus. Thus, basic homeostatic considerations (salt balance) manifested in thirst are conveyed to brain centers that provide salience to other sensory inputs and globalize priorities for seeking, or averting, specific stimuli. While the implications for neuroscience are profound, the implications for drug development are practical. One must ask: are there pharmacological targets in vasopressinergic cotransmitting neurons within the brain that are absent from vasopressinergic peripheral regulation conveyed hormonally?
Proof-of-Concept for CNS Peptide-centric Neuropharmacology and Therapeutics
As can be appreciated from the preceding section, the notion of proof-of-concept is not as straightforward for neurotherapeutics as it is for some other treatment arenas. Definitions of proof-of-concept can depend upon the disease or disorder in question and its genetic and physiological underpinnings. The absence of well-articulated criteria for proof-of-concept can in turn lead to disagreement over how to conceptualize drug targets at each stage of translation. As an example, a specific gene translocation leading to dysregulation of the disrupted gene and a specific kind of cancer, as for the Philadelphia chromosomal translocation and chronic mylogenous leukemia, provides a clear drug target for cancer treatment. Here, proof-of-concept involves interfering with a specific mechanism, unregulated tyrosine kinase activity, that drives disease.80 For gene mutations even very tightly correlated with clinical pathobiology of human brain disease with strong animal models, such as the DISC1 schizophrenia gene,81,82 or Shank-1 association with autism,83 this is not the case. Even the very striking ApoE4 allelic association with Alzheimer’s disease is not clearly identified as a gain or loss of a specific ApoE function, clearly hampering identification of drug targets.84 In others, mutation is associated with a very small subset of the vulnerable clinical population, in which case the demographics of drug targeting inhibits rather than encourages major drug development efforts. A key concept in establishing proof-of-concept also involves demonstration that the drug actually reaches its target in the brain, given the existence of a formidable biological barrier to entry of many drugs into the brain. In short, proof-of-concept is often rather proof of a succession of “concepts” that must be “proved” along the road to development of neurotherapeutics. Scott Young, from NIMH-IRP, chaired the final session in the NIMH Workshop on Regulatory Peptides and Neurotherapeutics, introducing a slate of speakers who addressed peptide-centric neuropharmacology from this perspective. The work of the Young laboratory, and others, exemplifies basic research focused on vasopressinergic regulation of social behavior, with a new target for vasopressin action, the CA2 region of the hippocampus, emerging as a result of traditional chemoanatomical, optogenetic, and behavioral experiments.76,85−87
The approval of Baqsimi nasal powder, a glucagon preparation with solubilizing and emulsifying excipients for emergency treatment of insulin-induced hypoglycemia (see https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf), highlights a new focus on peptide delivery systems in the pharmaceutical industry. Peptides, with their unique net charge, charge distribution, and primary sequences, interact with biological systems idiosyncratically. Thus, establishing criteria similar to Lipinski’s “rule of five” that defines desirable characteristics of small-molecule drugs, for peptides as pharmaceutical agents, is unlikely. However, a critical path for peptide subclasses does seem attainable. Mary Lee and colleagues from the NIDA/NIAAA-funded Clinical Psychoneuroendocrinology and Neuropsychopharmacology (CPN) Section have focused on intranasal administration of oxytocin for the potential treatment of alcohol and psychomotor stimulant abuse.88 Using deuterium-labeled oxytocin delivered to primates, this translational laboratory has been able to compare endogenous and exogenous levels of oxytocin in primate brain, to effectively “map” the brain distribution of oxytocin after intranasal administration and to predict its delivery to brain regions where it is likely to act at postsynaptic receptors.89 This work offers an important opportunity for reverse translation, as in a rodent model oxytocin delivered intravenously affects self-administration of methylphenidate.90 Repeating such experiments with various modes of administration, including the intranasal route, in this animal model should allow the correlation of delivery site with efficacy. Demonstrating functional receptor engagement by correlation of peptide delivery and efficacy has obvious translational relevance, which might be generalizable to other potential peptide neurotherapeutics.
Michael Brownstein of Azevan, Incorporated, has broadly considered the issue of drug purposing based on emerging basic research findings and their adaptation to clinical need. Recent laboratory findings, including those from the Young lab and others, point toward modulation of social behavior by vasopressin acting on the CA2 region of the hippocampus via the V1b receptor, while activation of V1a receptors in other brain areas may mediate aggression in mammals.91 Extrapolating from this work and that of many others, Brownstein and colleagues have devised an ongoing clinical trial, in which patients with Huntington’s chorea receive a vasopressin V1a receptor agonist, and are assessed for aggressive behavior by their caretakers. This study simultaneously representing both translation and reverse translation may provide guidance in tackling neurotherapeutics for other CNS disorders by focusing on quantifiable symptoms emerging in the activities of daily living of both patients and those who treat and care for them.
The laboratory of Ken Jacobson, Chief of the Molecular Recognition Section and the Laboratory of Bioorganic Chemistry, NIDDK, has studied ligand–receptor interactions in a group of nucleotide G protein-coupled receptors recognizing adenosine (the Adora1 (A1), A2A, A2B, and A3 receptors) and nucleotides (P2Y receptors). In the adenosine receptor series, it is now recognized that building out of functionality around individual domains (e.g., adenine, ribose) creates a series of congeners with constant properties in one domain with variation in the other.92 In the case of the purinergic receptors, this allows a measure of selectivity, in both agonist and antagonist compounds, for targeting inflammatory diseases, chronic pain, thrombosis, and cardiac ischemia, and other conditions.93 Computational modeling of purinergic GPCRs and their ligand interactions, for example through docking and molecular dynamics simulation, are effective in the discovery of both novel chemotypes by virtual screening, and the rational design of analogues of known chemotypes. When a closely related GPCR structure has been determined, but not that of the actual target receptor, validated homology modeling can be useful for drug discovery. Lessons learned in this arena clearly apply to peptide-liganded receptors, not only for recognition among closely related receptors (e.g., the PACAP/VIP receptors PAC1, VPAC1, and VPAC2; the glucagon peptide family and its receptors) but to engineer specificity for different signaling arms of the same receptor.
The NIMH-IRP Translational Neuropsychopharmacology Initiative, under the direction of NIMH-IRP’s Janet Clark, was developed in part as a response to recognition that the pharmaceutical industry had in recent years begun to deploy fewer resources for drug development into areas of CNS therapeutics due to expensive and difficult-to-recoup constrictions in the drug pipeline in its clinical phases. It seemed clear that micropharma contributions to the drug pipeline94 needed to be more explicitly recognized in the area of CNS neurotherapeutics. To date the program, primarily through its Translational Neuropsychopharmacology Task Force https://www.nimh.nih.gov/research/research-conducted-at-nimh/scientific-director/office-of-scientific-director/nimh-irp-translational-neuropsychopharmacology-initiative.shtml has had some signal successes in providing support to nascent drug development efforts in urgently needed areas including most notably major and postpartum depression. Janet Clark described at this NIMH Workshop how the Translational Neuropsychopharmacology Initiative fits into the concept of the “virtual drug pipeline” for neurotherapeutics. William Potter, formerly involved in drug development in the pharmaceutical sector, and currently advisor to the Office of the Director, NIMH, described further efforts in helping to shape the drug pipeline through precompetitive consortia for drug development, describing vividly how a failure to meet academia- and industry-wide standards for completion of discrete milestones in moving from concept to drug has been costly in terms of achieving success. Thus, identifying areas in which consortial efforts might yield better payoffs provides an alternative model whereby a single failure (for a plethora of reasons) does not “poison the well” of future drug development in otherwise promising areas. Precompetitive consortia, perhaps not only clinically but preclinically, can function to achieve some concordance about “milestones” for making a translational case for a given peptide without being at cross-purposes with fundamental research, the ultimate usefulness of which is very difficult to predict. The success of translational science we can and should predict in an actuarial sense, that of basic research we can refrain from prognosticating about, at least without specifying a specific domain immediately relevant to translation. A precedent for this important issue lies in the field of drugs for the treatment of stroke. Following thousands of failures to bring a stroke drug other than tissue plasminogen activator (tPA) into clinical practice, a working group was developed—the Stroke Therapy Academic Industry Roundtable (STAIR). This group created a checklist for clinical translation to avoid some of the most egregious errors, including the use of doses predicted to be ineffectual but used based on minimum-tolerated dose data; employment of unreplicated animal studies for human drug development; and insufficient clinical subtyping of potential study subjects.95−97 Both Potter and Clark pointed out that enabling investigators to see drug development implications of their work, and include others in the process at an early stage, contributes to the virtual drug pipeline in ways that have benefited research and development alike in the stroke field, and are likely to be helpful for assessing, and moving to first-in-human studies, with novel neurotherapeutics as they take their place beside emerging non-drug treatments for affective and other CNS-related illnesses.98
Epilogue: Integrating Knowledge along the Virtual Drug Pipeline
A virtual drug pipeline for peptide-based neurotherapeutics is being constructed and is finding application today. In the future, lncRNA-encoded “micropeptides”,99 aptamer technologies for laboratory evolution of spiegelmers100 and other peptide-interacting drugs, together with enhanced understanding of regulatory peptide receptors, will no doubt be incorporated into this pipeline. It will facilitate further progress in moving from receptor ligand to drug, using as a platform the oldest recognition system that nature has to offer—that existing between innate biological molecular dyads, encoded by living and still-evolving genomes. A short list of items to be incorporated in a future collective consideration of the virtual drug pipeline includes (1) continued attention to similarities and differences in GPCR structure and function among the widely diverse Gs-, Gq-, Gi-, and G11/12-coupled peptide-liganded receptors; (2) relevance to primate physiology of the flood of information being gained from the study of peptide and peptide receptor gain- and loss-of-function in rodent animal models, including close comparison of peptide and cognate peptide receptor knockout phenotypes; (3) identification of precompetitive consortia organized around specific peptide-liganded drug targets to rapidly close gaps in knowledge that impede drug development; (4) consideration of a STAIR-type neuropharmacologically oriented working group to evaluate “promise and peril” within the critical pathways established for promising drug candidates; closer attention to what constitutes proof-of-concept and specific target engagement within a specific drug development program; (5) reverse translation of behavioral outcome monitoring; and (6) integration of data gathering in patient and animal model telemetry, which can including smartphone data collection and bioinformatically aided data collation and evaluation according to the Research Domain Criteria (RDoC) model for organization of physiological and symptomatic read-outs for human mental disorders (https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/index.shtml) (Table 1).
Table 1. Virtual Drug Pipeline.
| 1. | GPCR structural analysis–cross-class generalizations for RAMPs; binding sites; allosteric sites |
| 2. | correlate basic knowledge of circuits, chemical anatomy across rodent and primate models |
| 3. | identify precompetitive consortia to validate drug targets |
| 4. | “promise and peril” assessment (academic-industrial working groups) |
| 5. | reverse translation needs and gaps identified during drug development |
| 6. | informatics-intensive integration of preclinical and clinical data gathering (RDoC model) |
Acknowledgments
We thank those who, in addition to the authors, participated in the NIMH Workshop organized by Lee E. Eiden (NIMH-IRP), Janet Clark (NIMH-IRP), and William Z. Potter (NIMH). We are grateful to Susan Amara (NIMH-IRP), Hugo Tejeda (NIMH-IRP), Eve Marder (Brandeis University), Ben White (NIMH-IRP), Patrick Sexton (Monash University), Samer Hattar (NIMH-IRP), James Collins (Fering Peptide Institute), Anish Konkar (Sanofi-Aventis Deutschland), Marc Ferrer (NCATS), Esther Sabban (New York Medical College), Mario Penzo (NIMH-IRP), Harvey Grill (University of Pennsylvania), Scott Young (NIMH-IRP), Michael Brownstein (Azevan Pharmaceuticals), Mary Lee (NIDA/NIAAA-IRP), and Mi Hillefors (NIMH) for their contributions distilled here. The authors acknowledge the support of joint funding by NIDA and NIAA IRPs (L.L.), UNAM (L.Z.), NIH extramural program (K.G.), NIDDK-IRP (K.J.), and NIMH-IRP (L.E.E.). We thank Dr. Veronica Salmaso, NIDDK, and Lilian Zavala, UNAM, for assistance in the preparation of the figures.
The authors declare no competing financial interest.
References
- Tejeda H. A.; Wu J.; Kornspun A. R.; Pignatelli M.; Kashtelyan V.; Krashes M. J.; Lowell B. B.; Carlezon W. A. Jr.; Bonci A. (2017) Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 93 (1), 147–163. 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum M. P.; Blitz D. M.; Marder E. (2017) Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 18 (7), 389–403. 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao F.; Elliott A. D.; Diao F.; Shah S.; White B. H. (2017) Neuromodulatory connectivity defines the structure of a behavioral neural network. eLife 6, 29797 10.7554/eLife.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T.; Barr M. M.; Bruchas M. R.; Ewer J.; Griffith L. C.; Maiellaro I.; Taghert P. H.; White B. H.; Monk K. R. (2015) Model Organisms in G Protein-Coupled Receptor Research. Mol. Pharmacol. 88 (3), 596–603. 10.1124/mol.115.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D.; Reynolds C. A.; Smith K. J.; Mobarec J. C.; Koole C.; Savage E. E.; Pabreja K.; Simms J.; Sridhar R.; Furness S. G.; Liu M.; Thompson P. E.; Miller L. J.; Christopoulos A.; Sexton P. M. (2016) The Extracellular Surface of the GLP-1 Receptor Is a Molecular Trigger for Biased Agonism. Cell 165 (7), 1632–43. 10.1016/j.cell.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfis M.; Christopoulos A.; Sexton P. M. (2003) RAMPs: 5 years on, where to now?. Trends Pharmacol. Sci. 24 (11), 596–601. 10.1016/j.tips.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Hay D. L.; Garelja M. L.; Poyner D. R.; Walker C. S. (2018) Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175 (1), 3–17. 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.; Goldfeld D. A.; Moo E. V.; Sexton P. M.; Christopoulos A.; McCammon J. A.; Valant C. (2016) Accelerated structure-based design of chemically diverse allosteric modulators of a muscarinic G protein-coupled receptor. Proc. Natl. Acad. Sci. U. S. A. 113 (38), E5675–84. 10.1073/pnas.1612353113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S.; Clydesdale L.; Dai A.; Cai X.; Feng Y.; Yang D.; Liang Y. L.; Koole C.; Zhao P.; Coudrat T.; Christopoulos A.; Wang M. W.; Wootten D.; Sexton P. M. (2018) Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism. J. Biol. Chem. 293 (24), 9370–9387. 10.1074/jbc.RA118.003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J.; Muller T. D.; DiMarchi R. D.; Tschop M. H.; Stemmer K. (2018) Peptide-based multi-agonists: a new paradigm in metabolic pharmacology. J. Intern. Med. 284 (6), 581–602. 10.1111/joim.12837. [DOI] [PubMed] [Google Scholar]
- Amara S. G.; Jonas V.; Rosenfeld M. G.; Ong E. S.; Evans R. M. (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298 (5871), 240–4. 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Shakhnovich V. (2018) It’s Time to Reverse our Thinking: The Reverse Translation Research Paradigm. Clin Transl Sci. 11 (2), 98–99. 10.1111/cts.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F. M.; Collins J. C.; Borin B.; Bradow J.; Liras S.; Limberakis C.; Mathiowetz A. M.; Philippe L.; Price D.; Song K.; James K. (2012) Biaryl-bridged macrocyclic peptides: conformational constraint via carbogenic fusion of natural amino acid side chains. J. Org. Chem. 77 (7), 3099–114. 10.1021/jo202105v. [DOI] [PubMed] [Google Scholar]
- Zhao T. J.; Liang G.; Li R. L.; Xie X.; Sleeman M. W.; Murphy A. J.; Valenzuela D. M.; Yancopoulos G. D.; Goldstein J. L.; Brown M. S. (2010) Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. U. S. A. 107 (16), 7467–72. 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P. S.; Harris-Warrick R. M. (1999) The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin. Neurobiol. 9, 628–633. 10.1016/S0959-4388(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Banks W. A.; Uchida D.; Arimura A.; Somgoyvari-Vigh A.; Shioda S. (1996) Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann. N. Y. Acad. Sci. 805, 270–277. 10.1111/j.1749-6632.1996.tb17489.x. [DOI] [PubMed] [Google Scholar]
- Banks W. A. (2015) Peptides and the blood-brain barrier. Peptides 72, 16–9. 10.1016/j.peptides.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrukol-Ak D.; Kumar V. B.; Ryerse J. S.; Farr S. A.; Verma S.; Nonaka N.; Nakamachi T.; Ohtaki H.; Niehoff M. L.; Edwards J. C.; Shioda S.; Morley J. E.; Banks W. A. (2009) Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J. Cereb. Blood Flow Metab. 29 (2), 411–22. 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- Henderson S. J.; Konkar A.; Hornigold D. C.; Trevaskis J. L.; Jackson R.; Fritsch Fredin M.; Jansson-Lofmark R.; Naylor J.; Rossi A.; Bednarek M. A.; Bhagroo N.; Salari H.; Will S.; Oldham S.; Hansen G.; Feigh M.; Klein T.; Grimsby J.; Maguire S.; Jermutus L.; Rondinone C. M.; Coghlan M. P. (2016) Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes, Obes. Metab. 18 (12), 1176–1190. 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers A.; Bossart M.; Pfeiffer-Marek S.; Elvert R.; Schreuder H.; Kurz M.; Stengelin S.; Lorenz M.; Herling A.; Konkar A.; Lukasczyk U.; Pfenninger A.; Lorenz K.; Haack T.; Kadereit D.; Wagner M. (2018) Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists Specifically Optimized for Multidose Formulations. J. Med. Chem. 61 (13), 5580–5593. 10.1021/acs.jmedchem.8b00292. [DOI] [PubMed] [Google Scholar]
- Simon G. M.; Niphakis M. J.; Cravatt B. F. (2013) Determining target engagement in living systems. Nat. Chem. Biol. 9 (4), 200–5. 10.1038/nchembio.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnage M. E.; Chekler E. L.; Jones L. H. (2013) Target validation using chemical probes. Nat. Chem. Biol. 9 (4), 195–9. 10.1038/nchembio.1197. [DOI] [PubMed] [Google Scholar]
- Hasan M. T.; Althammer F.; Silva da Gouveia M.; Goyon S.; Eliava M.; Lefevre A.; Kerspern D.; Schimmer J.; Raftogianni A.; Wahis J.; Knobloch-Bollmann H. S.; Tang Y.; Liu X.; Jain A.; Chavant V.; Goumon Y.; Weislogel J. M.; Hurlemann R.; Herpertz S. C.; Pitzer C.; Darbon P.; Dogbevia G. K.; Bertocchi I.; Larkum M. E.; Sprengel R.; Bading H.; Charlet A.; Grinevich V. (2019) A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Neuron 103 (1), 133–146. e8. 10.1016/j.neuron.2019.04.029. [DOI] [PubMed] [Google Scholar]
- Feldman R.; Braun K.; Champagne F. A. (2019) The neural mechanisms and consequences of paternal caregiving. Nat. Rev. Neurosci. 20 (4), 205–224. 10.1038/s41583-019-0124-6. [DOI] [PubMed] [Google Scholar]
- Grund T.; Tang Y.; Benusiglio D.; Althammer F.; Probst S.; Oppenlander L.; Neumann I. D.; Grinevich V. (2019) Chemogenetic activation of oxytocin neurons: Temporal dynamics, hormonal release, and behavioral consequences. Psychoneuroendocrinology 106, 77–84. 10.1016/j.psyneuen.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Allen W. E.; Chen M. Z.; Pichamoorthy N.; Tien R. H.; Pachitariu M.; Luo L.; Deisseroth K. (2019) Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364 (6437), 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormos V.; Gaszner B. (2013) Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47 (6), 401–19. 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Kohl J.; Babayan B. M.; Rubinstein N. D.; Autry A. E.; Marin-Rodriguez B.; Kapoor V.; Miyamishi K.; Zweifel L. S.; Luo L.; Uchida N.; Dulac C. (2018) Functional circuit architecture underlying parental behaviour. Nature 556 (7701), 326–331. 10.1038/s41586-018-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H. (2017) A Life of Neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 57, 1–11. 10.1146/annurev-pharmtox-010716-104511. [DOI] [PubMed] [Google Scholar]
- Girish C.; Manikandan S. (2007) Aprepitant: a substance P antagonist for chemotherapy induced nausea and vomiting. Indian J. Cancer 44 (1), 25–30. 10.4103/0019-509X.31164. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Liang Y. L.; Belousoff M. J.; Deganutti G.; Fletcher M. M.; Willard F. S.; Bell M. G.; Christe M. E.; Sloop K. W.; Inoue A.; Truong T. T.; Clydesdale L.; Furness S. G. B.; Christopoulos A.; Wang M. W.; Miller L. J.; Reynolds C. A.; Danev R.; Sexton P. M.; Wootten D. (2020) Activation of the GLP-1 receptor by a non-peptidic agonist. Nature 577 (7790), 432–436. 10.1038/s41586-019-1902-z. [DOI] [PubMed] [Google Scholar]
- Kawai T.; Tannino F.; Fukazawa M.; Ogawa K.; Nagao S.; Yoshino H.; Komatsu S.-I.; Suzuki H.; Kaqwabe Y. (2018) OWL833, an Orally Active Nonpeptide GLP-1 Receptor Agonist, Improves Glucose Tolerance by Increasing Insulin Secretion and Reduces Food Intake of Cynomolgus Monkeys. Diabetes 67, 1118-P. 10.2337/db18-1118-P. [DOI] [Google Scholar]
- Jazayeri A.; Rappas M.; Brown A. J. H.; Kean J.; Errey J. C.; Robertson N. J.; Fiez-Vandal C.; Andrews S. P.; Congreve M.; Bortolato A.; Mason J. S.; Baig A. H.; Teobald I.; Dore A. S.; Weir M.; Cooke R. M.; Marshall F. H. (2017) Crystal structure of the GLP-1 receptor bound to a peptide agonist. Nature 546 (7657), 254–258. 10.1038/nature22800. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. (2015) CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. British journal of clinical pharmacology 80 (2), 193–9. 10.1111/bcp.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninot A.; Collins J. C.; Nuss J. M. (2018) The Current State of Peptide Drug Discovery: Back to the Future?. J. Med. Chem. 61 (4), 1382–1414. 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Samal B.; Hamelink C. R.; Xiang C. C.; Chen Y.; Chen M.; Vaudry D.; Brownstein M. J.; Hallenbeck J. M.; Eiden L. E. (2006) Neuroprotection by endogenous and exogenous PACAP following stroke. Regul. Pept. 137 (1–2), 4–19. 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D.; Somogyvari-Vigh A.; Vigh S.; Maderdrut J. L.; Arimura A. (2000) Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann. N. Y. Acad. Sci. 921, 119–28. 10.1111/j.1749-6632.2000.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Rasmusson A. M.; Hauger R. L.; Morgan C. A.; Bremner J. D.; Charney D. S.; Southwick S. M. (2000) Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol. Psychiatry 47 (6), 526–39. 10.1016/S0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Serova L. I.; Tillinger A.; Alaluf L. G.; Laukova M.; Keegan K.; Sabban E. L. (2013) Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 236, 298–312. 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Sabban E. L.; Serova L. I.; Alaluf L. G.; Laukova M.; Peddu C. (2015) Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav. Brain Res. 295, 9. 10.1016/j.bbr.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Schmeltzer S. N.; Herman J. P.; Sah R. (2016) Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): A translational update. Exp. Neurol. 284 (Pt B), 196–210. 10.1016/j.expneurol.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz M.; Charney D. S.; Murrough J. W. (2017) Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci. Lett. 649, 164–169. 10.1016/j.neulet.2016.11.061. [DOI] [PubMed] [Google Scholar]
- Nahvi R. J.; Nwokafor C.; Serova L. I.; Sabban E. L. (2019) Single Prolonged Stress as a Prospective Model for Posttraumatic Stress Disorder in Females. Front. Behav. Neurosci. 13, 17. 10.3389/fnbeh.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftanovskaya E. M.; Ng H. H.; Soula M.; Rivas B.; Myhr C.; Ho B. A.; Cervantes B. A.; Shupe T. D.; Devarasetty M.; Hu X.; Xu X.; Patnaik S.; Wilson K. J.; Barnaeva E.; Ferrer M.; Southall N. T.; Marugan J. J.; Bishop C. E.; Agoulnik I. U.; Agoulnik A. I. (2019) Therapeutic effects of a small molecule agonist of the relaxin receptor ML290 in liver fibrosis. FASEB J. 33 (11), 12435–12446. 10.1096/fj.201901046R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.; Huang Z.; Chen C. Z.; Agoulnik I. U.; Southall N.; Hu X.; Jones R. E.; Ferrer M.; Zheng W.; Agoulnik A. I.; Marugan J. J. (2013) Identification and optimization of small-molecule agonists of the human relaxin hormone receptor RXFP1. Nat. Commun. 4, 1953. 10.1038/ncomms2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe X.; Darczak D.; Davis-Taber R. A.; Uchic M. E.; Scott V. E.; Jarvis M. F.; Stewart A. O. (2008) Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R). Bioorg. Med. Chem. Lett. 18 (6), 2162–6. 10.1016/j.bmcl.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Liang Y.-L.; Belousoff M. J.; Zhao P.; Koole C.; Fletcher M. M.; Truong T. T.; Julita V.; Christopoulos G.; Xu H. E.; Zhang Y.; Khoshouei M.; Christopouos A.; Danev R.; Sexton P. M.; Wootten D. (2020) Toward a structural understanding of class B GPCR peptide binding and activation. Mol. Cell 77 (3), 656–668. e5. 10.1016/j.molcel.2020.01.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi K.; Shihoya W.; Nishizawa T.; Kadji F. M. N.; Aoki J.; Inoue A.; Nureki O. (2020) Cryo-EM structure of the human PAC1 receptor coupled to an engineered heterotrimeric G protein. Nat. Struct. Mol. Biol. 27 (3), 274–280. 10.1038/s41594-020-0386-8. [DOI] [PubMed] [Google Scholar]
- Wang J.; Song X.; Zhang D.; Chen X.; Li X.; Sun Y.; Li C.; Song Y.; Ding Y.; Ren R.; Harrington E. H.; Hu L. A.; Zhong W.; Xu C.; Huang X.; Wang H. W.; Ma Y. (2020) Cryo-EM structures of PAC1 receptor reveal ligand binding mechanism. Cell Res. 0280-2 10.1038/s41422-020-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I.; Ogashi H.; Okada T.; Shimodaira A.; Hayakawa D.; Watanabe A.; Miyata A.; Kurihara T.; Gouda H.; Toyooka N. (2020) Synthesis of a novel and potent small-molecule antagonist of PAC1 receptor for the treatment of neuropathic pain. Eur. J. Med. Chem. 186, 111902. 10.1016/j.ejmech.2019.111902. [DOI] [PubMed] [Google Scholar]
- Takasaki I.; Watanabe A.; Yokai M.; Watanabe Y.; Hayakawa D.; Nagashima R.; Fukuchi M.; Okada T.; Toyooka N.; Miyata A.; Gouda H.; Kurihara T. (2018) In silico screening identified novel small-molecule antagonists of PAC1 receptor. J. Pharmacol. Exp. Ther. 365, 1. 10.1124/jpet.117.245415. [DOI] [PubMed] [Google Scholar]
- Martin B.; de Maturana R. L.; Brenneman R.; Walent T.; Mattson M. P.; Maudsley S. (2005) Class II G protein-coupled receptors and their ligands in neuronal function and protection. NeuroMol. Med. 7 (1–2), 3–36. 10.1385/NMM:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A. P.; Bonner T. I.; Foord S. M.; Harmar A. J.; Neubig R. R.; Pin J. P.; Spedding M.; Kojima M.; Kangawa K. (2005) International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 57 (4), 541–6. 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- Muller T. D.; Nogueiras R.; Andermann M. L.; Andrews Z. B.; Anker S. D.; Argente J.; Batterham R. L.; Benoit S. C.; Bowers C. Y.; Broglio F.; Casanueva F. F.; D’Alessio D.; Depoortere I.; Geliebter A.; Ghigo E.; Cole P. A.; Cowley M.; Cummings D. E.; Dagher A.; Diano S.; Dickson S. L.; Dieguez C.; Granata R.; Grill H. J.; Grove K.; Habegger K. M.; Heppner K.; Heiman M. L.; Holsen L.; Holst B.; Inui A.; Jansson J. O.; Kirchner H.; Korbonits M.; Laferrere B.; LeRoux C. W.; Lopez M.; Morin S.; Nakazato M.; Nass R.; Perez-Tilve D.; Pfluger P. T.; Schwartz T. W.; Seeley R. J.; Sleeman M.; Sun Y.; Sussel L.; Tong J.; Thorner M. O.; van der Lely A. J.; van der Ploeg L. H.; Zigman J. M.; Kojima M.; Kangawa K.; Smith R. G.; Horvath T.; Tschop M. H. (2015) Ghrelin. Mol. Metab. 4 (6), 437–60. 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L. B. (2019) Inventing Liraglutide, a Glucagon-Like Peptide-1 Analogue, for the Treatment of Diabetes and Obesity. ACS Pharmacology and Translastional Science 2, 468–484. 10.1021/acsptsci.9b00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J.; Hyman S. E. (2010) Animal models of neuropsychiatric disorders. Nat. Neurosci. 13 (10), 1161–9. 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B. N.; Kozak M. J. (2013) Constructing constructs for psychopathology: the NIMH research domain criteria. Journal of abnormal psychology 122 (3), 928–37. 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Insel T.; Cuthbert B.; Garvey M.; Heinssen R.; Pine D. S.; Quinn K.; Sanislow C.; Wang P. (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167 (7), 748–51. 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Fernandez D. C.; Chang Y. T.; Hattar S.; Chen S. K. (2016) Architecture of retinal projections to the central circadian pacemaker. Proc. Natl. Acad. Sci. U. S. A. 113 (21), 6047–52. 10.1073/pnas.1523629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan W. T.; Rupp A. C.; Ross R. A.; Somasundaram P.; Hiriyanna S.; Wu Z.; Badea T. C.; Robinson P. R.; Lowell B. B.; Hattar S. S. (2016) A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. eLife 5, 15392 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo M. A.; Robert V.; Tucciarone J.; De Bundel D.; Wang M.; Van Aelst L.; Darvas M.; Parada L. F.; Palmiter R. D.; He M.; Huang Z. J.; Li B. (2015) The paraventricular thalamus controls a central amygdala fear circuit. Nature 519 (7544), 455–9. 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M.; Hosoda H.; Date Y.; Nakazato M.; Matsuo H.; Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402 (6762), 656–60. 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M. (2008) The discovery of ghrelin--a personal memory. Regul. Pept. 145 (1–3), 2–6. 10.1016/j.regpep.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Dos-Santos R. C.; Reis L. C.; Perello M.; Ferguson A. V.; Mecawi A. S. (2019) The actions of ghrelin in the paraventricular nucleus: energy balance and neuroendocrine implications. Ann. N. Y. Acad. Sci. 1455, 81. 10.1111/nyas.14087. [DOI] [PubMed] [Google Scholar]
- Labarthe A.; Fiquet O.; Hassouna R.; Zizzari P.; Lanfumey L.; Ramoz N.; Grouselle D.; Epelbaum J.; Tolle V. (2014) Ghrelin-Derived Peptides: A Link between Appetite/Reward, GH Axis, and Psychiatric Disorders?. Front. Endocrinol. 5, 163. 10.3389/fendo.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. M.; Burgos-Robles A.; Liu E.; Correia S. S.; Goosens K. A. (2014) A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 19 (12), 1284–94. 10.1038/mp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufzai M.; Harmatz E. S.; Shah M.; Malik M. O.; Goosens K. A. (2018) Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl. Psychiatry 8 (1), 74. 10.1038/s41398-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsbad S. (2009) Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)--preclinical and clinical results. Best Pract Res. Clin Endocrinol Metab 23 (4), 463–77. 10.1016/j.beem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Hayes M. R.; Leichner T. M.; Zhao S.; Lee G. S.; Chowansky A.; Zimmer D.; De Jonghe B. C.; Kanoski S. E.; Grill H. J.; Bence K. K. (2011) Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 13 (3), 320–30. 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J.; Habener J. F.; Holst J. J. (2017) Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127 (12), 4217–4227. 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin E. M.; Mulvihill E. E.; Baggio L. L.; Koehler J. A.; Cao X.; Seeley R. J.; Drucker D. J. (2019) Distinct Neural Sites of GLP-1R Expression Mediate Physiological versus Pharmacological Control of Incretin Action. Cell Rep. 27 (11), 3371–3384. e3. 10.1016/j.celrep.2019.05.055. [DOI] [PubMed] [Google Scholar]
- Farokhnia M.; Faulkner M. L.; Piacentino D.; Lee M. R.; Leggio L. (2019) Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol. Behav. 204, 49–57. 10.1016/j.physbeh.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Buijs R. M. (1978) Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 192 (3), 423–35. 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs R. M.; De Vries G. J.; Van Leeuwen F. W.; Swaab D. F. (1983) Vasopressin and oxytocin: distribution and putative functions in the brain. Prog. Brain Res. 60, 115–22. 10.1016/S0079-6123(08)64379-4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perez O. R.; Hernandez V. S.; Nava-Kopp A. T.; Barrio R. A.; Seifi M.; Swinny J. D.; Eiden L. E.; Zhang L. (2019) A Synaptically Connected Hypothalamic Magnocellular Vasopressin-Locus Coeruleus Neuronal Circuit and Its Plasticity in Response to Emotional and Physiological Stress. Front. Neurosci. 13, 196. 10.3389/fnins.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Hernandez V. S. (2013) Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience 228, 139–62. 10.1016/j.neuroscience.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Hernandez V. S.; Hernandez O. R.; Perez de al Mora M.; Gomora J. J.; Fuxe K.; Eiden L. E.; Zhang L. (2016) Hypothalamic Vasopressinergic Projections Innervate Central Amygdala GABAergic Neurons: Implications for Anxiety and Stress Coping. Front. Neural Circuits 10, Article 92 10.3389/fncir.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Hernandez V. S.; Swinny J. D.; Verma A. K.; Giesecke T.; Emery A. C.; Mutig K.; Garcia-Segura L. M.; Eiden L. E. (2018) A GABAergic cell type in the lateral habenula links hypothalamic homeostatic and midbrain motivation circuits with sex steroid signaling. Transl. Psychiatry 8 (1), 50. 10.1038/s41398-018-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Lira E.; Kelly L.; Seifi M.; Jackson T. C.; Giesecke T.; Mutig K.; Koshimizu T.-A.; Hernandez V. S.; Zhang L.; Swinny J. D. (2018) Dynamic modulation of mouse locus coeruleus neurons by vasopressin 1a and 1b receptors. Front. Neurosci. 12, Article 919 10.3389/fnins.2018.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. (2005) SU11248: Genesis of a new cancer drug. Scientist April 11, 17–18. [Google Scholar]
- Li W.; Zhou Y.; Jentsch J. D.; Brown R. A.; Tian X.; Ehninger D.; Hennah W.; Peltonen L.; Lonnqvist J.; Huttunen M. O.; Kaprio J.; Trachtenberg J. T.; Silva A. J.; Cannon T. D. (2007) Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc. Natl. Acad. Sci. U. S. A. 104 (46), 18280–5. 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. K.; Pickard B. S.; Mackie S.; James R.; Christie S.; Buchanan S. R.; Malloy M. P.; Chubb J. E.; Huston E.; Baillie G. S.; Thomson P. A.; Hill E. V.; Brandon N. J.; Rain J. C.; Camargo L. M.; Whiting P. J.; Houslay M. D.; Blackwood D. H.; Muir W. J.; Porteous D. J. (2005) DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310 (5751), 1187–1191. 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Orefice L. L.; Zimmerman A. L.; Chirila A. M.; Sleboda S. J.; Head J. P.; Ginty D. D. (2016) Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 166 (2), 299–313. 10.1016/j.cell.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M.; Yu G. Q.; Shockley K.; Huang Y.; Jones B.; Masliah E.; Mallory M.; Yeo T.; Longo F. M.; Mucke L. (2002) Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J. Neurosci. 22 (24), 10539–10548. 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z.; Gerfen C. R.; Young W. S. 3rd (2013) Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 521 (8), 1844–66. 10.1002/cne.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani J. H.; Zhao M.; Cui Z.; Williams Avram S. K.; Caruana D. A.; Dudek S. M.; Young W. S. (2015) Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 20, 490. 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky I. F.; Hu S. B.; Szegda K. L.; Westphal H.; Young L. J. (2004) Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29 (3), 483–93. 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Lee M. R.; Rohn M. C.; Tanda G.; Leggio L. (2016) Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs 30 (2), 109–23. 10.1007/s40263-016-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. R.; Scheidweiler K. B.; Diao X. X.; Akhlaghi F.; Cummins A.; Huestis M. A.; Leggio L.; Averbeck B. B. (2018) Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol. Psychiatry 23 (1), 115–122. 10.1038/mp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. R.; Rohn M. C. H.; Zanettini C.; Coggiano M. A.; Leggio L.; Tanda G. (2019) Effect of systemically administered oxytocin on dose response for methylphenidate self-administration and mesolimbic dopamine levels. Ann. N. Y. Acad. Sci. 1455 (1), 173–184. 10.1111/nyas.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. F.; Lu S. F.; Messenger T.; Guillon C. D.; Heindel N.; Miller M.; Koppel G.; Robert Bruns F.; Simon N. G. (2006) Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol., Biochem. Behav. 83 (2), 169–74. 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A. (2015) New paradigms in GPCR drug discovery. Biochem. Pharmacol. 98 (4), 541–55. 10.1016/j.bcp.2015.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A.; Gao Z. G. (2006) Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discovery 5 (3), 247–64. 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden C. J.; Weaver D. F. (2010) The rise of micropharma. Drug Discovery Today 15 (3–4), 84–7. 10.1016/j.drudis.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Legos J. J.; Tuma R. F.; Barone F. C. (2002) Pharmacological interventions for stroke: failures and future. Expert Opin. Invest. Drugs 11 (5), 603–14. 10.1517/13543784.11.5.603. [DOI] [PubMed] [Google Scholar]
- Savitz S. I. (2007) A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp. Neurol. 205 (1), 20–25. 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Savitz S. I.; Fisher M. (2007) Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann. Neurol. 61 (5), 396–402. 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- Lisanby S. H. (2017) Noninvasive Brain Stimulation for Depression - The Devil Is in the Dosing. N. Engl. J. Med. 376 (26), 2593–2594. 10.1056/NEJMe1702492. [DOI] [PubMed] [Google Scholar]
- Choi S. W.; Kim H. W.; Nam J. W. (2019) The small peptide world in long noncoding RNAs. Briefings Bioinf. 20 (5), 1853–1864. 10.1093/bib/bby055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmling S.; Maasch C.; Eulberg D.; Buchner K.; Schroder W.; Lange C.; Vonhoff S.; Wlotzka B.; Tschop M. H.; Rosewicz S.; Klussmann S. (2004) Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. Proc. Natl. Acad. Sci. U. S. A. 101 (36), 13174–9. 10.1073/pnas.0404175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry G. M.; Adzhigirey M.; Day T.; Annabhimoju R.; Sherman W. (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 27 (3), 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graphics 14 (1), 33–8. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Fredriksson R.; Lagerstrom M. C.; Lundin L. G.; Schioth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63 (6), 1256–72. 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]