Abstract
G protein-coupled receptors (GPCRs) are cell surface receptors that for many years have been considered to function exclusively at the plasma membrane, where they bind to extracellular ligands and activate G protein signaling cascades. According to the conventional model, these signaling events are rapidly terminated by β-arrestin (β-arr) recruitment to the activated GPCR resulting in signal desensitization and receptor internalization. However, during the past decade, emerging evidence suggest that many GPCRs can continue to activate G proteins from intracellular compartments after they have been internalized. G protein signaling from intracellular compartments is in general more sustained compared to G protein signaling at the plasma membrane. Notably, the particular location closer to the nucleus is beneficial for selective cellular functions such as regulation of gene transcription. Here, we review key GPCRs that undergo compartmentalized G protein signaling and discuss molecular considerations and requirements for this signaling to occur. Our main focus will be on receptors involved in the regulation of important physiological and pathological cardiovascular functions. We also discuss how sustained G protein activation from intracellular compartments may be involved in cellular functions that are distinct from functions regulated by plasma membrane G protein signaling, and the corresponding significance in cardiovascular physiology.
Keywords: compartmentalized G protein signaling, sustained signaling, early endosomes, Golgi membranes, nuclear membranes, heart failure (HF), atherosclerosis, myocardial ischemia, cardioprotection, β-adrenergic receptors (βARs), vasopressin type 2 receptor (V2R), calcitonin gene-related peptide (CGRP) receptor, US28, sphingosine-1-phosphate 1 receptor (S1P1R)
Introduction
G protein-coupled receptors (GPCRs) represent the largest family of transmembrane receptors, encoded by about 800 genes in the human genome.1 These receptors play fundamental roles in nervous, cardiovascular, sensory, endocrine and immune systems, and they are popular drug targets with 30–50% of all prescribed drugs directly affecting GPCRs.2,3 Located at the cell surface, GPCRs detect extracellular stimuli such as light, neurotransmitters, peptides and hormones, and translate them into intracellular signals that lead to physiological responses.1,4
Agonist binding stabilizes active receptor conformations that interact with heterotrimeric G proteins, which are composed of Gα, Gβ, and Gγ subunits (Figure 1). This coupling results in guanosine diphosphate (GDP) for guanosine triphosphate (GTP) exchange in the Gα subunit and subsequent activation of the heterotrimeric G protein. G protein stimulation leads to dissociation of the Gα subunit and Gβγ dimer, which both modulate activity of membrane-localized effectors including adenylyl cyclase (AC), phospholipase Cβ (PLCβ), and ion channels. Upon activation, these effectors initiate signaling cascades that ultimately control cellular processes.
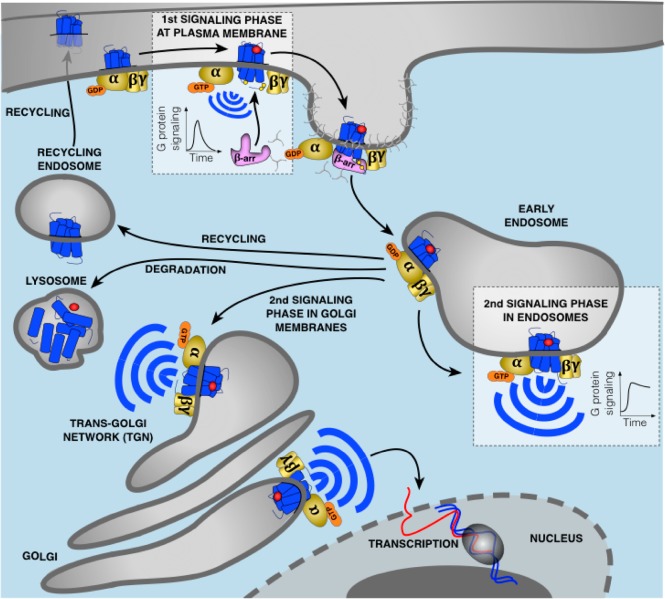
Figure 1.
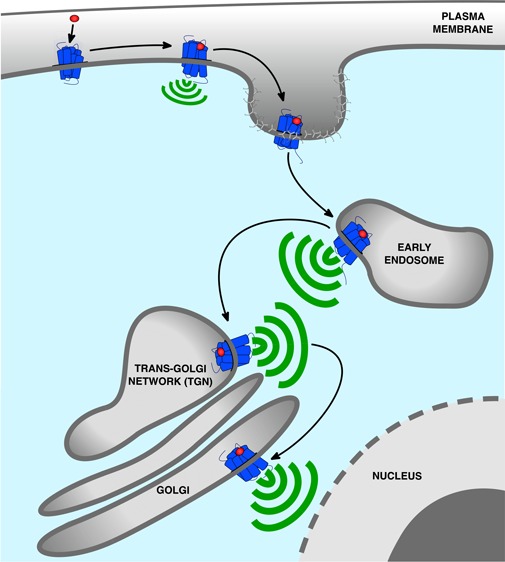
Updated model of G protein signaling. Agonist binding to GPCRs is detected by the Gα subunit of heterotrimeric G proteins. This induces exchange of GDP for GTP causing dissociation of the GTP-bound Gα from Gβγ which both activate membrane-localized effectors. This signaling is followed by the phosphorylation of the receptor by GRKs leading to recruitment of β-arr to the phosphorylated receptor. This event causes G protein uncoupling from the receptor and signaling desensitization. Recruitment of β-arr also promotes GPCR internalization into CCPs and receptor trafficking to early endosomes where desensitized receptors dissociate from β-arr and recycle back to the plasma membrane, are directed to lysosomes for degradation, or can undergo another round of G protein activation from early endosomes or Golgi membranes. In contrast to G protein signaling at the plasma membrane which is rapidly dampened by β-arr, this second activation upon GPCR internalization is generally more sustained. The particular duration and location of G protein signaling is critical for many cellular processes.
G protein signaling at the plasma membrane is often transient as it is rapidly followed by desensitization and receptor internalization. Upon agonist challenge, GPCR kinases (GRKs), protein kinases A (PKA) and/or C (PKC) phosphorylate serine and threonine residues located at the carboxy-terminal (CT) tail and/or in the third intracellular loop (ICL3) of the agonist-occupied receptor.5−7 This event triggers β-arrestin (β-arr) recruitment to the phosphorylated receptor (Figure 1). β-arr does not only bind the phosphorylated residues on the receptor but also to a region of the intracellular core that overlaps with heterotrimeric G protein binding site where it sterically blocks G protein binding to the receptor. Thus, β-arr recruitment to the receptor leads to G protein uncoupling and signaling desensitization.8−10 β-arr also recruits key proteins involved in the assembly of clathrin-coated pits (CCPs) such as clathrin heavy chain and the clathrin adapter protein-2 (AP2), which brings the desensitized receptor into CCPs followed by receptor internalization.1,11−16 Upon receptor internalization, dynamin, a GTPase, pinches off the membrane detaching and isolating CCPs from the plasma membrane.17 Following this step, GPCRs are then rapidly trafficked to early endosomes where they either dissociate from β-arr and recycle back to the membrane, or are directed to lysosomes for degradation (Figure 1).5,7,14,18 Furthermore, upon activation by GPCRs, β-arrs themselves serve as an alternative signaling system by acting as adaptors and scaffolds to interact with numerous signaling molecules.1
G protein signaling was originally believed to be restricted strictly to the plasma membrane. However, over the past decade many studies have demonstrated that some GPCRs continue to signal via G proteins after having been internalized into intracellular compartments (Table 1). This internalized G protein signaling has been difficult to incorporate within the aforementioned traditional understanding of GPCR signaling since β-arr plays a fundamental role in termination of G protein signaling. Despite the initial difficulties in understanding these observations, it has now become clear that internalized GPCRs can use several mechanisms to promote G protein signaling from internalized compartments. For example, some GPCRs bind β-arr in a specific conformation that allows simultaneous coupling to β-arr and G protein to form a “megaplex”. Therefore, the receptor in these megaplexes maintains its ability to activate G protein while being internalized by β-arr. In addition, receptors synthesized de novo may also stimulate G protein signaling from the Golgi network (Figure 1).8,10,18−29 The particular duration and location of G protein signaling is critical for many important cellular processes such as gene transcription and ion channel activity, and as explained in this review, fine-tuning of spatiotemporal feature of G protein signaling at intracellular sites is crucial for many aspects of normal and pathologic cardiovascular physiology.
Table 1. List of GPCRs Activating G Proteins in Intracellular Compartments.
| receptor | compartment | G protein | ref |
|---|---|---|---|
| α1A-adrenergic receptor (α1A-AR) | nucleus | Gαs | (300) |
| α1B-adrenergic receptor (α1B-AR) | nucleus | Gαs | (300) |
| β1-adrenergic receptor (β1AR) | Golgi | Gαs | (54) |
| β2-adrenergic receptor (β2AR) | early endosomes | Gαs | (25) |
| calcitonin-gene-related-peptide (CGRP) receptor | early endosomes | Gαs, Gαq | (79) |
| calcium-sensing receptor (CaSR) | early endosomes | Gαq | (301) |
| cannabinoid type 1 receptor 1 (CB1R) | mitochondria | Gαi | (302) |
| dopamine receptor type 1 (D1R) | early endosomes | Gαs | (24) |
| luteinizing hormone receptor (LHR) | early endosomes | Gαs | (304) |
| melatonin type 1 receptor (MT1R) | mitochondria | Gαi | (303) |
| neurokinin type 1 receptor (NK1R) | early endosomes | Gαq | (70) |
| parathyroid hormone receptor (PTHR) | early endosomes | Gαs | (22) |
| protease-activated receptor-2 (PAR2) | early endosomes | Gαq | (188) |
| sphingosine-1-phosphate 1 receptor (S1P1R) | Golgi | Gαi | (71) |
| thyroid stimulating hormone receptor (TSHR) | Golgi | Gαs | (23) |
| vasopressin type 2 receptor (V2R) | early endosomes | Gαs | (64) |
Role of Compartmentalized β-Adrenergic Receptor Signaling in Cardiovascular Pathophysiology
It is well-known that inappropriately increased sympathetic activity leads to the development of cardiovascular diseases such as heart failure (HF). This is mainly due to the prolonged activation of β-adrenergic receptors (βARs) by catecholamines. This prolonged activity results in changes in the size and shape of the heart leading to cardiac dysfunction.30 Of the three subtypes of βARs, β1ARs and β2ARs represent 70–80% and 20–30% (depending on the species) of total cardiomyocyte βARs, respectively. It has been widely accepted that β1AR and β2AR subtypes are both coupled to Gαs and stimulate cAMP production in cardiomyocytes; however, the β1AR subtype has greater functional effects in cardiomyocytes.31,32 A long-standing puzzle has been to decipher how β1AR and β2AR elicit significantly different effects on cardiac functions, despite having broadly overlapping tissue expression patterns and similar effectors. β1AR plays a dominant role in chronotropy, inotropy, and lusitropy responses in healthy cardiomyocytes through protein kinase A (PKA)-mediated phosphorylation of several key intracellular Ca2+ regulatory proteins such as L-type Ca2+ channels, ryanodine receptors, phospholamban, and troponin I, whereas β2AR produces modest chronotropic effect and no lusitropic responses.33,34 Similarly, during the development of HF these two receptor subtypes play significantly different roles: β1AR signaling promotes cardiomyocyte hypertrophy and apoptosis, whereas β2AR signaling prevents cardiomyocytes apoptosis and hypertrophy (Figure 2).35,36 While numerous attractive hypotheses have been offered to explain the molecular bases of these different outcomes, definitive answers have largely been elusive.
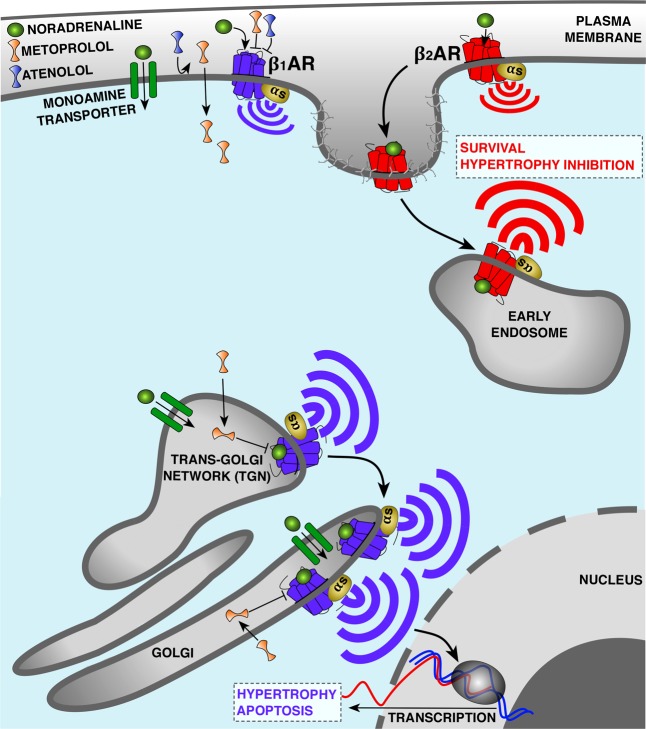
Figure 2.
Compartmentalized Gαs signaling by β-adrenerigic receptors (βAR) in cardiomyocytes. β1AR and β2AR are coupled to Gαs and stimulate cAMP production but elicit different effects on cardiac functions. In the case of heart failure (HF), β1AR promotes cardiomyocyte hypertrophy and apoptosis, whereas β2AR prevents these events. One hypothesis explaining this difference is the distinct subcellular localization of β1AR and β2AR. Both subtypes bind noradrenaline and activate Gαs at the plasma membrane. However, upon receptor internalization β2AR preferentially activates Gαs from early endosomes, while the biosynthetic pool of β1AR activates Gαs from the Golgi membranes. Proximity of active Golgi-localized β1AR with the nucleus allows transcription of genes mediating hypertrophy. Activation of Golgi-localized β1AR depends on the uptake 2 monoamine transporter, facilitating the transport of noradrenaline to the pool of receptors at the Golgi. β1AR antagonists are among the most widely used drugs to treat HF. Metoprolol, a hydrophobic and membrane-permeable β1AR selective antagonist, blocks both the plasma membrane and the Golgi pool of β1AR, whereas atenolol, a hydrophilic and membrane-impermeable antagonist, only inhibits β1AR signaling at the plasma membrane. Correlated with the differential abilities of these drugs to access internal sites of signaling, metoprolol is more efficient than atenolol at reducing cAMP response and heart rate as well as contraction/relaxation responses.
One explanation for this conundrum that has been extensively discussed in previous reviews is the existence of additional signaling pathways that modify the Gαs-mediated signaling.37−39 It has been shown that β2ARs in addition to Gαs can also couple to Gαi or activate β-arr-mediated pathways, both of which promote cardiomyocytes survival.40−42 An alternative model, first described by Buxton and Brunton in 1983, is that the βARs signaling complex is localized in particular cellular compartments.43 One of the first studies that reported the presence of compartmentalized βAR signaling came from cell fractionation studies in the heart tissues. Hayes et al. showed that activation of βAR by isopropanol leads to activation of cAMP/PKA in the particulate fraction, whereas activation of E-type prostaglandin receptors by PGE1 can only activate cAMP/PKA from the soluble fraction but not the particular fraction.44 Multiple lines of evidence indicate that key downstream effectors of PKA pathway such as ion channels, phospholamban, and phosphodiesterases are clustered into microdomains by A-kinase anchoring proteins (AKAP). This compartmentalization would allow signaling complexes to localize in the vicinity of specific effectors and control the local generation of cAMP at distinct subcellular compartments.45,46 In many of these studies, however, βARs have been assumed to only activate Gαs on the plasma membrane. Distinct signaling outcomes of βARs from the plasma membrane are thought to be due to localization of β2ARs, but not β1ARs, in distinct subdomains on the plasma membrane such as the caveolae and T-tubule localization in cardiomyocytes.47−52 Yet another model based on studies in the past decade posits that the site of GPCR signaling is not limited to the plasma membrane and that some receptors also activate downstream signaling pathways at various subcellular membrane compartments.8,18,22−27,53 Evidence for the presence of functional βARs at subcellular locations initially came from cell fractionation experiments in cardiomyocytes.54 Boivin et al. showed that β1AR but not β2AR is present on the nuclear fraction of adult cardiomyocytes and that this internal fraction stimulates Gαs-mediated cAMP response and ultimately regulates nuclear functions such as gene expression (Figure 2).54,55 More direct evidence for the model emerged from conformation-sensitive nanobody-based biosensors for βARs and Gαs. Application of these biosensors allowed for visualization of activated βARs as well as Gαs in living cells at previously unappreciated locations such as the endosomes and the Golgi membranes.25,26 These recent data also demonstrated that signaling from the endosomal and Golgi compartments constitute critical aspect of β2AR and β1ARs cellular responses, respectively, to external cues (Figure 2). While these studies provide a solution as how seemingly similar receptors lead to vastly different signaling outcomes, they also raise the question of how ligands access internal pools of these receptors. It has been previously demonstrated that for some receptors such as thyroid stimulating hormone receptor (TSHR), the internalized receptor and its ligand can traffic in a retrograde manner to the trans Golgi network and activate a second phase of cAMP/PKA signaling at the trans Golgi network.56 However, in the case of β1ARs, activation of Golgi-localized receptors depends on a transport mechanism mediated by the uptake 2 monoamine transporter, which allows membrane impermeable endogenous ligands of βARs such as epinephrine and norepinephrine to access compartments within the cell.26
Given the noted roles of βARs in the context of cardiac disease, small molecules that block β1ARs and small molecules that activate β2AR have been the goals of numerous drug development efforts. Indeed, β1AR antagonists are among the most widely used clinical drugs.57 Extensive clinical use has revealed obvious differences among the efficacies of various β1AR antagonists that cannot easily be explained by their receptor binding affinities, selectivity, or considerations of pharmacokinetics. There is an intriguing correlation between clinical efficacies of various of these antagonists and their abilities to access internal pools. For example, metoprolol, a β1AR-selective antagonist, has been shown to be more beneficial in reducing heart rate and contraction/relaxation responses when compared to other βAR antagonists such as atenolol (β1AR-selective antagonist) and sotalol (βAR-nonselective antagonist).58−60 The compartmentalized signaling of βARs might provide a potential solution to the conundrum of how these antagonists can elicit different physiological outcomes by showing that these differences correlate with differential abilities of these drugs to access internal sites of signaling. In support of this model is the observation that metoprolol (a hydrophobic and membrane-permeable β1AR antagonist) blocks both the plasma membrane and the Golgi pool of β1AR, whereas sotalol and atenolol (hydrophilic and membrane-impermeable β1AR antagonist) only inhibit the plasma membrane β1AR signaling and are less efficient in reducing cAMP response.26 Furthermore, it has been recently reported that Golgi-localized β1AR regulates cardiac hypertrophy through activation of mAKAP/PLCε/exchange protein directly activated by cAMP (EPAC) signaling complex in the vicinity of the receptor pool at the Golgi (Figure 2). More importantly, they demonstrated that inhibition of monoamine transporter or specific blockade of Golgi resident β1ARs by membrane permeable antagonist, such as metoprolol, prevents norepinephrine-dependent cardiomyocyte hypertrophy.61 This clearly demonstrates the functional importance of Golgi-localized β1ARs and presents a potential new paradigm for developing new HF drugs that deliberately targets internal pool of βARs.
In summary, the recognition that βARs also function and signal at internal membranes (endosomes, Golgi, and nuclear membranes) raises the need for understanding compartmentalized signaling in pathological conditions such as HF and how signaling from these internal compartments is regulated in healthy and diseased heart.
Endosomal G Protein Signaling by the Vasopressin Type 2 Receptor (V2R) as a Target for Heart Failure
Three types of vasopressin receptors exist (V1AR, V1BR, and V2R) that all are activated by the neurohypophysial hormone, arginine vasopressin (AVP). Vasopressin receptors regulate several physiological functions including renal water reabsorption, vasoconstriction, and myocyte biology. In HF, AVP levels are elevated, which leads to inappropriate changes in cardiovascular function as well as impaired renal solute-free water excretion that is associated with hyponatremia.62,63 Thus, vasopressin receptors, in particular V2R, have been popular targets to develop antagonists against as therapeutics for HF and/or hyponatremia.
V2R is predominantly expressed on the basolateral side of renal collecting duct principal cells where it regulates water reabsorption from the urine (Figure 3). Upon stimulation of V2R and subsequent Gαs–cAMP–PKA signaling, the aquaporin 2 (AQP2) proteins located in intracellular vesicles are phosphorylated at their CT tail.64,65 This phosphorylation triggers trafficking and long-lasting insertion of the AQP2-containing vesicles into the apical membrane allowing water from the urine to be reabsorbed into circulation.65 Thus, overstimulation of V2R as seen in HF and subsequent renal water reabsorption can lead to a pathological decrease in sodium serum levels.62
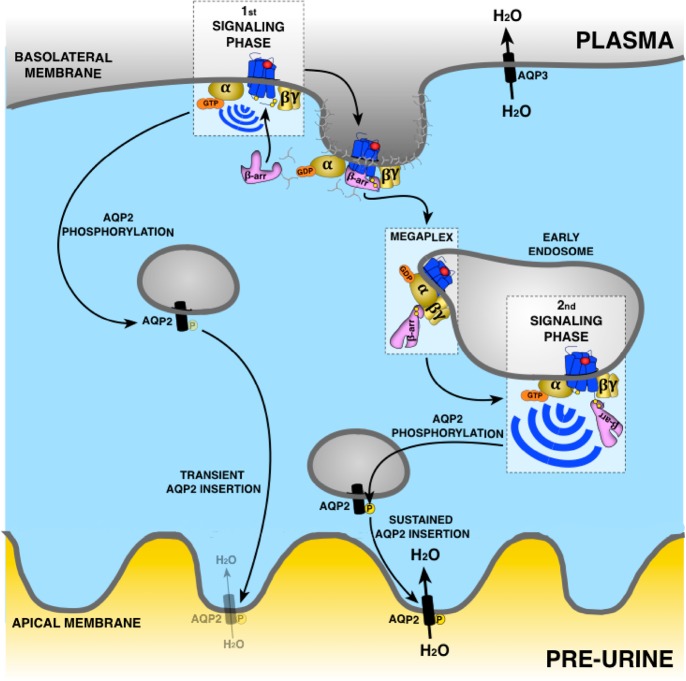
Figure 3.
Regulation of water transport in epithelial cells of the kidney by V2R. Vasopressin binding to V2R inactivates Gαs at the plasma membrane. This promotes phosphorylation of aquaporin-2 (AQP2) favoring its insertion into the apical membrane and entry of water. This is followed by a rapid recruitment of β-arr binding to V2R in core conformation leading to uncoupling from G proteins and internalization of the V2R-β-arr complex into early endosomes promoting the removal of AQP2 from the apical membrane. However, the change of β-arr from core to tail conformation in the endosome allows the core region of V2R to be available to interact with Gαs and activate this G protein in early endosomes. As β-arr in tail conformation does not uncouple V2R from G proteins, Gαs activation can occur causing a sustained AQP2 phosphorylation and insertion into apical membrane for a long-lasting entry of water. Aquaporin-3 (AQP3) allows water transport from epithelial cells to plasma to increase arterial volume and pressure and to decrease plasma sodium concentration.
V2R belong to a group of GPCRs that when activated and phosphorylated interact with β-arr in an exceptional stable manner.66,67 This stable interaction is maintained by clusters of phosphorylated serine/threonine sites at the receptor CT that make electrostatic interactions with positively charged arginine/lysine residues at the amino-terminal lobe of β-arrs.29,66−68 Thus, once these GPCRs have engaged with β-arr and been internalized, they stay associated and present in endosomes for prolonged periods of time. Counterintuitively, the strong GPCR-β-arr association does not seem to uncouple G protein from the receptor as would be expected from classical GPCR signaling paradigms. In fact, several of these GPCRs, including V2R, continue to stimulate G protein signaling for prolonged periods of time after having been internalized into endosomes by β-arr.8,64,69−71
The tight interaction between the phosphorylated CT and β-arr enables these GPCRs to form tail conformation GPCR−β-arr complexes where no association between β-arr and the receptor core takes place.9,10,28,72 This was recently demonstrated in a study using a mutant of β-arr1 that lacks the finger-loop region (FLR), which normally inserts itself into the receptor core to form a core conformation.10 Since this mutant β-arr (ΔFLR-β-arr1) does not interact with the receptor core region, it may only interact with GPCRs via the phosphorylated CT tail. Upon agonist stimulation, V2R recruits ΔFLR-β-arr1 robustly, whereas the β2AR, which lacks clusters of phosphorylation site on its CT tail, does not.10 Interestingly, exchanging the CT tail of β2AR with the CT tail of the V2R results in a β2V2R chimera receptor that now recruits equally well wild type β-arr1 and ΔFLR-βarr1 when activated by β2AR agonist.10 Furthermore, in the case of both V2R and β2V2R, ΔFLR-βarr1 maintains its ability to promote receptor internalization and β-arr-mediated signaling.10 Similar results were obtained in studies where the ICL3, which is essential for the receptor core interaction with β-arr, was removed from both V2R and β2V2R.28,72 Both of these receptor mutants only interact with β-arrs in the tail conformation. The selective recruitment of β-arrs in the tail conformation to both receptors was sufficient to stimulate β-arr-mediated receptor endocytosis and signaling.28,72
Since the entire V2R intracellular core is exposed in the tail conformation, heterotrimeric Gs can bind to the V2R−β-arr complex to form a V2R–Gs–β-arr “megaplex” (Figure 3).8 This megaplex configuration allows the receptor to activate G protein while being bound to β-arrs. Therefore, the existence of these megaplexes provides a mechanistic explanation of how certain GPCRs that bind to β-arrs strongly such as the V2R continue to stimulate G protein signaling while being internalized into endosomes by β-arrs. For these receptors, disrupting this interaction between the receptor CT tail and β-arr has been shown to strongly reduce endosomal G protein signaling.8,70 However, the exact molecular mechanism explaining this observation needs to be further investigated as the GPCRs having a lower affinity for β-arr such as β2AR do not require the presence of β-arr in endosomes to activate G protein in these intracellular compartments.23−25
The formation of megaplexes in cells is supported by a variety of fluorescence resonance energy transfer (FRET), bioluminescence resonance energy transfer (BRET), and fluorescence microscopy approaches that showed proximity of all three components within endosomes after receptor activation as well as activation of G protein by internalized GPCR-β-arr complexes.8,29,64,69,73 Using purified and reconstituted components, functional megaplexes form in an agonist-dependent manner in vitro, where the agonist-occupied receptor directly activates the G protein.8 Most recently, a high-resolution cryo-EM structure of a β2V2R–Gαs−β-arr1 megaplex was solved. The structure highlighted in atomic details how a single active GPCR can interact with and stabilize active conformation of G protein and β-arr simultaneously through its core and CT tail, respectively.29
In renal collecting duct principal cells, stimulation of the V2R with AVP at the basolateral side results in receptor internalization, megaplex formation, and prolonged endosomal cAMP signaling, which in turn leads to phosphorylation of AQP2 and long-lasting insertion into the apical membrane (Figure 3).8,64 The effect of AVP on AQP2 phosphorylation and trafficking is significantly more robust and prolonged compared to stimulation with oxytocin, an agonist that does not provoke endosomal V2R signaling. Since the majority of signaling promoted by endogenous AVP of the V2R in the renal collecting duct originates from internalized compartments, it might thus be favorable to target the receptor specifically at these sites to achieve more substantial antagonistic effect. Such drug delivery strategies have proven highly effective for other GPCR systems, and therefore, may prove to be a highly efficient way of treating HF and/or hyponatremia.74
Endosomal G Protein Signaling by CGRP Receptor: A Possible Role in the Cardioprotective Properties of CGRP?
The calcitonin gene-related peptide (CGRP) is a 37 amino acid peptide produced by alternative splicing of the calcitonin gene.75,76 The CGRP receptor is composed of calcitonin-like receptor (CLR) and a receptor activity modifying protein 1 (RAMP1), a single transmembrane protein required for ligand specificity and CLR expression at the plasma membrane (Figure 4A).77 CGRP is widely distributed in the central and peripheral nervous systems.75 It is released from the terminals of primary sensory neurons, especially the unmyelinated C fibers and thinly myelinated Aδ fibers, and in peripheral tissues in response to noxious stimuli to mediate nociception.78 Recently, Yarwood et al. showed that at the plasma membrane, CGRP receptor activates Gαs and Gαq mediating activation of cytosolic extracellular signal-regulated kinase (ERK) and protein kinase C (PKC), respectively (Figure 4A). Moreover, upon receptor internalization, both Gαs and Gαq are activated in endosomes leading to activation of nuclear ERK and PKC, respectively (Figure 4A).79 This Gαs-dependent activation of ERK was previously identified to be mediated by cAMP production as it was blocked by the PKA inhibitor H-89.80 Inhibition of endocytosis was shown to suppress CGRP-induced endosomal signaling, excitation of spinal neurons, and nociception, which suggests that endosomal G protein signaling is important for CGRP receptor-mediated nociception. This observation was also supported by the fact that a cholestanol-conjugated CGRP antagonist (CGRP8–37-Chol) that specifically targets the antagonist to endosomes prevented CGRP-induced activation of nuclear but not cytosolic ERK and inhibited CGRP-evoked excitation of spinal neurons and nociception.79
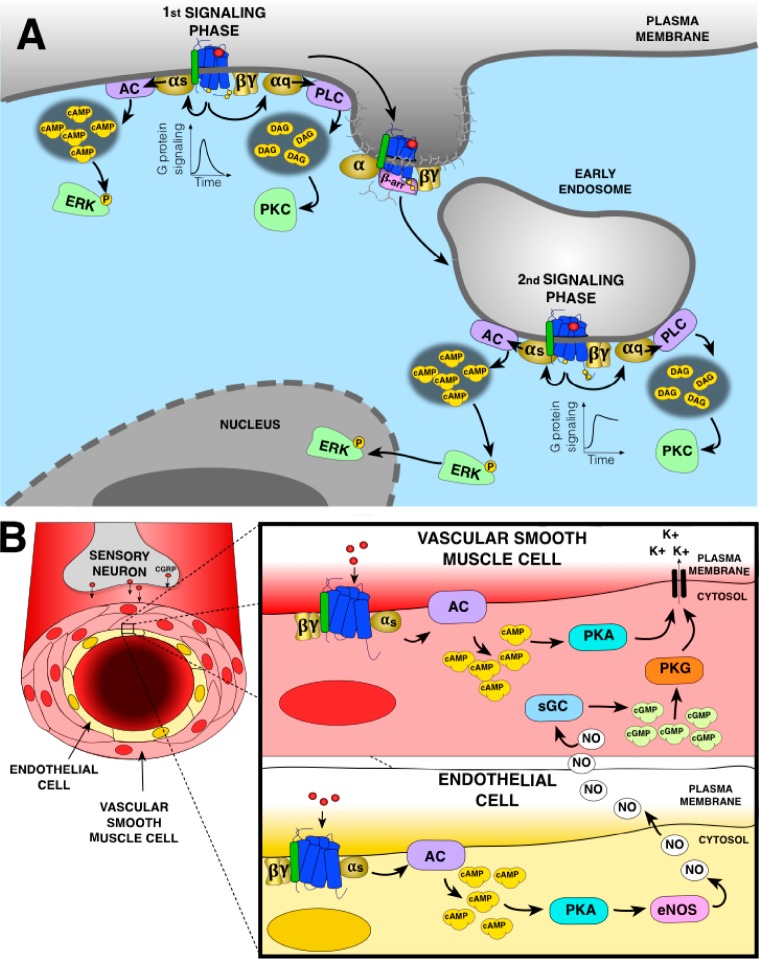
Figure 4.
(A) CGRP signaling at the plasma membrane and endosomes in sensory neurons. At the plasma membrane, CGRP receptor activates Gαs and Gαq. Gαs activation stimulates adenylyl cyclase (AC) to generate cAMP leading to phosphorylation of cytosolic ERK. Gαq activation stimulates phospholipase Cβ (PLC), production of second messengers, and activation of cytosolic protein kinase C (PKC). Recruitment of β-arr to the CGRP receptor induces receptor uncoupling from G proteins and internalization in early endosomes where the receptor can activate again G proteins. Endosomal Gαs activation induces sustained ERK phosphorylation and its translocation to the nucleus, while endosomal Gαq activation activate PKC in the cytosol. (B) Action of CGRP on peripheral vasculature. In the vascular smooth muscle cells (VSMC), activation of CGRP receptor induces Gαs activation, AC-mediated production of cAMP leading to activation of protein kinase A (PKA) opening the potassium channels to induce relaxation. In the endothelial cells, Gαs activation by CGRP receptor also lead to PKA activation. Although in these cells PKA activates the endothelial nitric oxide synthase (eNOS) responsible of nitric oxide (NO) production, NO diffuses into VSMC to mediate vasorelaxation by activating the soluble guanylate cyclase (sGC) producing cGMP-activating protein kinase G (PKG) also opening the potassium channels.
CGRP receptors are located not only in the central and peripheral nervous system but also in the cardiovascular system including the smooth muscle cells of coronary arteries, around peripheral arteries of the heart as well as in the atrium.81−83 These tissues are densely surrounded by CGRP-secreting nerves at the coronary arteries, ventricular muscle, and the conduction system that play an important role in the maintenance of cardiac homeostasis.78 CGRP is an extremely potent vasodilatator.84 It has been shown to prevent the onset of hypertension by regulating vascular resistance.85 Indeed, basal blood pressure is increased in many CGRP-knockout mouse models.86,87 CGRP exerts its action mainly on smooth muscle cells in the vascular wall of the microvasculature, which promote peripheral vascular resistance and affect the blood pressure. CGRP induces relaxation of smooth muscle cells due to Gαs activation mediating an increase in cAMP and activation of PKA, which phosphorylates and opens potassium channels (Figure 4B).88,89 CGRP also has the capacity to stimulate the endothelial NO synthase (eNOS) and production of nitric oxide (NO) via the Gαs–cAMP–PKA pathway that is stimulated by a receptor located in the endothelium. Eventually, NO diffuses into adjacent smooth muscle cells and activate guanylate cyclase (GC) leading to production of cGMP, activation of protein kinase G (PKG) that opens potassium channels leading to vasorelaxation (Figure 4B).90,91
CGRP also acts as a protective safeguard against myocardial ischemia.92−96 In humans, plasma CGRP expression is upregulated upon acute myocardial infarction.97 It is endogenously released in response to ischemia and potentially plays a role in preconditioning and protection against reperfusion injury in heart, the phenomenon in which a tissue is rendered resistant to the deleterious effects of prolonged ischemia.93,98−100 CGRP has been shown to decrease infarct size in a rat mesenteric artery occlusion model through myocardial PKC and to have antiapoptotic properties in cardiomyoblasts through inhibition of caspase 3 and increase of Bcl-2 mRNA expression.101,102 CGRP also protects against HF via positive chronotropic and ionotropic effects.103,104 It is naturally released in a compensatory manner in response to HF and acts in a protective manner. It is involved in the response to nitroglycerine in chronic HF and improves myocardial contractility by inducing vasodilatation, relieving cardiac hypertrophy and apoptosis.105−110
As opposed to the role of CGRP in nociception, the possible role of endosomal G protein signaling in CGRP-mediated vasodilatation and cardioprotection has not been addressed so far. It is tempting to hypothesize that the CGRP receptor has the same ability to signal via Gαs and Gαq from endosomes in cardiovascular cells and that this compartmentalized signaling mechanism is responsible for the CGRP-mediated cardioprotective effects. Additional work needs to be done to answer this question. The recent success of targeting endosomal compartments to block CGRP-mediated endosomal signaling and pain transmission highlights the importance of exploring a similar approach to potentially develop highly potent cardioprotective agents.
Intracellular Targeting of the Viral US28 Receptor as a Potential Treatment for Atherosclerosis
The human cytomegalovirus (HCMV) is a member of the β-herpesvirus family. This virus establishes life-long latent infections and is generally asymptomatic in healthy individuals as HCMV is controlled by a robust immune response. In immuno-compromised patients, HCMV infection can cause devastating diseases that are often lethal. However, despite the apparent lack of symptoms in the infected but immuno-competent population, there is strong evidence in the literature linking cardiovascular diseases to HCMV infection. Latent HCMV infection, which depends on US28 expression, is associated with increased risk of transplant-related vasculopathy, restenosis following angioplasty, atherosclerosis, and consequences thereof (thrombosis, stroke, and myocardial infection).111−113 Consequently, targeting US28 at the endosomes may represent a novel and efficacious strategy to treat these cardiovascular conditions. Both higher severity and mortality rate from atherosclerosis have been observed in situations of HCMV seropositivity.114,115 Atherosclerosis is characterized by an important endothelial dysfunction, monocyte recruitment, infiltration of macrophages to the arterial wall, and increased smooth muscle cell proliferation and migration. Inflammation of the vasculature leads to an increased expression of chemokines, adhesion molecules, and cytokines that promote adhesion and aggregation of inflammatory cells to the vascular walls.116−119 These events promote plaque formation and can result in thrombosis, stroke, or myocardial infection.120 HCMV seropositivity contributes to these events. In accordance with these studies, a recent work suggests that the link between HCMV and atherosclerosis could be explained by a dysregulation of cellular cholesterol metabolism by US28.121 HCMV, through US28 and cell division cycle 42 (CDC42) protein, has been shown to rearrange actin microfilaments and to modify lipid rafts creating new binding sites for apolipoprotein A-1 on the host plasma membrane, which results in enhanced cellular cholesterol efflux.
HCMV encodes four chemokine receptor homologues: UL33, UL78, US27, and US28.122,123 Among these receptors, only US28 has been reported to be required for latency.124,125 Interestingly, US28 has a high constitutive activity.126,127 The agonist-independent signaling of US28 is mainly mediated by Gαq, while its ligand-dependent signaling is predominantly occurring through Gαi and Gα12/13. The constitutive activation of Gαq by US28 has been reported in various cellular models such as monocytes, fibroblasts, hematopoietic, glioblastoma, endothelial, and smooth muscle cells.128−131 It has been estimated that a maximum of 20% of the total receptor population is expressed at the plasma membrane, while the vast majority (80%) is localized within endosomes.132 This prevalent endosomal localization of US28 has been explained by an abundance of GRK2, GRK5, PKC, and casein kinase-2 phosphorylation sites on the CT tail of US28 that promote constitutive clathrin-dependent US28 internalization.133−136 Interestingly, constitutive and agonist-induced internalization of US28 has been shown to be β-arr-independent as internalization of US28 is unaffected by the knockout of β-arrs in mouse embryonic fibroblasts (MEFs), while US28 internalization is inhibited in HeLa cells expressing siRNA against an AP-2 subunit, confirming the clathrin-dependent internalization of US28.135 Interestingly, truncation of the last 54 residues of the US28 CT tail (US28Δ317) has been shown to increase receptor surface expression by 500% compared to wild-type US28 but without any change of the constitutive inositol phosphate turnover in COS-7 cells.136 The fact that an increase of US28 expression at the plasma membrane did not correlate with a concomitant increase of constitutive signaling downstream of Gαq suggests that US28 may have the ability to activate Gαq independent of its cellular location.
The hypothesis that US28 constitutively activates Gαq signaling from endosomes is also supported by the recent work of Heukers et al.137 Previously, it has been shown that constitutive activation of US28 accelerates glioblastoma (GBM) tumor growth by inducing cell inflammation, migration, proliferation, and angiogenesis.130,138−140 With the objective to block US28-mediated GBM malignancy, Heukers et al. designed a high-affinity bivalent nanobody that targets the extracellular region of US28 thereby inhibiting agonist-induced and constitutive signaling of US28.137 Although this strategy reduced US28-mediated GBM cell growth and signaling, it only did so by only 50% in GBM cell lines and mice bearing GBM tumors. Given that the majority of US28 is localized intracellularly, the partial effect of the nanobody is not surprising as the nanobody cannot cross the plasma membrane and reach the main US28 pool. The partial inhibition of US28-mediated signaling and GBM malignancy in fact supports the idea that US28 is signaling from endosomes to promote GBM growth.
HCMV-associated diseases are treated with antivirals such as letermovir, ganciclovir, or foscarnet.141 These treatments are effective against lytically replicating virus but unfortunately are associated with viral resistance and drug toxicity.142−146 Furthermore, during the latent phase of infection, infected cells are masked from attack by a healthy immune response and are not targets of any current antiviral treatments. Consequently, targeting US28 signaling by designing cell-membrane-permeable inverse agonists or by directly targeting the drug to endosomes could offer more robust therapeutic results than those of the current antiviral drugs on the market. As latent HCMV infection that depends on US28 expression is associated with increased risk of transplant-related vasculopathy, restenosis following angioplasty, atherosclerosis, and consequences thereof (thrombosis, stroke and myocardial infection), specifically targeting US28 at endosomes may represent a novel and efficacious strategy to treat these cardiovascular conditions.
Is Sustained Gαi Activation by the Sphingosine-1-Phosphate (S1P)1 Receptor in the Golgi Responsible for the Cardioprotective Role of FTY720?
Sphingosine-1-phosphate (S1P) is an important bioactive lipid signaling molecule predominantly stored and released by red blood cells, platelets, fibroblasts, and vascular endothelial cells. In addition, S1P is widely present in the plasma and is a critical component of high-density lipoproteins (HDL).147−151 S1P interacts with five S1P receptors that all belong to the GPCR family (S1P1R–S1P5R). S1P1R, S1P2R, and S1P3R are found in a larger amount in the heart and blood vessels but are also present in the central nervous system and immune system.152 S1P1R is highly expressed in cardiomyocytes and endothelial cells, while S1P2R and S1P3R are the main S1P receptors in vascular smooth muscles, with S1P3R predominantly being distributed in fibroblasts.152−156 S1P4R is almost exclusive to lymphoid tissue, while S1P5R is mainly expressed in the central nervous system, natural killer cells, and spleen.157,158 The effects associated with activation of S1P receptors on different cell types are dictated by the specific G protein coupling of the S1P receptors expressed. S1P1R couples exclusively to Gαi, whereas S1P2R and S1P3R activate Gαi, Gαq, and Gα13. S1P4R and S1P5R couple to both Gαi and Gα13.159
S1P receptors regulate many cell biological processes, such as cell migration, survival, adhesion, and proliferation involved in modulation of important physiological functions.160−165 These functions are immune and inflammatory responses, heart rate, cardiac contractibility, and vascular tone. Furthermore, S1P receptors exert multiple cardioprotective effects including vasodilatation and inhibition of atherosclerosis. Alteration in S1P levels or activity has been associated with aberrant vascular maturation and development.155,166 Knockout mice for S1P1R have been recently characterized and show important ventricular septal defects and decreased myofibril organization leading to perinatal lethality.167 Cardiomyocyte-restricted deletion of S1P1R in mice results in progressive cardiomyopathy and compromised response to βAR stimulation.168 Changes in serum S1P is a predictive marker for the presence and severity of cardiovascular disease, as it is the case of obstructive coronary artery disease, atherosclerosis, myocardial infarction, and HF. S1P has been shown to decrease infarct size, protect against de novo acute HF, and improve functional recovery through the activation of signal transducer and activator of transcription 3 (STAT3) and protein kinase B (PKB).169,170 Unfortunately, after myocardial infarction, or in a situation of postischemic HF, the level of S1P1R has been shown to decrease.171,172 These observations suggest the high potential of S1P agonists as cardioprotective agents for patients suffering from HF or myocardial infarction.
Among S1P agonists, FTY720 (fingolimod) is the most common and is a structural analogue of S1P that is approved for the treatment of multiple sclerosis.173−175 FTY720 is phosphorylated by sphingosine kinases in vivo leading to the active metabolite FTY720P, a potent agonist of all S1P receptors except S1P2R.176−179 The action of FTY720P on multiple sclerosis results from its immunosuppressant action induced by sequestration of circulating mature lymphocytes.180−182 Interestingly, this molecule seems to interfere with the pathological processes of several other diseases, such as sepsis, inflammatory bowel disease, atherosclerosis, and myocardial infarction. FTY720P has been shown to reduce the formation of myocardial fibrosis by inhibiting oxygen free radicals to produce strong antioxidant anti-inflammatory properties, thus reducing the death of cardiomyocytes.183 FTY720P has also been reported to be an effective ischemic preconditioning agent which can reduce oxidative stress, inflammation, apoptosis, and myocardial fibrosis.184−187
Interestingly, FTY720P exhibits profound differences in the duration of signal transduction compared with that of the natural ligand S1P.71 Following transient exposure to FTY720P, S1P1R internalizes for long periods of time and localizes within the trans Golgi network. From this site, FTY720P but not the endogenous S1P continues to stimulate Gαi and ERK signaling in a sustained manner. Curiously, FTY720P-mediated activation of S1P3R only induced transient G protein activation suggesting that FTY720P-mediated sustained activation of S1P1R may be not be applicable for the other S1P receptor isoforms and thus may be unique to S1P1R. This sustained S1P1R-mediated G protein signaling in the trans Golgi network was also translated into an enhanced chemokinetic migration of endothelial cells. Given the cardioprotective effects associated with FTY720P and the ability of this molecule to promote a long-lasting Gαi protein signaling at the trans Golgi network, compartmentalized G protein signaling by S1P1R seems to provide a potentially interesting target for cardiovascular diseases. However, additional investigations need to be done to verify whether the cardioprotective effects of FTY720P are mediated by S1P1R-mediated sustained Gαi signaling or simply by transient activation of other S1P receptors at the plasma membrane.
Conclusion
In the present review, we have provided an overview of the current literature supporting the idea that G protein signaling in intracellular compartments such as early endosomes and the Golgi apparatus represents an emerging concept in the cardiovascular field. While solid evidence already supports a functional role of compartmentalized G protein signaling by βARs and V2R for cardiovascular pathophysiology, the pertinence of compartmentalized G protein signaling for cardiovascular functions by other GPCRs such as CGRP receptor, US28, and S1P1R needs to be further explored.
A considerable amount of work in the past decade has been done to identify GPCRs that activate G proteins from intracellular compartments, and efforts have been made to understand the physiological impact of this signaling far from the plasma membrane. Although many questions regarding the molecular mechanisms underlying compartmentalized G protein signaling remain to be answered, recent studies in the field of chronic pain have demonstrated the strong therapeutic potential of targeting endosomes to obtain highly specific and potent treatments associated with low toxicity and side effects. As discussed earlier, the conjugation of the transmembrane lipid cholestanol to a CGRP antagonist has been a particularly successful strategy to drive the antagonist toward early endosomes where CGRP is activating G proteins in a sustained manner mediating chronic nociception.79 A similar approach has been used to inhibit the substance P-mediated activation of the neurokinin 1 receptor (NK1R) G protein endosomal signaling using a cholestanol-conjugated NK1R antagonist spantide.70 Both conjugated antagonists selectively inhibit the sustained NK1R- and CGRP-receptor-induced excitation of spinal neurons, whereas their unconjugated counterparts were less effective. They were shown also to induce a remarkably long-lasting inhibition of nociceptive responses mediated by painful stimuli. Similarly, a cholestanol-conjugated antagonist of the protease-activated type 2 receptor (PAR2) has been shown to prevent the capacity of proteases to induce sustained hyperexcitability of nociceptors and therefore represents a promising treatment for patients suffering from irritable bowel syndrome.188 A pH-responsive nanoparticle approach has also been used recently to directly deliver NK1R antagonist, aprepitant, into endosomes.74 Aprepitant was incorporated into these nanoparticles and has been shown to successfully penetrate the plasma and endosomal membranes. In endosomes, the low pH triggers nanoparticle disassembly and delivery of its cargo of NK1R antagonist directly inside endosomes, as well as inhibiting NK1R-mediated excitation of spinal neurons leading to enhanced and persistent antinociceptive effects.
Although toxicity studies have not yet been completed, following the exciting success obtained by targeting endosomal G protein signaling mediated by GPCR involved in nociception, it seems that expanding these therapeutic approaches to other physiological systems such as the cardiovascular system may be a promising avenue to fight cardiovascular diseases. Blocking the G protein signaling of V2R, β1AR, or US28 from endosomes or Golgi would be an interesting approach to tackle hyponatremia-induced by HF, cardiac hypertrophy, as well as potentially atherosclerosis. Alternatively, targeting agonists in the endosomal compartment to enhance and maintain the cardioprotective effects of GPCRs such as the β2AR, as well as potentially the CGRP receptor or S1P1R, could also be a promising approach to exploit compartmentalized G protein signaling in a cardiovascular therapeutic context.
Acknowledgments
B.P. is funded by a Patrick G. Johnston Fellowship from Queen’s University Belfast, and a Wellcome Trust Seed Award in Science (215229/Z/19/Z). A.R.B.T. is funded by a LEO Foundation research grant (LF18043), and R.I. is funded by a NIH grant (NIH-R35GM133521).
The authors declare no competing financial interest.
References
- Pierce K. L.; Premont R. T.; Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650. 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Hauser A. S.; Attwood M. M.; Rask-Andersen M.; Schioth H. B.; Gloriam D. E. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discovery 16, 829–842. 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N.; Offermanns S. (2005) Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204. 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J. (2004) Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 25, 413–422. 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pitcher J. A.; Freedman N. J.; Lefkowitz R. J. (1998) G protein-coupled receptor kinases. Annu. Rev. Biochem. 67, 653–692. 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Pitcher J.; Lohse M. J.; Codina J.; Caron M. G.; Lefkowitz R. J. (1992) Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry 31, 3193–3197. 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- Krupnick J. G.; Benovic J. L. (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 38, 289–319. 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R. B.; Plouffe B.; Cahill T. J. 3rd; Shukla A. K.; Tarrasch J. T.; Dosey A. M.; Kahsai A. W.; Strachan R. T.; Pani B.; Mahoney J. P.; Huang L.; Breton B.; Heydenreich F. M.; Sunahara R. K.; Skiniotis G.; Bouvier M.; Lefkowitz R. J. (2016) GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell 166, 907–919. 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A. K.; Westfield G. H.; Xiao K.; Reis R. I.; Huang L. Y.; Tripathi-Shukla P.; Qian J.; Li S.; Blanc A.; Oleskie A. N.; Dosey A. M.; Su M.; Liang C. R.; Gu L. L.; Shan J. M.; Chen X.; Hanna R.; Choi M.; Yao X. J.; Klink B. U.; Kahsai A. W.; Sidhu S. S.; Koide S.; Penczek P. A.; Kossiakoff A. A.; Woods V. L. Jr.; Kobilka B. K.; Skiniotis G.; Lefkowitz R. J. (2014) Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222. 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill T. J. 3rd; Thomsen A. R.; Tarrasch J. T.; Plouffe B.; Nguyen A. H.; Yang F.; Huang L. Y.; Kahsai A. W.; Bassoni D. L.; Gavino B. J.; Lamerdin J. E.; Triest S.; Shukla A. K.; Berger B.; Little J. t.; Antar A.; Blanc A.; Qu C. X.; Chen X.; Kawakami K.; Inoue A.; Aoki J.; Steyaert J.; Sun J. P.; Bouvier M.; Skiniotis G.; Lefkowitz R. J. (2017) Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. U. S. A. 114, 2562–2567. 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte S. A.; Oakley R. H.; Zhang J.; Holt J. A.; Ferguson S. S.; Caron M. G.; Barak L. S. (1999) The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U. S. A. 96, 3712–3717. 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman O. B. Jr.; Krupnick J. G.; Santini F.; Gurevich V. V.; Penn R. B.; Gagnon A. W.; Keen J. H.; Benovic J. L. (1996) Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383, 447–450. 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu A. C.; von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568. 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Sorkin A.; von Zastrow M. (2009) Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622. 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M. T.; Shenoy S. K.; Lefkowitz R. J. (2006) Trafficking of G protein-coupled receptors. Circ. Res. 99, 570–582. 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- DeWire S. M.; Ahn S.; Lefkowitz R. J.; Shenoy S. K. (2007) Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510. 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Doherty G. J.; McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Irannejad R.; Tsvetanova N. G.; Lobingier B. T.; von Zastrow M. (2015) Effects of endocytosis on receptor-mediated signaling. Curr. Opin. Cell Biol. 35, 137–143. 10.1016/j.ceb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R. B.; Jensen D. D.; Hicks G. A.; Bunnett N. W. (2018) Therapeutic Targeting of Endosomal G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 39, 879–891. 10.1016/j.tips.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D.; Godbole A. (2018) Internalization of G-protein-coupled receptors: Implication in receptor function, physiology and diseases. Best Pract Res. Clin Endocrinol Metab 32, 83–91. 10.1016/j.beem.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Eichel K.; von Zastrow M. (2018) Subcellular Organization of GPCR Signaling. Trends Pharmacol. Sci. 39, 200–208. 10.1016/j.tips.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S.; Feinstein T. N.; Castro M.; Wang B.; Bouley R.; Potts J. T.; Gardella T. J.; Vilardaga J. P. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742. 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D.; Nikolaev V. O.; Gagliani M. C.; de Filippis T.; Dees C.; Tacchetti C.; Persani L.; Lohse M. J. (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7, e1000172 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski S. J.; Hopf F. W.; Seif T.; Bonci A.; von Zastrow M. (2011) Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290. 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R.; Tomshine J. C.; Tomshine J. R.; Chevalier M.; Mahoney J. P.; Steyaert J.; Rasmussen S. G.; Sunahara R. K.; El-Samad H.; Huang B.; von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538. 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R.; Pessino V.; Mika D.; Huang B.; Wedegaertner P. B.; Conti M.; von Zastrow M. (2017) Functional selectivity of GPCR-directed drug action through location bias. Nat. Chem. Biol. 13, 799–806. 10.1038/nchembio.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M.; Jullie D.; Lobingier B. T.; Laeremans T.; Steyaert J.; Schiller P. W.; Manglik A.; von Zastrow M. (2018) A Genetically Encoded Biosensor Reveals Location Bias of Opioid Drug Action. Neuron 98, 963–976. 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P.; Srivastava A.; Ghosh E.; Ranjan R.; Dogra S.; Yadav P. N.; Shukla A. K. (2017) Core engagement with beta-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Mol. Biol. Cell 28, 1003–1010. 10.1091/mbc.e16-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A. H.; Thomsen A. R. B.; Cahill T. J.; Huang R. III; Huang L. Y.; Marcink T.; Clarke O. B.; Heissel S.; Masoudi A.; Ben-Hail D.; Samaan F.; Dandey V. P.; Tan Y. Z.; Hong C.; Mahoney J. P.; Triest S.; Little J. t.; Chen X.; Sunahara R.; Steyaert J.; Molina H.; Yu Z.; des Georges A.; Lefkowitz R. J. (2019) Structure of an endosomal signaling GPCR-G protein-beta-arrestin megacomplex. Nat. Struct. Mol. Biol. 26, 1123. 10.1038/s41594-019-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. D.; Sun Y.; Bourque K.; Audet N.; Inoue A.; Tanny J. C.; Hebert T. E. (2018) Receptor- and cellular compartment-specific activation of the cAMP/PKA pathway by alpha1-adrenergic and ETA endothelin receptors. Cell. Signalling 44, 43–50. 10.1016/j.cellsig.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Gorvin C. M.; Rogers A.; Hastoy B.; Tarasov A. I.; Frost M.; Sposini S.; Inoue A.; Whyte M. P.; Rorsman P.; Hanyaloglu A. C.; Breitwieser G. E.; Thakker R. V. (2018) AP2sigma Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity. Cell Rep. 22, 1054–1066. 10.1016/j.celrep.2017.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Chatelain E.; Desprez T.; Serrat R.; Bellocchio L.; Soria-Gomez E.; Busquets-Garcia A.; Pagano Zottola A. C.; Delamarre A.; Cannich A.; Vincent P. (2016) A cannabinoid link between mitochondria and memory. Nature 539, 555–559. 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- Lyga S.; Volpe S.; Werthmann R. C.; Gotz K.; Sungkaworn T.; Lohse M. J.; Calebiro D. (2016) Persistent cAMP Signaling by Internalized LH Receptors in Ovarian Follicles. Endocrinology 157, 1613–1621. 10.1210/en.2015-1945. [DOI] [PubMed] [Google Scholar]
- Suofu Y.; Li W.; Jean-Alphonse F. G.; Jia J.; Khattar N. K.; Li J.; Baranov S. V.; Leronni D.; Mihalik A. C.; He Y. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U. S. A. 114, E7997–E8006. 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik J.; Wright P. T.; Lyon A. R.; Harding S. E. (2013) Spatial control of the betaAR system in heart failure: the transverse tubule and beyond. Cardiovasc. Res. 98, 216–224. 10.1093/cvr/cvt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom R. S.; Gregorian C.; Drenan R. M.; Xiang Y.; Regan J. W.; Insel P. A. (2001) Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 276, 42063–42069. 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- Bristow M. R.; Hershberger R. E.; Port J. D.; Minobe W.; Rasmussen R. (1989) Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol. Pharmacol. 35, 295–303. [PubMed] [Google Scholar]
- Chruscinski A. J.; Rohrer D. K.; Schauble E.; Desai K. H.; Bernstein D.; Kobilka B. K. (1999) Targeted disruption of the beta2 adrenergic receptor gene. J. Biol. Chem. 274, 16694–16700. 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Rohrer D. K.; Desai K. H.; Jasper J. R.; Stevens M. E.; Regula D. P. Jr.; Barsh G. S.; Bernstein D.; Kobilka B. K. (1996) Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc. Natl. Acad. Sci. U. S. A. 93, 7375–7380. 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communal C.; Singh K.; Sawyer D. B.; Colucci W. S. (1999) Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100, 2210–2212. 10.1161/01.CIR.100.22.2210. [DOI] [PubMed] [Google Scholar]
- Zhu W. Z.; Zheng M.; Koch W. J.; Lefkowitz R. J.; Kobilka B. K.; Xiao R. P. (2001) Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 98, 1607–1612. 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia C.; Eguchi A.; Koch W. J. (2018) New Insights in Cardiac beta-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 9, 904. 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A.; Rengo G.; Koch W. J. (2013) Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 113, 739–753. 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Gareri C.; Rockman H. A. (2018) G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 123, 716–735. 10.1161/CIRCRESAHA.118.311403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y.; Luttrell L. M.; Lefkowitz R. J. (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91. 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Xiao R. P.; Ji X.; Lakatta E. G. (1995) Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol. Pharmacol. 47, 322–329. [PubMed] [Google Scholar]
- Chesley A.; Lundberg M. S.; Asai T.; Xiao R. P.; Ohtani S.; Lakatta E. G.; Crow M. T. (2000) The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ. Res. 87, 1172–1179. 10.1161/01.RES.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Buxton I. L.; Brunton L. L. (1983) Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 258, 10233–10239. [PubMed] [Google Scholar]
- Hayes J. S.; Brunton L. L.; Mayer S. E. (1980) Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J. Biol. Chem. 255, 5113–5119. [PubMed] [Google Scholar]
- Musheshe N.; Schmidt M.; Zaccolo M. (2018) cAMP: From Long-Range Second Messenger to Nanodomain Signalling. Trends Pharmacol. Sci. 39, 209–222. 10.1016/j.tips.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Filadi R.; Basso E.; Lefkimmiatis K.; Pozzan T. (2017) Beyond Intracellular Signaling: The Ins and Outs of Second Messengers Microdomains. Adv. Exp. Med. Biol. 981, 279–322. 10.1007/978-3-319-55858-5_12. [DOI] [PubMed] [Google Scholar]
- Wright P. T.; Nikolaev V. O.; O’Hara T.; Diakonov I.; Bhargava A.; Tokar S.; Schobesberger S.; Shevchuk A. I.; Sikkel M. B.; Wilkinson R.; Trayanova N. A.; Lyon A. R.; Harding S. E.; Gorelik J. (2014) Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J. Mol. Cell. Cardiol. 67, 38–48. 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.; Gorelik J.; Spohr H. A.; Shevchuk A.; Lab M. J.; Harding S. E.; Vodyanoy I.; Klenerman D.; Korchev Y. E. (2002) High-resolution scanning patch-clamp: new insights into cell function. FASEB J. 16, 748–750. 10.1096/fj.01-1024fje. [DOI] [PubMed] [Google Scholar]
- Leroy J.; Richter W.; Mika D.; Castro L. R.; Abi-Gerges A.; Xie M.; Scheitrum C.; Lefebvre F.; Schittl J.; Mateo P.; Westenbroek R.; Catterall W. A.; Charpentier F.; Conti M.; Fischmeister R.; Vandecasteele G. (2011) Phosphodiesterase 4B in the cardiac L-type Ca(2)(+) channel complex regulates Ca(2)(+) current and protects against ventricular arrhythmias in mice. J. Clin. Invest. 121, 2651–2661. 10.1172/JCI44747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. B.; Rossow C. F.; Navedo M. F.; Westenbroek R. E.; Catterall W. A.; Santana L. F.; McKnight G. S. (2010) Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 107, 747–756. 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev V. O.; Moshkov A.; Lyon A. R.; Miragoli M.; Novak P.; Paur H.; Lohse M. J.; Korchev Y. E.; Harding S. E.; Gorelik J. (2010) Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657. 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Froese A.; Nikolaev V. O. (2015) Imaging alterations of cardiomyocyte cAMP microdomains in disease. Front. Pharmacol. 6, 172. 10.3389/fphar.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova N. G.; von Zastrow M. (2014) Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol. 10, 1061–1065. 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B.; Lavoie C.; Vaniotis G.; Baragli A.; Villeneuve L. R.; Ethier N.; Trieu P.; Allen B. G.; Hebert T. E. (2006) Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc. Res. 71, 69–78. 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Vaniotis G.; Del Duca D.; Trieu P.; Rohlicek C. V.; Hebert T. E.; Allen B. G. (2011) Nuclear beta-adrenergic receptors modulate gene expression in adult rat heart. Cell. Signalling 23, 89–98. 10.1016/j.cellsig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole A.; Lyga S.; Lohse M. J.; Calebiro D. (2017) Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat. Commun. 8, 443. 10.1038/s41467-017-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M. R. (2011) Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ. Res. 109, 1176–1194. 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- Zaca V.; Rastogi S.; Mishra S.; Wang M.; Sharov V. G.; Gupta R. C.; Goldstein S.; Sabbah H. N. (2009) Atenolol is inferior to metoprolol in improving left ventricular function and preventing ventricular remodeling in dogs with heart failure. Cardiology 112, 294–302. 10.1159/000159123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubarsch C.; Schneider R.; Pieske B.; Ruf T.; Hasenfuss G.; Fraedrich G.; Posival H.; Just H. (1995) Positive and negative inotropic effects of DL-sotalol and D-sotalol in failing and nonfailing human myocardium under physiological experimental conditions. Circulation 92, 2904–2910. 10.1161/01.CIR.92.10.2904. [DOI] [PubMed] [Google Scholar]
- Tuncer M.; Guntekin U.; Gunes Y.; Gumrukcuoglu H. A.; Eryonucu B. (2008) Differential effects of nebivolol and atenolol on transmitral diastolic filling parameters in patients with essential hypertension. Adv. Ther. 25, 619–626. 10.1007/s12325-008-0065-3. [DOI] [PubMed] [Google Scholar]
- Nash C. A.; Wei W.; Irannejad R.; Smrcka A. V. (2019) Golgi localized beta1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCepsilon to regulate cardiac hypertrophy. eLife 8, 8. 10.7554/eLife.48167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. J. t.; Konstam M. A.; Udelson J. E. (2008) Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation 118, 410–421. 10.1161/CIRCULATIONAHA.108.765289. [DOI] [PubMed] [Google Scholar]
- Goldsmith S. R. (2019) Arginine vasopressin antagonism in heart failure: Current status and possible new directions. J. Cardiol 74, 49–52. 10.1016/j.jjcc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Feinstein T. N.; Yui N.; Webber M. J.; Wehbi V. L.; Stevenson H. P.; King J. D. Jr.; Hallows K. R.; Brown D.; Bouley R.; Vilardaga J. P. (2013) Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 288, 27849–27860. 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert J. D.; Fenton R. A.; Moeller H. B.; Simons B.; Tchapyjnikov D.; McDill B. W.; Yu M. J.; Pisitkun T.; Chen F.; Knepper M. A. (2008) Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J. Biol. Chem. 283, 24617–24627. 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R. H.; Laporte S. A.; Holt J. A.; Barak L. S.; Caron M. G. (2001) Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J. Biol. Chem. 276, 19452–19460. 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Oakley R. H.; Laporte S. A.; Holt J. A.; Caron M. G.; Barak L. S. (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 275, 17201–17210. 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Shukla A. K.; Manglik A.; Kruse A. C.; Xiao K.; Reis R. I.; Tseng W. C.; Staus D. P.; Hilger D.; Uysal S.; Huang L. Y.; Paduch M.; Tripathi-Shukla P.; Koide A.; Koide S.; Weis W. I.; Kossiakoff A. A.; Kobilka B. K.; Lefkowitz R. J. (2013) Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141. 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbi V. L.; Stevenson H. P.; Feinstein T. N.; Calero G.; Romero G.; Vilardaga J. P. (2013) Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gbetagamma complex. Proc. Natl. Acad. Sci. U. S. A. 110, 1530–1535. 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. D.; Lieu T.; Halls M. L.; Veldhuis N. A.; Imlach W. L.; Mai Q. N.; Poole D. P.; Quach T.; Aurelio L.; Conner J.; et al. (2017) Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 9, eaal3447. 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F.; Zecri F.; Cetin C.; Billich A.; Guerini D.; Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434. 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- Kumari P.; Srivastava A.; Banerjee R.; Ghosh E.; Gupta P.; Ranjan R.; Chen X.; Gupta B.; Gupta C.; Jaiman D.; Shukla A. K. (2016) Functional competence of a partially engaged GPCR-beta-arrestin complex. Nat. Commun. 7, 13416. 10.1038/ncomms13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland C. T.; Salanga C. L.; Kawamura T.; Trejo J.; Handel T. M. (2013) The chemokine receptor CCR1 is constitutively active, which leads to G protein-independent, beta-arrestin-mediated internalization. J. Biol. Chem. 288, 32194–32210. 10.1074/jbc.M113.503797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Garcia P. D.; Retamal J. S.; Shenoy P.; Imlach W.; Sykes M.; Truong N.; Constandil L.; Pelissier T.; Nowell C. J.; Khor S. Y.; Layani L. M.; Lumb C.; Poole D. P.; Lieu T.; Stewart G. D.; Mai Q. N.; Jensen D. D.; Latorre R.; Scheff N. N.; Schmidt B. L.; Quinn J. F.; Whittaker M. R.; Veldhuis N. A.; Davis T. P.; Bunnett N. W. (2019) A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 14, 1150–1159. 10.1038/s41565-019-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G.; Mermod J. J.; Amara S. G.; Swanson L. W.; Sawchenko P. E.; Rivier J.; Vale W. W.; Evans R. M. (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304, 129–135. 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Amara S. G.; Jonas V.; Rosenfeld M. G.; Ong E. S.; Evans R. M. (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298, 240–244. 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Hay D. L.; Garelja M. L.; Poyner D. R.; Walker C. S. (2018) Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175, 3–17. 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F. A.; King R.; Smillie S. J.; Kodji X.; Brain S. D. (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142. 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood R. E.; Imlach W. L.; Lieu T.; Veldhuis N. A.; Jensen D. D.; Klein Herenbrink C.; Aurelio L.; Cai Z.; Christie M. J.; Poole D. P.; Porter C. J. H.; McLean P.; Hicks G. A.; Geppetti P.; Halls M. L.; Canals M.; Bunnett N. W. (2017) Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc. Natl. Acad. Sci. U. S. A. 114, 12309–12314. 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permpoonputtana K.; Porter J. E.; Govitrapong P. (2016) Calcitonin gene-related peptide mediates an inflammatory response in Schwann cells via cAMP-dependent ERK signaling cascade. Life Sci. 144, 19–25. 10.1016/j.lfs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S.; Saetrum Opgaard O.; Ekman R.; Costa Andrade N.; Wharton J.; Polak J. M.; Queiroz e Melo J.; Edvinsson L. (1993) Peptidergic innervation of human epicardial coronary arteries. Circ. Res. 73, 579–588. 10.1161/01.RES.73.3.579. [DOI] [PubMed] [Google Scholar]
- Uddman R.; Edvinsson L.; Ekblad E.; Hakanson R.; Sundler F. (1986) Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul. Pept. 15, 1–23. 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Sigrist S.; Franco-Cereceda A.; Muff R.; Henke H.; Lundberg J. M.; Fischer J. A. (1986) Specific receptor and cardiovascular effects of calcitonin gene-related peptide. Endocrinology 119, 381–389. 10.1210/endo-119-1-381. [DOI] [PubMed] [Google Scholar]
- Brain S. D.; Tippins J. R.; Morris H. R.; MacIntyre I.; Williams T. J. (1986) Potent vasodilator activity of calcitonin gene-related peptide in human skin. J. Invest. Dermatol. 87, 533–536. 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- Smillie S. J.; King R.; Kodji X.; Outzen E.; Pozsgai G.; Fernandes E.; Marshall N.; de Winter P.; Heads R. J.; Dessapt-Baradez C.; Gnudi L.; Sams A.; Shah A. M.; Siow R. C.; Brain S. D. (2014) An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension 63, 1056–1062. 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- Li J.; Carnevale K. A.; Dipette D. J.; Supowit S. C. (2013) Renal protective effects of alpha-calcitonin gene-related peptide in deoxycorticosterone-salt hypertension. Am. J. Physiol Renal Physiol 304, F1000–1008. 10.1152/ajprenal.00434.2012. [DOI] [PubMed] [Google Scholar]
- Mai T. H.; Wu J.; Diedrich A.; Garland E. M.; Robertson D. (2014) Calcitonin gene-related peptide (CGRP) in autonomic cardiovascular regulation and vascular structure. J. Am. Soc. Hypertens 8, 286–296. 10.1016/j.jash.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y.; Takagi Y.; Takata S.; Fukuda Y.; Yoshimi H.; Fujita T. (1988) Calcitonin gene-related peptide receptor in cultured vascular smooth muscle and endothelial cells. Biochem. Biophys. Res. Commun. 151, 1113–1121. 10.1016/S0006-291X(88)80481-9. [DOI] [PubMed] [Google Scholar]
- Crossman D. C.; Dashwood M. R.; Brain S. D.; McEwan J.; Pearson J. D. (1990) Action of calcitonin gene-related peptide upon bovine vascular endothelial and smooth muscle cells grown in isolation and co-culture. Br. J. Pharmacol. 99, 71–76. 10.1111/j.1476-5381.1990.tb14656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D.; Grant A. D. (2004) Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 84, 903–934. 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Gray D. W.; Marshall I. (1992) Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br. J. Pharmacol. 107, 691–696. 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S.; Kimura T.; Sakai S.; Yanagi K.; Miyauchi Y.; Aonuma K.; Miyauchi T. (2014) Calcitonin gene-related peptide protects the myocardium from ischemia induced by endothelin-1: intravital microscopic observation and (31)P-MR spectroscopic studies. Life Sci. 118, 248–254. 10.1016/j.lfs.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Chai W.; Mehrotra S.; Jan Danser A. H.; Schoemaker R. G. (2006) The role of calcitonin gene-related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur. J. Pharmacol. 531, 246–253. 10.1016/j.ejphar.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Song J.; Chen H.; Cao C.; Lee C. (2015) TRPV1 activation is involved in the cardioprotection of remote limb ischemic postconditioning in ischemia-reperfusion injury rats. Biochem. Biophys. Res. Commun. 463, 1034–1039. 10.1016/j.bbrc.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Li Y. J.; Peng J. (2002) The cardioprotection of calcitonin gene-related peptide-mediated preconditioning. Eur. J. Pharmacol. 442, 173–177. 10.1016/S0014-2999(02)01538-8. [DOI] [PubMed] [Google Scholar]
- Lei J.; Zhu F.; Zhang Y.; Duan L.; Lei H.; Huang W. (2016) Transient Receptor Potential Vanilloid Subtype 1 Inhibits Inflammation and Apoptosis via the Release of Calcitonin Gene-Related Peptide in the Heart after Myocardial Infarction. Cardiology 134, 436–443. 10.1159/000444439. [DOI] [PubMed] [Google Scholar]
- Roudenok V.; Gutjar L.; Antipova V.; Rogov Y. (2001) Expression of vasoactive intestinal polypeptide and calcitonin gene-related peptide in human stellate ganglia after acute myocardial infarction. Ann. Anat. 183, 341–344. 10.1016/S0940-9602(01)80176-X. [DOI] [PubMed] [Google Scholar]
- Li Y. J.; Xiao Z. S.; Peng C. F.; Deng H. W. (1996) Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. Eur. J. Pharmacol. 311, 163–167. 10.1016/0014-2999(96)00426-8. [DOI] [PubMed] [Google Scholar]
- Lu R.; Hu C. P.; Peng J.; Deng H. W.; Li Y. J. (2001) Role of calcitonin gene-related peptide in ischaemic preconditioning in diabetic rat hearts. Clin. Exp. Pharmacol. Physiol. 28, 392–396. 10.1046/j.1440-1681.2001.03467.x. [DOI] [PubMed] [Google Scholar]
- Peng J.; Xiao J.; Ye F.; Deng H. W.; Li Y. J. (2000) Inhibition of cardiac tumor necrosis factor-alpha production by calcitonin gene-related peptide-mediated ischemic preconditioning in isolated rat hearts. Eur. J. Pharmacol. 407, 303–308. 10.1016/S0014-2999(00)00702-0. [DOI] [PubMed] [Google Scholar]
- Wolfrum S.; Nienstedt J.; Heidbreder M.; Schneider K.; Dominiak P.; Dendorfer A. (2005) Calcitonin gene related peptide mediates cardioprotection by remote preconditioning. Regul. Pept. 127, 217–224. 10.1016/j.regpep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Sueur S.; Pesant M.; Rochette L.; Connat J. L. (2005) Antiapoptotic effect of calcitonin gene-related peptide on oxidative stress-induced injury in H9c2 cardiomyocytes via the RAMP1/CRLR complex. J. Mol. Cell. Cardiol. 39, 955–963. 10.1016/j.yjmcc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Gennari C.; Fischer J. A. (1985) Cardiovascular action of calcitonin gene-related peptide in humans. Calcif. Tissue Int. 37, 581–584. 10.1007/BF02554909. [DOI] [PubMed] [Google Scholar]
- Saetrum Opgaard O.; Hasbak P.; de Vries R.; Saxena P. R.; Edvinsson L. (2000) Positive inotropy mediated via CGRP receptors in isolated human myocardial trabeculae. Eur. J. Pharmacol. 397, 373–382. 10.1016/S0014-2999(00)00233-8. [DOI] [PubMed] [Google Scholar]
- Gennari C.; Nami R.; Agnusdei D.; Fischer J. A. (1990) Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc. Res. 24, 239–241. 10.1093/cvr/24.3.239. [DOI] [PubMed] [Google Scholar]
- Li J. Z.; Peng J.; Xiao L.; Zhang Y. S.; Liao M. C.; Li X. H.; Hu C. P.; Deng H. W.; Li Y. J. (2010) Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can. J. Physiol. Pharmacol. 88, 949–959. 10.1139/Y10-067. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M.; Hua Y.; Fredholm B. B. (1984) Capsaicin-induced stimulation of the guinea-pig atrium. Involvement of a novel sensory transmitter or a direct action on myocytes?. Naunyn-Schmiedeberg's Arch. Pharmacol. 325, 176–182. 10.1007/BF00506198. [DOI] [PubMed] [Google Scholar]
- Peng L. M.; Chen X. P.; Sun J.; Guo Y. J.; Li L.; Mo L.; Xie W.; Li Y. J.; Yang T. L.; Li C. C. (2012) Influence of ALDH2 Glu504Lys polymorphism on nitroglycerin response in chronic heart failure and involvement of Calcitonin Gene Related Peptide (CGRP). Int. J. Clin. Pharmacol. Ther. 50, 701–711. 10.5414/CP201635. [DOI] [PubMed] [Google Scholar]
- Bergdahl A.; Valdemarsson S.; Nilsson T.; Sun X. Y.; Hedner T.; Edvinsson L. (1999) Dilatory responses to acetylcholine, calcitonin gene-related peptide and substance P in the congestive heart failure rat. Acta Physiol. Scand. 165, 15–23. 10.1046/j.1365-201x.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- Katori T.; Hoover D. B.; Ardell J. L.; Helm R. H.; Belardi D. F.; Tocchetti C. G.; Forfia P. R.; Kass D. A.; Paolocci N. (2005) Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ. Res. 96, 234–243. 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- Lebedeva A. M.; Shpektor A. V.; Vasilieva E. Y.; Margolis L. B. (2018) Cytomegalovirus Infection in Cardiovascular Diseases. Biochemistry (Moscow) 83, 1437–1447. 10.1134/S0006297918120027. [DOI] [PubMed] [Google Scholar]
- Streblow D. N.; Dumortier J.; Moses A. V.; Orloff S. L.; Nelson J. A. (2008) Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Curr. Top. Microbiol. Immunol. 325, 397–415. 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Peng G.; Bai J.; He B.; Huang K.; Hu X.; Liu D. (2017) Cytomegalovirus Infection and Relative Risk of Cardiovascular Disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A Meta-Analysis of Prospective Studies Up to 2016. J. Am. Heart Assoc. 6, 6. 10.1161/JAHA.116.005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlestein J. B.; Horne B. D.; Carlquist J. F.; Madsen T. E.; Bair T. L.; Pearson R. R.; Anderson J. L. (2000) Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation 102, 1917–1923. 10.1161/01.CIR.102.16.1917. [DOI] [PubMed] [Google Scholar]
- Soderberg-Naucler C. (2006) Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer?. J. Intern. Med. 259, 219–246. 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Lamon B. D.; Hajjar D. P. (2008) Inflammation at the molecular interface of atherogenesis: an anthropological journey. Am. J. Pathol. 173, 1253–1264. 10.2353/ajpath.2008.080442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Vietinghoff S.; Koltsova E. K. (2019) Inflammation in atherosclerosis: A key role for cytokines. Cytokine+ 122, 154819. 10.1016/j.cyto.2019.154819. [DOI] [PubMed] [Google Scholar]
- Talepoor A. G., Fouladseresht H., Khosropanah S., and Doroudchi M. (2019) Immune-Inflammation in Atherosclerosis: A New Twist In An Old Tale. Endocr., Metab. Immune Disord.: Drug Targets, 19 10.2174/1871530319666191016095725. [DOI] [PubMed] [Google Scholar]
- Nguyen M. T.; Fernando S.; Schwarz N.; Tan J. T.; Bursill C. A.; Psaltis P. J. (2019) Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 8, 1109. 10.3390/jcm8081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J. (2000) Atherosclerosis. Nature 407, 233–241. 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low H.; Mukhamedova N.; Cui H. L.; McSharry B. P.; Avdic S.; Hoang A.; Ditiatkovski M.; Liu Y.; Fu Y.; Meikle P. J.; Blomberg M.; Polyzos K. A.; Miller W. E.; Religa P.; Bukrinsky M.; Soderberg-Naucler C.; Slobedman B.; Sviridov D. (2016) Cytomegalovirus Restructures Lipid Rafts via a US28/CDC42-Mediated Pathway, Enhancing Cholesterol Efflux from Host Cells. Cell Rep. 16, 186–200. 10.1016/j.celrep.2016.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S.; Bankier A. T.; Beck S.; Bohni R.; Brown C. M.; Cerny R.; Horsnell T.; Hutchison C. A. 3rd; Kouzarides T.; Martignetti J. A.; et al. (1990) Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154, 125–169. 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Gompels U. A.; Nicholas J.; Lawrence G.; Jones M.; Thomson B. J.; Martin M. E.; Efstathiou S.; Craxton M.; Macaulay H. A. (1995) The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209, 29–51. 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- Humby M. S.; O’Connor C. M. (2016) Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells. J. Virol. 90, 2959–2970. 10.1128/JVI.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna B. A.; Poole E. L.; Jackson S. E.; Smit M. J.; Wills M. R.; Sinclair J. H. (2017) Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection. mBio 8, 8. 10.1128/mBio.01754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomaske J.; Nelson J. A.; Streblow D. N. (2009) Human Cytomegalovirus US28: a functionally selective chemokine binding receptor. Infect. Disord.: Drug Targets 9, 548–556. 10.2174/187152609789105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg J. S.; Ingram J. R.; Venkatakrishnan A. J.; Jude K. M.; Dukkipati A.; Feinberg E. N.; Angelini A.; Waghray D.; Dror R. O.; Ploegh H. L.; Garcia K. C. (2015) Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science 347, 1113–1117. 10.1126/science.aaa5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. E.; Miller W. E. (2016) The HCMV US28 vGPCR induces potent Galphaq/PLC-beta signaling in monocytes leading to increased adhesion to endothelial cells. Virology 497, 233–243. 10.1016/j.virol.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. E.; Zagorski W. A.; Brenneman J. D.; Avery D.; Miller J. L.; O’Connor C. M. (2012) US28 is a potent activator of phospholipase C during HCMV infection of clinically relevant target cells. PLoS One 7, e50524 10.1371/journal.pone.0050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussang D.; Langemeijer E.; Fitzsimons C. P.; Stigter-van Walsum M.; Dijkman R.; Borg M. K.; Slinger E.; Schreiber A.; Michel D.; Tensen C. P.; van Dongen G. A.; Leurs R.; Smit M. J. (2009) The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 69, 2861–2869. 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- Minisini R.; Tulone C.; Luske A.; Michel D.; Mertens T.; Gierschik P.; Moepps B. (2003) Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J. Virol 77, 4489–4501. 10.1128/JVI.77.8.4489-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A.; Kledal T. N.; Pelchen-Matthews A.; Bowers K.; Schwartz T. W.; Marsh M. (2001) The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 12, 1737–1749. 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. E.; Houtz D. A.; Nelson C. D.; Kolattukudy P. E.; Lefkowitz R. J. (2003) G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 278, 21663–21671. 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Mokros T.; Rehm A.; Droese J.; Oppermann M.; Lipp M.; Hopken U. E. (2002) Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. J. Biol. Chem. 277, 45122–45128. 10.1074/jbc.M208214200. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A.; Kohout T. A.; Waldhoer M.; Marsh M. (2003) Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic 4, 243–253. 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Waldhoer M.; Casarosa P.; Rosenkilde M. M.; Smit M. J.; Leurs R.; Whistler J. L.; Schwartz T. W. (2003) The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J. Biol. Chem. 278, 19473–19482. 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- Heukers R.; Fan T. S.; de Wit R. H.; van Senten J. R.; De Groof T. W. M.; Bebelman M. P.; Lagerweij T.; Vieira J.; de Munnik S. M.; Smits-de Vries L.; van Offenbeek J.; Rahbar A.; van Hoorick D.; Soderberg-Naucler C.; Wurdinger T.; Leurs R.; Siderius M.; Vischer H. F.; Smit M. J. (2018) The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 37, 4110–4121. 10.1038/s41388-018-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L.; Matlaf L.; Bezrookove V.; Harkins L.; Martinez R.; Greene M.; Soteropoulos P.; Cobbs C. S. (2011) Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 71, 6643–6653. 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinger E.; Maussang D.; Schreiber A.; Siderius M.; Rahbar A.; Fraile-Ramos A.; Lira S. A.; Soderberg-Naucler C.; Smit M. J. (2010) HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci. Signaling 3, ra58 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- Maussang D.; Verzijl D.; van Walsum M.; Leurs R.; Holl J.; Pleskoff O.; Michel D.; van Dongen G. A.; Smit M. J. (2006) Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 103, 13068–13073. 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecke S. V.; Calcoen B.; Lagrou K.; Maertens J. (2019) Letermovir for prophylaxis of cytomegalovirus manifestations in adult allogeneic hematopoietic stem cell transplant recipients. Future Microbiol. 14, 175–184. 10.2217/fmb-2018-0250. [DOI] [PubMed] [Google Scholar]
- Yasri S.; Wiwanitkit V. (2019) Probability of Ganciclovir Resistance in Cytomegalovirus-Infected Pediatric Kidney Transplant Recipients after Cessation of Standard Antiviral Prophylaxis: Estimated Risk on Thai cases. Saudi J. Kidney Dis Transpl 30, 1000–1001. 10.4103/1319-2442.265448. [DOI] [PubMed] [Google Scholar]
- Lischka P.; Zimmermann H. (2008) Antiviral strategies to combat cytomegalovirus infections in transplant recipients. Curr. Opin. Pharmacol. 8, 541–548. 10.1016/j.coph.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Liang X.; Famure O.; Li Y.; Kim S. J. (2018) Incidence and Risk Factors for Leukopenia in Kidney Transplant Recipients Receiving Valganciclovir for Cytomegalovirus Prophylaxis. Prog. Transplant 28, 124–133. 10.1177/1526924818765798. [DOI] [PubMed] [Google Scholar]
- Varela-Fascinetto G.; Benchimol C.; Reyes-Acevedo R.; Genevray M.; Bradley D.; Ives J.; Silva H. T. Jr. (2017) Tolerability of up to 200 days of prophylaxis with valganciclovir oral solution and/or film-coated tablets in pediatric kidney transplant recipients at risk of cytomegalovirus disease. Pediatr. Transplant. 21, e12833. 10.1111/petr.12833. [DOI] [PubMed] [Google Scholar]
- Humar A.; Lebranchu Y.; Vincenti F.; Blumberg E. A.; Punch J. D.; Limaye A. P.; Abramowicz D.; Jardine A. G.; Voulgari A. T.; Ives J.; Hauser I. A.; Peeters P. (2010) The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transplant. 10, 1228–1237. 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- Hanel P.; Andreani P.; Graler M. H. (2007) Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 21, 1202–1209. 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Gellings Lowe N.; Swaney J. S.; Moreno K. M.; Sabbadini R. A. (2008) Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc. Res. 82, 303–312. 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman K.; Lee Y. M.; Michaud J.; Thangada S.; Ai Y.; Bonkovsky H. L.; Parikh N. S.; Habrukowich C.; Hla T. (2008) Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676. 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacimi R.; Vessey D. A.; Honbo N.; Karliner J. S. (2007) Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregulation and an enhanced proinflammatory response. J. Mol. Cell. Cardiol. 43, 85–91. 10.1016/j.yjmcc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Tani M.; Sano T.; Ito M.; Igarashi Y. (2005) Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J. Lipid Res. 46, 2458–2467. 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- Landeen L. K.; Aroonsakool N.; Haga J. H.; Hu B. S.; Giles W. R. (2007) Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am. J. Physiol Heart Circ Physiol 292, H2698–2711. 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Honbo N.; Goetzl E. J.; Chatterjee K.; Karliner J. S.; Gray M. O. (2007) Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am. J. Physiol Heart Circ Physiol 293, H3150–3158. 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- Landeen L. K.; Dederko D. A.; Kondo C. S.; Hu B. S.; Aroonsakool N.; Haga J. H.; Giles W. R. (2008) Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am. J. Physiol Heart Circ Physiol 294, H736–749. 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- Kerage D.; Brindley D. N.; Hemmings D. G. (2014) Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 35 (Suppl), S86–92. 10.1016/j.placenta.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Waeber C.; Blondeau N.; Salomone S. (2004) Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect. 17, 365–382. 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- Walzer T.; Jaeger S.; Chaix J.; Vivier E. (2007) Natural killer cells: from CD3(−)NKp46(+) to post-genomics meta-analyses. Curr. Opin. Immunol. 19, 365–372. 10.1016/j.coi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Pyne N. J.; Pyne S. (2010) Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 10, 489–503. 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Means C. K.; Brown J. H. (2008) Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200. 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers S. J.; Frias M.; Lacerda L.; Opie L. H.; Lecour S. (2012) Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc. Drugs Ther. 26, 227–237. 10.1007/s10557-012-6376-2. [DOI] [PubMed] [Google Scholar]
- Karliner J. S. (2009) Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J. Cardiovasc. Pharmacol. 53, 189–197. 10.1097/FJC.0b013e3181926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner J. S. (2013) Sphingosine kinase and sphingosine 1-phosphate in the heart: a decade of progress. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 1831, 203–212. 10.1016/j.bbalip.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavo A.; Liccardo D.; Komici K.; Corbi G.; de Lucia C.; Femminella G. D.; Elia A.; Bencivenga L.; Ferrara N.; Koch W. J.; Paolocci N.; Rengo G. (2017) Sphingosine Kinases and Sphingosine 1-Phosphate Receptors: Signaling and Actions in the Cardiovascular System. Front. Pharmacol. 8, 556. 10.3389/fphar.2017.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. L.; Alewijnse A. E. (2007) Sphingosine-1-phosphate signaling in the cardiovascular system. Curr. Opin. Pharmacol. 7, 186–192. 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Maceyka M.; Payne S. G.; Milstien S.; Spiegel S. (2002) Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 1585, 193–201. 10.1016/S1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wada R.; Yamashita T.; Mi Y.; Deng C. X.; Hobson J. P.; Rosenfeldt H. M.; Nava V. E.; Chae S. S.; Lee M. J.; Liu C. H.; Hla T.; Spiegel S.; Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961. 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H.; Wilsbacher L. D.; Wilson S. J.; Duong D. N.; McDonald M.; Lam I.; Park K. E.; Chun J.; Coughlin S. R. (2016) Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev. Biol. 418, 157–165. 10.1016/j.ydbio.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keul P.; van Borren M. M.; Ghanem A.; Muller F. U.; Baartscheer A.; Verkerk A. O.; Stumpel F.; Schulte J. S.; Hamdani N.; Linke W. A.; van Loenen P.; Matus M.; Schmitz W.; Stypmann J.; Tiemann K.; Ravesloot J. H.; Alewijnse A. E.; Hermann S.; Spijkers L. J.; Hiller K. H.; Herr D.; Heusch G.; Schafers M.; Peters S. L.; Chun J.; Levkau B. (2016) Sphingosine-1-Phosphate Receptor 1 Regulates Cardiac Function by Modulating Ca2+ Sensitivity and Na+/H+ Exchange and Mediates Protection by Ischemic Preconditioning. J. Am. Heart Assoc. 5, 5. 10.1161/JAHA.116.003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Laubscher R. F.; King J. C.; Hacking D.; Somers S.; Hastie S.; Stewart T.; Imamdin A.; Maarman G.; Pedretti S.; Lecour S. (2014) Cardiac preconditioning with sphingosine-1-phosphate requires activation of signal transducer and activator of transcription-3. Cardiovasc J. Afr 25, 118–123. 10.5830/CVJA-2014-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G. P.; Imamdin A.; Lecour S.; Opie L. H. (2018) Sphingosine-1-phosphate (S1P) activates STAT3 to protect against de novo acute heart failure (AHF). Life Sci. 196, 127–132. 10.1016/j.lfs.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Cannavo A.; Rengo G.; Liccardo D.; Pagano G.; Zincarelli C.; De Angelis M. C.; Puglia R.; Di Pietro E.; Rabinowitz J. E.; Barone M. V.; Cirillo P.; Trimarco B.; Palmer T. M.; Ferrara N.; Koch W. J.; Leosco D.; Rapacciuolo A. (2013) beta1-adrenergic receptor and sphingosine-1-phosphate receptor 1 (S1PR1) reciprocal downregulation influences cardiac hypertrophic response and progression to heart failure: protective role of S1PR1 cardiac gene therapy. Circulation 128, 1612–1622. 10.1161/CIRCULATIONAHA.113.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M.; Lisowska A.; Zabielski P.; Musial W.; Baranowski M. (2013) Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediators 106, 53–61. 10.1016/j.prostaglandins.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Chun J.; Kihara Y.; Jonnalagadda D.; Blaho V. A. (2019) Fingolimod: Lessons Learned and New Opportunities for Treating Multiple Sclerosis and Other Disorders. Annu. Rev. Pharmacol. Toxicol. 59, 149–170. 10.1146/annurev-pharmtox-010818-021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L.; Antel J.; Comi G.; Montalban X.; O'Connor P.; Polman C. H.; Haas T.; Korn A. A.; Karlsson G.; Radue E. W. (2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 355, 1124–1140. 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Pelletier D.; Hafler D. A. (2012) Fingolimod for multiple sclerosis. N. Engl. J. Med. 366, 339–347. 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- Billich A.; Bornancin F.; Devay P.; Mechtcheriakova D.; Urtz N.; Baumruker T. (2003) Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 278, 47408–47415. 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Zemann B.; Kinzel B.; Muller M.; Reuschel R.; Mechtcheriakova D.; Urtz N.; Bornancin F.; Baumruker T.; Billich A. (2006) Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458. 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- Mandala S.; Hajdu R.; Bergstrom J.; Quackenbush E.; Xie J.; Milligan J.; Thornton R.; Shei G. J.; Card D.; Keohane C.; Rosenbach M.; Hale J.; Lynch C. L.; Rupprecht K.; Parsons W.; Rosen H. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349. 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Brinkmann V.; Davis M. D.; Heise C. E.; Albert R.; Cottens S.; Hof R.; Bruns C.; Prieschl E.; Baumruker T.; Hiestand P.; Foster C. A.; Zollinger M.; Lynch K. R. (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457. 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Chiba K.; Yanagawa Y.; Masubuchi Y.; Kataoka H.; Kawaguchi T.; Ohtsuki M.; Hoshino Y. (1998) FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol 160, 5037–5044. [PubMed] [Google Scholar]
- Yanagawa Y.; Sugahara K.; Kataoka H.; Kawaguchi T.; Masubuchi Y.; Chiba K. (1998) FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J. Immunol 160, 5493–5499. [PubMed] [Google Scholar]
- Pinschewer D. D.; Ochsenbein A. F.; Odermatt B.; Brinkmann V.; Hengartner H.; Zinkernagel R. M. (2000) FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164, 5761–5770. 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- Aytan N.; Choi J. K.; Carreras I.; Brinkmann V.; Kowall N. W.; Jenkins B. G.; Dedeoglu A. (2016) Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer’s disease. Sci. Rep. 6, 24939. 10.1038/srep24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N.; Linardi D.; Muhammad N.; Chiamulera C.; Fumagalli G.; Biagio L. S.; Gebrie M. A.; Aslam M.; Luciani G. B.; Faggian G.; Rungatscher A. (2017) Sphingosine 1-Phosphate Receptor Modulator Fingolimod (FTY720) Attenuates Myocardial Fibrosis in Post-heterotopic Heart Transplantation. Front. Pharmacol. 8, 645. 10.3389/fphar.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Lu L.; Liu Y.; Gu G.; Tao R. (2014) FTY720 attenuates hypoxia-reoxygenation-induced apoptosis in cardiomyocytes. Exp. Mol. Pathol. 97, 218–224. 10.1016/j.yexmp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Santos-Gallego C. G.; Vahl T. P.; Goliasch G.; Picatoste B.; Arias T.; Ishikawa K.; Njerve I. U.; Sanz J.; Narula J.; Sengupta P. P.; Hajjar R. J.; Fuster V.; Badimon J. J. (2016) Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation 133, 954–966. 10.1161/CIRCULATIONAHA.115.012427. [DOI] [PubMed] [Google Scholar]
- Goltz D.; Huss S.; Ramadori E.; Buttner R.; Diehl L.; Meyer R. (2015) Immunomodulation by splenectomy or by FTY720 protects the heart against ischemia reperfusion injury. Clin. Exp. Pharmacol. Physiol. 42, 1168–1177. 10.1111/1440-1681.12465. [DOI] [PubMed] [Google Scholar]
- Jimenez-Vargas N. N.; Pattison L. A.; Zhao P.; Lieu T.; Latorre R.; Jensen D. D.; Castro J.; Aurelio L.; Le G. T.; Flynn B.; Herenbrink C. K.; Yeatman H. R.; Edgington-Mitchell L.; Porter C. J. H.; Halls M. L.; Canals M.; Veldhuis N. A.; Poole D. P.; McLean P.; Hicks G. A.; Scheff N.; Chen E.; Bhattacharya A.; Schmidt B. L.; Brierley S. M.; Vanner S. J.; Bunnett N. W. (2018) Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci. U. S. A. 115, E7438–E7447. 10.1073/pnas.1721891115. [DOI] [PMC free article] [PubMed] [Google Scholar]