Abstract

Class B G protein-coupled receptors are highly therapeutically relevant but challenges remain in identifying suitable small-molecule drugs. The calcitonin-like receptor (CLR) in particular is linked to conditions such as migraine, cardiovascular disease, and inflammatory bowel disease. The CLR cannot act as a cell-surface receptor alone but rather must couple to one of three receptor activity-modifying proteins (RAMPs), forming heterodimeric receptors for the peptides adrenomedullin and calcitonin gene-related peptide. These peptides have extended binding sites across their receptors. This is one reason why there are few small-molecule ligands that can modulate these receptors. Here we describe small molecules that are able to positively modulate the signaling of the CLR with all three RAMPs but are not active at the related calcitonin receptor. These compounds were selected from a β-arrestin recruitment screen, coupled with rounds of medicinal chemistry to improve their activity. Translational potential is shown as the compounds can positively modulate cAMP signaling in a vascular cell line model. Binding experiments do not support an extracellular domain binding site; however, molecular modeling reveals potential allosteric binding sites in multiple receptor regions. These are the first small-molecule positive modulators described for the CLR:RAMP complexes.
Keywords: adrenomedullin, calcitonin gene-related peptide, G protein-coupled receptor, allosteric modulator, receptor activity-modifying protein
G protein-coupled receptors (GPCRs) are important signaling proteins and drug targets.1 They are divided into classes based on common features. Class B GPCRs are a small family of 15 receptor genes, with pharmacological diversity increased by the ability of several family members to associate with receptor activity-modifying proteins (RAMPs).2,3 Several class B GPCRs have high therapeutic value, most notably for peptide drugs. For example, research on the glucagon-like peptide (GLP-1) receptor has yielded several peptide therapeutics for treating diabetes and obesity.4 The first therapeutic GPCR antibody to achieve regulatory approval (for migraine) was also directed at a class B GPCR: the calcitonin gene-related peptide (CGRP) receptor, comprising a GPCR (calcitonin-like receptor, CLR) with RAMP1.5
Class B GPCRs bind peptides between ∼30 and ∼50 amino acids in length. Accordingly, they have a binding mode that extends from the large N-terminal extracellular domain (ECD) through to the transmembrane (TM) bundle and extracellular loops (ECL), where several points of contact are made between the endogenous peptide ligand and receptor.6−10 This extended binding mode has made it challenging to identify small molecules that are capable of agonism or antagonism. Full-length ligand-bound receptor crystal structures have lagged behind those of class A, and consequently, there are limited numbers of molecules emerging from structure-based drug design approaches. Despite these challenges, there are a few examples of small molecules that bind to class B GPCRs. Most of these are allosteric modulators of the glucagon, corticotropin-releasing factor receptor 1, and GLP-1 receptors.11,12 A small-molecule agonist of the calcitonin receptor (CTR) has been reported.13 There are also antagonists, which may have an allosteric mechanism, that bind to the CLR:RAMP1 CGRP receptor complex.14,15 However, overall there is a paucity of small molecules directed against class B GPCRs.
The CGRP receptor is an important target for migraine, which led to the development of small-molecule antagonists (e.g., telcagepant, rimegepant, ubrogepant, atogepant, and vazegepant) and therapeutic antibodies (erenumab, galcanezumab, fremanezumab, and eptinezumab).16 However, its endogenous ligands, αCGRP and βCGRP, also have a range of other effects.17 CGRP is a protective factor in the cardiovascular system and a long-acting peptide agonist has shown a reduction in angiotensin II-induced hypertension.18 Adrenomedullin (AM) and adrenomedullin 2 (AM2) are related to CGRP, and both bind to the same GPCR, CLR, with relative agonist specificity driven by RAMP2 and RAMP3, creating AM1 and AM2 receptors.19 AM has effects in the blood and lymphatic vasculature that are either positive, such as wound-healing or reducing lymphedema, or are negative, in terms of pro-tumorigenic effects. AM administration appears to be beneficial in ulcerative colitis.20,21 At present, only a low-affinity peptide antagonist (AM22–52) exists for probing AM biology. Thus, there is a significant need for novel small molecules that could be used to further investigate CGRP, AM, and AM2 biology.
Here we describe the identification of novel positive modulators of CLR-based receptors that may prove useful as chemical biology tools or leads for further development.
Results and Discussion
Compound Selection
We set out to identify small-molecule tools for probing the biology of the AM and related receptors. We employed a medicinal chemistry approach complemented by virtual screening to identify potential modulators of AM1 receptor activity (see the Supporting Information compounds S1–S153; Tables S1 and S2). Using the structural information available at the time,22 we designed, by analogy with the binding mode of telcagepant (1) in a CGRP receptor ECD structure (Protein Databank (PDB) 3N7R) (Figure 1A), several series of molecules using the aza-benzimidazolone motif to direct binding into the CLR binding cleft.23 We coupled this motif to a variety of structural scaffolds designed to interact with the adjacent lipophilic pocket in the RAMP2 domain, rather than RAMP1 (Figure 1B). The targets were screened by molecular docking, then prioritized for synthesis and screening accordingly. This series included compounds with aryl, heteroaryl, fused aryl, benzyl, benzyl ether, and benzyl amine substituents on a pyridine or phenyl core (Figure 1B; compounds S1–S118; Table S1).
Figure 1.

Compounds were designed using a CLR-binding motif informed by CGRP receptor antagonists and both AM1 (CLR:RAMP2) and CGRP (CLR:RAMP1) receptor ECD structural information. (A) Structure of telcagepant (1) (yellow sticks) bound to the CGRP receptor ECD (PDB 3N7R). (B) Design of molecules targeting the CLR:RAMP2 interface based on 1. (C) Interaction of the C-terminal amide at AM peptide residue Y52 with the CLR:RAMP2 ECD (PDB 4RWF). (D) Design of indolinone analogues (e.g., 2) which mimic the RAMP2-AM interaction.
While this work was in progress, the crystal structure of the AM peptide bound to the AM1 receptor ECD complex was reported (PDB 4RWF(24)). Amino acid exchange experiments identified the RAMP2 E101 to AM Y52 hydrogen bond as important for AM1 receptor selectivity and provided us with new insights for the design of AM1-receptor-selective molecules (Figure 1C). We designed a small library of compound structures to mimic the binding of the C-terminal tyrosine of the AM peptide to the AM1 receptor. The structures were docked into the AM1 ECD (PDB 4RWF), and from these, we identified a 4-hydroxybenzylidene indolinone (2) as a suitable starting point (Figure 1D). We prepared a set of compounds to explore this hypothesis. These consisted of a series of 3-substituted benzimidazolones and indolinones with benzyl and benzylidene moieties variously substituted at the 3′- and 4′-positions (compounds S154–S240, Table S3). In order to broaden the chemical scope of AM1 modulators, we conducted a second virtual screen consisting of 205 000 compounds from the Chembridge library (MW 250–500) using the PDB 4RWF structure. The compounds were selected for specific hydrogen bond contacts and their ability to occupy a sphere defined for the aromatic ring on AM Y52. These compounds were purchased and evaluated in our primary screen (compounds S241–S318, Table S4). Overall, compound selection, synthesis, and priority for screening were performed iteratively, as informed by results from screening at defined receptors in cell-based assays; this workflow is shown in Figure 2.
Figure 2.

Workflow of compound screening and characterization process, indicating the number of compounds progressing at each step. (A) Compounds were synthesized in-house or sourced from commercial vendors. In a paired experimental design, primary and secondary screening measured the compounds’ effects on AM- or CGRP-induced β-arrestin recruitment in AM1- or CGRP receptor-expressing CHO-K1 cells. Compound activity in these screens informed further rounds of compound selection and screening. (B) On the basis of emerging structure–activity relationships from this iterative screening process, additional compounds from AstraZeneca were selected and screened. (C) A total of 9 compounds were more comprehensively evaluated in the same β-arrestin recruitment assay using a range of compound concentrations to generate a measure of modulatory activity (β value). Further compound characterization (D) employed additional signaling pathways and cell types as well as binding experiments and molecular modeling. For these studies, we predominantly focused on a single in-house compound (6), but in some assays, additional compounds supplemented the results. CRC: concentration–response curve. See also Figures 1, 3, 5, 6, 7, and 8 and Table 1.
Validation of Primary Screening Assay
For screening, we selected the DiscoveRx PathHunter β-arrestin assay, which quantifies receptor activation by measuring β-arrestin recruitment, using an enzyme complementation approach. However, as the PathHunter β-arrestin cell lines for the AM1 receptor (CLR:RAMP2; CHO-AM1 cells) and the CGRP receptor (CLR:RAMP1; CHO-CGRP cells) have had limited use, so their pharmacology has not been validated. This is particularly important as the cells express heterodimeric receptors, which are unusual in this assay system. Therefore, our screen was preceded by thorough characterization of the two cell lines using control agonists and antagonists for both receptors, as described in Figures S1 and S2 and Table S5. Overall, the pharmacology of the cells was consistent with the known pharmacology of these receptors, which enabled their use in screening.3
For validation of our screening approach, inhibition curves were generated using AM22–52, telcagepant (compound 1) and olcegepant, as presented in Figure S3. An approximate EC70 concentration of AM and CGRP (20 nM) was used to detect compounds capable of affecting β-arrestin recruitment to the AM1 and CGRP receptors, respectively. As expected, telcagepant and olcegepant showed little to no activity at the AM1 receptor at concentrations up to 100 μM, while AM22–52 gave a pIC50 value of 6.26 ± 0.14 (n = 8). In the CHO-CGRP cells, olcegepant and telcagepant were potent antagonists, while AM22–52 was not an effective antagonist (Figure S3). Overall, we concluded that assays using the DiscoveRx β-arrestin cells could detect both agonism and antagonism and therefore we proceeded to cell-based screening of compounds (see the Methods section). Compounds were screened using the same automated method used to generate the data in Figure S3. Each receptor acted as a counter screen for the other in a paired experimental design, and compounds were screened in duplicate.
β-Arrestin Screening Results
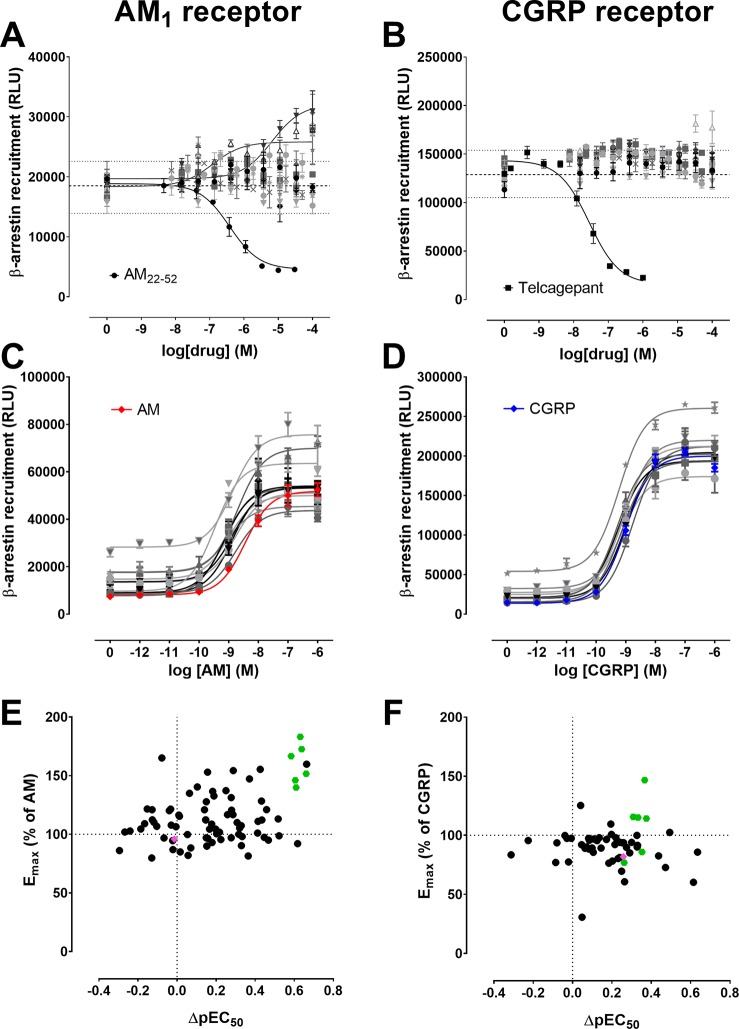
Overall, 318 small molecules from synthesized series and the virtual screens (Tables S1–S4) were tested in the primary β-arrestin screen. Figure 3A,B shows the results of typical screens using the CHO-AM1 and CHO-CGRP cells, respectively. The range and mean of the β-arrestin response in the absence of compound were used as a guide to identify the compounds with activity that departed markedly from vehicle control at two or more concentrations. These potentially active compounds were progressed to a secondary screen whereby concentration–response curves to AM in CHO-AM1 cells were constructed in the absence or presence of a single concentration of compound, within individual compound solubility constraints. As before, the CGRP receptor was used as a counter screen. Figure 3C,D shows examples of typical secondary screening assays. Figure 3E,F summarizes the secondary screen data in the form of scatter plots.
Figure 3.
(A, B) Typical primary screens are shown for (A) CHO-AM1 (CLR:RAMP2) cells or (B) CHO-CGRP (CLR:RAMP1) cells, measuring compound modulation of β-arrestin recruitment stimulated by 20 nM of AM or CGRP respectively. The range and mean of vehicle control wells are shown with dotted lines. Control antagonists (AM22–52 and telcagepant) are fitted with a three-parameter logistic equation, as are selected test compounds. (C, D) Secondary screens measured the ability of compounds to shift the β-arrestin recruitment concentration–response curve stimulated by (C) AM in CHO-AM1 cells or (D) CGRP in CHO-CGRP cells. A representative screening assay is shown for each receptor. Assays were performed in triplicate wells, and the mean ± s.e.m. is shown. (E, F) Secondary screening data for all compounds are summarized in scatter plots showing compounds’ relative effects on Emax and pEC50 of (E) AM- or (F) CGRP-induced β-arrestin recruitment, compared to AM or CGRP, respectively (indicated by dotted lines). The highest concentration tested for each compound is plotted; where compounds were tested multiple times, the mean response is shown. A compound cluster is evident for the AM1 receptor, with selected compounds highlighted in green on both scatter plots. A structurally related negative control compound chosen is shown in pink. RLU: relative luminescence units.
Most of the synthesized and virtual screen compounds targeting the CLR:RAMP2 interface (Tables S1 and S2) did not show any consistent effect against AM1 receptor activity. The aryl-substituted pyridinones based on a series of telcagepant analogues25 displayed no activity at the AM1 receptor. The introduction of more flexibility (i.e., substituted benzylpyridones) weakly enhanced AM-induced β-arrestin recruitment at the AM1 receptor and inconsistently modulated the CGRP-induced β-arrestin response at the CGRP receptor. Simpler aryl benzyl ethers did show increased β-arrestin recruitment in the presence of AM, suggestive of positive modulation of the AM1 receptor. However, we were unable to improve upon this activity with successive analogues.
The compounds designed to mimic the interaction between the terminal tyrosine residue of the AM ligand and the AM1 receptor did not produce antagonism at either the AM1 or CGRP receptors, but surprisingly, compound 3 was found to be a positive modulator of the AM1 receptor (Figure 4). This unexpected finding prompted us to prepare the set of compounds S154–S240 (Table S3) to explore the structure–activity relationships (SAR) for positive modulation around compound 3. These compounds were screened in the primary and secondary screens as before (Figure 2).
Figure 4.
Indolinone structures of selected active in-house compounds (3–8), an inactive analogue (9) and selected active AstraZeneca compounds (10–12).
Overall, a cluster of active compounds emerged from the screens that produced consistent positive modulation of the CHO-AM1 β-arrestin response to AM, but were relatively inactive in altering CGRP-induced β-arrestin recruitment in the CHO-CGRP cells (Figure 3E,F). Compounds 3–8 were selected from this cluster. These compounds consisted of benzylidene indolinones featuring 3′-methyl and various 4′-alkoxy substituents on the benzylidine ring and were isolated as mixtures of E/Z isomers with the E-isomer predominating (as drawn in Figure 4). We also identified an N-substituted analogue (9) that was inactive in modulating CHO-AM1 β-arrestin recruitment, enabling its use as a negative control (Figure S4).
We were able to assign SAR for the wider series of indolinone analogs (Figure 4, Table S3). The NH of the indolinone was required for activity with alkyl substitution resulting in loss of activity. Interestingly, compounds with a 7-aza indolinone group (e.g., the telcagepant CLR-binding motif) were inactive suggesting that these compounds may not act through interaction with the CLR pocket as we had initially designed. We found limited steric tolerance at the 5-position on the indolinone with halo substituents tolerated, but not amide or extended alkyl substituents. There appeared to be more steric tolerance at the 6-position with small amine, amide or carbamate groups tolerated, but not phenyl or extended systems. The benzylidene double bond was also required for activity with additional substitution on the bond reducing activity or a saturated bond linked by carbon (indolinone) or nitrogen (benzimidazolone) being disfavored. Electron-donating substituents at both 3′ and 4′ positions provided the best activity, with further substitution providing no benefit. Although single electron-donating substituents were acceptable, activity was lost with electron-withdrawing substituents. The modulatory activity of these compounds at the AM1 or CGRP receptors has not been previously identified.
Compounds 3–8 showed a trend toward an increase in Emax and in most cases a leftward shift in the AM concentration–response curve. Given this activity profile, we tested these compounds further using multiple concentrations of compound, measuring AM-induced β-arrestin recruitment in CHO-AM1 cells (Figure 5). This generally enabled analysis of the compound activity using an operational model of allostery to obtain a measure of the effect of the compound on efficacy (β value).26 Details of the equation and parameters used are provided in the Supporting Information. Compounds were tested in the presence of CGRP in the CHO-CGRP cells, as shown in Figure S5. Compounds 3–8 were weaker at the CGRP receptor, and β values could not be calculated from these data sets, though some modulation was evident. Pharmacological parameters (pEC50, Emin, and Emax) from AM1 and CGRP receptor data sets, fitted with three-parameter logistic equations, are presented in Tables S6 and S7, respectively. Interestingly, some compounds displayed weak intrinsic agonist properties at higher concentrations.
Figure 5.
Compound activity at the AM1 (CLR:RAMP2) receptor fitted with an operational model of allostery. The ability of compounds to modulate AM-induced β-arrestin recruitment in CHO-AM1 cells was measured. Concentration–response curves are the combined mean data from three independent experiments, in triplicate wells, normalized to the response with AM + vehicle. The mean ± s.e.m. has been plotted. Where possible, the normalized data have been fitted with an operational model of allostery (see the Supporting Information), allowing log β values to be determined (B–H). Data from compounds 3 and 12 (A, I) could not be fitted with the same parameters and are therefore fitted with a three parameter logistic equation.
As positive modulation of the CLR-based receptors by this class of small molecules was novel and unexpected, we were prompted to further validate this finding. On the basis of the pharmacophore and SAR emerging from our compound set (Table S3), we undertook an additional screen to further supplement our SAR using structurally similar compounds curated by AstraZeneca. These compounds were screened as before (Figure 2) although this time experimenters were blinded to the compound structures. Unblinding of active compound structures by AstraZeneca only occurred postscreening and postanalysis. Potent positive modulation of β-arrestin recruitment at the AM1 receptor by three major hits was observed (compounds 10–12). These compounds were further characterized using multiple concentrations of compound to determine β values (Figure 5, Table S6). As for compounds 3–8, weaker modulation of the CGRP response in CHO-CGRP cells was seen (Figure S5 and Table S7). For compounds 10–12, we also cross-checked the ability of each compound to modulate β-arrestin by exchanging the ligand for each receptor (i.e., CGRP at the AM1 receptor and AM at the CGRP receptor). Positive modulation was seen for both of these ligand/receptor combinations (Figure S6). These hits were also 3-benzylidene indolinones with 3′- or 4′-substituents, bearing an additional 6-chloro substituent on the indolinone moiety (Figure 4).
Compound Activity in cAMP Assays
The DiscoveRx β-arrestin assay is a convenient screening tool but this involves a modified receptor and β-arrestin. Furthermore, cAMP is the canonical signaling pathway for CLR-based receptors.19 Therefore, we selected the in-house compound with a high log β value (6, log β = 1.305) and measured its effect on cAMP production in the CHO-AM1 cells. Compound 6 was able to positively modulate the AM-induced cAMP response in these cells (Figure S7). To further investigate this result, a second cell background was chosen (transiently transfected Cos7 cells). This cell line is a well-validated model for studying calcitonin-family receptors. Again, positive modulation of AM-induced AM1 cAMP production by 25 μM of compound 6 was observed (Figure 6A) with effects on both pEC50 and Emax (Table S8). Enhancement of cAMP production was similarly observed with compound 6 at the CGRP receptor using either CGRP or AM as the agonist (Figure 6B,E, Table S8).
Figure 6.
Compound 6 positively modulates ligand-induced cAMP production by CLR-based receptors but not other class B GPCRs. Effect of 25 μM of compound 6 on ligand-induced cAMP production by cognate ligands at the (A, D) AM1 receptor (CLR:RAMP2), (B, E) CGRP receptor (CLR:RAMP1), (C) AM2 receptor (CLR:RAMP3), (F) AMY1 receptor (CTR:RAMP1), (G) PAC1n receptor, (H) CRF1 receptor, or (I) CT(a) receptor (CTR). Concentration–response curves are the combined mean data from 4 to 14 independent experiments in duplicate or triplicate wells. The mean ± s.e.m. has been plotted, and data are fitted with a four parameter (CT, AMY1 receptors) or three parameter logistic equation (others). pEC50, Emax, and n values are shown in Table S8.
This assay system gave us the opportunity to test compound 6 at the related AM2 receptor (CLR:RAMP3), which gave similar results (Figure 6C). In addition, the CTR is the closest relative to CLR, and it was important to determine whether compound 6 is also active at this receptor. At the highest concentration we could test given solubility constraints (25 μM), no activity was observed at the CTR alone or at the CTR coexpressed with RAMP1 (AMY1 receptor), suggesting that this molecule has a degree of selectivity for CLR-based receptors (Figure 6F,I). Compound 6 was also tested against two other class B GPCRs with their cognate ligands (corticotrophin-releasing factor 1 (CRF1) and pituitary adenylate cyclase-activating polypeptide receptor type 1n (PAC1n) receptors), and no modulatory activity was observed (Figure 6G,H).
Compound Activity in Endothelial Cells
Robust positive modulation of peptide responses at CLR-based receptors prompted us to explore the possibility that compound 6 has sufficient activity to affect receptors endogenously expressed in cells and not only in heterologous expression systems. For these experiments, we used a human microvascular endothelial cell line (HMEC-1), which has previously been reported to contain AM-responsive receptors and is relevant to the known vascular biology of this peptide.27−29Figure 7 shows that 25 μM of compound 6 produced robust enhancement of AM- or CGRP-stimulated cAMP production in HMEC-1 cells.
Figure 7.

Compound 6 (25 μM) positively modulates both AM- and CGRP-induced cAMP production in HMEC-1 cells. Concentration–response curves are the mean data from three (CGRP) or six (AM) independent experiments in triplicate wells, normalized to the maximal response with AM (as a full curve could not be fitted to the CGRP data). The mean + s.e.m. has been plotted. pEC50 values are significantly different by paired t-test (p < 0.05). See Table S8.
Mechanisms
To investigate possible molecular mechanisms of action by which our compounds positively modulate AM and CGRP responses, we measured the effect of compound 6 in a fluorescence polarization/anisotropy peptide competition binding assay using receptor/RAMP ECD complexes. Telcagepant (1) could bind to the CGRP receptor ECD complex as expected, as shown by the displacement of the peptide probe (Figure S8). However, compound 6 did not displace or enhance binding of the peptide probe at either the CGRP or AM1 receptor ECD complexes (Figure S8). This raised the question of whether the compounds actually bind to the canonical CGRP antagonist ECD binding site as designed or a different site elsewhere in the receptor complex. The publication of a cryo-electron microscopy structure for the full-length CGRP receptor provided us with the opportunity to explore the presence of alternative binding sites using molecular modeling.10 This allowed us to explore possible alternative binding sites instead of the established CGRP antagonist site in the ECD.23
We employed sequence conservation (ConSurf) in combination with a topology-based local connectivity approach to probe for potential binding sites.30,31 Sequence conservation analysis provides us with an opportunity to discover reasonably conserved allosteric binding sites, while the topology-based local connectivity approach is known to provide a good description of binding sites.31
We then cross-checked the presence of potential binding sites with FTMap.32 We found general consensus between the two methods but found that FTMap did not identify sites on the external face of the TM domain as potential binding sites. We also used docking (Autodock Vina and Glide) to assess the ligand binding potential of these sites.
Overall, 10 potential binding sites were identified (with some sites combined as a cluster of several possible binding modes) and ranked by predicted binding propensity (using ConSurf/local connectivity) (Figure 8, Table 1). Ligand docking, carried out in the presence of the bound peptide, identified ligand binding poses in each of the potential binding sites, with the exception of site 2 located deep within the TM bundle. The most favorable compound from 3–12 (by docking score) is shown bound to each potential binding site (Figure 8A–E, Table 1).
Figure 8.

Potential CGRP receptor allosteric sites. (A) Cartoon representation indicating CLR (cyan), RAMP1 (gray), and the CGRP peptide (magenta). Numbers indicate the binding site; the FTmap ranking of these cluster sites is given in Table 1. (B) The surface of the CGRP receptor color-coded according to predicted binding propensity (red, high; blue, low). The sites in B–E are numbered 1–10 according to Table 1. (C) The opposite surface of the CGRP receptor color-coded according to predicted binding propensity. (D) and (E) The top and bottom surfaces of the CGRP receptor, respectively, are color-coded according to the predicted binding propensity. The most favorable ligand, as determined by the Glide docking score, is shown in each binding site (see Table 1). (F) Binding of compound 6 to site 4. The CLR surface is colored according to the binding propensity, with RAMP1 shown in gray. (G) Molecular interactions of compound 6 in site 4, as predicted by Glide.
Table 1. Potential Compound Binding Sites Identified Using Molecular Modeling of the CGRP Receptorb.
| site | FT map cluster ranking | centre of glide docking box | mean docking score | best docking score | best ligand | description |
|---|---|---|---|---|---|---|
| 1 | 0, 2, 6 | P97, Cβ | –4.50 | –5.24 | 3 | between peptide and ECL1, ECL2, P97R (ECD), and V111RAMP1 (ECD) |
| V111RAMP1, Cγ | –4.94 | –6.06 | 11 | |||
| 2 | 1, 5 | Y227, OH | N/A | deep within TM helix | ||
| 3 | 3 | D366, Oδ2 | –3.97 | –4.54 | 3 | between peptide and D366 (top of TM7) |
| 4 | 4 | W69, CH2 P97, Cβ V111RAMP1, Cγ2 | –4.76 | –6.55 | 11 | Near-CLR N-terminal helix, opposite side of ECD to CGRP C-terminus, there is overlap between the W69 and V111RAMP1sites. |
| 4a | W69, CH2 | –4.16 | –4.57 | 11 | as isolated ECD (3N7R)a | |
| 5 | 7 | R173, Cγ | –4.91 | –4.87 | 8 | G protein binding site |
| 6 | 8 | W72, Nε1 | –4.09 | –4.58 | 10 | adjacent to F37 of CGRP peptide, between CLR and RAMP1 |
| 7 | 9 | D90, Oδ1 | –5.24 | –6.20 | 10 | Between ECL1, the N-terminal end of the N-terminal helix, the C-terminal end of the peptide helix; overlaps the W69 site. |
| 8 | 10 | D90RAMP1, Oδ2 | –4.40 | –5.45 | 6 | top of ECD between CLR and RAMP1 |
| 9 | N/A | P343, Cβ | –4.32 | –5.45 | 6 | External face of TM7/TM7 |
| 10 | N/A | V304, Cγ1 | –4.50 | –5.03 | 11 | external face of TM5, adjacent to RAMP1 |
Site 4 when modeled as the isolated ECD (3N7R), which gave weaker docking scores. Sites close to the CLR:RAMP1 interface are described in italics.
Correspondence between the FTmap cluster and the center of the Glide docking boxes is shown. FTmap scores are ranked from highest (0) to lowest (10). N/A indicates sites were not identified by FTmap. Residue numbers indicate amino acids in CLR unless stated.
The docking and scoring process predicted that compounds would bind most favorably to the recognized CGRP antagonist site (in the full-length receptor) to which they were designed, provided that the peptide was absent. The compounds appear to make similar interactions to telcagepant (1) and olcegepant (i.e., accepting a hydrogen bond from residue T122 in CLR to the ligand carbonyl group and an aromatic ring replacing molecular contacts usually made by F37 of the CGRP peptide). However, these interactions seem more consistent with bound ligands generating an antagonistic effect at the receptor, by interfering with peptide-receptor interactions, as opposed to the positive modulatory effects we observed.
The wide variety of potential allosteric binding pockets observed is partly a function of the way the RAMP creates indentations and crevices at the CLR:RAMP1 interface. The allosteric effects of RAMPs are well-reported,33,34 and it is possible that sites close to the CLR:RAMP1 domain boundary (e.g., sites 1, 4, 6, and 7) could moderate the allosteric effects of the RAMP, resulting in receptor activity modulation. Of particular interest is site 4, which sits above the peptide helix interaction site centered on W69/D90 and had the best docking score within the allosteric sites, along with ranking highly in FTMap and ConSurf/local connectivity scores. The noncanonical site 4 is occupied by ligand 3N7 in the X-ray structure of the CLR/RAMP1 ECD complex (3N7S) that binds olcegepant in the canonical binding site. A possible binding pose for compound 6 at this site is shown in Figure 8F,G. This site is consistent with the inactive N-methyl analogue (9), which could not hydrogen bond to D90. The proposed CTR small-molecule agonist SUN-B8155 could form a similar hydrogen bond to D97 in the equivalent ECD site in CTR, but compound 6 failed to bind to this CTR site in line with our experiments (modeling not shown).35,36 The putative interactions shown in Figure 8 are largely isolated to the receptor ECD, which would be inconsistent with the lack of binding to the isolated ECD (Figure S8) except that site 4 is close to the RAMP linker, which may have a direct or indirect effect on binding. Site-directed mutagenesis experiments would be useful to support these proposed binding sites but are not always feasible. For example, in the case of site 4, W69 mutated to alanine produces an effectively nonfunctional receptor.24
More unusual binding sites included a site at the G protein interface (site 5) and another site (site 2) within the TM bundle similar to that of the antagonist CP-376395 in the human CRF1 receptor.37 These sites should not be disregarded given that it is now evident that allosteric ligands can bind through the entire structure of a GPCR to produce their effects, not just in proximity to an orthosteric binding site.11,38 Additional work will be required to define precisely which sites may be exploited by these compounds, including structural studies.
In summary, we have identified a class of compounds that act as the first small-molecule positive modulators of CLR-based receptors. They are also one of the few examples of positive modulators of class B GPCRs. Compound 6 exhibited robust enhancement of both cAMP production and β-arrestin recruitment in response to peptide ligands for the CLR-based receptors, while ligand-induced cAMP responses at the other class B GPCRs we tested were unaffected. Several related compounds had robust activity and are excellent leads for future SAR studies. Further investigation of this compound series to improve activity and selectivity is warranted, for the development of useful chemical probes/tools, at targets with strong therapeutic potential.
Methods
Materials
Human AM, αCGRP, AM22–52, amylin, and calcitonin were purchased from American Peptide (Sunnyvale, CA, USA). Human corticotrophin-releasing factor (CRF) was purchased from Bachem (Bubendorf, Switzerland). Human PACAP-38, human αCGRP, and AM22–52 were also synthesized in-house.33,39 No differences in activity were observed between in-house and commercially available peptides (data not shown). Telcagepant (MK-0974) was purchased from Suzhou Rovathin Pharmatech Co. (Jiangsu, China) and dissolved in DMSO. Olcegepant (BIBN4096BS) was kindly provided by Henri Doods (Boehringer Ingelheim) and prepared as described previously.40 Both were stored as frozen 10 mM aliquots (−30 °C).
CHO-K1 cells expressing human CLR, RAMP1, and β-arrestin-2 (CHO-CGRP cells; catalog number 93-0269C2) or human CLR, RAMP2 and β-arrestin-2 (CHO-AM1; catalog number 93-0270C2) as well as AssayComplete growth medium, Cell Detachment Reagent, and PathHunter detection reagents were purchased from DiscoveRx (Fremont, CA, USA). Phosphate-buffered saline (PBS), Dulbecco’s PBS (DPBS), Dulbecco’s modified Eagle’s medium (DMEM), MCDB-131 medium, and phenol red-free OptiMEM were obtained from Life Technologies (Carlsbad, CA). Bovine serum albumin (BSA) was sourced from MP Biomedicals (Auckland, New Zealand) and 3-isobutyl-1-methylxanthine (IBMX) was from Sigma-Aldrich (St. Louis, MO, USA).
Chemical Synthesis
General procedures for chemical synthesis of compounds are reported in Supporting Information.
(E)-3-(4-Hydroxy-3-methylbenzylidene)indolin-2-one (2).41
Oxindole (657 mg, 4.93 mmol), 4-hydroxy-3-methylbenzaldehyde (739 mg, 5.42 mmol), and piperidine (48 μL, 0.49 mmol) were stirred in EtOH (20 mL) at 80 °C for 3 h. Solvent was removed and the residue triturated in 50% EtOAc/petroleum ether to obtain phenol 2 (1.05 g, 85%) as a yellow solid: mp 234–237 °C. 1H NMR [(CD3)2SO] δ 10.50 (s, 1H, NH-1), 7.71 (d, J = 7.6 Hz, 1H, H-4), 7.51 (s, 1H, =CH), 7.44–7.50 (m, 2H, H-2′ and H-6′), 7.20 (td, J = 7.7, 1.0 Hz, 1H, H-6), 6.92 (d, J = 8.1 Hz, 1H, H-5′), 6.84–6.90 (m, 2H, H-5 and H-7), 2.18 (s, 1H, Me-3′). 13C NMR [(CD3)2SO] δ 169.1 (C-2), 157.6 (C-4′), 142.2 (C-7a), 136.9 (=CH), 132.7 (C-2′), 129.3 (C-6), 129.1 (C-6′), 124.9 (C-3′), 124.4 (C-3 and C-1′), 122.0 (C-4), 121.4 (C-3a), 121.0 (C-5), 114.8 (C-5′), 109.9 (C-7), 15.9 (Me-3′). MS m/z 252.2 (MH+, 100%). Anal. Calcd for C16H13NO2: C, 76.48; H, 5.21; N, 5.57. Found: C, 76.65; H, 5.32; N, 5.59%.
(E/Z)-3-(4-Methoxy-3-methylbenzylidene)indolin-2-one (3)
Oxindole (500 mg, 3.76 mmol), 3-methyl-p-anisaldehyde (1.14 mL, 7.51 mmol), and piperidine (37 μL, 0.38 mmol) were dissolved in EtOH (10 mL). The reaction mixture was purged with N2, sealed, and heated at 80 °C for 3 h. The reaction mixture was cooled to room temperature (r.t.), and the resulting yellow solid was purified by silica gel column chromatography. The product eluted with 20–50% EtOAc/petroleum ether. Solvent was removed under reduced pressure, and the product was triturated with EtOAc/petroleum ether to obtain the alkene (E/Z)-3 (942 mg, 95%) as a yellow solid: mp 192–195 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.54 (s, 1H, NH-1), 7.67 (obscured dd, J = 8.0, 1.0 Hz, 1H, H-4), 7.61 (dd, J = 8.5, 2.2 Hz, 1H, H-6′), 7.55 (s, 1H, =CH), 7.52–7.53 (m, 1H, H-2′), 7.21 (dt, J = 7.7, 1.0 Hz, 1H, H-6), 7.09 (d, J = 8.5 Hz, 1H, H-5′), 6.84–6.90 (m, 2H, H-5 and H-7), 3.87 (s, 3H, OMe-4′), 2.20 (s, 3H, Me-3′). Z-isomer (minor) 10.54 (s, 1H, NH-1), 8.40 (dd, J = 8.7, 2.1 Hz, 1H, H-6′), 8.31 (d, J = 2.1 Hz, 1H, H-2′), 7.70 (s, 1H, =CH), 7.64–7.67 (m, 1H, H-4), 7.17 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.05 (d, J = 8.7 Hz, 1H, H-5′), 6.97 (td, J = 7.6, 1.0 Hz, 1H, H-5), 6.81 (d, J = 7.6 Hz, 1H, H-7), 3.87 (s, 3H, OMe-4′), 2.19 (s, 3H, Me-3′). 13C NMR [(CD3)2SO] δ E-isomer (major) 168.9 (C-2), 158.7 (C-4′), 142.6 (C-7a), 136.3 (=CH), 132.0 (C-2′), 129.6 (C-6), 129.2 (C-6′), 126.2 (C-1′), 126.0 (C-3′), 125.4 (C-3), 122.0 (C-4), 121.3 (C-3a), 121.0 (C-5), 110.4 (C-5′), 110.0 (C-7), 55.5 (OMe), 16.0 (Me). Z-isomer (minor) 167.4 (C-2), 159.5 (C-4′), 140.2 (C-7a), 137.1 (=CH), 134.8 (C-2′), 132.5 (C-6′), 128.1 (C-6), 126.6 (C-1′), 125.4 (C-3a), 125.2 (C-3′), 123.8 (C-3), 120.9 (C-5), 119.1 (C-4), 110.0 (C-5′), 109.2 (C-7), 55.5 (OMe), 16.1 (Me). MS m/z 266.6 (MH+, 100%). Anal. Calcd for C17H15NO2: C, 76.96; H, 5.70; N, 5.28. Found: C, 76.85; H, 5.61; N, 5.26%.
(E/Z)-3-(4-Ethoxy-3-methylbenzylidene)indolin-2-one (4)
Phenol 2 (200 mg, 0.80 mmol), K2CO3 (220 mg, 1.59 mmol), and ethyl iodide (64 μL, 0.80 mmol) were stirred in DMF (5 mL) at r.t. overnight (19 h). The resulting mixture was diluted with EtOAc (50 mL), washed with water (2 × 50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography, eluting in 20% EtOAc/petroleum ether. Solvent was removed, and the residue was triturated in 10% EtOAc/petroleum ether to give (E/Z)-4 (128 mg, 58%) as a yellow solid: mp 236–239 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.53 (s, 1H, NH), 7.65–7.69 (m, 1H, H-4), 7.58 (dd, J = 8.5 Hz, 1H, H-6′), 7.53–7.54 (m, 2H, =CH and H-2′), 7.21 (td, J = 7.7, 1.0 Hz, 1H, H-6), 7.08 (d, J = 8.5 Hz, 1H, H-5′), 6.86–6.89 (m, 2H, H-5 and H-7), 4.10–4.16 (m, 2H, OCH2), 2.20 (s, 3H, Me), 1.36–1.40 (m, 3H, CH2CH3). Z-isomer (minor) 10.53 (s, 1H, NH), 8.40 (dd, J = 8.7, 2.1 Hz, 1H, H-6′), 8.30 (d. J = 2.1 Hz, 1H, H-2′), 7.65–7.69 (m, 2H, H-4 and =CH), 7.17 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.03 (d, J = 8.7 Hz, 1H, H-5′), 6.97 (td, J = 7.6, 1.0 Hz, 1H, H-5), 6.81 (d, J = 7.6 Hz, 1H, H-7), 4.10–4.16 (m, 2H, OCH2), 2.19 (s, 3H, Me), 1.36–1.40 (m, 3H, CH2CH3). 13C NMR [(CD3)2SO] δ E-isomer (major) 169.0 (C=O), 158.1 (C-4′), 142.6 (C-7a), 136.3 (=CH), 132.1 (C-2′), 129.6 (C-6), 129.1 (C-6′), 126.1 (C-1′), 126.0 (C-3′), 125.3 (C-3), 122.0 (C-4), 121.3 (C-3a), 121.0 (C-5), 111.2 (C-5′), 110.0 (C-7), 63.4 (OCH2), 15.9 (Me), 14.7 (CH2CH3). Z-isomer (minor) 167.4 (C=O), 158.8 (C-4′), 140.2 (C-7a), 137.1 (=CH), 134.9 (C-2′), 132.4 (C-6′), 128.1 (C-6), 126.4 (C-3′), 125.5 (C-3a), 125.3 (C-1′), 123.7 (C-3), 120.8 (C-5), 119.1 (C-4), 110.8 (C-5′), 109.1 (C-7), 63.4 (OCH2), 16.1 (Me), 14.7 (CH2CH3). m/z 280.2 (MH+, 100%). Anal. Calcd for C18H17NO2: C, 77.40; H, 6.13; N, 5.01. Found: C, 77.33; H, 6.30; N, 5.02%.
(E/Z)-3-(3-Methyl-4-propoxybenzylidene)indolin-2-one (5)
Phenol 2 (272 mg, 1.08 mmol), propyl iodide (106 μL, 1.08 mmol), and K2CO3 (300 mg, 2.17 mmol) were stirred in DMF (5 mL) at r.t. overnight (23 h). The resulting mixture was diluted with EtOAc (50 mL), washed with water (2 × 50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography, eluting in 30–40% EtOAc/petroleum ether to give (E/Z)-5 (172 mg, 54%) as a yellow solid: mp 130–132 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.53 (s, 1H, NH), 7.65–7.70 (m, 1H, H-4), 7.59 (dd, J = 8.5, 2.0 Hz, 1H, H-6′), 7.53–7.54 (m, 2H, =CH and H-2′), 7.20 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.07 (d, J = 8.5 Hz, 1H, H-5′), 6.86–6.89 (m, 2H, H-5 and H-7), 4.02–4.06 (m, 2H, OCH2), 2.21 (s, 3H, Me), 1.74–1.83 (m, 2H, CH2), 1.00–1.04 (m, 3H, CH2CH3). Z-isomer (minor) 10.53 (s, 1H, NH), 8.39 (dd, J = 8.7, 2.0 Hz, 1H, H-6′), 8.31 (d, J = 2.0 Hz, 1H, H-2′), 7.65–7.70 (m, 2H, H-4 and =CH), 7.17 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.03 (d, J = 8.7 Hz, 1H, H-5′), 6.97 (dt, J = 7.6, 1.0 Hz, 1H, H-5), 6.81 (d, J = 7.6 Hz, 1H, H-7), 4.02–4.06 (m, 2H, OCH2), 2.20 (s, 3H, Me), 1.74–1.83 (m, 2H, CH2), 1.00–1.04 (m, 3H, CH2CH3). 13C NMR [(CD3)2SO] δ (both isomers) 168.9, 167.4, 158.9, 158.2, 142.6, 140.2, 137.1, 136.3, 134.8, 132.4, 132.0, 129.6, 129.1, 128.0, 126.4, 126.2, 126.0, 125.5, 125.3 (2), 123.6, 122.0, 121.3, 121.0, 120.8, 119.1, 111.2, 110.8, 110.0, 109.1, 69.2, 69.1, 22.1 (2), 16.0, 15.9, 10.5, 10.4. m/z 294.2 (MH+, 100%). Anal. Calcd for C19H19NO2: C, 77.79; H, 6.53; N, 4.77. Found: C, 77.65; H, 6.32; N, 4.81%.
(E/Z)-3-(4-Isopropoxy-3-methylbenzylidene)indolin-2-one (6)
Phenol 2 (200 mg, 0.80 mmol), K2CO3 (110 mg, 0.80 mmol), and 2-bromopropane (75 μL, 0.80 mmol) were stirred in DMF (5 mL) at r.t. overnight (24 h), then at 60 °C for 5 h. Once cooled, the mixture was diluted with EtOAc (50 mL), washed with water (2 × 50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography, eluting in 20% EtOAc/petroleum ether. Solvent was removed to give (E/Z)-6 (109 mg, 47%) as a yellow solid: mp 136–139 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.53 (s, 1H, NH), 7.68–7.70 (m, 1H, H-4), 7.57 (dd, J = 8.5 Hz, 1H, H-6′), 7.53–7.54 (m, 2H, =CH), 7.21 (td, J = 7.7, 1.0 Hz, 1H, H-6), 7.10 (d, J = 8.5 Hz, 1H, H-5′), 6.86–6.90 (m, 2H, H-5 and H-7), 4.69–4.77 (m, 1H, CH), 2.18 (s, 3H, Me), 1.32 (d, J = 6.02 Hz, 6H, CH(Me)2). Z-isomer (minor) 10.53 (s, 1H, NH), 8.40 (dd, J = 8.8, 2.1 Hz, 1H, H-6′), 8.28 (d, J = 2.1 Hz, 1H, H-2′), 7.68–7.70 (m, 1H, =CH), 7.65 (d, J = 7.6 Hz, 1H, H-4), 7.16 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.06 (d, J = 8.8 Hz, 1H, H-5′), 6.96 (td, J = 7.6, 1.0 Hz, 1H, H-5), 6.81 (d, J = 7.6 Hz, 1H, H-7), 4.69–4.77 (m, 1H, CH), 2.16 (s, 3H, Me), 1.32 (d, J = 5.99 Hz, 6H, CH(Me)2). 13C NMR [(CD3)2SO] δ (both isomers) 169.0, 167.4, 157.9, 157.2, 142.6, 140.1, 137.1, 136.3, 135.1, 132.3 (2), 129.5, 129.1, 128.0, 126.9, 126.2, 126.0, 125.8, 125.5, 125.2, 123.6, 122.0, 121.3, 121.0, 120.8, 119.1, 112.5, 112.0, 110.0, 109.1, 69.8, 69.7, 22.0 (4), 16.2, 16.1. m/z 294.2 (MH+, 100%). Anal. Calcd for C19H19NO2: C, 77.79; H, 6.53; N, 4.77. Found: C, 77.51; H, 6.27; N, 4.74%.
(E/Z)-3-(4-(Benzyloxy)-3-methylbenzylidene)indolin-2-one (7)
Phenol 2 (200 mg, 0.80 mmol), K2CO3 (110 mg, 0.80 mmol), and benzyl bromide (90 μL, 0.76 mmol) were stirred in DMF (5 mL) at r.t. for 3 h. Once cooled, the mixture was diluted with EtOAc (50 mL), washed with water (2 × 50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography, eluting in 20–30% EtOAc/petroleum ether. Solvent was removed to give (E/Z)-7 (130 mg, 48%) as a yellow solid: mp 143–145 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.55 (br s, 1H, NH), 7.66–7.70 (m, 1H, H-4), 7.55–7.60 (m, 3H, =CH, H-2′ and H-6′), 7.48–7.51 (m, 2H, H-2″ and H-6″), 7.39–7.44 (m, 2H, H-3″ and H-5″), 7.32–7.37 (m, 1H, H-4″), 7.20 (td, J = 7.7, 1.0 Hz, H-6), 7.16–7.18 (d obscured, H-5′), 6.85–6.89 (m, 2H, H-5 and H-7), 5.22 (s, 2H, CH2), 2.25 (s, 3H, Me). Z-isomer (minor) 10.55 (br s, 1H, NH), 8.40 (dd, J = 8.7, 2.0 Hz, 1H, H-6′), 8.31 (d, J = 2.0 Hz, 1H, H-2′), 7.64–7.66 (m, 2H, H-4 and =CH), 7.48–7.51 (m, 2H, H-2″ and H-6″), 7.39–7.44 (m, 2H, H-3″ and H-5″), 7.32–7.37 (m, 1H, H-4″), 7.12–7.17 (m, 2H, H-6 and H-5′), 6.97 (td, J = 7.6, 0.9 Hz, 1H, H-5), 6.81 (d, J = 7.60 Hz, 1H, H-7), 5.23 (s, 2H, CH2), 2.24 (s, 3H, Me). 13C NMR [(CD3)2SO] δ (both isomers) 168.9, 167.3, 158.4, 157.8, 142.6, 140.2, 137.0, 136.2, 134.9, 132.2, 132.0, 129.6, 129.1, 128.5, 128.1, 127.8, 127.4, 127.4, 126.8, 126.5, 126.4, 125.6, 125.5, 125.4, 123.9, 122.0, 121.2, 121.0, 120.9, 119.1, 111.8, 111.4, 110.0, 109.2, 69.4, 69.3, 16.2, 16.1. m/z 342.2 (MH+, 100%). Anal. Calcd for C23H19NO2: C, 80.92; H, 5.61; N, 4.10. Found: C, 80.78; H, 5.47; N, 4.17%.
(E/Z)-3-(4-(2-Hydroxyethoxy)-3-methylbenzylidene)indolin-2-one (8)
4-(2-Hydroxyethoxy)-3-methylbenzaldehyde (8a)
4-Hydroxy-3-methylbenzaldehyde (200 mg, 1.47 mmol) and 2-bromoethanol (160 μL, 2.20 mmol) were stirred with K2CO3 (609 mg, 4.41 mmol) in DMF (5 mL) in a sealed tube at 60 °C for 20 h. A second portion of 2-bromoethanol (160 μL, 2.20 mmol) was added, and the mixture was heated at 60 °C for another 6 h. Once cooled, the resulting mixture was diluted with water (50 mL) and extracted with EtOAc (50 mL). The organic layer was washed with water (50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography, eluting in 40–50% EtOAc/petroleum ether to give aldehyde 8a (134 mg, 51%) as a yellow solid: 1H NMR δ 9.87 (s, 1H, CHO), 7.70–7.72 (m, 2H, H-2 and H-6), 6.93–6.95 (m, 1H, H-5), 4.18–4.20 (m, 2H, OCH2), 4.02–4.06 (m, CH2OH), 2.30 (s, 3H, Me), 1.96 (t, J = 6.3 Hz, 1H, OH). MS m/z 181.2 (MH+, 100%).
(E/Z)-3-(4-(2-Hydroxyethoxy)-3-methylbenzylidene)indolin-2-one (8)
Oxindole (98 mg, 0.74 mmol), aldehyde 8a (133 mg, 0.74 mmol), and piperidine (a drop) were dissolved in EtOH (5 mL). The reaction mixture was purged with N2, sealed, and heated at 80 °C for 3 h. Solvent was removed and the residue triturated in 50% EtOAc/petroleum ether and washed with EtOAc to give alcohol (E/Z)-8 (191 mg, 88%) as an orange solid: mp 202–205 °C. 1H NMR [(CD3)2SO] δ E-isomer (major) 10.53 (s, 1H, NH), 7.65–7.68 (m, 1H, H-4), 7.58 (dd, J = 8.4, 2.3 Hz, 1H, H-6′), 7.53–7.54 (m, 2H, =CH and H-2′), 7.21 (td obscured, J = 6.9, 1.0 Hz, 1H, H-6), 7.09 (d, J = 8.4 Hz, 1H, H-5′), 6.86–6.89 (m, 2H, H-5 and H-7), 4.88 (t, J = 5.3 Hz, 1H, OH), 4.10 (t, J = 5.0 Hz, 2H, OCH2), 3.76 (dt, J = 5.3, 5.0 Hz, 2H, CH2OH). Z-isomer (minor) 10.53 (s, 1H, NH), 8.40 (dd, J = 8.7, 2.0 Hz, 1H, H-6′), 8.30 (d, J = 2.0 Hz, 1H, H-2′), 7.70 (s, 1H, =CH), 7.65–7.68 (m, 1H, H-4), 7.17 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.04 (d, J = 8.7 Hz, 1H, H-5′), 6.97 (td, J = 7.6, 1.0 Hz, 1H, H-5), 6.81 (d, J = 7.6 Hz, 1H, H-7), 4.88 (t, J = 5.3 Hz, 1H, OH), 4.10 (t, J = 5.0 Hz, 2H, OCH2), 3.76 (dt, J = 5.3, 5.0 Hz, 2H, CH2OH). 13C NMR [(CD3)2SO] δ (both isomers) 168.9, 167.4, 158.9, 158.2, 142.6, 140.2, 137.1, 136.3, 134.9, 132.4, 132.0, 129.6, 129.1, 128.1, 126.5, 126.4, 126.1, 125.5 (2), 125.4, 123.7, 122.0, 121.3, 121.0, 120.8, 119.1, 111.4, 110.9, 110.0, 109.1, 69.9, 69.8, 59.6 (2), 16.1, 15.9. MS m/z 296.2 (MH+, 100%). (+)-HRESIMS m/z [M + H]+ 296.1284 (calcd for C18H18NO3, 296.1281).
(E)-3-(4-Methoxy-3-methylbenzylidene)-1-methylindolin-2-one (9)
Indolin-2-one 3 (207 mg, 0.78 mmol) was stirred with K2CO3 (324 mg, 2.34 mmol) in DMF (10 mL). MeI (73 μL, 1.17 mmol) was added to the reaction mixture, which was then stirred at 40 °C for 21 h and at r.t. for another 42 h. The crude reaction mixture was diluted with water (50 mL) and extracted with EtOAc (50 mL). The organic layer was washed with water (50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography eluting in 5% EtOAc/DCM to give E-isomer 9 (86 mg, 39%) as a yellow solid: mp 89–91 °C. 1H NMR [(CD3)2SO] δ 7.71 (d, J = 7.6 Hz, 1H, H-4), 7.62–7.64 (m, 2H, =CH and 6′), 7.54–7.55 (m, 1H, H-2′), 7.32 (td, J = 7.6, 1.0 Hz, 1H, H-6), 7.11 (d, J = 8.5 Hz, 1H, H-5′), 7.04 (d, J = 7.6 Hz, 1H, H-7), 6.96 (td, J = 7.6, 1.0 Hz, 1H, H-5), 3.88 (s, 3H, OMe), 3.21 (s, 3H, NMe), 2.21 (s, 3H, Me). 13C NMR [(CD3)2SO] δ 167.5, 165.5, 159.6, 158.8, 143.7, 141.3, 137.4, 136.9, 134.7, 132.7, 132.1, 129.6, 129.2, 128.1, 126.5, 126.1, 126.0, 125.2, 124.4, 124.3, 122.6, 121.7, 121.6, 121.5, 120.5, 118.8, 110.4, 110.0, 108.8, 108.1, 55.5 (2), 25.9, 25.8, 16.1, 15.9. MS m/z 280.2 (MH+, 100%). (+)-HRESIMS m/z [M + H]+ 280.1338 (calcd for C18H18NO2, 280.1332).
Compounds Provided By AstraZeneca
Key compounds identified by AstraZeneca (10–12) were purchased from Enamine (Kyiv, Ukraine).
Docking Study and Virtual Screening
Telcagepant-Type Compounds (Table S1)
The X-ray crystal structure of the AM1 receptor (PDB 3AQF) was used for the initial docking work (Tables S1 and S2).22 Hydrogen atoms were added, and the side-chain orientations were modified for the following residues: Chain A/RAMP2: Asn60, His124, Asn130; Chain B/CLR: Asn39, Gln86, Asn128, and His132 based on MolProbity analysis.42 Water molecules were removed prior to docking. Molecular docking was performed using GOLD v5.2 (Genetic Optimization for Ligand Docking),43 and the scoring function used was ChemPLP. The docking cavity was defined as a sphere with a radius of 13 Å, centered on Trp72 (side chain NH, atom file index 2337). No protein hydrogen bond constraints were applied during docking.
Ligands for docking were prepared in SYBYLX2.1.1 (Tripos), and protoplex was used to generate the protonation state at pH 7. Stereocenter enumeration was performed using stereoplex, and a single low-energy 3D conformer was generated using CONCORD v6.1.3 with the ccr limit set at 0.4 and anilinic nitrogens treated as planar.
Docking results were analyzed in Hermes v1.6. Only the top-ranking solution was considered. Hydrogen bond contacts were calculated as follows: RAMP2: Arg97 (side chain NH), Glu101 (side chain CO), and Phe111 (backbone CO); CLR: Asp70 (backbone CO), Trp72 (side chain NH), Thr122 (backbone CO), and Thr122 (backbone NH). A sphere volume descriptor with a radius of 1.8 Å was used to define the hydrophobic pocket created by Ile41, Met42, Gln45, and Trp69. Only compounds predicted to form a hydrogen bond contact to the residues above and that occupied >95% of the defined sphere volume were visually inspected and prioritized for synthesis.
Virtual Screening (Table S2)
The X-ray crystal structure of the AM1 receptor corresponding to PDB entry 3AQF was used for molecular docking.22 Docking was performed using GOLD v5.0.143 using a docking cavity with a 16 Å radius centered on the side chain CD atom of Pro112. The ChemPLP scoring function was used with the search efficiency set at 0.3, the Diverse Solutions RMSD set at two with the cluster size set at one, and the number of GA runs set to five. The top three solutions per compound were kept for all compounds with a fitness score above zero. A protein hydrogen bond restraint was included using the default settings to drive a hydrogen bond with the indole nitrogen of Trp72. The poses were then rescored using the same settings with local optimization and the rotated protein atom positions. Vendor libraries for Asinex, ChemDiv, ChemBridge, InterBioSci, Life Chemicals, Maybridge, Matrix, NCI, Ryan Scientific, and Specs were obtained from ZINC844 and filtered for the lead-like properties defined in the OpenEye filter program, with a modified molecular weight range of 250–440. Duplicate compounds were removed using SYBYL. One low-energy conformer was generated for each compound, and up to 10 stereocenters were enumerated using Omega2 (OpenEye) using the mmff94 force field, with the dielectic constant and exponent set at 1.
Docking results were analyzed in Hermes v1.6. Only the top-ranking solution was considered. Hydrogen bond contacts were calculated as follows: RAMP2: Arg97 (side chain NH), Glu101 (side chain CO), and Phe111 (backbone CO); CLR: Asp70 (backbone CO), Trp72 (side chain NH), Thr122 (backbone CO), and Thr122 (backbone NH). A sphere volume descriptor with a radius of 1.8 Å was used to define the hydrophobic pocket created by Ile41, Met42, Gln45, and Trp69. Only compounds predicted to form a hydrogen bond contact to the residues above and occupied >95% of the defined sphere volume were visually inspected and selected for screening.
Tyrosine Mimetics (Table S3)
The X-ray crystal structure of the AM1 receptor corresponding to PDB entry 4RWF was used for molecular docking.24 This series of compounds were designed to mimic the interaction of the C-terminal amide at AM peptide residue Y52 with the CLR:RAMP2 ECD (Tables S3 and S4). The maltose sugar and any water molecules found within the receptor binding site were removed. The binding site was defined as a sphere with a radius of 10 Å centered on C-1 at AM-Y52. Two histidines were found within the binding site and the protonation on His114 was swapped. Molecular docking was performed using GOLD v5.2 (Genetic Optimization for Ligand Docking), and the scoring function used was ChemPLP. Protein hydrogen bond constraints were applied as follows: RAMP2: Glu101 (side chain CO); CLR: Thr122 (backbone CO) and Thr122 (backbone NH).43
Ligands for docking were prepared in SYBYLX2.1.1 (Tripos) with 3D conformers generated using CONCORD v6.1.3 (protonation using protoplex, stereocenter enumeration using stereoplex, and generation of a single low-energy 3D conformer with the ccr limit set at 0.4 and anilinic nitrogens treated as planar).
Docking results were analyzed in Hermes v1.6. Only the top-ranking solution was considered. Hydrogen bond contacts were calculated as follows: RAMP2: Glu101 (side chain CO); CLR: Thr122 (backbone CO) and Thr122 (backbone NH). A sphere volume descriptor with a radius of 2.0 Å was used to define site occupancy around the aromatic ring on AM Y52. Only compounds that delivered a hydrogen bond contact to the residues above and occupied >80% of the defined sphere volume were visually inspected and prioritized for synthesis.
Virtual Screening Round 2 (Table S4)
The X-ray crystal structure of the AM1 receptor corresponding to PDB 4RWF prepared above (tyrosine mimetics, Table S3)24 was used in a molecular docking screen of the ChemBridge Express Pick collection.
Compounds were selected from the ChemBridge Express Pick collection with molecular weight range of 350–500, a ring count between one and four, and chiral centers limited to three, and that did not possess the unwanted functional groups or PAINs substructures defined by Baell and Holloway.45 The set was further restricted to only those with a cLogP value between −4 and 5, hydrogen bond donors between zero and three, hydrogen bond acceptors between zero and six, and a maximum of 10 rotatable bonds. Compounds were prepared for docking by protonation using protoplex and stereocenters were enumerated using stereoplex as implemented in SYBYLX2.1.1. CONCORDv6.1.3 was used to generate the protonation state at pH 7. Stereocenter enumeration was performed using stereoplex, and a single low-energy 3D conformer was generated using CONCORD v6.1.3 with the ccr limit set at 0.4 and anilinic nitrogens treated as planar.
Docking results were analyzed in Hermes v1.6. Only the top ranking solution was considered. Hydrogen bond contacts were calculated as follows: RAMP2: Glu101 (side chain CO); CLR: Thr122 (backbone CO) and Thr122 (backbone NH). A sphere volume descriptor with a radius of 2.0 Å was used to define site occupancy around the aromatic ring on AM Y52. Only compounds predicted to form a hydrogen bond contact to the residues above and that occupied >80% of the defined sphere volume were considered. These were clustered, then visually inspected and selected for screening.
Virtual screening compounds were purchased from ChemBridge corp (San Diego, CA) and were tested as received. The minimum purity for these compounds is stated as 85%.
Cell Culture
All cells were maintained as adherent monolayers in T75 cm2 or T175 cm2 flasks and incubated in humidified incubators at 37 °C with 5% CO2. Cell counting used a Countess cell counter (Life Technologies).
CHO-AM1 and CHO-CGRP cells were passaged twice weekly. For a T75 cm2 flask, cells were washed with 5 mL of DPBS, dissociated with 2 mL of Cell Detachment Reagent, and 1 mL of OptiMEM with 1% fetal bovine serum (FBS) was added. For maintenance of cultures, 1 mL of cells was added to a new flask containing growth medium. The remaining cells were counted, diluted further using OptiMEM/1% FBS, then transferred into microplates. For β-arrestin assays, 5000 cells per well were seeded into a white 384-well plate with clear-bottomed wells (Corning, NY, USA). For cAMP assays, 20 000 cells per well were plated into clear 96-well SpectraPlates (PerkinElmer) before assays were completed the following day.
Cos7 cells were cultured and transiently transfected as previously described.33,46 These cells do not endogenously express RAMPs or receptors of interest.46 Cells were plated into 96-well SpectraPlates at 20 000 cells per well then transfected 1 day later using polyethylenimine and relevant plasmids (pcDNA3.0 or pcDNA3.1 expressing human receptor components). HA-tagged human CLR, myc-tagged human RAMP1, FLAG-tagged human RAMP2, untagged-human RAMP3, and HA-tagged CTR have been previously described.47,48 N-Terminally tagged receptors have been shown to retain function.33 PAC1n and CRF1 receptors were sourced from cDNA.org.
HMEC-1 cells (kindly provided by Dr. Katheryn Askelund, University of Auckland) were grown in MCDB-131 medium supplemented with heat-inactivated FBS, 50 μg/mL endothelial growth supplement (Merck, MA, USA), 1 μg/mL hydrocortisone, and 5% v/v penicillin/streptomycin/glutamine. Cells were plated at 20 000 cells per well into 96-well SpectraPlates and left for 48 h before assays.
β-Arrestin Assays
Pharmacological Characterization
The day after cell seeding, control agonists and antagonists were prepared in assay medium (OptiMEM with 0.1% BSA). DMSO was also added to the assay media to give a final concentration of 1%. No effects were observed with the addition of DMSO (data not shown). For concentration–response curves, final concentrations of agonist ranged from 10 μM to 1 pM in the wells. Cells were incubated with 5 μL of agonist for 90 min at 37 °C. For antagonist assays, 5 μL of the seeding media was removed and replaced with 5 μL of antagonist for 30 min at 37 °C prior to the addition of agonists for 90 min at 37 °C. After stimulation, 12 μL of DiscoveRx PathHunter detection reagent was added to each well. Plates were incubated at room temperature for 60 min and luminescence was read on an EnVision plate reader using a 1 s read time (PerkinElmer).
Screening
Compounds were weighed and dissolved in DMSO at 10 mM for each experiment, then further diluted in assay media. Solubility of compounds was confirmed visually and reduced concentrations were used if obvious precipitation occurred. The remaining steps were completed with the aid of a Janus liquid handing robot (PerkinElmer). The compounds were serially diluted, and 2.5 μL/well diluted compounds was added in duplicate to a 384-well plate of CHO-AM1 or CHO-CGRP cells. Compounds were preincubated for 30 min at 37 °C or 10 min at room temperature before 2.5 μL of AM or CGRP was added to give a final concentration of 20 nM in the wells. No differences in screening results were observed between the two preincubation periods. The screening method then followed the procedure described above: 90 min of incubation at 37 °C, addition of 12 μL/well detection reagent, further incubation at room temperature for 60 min, and luminescence signal measurement. On each 384-well plate, 32 wells provided a minimum response (no agonist), and 32 wells were used as maximum response (agonist only). The peptide antagonist AM22–52 was used as a positive control to inhibit AM responses in the CHO-AM1 screens. Similarly, the small-molecule antagonist telcagepant was the positive control for CHO-CGRP screens using CGRP as an agonist. Compounds were tested in duplicate wells.
cAMP Assays
The cAMP stimulation and detection method was similar across all three cell types in this study, and has been previously described.33 Details for Cos7 and HMEC-1 cells are provided below, while assays using CHO-K1 cells are detailed in the Supporting Information.
On the day of assay, growth medium was replaced with assay medium (DMEM with 0.1% BSA and 1 mM 3-isobutyl-1-methylxanthine) containing compound or vehicle. Cells were pretreated with compound for 30 min at 37 °C, followed by incubation with agonists for 15 min at 37 °C. Wells were then aspirated, and 50 μL of ice-cold absolute ethanol was added. Plates were stored at −30 °C before processing continued using PerkinElmer LANCE (Cos7) or LANCE Ultra assays (HMEC-1). Manufacturer’s directions were followed with modifications for adherent cell lines. The ethanol in plates was evaporated, and 50 μL/well lysis buffer was added. Plates were gently shaken for 15 min, then 10 μL/well cell lysate was transferred to a white 384-well OptiPlate (PerkinElmer). cAMP detection reagents were added, and the plate was incubated for 1–24 h. Signal was read using an EnVision plate reader (PerkinElmer) and compared to a standard curve generated for each assay.
Data Analysis
Data were analyzed using GraphPad Prism version 6.0–8.0 (GraphPad Software, La Jolla, CA). Concentration–response curves were fitted with a four-parameter logistic equation. An F-test was conducted to determine whether the Hill slope was significantly different from 1. In most experiments, it was not different from 1 and was therefore constrained to 1. Concentration–response data were normalized to the minimum and maximum of the control agonist curve (without compound), and curves were constrained where appropriate. The inhibition curve data were normalized to the mean of minimum response and maximum response controls for each plate. The independent experiments (performed in triplicate) were then combined. Data are presented as the mean ± standard error of the mean (s.e.m.). Screens with a negative Z′ value were repeated.49
In order to estimate an efficacy co-operativity factor for the modulators, concentration–response curves using a range of compound concentrations were plotted using an operational model of allostery.26 Individual experiments were normalized and combined as before, using the top and bottom of the three-parameter logistic equation fitted. The mean values of independent experiments were combined, then the model applied. (Details of the parameters are described in the Supporting Information). The resulting log β value was used as a guide to compare compound activity.
Computational Modeling: Structural Cheminformatics (Compounds 3–12)
Several methods have been proposed for identifying binding sites (e.g., methods based on sequence conservation,50−54 methods based on topology,55−59 and methods based on energetics).32,60−64 Given its proven track record, we have used the ConSurf sequence-based method.30 However, we note that one advantage of allosteric binding sites is the lower conservation that offers an increased prospect of subtype-specific drugs over the orthosteric binding site. Consequently, we have combined our results with our topology-based local connectivity approach that has been shown to give a good description of ligand binding sites, including allosteric binding sites.31 In addition, we have tested these putative binding sites for the presence of hot spots using FTMap; these hot spots were determined by docking and clustering 16 small molecules to the target. Glide was used for docking selected active compounds (Figures 4 and 5) into the predicted sites, supplemented by AutoDock Vina.65
The binding sites were identified on the full model of the CGRP receptor (PDB code 6E3Y), with missing regions added as described by Liang et al.10 The CLR and RAMP multiple sequence alignments were generated by carrying out a BLASTp search of the nr database and filtering the hits by a keyword search to retrieve only CLR or RAMP sequences. The sequences were aligned using Clustal Omega66 refined by hand-editing using jalview.67 The resultant multiple sequence alignments were submitted to the ConSurf Web server,30 and the resultant conservation data was scaled between 0 and 1, with 1 being most conserved. The local connectivity31 for the CGRP receptor structure was calculated using in-house software and the results scaled between 0 and 1, with 1 being the most connected residue(s). The two sets of results were multiplied together on a residue by residue basis, inserted into the B-factor column of the PDB file, and plotted using Pymol.68 The multiplication helps to eliminate highly conserved flat regions and pockets with low conservation that are therefore unlikely to have an allosteric affect.
Pymol was also used to determine the RMSD (over C atoms only); the experimental ligand pose for olcegepant and telcagepant (1) was determined by superimposition of the CLR/RAMP1 ECD X-ray structures (PDB codes 3N7S and 3N7R).23 Pymol was also used to determine the RMSD (over C atoms only; the experimental ligand pose for telcagepant (1), etc. was determined by superimposition of the CLR/RAMP1 ECD X-ray structures (PDB codes 3N7S and 3N7R) onto the full-length CGRP receptor). Olcegepant and telcagepant (1) docked to the ECD with a low RMSD to experiment of 1.5 Å (2.4 Å including the highly flexible butamine chain) and 1.8 Å respectively, justifying the use of Glide for docking, at least at the canonical ECD binding site.
Nine compounds (3–8, 10–12), along with telcagepant (1) and olcegepant were docked into the full CGRP receptor structure using Glide SP65 with a 20 Å docking box centered on key residues in the putative allosteric sites; the center of the 5 CLR allosteric sites were CH2 of W72, Cβ of P97, Cγ1 of V304, Cβ of P343, and Oδ of D90 and Cγ2 of V111. In addition, two sites were centered on RAMP1 residues, namely Oδ of D90 and Cγ2 of V111.
Acknowledgments
The authors acknowledge funding from the Health Research Council of New Zealand, University of Auckland BioPharma Thematic Research Initiative, and the Lottery Health Commission of New Zealand. The authors acknowledge Richard Bailey, Manupriya Vishnoi, and Michael Garelja. The authors also thank Patrick Sexton for advice on the operational model and acknowledge the technical support provided by Sisira Kumara and Karin Tan. E.R.H. is supported by the Auckland Medical Research Foundation Barbara Basham Doctoral Scholarship. C.A.R. acknowledges support from the Biotechnology and Biological Sciences Research Council (BB/M006883/1); C.A.R. is a Royal Society Industry Fellow. C.A.R. acknowledges Miles Congrieve and Connor Scully for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.9b00108.
Synthesis details, structures, and purity values for screened compounds and supplemental biology: supplementary methods, additional compound characterization (PDF)
Author Contributions
□ E.R.H. and L.P.L. contributed equally to this work. The manuscript was written through contributions of all authors. L.P.L and M.B. performed synthesis of compounds. L.P.L. and J.U.F performed virtual screening of compounds. M.P.H. was the chemistry lead for design and synthesis of compounds. E.R.H., R.L.B., M.A.J., N.P., K.D.R., and J.M.B. performed biological experiments. G.P. and D.M.S. selected and provided compounds. R-M.R., R.S., and C.A.R. performed molecular modeling. E.R.H., L.P.L., C.S.W., C.A.R., A.A.P., J.U.F., M.P.H., and D.L.H. interpreted experiments and wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sriram K.; Insel P. A. (2018) GPCRs as targets for approved drugs: How many targets and how many drugs?. Mol. Pharmacol. 117, 111062. 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. L.; Pioszak A. A. (2016) Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu. Rev. Pharmacol. Toxicol. 56, 469. 10.1146/annurev-pharmtox-010715-103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. L.; Garelja M. L.; Poyner D. R.; Walker C. S. (2018) Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175, 3–17. 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J. (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756. 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Dolgin E. (2018) First GPCR-directed antibody passes approval milestone. Nat. Rev. Drug Discovery 17, 457–459. 10.1038/nrd.2018.103. [DOI] [PubMed] [Google Scholar]
- Liang Y.-L.; Khoshouei M.; Radjainia M.; Zhang Y.; Glukhova A.; Tarrasch J.; Thal D. M.; Furness S. G.; Christopoulos G.; Coudrat T.; et al. (2017) Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature 546, 118. 10.1038/nature22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Sun B.; Feng D.; Hu H.; Chu M.; Qu Q.; Tarrasch J. T.; Li S.; Sun Kobilka T.; Kobilka B. K.; Skiniotis G. (2017) Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248. 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Qiao A.; Yang D.; Yang L.; Dai A.; De Graaf C.; Reedtz-Runge S.; Dharmarajan V.; Zhang H.; Han G. W.; et al. (2017) Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 546, 259. 10.1038/nature22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf C.; Song G.; Cao C.; Zhao Q.; Wang M.-W.; Wu B.; Stevens R. C. (2017) Extending the structural view of class B GPCRs. Trends Biochem. Sci. 42, 946–960. 10.1016/j.tibs.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Liang Y.-L.; Khoshouei M.; Deganutti G.; Glukhova A.; Koole C.; Peat T. S.; Radjainia M.; Plitzko J. M.; Baumeister W.; Miller L. J.; et al. (2018) Cryo-EM structure of the active, G s-protein complexed, human CGRP receptor. Nature 561, 492. 10.1038/s41586-018-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D. M.; Glukhova A.; Sexton P. M.; Christopoulos A. (2018) Structural insights into G-protein-coupled receptor allostery. Nature 559, 45. 10.1038/s41586-018-0259-z. [DOI] [PubMed] [Google Scholar]
- Hoare S. R.J.; Grigoriadis D. E. (2017) Non-peptide CRF-Receptor Antagonists: Allosterism, Kinetics and Translation to Efficacy in Human Disease. CMP 10, 282–295. 10.2174/1874467210666170110124539. [DOI] [PubMed] [Google Scholar]
- Katayama T.; Furuya M.; Yamaichi K.; Konishi K.; Sugiura N.; Murafuji H.; Magota K.; Saito M.; Tanaka S.; Oikawa S. (2001) Discovery of a non-peptide small molecule that selectively mimics the biological actions of calcitonin. Biochim. Biophys. Acta, Gen. Subj. 1526, 183–190. 10.1016/S0304-4165(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Hay D. L.; Christopoulos G.; Christopoulos A.; Sexton P. M. (2006) Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl] carbonyl] pentyl] amino]-1-[(3, 5-dibromo-4-hydroxyphenyl) methyl]-2-oxoethyl]-4-(1, 4-dihydro-2-oxo-3 (2H)-quinazolinyl)(BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors—the role of receptor activity modifying protein 1. Mol. Pharmacol. 70, 1984–1991. 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Archbold J. K.; Flanagan J. U.; Watkins H. A.; Gingell J. J.; Hay D. L. (2011) Structural insights into RAMP modification of secretin family G protein-coupled receptors: implications for drug development. Trends Pharmacol. Sci. 32, 591–600. 10.1016/j.tips.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Rujan R.-M.; Reynolds C. A. (2018) Calcitonin Gene-Related Peptide Antagonists and Therapeutic Antibodies. Handb. Exp. Pharmacol. 255, 169–192. 10.1007/164_2018_173. [DOI] [PubMed] [Google Scholar]
- Russell F. A.; King R.; Smillie S. J.; Kodji X.; Brain S. D. (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099. 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubdool A. A.; Thakore P.; Argunhan F.; Smillie S.-J.; Schnelle M.; Srivastava S.; Alawi K. M.; Wilde E.; Mitchell J.; Farrell-Dillon K.; et al. (2017) A novel α-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failure. Circulation 136, 367–383. 10.1161/CIRCULATIONAHA.117.028388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. L.; Poyner D. R.; Walker C. S. (2019) Calcitonin receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. GtoPdb CITE F11. 10.2218/gtopdb/F11/2019.4. [DOI] [Google Scholar]
- Ashizuka S.; Inatsu H.; Kita T.; Kitamura K. (2016) Adrenomedullin therapy in patients with refractory ulcerative colitis: a case series. Dig. Dis. Sci. 61, 872–880. 10.1007/s10620-015-3917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashizuka S.; Kita T.; Inatsu H.; Kitamura K. (2013) Adrenomedullin: a novel therapy for intractable ulcerative colitis. Inflamm. Bowel Dis. 19, E26–E27. 10.1002/ibd.22891. [DOI] [PubMed] [Google Scholar]
- Kusano S.; Kukimoto-Niino M.; Hino N.; Ohsawa N.; Okuda K. i.; Sakamoto K.; Shirouzu M.; Shindo T.; Yokoyama S. (2012) Structural basis for extracellular interactions between calcitonin receptor-like receptor and receptor activity-modifying protein 2 for adrenomedullin-specific binding. Protein Sci. 21, 199–210. 10.1002/pro.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E.; Koth C. M.; Abdul-Manan N.; Swenson L.; Coll J. T.; Lippke J. A.; Lepre C. A.; Garcia-Guzman M.; Moore J. M. (2010) Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 18, 1083–1093. 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Booe J. M.; Walker C. S.; Barwell J.; Kuteyi G.; Simms J.; Jamaluddin M. A.; Warner M. L.; Bill R. M.; Harris P. W.; Brimble M. A.; et al. (2015) Structural basis for receptor activity-modifying protein-dependent selective peptide recognition by a G protein-coupled receptor. Mol. Cell 58, 1040–1052. 10.1016/j.molcel.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. N.; Paone D. V.; Shaw A. W.; Burgey C. S.; Mosser S. D.; Johnston V.; Salvatore C. A.; Leonard Y. M.; Miller-Stein C. M.; Kane S. A.; et al. (2008) Calcitonin gene-related peptide (CGRP) receptor antagonists: Investigations of a pyridinone template. Bioorg. Med. Chem. Lett. 18, 755–758. 10.1016/j.bmcl.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Leach K.; Sexton P. M.; Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 28, 382–389. 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Schwarz N.; Renshaw D.; Kapas S.; Hinson J. P. (2006) Adrenomedullin increases the expression of calcitonin-like receptor and receptor activity modifying protein 2 mRNA in human microvascular endothelial cells. J. Endocrinol. 190, 505–514. 10.1677/joe.1.06806. [DOI] [PubMed] [Google Scholar]
- Hinson J. P.; Kapas S.; Smith D. M.; et al. (2000) Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 21, 138–167. 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- Schönauer R.; Els-Heindl S.; Beck-Sickinger A. G. (2017) Adrenomedullin–new perspectives of a potent peptide hormone. J. Pept. Sci. 23, 472–485. 10.1002/psc.2953. [DOI] [PubMed] [Google Scholar]
- Armon A.; Graur D.; Ben-Tal N. (2001) ConSurf: an algorithmic tool for the identification of functional regions in proteins by surface mapping of phylogenetic information. J. Mol. Biol. 307, 447–463. 10.1006/jmbi.2000.4474. [DOI] [PubMed] [Google Scholar]
- Illingworth C. J.; Scott P. D.; Parkes K. E.; Snell C. R.; Campbell M. P.; Reynolds C. A. (2010) Connectivity and binding-site recognition: Applications relevant to drug design. J. Comput. Chem. 31, 2677–2688. 10.1002/jcc.21561. [DOI] [PubMed] [Google Scholar]
- Kozakov D.; Grove L. E.; Hall D. R.; Bohnuud T.; Mottarella S. E.; Luo L.; Xia B.; Beglov D.; Vajda S. (2015) The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 10, 733. 10.1038/nprot.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower R. L.; Yule L.; Rees T. A.; Deganutti G.; Hendrikse E. R.; Harris P. W.; Kowalczyk R.; Ridgway Z.; Wong A. G.; Swierkula K.; et al. (2018) Molecular signature for receptor engagement in the metabolic peptide hormone amylin. ACS Pharmacol. Transl. Sci. 1, 32–49. 10.1021/acsptsci.8b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H. A.; Chakravarthy M.; Abhayawardana R. S.; Gingell J. J.; Garelja M.; Pardamwar M.; McElhinney J. M.; Lathbridge A.; Constantine A.; Harris P. W.; et al. (2016) Receptor activity-modifying proteins 2 and 3 generate adrenomedullin receptor subtypes with distinct molecular properties. J. Biol. Chem. 291, 11657–11675. 10.1074/jbc.M115.688218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dal Maso E.; Glukhova A.; Zhu Y.; Garcia-Nafria J.; Tate C. G.; Atanasio S.; Reynolds C. A.; Ramírez-Aportela E.; Carazo J.-M.; Hick C. A.; et al. (2019) The Molecular Control of Calcitonin Receptor Signaling. ACS Pharmacol. Transl. Sci. 2, 31–51. 10.1021/acsptsci.8b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.; Guo S.; Yang C.; Gong Z.; Wang Y.; Jia Y.; Jiang X.; Xu L.; Shi L.; Yu X.; et al. (2020) Cell active and functionally-relevant small-molecule agonists of calcitonin receptor. Bioorg. Chem. 96, 103596. 10.1016/j.bioorg.2020.103596. [DOI] [PubMed] [Google Scholar]
- Hollenstein K.; Kean J.; Bortolato A.; Cheng R. K.; Doré A. S.; Jazayeri A.; Cooke R. M.; Weir M.; Marshall F. H. (2013) Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 499, 438. 10.1038/nature12357. [DOI] [PubMed] [Google Scholar]
- Wootten D.; Miller L. J. (2020) Structural Basis for Allosteric Modulation of Class B G Protein–Coupled Receptors. Annu. Rev. Pharmacol. Toxicol. 60, 89. 10.1146/annurev-pharmtox-010919-023301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H. A.; Rathbone D. L.; Barwell J.; Hay D. L.; Poyner D. R. (2013) Structure-activity relationships for α-calcitonin gene-related peptide. Br. J. Pharmacol. 170, 1308–1322. 10.1111/bph.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. L.; Howitt S. G.; Conner A. C.; Doods H.; Schindler M.; Poyner D. R. (2002) A comparison of the actions of BIBN4096BS and CGRP8–37 on CGRP and adrenomedullin receptors expressed on SK-N-MC, L6, Col 29 and Rat 2 cells. Br. J. Pharmacol. 137, 80–86. 10.1038/sj.bjp.0704844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. M.; Khan M.; Ambreen N.; Taha M.; Rahim F.; Rasheed S.; Saied S.; Shafi H.; Perveen S.; Choudhary M. I. (2013) Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 9, 681–688. 10.2174/1573406411309050007. [DOI] [PubMed] [Google Scholar]
- Chen V. B.; Arendall W. B.; Headd J. J.; Keedy D. A.; Immormino R. M.; Kapral G. J.; Murray L. W.; Richardson J. S.; Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 66, 12–21. 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.; Willett P.; Glen R. C.; Leach A. R.; Taylor R. (1997) Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267, 727–748. 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- Shoichet B. K., and Irwin J. J.. ZINC8 Database. https://zinc.docking.org/.
- Baell J. B.; Holloway G. A. (2010) New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53, 2719–2740. 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- Bailey R. J.; Hay D. L. (2006) Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides 27, 1367–1375. 10.1016/j.peptides.2005.11.014. [DOI] [PubMed] [Google Scholar]
- McLatchie L. M.; Fraser N. J.; Main M. J.; Wise A.; Brown J.; Thompson N.; Solari R.; Lee M. G.; Foord S. M. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339. 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Qi T.; Dong M.; Watkins H. A.; Wootten D.; Miller L. J.; Hay D. L. (2013) Receptor activity-modifying protein-dependent impairment of calcitonin receptor splice variant Δ(1–47)hCT(a) function. Br. J. Pharmacol. 168, 644–657. 10.1111/j.1476-5381.2012.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-H.; Chung T. D.; Oldenburg K. R. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screening 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Lichtarge O.; Yamamoto K. R.; Cohen F. E. (1997) Identification of functional surfaces of the zinc binding domains of intracellular receptors. J. Mol. Biol. 274, 325–337. 10.1006/jmbi.1997.1395. [DOI] [PubMed] [Google Scholar]
- Sowa M. E.; He W.; Slep K. C.; Kercher M. A.; Lichtarge O.; Wensel T. G. (2001) Prediction and confirmation of a site critical for effector regulation of RGS domain activity. Nat. Struct. Biol. 8, 234. 10.1038/84974. [DOI] [PubMed] [Google Scholar]
- Dean M. K.; Higgs C.; Smith R. E.; Bywater R. P.; Snell C. R.; Scott P. D.; Upton G. J.; Howe T. J.; Reynolds C. A. (2001) Dimerization of G-protein-coupled receptors. J. Med. Chem. 44, 4595–4614. 10.1021/jm010290+. [DOI] [PubMed] [Google Scholar]
- Thummer R. P.; Campbell M. P.; Dean M. K.; Frusher M. J.; Scott P. D.; Reynolds C. A. (2005) Entropy and oligomerization in GPCRs. J. Mol. Neurosci. 26, 113–122. 10.1385/JMN:26:2-3:113. [DOI] [PubMed] [Google Scholar]
- Filizola M.; Olmea O.; Weinstein H. (2002) Prediction of heterodimerization interfaces of G-protein coupled receptors with a new subtractive correlated mutation method. Protein Eng., Des. Sel. 15, 881–885. 10.1093/protein/15.11.881. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A.; Luscombe N. M.; Swindells M. B.; Thornton J. M. (1996) Protein clefts in molecular recognition and function. Protein Sci. 5, 2438–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendlich M.; Rippmann F.; Barnickel G. (1997) LIGSITE: automatic and efficient detection of potential small molecule-binding sites in proteins. J. Mol. Graphics Modell. 15, 359–363. 10.1016/S1093-3263(98)00002-3. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M.; Jiang X.; Oldfield T.; Waldman M. (2003) LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J. Mol. Graphics Modell. 21, 289–307. 10.1016/S1093-3263(02)00164-X. [DOI] [PubMed] [Google Scholar]
- Wangikar P. P.; Tendulkar A. V.; Ramya S.; Mali D. N.; Sarawagi S. (2003) Functional sites in protein families uncovered via an objective and automated graph theoretic approach. J. Mol. Biol. 326, 955–978. 10.1016/S0022-2836(02)01384-0. [DOI] [PubMed] [Google Scholar]
- Amitai G.; Shemesh A.; Sitbon E.; Shklar M.; Netanely D.; Venger I.; Pietrokovski S. (2004) Network analysis of protein structures identifies functional residues. J. Mol. Biol. 344, 1135–1146. 10.1016/j.jmb.2004.10.055. [DOI] [PubMed] [Google Scholar]
- Goodford P. J. (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 28, 849–857. 10.1021/jm00145a002. [DOI] [PubMed] [Google Scholar]
- Laurie A. T.; Jackson R. M. (2005) Q-SiteFinder: an energy-based method for the prediction of protein–ligand binding sites. Bioinformatics 21, 1908–1916. 10.1093/bioinformatics/bti315. [DOI] [PubMed] [Google Scholar]
- Harris M. R.; Kihlen M.; Bywater R. P. (1993) PLIM: A protein–ligand interaction modeller. J. Mol. Recognit. 6, 111–115. 10.1002/jmr.300060303. [DOI] [PubMed] [Google Scholar]
- Glick M.; Robinson D. D.; Grant G. H.; Richards W. G. (2002) Identification of ligand binding sites on proteins using a multi-scale approach. J. Am. Chem. Soc. 124, 2337–2344. 10.1021/ja016490s. [DOI] [PubMed] [Google Scholar]
- Ghanakota P.; Carlson H. A. (2016) Moving beyond active-site detection: MixMD applied to allosteric systems. J. Phys. Chem. B 120, 8685–8695. 10.1021/acs.jpcb.6b03515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner R. A.; Banks J. L.; Murphy R. B.; Halgren T. A.; Klicic J. J.; Mainz D. T.; Repasky M. P.; Knoll E. H.; Shelley M.; Perry J. K.; et al. (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749. 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Sievers F.; Wilm A.; Dineen D.; Gibson T. J.; Karplus K.; Li W.; Lopez R.; McWilliam H.; Remmert M.; Söding J. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp M.; Cuff J.; Searle S. M.; Barton G. J. (2004) The jalview java alignment editor. Bioinformatics 20, 426–427. 10.1093/bioinformatics/btg430. [DOI] [PubMed] [Google Scholar]
- Schrodinger, LLC . (2015) The PyMOL Molecular Graphics System, version 1.8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.