Summary
Engineering protein-based biomaterials is extremely challenging in bioelectronics, medicine, and materials science, as mechanical, electrical, and optical properties need to be merged to biocompatibility and resistance to biodegradation. An effective strategy is the engineering of physiological processes in situ, by addition of new properties to endogenous components. Here we show that a green fluorescent semiconducting thiophene dye, DTTO, promotes, in vivo, the biogenesis of fluorescent conductive protein microfibers via metabolic pathways. By challenging the simple freshwater polyp Hydra vulgaris with DTTO, we demonstrate the stable incorporation of the dye into supramolecular protein-dye co-assembled microfibers without signs of toxicity. An integrated multilevel analysis including morphological, optical, spectroscopical, and electrical characterization shows electrical conductivity of biofibers, opening the door to new opportunities for augmenting electronic functionalities within living tissue, which may be exploited for the regulation of cell and animal physiology, or in pathological contexts to enhance bioelectrical signaling.
Subject Areas: Supramolecular Chemistry, Bioelectronics, Bioengineering
Graphical Abstract

Highlights
-
•
The oligothiophene DTTO promotes the synthesis of microfibers in Hydra vulgaris
-
•
DTTO co-assembles with proteins giving rise to fluorescent and conductive microfibers
-
•
The biofiber synthesis is an active process, based on protein synthesis
-
•
In situ produced hybrid microfibers have great potential in biolectronics and biomedicine
Supramolecular Chemistry; Bioelectronics; Bioengineering
Introduction
Protein-based nano/microfibers are currently matter of great interest as advanced biomaterials for a variety of applications, from tissue regeneration to drug delivery and bioelectronics. Several studies have been reported describing the de novo engineering and in vitro preparation of these systems (Frezzo and Montclare, 2015, Hume et al., 2014, Kamada et al., 2017, McNamara et al., 2017, Xu et al., 2015). Studying the properties of protein-based nano-/microfibers and the mechanisms of formation of their hierarchical supramolecular structures is expected, on one side, to extend our capability to design high-performance biomaterials and, on the other, to improve our knowledge on the formation of protein aggregates related to neurodegenerative pathologies such as Parkinson, Alzheimer, and prion diseases. Recently, microfibers of collagen and elastin have been prepared by self-assembly of precursor nanofibrils using a microfluidic set up, the assembly mechanism elucidated, and the microfibers used as building blocks for the fabrication of biomaterials for tissue engineering (Kamada et al., 2017). A few de novo proteins capable to self-assemble into nano-/microfibers have been prepared and their secondary structure analyzed by a variety of techniques; the engineered protein coiled-coil secondary structure was found to be capable to incorporate hydrophobic small molecules such as curcumin, a cancer therapeutic agent (Hume et al., 2014), and the protein microfibers were checked for drug delivery (Frezzo and Montclare, 2015). Micron-sized silk fibers embedded into a chitosan membrane showed improved repair efficiency for wound healing in vivo (Xu et al., 2015). Nevertheless, although it is proved that it is possible to prepare in vitro proteins with programmed structural motifs, capable to self-assemble into desired supramolecular structures, it is still a major challenge to achieve the same level of structural variety, precision, and specificity as native proteins in living organisms. One way to attain the objective would be to find the means to induce living organisms to physiologically form in situ biomolecules with new properties via recognition and incorporation of specific nontoxic small molecules introduced from outside. Recently, the complex internal structure of plants has been used as a template for in situ fabrication of electronic circuits by means of conducting polymers and hybrid oligothiophenes inspiring novel bioengineering concepts (Stavrinidou et al., 2015, Stavrinidou et al., 2017). Naturally derived proteins are an exceptional alternative to synthetic materials, as they offer favourable sustainability and biocompatibility, in the form of fibers, films, and scaffolds. However, proteins properties and functions are strictly related to the characteristics of their hierarchical supramolecular structure, so the introduction of exogenous elements in living organisms should not perturb the assembly mechanism. In recent years, we have reported that treating different lines of living cells (NIH 3T3, HeLa, B104) with a dilute solution of the fluorescent semiconducting dye 3,5-dimethyl-2,3′-bis(phenyl)dithieno[3,2-b;2′,3′-d]thiophene-4,4-dioxide (DTTO, Figure 1A), capable to spontaneously cross the cell membrane and be recognized by specific intracellular proteins, the physiological formation of fluorescent conductive protein microfibers takes place without causing any significant effect on cell viability and proliferation (Palama et al., 2011, Viola et al., 2013). The physiologically produced protein microfibers, used as biomaterials to seed living cells, induced different fate in terms of cellular morphology, viability, and cytoskeleton rearrangement (Palama et al., 2015). We report here a further and more important step in our search for the production of functional microfibers in vivo, in a noninvasive way, namely the production of protein-DTTO co-assembled fluorescent conducting microfibers in the small freshwater model Hydra vulgaris. At the base of metazoan evolution the polyp Hydra vulgaris is a very simple animal, shaped as a hollow tube whose walls are made by a two-cell thick layer, i.e. an outer epithelial cell sheet, the ectoderm, and an inner epithelial cell sheet, the endoderm (Galliot and Schmid, 2002). Stem cells interspersed between these two layers ensure continuous cell turnover and differentiation of only a few types of specialized cells (neurons, gland cells, nematocytes) (Hobmayer et al., 2012). The tissue plasticity and the capability to regenerate amputated body parts made this organism a well-established model for developmental and stem cell biology (Holstein et al., 2003). Recently, the tissue-like organization, the absence of organs, and biological fluids posed the bases to use Hydra to test toxicity and bioactivity of a variety of nanomaterials and nanodevices (Allocca et al., 2019, Ambrosone et al., 2016, Moros et al., 2018, Tortiglione et al., 2017). With the aim to translate the in vivo capability of DTTO monomers to self-organize into supramolecular structures, which could functionally affect/modulate the overall physiology, here we soaked Hydra polyps in their culture solution containing DTTO and found in a few days the spontaneous production of microfibers that were in part secreted into the medium. A detailed physico-chemical characterization of these fibers demonstrated their protein-based structure and, more importantly, their conductive behavior, which could be exploited as a novel biocompatible source of endogenously produced materials for bioelectronics. Overall we propose a simple and effective strategy to manipulate physiological processes in situ, by spontaneous engineering of endogenous components, leading to a new class of hybrid-protein-based materials with electrical functionality. The introduction of a new electronic functionality into a living animal may represent an unprecedented tool to regulate physiological function, from cell signaling to tissue regeneration and neuronal transmission and a valid alternative to genetic manipulation. Tissues with integrated biocompatible electronics, manufactured in vivo in localized regions, may inspire new devices to manipulate biological functions by adding or augmenting conductivity in physiological or pathological contexts with spatiotemporal control, paving the way to new bioengineering concepts.
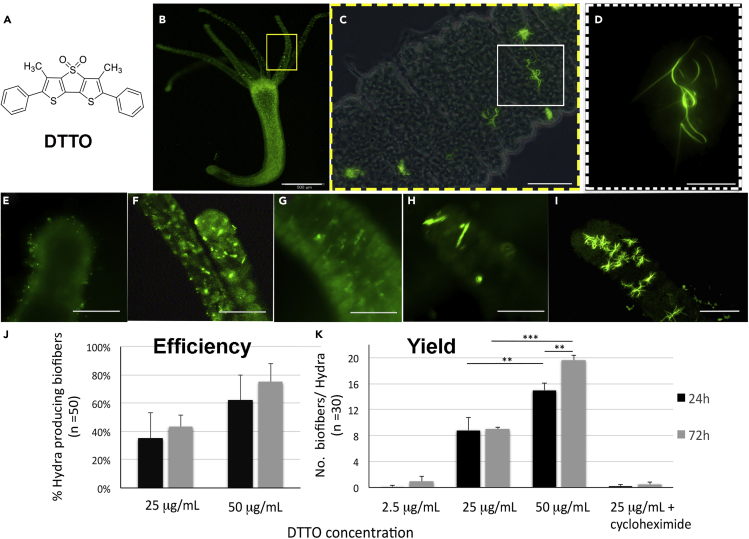
Figure 1.
In Vivo Production of Fluorescent Biofibers from DTTO
(A) Molecular structure of the green fluorescent semiconducting dye DTTO.
(B–D) (B) In vivo fluorescence image of a polyp incubated with DTTO in Hydra medium for 24 h. Both a diffuse- and a granular-patterned fluorescence label the whole animal. The tentacle region within the yellow rectangle is shown at higher magnification in (C) optical and fluorescence-merged image of the tentacle showing highly fluorescent green fibers. See also Figure S6 and Videos S1 and S2. An example of a single biofiber (as that inside the white rectangle) is shown at high magnification in (D) a fluorescent biofiber presenting linear, bent, and coiled regions. (E–H) Dynamics of biofiber production. Animals were pulsed 5 h with DTTO (25 μg/mL) and continuously monitored.
(E–I) (E) At time zero p.t only granular spots are detectable, whereas after (F) 24 h linear fibers are produced inside the tentacles, of length progressively increasing after (G) 3 days and (H) 6 days p.t. (I) DTTO concentration equal to 50 μg/mL increases the average number of biofiber detected into the tentacles. Scale bars, 500 μm in B; 20 μm in C and D; 100 μm in E–I.
(J) Efficiency of biofiber production in relation to DTTO concentration and incubation time. Data represent the average ±SD of three independent experiments (n = 50). Statistical evaluation performed using Student t-test indicates no significance.
(K) DTTO-treated polyps were fixed and mounted on microscopy slides for biofiber detection. Data represent the average ±SD of three independent experiments (n = 30). Statistical comparisons were performed using unpaired t test; ∗p< 0.05; ∗∗p< 0.01; ∗∗∗p< 0.001. p values are reported for all conditions in Table S1.
Results
In Vivo Biosynthesis of Biofibers from Green Fluorescent Semiconducting Thiophene Dye DTTO
Preliminary analyses were performed to test potential toxic effects played by DTTO on living Hydra. The dose range was initially selected on the bases of our previous results obtained in cell cultures (Palama et al., 2011, Palama et al., 2015), where a 25 μg/mL dose supplied for 5 h was found biocompatible and effective for fiber formation. Dose response evaluations were performed under chronic or acute conditions, up to 72 h. Figure S1 shows dose-response curves and in vivo fluorescence imaging obtained in chronic condition, i.e. by continuously treating animals with DTTO or with ECB04, another thiophene-based compound previously shown unable to induce fiber formation in cells (Palama et al., 2011). Both compounds showed similar toxicological profile, i.e. the appearance of behavioral and morphological alterations from 24 h of continuous incubation onwards, while higher doses were lethal at this time point. Next we analyzed the effect induced by increasing doses of DTTO (2.5, 25, and 50 μg/mL) exposed for a limited period (5 h, acute condition) to Hydra by using several in vivo and in vitro approaches (Figures S2 and S3) and detected slight effects only at the highest dose, when the morphology was transiently affected. The 25 μg/mL dose was confirmed fully biosafe, as previously observed in cells, and was used for following analyses. At this dose, a spotted granular-like fluorescence was detected inside animal tissues (Figure 1B), probably due to dye accumulation into storage vacuoles, as observed for other microbeads and inorganics nanoparticles (Marchesano et al., 2013, Tortiglione et al., 2009). A similar pattern was observed in presence of ECB04 and two other fluorophores (Figures S1, S4, and S5) presenting similar thiophene-based backbone but different lateral moieties replacing the phenyl group in position X (Scheme S1). These fluorophores, shown to induce fluorescent fiber formation in NIH3T3cells (Palama et al., 2011), accumulated into Hydra tissues producing intense and diffuse staining. Remarkably, only in the tentacles of animals treated with DTTO long and highly fluorescent fibers (from here biofibers, i.e. produced in Hydra), mostly aligned perpendicular to the tentacle axis, were found. The biofibers detected on the external surface of the tentacle cells and embedded between the cells showed diverse shapes, length, and structures—either linear, branched or hooked (Figures 1C, 1D, and S6, Videos S1 and S2). Spontaneous aggregation of DTTO in Hydra medium was indicated by the presence of round–short fibril-shaped fluorescent aggregates (Figure S7), remarkably different from those detected in Hydra tissue and never presenting coiled structures. In order to study the dynamics of biofiber formation the animals were pulsed for 5 h with DTTO, extensively washed to remove any dye from the medium, and then continuously inspected. At the beginning of the monitoring, a diffuse fluorescence was detected in the external layer of the Hydra together with green granular spots (Figure 1E), progressively increasing with the incubation time and detectable inside ectodermal cells. At 24 h post-treatment (p.t.), in addition to this punctuated pattern, microfibers of about 20 μm length were found in the tentacles (Figure 1F) and could be observed up to 6 days p.t. (Figures 1G and 1H), whose length increased with time. In the rest of the body only the punctuated fluorescence but not the fibers was detectable. The formation of long microfibers observed with DTTO and not with other thiophene-based compounds, such as ECB04 and compounds 2–3, with backbones similar to those of DTTO, suggests specific bioactivity of the latter in promoting endogenous fiber production. Clearly, the presence of two lateral phenyl groups in the molecular structure appears to be a necessary requirement to promote dye-protein co-assembly.
After treatment, animals were fixed with 4% paraformaldehyde and mounted on a microscopy slide. The movie shows the complex structure of fluorescent biofibers located at the base and at the tip of a tentacle. The movie was acquired on 200 Z-stacks, using a Leica THUNDER Imager 3D Cell Culture microscope, 40x
dry objective, NA = 0.95.
After treatment, animals were fixed with 4% paraformaldehyde and mounted on a microscopy slide. The movie shows green fluorescent biofibers located on two tentacles, presenting both linear and bent structure. The movie was acquired on 200 Z-stacks, using a Leica THUNDER Imager 3D Cell Culture microscope, 40x dry objective, NA = 0.95.
The efficiency of the biofiber production ranged from 35% (lowest dose, 24 h p.t.) up to 75% (highest dose, 72 h p.t.), indicating a variability among individuals and suggesting at the same time that the supramolecular dye-protein co-assembly could rely on an active mechanism (Figure 1J). The yield of this process (number of biofibers/Hydra) was shown as concentration and time dependent, ranging from less than 1 biofiber/Hydra produced by lowest DTTO dose (2.5 μg/mL) up to 19 ± 0.7 biofibers produced at 72 h in the presence of 50 μg/mL (Figure 1K and Table S1 for evaluation of statistical significance between all conditions). Relative images are shown in Figure S8. These data prompted us to investigate whether or not the mechanism of biofiber production involved any active metabolic pathway, such as the protein synthesis. Hydra polyps were pre-treated 2 h with cycloheximide, a known inhibitor of the protein synthesis (Obrig et al., 1971), before DTTO addition. The graph of Figure 1K shows a strong inhibition of the biofibers production, as only a few biofibers could be detected in treated polyps, suggesting the protein-based structure of the DTTO biofibers (Figure S9). Figure 2 displays the fluorescence microscopy images of cell suspensions obtained by maceration of treated animals. This procedure allows dissociating the animal in single cells, preserving their morphology for microscopic analysis. Short fluorescent fibers were detected inside nematocytes (Figure 2A), the stinging cells located in the tentacles, and body ectodermal cells (Figure 2B) indicating the capability of diverse cell types to initiate the assembling of these structures. Maceration of animals treated with compounds 2 and 3 did not reveal the presence of any biofiber but only fluorophore accumulation into vesicle-like structures, representing stable structures devoted to garbage storage, before external secretion (Figure S5). Long and highly structured biofibers were by contrast observed upon treatment with DTTO into the macerated solution but never included into the cells, suggesting either their degradation into small pieces due to the mechanical forces used for tissue maceration or that their growth and sterical structuring occurs during the secretion pathway. In Figure 2C laser scanning confocal microscopy images of several biofibers are reported, showing straight and bent motifs alternating and interlaced into the same biofiber, displaying large aspect ratios and an average length of 59 μm, in contrast with the structures formed by DTTO self-assembled in Hydra medium (13 μm), as determined in the graph of Figure S7. The two microstructures also showed a different photobehavior, biofibers being more stable (maintained their shape and high fluorescence up to two years from biosynthesis) and resistant to UV illumination, compared with structures spontaneously formed by DTTO in Hydra medium (Figure S7). Beside morphological characterization by fluorescence microscopy, optical and electrical properties of biofibers were characterized by a variety of techniques, including atomic force microscopy (AFM), electrostatic force microscopy (EFM), synchrotron fourier transform infrared micro-spectroscopy (IMS), X-ray photoelectron spectroscopy (XPS), UV-Vis, photoluminescence (PL), and circular dichroism (CD). Cells and floating fibers obtained by maceration of treated animals (5 h DTTO treatment and 72 h p.t.), concentrated by centrifugation, were used for all approaches and compared with macerates from untreated Hydra (control) and with supramolecular structures formed spontaneously by DTTO in animal-free medium.
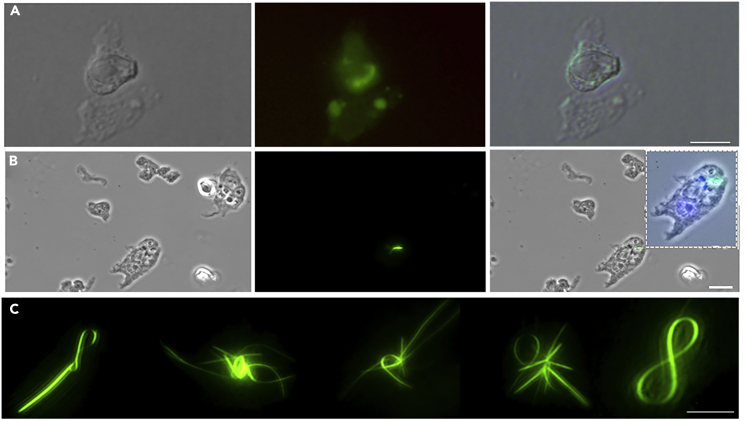
Figure 2.
Characterization of Fluorescent Biofibers from Macerated Hydra
Animals 72 h p.t. were dissociated into a suspension of single cells, fixed and imaged using phase contrast (left column) and fluorescence microscopy (central column). Right column displays the overlay of both images.
(A and B) (A) Picture of a nematocyte containing a green emitting fiber and (B) a group of cells, showing a fiber inside an ectodermal epithelial cell. Nuclei counterstained with DAPI appear in blue in the inset. Scale bars, 10 μm in A, 20 μm in B.
(C) Fluorescence microscopy of fibers present into the macerated solution. Several structures are formed, presenting both linear and bent motifs, of average length higher than biofibers.
See also Figure S7.
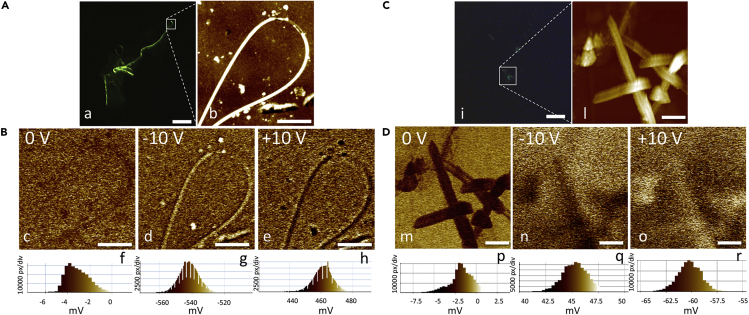
Atomic and Electrostatic Force Microscopy Characterization
EFM measures local Coulomb electrostatic interaction between the conductive tip and the sample, when a bias voltage is applied on the tip. Such a local interaction can be detected as oscillation amplitude and phase of the EFM probe (Rea et al., 2016, Kader et al., 2005). Green fluorescent fibers obtained from macerates were first localized by fluorescence microscopy and then the region of interest examined by AFM (Figure 3A, section a, b) and EFM (Figure 3B). AFM topographies on a 1 × 1 μm2 field are shown in Figure S10, where the morphology, a line profile, and pointed properties are presented for both a biofiber and a DTTO aggregate. For the biofiber the estimated sizes were about 60 nm high and 235 nm wide (Figure S10I). The EFM was performed on the same field of AFM, applying a bias voltage 0 V,−10 V, and +10 V. The amplitude EFM image without voltage application identifies shadowed features mirroring sample morphology (Figure 3B, section c, Figure S10), whereas, when bias voltage of −10 V and +10 V is applied a clear contrasted morphology becomes evident, with bright and dark zones mirroring the fiber morphology (Figure 3B, section d and e, respectively). The EFM amplitudes showed Gaussian distributions (Figure 3B, sections f–h) centered on an average level, which is directly proportional to the applied bias voltage. These features indicate the current flow correlated to a conducting or semiconducting behavior of the sample. For comparison, also the structures formed by aggregation of DTTO alone in Hydra medium at the same concentration over the same incubation time were examined. The results are reported in Figures 3C, 3D,and S10. These fibers show completely different features and electrical behavior. DTTO tends to self-assembly in round fibril-shaped aggregates. Figure 3C, image i, shows a very short fluorescent fibril and the region where the AFM and EFM characterizations were performed. AFM topography (Figure 3C, section l) shows shorter but wider fibrils about 3–5 μm long, 130 nm high, and 420 nm wide (see also Figure S10). Also in this case, the EFM images have been performed with a bias voltage 0 V,−10 V, and +10 V, respectively (Figure 3D, sections m, n, o, and Figure S10). In the amplitude EFM (Figure 3D, section m) the morphology of the control sample is clearly identified at 0 V bias voltage, whereas, when bias voltage of −10 V and +10 V is applied, the morphology becomes less clear and shows a kind of inverse polarization (Figure 3D, sections n, o), probably due to the opposite polarization of dipole distribution in an insulating behavior. Moreover, the EFM amplitude distributions for −10 V and +10 V are centered on average levels that are only slightly different from the average level at 0 V (Figure 3D, sections p, q, and r), indicating that the flowing current is similar for all applied voltages. Altogether this evidence shows a DTTO unique behavior featuring the biofibers, clearly distinguishable by the self-assembled DTTO, indicating a specific property added to the fiber by the cell machinery, in vivo.
Figure 3.
Morphological and Electrical Characterization of Biofibers
(A) Fluorescence image of (a) a biofiber deposited on a conductive substrate (scale bar, 20 μm). The white square shows the sub-region selected for AFM and EFM characterizations. (b) AFM topography showing a typical loop structure found in the biofibers (scale bar, 2 μm).
(B) EFM amplitude of the biofiber at bias voltage (c) 0 V, (d) −10 V, and (e) +10 V, respectively (scale bar, 2 μm). The morphology of the sample appears when a bias voltage is applied. EFM amplitude distribution at bias voltage: (f) 0 V, (g) −10 V, and (h) +10 V, respectively. The Gaussian distribution shows that the average level is proportional to the applied voltage, indicating the conducting or semiconducting nature of the sample.
(C) Fluorescence image (i) of a control fibril fragment, formed by aggregation of DTTO alone in Hydra medium, deposited on a conductive substrate (scale bar, 10 μm). The white square shows the sub-region selected for AFM and EFM characterizations. (l) AFM topography (scale bar, 1 μm).
(D) EFM amplitude of the control at bias voltage (m) 0 V, (n) −10 V, and (o) +10 V, respectively (scale bars, 1 μm). The morphology of the sample is clearer when bias voltage does not apply. EFM amplitude distribution at bias voltage: (p) 0 V, (q) −10 V, and (r) +10 V, respectively. The average level is quite the same for all applied voltage, indicating the insulating nature of the sample.
See also Figure S10.
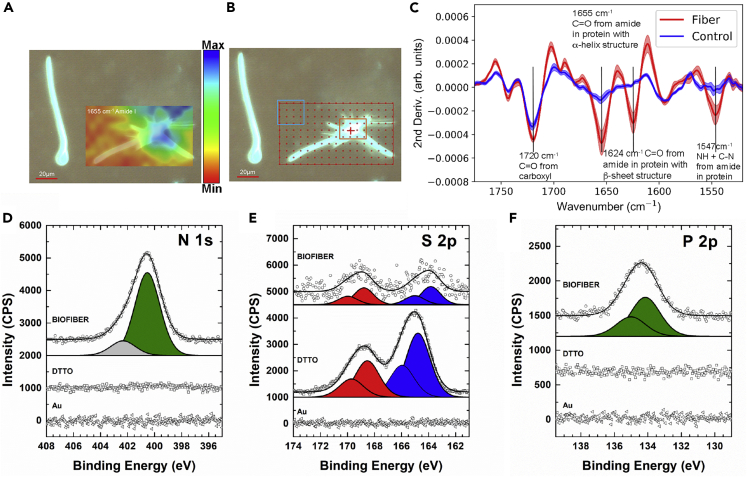
Fourier Transform Infrared Micro-spectroscopy
A macerated solution from DTTO-treated animals, containing fixed cells and floating biofibers, was analyzed by IMS using synchrotron light as source (Ling et al., 2011). The infrared spectrum of protein contains two characteristic absorption bands of particular pertinence to protein secondary structure, the absorptions associated to C=O stretching denoted as amide I (≈1655–1620 cm−1) and those associated to N-H bending denoted as amide II (1547 cm−1). The C=O and N-H functional groups involved in hydrogen bonding between protein moieties influence the positions of both amide I and amide II, hence these bands are sensitive to secondary structure composition. In particular, the position of amide I is a very useful predictor of protein secondary structure with band assignment regions for α-helix structure (1648–1657 cm−1) and β-sheet (1623-1641 cm−1) (Miller et al., 2013). The IMS results showed the protein-based nature of a biofiber, giving the characteristic amide I and amide II absorption bands. From Figure 4A it can be observed that the amide I absorbance (1655 cm−1) corresponds to the location of the biofibers. In Figure 4C the comparison of extracted IR spectra from fiber and outside the fiber provides more details about the protein structure composed of mixed α-helix and β-sheet as evidenced by the intense absorptions in the fiber spectrum at 1655 cm−1 and 1624 cm−1. IMS has been successfully used for investigations in cells and tissue where the same absorption bands from amides identified in this study (in particular amide I) have been used to determine protein misfolding and aggregation of amyloid plaques, infectious prion proteins, α-synuclein, and tau proteins (Miller et al., 2013).
Figure 4.
Synchrotron Infrared Microspectroscopy and X-ray Photoelectron Spectroscopy of a Single Green Fluorescent Biofiber
(A) Image of a UV illuminated biofiber and IMS image at 1655cm−1 showing the distribution of amide I bond from protein. For interpretation of the amide I bond signal intensity, a pseudocolor scale bar is introduced, where blue represents the highest intensity and red the lowest. Scale bar, 20 μm.
(B) The red box is the mesh used for IR mapping, the blue box is the control region, and the orange box is the biofiber region where IR spectra were extracted. Scale bar, 20 μm.
(C) Averaged second derivative IR spectra of the control and fiber regions.
(D–F) (D) N 1s, (E) S 2p, and (F) P 2p XPS spectra of Ar+ sputtered Au (triangles), DTTO on Au (squares), and biofibers (circles). S 2p presents two doublets associated to S=O bond (red) and S-C bond (blue). In all peaks the background was subtracted and a constant value added to order the samples. S 2p and P 2p were fitted by doublets in fixed energy shift (2p3/2 and 2p1/2). Signal of S 2p of biofibers was multiplied by a factor 10. See also Table S2.
X-Ray Photoelectron Spectroscopy
In order to have evidence of the supramolecular incorporation of DTTO inside proteins XPS measurements were performed on pure DTTO and biofibers from Hydra macerates. The results are reported in Figure 4 and Table S2. The S 2p peak is a fingerprint for DTTO on Au substrates and the presence of two doublets of S 2p3/2, i.e. S=O group at 168.5 eV and S-C group at 164.8 eV, is in excellent agreement with the spectra reported in previous work on oxidized thiophene-based materials (Figure 4E) (Di Maria et al., 2017). Moreover, the stoichiometric ratio between Sulphur-Carbon (S-C)- and Sulphur-Oxygen (S=O)-bonded species was close to the expected value of 2 (1.8 ± 0.2). The XPS spectrum of the biofibers from Hydra macerates deposited on a gold substrate confirmed the presence of the S 2p3/2 peak, with the S=O component at 168.5 eV and the S-C component slightly reduced at 163.8 eV probably due to fiber formation. Additionally, the chemical shift of N 1s at 400.5 eV, related to the protein material contained in the fiber, was observed together with that of P 2p3/2 at 134.2 eV, compatible with phosphate groups (P=O) associated to the phospholipid bilayer (Figures 4D and 4F) (Wagner et al., 1997). The presence of significant amounts of protein and phospholipid materials justifies the low S 2p signal from biofibers (0.25%, Table S2). This suggests that inside biofibers DTTO is surrounded by a layer of protein and phospholipid materials with a thickness in the same order of magnitude of the XPS probe depth (up to 10 nm).
Optical Characterization
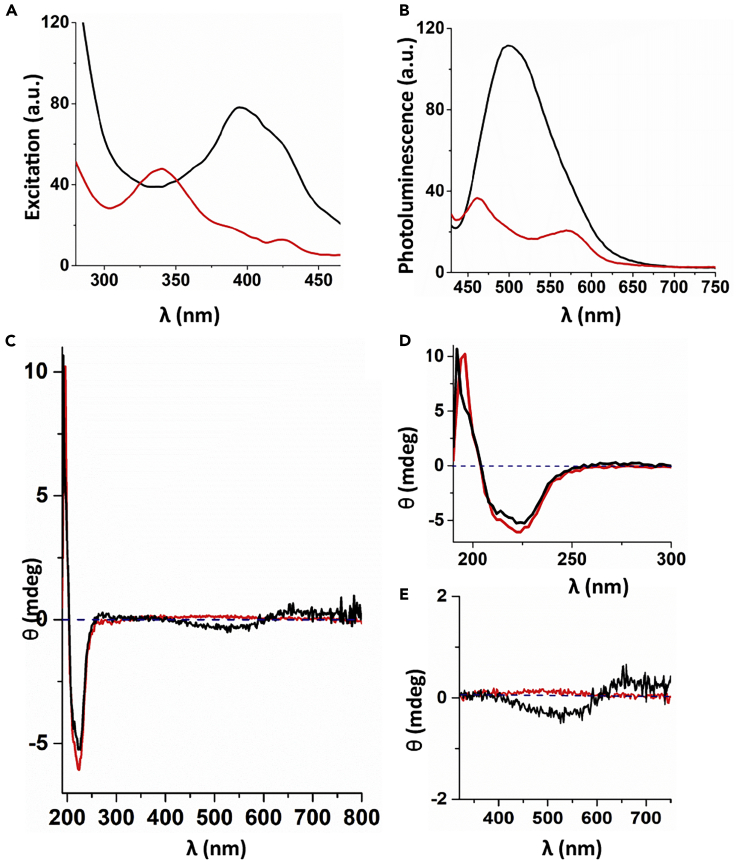
The photophysical characterization of the biofibers produced by living animals was obtained by UV-vis, photoluminescence (PL), and circular dichroism spectroscopy (CD) and compared with those produced by DTTO self-aggregated in organic solvent and Hydra medium. Table 1 shows that λexc,λPL and the lifetimes τ1 and τ2 of DTTO self-assembled in Hydra medium are deeply different from those measured in macerates containing isolated biofibers. Particularly significant is the value of the fluorescence anisotropy, which passes from 0 for the former to 0.25 for the latter. The large value of fluorescence anisotropy in sample A (biofiber) suggests that DTTO is embedded into a highly anisotropic supramolecular structure. Figure 5 reports the excitation, PL, and CD spectra of macerates containing isolated biofibers (black plot) and the macerates of untreated Hydra used as a control (red plot). The absorption spectra of the samples are characterized by a large amount of scattering hiding the absorption signals (Figure S10). Thus, the excitation spectra—displaying the frequencies generating the photoluminescence spectrum and corresponding to the frequencies absorbed by the system—were measured applying a fixed excitation wavelength of 500 nm (Figure 5A). The comparison of DTTO parameters relative to the biofibers with those of DTTO in methylene chloride (Figure S10) shows unambiguously the signature of the dye in the fibers. In particular, the photoluminescence spectra of the samples indicate a Stokes shift between absorption and emission spectra amounting up to 100 nm, a value in line with what has already been observed for DTTO in organic solution (Palama et al., 2011).
Table 1.
Photophysical Characterization of Biofibers Formed in Hydra and DTTO Aggregates Formed in Culture Medium
| Sample | λabs (nm) | λexc (nm)a | λPL (nm)b | τ1 (ns)c | τ2 (ns)c | Fluorescenced Anisotropy |
|---|---|---|---|---|---|---|
| Ae | – | 395 | 500 | 2.2 | 8.1 | 0.25 |
| Bf | 408 | – | 514 | 16.17 | – | ~0 |
| Cg | – | 350 | 425;575 | 1.2 | 4.4 | ~0 |
Characterization was performed on (A) biofibers, (B) DTTO in methylene chloride, and (C) DTTO aggregated in Hydra medium.
Excitation spectrum obtained with a fixed emission wavelength of 500 nm.
Maximum of photoluminescence emission (λexc = 395 nm).
Fluorescence lifetimes.
Steady state fluorescence anisotropy.
Cell suspension and floating biofibers.
DTTO in methylene chloride (molar extinction coefficient ε = 18.000 M−1cm−1; fluorescence quantum yield = 0.83).
DTTO self-assembled in Hydra medium (CaCl2 1mM, 0.1mM NaHCO3).
Figure 5.
Optical Characterization of DTTO-Based Biofibers
(A) Excitation spectra obtained by irradiating at 500 nm.
(B and C) (B) Photoluminescence spectra and (C) CD spectra relative to a cell suspension of biofibers (black line) and macerates of untreated Hydra (red line).
(D and E) (D) Enlargements of CD spectra (C) in the 180–300 nm and (E) in the 350–750 nm regions.
See also Figure S10.
Figure 5C shows the CD spectrum of the macerates containing biofibers presenting a signal with negative Cotton effect in the region 190–240 nm and a less intense signal in the region 400–600 nm, indicating chiral content in both regions of the sample. On the contrary, the control sample (untreated Hydra) displayed only one signal with negative Cotton effect in the region 200–220 nm superimposable to that of the treated Hydra. The CD signal in the 190–240 nm region (see magnification Figure 5D) is mainly due to protein peptide bond with an n→π∗ transition centered around 220 nm and a π→π∗ transition around 190 nm (Greenfield, 2006, Holzwarth and Doty, 1965, Kelly et al., 2005). The less intense signal observed in DTTO treated Hydra in the region 400–600 nm (see magnification Figure 5E) corresponds to the absorption region of DTTO. CD spectroscopy is extensively used to study the secondary structure of proteins in solution, which is very sensitive to the environment, temperature, or binding interactions with other molecules and can furnish structural, kinetic, and thermodynamic information (Kelly et al., 2005, Greenfield, 2006, Holzwarth and Doty, 1965). The CD signal in the region 190–220 nm is characteristic of α-helix protein secondary structure, whereas the signal in the region 400–600 nm is a strong indication of the presence of DTTO embedded into the supramolecular structure of some proteins. Indeed, it is well known that in addition to the intrinsic CD of the protein backbone, small molecules interacting with the proteins show extrinsic CD bands indicative of such an interaction (Kelly et al., 2005, Greenfield, 2006, Holzwarth and Doty, 1965, Di Maria et al., 2014). Thus, although DTTO is an intrinsically achiral molecule, the proximity of the proteins causes the appearance of an induced CD signal pertaining to the fluorophore. Moreover, it cannot be excluded that such a signal also reflects the chiral supramolecular organization of the dye driven by the proteins themselves.
In an attempt to identify the protein composition of the biofiber we used Hydra anti-collagen-specific antibody in immunolocalization experiments on macerates from DTTO-treated Hydra. Results failed to detect any cross-reactivity (Figure S6C), opening the possibility that other proteins may be bound to the DTTO monomer structuring the fluorescent biofiber. We cannot rule out the possibility that the epitope can be masked due to DTTO bonding or due to the tissue maceration process.
Discussion
Our data unambiguously indicate that DTTO is biocompatible and inside Hydra tissue spontaneously co-assembles with proteins giving rise to the formation of fluorescent and conductive microfibers. Interestingly, the biofiber formation is specific for DTTO, as other oligotiophene derivatives were not able to produce them. We have already observed the spontaneous co-assembly of DTTO with proteins inside specific cell lines such as living fibroblasts of different origin, where DTTO is incorporated within type-I collagen's triple helix (Palama et al., 2011), and mouse neuroblastoma cells (B104), where the fluorophore is incorporated within vimentin's supramolecular structure (Palama et al., 2015). Most probably, in the present case bundles of microfibers are spontaneously formed where DTTO is incorporated within different types of cells. This hypothesis is supported by the Hydra anatomy, composed of three stem cell lineages, i.e. ectoderm, endoderm, and interstitial stem cell lineage, including self-renewing cells and differentiating products, such as gland cells, gametes, nematocytes, and neurons. After maceration of DTTO-treated animals the fluorescent microfibers could be detected in epitheliomuscular cells and nematocytes, suggesting that they might be formed by a variety of proteins coming from different cellular types rather than from a single cell type, and this hampers the identification of a unique protein into the fiber. This raises questions about the mechanism of formation of the microfibers, in particular about (1) the recognition process of the dye by the different proteins and (2) about the molecular and supramolecular processes taking place separately or simultaneously as in the case of spider silk and live cells (Rising and Johansson, 2015, Palama et al., 2011). As to the first point, a primary role is certainly played by the SO2 group of the fluorophore with two oxygens capable of multiple interactions with neighboring hydrogens, as suggested by previous calculations on a model simulating a collagen strand (Palama et al., 2011). As to the second point, the biofibers showing higher complexity and size were detected in Hydra macerates either as floating fibers, or bound to the external part of tissue fragments deriving from tentacles, suggesting that the growth and supramolecular structuring may take place specifically in the tentacles and during the secretion process. However, further investigations are required before a satisfying interpretation can be given on both points. Inside cells only short fluorescent fibers were found inside cells; however, we could not rule out that mechanic forces employed for animal dissociation affect stability of supramolecular folding and cause fiber fragmentation. Due to the lack of protocols to immortalize and culturing Hydra cells in vitro, the maceration of whole animal represents, up to date, a valuable and broadly used method to analyze single cells and structures released from the tissue bilayer architecture. Following this approach, we performed accurate characterization of fibrils, both inside cells and on the external side of cells or tentacles, yet preserving their spectacular fluorescence. The conductive behavior of biofibers makes this spontaneous process very fascinating and suggests our model as a new living bioreactor for the production of electroactive materials.
It would be difficult to assign a precise organization at the nanoscale to the proteins’ supramolecular structure embedding DTTO. Coexistence of α-helix and β-sheet secondary structures is common in natural fibrillar proteins (Rising and Johansson, 2015), and our IMS data indicate that both secondary structures are present in the fluorescent biofibers. Although our previous work on live cells indicates that DTTO is embedded within the α-helix, we do not have any experimental evidence that DTTO can also be embedded into β-sheets. So we cannot say whether the observed fluorescence comes exclusively from α helices or also from β-sheet configuration.
By using the protein synthesis inhibitor cycloheximide we could prevent biofiber production demonstrating that DTTO drives the formation of hybrid protein-dye microfibers through protein synthesis cell machinery. The microfibers obtained by DTTO self-assembly in Hydra medium are morphologically and electrically deeply different from the biofibers. In particular, they are fluorescent but insulating supramolecular structures.
DTTO is a semiconducting conjugated molecule and as most thiophene-based oligomers may display electrical conductivity when organized into appropriate supramolecular structures. Recent evidence reports on hybrid oligothiophenes self-organized in conducting wires along the vascular tissue of a rose plant (Stavrinidou et al., 2017). Here, the supramolecular structure achieved in a living animal by DTTO monomers shows electrical conductivity, as demonstrated by EFM measurements. The incorporation of fluorescence and conductive properties into endogenous building blocks, in part or totally made of proteins, led to the production of novel structures, according to precise dose and time. Under these conditions the DTTO does not interfere with animal viability, suggesting that the fluorophore does not affect biomolecule function.
In conclusion, we show that a simple invertebrate presenting a tissue grade of organization when soaked with a non-toxic fluorescent/semiconducting dye produces fluorescent electroactive protein-dye microfibers having prevalently coiled-coil and a significant contribution of β-sheet secondary structure. By merging multiple techniques, from in vivo imaging and single cell analysis, up to optical, spectroscopic, and electrical characterization we demonstrate the possibility to modify endogenous biomolecules into novel hybrid structures showing superior properties. One of the most important applications we foresee for these fluorescent microfibers is their use as biocompatible electroactive scaffolds for bioengineering and tissue regeneration. In particular, biocompatible and biodegradable scaffolds with protein fibers can be used as potentially implantable devices and may contribute to stimulating and controlling neural or muscle activities under electrical stimulation and effectively guide tissue repair. To the best of our knowledge, only a few examples have been reported in which the spontaneous assembly of exogenous organic components has been triggered in whole animals in very complicated environments (Zhao et al., 2018, Zhang et al., 2015, He et al., 2019). The possibility to chemically control, in vivo, the formation of micrometer-sized supramolecular architectures provides a new way to bestow additional properties upon the system and has important fundamental as well as applicative implications.
Limitation of the Study
Model organisms allow prediction studies, testing of chemical compounds, and discovering processes and mechanisms of action but also present innate constrains that limit their use. We demonstrated with Hydra the possibility to produce new microstructures modifying in situ endogenous proteins, but more functional studies are necessary to evaluate the impact on physiological processes, with or without electrical stimulation, or the induction of new ones. Moreover, both fiber purification and homogeneity need to be implemented. Large-scale purification of the microfibers from the animal tissues is hampered by the tight embedding into membrane/external cuticle, possibly due to chemical composition preventing biofiber release into the medium. Moreover, homogeneity is not possible as biofibers are produced by different cell types, possibly co-assembling with different proteins. Commercially available antibodies raised against vertebrates and antigens would not easily cross-react with Hydra proteins, which justified our use of Hydra-specific antibody for immunolocalization. In a future perspective the development of DTTO delivery methods alternative to soaking may drive the homogeneous biofiber production from a specific tissue/cell type.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
M.M. acknowledges financial support from the European Union’s Horizon 2020 research and Innovation program Marie Sklodowska-Curie grant agreement No. 660228 and the Spanish Juan de la Cierva program. C.T. and H.C. acknowledge European Synchrotron Radiation Facility for access to beam time on the ID21 beam line (experiment MA3261). C.T. acknowledges Prof. Xiaoming Zhang (Baylor College of Medicine, Houston) for the kind gift of Hydra anti-collagen type I antibody. F.D.M. acknowledges financial support from the project Molecular Nanotechnologies for Human Health and Environment (PON R&C 611 2007–2013, code PON02_00563_3316357) and the EU project INFUSION (Proposal number: 734834).
Author Contributions
C.T., A.T., M.M., and G.B. conceived this project and provided general supervision. M.M., A.T., G.T., and M.B. performed all experiments with Hydra vulgaris. F.D.M. and M.Z. performed optical characterization and data analysis, P.D. and L.D.S. performed electrical characterization, H.C.M. and A.K. performed spectroscopic characterization. All authors analyzed and discussed the data and contributed to drafting and editing the manuscript. C.T., M.M., and G.B. finalized the manuscript.
Declaration of Interests
The authors declare no competing financial interest.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101022.
Supplemental Information
References
- Allocca M., Mattera L., Bauduin A., Miedziak B., Moros M., De Trizio L., Tino A., Reiss P., Ambrosone A., Tortiglione C. An integrated multilevel analysis profiling biosafety and toxicity induced by indium- and cadmium-based quantum dots in vivo. Environ. Sci. Technol. 2019;53:3938–3947. doi: 10.1021/acs.est.9b00373. [DOI] [PubMed] [Google Scholar]
- Ambrosone A., Marchesano V., Carregal-Romero S., Intartaglia D., Parak W.J., Tortiglione C. Control of Wnt/beta-catenin signaling pathway in vivo via light responsive capsules. ACS Nano. 2016;10:4828–4834. doi: 10.1021/acsnano.5b07817. [DOI] [PubMed] [Google Scholar]
- Di Maria F., Fabiano E., Gentili D., Biasiucci M., Salzillo T., Bergamini G., Gazzano M., Zanelli A., Brillante A., Cavallini M. Polymorphism in crystalline microfibers of achiral octithiophene: the effect on charge transport, supramolecular chirality and optical properties. Adv. Funct. Mater. 2014;24:4943–4951. [Google Scholar]
- Di Maria F., Zanelli A., Liscio A., Kovtun A., Salatelli E., Mazzaro R., Morandi V., Bergamini G., Shaffer A., Rozen S. Poly(3-hexylthiophene) nanoparticles containing thiophene-S,S-dioxide: tuning of dimensions, optical and redox properties, and charge separation under illumination. ACS Nano. 2017;11:1991–1999. doi: 10.1021/acsnano.6b08176. [DOI] [PubMed] [Google Scholar]
- Frezzo J.A., Montclare J.K. Exploring the potential of engineered coiled-coil protein microfibers in drug delivery. Ther. Deliv. 2015;6:643–646. doi: 10.4155/tde.15.19. [DOI] [PubMed] [Google Scholar]
- Galliot B., Schmid V. Cnidarians as a model system for understanding evolution and regeneration. Int. J. Dev. Biol. 2002;46:39–48. [PubMed] [Google Scholar]
- Greenfield N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006;1:2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.P., Li X.D., Wang L., Wang H. Bispyrene-based self-assembled nanomaterials: in vivo self-assembly, transformation, and biomedical effects. Acc. Chem. Res. 2019;52:367–378. doi: 10.1021/acs.accounts.8b00398. [DOI] [PubMed] [Google Scholar]
- Hobmayer B., Jenewein M., Eder D., Eder M.K., Glasauer S., Gufler S., Hartl M., Salvenmoser W. Stemness in Hydra - a current perspective. Int. J. Dev. Biol. 2012;56:509–517. doi: 10.1387/ijdb.113426bh. [DOI] [PubMed] [Google Scholar]
- Holstein T.W., Hobmayer E., Technau U. Cnidarians: an evolutionarily conserved model system for regeneration? Dev. Dyn. 2003;226:257–267. doi: 10.1002/dvdy.10227. [DOI] [PubMed] [Google Scholar]
- Holzwarth G., Doty P. The ultraviolet circular dichroism of polypeptides. J. Am. Chem. Soc. 1965;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- Hume J., Sun J., Jacquet R., Renfrew P.D., Martin J.A., Bonneau R., Gilchrist M.L., Montclare J.K. Engineered coiled-coil protein microfibers. Biomacromolecules. 2014;15:3503–3510. doi: 10.1021/bm5004948. [DOI] [PubMed] [Google Scholar]
- Kader M.A., Choi D., Lee S.K., Nah C. Morphology of conducting filler-reinforced nitrile rubber composites by electrostatic force microscopy. Polym. Test. 2005;24:363–366. [Google Scholar]
- Kamada A., Mittal N., Soderberg L.D., Ingverud T., Ohm W., Roth S.V., Lundell F., Lendel C. Flow-assisted assembly of nanostructured protein microfibers. Proc. Natl. Acad. Sci. U S A. 2017;114:1232–1237. doi: 10.1073/pnas.1617260114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.M., Jess T.J., Price N.C. How to study proteins by circular dichroism. Biochim.Biophys. Acta Proteins Proteom. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Ling S.J., Qi Z.M., Knight D.P., Shao Z.Z., Chen X. Synchrotron FTIR microspectroscopy of single natural silk fibers. Biomacromolecules. 2011;12:3344–3349. doi: 10.1021/bm2006032. [DOI] [PubMed] [Google Scholar]
- Marchesano V., Hernandez Y., Salvenmoser W., Ambrosone A., Tino A., Hobmayer B., M De La Fuente J., Tortiglione C. Imaging inward and outward trafficking of gold nanoparticles in whole animals. ACS Nano. 2013;7:2431–2442. doi: 10.1021/nn305747e. [DOI] [PubMed] [Google Scholar]
- McNamara M.C., Sharifi F., Wrede A.H., Kimlinger D.F., Thomas D.G., Vander Wiel J.B., Chen Y.F., Montazami R., Hashemi N.N. Microfibers as physiologically relevant platforms for creation of 3D cell cultures. Macromol. Biosci. 2017;17:10. doi: 10.1002/mabi.201700279. [DOI] [PubMed] [Google Scholar]
- Miller L.M., Bourassa M.W., Smith R.J. FTIR spectroscopic imaging of protein aggregation in living cells. Biochim. Biophys. Acta. 2013;1828:2339–2346. doi: 10.1016/j.bbamem.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moros M., Kyriazi M.E., El-Sagheer A.H., Brown T., Tortiglione C., Kanaras A.G. DNA-coated gold nanoparticles for the detection of mRNA in live Hydra vulgaris animals. ACS Appl. Mater. Interfaces. 2018;11:13905–13911. doi: 10.1021/acsami.8b17846. [DOI] [PubMed] [Google Scholar]
- Palama I., Di Maria F., Viola I., Fabiano E., Gigli G., Bettini C., Barbarella G. Live-cell-permeant thiophene fluorophores and cell-mediated formation of fluorescent fibrils. J. Am. Chem. Soc. 2011;133:17777–17785. doi: 10.1021/ja2065522. [DOI] [PubMed] [Google Scholar]
- Obrig T.G., Culp W.J., Mckeehan W.L., Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J. Biol. Chem. 1971;246:174–181. [PubMed] [Google Scholar]
- Palama I.E., Di Maria F., D'amone S., Barbarella G., Gigli G. Biocompatible and biodegradable fluorescent microfibers physiologically secreted by live cells upon spontaneous uptake of thiophene fluorophore. J. Mater. Chem. B. 2015;3:151–158. doi: 10.1039/c4tb01562b. [DOI] [PubMed] [Google Scholar]
- Rea I., Terracciano M., Chandrasekaran S., Voelcker N.H., Dardano P., Martucci N.M., Lamberti A., De Stefano L. Bioengineered silicon diatoms: adding photonic features to a nanostructured semiconductive material for biomolecular sensing. Nanoscale Res. Lett. 2016;11:405. doi: 10.1186/s11671-016-1624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rising A., Johansson J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015;11:309–315. doi: 10.1038/nchembio.1789. [DOI] [PubMed] [Google Scholar]
- Stavrinidou E., Gabrielsson R., Gomez E., Crispin X., Nilsson O., Simon D.T., Berggren M. Electronic plants. Sci. Adv. 2015;1:e1501136. doi: 10.1126/sciadv.1501136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrinidou E., Gabrielsson R., Nilsson K.P., Singh S.K., Franco-Gonzalez J.F., Volkov A.V., Jonsson M.P., Grimoldi A., Elgland M., Zozoulenko I.V. In vivo polymerization and manufacturing of wires and supercapacitors in plants. Proc. Natl. Acad. Sci. U S A. 2017;114:2807–2812. doi: 10.1073/pnas.1616456114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortiglione C., Antognazza M.R., Tino A., Bossio C., Marchesano V., Bauduin A., Zangoli M., Morata S.V., Lanzani G. Semiconducting polymers are light nanotransducers in eyeless animals. Sci. Adv. 2017;3:e1601699. doi: 10.1126/sciadv.1601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortiglione C., Quarta A., Malvindi M.A., Tino A., Pellegrino T. Fluorescent nanocrystals reveal regulated portals of entry into and between the cells of Hydra. PLoS One. 2009;4:e7698. doi: 10.1371/journal.pone.0007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola I., Palama I.E., Coluccia A.M.L., Biasiucci M., Dozza B., Lucarelli E., Di Maria F., Barbarella G., Gigli G. Physiological formation of fluorescent and conductive protein microfibers in live fibroblasts upon spontaneous uptake of biocompatible fluorophores. Integr. Biol. 2013;5:1057–1066. doi: 10.1039/c3ib40064f. [DOI] [PubMed] [Google Scholar]
- Wagner C.D., Allison J.W., Rumble J.R., Jr, Powell C.J. National Institute of Standards and Technology (NIST); Gaithersburg, MD: 1997. SRD-20 X-ray Photoelectron Spectroscopy Database. (version 2.0) [Google Scholar]
- Xu Z.P., Shi L.Y., Yang M.Y., Zhang H.P., Zhu L.J. Fabrication of a novel blended membrane with chitosan and silk microfibers for wound healing: characterization, in vitro and in vivo studies. J. Mater. Chem. B. 2015;3:3634–3642. doi: 10.1039/c5tb00226e. [DOI] [PubMed] [Google Scholar]
- Zhang D., Qi G.B., Zhao Y.X., Qiao S.L., Yang C., Wang H. In situ formation of nanofibers from purpurin18-peptide conjugates and the assembly induced retention effect in tumor sites. Adv. Mater. 2015;27:6125–6130. doi: 10.1002/adma.201502598. [DOI] [PubMed] [Google Scholar]
- Zhao M.Z., Cheng D.B., Shang Z.R., Wang L., Qiao Z.Y., Zhang J.P., Wang H. An “in vivo self-assembly” strategy for constructing superstructures for biomedical applications. Chin. J. Polym. Sci. 2018;36:1103–1113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After treatment, animals were fixed with 4% paraformaldehyde and mounted on a microscopy slide. The movie shows the complex structure of fluorescent biofibers located at the base and at the tip of a tentacle. The movie was acquired on 200 Z-stacks, using a Leica THUNDER Imager 3D Cell Culture microscope, 40x
dry objective, NA = 0.95.
After treatment, animals were fixed with 4% paraformaldehyde and mounted on a microscopy slide. The movie shows green fluorescent biofibers located on two tentacles, presenting both linear and bent structure. The movie was acquired on 200 Z-stacks, using a Leica THUNDER Imager 3D Cell Culture microscope, 40x dry objective, NA = 0.95.