Abstract
Multi-target drugs can better address the cascade of events involved in oxidative stress and the reduction in cholinergic transmission that occur in Alzheimer’s disease than cholinesterase inhibitors alone. We synthesised a series of 3-arylbenzofuranone derivatives and evaluated their antioxidant activity, cholinesterase inhibitory activity, and monoamine oxidase inhibitory activity. 3-Arylbenzofuranone compounds exhibit good antioxidant activity as well as selective acetylcholinesterase inhibitory activity. The IC50 value of anti-acetylcholinesterase inhibition of Compound 20 (0.089 ± 0.01 μM) is similar to the positive drug donepezil (0.059 ± 0.003 μM). According to the experimental results, Compounds 7, 13 show a certain effect in the in vitro evaluation performed and have the potential as drug candidates for the treatment of Alzheimer’s disease.
Keywords: 3-Arylbenzofuranone derivatives, Alzheimer’s disease, cholinesterase inhibitors, monoamine oxidase inhibitors, antioxidant activity
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder characterised by the destruction of nerve cells, the rapid deterioration of memory and other important cognitive functions1. The two major hallmarks of AD are intracellular neurofibrillary tangles composed of hyperphosphorylated T protein2 and senile plaques containing aggregated amyloid β-peptide (Aβ)3. There are other factors that cause synaptic dysfunction and neurodegeneration, which leads to a decrease in neurotransmitter acetylcholine (ACh) levels4, leading to memory and cognitive deficits5. Monoamine oxidase (MAO) is one of the several enzymes that contribute to the behavioural and psychological symptoms of dementia in AD6. Accumulating evidence indicates a close contact between several enzymes and AD. Cholinesterases (ChEs) and MAOs are closely related to the disease symptomatology and progression.
ChEs, enzymes that terminate cholinergic neurotransmission in the brain, act by catalysing the hydrolysis of ACh, so their inhibition can be used to alleviate memory and cognitive deficits in AD. Two types of ChEs, acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), are known. The difference between the two types is related to their respective preference for the substrate: the former hydrolyses ACh more rapidly; the latter hydrolyses butyrylcholine more rapidly. Experimental studies have found that the maintenance of AChE/BuChE activity ratio in the healthy brain can improve the symptoms of AD7. Inhibition of AChE can result in an increase in BuChE activity, which causes hydrolysis of AChE in a novel manner. Therefore, a double ChE inhibitor may be a more effective anti-AD drug. MAO has a major role in brain development and function, and its inhibitors have found clinically as antidepressant and anti-Parkinsonian drugs6. MAO regulates the concentration of exogenous and endogenous amines (including neurotransmitters) in the central nervous system and peripheral tissues, which exacerbates subsequent oxidative stress and neurodegeneration.
Benzofuranone and its derivatives are important heterocyclic compounds and are widely used in pesticides, dyes, foods, polymer processing, and other fields. Benzofuranone and its derivatives have rich biological activities, including anticholinesterase8, antibacterial9, anti-HIV10, anti-allergic and anti-inflammatory activity11, antioxidant, antinociceptive activity12, and other activity, it can be used as a substrate for β-lactamase13 and insulin amyloid fibrosis inhibitor14 and is also potential antipsychotics15. The current study describes the preparation and in vitro activity of 3-arylbenzofuranone derivatives as ChE inhibitors, MAO B inhibitors, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavengers. The purpose of the research is to screen compounds that inhibit ChE and MAO, which have the potential for the treatment of AD and other neurodegenerative diseases.
Experimental
Synthesis
Materials and methods
Melting points were determined using a Thiele tube and were uncorrected. The 1HNMR and 13CNMR spectra were recorded with a Bruker AM-600 spectrometer (Billercia, MA, USA) with TMS as the internal standard. Chemical shifts were reported at room temperature on a scale (ppm) with DMSO-d6 as the solvents and J values are given in Hertz. Mass spectra were obtained with an Agilent Trap VL LC/MS spectrometer (Santa Clara, CA, USA). The absorbance was recorded by RZ-9618 Microplate Reader. Unless otherwise noted, all solvents and reagents were commercially available and used without further purification.
General method for synthesis of compounds 3a-3d
Taking the synthesis of 3, 4, 5-trimethoxy mandelic acid as an example. Other mandelic acids were obtained using the same procedures. 3,4,5-Trimethoxybenzaldehyde 39.2 g (0.2 mol), TBAB 3.2 g (10 mmol), and chloroform 240 ml were added to a 500 ml three-necked flask equipped with a dropping funnel and a reflux condenser. The mixture was thoroughly stirred to completely dissolve, and the temperature was raised to 40 °C. A 50% NaOH solution (40 g of NaOH dissolved in 40 g of water) was slowly added dropwise through a dropping funnel to maintain a temperature of 45–50 °C. After the TLC detection reaction was completed, it was allowed to stand for cooling and suction filtration. The filter cake was washed with chloroform 40 ml × 3. The resulting solid mixture was acidified with hydrochloric acid, extracted with ethyl acetate, dried over anhydrous sodium sulphate, concentrated, and recrystallisation from ethyl acetate/petroleum ether gave a white solid (yield: 70.5%).
General method for the synthesis of 3-arylbenzofuranone 1–23
Taking the synthesis of 6-hydroxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone as an example. Other 3-arylbenzofuranone compounds were obtained using the same procedures. 3,4,5-Trimethoxymandelic acid 4.84 g (20 mmol), resorcin 2.64 g (24 mmol), and boron trifluoride-diethyl ether 20 ml were added to a 100 ml three-necked flask equipped with a reflux condenser and a drying tube. The raw material was stirred well to completely dissolve, and maintain the temperature at 30–35 °C continuous stirring. After the TLC detection reaction was completed, the reaction was allowed to stand for cooling. The reaction solution was poured into a beaker containing 100 ml of ice water and thoroughly stirred. After a large amount of white solid was precipitated, it was allowed to stand, and suction filtered. The filter cake was washed with saturated sodium bicarbonate solution, then washed with distilled water until near neutral, dried to give a pale pink solid, and recrystallised from methanol to yield white solid (yield: 92.72%).
6-Hydroxyl-3–(4′-methoxyphenyl)-benzofuranone (1). White solid, yield 94.14%, m.p. 188–190 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 7.11 (s, 2H), 7.02 − 6.86 (m, 3H), 6.63 (d, J = 46.1 Hz, 2H), 5.13 (s, 1H), 3.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.19 (s), 158.82 (s), 158.30 (s), 154.05 (s), 129.30 (s), 128.36 (s), 125.61 (s), 117.82 (s), 114.38 (s), 111.32 (s), 98.24 (s), 55.14 (s), 47.84 (s), 39.94 (s), 39.80 (s), 39.66 (s), 39.60 − 39.11 (m), 39.10 (s), 39.09 − 38.90 (m). MS: m/z (%) [M + Na]+ 278.9.
5-Hydroxyl-3–(4′-methoxyphenyl)-benzofuranone (2). White solid, yield 95.12%, m.p. 164–165 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.38 (s, 1H), 7.13 − 7.08 (m, 3H), 6.96 − 6.93 (m, 2H), 6.74 (ddd, J = 8.7, 2.6, 0.7 Hz, 1H), 6.55 (dd, J = 2.5, 0.9 Hz, 1H), 5.23 (s, 1H), 3.75 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.09 (s), 158.85 (s), 154.32 (s), 145.74 (s), 129.40 (s), 128.99 (s), 127.94 (s), 115.10 (s), 114.44 (s), 111.74 (s), 111.02 (s), 55.15 (s), 48.89 (s), 40.02 (d, J = 11.3 Hz), 39.98 − 39.62 (m), 39.55 (s), 39.52 (s), 39.38 (s), 39.29 (s), 39.17 (d, J = 21.0 Hz). MS: m/z (%) [M + Na]+ 279.0.
6-Methoxy-3–(4′-methoxyphenyl)-benzofuranone (3). White solid, yield 85.32%, m.p. 156–157 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.10 (dd, J = 11.4, 4.6 Hz, 3H), 6.96 − 6.92 (m, 3H), 6.75 (dd, J = 8.3, 2.3 Hz, 1H), 5.20 (s, 1H), 3.79 (s, 3H), 3.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.03 (s), 160.14 (s), 158.87 (s), 154.16 (s), 129.33 (s), 128.13 (s), 125.62 (s), 119.61 (s), 114.42 (s), 110.19 (s), 97.22 (s), 55.66 (s), 55.15 (s), 47.86 (s), 39.87 (d, J = 21.0 Hz), 39.66 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 293.0, [M + H]+ 271.0.
5-Methoxy-3–(4′-methoxyphenyl)-benzofuranone (4). White solid, yield 76.32%, m.p. 126–127 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.23 (d, J = 8.8 Hz, 1H), 7.12 (d, J = 8.7 Hz, 2H), 6.97 − 6.92 (m, 3H), 6.79 − 6.74 (m, 1H), 5.28 (s, 1H), 3.75 (s, 3H), 3.70 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.92 (s), 158.90 (s), 156.37 (s), 146.97 (s), 129.46 (s), 129.13 (s), 127.74 (s), 114.47 (s), 114.22 (s), 111.11 (s), 110.70 (s), 55.68 (s), 55.15 (s), 48.95 (s), 39.94 (s), 39.73 (d, J = 21.0 Hz), 39.52 (s), 39.39 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 293.0, [M + H]+ 271.0.
6,7-Dihydroxy-3–(4′-methoxyphenyl)-benzofuranone (5). White solid, yield 91.91%, m.p. 138–140 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.12 − 7.09 (m, 2H), 6.94 − 6.91 (m, 2H), 6.59 (d, J = 8.0 Hz, 1H), 6.43 (dd, J = 8.0, 1.1 Hz, 1H), 5.15 (s, 1H), 3.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.16 (s), 158.81 (s), 146.82 (s), 141.77 (s), 129.93 (s), 129.35 (s), 128.56 (s), 119.11 (s), 114.67 (s), 114.39 (s), 111.45 (s), 55.18 (s), 48.53 (s), 39.94 (s), 39.81 (s), 39.80 − 39.52 (m), 39.39 (s), 39.31 (d, J = 21.0 Hz), 39.10 (s). MS: m/z (%) [M + Na]+ 295.0, [M + H]+ 273.0.
6-Hydroxy-3–(3′,4′-dimethoxyphenyl)-benzofuranone (6). White solid, yield 60.47%, m.p. 171–172 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 7.00 (dd, J = 8.2, 0.9 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 6.81 (d, J = 2.0 Hz, 1H), 6.67 (d, J = 2.2 Hz, 1H), 6.64 (dd, J = 8.3, 2.0 Hz, 1H), 6.59 (dd, J = 8.2, 2.2 Hz, 1H), 5.12 (s, 1H), 3.73 (s, 3H), 3.71 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.29 (s), 154.03 (s), 148.95 (s), 148.44 (s), 128.68 (s), 125.64 (s), 120.14 (s), 117.76 (s), 112.08 (d, J = 15.6 Hz), 111.31 (s), 98.25 (s), 55.54 (d, J = 3.3 Hz), 48.19 (s), 39.87 (d, J = 20.9 Hz), 39.67 (s), 39.59 (d, J = 21.0 Hz), 39.41 (s), 39.38 (s), 39.17 (d, J = 21.0 Hz). MS: m/z (%) [M + Na]+ 308.9.
5-Hydroxy-6-methoxy-3–(4′-methoxyphenyl)-benzofuranone (7). White solid, yield 83.92%, m.p. 151–153 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.92 (s, 1H), 7.11 − 7.08 (m, 2H), 7.00 (s, 1H), 6.95 − 6.92 (m, 2H), 6.57 (d, J = 0.7 Hz, 1H), 5.14 (s, 1H), 3.81 (s, 3H), 3.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.46 (s), 158.82 (s), 148.21 (s), 145.91 (s), 143.58 (s), 129.30 (s), 128.29 (s), 118.50 (s), 114.39 (s), 111.34 (s), 96.47 (s), 56.13 (s), 55.15 (s), 48.54 (s), 40.00 (d, J = 16.4 Hz), 39.87 (d, J = 21.0 Hz), 39.66 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 309.0.
6-Methoxy-7-hydroxy-3–(4′-methoxyphenyl)-benzofuranone (8). White solid, yield 86.36%, m.p. 138–139 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.44 (s, 1H), 7.11 (d, J = 8.5 Hz, 2H), 6.93 (d, J = 8.6 Hz, 2H), 6.77 (d, J = 8.2 Hz, 1H), 6.57 (d, J = 8.2 Hz, 1H), 5.20 (s, 1H), 3.80 (s, 3H), 3.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.87 (s), 158.82 (s), 148.87 (s), 141.31 (s), 130.99 (s), 129.37 (s), 128.31 (s), 121.27 (s), 114.43 (d, J = 13.2 Hz), 108.07 (s), 56.27 (s), 55.16 (s), 48.44 (s), 39.94 (s), 39.80 (s), 39.66 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 309.0, [M + H]+ 287.0.
6-Methoxy-3–(3′,4′-dimethoxyphenyl)-benzofuranone (9). White solid, yield 62.81%, m.p. 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.12 (d, J = 7.7 Hz, 1H), 6.99 − 6.89 (m, 2H), 6.83 (s, 1H), 6.75 (d, J = 7.4 Hz, 1H), 6.65 (d, J = 7.0 Hz, 1H), 5.18 (s, 1H), 3.80 (s, 3H), 3.76 − 3.68 (m, 6H). 13C NMR (151 MHz, DMSO-d6) δ 175.87 (s), 160.11 (s), 154.13 (s), 148.73 (d, J = 72.9 Hz), 128.42 (s), 125.62 (s), 120.16 (s), 119.53 (s), 112.10 (d, J = 11.7 Hz), 110.16 (s), 97.20 (s), 55.59 (d, J = 16.4 Hz), 48.19 (s), 39.97 − 39.30 (m), 39.17 (d, J = 21.0 Hz). MS: m/z (%) [M + Na]+ 323.0, [M + H]+ 301.1, [2M + Na]+ 623.1.
5,6-Dimethoxy-3–(4′-methoxyphenyl)-benzofuranone (10). White solid, yield 91.11%, m.p. 128–130 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.12 (d, J = 8.7 Hz, 2H), 7.07 (s, 1H), 6.94 (d, J = 8.8 Hz, 2H), 6.79 (d, J = 0.5 Hz, 1H), 5.18 (s, 1H), 3.81 (s, 3H), 3.75 (s, 3H), 3.67 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.34 (s), 158.87 (s), 149.74 (s), 147.12 (s), 146.14 (s), 129.38 (s), 128.13 (s), 117.96 (s), 114.43 (s), 108.67 (s), 96.44 (s), 56.28 (s), 56.09 (s), 55.15 (s), 48.66 (d, J = 15.2 Hz), 40.00 (d, J = 18.1 Hz), 39.80 (s), 39.59 (d, J = 21.0 Hz), 39.43 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 323.0, [M + H]+ 301.1, [2M + Na]+ 623.1.
6-Hydroxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (11). White solid, yield 92.72%, m.p. 191–193 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.90 (s, 1H), 7.04 (dd, J = 8.2, 1.0 Hz, 1H), 6.67 (d, J = 2.2 Hz, 1H), 6.60 (dd, J = 8.2, 2.2 Hz, 1H), 6.47 (s, 2H), 5.14 (s, 1H), 3.72 (s, 6H), 3.65 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.71 (s), 158.35 (s), 154.04 (s), 153.17 (s), 137.05 (s), 131.93 (s), 125.68 (s), 117.42 (s), 111.35 (s), 105.53 (s), 98.31 (s), 60.00 (s), 55.92 (s), 48.76 (s), 39.94 (s), 39.80 (s), 39.66 (s), 39.52 (s), 39.31 (d, J = 21.0 Hz), 39.24 − 39.23 (m), 39.10 (s). MS: m/z (%)[M + Na]+ 339.3.
6-Methoxy-7-hydroxy-3–(3′,4′-dimethoxyphenyl)-benzofuranone (12). White solid, yield 78.13%, m.p. 135–137 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.43 (s, 1H), 6.93 (d, J = 8.3 Hz, 1H), 6.82 (d, J = 1.9 Hz, 1H), 6.77 (d, J = 8.3 Hz, 1H), 6.66 (dd, J = 8.3, 1.9 Hz, 1H), 6.60 (d, J = 8.2 Hz, 1H), 5.18 (s, 1H), 3.81 (s, 3H), 3.74 (s, 3H), 3.71 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.72 (s), 148.91 (d, J = 13.3 Hz), 148.45 (s), 141.30 (s), 130.95 (s), 128.60 (s), 121.17 (s), 120.25 (s), 114.49 (s), 112.10 (d, J = 8.6 Hz), 108.00 (s), 56.25 (s), 55.55 (s), 48.78 (s), 48.62 (s), 39.87 (d, J = 21.0 Hz), 39.66 (s), 39.64 − 39.62 (m), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 339.0, [M + H]+ 317.1, [2M + Na]+ 655.1.
5-Hydroxy-6-methoxy-3–(3′,4′-dimethoxyphenyl)-benzofuranone (13). White solid, yield 79.11%, m.p. 154–155 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.91 (d, J = 1.5 Hz, 1H), 7.00 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.3, 1.8 Hz, 1H), 6.81 (s, 1H), 6.63 (d, J = 8.2 Hz, 1H), 6.59 (s, 1H), 5.12 (s, 1H), 3.81 (d, J = 1.8 Hz, 3H), 3.73 (dd, J = 10.9, 1.8 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 176.31 (s), 148.96 (s), 148.45 (s), 148.19 (s), 145.89 (s), 143.53 (s), 128.61 (s), 120.13 (s), 118.43 (s), 112.10 (d, J = 13.5 Hz), 111.35 (s), 96.43 (s), 56.11 (s), 55.55 (s), 48.88 (s), 39.94 (s), 39.90 − 39.44 (m), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 339.0, [2M + Na]+ 655.1.
5-Hydroxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (14). White solid, yield 50.68%, m.p. 188–189 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.38 (s, 1H), 7.10 (d, J = 8.7 Hz, 1H), 6.75 (ddd, J = 8.7, 2.6, 0.7 Hz, 1H), 6.61 (dd, J = 2.5, 0.9 Hz, 1H), 6.49 (s, 2H), 5.23 (s, 1H), 3.73 (s, 6H), 3.66 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.61 (s), 154.29 (s), 153.21 (s), 145.70 (s), 137.14 (s), 131.52 (s), 128.65 (s), 115.23 (s), 111.74 (s), 111.08 (s), 105.67 (s), 60.02 (s), 55.97 (s), 49.83 (s), 39.87 (d, J = 21.0 Hz), 39.68 (s), 39.66 (s), 39.52 (s), 39.38 (s), 39.17 (d, J = 21.0 Hz). MS: m/z (%) [M + H]+ 317.1, [M + Na]+ 339.1, [2M + Na]+ 655.2.
5-Methoxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (15). White solid, yield 78.79%, m.p. 141–142 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.23 (d, J = 8.8 Hz, 1H), 6.95 (ddd, J = 8.8, 2.7, 0.7 Hz, 1H), 6.84 (dd, J = 2.7, 1.0 Hz, 1H), 6.49 (s, 2H), 5.28 (s, 1H), 3.73 (s, 6H), 3.72 (s, 3H), 3.66 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.47 (s), 156.38 (s), 153.23 (s), 146.95 (s), 137.16 (s), 131.26 (s), 128.71 (s), 114.43 (s), 111.18 (s), 110.71 (s), 105.72 (s), 60.01 (s), 55.97 (s), 55.76 (s), 49.89 (s), 39.94 (s), 39.80 (s), 39.66 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + H]+ 330.9.
6-Methoxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (16). White solid, yield 90.16%, m.p. 153–154 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.17 (d, J = 8.4 Hz, 1H), 6.96 (d, J = 2.2 Hz, 1H), 6.76 (dd, J = 8.3, 2.2 Hz, 1H), 6.49 (s, 2H), 5.20 (s, 1H), 3.80 (s, 3H), 3.72 (s, 6H), 3.65 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.54 (s), 160.18 (s), 154.15 (s), 153.20 (s), 137.12 (s), 131.66 (s), 125.67 (s), 119.18 (s), 110.19 (s), 105.59 (s), 97.24 (s), 59.99 (s), 55.93 (s), 55.65 (s), 48.78 (s), 39.94 (s), 39.73 (d, J = 21.0 Hz), 39.57 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + H]+ 331.1, [M + Na]+ 353.1, [2M + Na]+ 683.2.
5-Methoxy-3–(2′,3′,4′-trimethoxyphenyl)-benzofuranone (17). White solid, yield 84.85%, m.p. 132–135 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.17 (d, J = 8.7 Hz, 1H), 7.13 (d, J = 8.4 Hz, 1H), 6.89 − 6.86 (m, 1H), 6.84 (d, J = 8.6 Hz, 1H), 6.59 − 6.57 (m, 1H), 5.14 (s, 1H), 3.81 (s, 3H), 3.69 (s, 3H), 3.67 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.56 (s), 156.57 (s), 154.30 (s), 147.54 (s), 142.16 (s), 130.53 (s), 125.91 (s), 122.95 (s), 114.01 (s), 111.07 (s), 110.34 (s), 108.04 (s), 60.69 (s), 60.42 (s), 56.36 (s), 56.11 (s), 43.89 (d, J = 1014.6 Hz), 40.52 (s), 40.57 − 40.18 (m), 40.18 − 39.79 (m), 39.81 − 39.79 (m), 39.69 (s), 39.55 (s). MS: m/z (%) [M + Na]+ 353.1, [2M + Na]+ 683.2.
6-Methoxy-3–(2′,3′,4′-trimethoxyphenyl)-benzofuranone (18). White solid, yield 75.76%, m.p. 100–101 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.12 (d, J = 8.5 Hz, 1H), 6.92 (dd, J = 9.6, 1.6 Hz, 2H), 6.83 (d, J = 8.6 Hz, 1H), 6.66 (dd, J = 8.3, 2.4 Hz, 1H), 5.06 (s, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.69 (s, 3H), 3.33 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.68 (s), 160.31 (s), 154.61 (s), 154.22 (s), 151.07 (s), 142.21 (s), 125.71 (s), 124.98 (s), 123.39 (s), 121.12 (s), 109.91 (s), 108.05 (s), 97.47 (s), 60.68 (s), 60.44 (s), 56.36 (s), 56.04 (s), 46.15 (s), 40.33 (d, J = 21.0 Hz), 40.12 (s), 39.98 (s), 39.84 (s), 39.70 (s), 39.56 (s). MS: m/z (%) [M + Na]+ 353.1, [2M + Na]+ 683.2.
5,6-Dimethoxy-3–(3′,4′-dimethoxyphenyl)-benzofuranone (19). White solid, yield 84.85%, m.p. 149–151 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.06 (s, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.83 − 6.80 (m, 2H), 6.66 (d, J = 8.2 Hz, 1H), 5.17 (s, 1H), 3.81 (s, 3H), 3.74 (s, 3H), 3.72 (s, 3H), 3.68 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 176.18 (s), 149.71 (s), 148.95 (s), 148.49 (s), 147.10 (s), 146.09 (s), 128.39 (s), 120.24 (s), 117.85 (s), 112.11 (d, J = 11.0 Hz), 108.68 (s), 96.40 (s), 56.29 (s), 56.06 (s), 55.53 (s), 54.91 (s), 49.03 (s), 39.87 (d, J = 21.0 Hz), 39.66 (s), 39.52 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + Na]+ 353.1, [2M + Na]+ 683.2.
6,7-Dihydroxy−3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (20). White solid, yield 87.65%, m.p. 170–172 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.49 (s, 1H), 9.26 (s, 1H), 6.60 (d, J = 8.1 Hz, 1H), 6.50 (dd, J = 8.1, 1.1 Hz, 1H), 6.47 (s, 2H), 5.15 (s, 1H), 3.72 (s, 6H), 3.65 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.61 (s), 153.15 (s), 146.86 (s), 141.74 (s), 132.06 (s), 129.89 (s), 118.62 (s), 114.72 (s), 111.45 (s), 105.53 (s), 60.01 (s), 55.91 (s), 49.41 (s), 39.87 (d, J = 21.0 Hz), 39.76 − 39.72 (m), 39.66 (s), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + H]+ 332.9.
5-Hydroxy-6-methoxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (21). White solid, yield 83.24%, m.p. 178–180 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.92 (s, 1H), 7.00 (s, 1H), 6.63 (d, J = 0.9 Hz, 1H), 6.47 (s, 2H), 5.14 (s, 1H), 3.81 (s, 3H), 3.72 (s, 6H), 3.66 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.97 (s), 153.19 (s), 148.27 (s), 145.90 (s), 143.52 (s), 137.09 (s), 131.88 (s), 118.11 (s), 111.33 (s), 105.55 (s), 96.44 (s), 60.01 (s), 56.09 (s), 55.95 (s), 49.49 (s), 39.94 (s), 39.73 (d, J = 21.0 Hz), 39.52 (s), 39.38 (s), 39.24 (s), 39.10 (s). MS: m/z (%) [M + H]+ 347.1, [M + Na]+ 369.1, [2M + Na]+ 715.2.
6-Methoxy-7-hydroxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (22). White solid, yield 76.59%, m.p. 200–202 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.44 (s, 1H), 6.78 (d, J = 8.3 Hz, 1H), 6.64 (dd, J = 8.2, 1.1 Hz, 1H), 6.49 (s, 2H), 5.21 (d, J = 0.7 Hz, 1H), 3.81 (s, 3H), 3.72 (s, 6H), 3.65 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.83 (s), 153.62 (s), 149.38 (s), 141.75 (s), 137.53 (s), 132.27 (s), 131.39 (s), 121.24 (s), 115.00 (s), 108.41 (s), 106.08 (s), 60.45 (s), 56.67 (s), 56.39 (s), 49.82 (s), 40.39 (s), 40.25 (s), 40.11 (s), 39.90 (d, J = 21.0 Hz), 39.69 (s), 39.55 (s). MS: m/z (%) [M + H]+ 347.1044, [M + Na]+ 369.0866, [2M + Na]+ 715.1836.
5-Hydroxy-6-methoxy-3–(3′,4′,5′-trimethoxyphenyl)-benzofuranone (23). White solid, yield 84.26%, m.p. 183–184 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.07 (s, 1H), 6.86 (d, J = 0.6 Hz, 1H), 6.49 (s, 2H), 5.19 (s, 1H), 3.81 (s, 3H), 3.73 (s, 6H), 3.69 (d, J = 4.3 Hz, 3H), 3.66 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 175.83 (s), 153.19 (s), 149.79 (s), 147.15 (s), 146.12 (s), 137.10 (s), 131.57 (s), 117.40 (s), 108.74 (s), 105.61 (s), 96.40 (s), 59.97 (s), 56.34 (s), 55.98 (d, J = 18.2 Hz), 49.58 (s), 39.87 (d, J = 21.0 Hz), 39.67 (s), 39.59 (d, J = 21.0 Hz), 39.38 (s), 39.25 (s), 39.17 (d, J = 21.0 Hz). MS: m/z (%) [M + H]+ 361.1, [M + Na]+ 383.1, [2M + Na]+ 743.2.
Biological activity
Animals
Wistar rat, weight 200–250 g, were obtained from Jinan Peng Yue Experimental Animal Co. (License number: SCXK (Lu) 2014–0007), Ltd. The animals were housed under standard laboratory conditions and maintained on a standard pellet diet and water ad libitum. All experiments involving living animals and their care were performed in strict accordance with the National Care and Use of Laboratory Animals by the National Animal Research Authority (China) and guidelines of Animal Care and Use issued by University of Jinan Institutional Animal Care and Use Committee. The experiments were approved by the Institutional Animal Care and Use Committee of the School of Medicine and Life Sciences, University of Jinan. All efforts were made to minimise animal’s suffering and to reduce the number of animals used.
In vitro ChE inhibitory activity
The anticholinesterase activity of the 3-arylbenzofuranone compounds was determined by the method of Ellman et al.16 with slight modifications. The in vitro inhibition assays of AChE from electric eel and BChE from equine serum were run in phosphate buffer 0.1 M, at pH 8.0. Acetylthiocholine iodide and butyrylthiocholine iodide were used as substrates respectively. 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) was used as the chromophoric reagent. To a 96-well plate, 120 µL of phosphate buffer solution (0.1 M, pH = 8.0, PBS), 20 µL of DTNB (3.3 mM in 0.1 M PBS, pH = 8.0) were added sequentially, 20 µL AChE solution (0.2 U/mL in 0.1 M PBS, pH = 8.0), 20 µL of different concentrations of the sample solution, shaken well, and incubated at 37 °C for 5 min. Then, 20 µL of substrate (5 mM in 0.1 M PBS, pH = 8.0) were added, shaken well, and incubated at 37 °C for 20 min. The absorbance at 412 nm of the samples was measured using a spectrophotometer, and the inhibition rate of ChE and the IC50 value of each sample were calculated according to the formula. BChE inhibitory activity was assessed similarly using butyrylthiocholine iodide as the substrate. The sample solution was set to five concentration gradients and the experiment was repeated three times. Donepezil was used as a positive control.
where A0 is the absorbance of blank group; A1 is the absorbance of sample group; A2 is the absorbance of sample blank group.
In vitro MAO inhibitory activity
The MAO inhibitory activity of the 3-arylbenzofuranone compounds was determined by the method of Holt et al.17 with slight modifications. The crude enzyme was extracted from the liver of 200–250 g of Wistar rats according to literature methods18,19. The crude enzyme protein content was determined by the Bradford method using a Bradford Protein Assay Kit (Beyotime). The assay was performed in 0.2 M potassium phosphate buffer pH 7.6 on 96-well plates in 240 μl total volume. The chromogenic solution containing 1 mM vanillic acid, 0.5 mM 4-aminoantipyrine, and 4 U/ml horseradish peroxidase in 0.2 M potassium phosphate buffer pH 7.6 was mixed anew for each measurement. 40 μL of enzyme solution and 40 μL of sample solution were added to a 96-well plate. The solution was then incubated at 37 °C for 20 min. 120 μL of the 4-(trifluoromethyl) benzylamine solution and 40 μL of the chromogenic solution were added subsequently and incubated at 37 °C for 90 min. The absorbance was measured at 490 nm using a microplate reader, and the inhibition rate of MAO and the IC50 value of each sample were calculated according to the formula. The control group replaced the sample solution with PBS (0.2 M, pH = 7.6), the positive control replaced the sample with the positive drug, and the blank group replaced the substrate with PBS, and each group was measured three times in parallel to average.
where AC is the absorbance of control group; AB is the absorbance of blank group; AS is the absorbance of sample group; ASB is the absorbance of sample blank group.
In vitro antioxidant activity
The assay provides an assessment of the antioxidant activity due to free radical scavenging by measuring hydrogen atom (or one electron) donating activity. DPPH is a purple stable free radical that is reduced to a yellow-coloured diphenylpicryl hydrazine by an antioxidant. The spectrophotometric assay was carried out as described by the literature method with a slight modification20. 100 μL of a sample solution was added to 100 μL of DPPH solution. Following 30 min of incubation at 37 °C and protection from light, the absorbance at 517 nm was determined using a microplate reader. Each group was measured three times in parallel to average. IC50 values were calculated. The percentage of DPPH radical scavenging rate of the target compound is calculated as follows:
where A0 is the absorbance of blank group; A1 is the absorbance of sample group; A2 is the absorbance of sample blank group.
Kinetic characterisation of ChEs inhibition
To obtain the mechanism of action of 13, reciprocal plots of 1/velocity versus 1/[substrate] were constructed at different concentrations of the substrate thiocholine iodide by using Ellman’s method. Compound 13 was added to the assay solution and preincubated with the enzyme at 37 °C for 5 min, followed by the addition of the substrate. Kinetic characterisation catalysed by enzyme was achieved spectrometrically at 412 nm. A parallel control experiment was carried out without compound 13 in the mixture. Lineweaver–Burk plot was made based on the reaction rate and substrate concentration to determine the type of inhibition of the ChE by the compound.
Molecular modelling
The docking of the compounds was performed using the Accelrys Discovery Studio 2019 (DS, Accelrys Software Inc., San Diego, CA, USA) CDOCKER docking protocol. A simulation system was constructed based on the structure obtained from the protein database (PDB: 6O4W for AChE; 6EP4 for BuChE; 4A79 for MAO-B). Preparing the protein using prepare protein method building in DS retains water in the protein-binding pocket. The active site is defined by the ligand of the protein crystal structure, and then the original crystal ligand is docked again, and the RMSD value obtained is less than 1. Prior to the docking calculation, the original ligand was removed. The 3D structure of the compound was generated and optimised using DS.
TOPKAT prediction
TOPKAT protocol in DS was also used to predict the rat oral LD50 and potential toxicity (i.e. carcinogenicity and mutagenicity) of the compounds (Table 3).
Table 3.
TOPKAT prediction results
| Compound | Mutagenicity | TD50 value (mg/kg) |
LD50 value(g/kg) | |
|---|---|---|---|---|
| Carcinogenicity (Mouse) | Carcinogenicity (Rat) | Acute oral toxicity (Rat) | ||
| 1 | 0.495 | 184 | 7.9 | 0.719 |
| 2 | 0.535 | 184 | 7.9 | 0.998 |
| 3 | 0.631 | 89 | 1.49 | 0.784 |
| 4 | 0.586 | 89 | 2.08 | 1.95 |
| 5 | 0.569 | 83.6 | 7.11 | 0.842 |
| 6 | 0.406 | 252 | 11.8 | 2.62 |
| 7 | 0.583 | 209 | 5.81 | 0.488 |
| 8 | 0.571 | 127 | 5.81 | 0.732 |
| 9 | 0.522 | 100 | 2.09 | 2.89 |
| 10 | 0.528 | 100 | 2.09 | 1.62 |
| 11 | 0.211 | 268 | 6.87 | 1.76 |
| 12 | 0.522 | 268 | 8.55 | 1.83 |
| 13 | 0.533 | 268 | 8.55 | 1.75 |
| 14 | 0.274 | 268 | 9.24 | 1.37 |
| 15 | 0.323 | 106 | 1.64 | 1.76 |
| 16 | 0.33 | 106 | 2.08 | 1.73 |
| 17 | 0.443 | 106 | 2.08 | 3.63 |
| 18 | 0.474 | 106 | 1.49 | 1.73 |
| 19 | 0.516 | 128 | 2.19 | 2.75 |
| 20 | 0.293 | 198 | 6.11 | 2.59 |
| 21 | 0.336 | 158 | 3.89 | 1.07 |
| 22 | 0.292 | 282 | 8.46 | 1.95 |
| 23 | 0.275 | 75.4 | 0.994 | 1.67 |
| Donepezil | 0.22 | 99.8 | 1.56 | 0.392 |
| Rasagiline | 0.598 | 94.9 | 20.2 | 0.427 |
Statistical analysis
Data were shown as mean ± SD Differences between individual groups were analysed by using ANOVA followed by Dunett’s test. A difference with a p values of <0.05 was considered to be significant.
Results and discussion
Chemistry
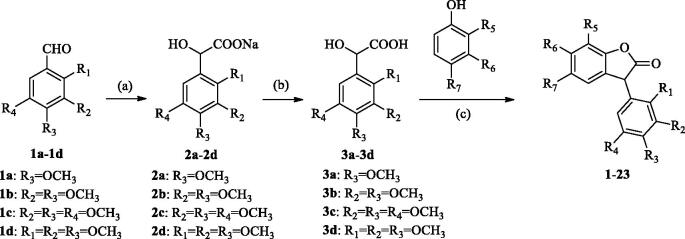
The routes for the synthesis of 3-arylbenzofuranone derivatives are shown in Scheme 1 and the final products are listed in Table 1. The synthesis method of the 3-arylbenzofuranone compound was based on Lv et al. and optimised slightly21. Substituted mandelic acid 3a–3d were synthesised with substituted benzaldehyde 1a–1d. The substituted mandelic acid and substituted phenol were used as starting materials to obtain the corresponding compounds 1–23 by esterification and intramolecular alkylation reaction. Details on the chemical and spectroscopic characterisations of compounds 1–23 were described in the Supporting Information.
Scheme 1.
General synthetic route to 3-arylbenzofuranone, reagents and conditions: (a) CHCl3, TBAB, NaOH, 40–50 °C; (b) H3O+; (c) BF3·Et2O, 30 °C.
Table 1.
Compounds 1–23.
| Product | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 1 | H | H | OCH3 | H | H | OH | H |
| 2 | H | H | OCH3 | H | H | H | OH |
| 3 | H | H | OCH3 | H | H | OCH3 | H |
| 4 | H | H | OCH3 | H | H | H | OCH3 |
| 5 | H | H | OCH3 | H | OH | OH | H |
| 6 | H | OCH3 | OCH3 | H | H | OH | H |
| 7 | H | H | OCH3 | H | H | OCH3 | OH |
| 8 | H | H | OCH3 | H | OH | OCH3 | H |
| 9 | H | OCH3 | OCH3 | H | H | OCH3 | H |
| 10 | H | H | OCH3 | H | H | OCH3 | OCH3 |
| 11 | H | OCH3 | OCH3 | OCH3 | H | OH | H |
| 12 | H | OCH3 | OCH3 | H | OH | OCH3 | H |
| 13 | H | OCH3 | OCH3 | H | H | OCH3 | OH |
| 14 | H | OCH3 | OCH3 | OCH3 | H | H | OH |
| 15 | H | OCH3 | OCH3 | OCH3 | H | H | OCH3 |
| 16 | H | OCH3 | OCH3 | OCH3 | H | OCH3 | H |
| 17 | OCH3 | OCH3 | OCH3 | H | H | H | OCH3 |
| 18 | OCH3 | OCH3 | OCH3 | H | H | OCH3 | H |
| 19 | H | OCH3 | OCH3 | H | H | OCH3 | OCH3 |
| 20 | H | OCH3 | OCH3 | OCH3 | OH | OH | H |
| 21 | H | OCH3 | OCH3 | OCH3 | H | OCH3 | OH |
| 22 | H | OCH3 | OCH3 | OCH3 | OH | OCH3 | H |
| 23 | H | OCH3 | OCH3 | OCH3 | H | OCH3 | OCH3 |
Compared with the original method, the ratio of mandelic acid compounds to phenolic compounds was adjusted from 1:1 to 1:1.2 to ensure that the mandelic acid compounds fully reacted. The completion of the reaction is monitored by thin layer chromatography (TLC), not just the reaction time. Compounds with different substituents differ greatly in reaction time. Excessive reactions can affect compound yield and purity. Several polyhydroxy compounds were synthesised by microwave reactions. The previous reaction by heating in an oil bath required a reaction for 8 h, and now the reaction time is shortened to 15 min by 200 W microwave heating. The yield of the compound obtained by microwave heating is also slightly improved. Most 3-arylbenzofuranone compounds can be purified by methanol recrystallisation, which is efficient and fast.
Biology
In vitro ChE inhibitory activity
AChE is responsible for the hydrolysis of ACh in the synaptic cleft, and AChE inhibitors may help increase ACh levels in damaged cholinergic neurons22. Some of the most common untoward effects of ChEs inhibition therapy are gastrointestinal complaints resulting from stimulation of peripheral autonomic cholinergic systems. The pre-clinical data indicate that more selective AChE inhibitors produce fewer peripheral cholinergic signs than non-selective ChE inhibitors23. Dual ChE inhibitors may increase the incidence of adverse reactions.
The ChE inhibitory activity of all compounds was evaluated by the method of Ellman16. Donepezil was used as a reference compound in this assay. As shown in Table 2, all compounds presented AChE inhibitory activity. Notably, compound 20 (IC50 = 0.089 ± 0.005 μM) had relatively strong activity, which displayed little weaker capacity than donepezil (IC50 = 0.059 ± 0.003 μM). Compounds 4, 5, 7, 9, 13, 20 have IC50 values less than 1 μM. Compound 1 (IC50 = 50.58 ± 3.12 μM) and 11 (IC50 = 48.24 ± 0.34 μM) were respectively lacking one hydroxyl group at R5 than compound 5 (IC50 = 0.48 ± 0.05 μM) and 20 (IC50 = 0.089 ± 0.005 μM), so we speculated that the R5, R6-dihydroxy substituted compound is better than the R6 monosubstituted compound at AChE inhibitory activity. The activities of the compounds 7 (IC50 = 0.52 ± 0.07 μM), 13 (IC50 = 0.28 ± 0.01 μM) are respectively superior to those of the compounds 10 (IC50 = 28.65 ± 1.44 μM), 19 (IC50 = 36.50 ± 0.51 μM), so we presumed that the R6 methoxy-substituted and the R7 hydroxy-substituted compound is superior to that of the R6, R7-dimethoxy-substituted compound. Nearly half of the compounds have BuChE inhibitory activity. The IC50 value of compound 13 (IC50 = 9.91 ± 0.48 μM) is similar to positive drug donepezil (IC50 = 4.67 ± 0.16 μM). Compounds 2, 7, and 13 have IC50 values less than 50 μM. As shown in Table 2, most of the compounds have selective AChE inhibitory activity, which may have a lower incidence of adverse reactions than the double ChE inhibitor.
Table 2.
Biological evaluation in vitro.
| Product | IC50 value (μM) |
|||
|---|---|---|---|---|
| AChE inhibitory activity | BuChE inhibitory activity | MAO-B inhibitory activity | Antioxidant activity | |
| 1 | 50.58 ± 3.12 | >200 | 61.26 ± 0.79 | 13.28 ± 0.27 |
| 2 | 4.15 ± 1.02 | 24.92 ± 0.96 | 14.67 ± 0.78 | >100 |
| 3 | 80.48 ± 3.41 | >200 | 298.43 ± 5.4 | 11.78 ± 0.18 |
| 4 | 0.74 ± 0.06 | >200 | 383.45 ± 5.08 | 33.07 ± 1.45 |
| 5 | 0.48 ± 0.05 | >200 | 22.48 ± 0.22 | 5.51 ± 0.20 |
| 6 | 24.38 ± 1.21 | >200 | 48.59 ± 2.02 | / |
| 7 | 0.52 ± 0.07 | 32.92 ± 0.76 | 91.13 ± 0.70 | 2.62 ± 0.21 |
| 8 | 9.24 ± 0.62 | >200 | 40.62 ± 1.92 | 5.96 ± 0.14 |
| 9 | 0.35 ± 0.03 | >200 | 354.19 ± 5.05 | 17.17 ± 0.58 |
| 10 | 28.65 ± 1.44 | >200 | 493.39 ± 7.10 | 13.89 ± 0.11 |
| 11 | 48.24 ± 0.34 | >200 | 38.61 ± 0.42 | 11.22 ± 0.24 |
| 12 | 5.25 ± 0.44 | 180.91 ± 1.48 | 22.85 ± 0.73 | 1.60 ± 0.10 |
| 13 | 0.28 ± 0.01 | 9.91 ± 0.48 | 11.24 ± 0.72 | 5.33 ± 0.08 |
| 14 | 3.37 ± 0.17 | 128.33 ± 4.65 | 69.65 ± 1.26 | 5.11 ± 0.13 |
| 15 | 2.54 ± 0.19 | >200 | 215.22 ± 2.27 | 32.58 ± 0.42 |
| 16 | 4.07 ± 0.19 | >200 | >500 | 18.01 ± 0.48 |
| 17 | 42.26 ± 4.01 | >200 | 82.68 ± 1.31 | 29.17 ± 0.31 |
| 18 | 123.88 ± 2.17 | >200 | 180.34 ± 1.32 | 64.48 ± 1.29 |
| 19 | 36.50 ± 0.51 | >200 | 145.56 ± 1.83 | 12.56 ± 0.14 |
| 20 | 0.089 ± 0.01 | >200 | 149.21 ± 3.39 | 42.56 ± 2.58 |
| 21 | 2.38 ± 0.20 | 145.89 ± 3.05 | >500 | 5.35 ± 0.33 |
| 22 | 2.03 ± 0.09 | >200 | 30.27 ± 0.65 | 6.44 ± 0.12 |
| 23 | 25.96 ± 1.26 | >200 | 43.89 ± 1.26 | 11.94 ± 0.16 |
| Donepezil | 0.059 ± 0.003 | 4.67 ± 0.16 | ||
| Rasagiline | 0.104 ± 0.002 | |||
| Ascorbic acid | 7.69 ± 0.10 | |||
Each value represents the mean ± SD (n = 3).
In vitro MAO inhibitory activity
MAO plays a major role in brain development and function, and its inhibitors are used clinically as antidepressants and anti-Parkinson’s drugs. MAO-B activity is increased in the brains of Alzheimer’s patients24. All the synthesised compounds were evaluated for their MAO inhibitory activity in the way of Holt by references17. Among them, compound 13 (IC50 = 11.24 ± 0.72 μM) had the best inhibitory activity of MAO-B but was still weaker than the positive drug rasagiline (IC50 = 0.104 ± 0.002 μM). Compounds 8, 12, 22 all have a hydroxyl group at R5 and a methoxy group at the R6 position, all exhibiting good activity. All of the tested compounds showed good activity in the substitution of R5 with a hydroxyl group, so we speculated that the hydroxyl substitution at the R5 position may contribute to an increase in the MAO-B inhibitory activity of the compound.
In vitro antioxidant activity
Alzheimer’s patients exhibit extensive oxidative stress throughout the body. Oxidative stress is an early and prominent symptom of AD, which plays an important role in AD20. All the synthesised compounds were evaluated for their antioxidant activities in the way of scavenging DPPH25. The 3-arylbenzofuranone compounds exhibit good antioxidant capacity. The antioxidant activity of the compounds 5, 7, 8, 12, 13, 14, 21, 22 was superior to the positive drug vitamin C (IC50 = 7.69 ± 0.10 μM). Compounds 8, 12, 22 are substituted at the R5 position with a hydroxy group and at the R6 position with a methoxy group. Compounds 7, 13, and 21 are substituted at the R6 with a methoxy group and at the R7 position with a hydroxy group. Therefore, we speculated that the hydroxymethoxy ortho-substitution facilitates the improvement of the antioxidant capacity of the compound.
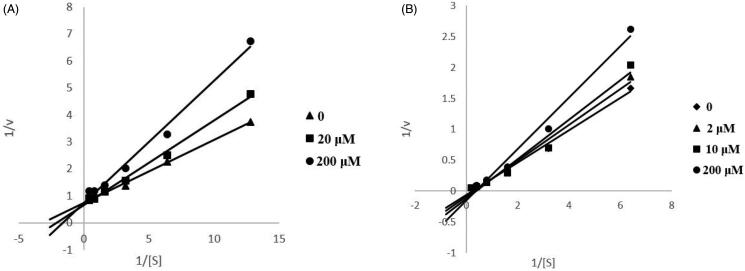
Kinetic study of ChEs inhibition
In order to gain insight into the mechanism of action of these derivatives on ChEs, compound 13, showing good inhibitory activity against ChEs, was selected for kinetic measurements. The graphical analysis of the steady-state inhibition data of 13 against ChEs is shown in Figure 1. According to the figure, it can be judged that the inhibition mode of compound 13 against ChEs is reversible inhibition.
Figure 1.
Kinetic study of the mechanism of ChEs inhibition by compound 13. Overlaid Lineweaver–Burk reciprocal plots of ChEs initial velocity at increasing substrate concentration in the absence of inhibitor and in the presence of 13 are shown. A is a double reciprocal plot of compound 13 inhibition of AChE. B is a double reciprocal plot of compound 13 inhibition of BuChE.
Molecular modelling
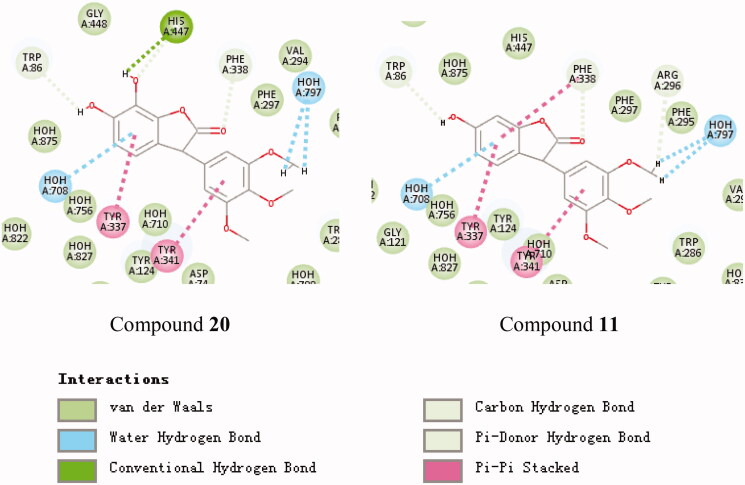
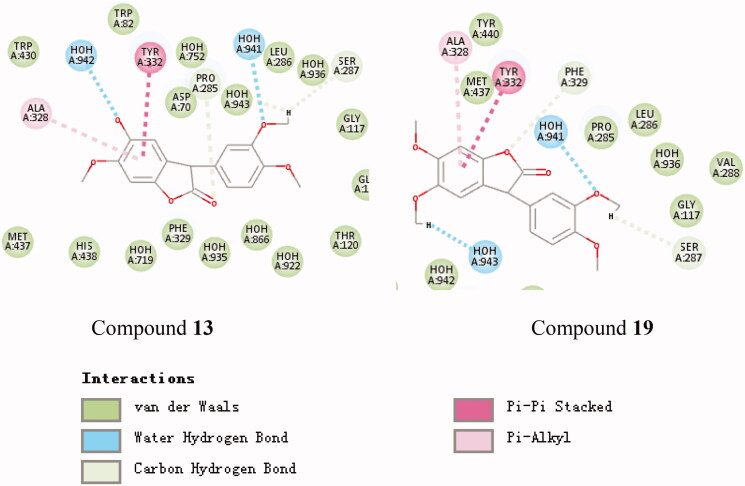
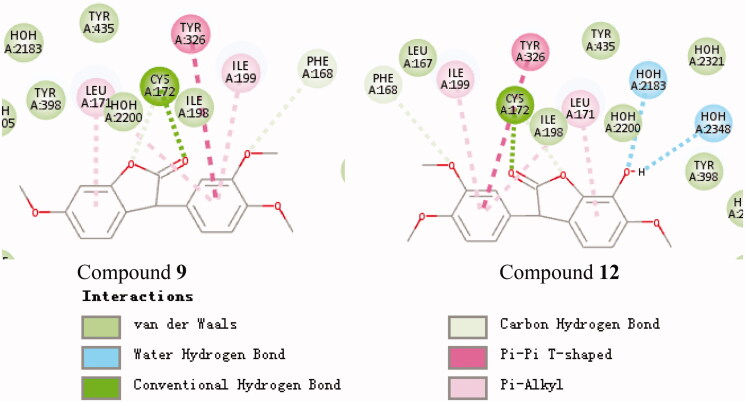
To clarify the mechanism of the compound’s inhibitory activity on the enzyme, the binding model of the active and inactive compounds to the active site was compared. The active site binding patterns of compounds 20, 11, and 4A79 are shown in Figure 2, respectively. It can be seen from Figure 2 that the main difference between the two compounds and the 4A79 binding model is the interaction between compound 20 and His447. This supports the R5, R6-dihydroxy substituted compound is better than the R6 monosubstituted compound at AChE inhibitory activity. The active site binding patterns of compounds 13, 19, and 6EP4 are shown in Figure 3, respectively. The effect of substituents on BuChE inhibition cannot be summarised by IC50 value and molecular docking results. The active site binding patterns of compounds 9, 12, and 4A79 are shown in Figure 4, respectively. It can be seen from Figure 4 that the main difference between the two compounds and the 6O4W binding model is the interaction between the hydroxyl group at the R5 position of compound 12 and two molecules of water. This supports the hydroxyl substitution at the R5 position may contribute to an increase in the MAO-B inhibitory activity of the compound.
Figure 2.
Schematic presentations of the putative AChE binding modes with compound 20 and compound 11.
Figure 3.
Schematic presentations of the putative BuChE binding modes with compound 13 and compound 19.
Figure 4.
Schematic presentations of the putative MAO-B binding modes with compound 9 and compound 12.
TOPKAT prediction
Toxicity Prediction by Komputer assisted technology (TOPKAT) protocol in DS was used to predict the potential toxicity of the compound and the acute oral toxicity effects in rats. According to the analysis of the results in Table 3, all compounds were non-mutagenic. Table 3 summarises the acute oral toxicity effects of rats, indicating that a low level of toxicity with values ranging from 0.488 to 3.63 g/kg, which are all greater than the positive drug.
Conclusion
In conclusion, a series of 3-arylbenzofuranone derivatives were designed, synthesised, and evaluated as multi-targeting anti-AD agents, which have inhibitory activity against ChEs and MAO-B and antioxidant activity. All the synthesised compounds were evaluated for their antioxidant activities in the way of DPPH free radical scavenging experiment. Most compounds demonstrated moderate to high activity. According to the results of in vitro ChEs inhibition assay, we selected compound 13 with better ChE inhibition to study the kinetics of ChE inhibition. According to the kinetic experiments, the type of action of compound 13 on ChE inhibition is reversible inhibition, indicating that our design strategy is reasonable. Previously, our research group also carried out anti-AD research on 3-arylcoumarin compounds, and most of them have double ChE inhibitory activity26. Compared with 3-arylcoumarin compounds, 3-arylbenzofuranone compounds have better AChE and MAO-B inhibitory activity than 3-arylcoumarin compounds and have selective AChE inhibitory activity. Selective AChE compounds have fewer adverse reactions than double ChE inhibition, so screening for compounds with better selective AChE inhibitory activity also has a certain significance. Multi-target anti-AD compounds can modulate multiple signalling pathways or targets associated with AD, potentially producing significant clinical effects. All in all, the multifunctional effects of these 3-arylbenzofuranone derivatives qualify them as potential anti-AD agents, and they are more promising than 3-arylcoumarin compounds in anti-AD.
Supplementary Material
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
The authors are grateful to support from the Science and Shandong Provincial Natural Science Foundation [ZR2018LH021], the Innovation Project of Shandong Academy of Medical Sciences and Academic promotion programme of Shandong First Medical University [No. 2019LJ003].
Ethical statement
All experiments involving living animals and their care were performed in strict accordance with the National Care and Use of Laboratory Animals by the National Animal Research Authority (China) and guidelines of Animal Care and Use issued by University of Jinan Institutional Animal Care and Use Committee. The experiments were approved by the Institutional Animal Care and Use Committee of the School of Medicine and Life Sciences, University of Jinan. All efforts were made to minimise animal’s suffering and to reduce the number of animals used.
Disclosure statement
The authors declare that they have no competing interests.
References
- 1.Burns A, Iliffe S.. Alzheimer's disease. BMJ 2009;338:b158. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Knudsen GM, Maeda S, et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci 2015;18:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glabe CC. Amyloid accumulation and pathogensis of Alzheimer’s disease: significance of monomeric, oligomeric and fibrillar Aβ. Alzheimer’s Dis 2005;38:167–77. [DOI] [PubMed] [Google Scholar]
- 4.Scarpini E, Schelterns P, Feldman H.. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol 2003;2:539–47. [DOI] [PubMed] [Google Scholar]
- 5.Perry EK, Tomlinson BE, Blessed G, et al. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 1978;2:1457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youdim MB, Edmondson D, Tipton KF.. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 2006;7:295–309. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson D, Fallarero A, Brunhofer G, et al. The exploration of thienothiazines as selective butyrylcholinesterase inhibitors. Eur J Pharm Sci 2012;47:190–205. [DOI] [PubMed] [Google Scholar]
- 8.Nadri H, Pirali-Hamedani M, Moradi A, et al. 5,6-Dimethoxybenzofuran-3-one derivatives: a novel series of dual acetylcholinesterase/butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety. Daru J Pharm Sci 2013;21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaleghi M, Navidpour L, Sepehrizadeh Z, et al. The sensitization of legionella pneumophila to some antibiotics by reserpine and anti-legionella effects of different benzofuranone derivatives. J Med Bacteriol 2013;2:35–40. [Google Scholar]
- 10.Gu Q, Wang RR, Zhang XM, et al. A new benzofuranone and anti-HIV constituents from the stems of Rhus chinensis. Planta Med 2007;73:279–82. [DOI] [PubMed] [Google Scholar]
- 11.de Souza Nunes JP, Da Silva KA, Da Silva GF, et al. The antihypersensitive and antiinflammatory activities of a benzofuranone derivative in different experimental models in mice: the importance of the protein kinase C pathway. Anesth Analg 2014;119:836–46. [DOI] [PubMed] [Google Scholar]
- 12.Padaratz P, Fracasso M, De Campos-Buzzi F, et al. Antinociceptive activity of a new benzofuranone derived from a chalcone. Basic Clin Pharmacol Toxicol 2009;105:257–61. [DOI] [PubMed] [Google Scholar]
- 13.Adediran SA, Cabaret D, Drouillat B, et al. The synthesis and evaluation of benzofuranones as β-lactamase substrates. Bioorg Med Chem 2001;9:1175–83. [DOI] [PubMed] [Google Scholar]
- 14.Rabiee A, Ebrahim-Habibi A, Navidpour L, et al. Benzofuranone derivatives as effective small molecules related to insulin amyloid fibrillation: a structure-function study. Chem Biol Drug Des 2011;78:659–66. [DOI] [PubMed] [Google Scholar]
- 15.Aranda R, Villalba K, Raviña E, et al. Synthesis, binding affinity, and molecular docking analysis of new benzofuranone derivatives as potential antipsychotics. J Med Chem 2008;51:6085–94. [DOI] [PubMed] [Google Scholar]
- 16.Ellman GL, Courtney KD, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95. [DOI] [PubMed] [Google Scholar]
- 17.Holt A, Sharman DF, Baker GB, et al. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem 1997;244:384–92. [DOI] [PubMed] [Google Scholar]
- 18.Youdim MBH, Gross A, Finberg JPM.. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br J Pharmacol 2001;132:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stafford GI, Pedersen PD, Jäger AK, et al. Monoamine oxidase inhibition by southern African traditional medicinal plants. S Afr J Bot 2007;73:384–90. [Google Scholar]
- 20.Wang XL, Wang WZ, Li L, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 2014;1842:1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv ZL, Gao Y, Li J, et al. Synthesis and antitumor activities of 3-arylbenzofunanone analogues. Chem J Chin Univ 2013;34:2531–9. [Google Scholar]
- 22.Richman DP, Agius MA.. Treatment of autoimmune myasthenia gravis. Neurology 2003;61:1652–61. [DOI] [PubMed] [Google Scholar]
- 23.Liston DR, Nielsen JA, Villalobos A, et al. Pharmacology of selective acetylcholinesterase inhibitors: implications for use in Alzheimer’s disease. Eur J Pharmacol 2004;486:9–17. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy BP, Ziegler MG, Alford M, et al. Early and persistent alterations in prefrontal cortex MAO A and B in Alzheimer’s disease. J Neural Transm 2003;110:789–801. [DOI] [PubMed] [Google Scholar]
- 25.Liang XL, Wang XL, Li Z, et al. Improved in vitro assays of superoxide anion and 1,1-diphenyl- 2-picrylhydrazyl (DPPH) radical-scavenging activity of isoflavones and isoflavone metabolites. J Agric Food Chem 2010;58:11548–52. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Zhang PP, Hu YH, et al. Synthesis and biological evaluation of 3-arylcoumarins as potential anti-Alzheimer’s disease agents. J Enzyme Inhib Med Chem 2019;34:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.