Abstract
Background
Solitary tracheobronchial papilloma (STBP) is a rare benign tumor. Human papilloma virus (HPV) infection is associated with dysplasia and a high risk of carcinoma.
Case 1
Sixty five year old male with hemoptysis and with coilocytic atypia, indicating the presence of HPV.
Case 2
Thirty two year old female with a polypoid villoglandular bronchial structure and no cytoplasmic or nuclear atypia but prominent microvilli.
Discussion
Tissue sample is the best sample in order to determine and distinguish the two entities, local treatment should be considered as first option when possible.
Keywords: Bronchoscopy, hpv, Argon plasma, Surgery, Cruotherapy, YAG-Laser, Jet-ventilation, EBUS
1. Introduction
Solitary endobronchial papillomas (SEP) are rare tumors and are mostly presented as case series instead of clinical studies. A misdiagnosis is common with viral related papillomas [1]. A histopathological classification has recently permitted a major advancement in the understanding of the disease. Solitary tracheobronchial papilloma (STBP) is known to be a rare benign tumor. Human papilloma virus (HPV) infection is associated with dysplasia and a high risk of carcinoma. The best material in order to distinguish these two entities is tissue biopsies, although there are cases where we have only bronchial aspirates or cells from a fine needle aspiration [2]. We have the capability to Detect of human papillomaviruses type 16, 18 and 33 in bronchial aspirates of lung carcinoma patients by polymerase chain reaction and Detection of human papillomavirus genotypes in bronchial cancer using sensitive multimetrix assay [3,4]. Moreover; there are mixed evidence that HPV infection could be involved in lung cancer. In the study by Syrjänen K. et al. [4] it was suggested that HPV infection could be a substitute of smoking habit, while in the study by Branica BV. Et. al [3]. no correlation between HPV and lung cancer could be established. These two studies used different methods for HPV SEP vs lung cancer diagnosis. There are also two studies with clear association between specific strains of HPV associated with lung cancer [5,6] Exhaled breath could also be a method for HPV investigation in lung cancer patients [7]. There is also the case were both entities were discovered and there is no clear clinical evidence whether glandular papilloma induced squamous cell carcinoma [8]. Depending of the lesion, and place within the bronchial tree local treatment is usually proposed with argon plasma coagulation (APC), cryotherapy, local drug therapy with interferon-α [9].
2. Cases
2.1. Case 1
A Sixty five year old male came to the outpatient ward referring with a small amount of hemoptysis. Upon CT of the thorax there were no signs of lesion inside the lung parenchyma. Bronchoscopy and endobronchial ultrasound with convex probe revealed a lesion in the carina between the upper and lower lobe of the left lung. Also, we could see the depth of the lesion with the convex probe (EBUS) measurement [10,11]. Fig. 1. Pathological examination revealed a exophytic polyloid structure of multilayerd squamous epithelium. The coilocytic atypia observed, indicated the presence of HPV. Fig. 2, Fig. 3, Fig. 4, Fig. 5. The patient was initially treated with local treatment with argon plasma and cryotherapy, the patient is free of local disease for six months. The treatment was performed under general anesthesia with a rigid STORZ bronchoscope 12mm outer rim and 11mm inner tube, under jet-ventilation model and with the use of an Olympus bronchoscope (semi rigid technique).
Fig. 1.
Left blue arrows indicate the lesions and right the convex probe endobronchial ultrasound indicates the depth of the lesions and surrounding vessels. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Hematoxylin/Eosin x 40, Among the bronchial mucus producing epithelium, revealed a exophytic polyloid structure of multilayerd squamous epithelium.
Fig. 3.
Hematoxylin/Eosin x 200, The polypoid structure exhibit hyperplastic basal layer composed of small cells with dark nucleus with minimal atypia and small number of mitosis. The parabasal layer was also hyperplastic and the inner layer was paraceratotic.
Fig. 4.
Hematoxylin/Eosin x 400, In the acanthotic layer there were numerous cells with small, sringed nucleus sorrounden by perinuclear halo and evidence of coilocytic atypia.
Fig. 5.
Immunostain for p16 antibody developed nuclear and cytoplasmic positivity in cells with coilocytic atypia, indicating the presence of HPV.
2.2. Case 2
A thirty two year old female came to the outpatient ward with persistent cough for more than a month. A CT of the thorax did not reveal and lesions and bronchoscopy was performed. Fig. 6. Pathological findings from the biopsies revealed a polypoid villoglandular bronchial structure composed of long columnar respiratory type cells with no cytoplasmic or nuclear atypia but prominent microvilli. Fig. 7, Fig. 8. Local therapy was performed with cryotherapy and YAG-laser, the patient is disease free for more than a year. The treatment was performed under general anesthesia with a rigid STORZ bronchoscope 12mm outer rim and 11mm inner tube, under jet-ventilation model and with the use of an Olympus bronchoscope (semi rigid technique). Fig. 9, Fig. 10. The watts that we used were 20W and the cryotherapy sessions lasted 5–10seconds each in both cases. Regarding the YAG-Laser an initial power setting of 20–40 Watts with a pulse duration of 0.5–1 s was used a safe initial setting to obtain devascularisation.
Fig. 6.
Left lesion in the trachea, right lesion in the left main stem bronchus.
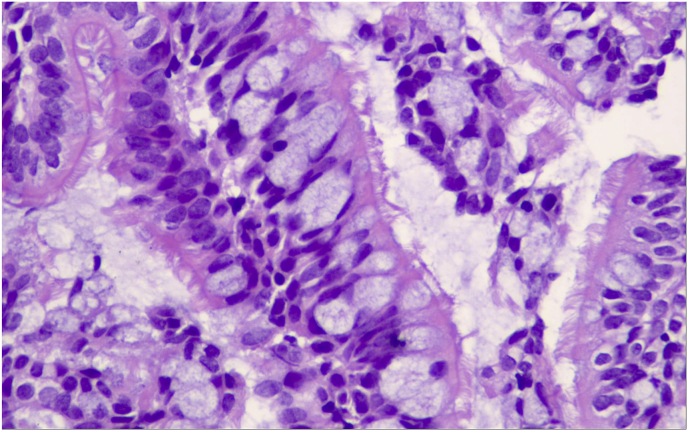
Fig. 7.
Hematoxylin/Eosin x 200, This is a polypoid villoglandular bronchial structure, composed of long columnar respiratory type cells. There is no cytoplasmic or nuclear atypia but prominent microvilli.
Fig. 8.
Hematoxylin/Eosin x 400, The fibrovascular cores were delicate and thin, composed of numerous mucin producting cells reminisced “Goblet cells”.
Fig. 9.
From left to right; STORZ rigid bronchoscope with 12mm outer rim and 11mm inner rim, argon plasma coagulation system (APC), cryotherapy system, YAG-laser system.
Fig. 10.
Patient under general anesthesia, semi rigid technique with the endobronchial ultrasound bronchospe EBUS convex probe inserted through the rigid bronchoscope and Jet-Ventilation model.
3. Discussion
Based on previously published experience and current laboratory methods. The best sample in order to identify solitary tracheobronchial papilloma and Human papilloma virus (HPV) infection/dysplasia is tissue. Tissue samples can be obtained either with forceps or biopsy needle 22G/21G (FNA) and 19G (FNB) [2,5,12]. We can identify the HPV different strains with gene sequencing [13]. Based on the HPV viral strain dysplasia might be formed and cancer might be induced as it has been previously reported [8]. Therapy is based on whether the lesion is benign as in the case of solitary tracheobronchial papilloma or HPV infection with malignant transformation. Usually for SEP local treatment with argon plasma coagulation, laser, cryotherapy or a combination of those treatments is enough. However; in the case of HPV infection and dysplasia and based on the extent of the lesion additionally to interventional local therapies we can add immunomodulatory drugs such as; interferon-α [9,14]. In the case of mixed HPV with lung cancer (e.g squamous) then we should evaluate the stage of the disease and decide surgery or therapy (based on the type of lung cancer). There is also the case that dual drug treatment can be used efficiently without local interventional treatment with laser, APC or Cryotherapy [15]. Early detection is crucial for early treatment, since surface lesions are easier to treat in both situations. Rigid bronchoscope with general anesthesia is a usual practice for the interventional pulmonary treatment of these patients for safety and higher treatment efficiency. We present two cases one benign and one with malignant transformation.
Declaration of competing interest
None to declare.
References
- 1.Harris K., Chalhoub M. Tracheal papillomatosis: what do we know so far? Chron. Respir. Dis. 2011;8(4):233–235. doi: 10.1177/1479972311416381. [DOI] [PubMed] [Google Scholar]
- 2.Lang T.U., Khalbuss W.E., Monaco S.E., Pantanowitz L. Solitary Tracheobronchial Papilloma: cytomorphology and ancillary studies with histologic correlation. CytoJournal. 2011;8:6. doi: 10.4103/1742-6413.77286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branica B.V., Smojver-Jezek S., Juros Z., Grgic S., Srpak N., Mitrecic D., Gajovic S. Detection of human papillomaviruses type 16, 18 and 33 in bronchial aspirates of lung carcinoma patients by polymerase chain reaction: a study of 84 cases in Croatia. Coll. Antropol. 2010;34(1):159–162. [PubMed] [Google Scholar]
- 4.Syrjanen K., Silvoniemi M., Salminen E., Vasankari T., Syrjanen S. Detection of human papillomavirus genotypes in bronchial cancer using sensitive multimetrix assay. Anticancer Res. 2012;32(2):625–631. [PubMed] [Google Scholar]
- 5.Munoz J.P., Gonzalez C., Parra B., Corvalan A.H., Tornesello M.L., Eizuru Y., Aguayo F. Functional interaction between human papillomavirus type 16 E6 and E7 oncoproteins and cigarette smoke components in lung epithelial cells. PloS One. 2012;7(5):e38178. doi: 10.1371/journal.pone.0038178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani L., Jaxmar T., Casadio C., Gariglio M., Manna A., D'Antonio D., Syrjanen K., Favalli C., Ciotti M. Detection of oncogenic viruses SV40, BKV, JCV, HCMV, HPV and p53 codon 72 polymorphism in lung carcinoma. Lung Canc. 2007;57(3):273–281. doi: 10.1016/j.lungcan.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Carpagnano G.E., Koutelou A., Natalicchio M.I., Martinelli D., Ruggieri C., Di Taranto A., Antonetti R., Carpagnano F., Foschino-Barbaro M.P. HPV in exhaled breath condensate of lung cancer patients. Br. J. Canc. 2011;105(8):1183–1190. doi: 10.1038/bjc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabuki K., Matsuyama A., Obara K., Takenaka M., Tanaka F., Nakatani Y., Hisaoka M. A unique case of a huge mixed squamous cell and glandular papilloma of non-endobronchial origin with a peripheral growth. Respir. Med. Case Rep. 2018;24:108–112. doi: 10.1016/j.rmcr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildirim F., Turk M., Demircan S., Akyurek N., Yurdakul A.S. Tracheal papilloma treated with cryotherapy and interferon-alpha: a case report and review of the literature. Case Rep. Pulmonol. 2015;2015:356796. doi: 10.1155/2015/356796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarogoulidis P., Huang H., Bai C., Kosmidis C., Porpodis K., Kallianos A., Veletza L., Trakada G., Benhassen N., Hohenforst-Schmidt W. A new mode of ventilation for interventional pulmonology. A case with EBUS-TBNA and debulking. Respir. Med. Case Rep. 2018;23:38–42. doi: 10.1016/j.rmcr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarogoulidis P., Huang H., Bai C., Kosmidis C., Trakada G., Veletza L., Tsiouda T., Barbetakis N., Paliouras D., Athanasiou E., Hatzibougias D., Kallianos A., Panagiotopoulos N., Papaemmanouil L., Hohenforst-Schmidt W. Endobronchial ultrasound convex probe for lymphoma, sarcoidosis, lung cancer and other thoracic entities. Case Ser. Respir. Med. Case Rep. 2017;22:187–196. doi: 10.1016/j.rmcr.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soldatski I.L., Onufrieva E.K., Steklov A.M., Schepin N.V. Tracheal, bronchial, and pulmonary papillomatosis in children. Laryngoscope. 2005;115(10):1848–1854. doi: 10.1097/01.mlg.0000173155.57491.2a. [DOI] [PubMed] [Google Scholar]
- 13.Martinez S.B., Palomares J.C., Artura A., Parra M., Cabezas J.L., Romo J.M., Martin-Mazuelos E. Comparison of the cobas 4800 human papillomavirus test against a combination of the amplicor human papillomavirus and the linear array tests for detection of HPV types 16 and 18 in cervical samples. J. Virol Methods. 2012;180(1–2):7–10. doi: 10.1016/j.jviromet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Aaron S., Wong E., Tyrrell D., Duggan M., Vallieres E., Jewell L., Romanowski B., Doe P.J. Interferon treatment of multiple pulmonary malignancies associated with papilloma virus. Can. Respir. J. J. Can. Thorac. Soc. 2004;11(6):443–446. doi: 10.1155/2004/327431. [DOI] [PubMed] [Google Scholar]
- 15.Armbruster C., Kreuzer A., Vorbach H., Huber M., Armbruster C. Successful treatment of severe respiratory papillomatosis with intravenous cidofovir and interferon alpha-2b. Eur. Respir. J. 2001;17(4):830–831. doi: 10.1183/09031936.01.17408300. [DOI] [PubMed] [Google Scholar]