Abstract
Background:
The present study evaluates the possible prognostic benefits of 7 T susceptibility weighted imaging (SWI) of traumatic cerebral microbleeds (TMBs) over 3 T SWI to predict the acute clinical state and subjective impairments, including health-related quality of life (HRQOL), after closed head injury (CHI).
Methods:
The study group comprised 10 participants with known TMBs All subjects underwent 3 T magnetic resonance imaging (MRI) and 7 T MRI, respectively. Location and count of TMBs were independently evaluated by two neuroradiologists. The initial Glasgow Coma Scale (GCS), the duration of coma and further clinical data were taken from the patients records. HRQOL was assessed by means of a questionnaire. Memory complaints and neurological symptoms were inquired at the time of the MRI examinations.
Results:
SWI revealed a total of 485 TMBs at 3 T, 584 TMBs at 7 T with similar spatial resolution, and 684 TMBs at 7 T with a factor of 10 higher spatial resolution. The TMBs depicted by 7 T high-resolution SWI were correlated with the duration of coma (Spearman’s rho of 0.77). The corresponding association with TMBs in 3 T MRI SWI showed a Spearman’s rho of 0.71. The initial GCS score and TMBs correlated with a Spearman’s rho of −0.35 at 3 T SWI MRI and a rho of −0.33 at 7 T high-resolution SWI, respectively. The physical aspect of HRQOL correlated substantially with the count of TMBs (rho = 0.44 for 3 T SWI and rho = 0.35 for both 7 T SWI sequences, respectively).
Conclusions:
The number of TMBs showed a substantial association with indicators of the acute clinical state and chronic neurobehavioral parameters after CHI, but there was no additional advantage of 7 T MRI. These preliminary findings warrant a larger prospective study for the future.
Keywords: traumatic brain inury, traumatic cerebral microbleeds, diffuse axonal injury, susceptibility weighted imaging, 7 Tesla MRI, clinical significance, acute clinical state, health-related quality of life
Introduction
The annual incidence of traumatic brain injury (TBI) in Europe is reported to be 235–262/100,000.1,2 Many patients present with chronic neurological and neuropsychological impairment after TBI.3–8 Diffuse axonal injury (DAI) can be found in 72% of patients with moderate or severe head injury9–12 and is regarded as an important factor for neurological and neuropsychological disorders in the acute and chronic phase after TBI.13–17 First described in 1956, DAI has been defined as small hemorrhagic brain lesions of less than 15 mm maximum diameter located in the gray–white matter junction and midline structures.18,19 Axonal degeneration is not only limited to the acute and subacute phase of TBI, but may be followed by a progressive, long-term neurodegeneration with the disconnection of brain networks.20–22 In the last few decades, MRI evolved as the imaging modality of choice to detect traumatic microbleedings (TMBs) following TBI.23–26 However, TMBs as a consequence of damage to vessels are only an indirect marker of DAI as damage to axons. Therefore, TMBs can only be regarded as implicit indicators of DAI while their relationship remains rather hazy.27–32 Some authors suggest that these two are not necessarily closely related.28–32 Only in pediatric patients do correlations between TMBs and neuropsychological functioning including other aspects of outcome occur consistently.33–35 In contrast, studies on adults after TBI especially focusing on TMBs and their prognostic significance are far from being conclusive.28–32 Currently, T*2 weighted gradient echo (GRE) sequences and susceptibility weighted imaging (SWI) sequences are used for the detection of TMBs23–26,36–38 whereas 3 T T*2 weighted sequences have been demonstrated to be twice as sensitive compared with 1.5 T MRI, with the highest sensitivity for the detection of hemosiderin for SWI sequences.22–24,26,39–41 As a consequence, SWI is increasingly applied in the clinical routine after TBI.38,42,43 An improved depiction of small TMBs at 7 T may be helpful to better predict the acute state and the later outcome in patients after TBI. In an earlier publication, we focused on the significance of different field strengths on the detection of small TMBs demonstrating the superiority of 7 T ultra-high-field (UHF) SWI.44 The purpose of the present study was to correlate the number of TMBs at 3 and 7 T with the acute clinical state and their later impairments of health-related quality of life (HRQOL) in order to evaluate the clinical significance of 7 T SWI over 3 T MRI in patients after TBI. The present publication is based on the same patients and neuroradiological data as the earlier work.44
Patients and methods
Study sample
This retrospective study was performed between July 2012 and April 2014. The study was conducted in conformance with the Declaration of Helsinki and approved by the Ethics Commission of the Medical Faculty of the University Duisburg-Essen (study number 11–4898-BO). Inclusion criteria were as follows: treatment for closed head injury (CHI) at the Department of Neurosurgery of the University Hospital Essen, aged between 18 and 75 years, TMB confirmed by 1.5 T MRI performed between 3 and 8 weeks after the acute trauma, and residence in the catchment area of the city of Essen. Exclusion criteria were: contraindications against MRI examinations (e.g. pacemaker, metallic implants with unknown compatibility at 7 T, claustrophobia), neurological disease other than TBI (including history of stroke or migraine), psychiatric disease, uncontrolled hypertension, diabetes mellitus, alcohol or drug abuse, anticoagulant or antiplatelet therapy, and a neurological result according to the Glasgow Outcome Scale (GOS) at the time of the 6-month follow-up examination from grade I to grade II. In addition, patients had to be excluded from the study if the neurological exam performed before the first MRI exam revealed any information suggesting the presence of one of the above-mentioned exclusion criteria. According to these criteria, 72 patients were eligible for the present study. Of these, 16 (22%) were ready to take part in the study and gave written informed consent. The neurological exam necessitated the exclusion of three patients. A further three patients left the study during the period of MRI examinations. Therefore, the study group comprised 10 patients (4 men, 6 women) with TMBs after CHI. The median age of the study participants was 41.5 (range 54 years). The median time elapsing between TBI and MRI examinations was 18.5 months (range 107 months). The trauma severity was evaluated at the time of admission to the neurosurgical unit by means of the Glasgow Coma Scale (GCS).45 Table 1 lists the clinical and sociodemographic data of the study participants.
Table 1.
Clinical and sociodemographic data of the patients.
| Patient no. | Sex | Age | Initial GCS | TMB grade (MRI) | Coma duration (h) | MRI after trauma (months) | GOS | Anosmia | Epilepsy | Kind of trauma |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 20 | 4 | 3 | 48 | 45 | I | No | No | TA (cyclist) |
| 2 | f | 23 | 5 | 3 | 120 | 38 | II | No | No | fall |
| 3 | f | 39 | 7 | 1 | 72 | 96 | II | No | No | fall |
| 4 | f | 74 | 13 | 1 | 6 | 6 | II | No | No | TA (pedestrian) |
| 5 | m | 44 | 8 | 3 | 12 | 240 | I | No | No | TA (cyclist) |
| 6 | f | 57 | 10 | 1 | 24 | 26 | I | No | Yes | fall |
| 7 | f | 25 | 12 | 2 | 96 | 11 | II | Yes | No | TA (car driver) |
| 8 | f | 20 | 5 | 3 | 504 | 14 | II | No | Yes | TA (car driver) |
| 9 | m | 61 | 9 | 1 | 12 | 17 | I | No | No | fall |
| 10 | m | 59 | 5 | 1 | 24 | 3 | II | No | No | TA (cyclist) |
f, female; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; m, male; MRI, magnetic resonance imaging; TA, traffic accident; TMB, traumatic microbleeding.
According to the criteria of the GCS, six patients exhibited a severe, three a moderate, and one patient a mild TBI. The duration of coma was protocolled in hours during the stay at the intensive care unit. Coma was diagnosed in intubated and sedated patients if the patient did not show any sign of awakening after a substantial reduction of the sedation. From the beginning of the stay at the intensive care unit of the Department of Neurosurgery at the University Hospital Essen, this procedure was repeated every 6 h in order to control for the neurological state including consciousness in every patient. The median duration of coma was 60 h with a range of 6–504 h (Table 1). The patient with an initial GCS of 13 who became comatose for 6 h (Table 1) had sustained a sharp short increase of intracranial pressure that declined quickly. Computed tomography (CT) scanning excluded any kind of intracranial bleeding in this patient. A total of six patients were hurt in traffic accidents, the other four had sustained a fall from great height (Table 1). Of our 10 study participants, 6 had sustained a polytrauma encompassing fractures of the skull, bones, and the lumbar spine and abdominal contusions. In all patients a CT of the head was performed routinely immediately after admission to the hospital. For the assessment of brain damage the last CT of those obtained within the first 3 days after admission was evaluated. At that point of time, in three patients a traumatic subarachnoid hemorrhage (tSAH) could be detected, while one patient presented with a left parietal contusion. The neurological state at the time of the 6-month follow-up examination was assessed according to the GOS.46 At that time, four patients had a good neurological result (GOS = I) and six were slightly to moderately impaired (GOS = II) (Table 1).
Study design
The study design encompassed two different appointments for data acquisition. The MRI examinations were performed at two different days with a scan order of 3 T followed by 7 T within 1 week in 9 of 10 patients. One patient was examined for organizational reasons at both field strengths with a delay of 8 weeks. The patients received a set of questionnaires via mail with the request to fill them out at home and bring them back to their first MRI exam. On the first day before the MRI scanning, all patients received a comprehensive neurological examination in order to ensure that they currently fulfill all required criteria. After that, one coauthor of the present study (J.A.) checked whether the questionnaires had been completed correctly and interviewed the patients about the circumstances of the trauma, presence of anosmia since the traumatic event, and subjective memory problems as frequent typical late sequelae of CHI that were coded dichotomously as present or not present. Furthermore, the patients were informed about details of the scanning procedures and the presence of any contraindications was excluded.
MRI examinations
The MRI examinations and their methods have been described in detail in an earlier publication.44 The SWI 7 T acquisition time was about 13 min. UHF MR examinations were performed on a 7 T whole-body research system (Magnetom 7T, Siemens Healthcare, Germany). Imaging at 3 T was performed on a clinical MR system (Magnetom Skyra, Siemens Healthcare, Germany). Both MR systems were used in combination with 32-channel radiofrequency head coils. Further details of image acquisition were given previously.44
Image analysis
The radiological grading of TMB (grade 1–3) is based on the cerebral location of TMBs. Three grades of TMB were differentiated: in grade 1 TMB is found in the white matter of the cerebral hemispheres; in grade 2 additional focal lesions in the corpus callosum can be detected; and in grade 3 further brain stem lesions are present.11 For TMB grading, both 3 and 7 T (including UHF) sequences were employed. Table 1 gives the TMB grades of the study participants. More details of image analysis and lesion definition have been published in an earlier paper.44 Image analysis was performed by two experienced neuroradiologists (C.M., M.S.) who had been blinded regarding the patients characteristics. Both observers rated SWI data sets for frequency of TMBs independently. Ratings by both observers differed in six subjects. Therefore, scans were reevaluated in consensus in order to define the final rating. More details about image analysis can be found in the precursor publication.44
Assessment of HRQOL
HRQOL was measured by means of the Aachen Life Quality Inventory (ALQI), which is based on the modified German version of the Sickness Impact Profile (SIP).47–49 The original American SIP has also been shown to be applicable in patients after TBI.50 The ALQI was developed in particular for the use in patients with brain damage.49 The psychometric properties of the ALQI were examined in a sample of 231 patients with brain damage of different etiology including TBI.49 The internal consistency (Cronbach’s alpha) ranged from 0.79 to 0.94 for the individual subscales, whereas the respective scores for the ALQI summary scales ranged between 0.93 and 0.97.49 The ALQI consists of 11 subscales, the first 10 of which contain 10 items each, whereas the subscale Cognitive capacity contains 17 items. The ALQI assesses the following areas of HRQOL: 1. Activation; 2. Mobility; 3. Housework; 4. Social contact; 5. Family relations; 6. Ambulation; 7. Work; 8. Free-time activities; 9. Autonomy; 10. Communication; 11. Cognitive capacity. The ALQI encompasses exclusively items covering illness-related physical and psychosocial restrictions in daily life on a concrete behavioral level that are objectively observable (e.g. for the most of the day, I lay down to relax). All 117 items have dichotomous response categories (yes/no). The ALQI allows the calculation of summary scores of total impairment (ALQI Total Score), psychosocial (ALQI Psycho-social Score), and physical impairment (ALQI Physical Score). The ALQI Psycho-social Score consists of the following subscales: Social contact, Family relations, Free-time activities, Communication, and Cognitive capacity. The ALQI Physical Score encompasses the subscales Activation, Mobility, Housework, Ambulation, and Autonomy. The subscale Work stands alone and will not be added to any summary score. A higher score, whether in the subscales or in the summary scores, means a higher degree of impairment indicating worse HRQOL.
Statistical analysis
The data were statistically analyzed using SPSS version 19 in order to compute descriptive statistics if adequate and Spearman rank-ordered correlations. Median and range are given as descriptives in the case of small group size or deviations from the assumption of normal distribution. Owing to the small sample size, we did not compute descriptives for most variables and instead gave the raw scores for every single patient. Again owing to the small sample size, correlation coefficients obtained were not tested for statistical significance. Only coefficients greater than 0.32 were regarded as substantial and thus reported as they explain more than 10% of the shared variance. Scatterplots illustrate the associations, which met the previously mentioned criteria. In particular, the ALQI summary scores were not normally distributed. Owing to the problematic data (small group size and deviations from the assumption of normal distribution) we computed specially developed statistical procedures adjusted to the current problematic data (program package StatXact encompassing algorithms for small n analyses from Cytel Software). We applied for ordinally scaled figures the Permutation test using an algorithm adjusted particularly to small n analyses. In order to underline the reproducibility of the rank-ordered correlations obtained between the physical score and the TMB count we computed Somers’ D as a conservative nonparametric measure of association between pairs of ordinal variables applying an algorithm adjusted for small n. The permutation tests and the Somers’ D were computed using the software CytelStudio version 9.0.0 (program package StatXact encompassing algorithms for small n analyses from Cytel Software). For these analyses asymptotic p values were calculated.
Results
Associations between TMB count and neurological parameters
Permutation tests revealed no substantial associations between the number of TMBs counted in 3 T SWI, 7 T SWI, or high-resolution 7 T SWI scans and the presence of tSAH in the acute stage (p > 0.05). Therefore, a confounding effect of hemosiderin deposits stemming possibly from tSAH can be excluded. However, the number of TMBs as identified in 7 T MRT high-resolution SWI correlated with a Spearman’s rho of 0.77 with the duration of coma. The corresponding association with the TMB count in 3 T MRT SWI showed a Spearman’s rho of 0.71. The significant relationship between a higher TMB grading and a longer duration of coma could be shown by permutation testing (asymptotic statistic −1.869; p = 0.03). Furthermore, substantial relationships could be detected between the initial GCS score and the number microbleeds in the chronic state after TBI with a Spearman’s rho of −0.35 (3 T SWI sequences) and a Spearman’s rho of −0.33 at 7 T high-resolution SWI. There were no substantial relationships between the delay since trauma/MRI examination (Table 1) and the number of TMBs in 3 T SWI, 7 T SWI, and high-resolution 7 T SWI. Spearman’s rho coefficients were markedly below 0.33.
Associations between TMB count, HRQOL, and subjective memory complaints
All patients filled in their questionnaires at home answering all questions with no missing items. Table 2 lists scores for ALQI summary scales of individual patients and whether they complained of subjective memory problems. There were large differences in the degree of impairment between the patients (Table 2). In the ALQI subscales, the most frequent complaints were reported in the areas of Cognitive capacity, Free-time activities, Family relations, and Housework. On the other hand, according to the ALQI subscale Autonomy, no single patient felt any restriction concerning these items. Given the small sample size, the other ALQI subscale scores were too low to give them here in detail. However, the summary scores showed a larger span and were therefore employed for further analyses.
Table 2.
Scores of the ALQI summary scales and subjective memory impairment.
| Patient no. | ALQI psycho-social score | ALQI physical score | ALQI total score | Subjective memory impairment |
|---|---|---|---|---|
| 1 | 11 | 2 | 13 | Yes |
| 2 | 9 | 2 | 11 | Yes |
| 3 | 0 | 0 | 0 | Yes |
| 4 | 12 | 1 | 13 | No |
| 5 | 5 | 0 | 5 | No |
| 6 | 1 | 0 | 1 | Yes |
| 7 | 24 | 14 | 38 | No |
| 8 | 17 | 5 | 22 | Yes |
| 9 | 26 | 2 | 28 | Yes |
| 10 | 32 | 11 | 43 | Yes |
ALQI, Aachen Life Quality Inventory.
The degree of impairments in the chronic state after TBI showed a large variance, ranging from completely unrestricted (a total score of impairment of 0) to substantially impaired with a total score of 43 (Table 2). The delay between the acute trauma and the MRI follow-up correlated negatively at a modest degree (Spearman’s rho −0.44 to −0.58) with the three summary scores of the ALQI indicating a decrease of impairment over time. Table 3 shows the rank-ordered correlations between ALQI summary scores and counts of microbleeds at 3 T SWI, 7 T SWI, and high-resolution 7 T SWI, respectively.
Table 3.
Spearman rank-ordered correlations between the ALQI summary scores and the frequency of microbleeds in MRI.
| 3 T SWI | 7 T SWI | 7 T SWI HR* | |
|---|---|---|---|
| Physical score | 0.44 | 0.35 | 0.35 |
| Psycho-social score | 0.21 | 0.12 | 0.15 |
| Total score | 0.10 | –0.01 | 0.01 |
ALQI, Aachen Life Quality Inventory; HR, high-resolution; MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging.
HR 7 T SWI; bold figures, substantial associations of >10% shared variance.
Only the physical score consistently exhibited substantial correlations with the TMB count (Table 3). Spearman’s rho was 0.44 for 3 T SWI imaging and 0.35 for both 7 T SWI sequences. (Table 3). Figures 1–3 show the respective scatterplots of the associations regarded as substantial.
Figure 1.
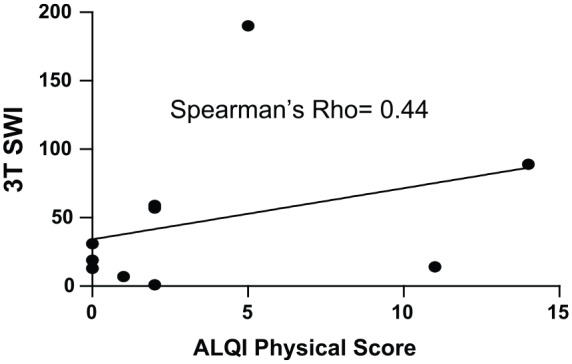
Scatterplot 3 T SWI: ALQI physical impairment.
ALQI, Aachen Life Quality Inventory; SWI, susceptibility weighted imaging.
Figure 2.
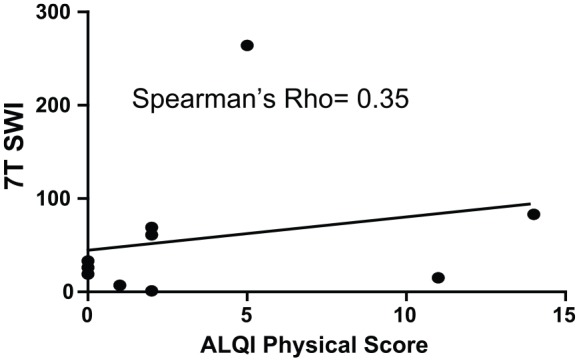
Scatterplot 7 T SWI: ALQI physical impairment.
ALQI, Aachen Life Quality Inventory; SWI, susceptibility weighted imaging.
Figure 3.
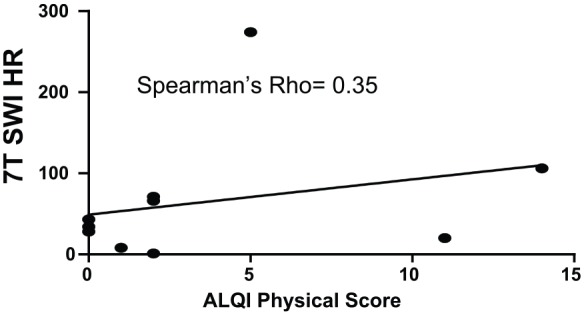
Scatterplot high-resolution 7 T SWI: ALQI physical impairment.
ALQI, Aachen Life Quality Inventory; SWI, susceptibility weighted imaging.
The Somers’ D between the number of TMB counts in 3 T SWI and the physical score was 0.333 [95% confidence interval (CI) –0.081 to 0.751]. The respective Somers’ D for the strength of association between the DAI-associated microbleeds in 7 T SWI and the physical summary scale was 0.281 (95% CI –0.192 to 0.756) while it was 0.282 (95% CI –0.192 to 0.756) for the high-resolution 7 T SWI sequence.
Discussion
General
The clinical significance of 7 T SWI high-field imaging seems to be questionable according to the present findings. This is in apparent contradiction to a previous publication in which we could demonstrate the superiority of 7 T SWI over 3 T SWI in detecting TMBs in the chronic state after TBI.44 However, the inflated error variance when detecting an increased number of small and clinically irrelevant TMBs using 7 T SWI may attenuate the association of microbleedings with clinical and functional parameters. In detail, the duration of comas and the number of TMBs showed a substantial association without further diagnostic advantage for higher magnetic field strength SWI. Park and colleagues51 also showed a significant relationship between the count of TMBs and the duration of comas in patients after CHI. The strongest relationship between the duration of coma and the TMB grade in our patients, sharing about 50% of the common variance, may appear large on first view. However, from a statistical point of view, the strength of this association could only be regarded as modest. On the other hand, complex pathophysiological processes initiated by TBI and a variety of other factors make these results appear differently. In fact, the number of TMBs is not a very strong prognostic factor and other clinical factors, in particular the anatomical location of the TMBs, had to be taken into account.31,32,52 Furthermore, SWI data may also be related to extracranial factors and not to axonal pathology.27,29 In pediatric patients after CHI, the SWI TMBs in terms of number, volume, and location were shown in several studies to be reliable correlations with neuropsychological function and the outcome in general.33–35 In contrast, in adult patients and especially in studies focusing strictly on TMBs the results are at best mixed.29,30,32,52–54 Van der Horn and colleagues30 could not find any differences in terms of number, depth, and anatomical location of TMBs in patients after CHI with or without posttraumatic complaints. Furthermore, a substantial relationship between the initial GCS score and trauma-associated microbleeds emerged in the present study. This is in line with the results of Scheid and colleagues25 who reported an initially worse GCS score to be a good predictor for an unfavorable late outcome that was associated with increased microbleeds in their patients. However, we could not find any advantage of 7 T SWI, using equal or higher spatial resolution, in comparison with a conventional 3 T SWI to predict the initial GCS, the duration of coma, subjective memory problems in the chronic state, and late HRQOL. Only the physical aspects of HRQOL showed a substantial relationship with the TMB counts at 3 T SWI and 7 T SWI with a proportion of 10% of shared variance at both field strengths. This raises the question about the diagnostic benefit of using 7 T SWI after CHI. Yuh and colleagues55 reported that TMBs were a significant predictor of poor outcome. In addition, Spitz and colleagues56 found a similar association between lesion load (TMBs) and clinical outcome. Griffin and colleagues53 identified TMBs beyond other clinical factors as independent predictors of disability after TBI. A retrospective analysis of 3 T MRI examinations including 274 patients with TBI revealed TMBs in 148 (46%) patients without chronic signs of traumatic tissue alterations in 76 (23.8%) patients.57 In this study, a relationship between traumatic structural and functional brain alterations could not be revealed after comparing neuroradiological findings and neuropsychological exams.57 De Haan and colleagues31 could not find a significant correlation between the number of TMBs and the number of posttraumatic complaints. However, a significant association explaining 44% of the variance emerged between the number of TMBs in the corticotemporal area and an unfavorable outcome.31 Recent work suggests that TMB localization is much more important than pure lesion count or volumetry.29,31,32,52 It would be interesting for future work to analyze whether 7 T reveals lesions in more regions than 3 T and to analyze the clinical significance of a potential difference. However, the present sample size was too small to make such analyses on a statistically sound basis. Beyond the number of TMBs, the localization of TMBs is relevant for the prognosis after TBI.33,54 In the study by Park and colleagues54 TMBs exhibited characteristic anatomical localizations and were related to initial states and prognosis after CHI. The presence and quantity of TMBs were closely related to a lower GCS score and a worse outcome 1 year later according to the GOS.54 Izzy and colleagues52 found that the presence of TMBs in the brainstem is not, per se, prognostically significant. Rather the exact anatomical location of TMBs in the brainstem correlated substantially with the 1-year outcome.52 Recent work demonstrates that diffusion tensor imaging (DTI) and DTI fiber tractography are sensitive and useful methods to visualize and quantify DAI-related white matter lesions at field strengths up to 3 T.58–66 Several studies have already demonstrated the feasibility of DTI at 7 T.67–69 Several studies included DTI beyond identification of TMBs in order to compare direct and indirect indicators of DAI.28,29,32 In general, TMBs can only be regarded as indirect markers of DAI but the exact relationship is unclear as of yet and needs to be elucidated. An increasing body of evidence shows that TMBs are only precariously related to DAI.28,29,32 Toth and colleagues29 studied a sample of 38 patients after CHI traumatic bleedings by means of SWI imaging and white matter integrity by DTI. They found that DAI was associated with TMBs as well as with nonhemorrhagic lesions.29 On the other hand, Studerus-Germann and colleagues28 found that the amount of TMBs in the acute phase was significantly associated with cognitive symptoms 1 year later. However, structural integrity as measured by DTI at the acute stage was not. DTI may be prognostically significant only in a later postacute more stable phase. Andreasen and colleagues32 found in 14 patients in the subacute phase after severe TBI that TMBs were not strictly related to DAI in the midsagittal region as assessed by DTI. TMBs and DTI-based DAI were only able to predict individual disturbances of consciousness in deep but not in superficial regions.32
Limitations of the present study
The small number of study participants is the primary limitation of this study. Furthermore, the variable time span between trauma and examination biases the results and was intrinsically related to the retrospective design. This led to error variance that may have attenuated the associations in the present study. We could identify one single outlier (case #8) with a TMB count substantially deviating from the other patients. Rank-order correlation mitigates this issue. We reanalyzed the data after exclusion of this outlier but found rather similar figures, with rhos becoming gradually smaller between TMBs and the physical life quality, and greater with the acute clinical state. The delay between trauma and MRI is very variable in the present study. Furthermore, this time span is highly related to the late HRQOL. Other predictors (such as coma duration, initial GCS score, etc.) in combination with delay might explain HRQOL much better than the TMB count alone. However, because of the small sample size, we chose not to perform such multivariate analyses. This issue should be pursued in future work by a prospective study including more TBI patients with a homogeneous delay between trauma and follow-up. Another bias stems from the heterogeneous age distribution in the study sample. On the other hand, all MRI examinations have been performed in the same group of patients that might have substantially reduced the interindividual variability by minimizing this source of error variance. Since our most important conclusions concerning the clinical significance of 7 T as compared with 3 T imaging hinge on Spearman correlations, we tried to confirm our figures vicariously by means of the Somers’ D nonparametric association test for small n. Owing to the narrow data base, only the fact that the empirical rho does not exceed the estimated confidence interval can be regarded as a bland validation of the correlations found. However, the large width of the estimated intervals underlines the relative uncertainty in our findings resulting from the small sample size. The so-called ‘blooming effect’ causes earlier dephasing of the surrounding tissue signal and increases with higher field strengths and longer echo time.70–72 This effect additionally improves the depiction of very small TMB and venous vessels, which thus become detectable. On the other hand, adjacent microbleeds merge with each other at higher field strengths, which reduces their discriminability. Furthermore, the MR sequence protocols were adapted to both field strengths to ensure high diagnostic image quality and spatial resolution in a reasonable acquisition time. Adapting sequence parameters at 3 and 7 T was not possible under the circumstances of the present study and might have led to further bias.73 In the present study, 3 and 7 T were used in combination for the grading of TMB, but it would be interesting to explore in larger patient samples whether 3 or 7 T reveal a different grade of TMB and if this has a different influence on the acute and chronic prognosis after TBI. Moreover, we only used the count of microbleeds as an indirect indicator of fiber disruption and did not perform fiber tracking (DTI) to directly detect fiber damage. This should be implemented in forthcoming work. A comprehensive neuropsychological exam is also missing in the present study and should be included in the future. Therefore, the current pilot study can only be regarded exploratory with preliminary findings, which can help to state clear hypotheses for future investigations.
Conclusion
The present findings, even though preliminary, imply that the prognostic power of 3 T SWI is comparable with that of 7 T SWI even in the case of UHF sequences. Larger prospective studies are warranted to confirm the present findings in larger, more homogenous patient samples. Furthermore, it would be interesting to study if a TMB is diagnosed by 7T and not by 3T. Also, it would be a question for future work if 3 and 7 T differ in the localization of TMBs and if this is relevant for the acute state and the late complaints after TBI. Therefore, the analysis of possible differences in the anatomical pattern of TMB as revealed by 3 and 7 T is promising for the future.
Acknowledgments
The authors thank all patients for their voluntary study participation and the involved radiological technicians for their excellent help to perform all MRI examinations.
Footnotes
Author contributions: BOH, CM, MS, and OK conceived and designed the experiments. CM and JA performed the experiments. BOH, CM, KHW, PD, and AR analyzed the data. JMT, SM, HHQ, MS, and BOH contributed reagents/materials/analysis tools. BOH, CM, HHQ, and KHW wrote the paper.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ORCID iD: Bernd-Otto Hütter  https://orcid.org/0000-0001-7034-0420
https://orcid.org/0000-0001-7034-0420
Contributor Information
Bernd-Otto Hütter, Department of Neurosurgery, University Hospital Essen, Hufelandstr. 55, Essen, 45147, Germany.
Jan Altmeppen, Institute of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Oliver Kraff, Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany.
Stefan Maderwald, Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany.
Jens M. Theysohn, Institute of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany
Adrian Ringelstein, Institute of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Karsten H. Wrede, Department of Neurosurgery, University Hospital Essen, Essen, Germany Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany.
Philipp Dammann, Department of Neurosurgery, University Hospital Essen, Essen, Germany.
Harald H. Quick, Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany High Field and Hybrid MR Imaging, University Hospital Essen, Essen, Germany.
Marc Schlamann, Department of Neuroradiology, University Hospital Giessen, Giessen, Germany.
Christoph Moenninghoff, Institute of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
References
- 1. Tagliaferi F, Compagnone C, Korsic MS, et al. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006; 148: 255–268. [DOI] [PubMed] [Google Scholar]
- 2. Peters W, van den Brande R, Polinder S, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien) 2015; 157: 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma 2002; 19: 503–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niogi SN, Mukherjee P, Ghajar J, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 2008; 131: 3209–3221. [DOI] [PubMed] [Google Scholar]
- 5. Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011; 134: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fork M, Bartels C, Ebert AD, et al. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj 2005; 19: 101–108. [DOI] [PubMed] [Google Scholar]
- 7. Satz P, Alafano MS, Light R, et al. Persistent post-concussive syndrome: a proposed methodology and literature review to determine the effects, if any, of mild head and other body injury. J Clin Experimental Neuropsychol 1999; 21: 620–628. [DOI] [PubMed] [Google Scholar]
- 8. Scheid R, Walther K, Guthke T, et al. Cognitive sequelae of diffuse axonal injury. Arch Neurol 2006; 63: 418–424. [DOI] [PubMed] [Google Scholar]
- 9. Skandsen T, Kvistad KA, Solheim O, et al. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg 2010; 113: 556–563. [DOI] [PubMed] [Google Scholar]
- 10. Wallesch CW, Curio N, Kutz S, et al. Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Inj 2001; 15: 401–412. [DOI] [PubMed] [Google Scholar]
- 11. Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, Diagnosis and grading. Histopathology 1989; 15: 49–59. [DOI] [PubMed] [Google Scholar]
- 12. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paterakis K, Karantanas AH, Komnos A, et al. Outcome of patients with diffuse axonal injury: the significance and prognostic value of MRI in the acute phase. J Trauma 2000; 49: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 14. Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 1994; 15: 1583–1589. [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss N, Galanaud D, Carpentier A, et al. Clinical review: Prognostic value of magnetic resonance imaging in acute brain injury and coma. Crit Care 2007; 11: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirov II, Tal A, Babb JS, et al. Proton MR spectroscopy correlates diffuse axonal abnormalities with post-concussive symptoms in mild traumatic brain injury. J Neurotrauma 2013; 30: 1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esbjörnsson E, Skoglund T, Mitsis MK, et al. Cognitive impact of traumatic axonal injury (TAI) and return to work. Brain Inj 2013; 27: 521–528. [DOI] [PubMed] [Google Scholar]
- 18. Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry 1956; 119: 163–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parizel PM, Ozsarlak Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 1998; 8: 960–965. [DOI] [PubMed] [Google Scholar]
- 20. Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol 2014; 10: 156–166. [DOI] [PubMed] [Google Scholar]
- 21. Monaco EA, III, Tempel Z, Friedlander RM. Inflammation triggered by traumatic brain injury may continue to harm the brain for a lifetime. Neurosurgery 2013; 72: 19–20. [DOI] [PubMed] [Google Scholar]
- 22. Warner MA, Marquez de la Plata C, Spence J, et al. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma 2010; 27: 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheid R, Ott DV, Roth H, et al. Comparative magnetic resonance imaging at 1.5 and 3 Tesla for the evaluation of traumatic microbleeds. J Neurotrauma 2007; 24: 1811–1816. [DOI] [PubMed] [Google Scholar]
- 24. Luccichenti G, Giugni E, Peran P, et al. 3 Tesla is twice as sensitive as 1.5 Tesla magnetic resonance imaging in the assessment of diffuse axonal injury in traumatic brain injury patients. Funct Neurol 2010; 25: 109–114. [PubMed] [Google Scholar]
- 25. Scheid R, Preul C, Gruber O, et al. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol 2003; 24: 1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 26. Allkemper T, Schwindt W, Maintz D, et al. Sensitivity of T2-weighted FSE sequences towards physiological iron depositions in normal brains at 1.5 and 3.0 T. Eur Radiol 2004; 14: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 27. Studerus-Germann AM, Thiran JP, Daducci A, et al. Diagnostic approaches to predict persistent post-traumatic symptoms year after mild traumatic brain injury - a literature review. Int J Neurosci 2016; 126: 289–298. [DOI] [PubMed] [Google Scholar]
- 28. Studerus-Germann AM, Gautschi OP, Bontempi P, et al. Central nervous system microbleeds in the acute phase are associated with structural integrity by DTI one year after mild traumatic brain injury: a longitudinal study. Neurol Neurochir Pol 2018; 52: 710–719. [DOI] [PubMed] [Google Scholar]
- 29. Toth A, Kornyei B, Kovacs N, et al. Both hemorrhagic and non-hemorrhagic traumatic MRI lesions are associated with the microstructural damage of the normal appearing white matter. Behav Brain Res 2018; 340: 106–116. [DOI] [PubMed] [Google Scholar]
- 30. van der Horn HJ, de Haan S, Spikman JM, et al. Clinical relevance of microhemorrhagic lesions in subacute mild traumatic brain injury. Brain Imaging Behav 2018; 12: 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Haan S, de Groot JC, Jacobs B, et al. The association between microhaemorrhages and post-traumatic functional outcome in the chronic phase after mild traumatic brain injury. Neuroradiology 2017; 59: 963–969. [DOI] [PubMed] [Google Scholar]
- 32. Andreasen SH, Andersen KW, Conde V, et al. Limited co-localization of microbleeds and microstructural changes after severe traumatic brain injury. J Neurotrauma. Epub ahead of print 20 November 2019. DOI: 10.1089/neu.2019.6608 [DOI] [PubMed] [Google Scholar]
- 33. Tong KA, Ashwal S, Obenaus A, et al. Susceptibility weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol 2008; 29: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Babikian T, Freier MC, Tong KA, et al. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr Neurol 2005; 33: 184–194. [DOI] [PubMed] [Google Scholar]
- 35. Beauchamp MH, Beare R, Ditchfield M, et al. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex 2013; 49: 591–598. [DOI] [PubMed] [Google Scholar]
- 36. Theysohn JM, Kraff O, Maderwald S, et al. 7 tesla MRI of microbleeds and whitematter lesions as seen in vascular dementia. J Magn Reson Imaging 2011; 33: 782–791. [DOI] [PubMed] [Google Scholar]
- 37. Hilal S, Saini M, Tan CS, et al. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord 2014; 28: 106–112. [DOI] [PubMed] [Google Scholar]
- 38. Haacke EM, Mittal S, Wu Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009; 30: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Akter M, Hirai T, Hiai Y, et al. Detection of hemorrhagic hypointense foci in the brain on susceptibility-weighted imaging clinical and phantom studies. Acad Radiol 2007; 14: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 40. Lupo JM, Chuang CF, Chang SM, et al. 7-Tesla susceptibility weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys 2012; 82: e493–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 2003; 227: 332–339. [DOI] [PubMed] [Google Scholar]
- 42. Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 2009; 30: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reichenbach JR, Venkatesan R, Schillinger DJ, et al. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 1997; 204: 272–277. [DOI] [PubMed] [Google Scholar]
- 44. Mönninghoff C, Kraff O, Maderwald S, et al. Diffuse axonal injury at ultra-high field MRI. PLoS One 2015; 10: e0122329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tesadale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2: 81–84. [DOI] [PubMed] [Google Scholar]
- 46. Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975; 1: 480–484. [DOI] [PubMed] [Google Scholar]
- 47. Bergner M, Bobbitt RA, Carter WB, et al. The sickness impact profile: development and final revision of a health status measure. Med Care 1981; 19: 787–805. [DOI] [PubMed] [Google Scholar]
- 48. Hütter BO, Würtemberger G. Reliability and validity of the German version of the sickness impact profile in patients with chronic obstructive pulmonary disease. Psychol Health 1997; 12: 149–159. [Google Scholar]
- 49. Hütter BO, Gilsbach JM. Grundlagen und erste Ergebnisse zur methodischen Eignung des Aachener Lebensqualitätsinventars. Zentralblatt für Neurochirurgie 2002; 63: 37–42. [DOI] [PubMed] [Google Scholar]
- 50. Klonoff PS, Snow WG, Costa LD. Quality of life in patients 2 to 4 years after closed head injury. Neurosurgery 1985; 19: 735–743. [DOI] [PubMed] [Google Scholar]
- 51. Park SJ, Hur JW, Kwon KY, et al. Time to recover consciousness in patients with diffuse axonal injury: assessment with reference to magnetic resonance grading. J Korean Neurosurg Soc 2009; 46: 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Izzy S, Mazwi NL, Martinez S, et al. Revisiting grade 3 diffuse axonal injury: not all brainstem microbleeds are prognostically equal. Neurocrit Care 2017; 27: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griffin AD, Turtzo LC, Parikh GY, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019; 142: 3550–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park JH, Park SW, Kang SH, et al. Detection of traumatic cerebral microbleeds by susceptibility–weighted image of MRI. J Korean Neurosurg 2009; 46: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013; 73: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spitz G, Maller JJ, Ng A, et al. Detecting lesions after traumatic brain injury using susceptibility weighted imaging: a comparison with fluid-attenuated inversion recovery and correlation with clinical outcome. J Neurotrauma 2013; 30: 2038–2050. [DOI] [PubMed] [Google Scholar]
- 57. Scheid R, von Cramon DY. Clinical findings in the chronic phase of traumatic brain injury: data from 12 years’ experience in the cognitive neurology outpatient clinic at the university of Leipzig. Dtsch Ärztebl Int 2010; 107: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang MC, Jang SH. Corpus callosum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. Neuro Rehabilitation 2010; 26: 339–345. [DOI] [PubMed] [Google Scholar]
- 59. Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004; 25: 370–376. [PMC free article] [PubMed] [Google Scholar]
- 60. Kasahara K, Hashimoto K, Abo M, et al. Voxel-and atlas-based analysis of diffusion tensor imaging may reveal focal axonal injuries in mild traumatic brain injury—comparison with diffuse axonal injury. Magn Reson Imaging 2012; 30: 496–505. [DOI] [PubMed] [Google Scholar]
- 61. Sugiyama K, Kondo T, Oouchida Y, et al. Clinical utility of diffusion tensor imaging for evaluating patients with diffuse axonal injury and cognitive disorders in the chronic stage. J Neurotrauma 2009; 26: 1879–1890. [DOI] [PubMed] [Google Scholar]
- 62. Kumar R, Husain M, Gupta RK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma 2009; 26: 481–495. [DOI] [PubMed] [Google Scholar]
- 63. Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007; 130: 2508–2519. [DOI] [PubMed] [Google Scholar]
- 64. Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008; 29: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gu L, Li J, Feng DF, et al. Detection of white matter lesions in the acute stage of diffuse axonal injury predicts long-term cognitive impairments: a clinical diffusion tensor imaging study. J Trauma Acute Care Surg 2013; 74: 242–247. [DOI] [PubMed] [Google Scholar]
- 66. Miles L, Grossman RA, Johnson G, et al. Short-term DTI predictors of cognitive function in mild traumatic brain injury. Brain Inj 2008; 22: 115–122. [DOI] [PubMed] [Google Scholar]
- 67. Polders DL, Leemans A, Hendrikse J, et al. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J Magn Reson Imaging 2011; 33: 1456–1463. [DOI] [PubMed] [Google Scholar]
- 68. Soria G, De Notaris M, Tudela R, et al. Improved assessment of ex vivo brainstem neuroanatomy with high-resolution MRI and DTI at 7 Tesla. Anat Rec (Hoboken) 2011; 294: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 69. Wargo CJ, Gore JC. Localized high-resolution DTI of the human midbrain using single-shot EPI, parallel imaging, and outer-volume suppression at 7T. Magn Reson Imaging 2013; 31: 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tatsumi S, Ayaki T, Shinohara M, et al. Type of gradient recalled-echo sequence results in size and number change of cerebral microbleeds. AJNR Am J Neuroradiol 2008; 29: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schlamann M, Maderwald S, Becker W, et al. Cerebral cavernous hemangiomas at 7 Tesla: initial experience. Acad Radiol 2010; 17: 3–6. [DOI] [PubMed] [Google Scholar]
- 72. Conijn MM, Geerlings MI, Biessels GJ, et al. Cerebral microbleeds on MR imaging: comparison between 1.5 and 7T. AJNR Am J Neuroradiol 2011; 32: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reichenbach JR, Venkatesan R, Yablonskiy DA, et al. Theory and application of static field inhomogeneity effects in gradient-echo imaging. J Magn Reson Imaging 1997; 7: 266–279. [DOI] [PubMed] [Google Scholar]


