Abstract
Objective:
We investigated the coexistence of sarcopenia and obesity in older adults≥65 years diagnosed with osteoporosis and the association with Quality of Life (QoL)
Methods:
A Cross-sectional survey has been performed on a randomized sample of 50 diagnosed osteoporotic elderly people from both sexes (Men=16; Women=34)
Measurements:
Quantitative ultrasound was conducted to identify osteoporosis and defined with a T score ≤2.5. Validated anthropometric equations were used in order to estimate body fat percentage and skeletal muscle mass so as to detect the reallocation of body fat and lean muscle. 10m gait speed and hand grip strength was measured in order to diagnose sarcopenia according to European Society for Clinical Nutrition and Metabolism (ESPEN) algorithm. The evaluation of QoL was conducted using a QoL questionnaire specific to osteoporosis. The data were analyzed with descriptive statistics and a chi-square test was performed to examine if Osteosarcopenic Obesity (OSO) is sex related and the correlation between OSO and QoL
Results:
From the 50 participants, 40%(n=19) were classified as people with OSO and 60%(n=31) without OSO. From n=19 people that experienced OSO women represent 20% (n=9) and men 18% (n=9); with the latter had a greater decline in muscle mass than women, while women had lower BMD than men according to the z score. OSO is not related with sex (p>.05) and there is no significant association between OSO and QoL (p> .05 for all the domains of QoL questionnaire)
Conclusion:
Osteoporosis in the elderly often coexists with reduced muscle mass and muscle strength as well as an increase in adiposity and was independently associated with QoL. People that experience OSO presenting lower functionality that increases the risk for falls and bone fractures originated from the decline in bone and muscle mass, and increased adiposity. Increased awareness of OSO may help develop efficient interventions and public health policies for healthier and more active elderly people.
Keywords: Sarcopenic obesity, Osteopenic obesity, Obesity, Frailty, Quality of life
Introduction
Nowadays, there is a great concern about a new clinical condition that can affect functional capacity, as well as, the quality of life (QoL) of older adults, named osteosarcopenic obesity (OSO), as described by Illich and colleagues[1]. OSO was first described as sarcopenic obesity, namely a condition that is characterized by excess body fat with the concurrent loss of skeletal muscle[2]. Thereafter, the term ‘osteopenic obesity’ emerged, describing the condition with bone loss and the concurrent excess adiposity[1]. OSO was later defined by the concurrent incidence of three different conditions; osteoporosis, sarcopenia and obesity[1,3], and described by the concurrent coexistence of low bone mass (T-score ≤-2.5 defining osteoporosis), low muscle mass, low muscle strength, and increase of adipose tissue[1,3]. These abnormalities in body composition are often met in elderly people as body composition is altered with age; people begin to lose around 3% to 5% of muscle mass per decade after the 3rd decade of life[4,5]. Specifically, there are changes in the musculoskeletal system as aging commences, including a decrease in muscle and bone mass with a simultaneous increase in fat mass; this combination has a grave impact on the QoL[6] and constitutes OSO a great clinical concern in older adults as it causes serious metabolic changes -including inflammation, insulin resistance, decrease production of anabolic hormones-and clinical implications related to the coexistence of the three conditions[3]. It appears that individuals who have all three conditions will present worse clinical outcomes as a result of the metabolic aberration related to the changes in the musculoskeletal system[7,8]. The prevalence of these diseases will grow and will burden healthcare and the public health cost[11,12], as an upward trend of life expectancy has been reported in Western societies during the last century and the number of people over the age of 65 has been increasing[9,10].
Relationship between bone and fat
There is the opinion that weight greater than the ideal is associated with better bone density and more strength as a result of ‘increasing mechanical loading[13,14]. As a matter of fact, body mass is assumed to be a respected predictor of bone mass for both sexes; the higher the body mass the lower the risk of osteoporotic vertebral and hip fractures[15]. On the other hand, increased weight is usually associated with obesity and higher levels of adiposity. New data suggests that there is a 33 percent body fat threshold where adiposity is not salutary anymore and starts having unpropitious effects since visceral fat produce multifarious adipokines and other molecules that could cause damage to the bone microenvironment[16-18]. Especially in obese older adults there is a high level (nearly 50%) of fat infiltration in muscles[19,20], and in bone marrow adipocytes as a result of the augment expression of peroxisome proliferator-activated receptor gamma (PPARγ) in the bone marrow, therefrom fostering adipogenesis of Mesenchymal stem cells whilst subdue osteogenesis. Moreover, marrow fat is believed to be a filler of the empty space occurred from the trabecular bone loss[21]. Both conditions can lead to a damage of muscle tissue and bone[20,22]. Furthermore, according to Liu and colleagues[22], excess body fat in females (33-38%) has negative relationship with bone mineral density (BMD) on most of the skeletal system. In addition, adiposity over this threshold has been proved to be relevant to type 2 diabetes, sleep apnea, osteoarthritis, heart disease, certain cancers, dyslipidemia, high blood pressure, stroke, and liver/gallbladder disease[23].
Sarcopenia
According to the three consensus papers[32] sarcopenia can be defined as ‘The presence of low skeletal muscle mass and either low muscle strength (e.g., handgrip) or low muscle performance (e.g., walking speed or muscle power); when all three conditions are present, severe sarcopenia may be diagnosed (European Working Group on Sarcopenia in Older People-first version)’ or ‘The presence of low skeletal muscle mass and low muscle strength (which they advised could be assessed by walking speed)’. The consensus group criteria utilize three established stages of sarcopenia classification; Presarcopenia is defined as having low Appendicular Lean Mass (ALM)/height[2] only, sarcopenia was defined with low Appendicular Lean Mass/height[2] (<5.67 kg/m[2]) and the presence of low gait speed (≤0.8 m/s) or low grip strength (<20 kg) and severe sarcopenia classification requires low ALM/height[2], gait speed, and grip strength. Gait speed test is used to a large degree in clinical practice to measure a plethora of adverse health outcomes, such as mobility disability, frailty, general physical condition, falls, loss of independence and poor general health[61-64]. Bohannon and Andrews[65] stated that “gait speed has been recommended as a ‘vital sign” for assessing health related risks in elderly. Older adults with osteoporosis have atrophy of type II muscle fibers and a reduction of the myosin content per half-sarcomere; this atrophy is proportional to the extent of BMD loss[24,25]. This hastens the loss of type II fast glycolytic muscle fibers considering that they become thinner and atrophic. Also, there is deregulation of the energy alteration from ATP into mechanical energy effectuating reduction in muscle tension[36]. The muscle loading and the ensuing tension helps in the maintenance of BMD; dysfunction upon this mechanism leads to lessening of BMD, with thinner and fragile bones[24,25]. Sarcopenia means loss of muscle mass and the word comes from the ancient Greek words ‘sarx’ and ‘penia’ which means ‘flesh’ and ‘loss’; by this time the term has been originally used to describe the concern towards the phenomenon of age-related decline of muscle mass as well as muscle function and strength[26]. Muscle mass and muscle function have a critical role for mobility and autonomous living especially in elderly[27].
In sarcopenia there is a decrease in both muscle fiber number-motor units and muscle fiber size (atrophy) with the consequence in the diminution in the muscle efficiency to generate force; in everyday life this force is translated into lower muscle strength[35]. This decrease in muscle mass that entail both lower motor unit numbers and atrophy is what make sarcopenia unique and shows how different it is from disuse atrophy that entails only reduction in fiber size[35]. The major pathway that causes loss of muscle mass is the lack of equilibrium between anabolism and catabolism, with main responsible for this the protein loss and main intercessors an excess of endocrine and inflammatory factors. Muscle loss is not only age related but also is a consequence of muscle disuse, a state that can occur when a muscle is no longer active; in elderly this may be an after effect of a mobility limitation[35,36]. This condition could lead to the slow recovery of the muscle fibers and eventually to reduced strength and physical frailty; as a result, there is a much greater risk regarding fall-related injuries[36,37]. Frailty is correlated with sarcopenia and is a biological syndrome, resulting from age and is associated with weight loss, low activity, weakness, exhaustion and slowness and may lead to falls, functional decline disability, hospitalization and death[38,39]. The risk of fall can be very critical in elderly people who suffer from osteoporosis and experience increased vulnerability to fractures, in particular in the spine, hip (femur), and wrist[37,40].
Relationships between bone, muscle, and adipose tissue
The deregulation of major metabolic pathways caused by pro-inflammatory factors and endocrine imbalance can lead to the reallocation of fat in muscle tissue and bone mass (Figure 1). This condition, which comes as a consequence of ageing, can also determine obesity[1,42,43]. Sarcopenia comes along with fat infiltration in skeletal muscles known as myosteatosis. Resulting in a smaller number of contractile muscle fibers and a higher percentage of non-contractile tissue[44,45]. Myosteatosis occurs from various pathways; an instant way is via the accumulation of intramuscular lipid (inside muscle fibers). Intramuscular fat is associated with insulin resistance, inflammation and decline in skeletal muscle function which will cause decrease to force and muscle quality inducing immobilization[20,45]. Another way is the intermuscular fat (between muscle fibers); the accumulation of bone marrow adipocytes within skeletal muscle where a plethora of stem cells exists, and predominantly muscle satellite cells(SCs) which are the main cells responsible for myogenesis and muscle regeneration after muscle trauma[20,46]. In Sarcopenia there is a reduction in muscle SCs as a result of the muscle loss and the fat infiltration[35,47].
Figure 1.
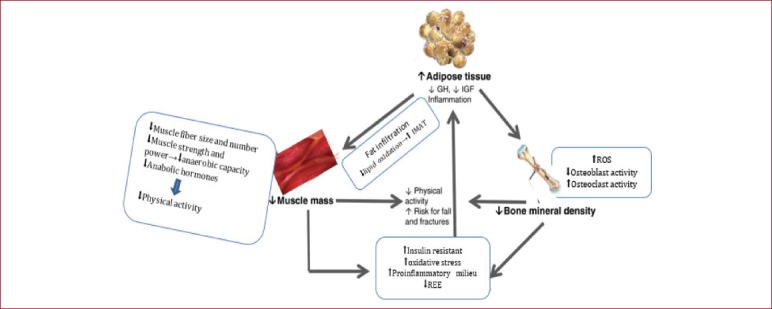
Interaction between adipose tissue, muscle and bone.
Osteosarcopenic obesity is an up to date topic with most of the surveys focusing on menopausal women. OSO is consisted of sarcopenic obesity and osteopenic obesity; it is not clear if both conditions could be equally traced in men and women from the age of 65 years or above. Therefore, one of the objectives of this research was the statistical difference between the two sexes in prevalence of osteosarcopenic obesity. Our concern was also to investigate whether reduced muscle mass and muscle strength coexists with the increase in adiposity, in older adults equal or over 65 years-old with osteoporosis. In the recent years the term QoL has become benchmark, in a slew of research specialized in geriatrics. Nowadays the term is in vogue especially the relationship between QoL and OSO as the literature suggests that osteoporosis and sarcopenia have negative effect on QoL. European population experience an ageing process as an effect of increased life expectancy that never seen before. Ageing is inseparable with NCDs and diseases of the musculoskeletal system[69], diseases that induce deterioration of the QoL in elderly population. Under this context, a significant pillar of research is the investigation of the QoL people with OSO may experience and the limitations they may encounter in everyday life.
Materials and Methods
Participants
A cross-sectional study was performed assessing 34 women and 16 men equal or over 65 years old from the patient support group ‘Butterfly’ between February 2018- May 2018; this club is for elderly people who suffer from osteoporosis. Advertisements were posted in several community Centers for the Open Care for the Elderly where osteoporosis preventive days has taken place from the “Butterfly” patient support group; the sample were collecting using random sampling in the elderly where Quantitative Ultrasound were performed and with a T score <-1. All procedures have been approved by the Human Research Ethics Committee of Queen Margaret University (QMU Code of Good Practice in Research- approved 2 May 2018). People entitled to participate should meet the following criteria: a) aged over 65-years old and b) are diagnosed with osteoporosis (osteoporosis must be diagnosed by a physician). Excluding criteria have been: a) if people were then or earlier under cancer treatment or under any treatment that may cause osteoporosis and b) people with kidney failure and with GFR< 60c.
Diagnosis of sarcopenia
Clinicians use hand grip strength and walking speed over a short distance in order to estimate muscle strength and physical performance[51,52] which are interwoven with ESPEN[32] algorithm (first version guidelines) to diagnose sarcopenia based on loss of muscle mass and strength (Hand grip) and/or function (gait speed)[57]. The cut-offs for gait-speed were <0.8 m/s and for strength suggested cut-off points were <20 kg for women and <30 kg for men.
Anthropometric Measurements
In order to detect the reallocation of body fat and lean muscle, upper-arm anthropometry was used as it is a recognized technique in clinical diagnosis and disease prevalence[48-50]. Anthropometric measurements, height, weight, Body Mass Index (BMI), Mid Upper Arm Circumference (MUAC), and Triceps Skin Fold (TSF) have been measured with standard procedures[53]. Additionally, waist and hip, circumference were measured as well and Waist to hip ratio was calculated. Hip and waist circumferences were measured to the nearest millimeter with a flexible no elastic measuring tape[53] (Seca 201). TSF was measured (to the nearest 0,1 mm) at the upper arm midpoint mark on the posterior surface of the right upper arm with Harpenden Skinfold Caliper[53]. Furthermore, Mid-arm muscle area (MAMA) has been calculated as follows: MAMA(cm[2])=[MUAC-3,14(TSF)]2/4*3,14. Because of the overestimation of MAMA we calculate corrected MAMA (cMAMA) as follow for men and women respectively: [(MUAC-3,14(TSF))2/4*3,14] -10 and [(MUAC-3,14(TSF))2/4*3,14] -6,5 [82,83]. Body fat (BF) percentage was calculated from Lean and colleagues[56] equation: BF%(male)=0,353 *waist+0,756*TSF+0,235*age-5,5 and BF%(female)=0,232*waist+ 0,657*TSF+ 0,215*age -9,4. This equation is the most accurate prediction with the least bias that can detect fat mass reallocation[56]. Additionally, muscle mass was calculated as follow: Muscle Mass (kg)=(Height in cm[2]) * (0,0264+0,0029*cMAMA)[54]. The appendicular skeletal muscle mass cut off points to identify sarcopenia according to European Working Group on Sarcopenia in Older people (EWGSOP)[57] for men and women respectively are 9,2 kg/m[2] and 7,4 kg/m[2]; to this end we convert Height in cm to Height in m[2] and we calculate Height adjusted Muscle mass as follow: Muscle mass/height in m[2]. Weight and height were measured using a Digital column scale with height rode (Tanita, WB-800H). Both measurements were carried out with standard procedure[53]. All anthropometric measurements were performed three times in standardized way by the same investigator, a trained registered dietitian.
Hand Grip Strength
Muscle strength and muscle mass were assessed with Baseline Pneumatic Bulb Hand Dynamometer. Hand Grip Strength (HGS) is a fast, reliable and simple measurement and it is considered to be an important index for the diagnosis of sarcopenia since low HGS is a clinical marker of poor mobility and low muscle mass[59,60]. The position that participants had as they performed the hand grip was in accordance with several papers; participants were at standing posture, using only the dominant hand with an angle of 90 degree at elbow joint and 180 degrees at shoulder joint, additionally the wrist and trunk were at neutral positions Participants conducted HGS three times (with 30 s rest between them) and the best of these three attempts has been recorded.
Gait speed
In the present study a 10-meter unobstructed and flat ground was used to perform gait speed test. Two end lines were taped in the ground; one in the beginning and one in the end of the 10m. Participants were asked to walk at a self-selected pace and a stopwatch (MARATHON Adanac 3000 Digital Stopwatch Timer) was used to record the time. Participants repeated the test twice with the average of the two trials used for grading purposes; the variance between the two efforts were between 1 and 4 tenths of a second.
Heel ultrasound
We assessed BMD and bone stiffness by measuring stiffness index in the calcaneus bone with an ultrasound device (Achilles InSight™ bone ultrasonometer, GE Healthcare), grounded on quantitative ultrasound technique. Cut off points for osteopenia defined as -1<T score<-2,5 and osteoporosis T score>-2,5. Heel ultrasound is a bone health assessment technique which is widely acceptable for estimating the bone health status both in men and women[66-68].
Quality of Life
In order to assess the health-related quality of life outcome in the participants the QoL questionnaire of the European Foundation for Osteoporosis -41 (QUALEFFO-41) was used as it is the most often used questionnaire in people with osteoporosis and vertebral fractures[70]. QUALEFFO-41 is translated in Greek by official interpreters[71], consists of 41 questions and comprises of five domains: pain, physical function, social function, general health perception, and mental function[72].
Data analysis
Quantitative analysis was used to process the experimental data. The data were encoded, and subsequently statistical processing and analyses of the results were conducted with IBM SPSS statistics version 22.0 (IBM Co., Armonk, NY, USA). All the quantitative parameters were expressed as means +/- SD, unless otherwise stated. All variables were tested using the Kolmogorov-Smirnov test for normality; when the distribution was not normal the record was transformed. Variables from gait speed, hand grip strength, Z-score and muscle mass measurements were compared between people with OSO and people without OSO using analysis of Variance (ANOVA) controlled for age, followed by Bonferroni corrections. Qualitative parameters (qualeffo-41 scores) were expressed in a Box plot as medians and inter-quartile range. To determine the percentage of people who experience OSO we used descriptive statistics while to examine the frequency of men and women that experience OSO we performed a chi-square test. To assess the correlation of OSO with functional deficits, leading to lower QoL, we performed chi squares for various parameters that were evaluated through the questionnaires. The significance threshold set at p< .05.
Results
Prevalence of OSO
The research included a total of n=50 osteoporotic/osteopenic older adults of both sexes; women n=34 and men n=16. Table 1 depicts the descriptive characteristics of subjects inclusively age, anthropometrics, body composition estimations, bone density as defined by participants, Z score and physical performance parameters (gait speed and hand grip strength). Statistically significant differences were observed between sexes and triceps skinfold, Waist to Hip Ratio (WHR), body fat percentage and hand grip strength (p<.05). Regarding physical performance parameters, males had a better score in hand grip strength while gait speed and z score had no significant difference.
Table 1.
Descriptive characteristics of 50 osteoporotic/osteopenic older adults.
| Variables | Women (n=34) | Men (n=16) | ||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean±SD | Minimum | Maximumx | Mean±SD | |
| Age (yrs) | 65,00 | 83,00 | 72,50±5,15 | 65,00 | 81,00 | 73,25±5,13 |
| Mid Upper Arm Circumference (cm) | 23 | 49 | 32,34±4,6 | 26 | 37 | 3,79±3,79 |
| Tricep_Skinfold(mm) | 14,00 | 54,00 | 23,47±7,17 | 7,00 | 32,00 | 16,56±5,60 |
| Waist to Hip Ratio | ,09 | ,99 | ,83± ,144 | ,81 | 1,09 | ,94± ,082 |
| Corrected Mid Arm Muscle Area(mm) | 16,60 | 75,20 | 43,76±11,92 | 25,00 | 65,50 | 42,99±12.83 |
| Body Fat % | 37 | 74 | 47,64±6,75 | 25 | 58 | 39,01±8,29 |
| Muscle_Mass (kg) | 4,70 | 16,52 | 9,78±2,40 | 5,87 | 13,43 | 8,87±2,27 |
| Hand_Grip Strenght (bar) | ,15 | ,50 | ,28± ,08 | ,22 | ,65 | ,36± ,14 |
| Gait_Speed (m/s) | 7,00 | 16,00 | 10,09±2,74 | 6,30 | 13,50 | 10,34±2,42 |
| Heel_Ultrasound Bone Mineral Densi-ty (T Score) | -4,20 | -1,00 | -2,70± ,79 | -4,00 | -1,30 | -2,59± ,69 |
The prevalence of OSO in the 50 osteoporotic/osteopenic participants, where almost 40% (n=19) have experienced OSO and just above 60% (n=31) have not experienced OSO. Almost the half of the participants (n=24) that have not experienced OSO accounts for women and a 14% (n=7) accounts for men; regarding the 19 people (38%) that experienced OSO women represent 20% (n=10) and men 18% (n=9).
Based on the results of Chi-square test (Figure 2) we can state that there was not a significant association between sex and prevalence of OSO (Χ[2](1)=3,326, p>.05). The clustered bar chart from the Crosstabs procedure emphasizes that there are not differences within the two sexes groups. Almost the same number of men and women have experienced OSO, with n=9 and n=10 respectively.
Figure 2.
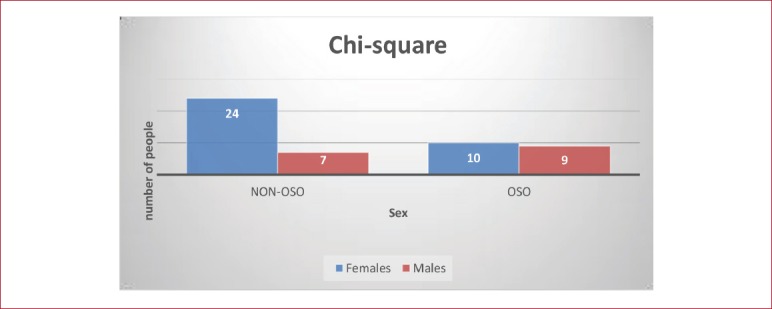
Chi-square clustered bar chart. Differences within the males and females’ group.
Furthermore, there were not significant differences in the QoL total score from QUALEFFO-41 between the 2 groups (Figure 3). P value (0,878) is greater than the significance level we set α=0.05 and we accept the null hypothesis that there was not a significant association between quality of life and people with OSO or without OSO (Χ[2](2)=0,261, p>.05).
Figure 3.
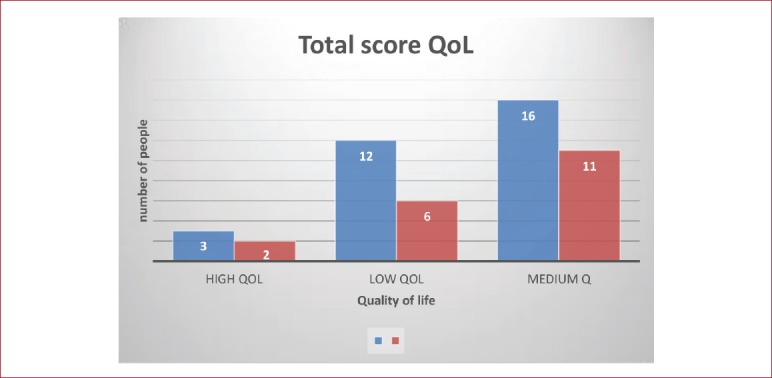
The total score in Quality of Life and the association with OSO.
Discussion
The objective of the present study was to identify the coexistence of obesity and sarcopenia in a generally healthy osteoporotic/osteopenic population of older adults aged ≥65 years from both sexes, and then to evaluate the correlation between people with OSO and the quality of life. There is a plethora of compounds and cut-off points in use for the identification of OSO as there are not standardized diagnostic criteria[73]. Ilich and colleagues[74] have recommended a combination of the diagnostic criteria used for the definition of osteoporosis, sarcopenia and obesity so as to define OSO. In this study, we didn’t define obesity using BMI, as it is an inadequate marker to identify adiposity in elderly and cannot detect fat infiltration into bones and muscles[75-78]. Dufour and colleagues[79] suggested the cutoff point of total body fat for obesity to be >30% for males and >40% for females. Recent findings point out a threshold of fat mass between 33-38% at which BMD for most skeletal sites starts to decline, however the findings were only for overweight and obese women[74]. The most recent cutoffs from the American Society of Bariatric Physicians board[80]; were utilized to classify obesity they recommend cutoffs of 25% and 30% of total body fat measured by dual-energy X-ray absorptiometry for males and females respectively. Ilich and colleagues[81] use the cutoff ≥35 % of total body fat measured by DXA; on the grounds that in this study we use validated equation to estimate body fat percentage with a small error in comparison with DXA[57] the ASBP cutoffs was used to avoid underestimating the BF percentage which originates from this error. In the present study all of our participants (n=50) were obese; however, the result would be the same in both cases of cutoff points (ASBP cutoffs or ≥35%). For the diagnosis of osteoporosis/osteopenia we used the World Health Organization[82] diagnostic criteria based on BMD measurement where osteopenia defined as -1<T score<-2,5 and osteoporosis T score>-2,5. Touching the definition of sarcopenia we follow the first version of ESPEN[53] algorithm and we used the muscle mass cut off points according to European Working Group on Sarcopenia in Older people (EWGSOP)[58].
This study was based in validated anthropometric predictive equations for estimating body composition. Anthropometric method has been a non-invasive, valid, precise, harmless, and inexpensive alternative to measure body composition in clinical practice and research[83-85]. Kanellakis & Manios[84] executed the validation of five anthropometric models in Greek postmenopausal women, one of which we used in our research with the advantages and disadvantages that an equation may have. Furthermore, Pereira and colleagues[86] suggested the use of a combination with anthropometric models and valid equations to assess muscle mass and body fat percentage in elderly as a strategy to identify people with sarcopenic obesity, caused by fat infiltration.
Vital part for the identification of sarcopenia is not only the measuring of body composition and skeletal muscle mass, but the measurement of physical performance and strength[87], which used in the suggested algorithm for sarcopenia case finding in older individuals[53]. In this algorithm the suggested cut-off points for hand grip strength are <20 kg for women and <30 kg for men when measured with Jamar dynamometer. In this research, we did not employ Jamar hand dynamometer (Lafayette Instrument Company, USA) which is accepted as the golden standard to measure hand grip strength. Moreover, it is the most used and cited dynamometer in the literature. However, we have used Dynatest Riester Pneumatic Bulb Hand Dynamometer while some studies used it to measure hand grip strength in elderly[89]. No cutoff points were published for this specific dynamometer; for the needs of our research we choose cut off points as follows: quartiles of grip strength were calculated on our sample and we set as cut off point the median of the first quartile separated for each sex which corresponded to a cut-off point of 0,21 bars and 0,25 bars for females and males respectively. The cutoffs we set conform to González and colleagues[89] cut-off point using the same dynamometer.
In the present study women had higher percentage of body fat and muscle mass than men since women had greater MUAC than men and this affected the predictive equations we used. The results are in accordance to the other findings of our study that men have a greater percentage of sarcopenia (56,25%) than women (29,4%). Epidemiological studies have been conflicting regarding the prevalence of sarcopenia between the two sexes[11,90,91]. Recently Tay and colleagues[92] (p. 121) stated that “data from the Framingham Heart Study had suggested that longitudinal decline in fat-free mass was consequent to a withdrawal of anabolic stimuli in men but reflecting an increase in catabolic stimuli represented by interleukin-6 (IL-6) in women” which explain our results for the lower muscle mass in males as they have lower percentage of body fat which leads on faster rate of muscle loss. Regarding the physical performance parameters, men had better hand grip strength (0,36 bars) than women (0,28 bars) but the gait speed time was almost the same for both sexes. Several studies[62,93,94] claim that regardless of age, men have better hand grip strength and muscle strength than women while gait speed (physical activity) is not related to sex[95]. It is well established that prevalence of osteoporosis is higher in women than in men[96], in our study women had lower BMD than men according to the z score results from heel ultrasound. Alswat[97] states that osteoporosis can affect both sexes but at different rates and at different ages; there is a rapid increase of osteoporosis in women after the 6th decade of life while in men this considerable change takes place after the age of 7th decade. Furthermore, women tend to have four times further up rates in osteoporosis than men[97].
We evaluated quality of life with the questionnaire QUALEFFO-41 and we separated the results into three categories: high, medium and low quality of life. This questionnaire is validated to evaluate the quality of life in osteoporotic/osteopenic people. Even though no association has been observed between the total score of quality of life and people with OSO, the mean score in some domains of QUALEFFO-41 was lower in people experience OSO but no statistically significant difference was found between domains of QUALEFFO-41 and people with OSO. The results were in contrary to the literature- probably the questionnaire was not suitable and the sample size was small to reflect the differences in QoL between the two groups- as OSO affecting quality of life and is related with mobility limitations, increased risk of falls, NCDs and mortality[3,18,73]. When we compare each domain of the questionnaire between people with OSO and without OSO it was observed that in some cases people who experience OSO had better quality of life in this specific domain. Although there are published studies evaluating QoL and osteoporosis or QoL and sarcopenia, no studies have been found regarding quality of life evaluation with questionnaire in OSO people to compare our findings. Silva Neto and colleagues[98] investigated the relationship between sarcopenic obesity, sarcopenia and QoL in elderly women using SF-36 questionnaire. No association was found between sarcopenia, sarcopenic obesity and QoL. Studies that have used general questionnaires to evaluate QoL in sarcopenic individuals showing no difference in quality of life between sarcopenic and non-sarcopenic people[99]. QUALEFFO-41 is a validated questionnaire for osteoporotic people with vertebral deformities[72] and probably the questions are not adequate for people who suffer from OSO even though osteoporosis exists, but without investigate if the participants address any vertebral deformities. It is more likely that none of the other validated QoL questionnaires would be proper to assess QoL in our participants. As Silva Neto and colleagues[98] (p. 365) state “….the general concept of QoL is broad and subjective, involving the individual’s perception of life as well as his expectations and concerns” and does not involve functioning and kinetic findings after medical assessment”. Beaudart and colleagues[99] claim that it is important to use specific questionnaire proper to sarcopenia in order to evaluate the QoL and people who suffer from sarcopenia. Probably, to evaluate the QoL and individuals with OSO is important to develop questionnaire that contains a blend of questions suitable both for osteoporosis and sarcopenia.
Osteoporosis, Sarcopenia and obesity are three metabolic diseases which share the same pathophysiological paths[3], the term OSO is the combination of these three conditions[18] and according to several papers postmenopausal women have more chances to develop OSO due to their estrogen depletion[73]. Although there is a wealth of studies for the prevalence of sarcopenic obesity with participants from both sexes, there are not published studies regarding the prevalence of OSO including both sexes; all the studies thus far have been focusing only on women participants. This is mainly due to the fact that osteoporosis is characterizing OSO and is considered to be a skeletal disease of postmenopausal women[12]; on the other hand, osteoporosis in males is rarely diagnosed through BMD screening and when it is diagnosed is after a fracture[100], which is also the main reason for our small male sample. No association was found in this study between gender and prevalence of OSO, apparently OSO exists in both sexes and it is not a postmenopausal women syndrome as it is mistakenly believed.
The present research had some limitations. Since this was a cross-sectional study it did not allow us to define causal-effect relationship and also the sample size was small. Another limitation has been the use of anthropometric equations to assess body composition; DXA and BIA are the most often used methods to assess wholebody fat or muscle mass. Moreover, a third limitation was the use of QUS to determine z-score which presents a drawback owing to the anthropometric parameters and the soft tissue thickness and temperature which may lead to a no so precise result as DXA. Regardless of the DXA limitations it is still the gold standard to define osteoporosis and also can be readily used in routine clinical practice to assess body composition; in studies like this where z-score, muscle mass and body fat percentage are vital parameters of the research it is crucial to evaluate them with the more precise method. One additional limitation has been the use of the Pneumatic Bulb Hand Dynamometer instead of the Jamar hand grip dynamometer. There are not any published cut offs for dynamometers that use psi as measurement unit of strength and beyond that the published cutoffs to identify sarcopenia from ESPEN use kg as the measurement unit of strength. As a limitation can be account also the unbalanced sample size regarding the sex. The last limitation has been the application of QUALEFFO-41 to assess the QoL in OSO people. More appropriate questionnaires in OSO condition are deemed necessary in order to evaluate the QoL of these people.
Future research should confirm these results by considering participants (men and women) from all over the country in order to achieve a more concrete image about the prevalence of OSO. Additionally, more accurate measures (DXA) should be used for total body composition alongside a hand grip dynamometer that measures strength in kg so as to be more precise with the publisher cutoffs both in hand grip strength and body composition (body fat and muscle mass). Furthermore, future studies that evaluate the QoL and OSO should contain a more suitable and pitched to this condition questionnaire.
Conclusions
Despite the differences between existing studies, regarding the diagnostic tools used to measure total body composition and BMD as well as cutoffs, this cross-sectional study can be used as a pilot study for further research since it appears that there is a remarkable percentage with people that experience OSO regardless of sex. OSO as a consequence of sarcopenic obesity and osteoporosis has great impact in health and in clinical implication, reducing the expectancy of life and the QoL. To detect this multifactorial condition a multifarious approach with precise diagnostic methods is deemed necessary. In literature the knowledge on OSO’s etiology, prevalence, and consequences is very limited and future research is needed to understand better the pathophysiology of these conditions.
Footnotes
Edited by: Dawn Skelton
References
- 1.Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone:connecting the dots on cellular, hormonal, and whole-body levels. Ageing Research Review. 2014;15(51):51–60. doi: 10.1016/j.arr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Stenholm S, Harris T.B, Rantanen T, Visser M, Kritchevsky SB, Ferrucci l. Sarcopenic obesity - definition, etiology and consequences. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, Panton L. Osteosarcopenic obesity:the role of bone, muscle, and fat on health. Journal of Cachexia, Sarcopenia and Muscle. 2014;5(3):183–192. doi: 10.1007/s13539-014-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ST-Onge M-P, Gallagher D. Body composition changes with aging:The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26(2):152–155. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demontiero O, Vidal C, Duque G. Aging and bone loss:new insights for the clinician. Therapeutic advances in musculoskeletal disease. 2012;4(2):61–76. doi: 10.1177/1759720X11430858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Digirolamo DJ, Kiel DP, Esser KA. Bone and Skeletal Muscle:Neighbors with Close Ties. Journal of Bone and Mineral Research:The Official Journal of the American Society for Bone and Mineral Research. 2013;28(7):1509–1518. doi: 10.1002/jbmr.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE. Assessment of nutritional status in cancer--the relationship between body composition and pharmacokinetics. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(8):1197–1203. doi: 10.2174/18715206113139990322. [DOI] [PubMed] [Google Scholar]
- 8.Steves CJ, Bird S, Williams FMK, Spector TD. The Microbiome and Musculoskeletal Conditions of Aging:A Review of Evidence for Impact and Potential Therapeutics. Journal of Bone and Mineral Research. 2015;31(2):261–269. doi: 10.1002/jbmr.2765. [DOI] [PubMed] [Google Scholar]
- 9.World population ageing -Highlights. New York: United Nations; 2017. United Nations-Department Of Economic And Social Affairs. ST/ESA/SER.A/397. [Google Scholar]
- 10.WHO. Ageing and health. Key facts [online] Geneva: World Health Organization; 2018. [[viewed 20 April 2018]]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/ageing-and-health . [Google Scholar]
- 11.Patel HP, Clift E, Lewis L, Cooper C. Epidemiology of Sarcopenia and Frailty. In: Dionyssiotis Y, editor. Frailty and Sarcopenia - Onset, Development and Clinical Challenges [online] Vienna: Intech; 2017. Available from: https://www.intechopen.com/books/frailty-and-sarcopenia-onset-development-and-clinical-challenges/epidemiology-of-sarcopenia-and-frailty . [Google Scholar]
- 12.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, Mccloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union:Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Archives of Osteoporosis. 2013;8(136) doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travison TG, Araujo AB, Esche GR, Mckinlay JB. The relationship between body composition and bone mineral content:threshold effects in a racially and ethnically diverse group of men. Osteoporosis International. 2008;19(1):29–38. doi: 10.1007/s00198-007-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein-Nulend J, Bacabac RG, Bakker AD. 2012. Mechanical loading and how it affects bone cells:the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater. 2012;24(24):278–91. doi: 10.22203/ecm.v024a20. [DOI] [PubMed] [Google Scholar]
- 15.King GA, Deemer SE, Thompson DL. Relationship between leptin, adiponectin, bone mineral density, and measures of adiposity among pre-menopausal Hispanic and Caucasian women. Endocrine Research. 2010;35(3):106–117. doi: 10.3109/07435800.2010.496090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. The Journal of Clinical Endocrinology & Metabolism. 2008;93(11Suppl 1):S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis:Effect of fat mass on the determination of osteoporosis. Journal of Bone and Mineral Research. 2008;23(1):17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafarinasabian P, Inglis JE, Kelly OJ, Ilich JZ. Osteosarcopenic obesity in women:impact, prevalence, and management challenges. International Journal of Women's Health. 2017;9:33–42. doi: 10.2147/IJWH.S106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration:impact of age, inactivity, and exercise. The journal of nutrition, health &aging. 2010;14(5):362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamrick MW, Mcgee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle:Mechanisms and Comparisons with Bone Marrow Adiposity. Frontiers in Endocrinology. 2016;7(69) doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai M, De Paula FJA, Rosen CJ. New Insights into Osteoporosis:The Bone-Fat Connection. Journal of Internal Medicinen. 2012;272(4):317–329. doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu PY, Ilich JZ, Brummel-Smith K, Ghosh S. New insight into fat, muscle and bone relationship in women:determining the threshold at which body fat assumes negative relationship with bone mineral density. International Journal of Preventive Medicine. 2014;5(11):1452–1463. [PMC free article] [PubMed] [Google Scholar]
- 23.Berryman DE, List EO. Growth Hormone's Effect on Adipose Tissue:Quality versus Quantity. International Journal of Molecular Sciences. 2017;18(8):1621. doi: 10.3390/ijms18081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrucci L, Baroni M, Ranchelli A, Lauretani F, Maggio M, Mecocci P, Ruggiero C. Interaction Between Bone and Muscle in Older Persons with Mobility Limitations. Current Pharmaceutical Design. 2014;20(19):3178–3197. doi: 10.2174/13816128113196660690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terracciano C, Celi M, Lecce D, Baldi J, Rastelli E, Lena E, Massa R, Tarantino U. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporosis International. 2013;24(3):1095–1100. doi: 10.1007/s00198-012-1990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhillon RJS, Hasni S. Pathogenesis and Management of Sarcopenia. Clinics in Geriatric Medicine. 2017;33(1):17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narici MV, Maffulli N. Sarcopenia:characteristics, mechanisms and functional significance. British Medical Bulletin. 2010;95(1):139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 28.Woo T, Yu S, Visvanathan R. Systematic Literature Review on the Relationship Between Biomarkers of Sarcopenia and Quality of Life in Older People. The Journal of Frailty &Aging. 2016;5(2):88–99. doi: 10.14283/jfa.2016.93. [DOI] [PubMed] [Google Scholar]
- 29.Ishii S, Tanaka T, Akishita M, Ouchi Y, Tuji T, Iijima K For The Kashiwa Study Investigators. Metabolic Syndrome, Sarcopenia and Role of Sex and Age:Cross-Sectional Analysis of Kashiwa Cohort Study. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0112718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KM. Sarcopenia and sarcopenic obesity. The Korean Journal of Internal Medicine. 2016;31(6):1054–1060. doi: 10.3904/kjim.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, ligaments and tendons journal. 2014;3(4):346–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, De Van Der Schueren MAE, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clinical nutrition. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH For the health, aging, and body composition study. Longitudinal study of muscle strength, quality and adipose tissue infiltration. The American Journal of Clinical Nutrition. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keaveny T M, Kopperdahl DL, Melton LJ, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-Dependence of Femoral Strength in White Women and Men. Journal of Bone and Mineral Research. 2010;25(5):994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanick M, Brown-Borg HM. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochimica et Biophysica Acta. 2013;1832(9):1410–1420. doi: 10.1016/j.bbadis.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, Atherton PJ. Human Skeletal Muscle Disuse Atrophy:Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance - A Qualitative Review. Frontiers in Physiology. 2016;7(361) doi: 10.3389/fphys.2016.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults:A Systematic Review. Rejuvenation Research. 2013;16(2):05–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernabei R, Martone AM, Vetrano DL, Calvani R, Landi F, Marzetti E. Frailty, Physical Frailty, Sarcopenia:A New Conceptual Model. In: Giuseppe R, Marsan PA, Grassi C, editors. Active Ageing and Healthy Living:A Human Centered Approach in Research and Innovation as Source of Quality of Life. Amsterdam: IOS Press; 2014. pp. 78–84. [Google Scholar]
- 39.Makizako H, Shimada H, Doi T, Tsutsumimoto K, Suzuki T. Impact of physical frailty on disability in community-dwelling older adults:a prospective cohort study. BMJ Open. 2015;5(9) doi: 10.1136/bmjopen-2015-008462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu WL, Chen CY, Tsauo JY, Yang RS. Balance control in elderly people with osteoporosis. Journal of the Formosan Medical Association. 2014;113(6):334–339. doi: 10.1016/j.jfma.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Ricciardi A, Geraci A, Montagner IM, Alongi GD, Marinato L, Corso L. The Role of Osteoporosis in Hip Fractures in Two Italian Hospitals. SM Journal of orthopedics. 2016;2(4) [Google Scholar]
- 42.Inglis JE, Ilich JZ. The microbiome and osteosarcopenic obesity in older individuals in long-term care facilities. Current Osteoporosis Reports. 2015;13(5):358–362. doi: 10.1007/s11914-015-0287-7. [DOI] [PubMed] [Google Scholar]
- 43.Ilich JZ, Kelly OJ, Inglis JE. Osteosarcopenic obesity syndrome:what is it and how can it be identified and diagnosed? Current Gerontology and Geriatric Research. 2016;2016(2016):1–7. doi: 10.1155/2016/7325973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. Journal of Biomechanics. 2011;44(12):2299–2306. doi: 10.1016/j.jbiomech.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miljkovic I, Kuipers A, Cvejkus R, Bunker C, Patrick A, Gordon C, Zmuda J. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity. 2016;24(2):476–482. doi: 10.1002/oby.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H, Price F, Rudnicki MA. Satellite Cells and the Muscle Stem Cell Niche. Physiological Reviews. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alway SE, Myers MJ, Mohamed JS. Regulation of Satellite Cell Function in Sarcopenia. Frontiers in Aging Neuroscience. 2014;6(246) doi: 10.3389/fnagi.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conn PM. Handbook of models for human aging. USA: Elsevier; 2006. pp. 981–982. [Google Scholar]
- 49.Coqueiro Rda S, Barbosa AR, Borgatto AF. Anthropometric measurements in the elderly of Havana, Cuba:age and sex differences. Nutrition. 2009;25(1):33–9. doi: 10.1016/j.nut.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Jaswant S, Nitish M. Use of Upper-Arm Anthropometry as Measure of Body-Composition and Nutritional Assessment in Children and Adolescents (6-20 Years) of Assam, Northeast India. Ethiopian Journal of Health Sciences. 2014;24(3):243–252. doi: 10.4314/ejhs.v24i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pahor M, Manini T, Cesari M. Sarcopenia:clinical evaluation, biological markers and other evaluation tools. The Journal of Nutrition, Health & Aging. 2009;13(80):724–728. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cawthon PM. Assessment of Lean Mass and Physical Performance in Sarcopenia. Journal of Clinical Densitometry. 2015;18(4):467–71. doi: 10.1016/j.jocd.2015.05.063. [DOI] [PubMed] [Google Scholar]
- 53.NHANES. Anthropometry Procedures Manual [online] USA: CDC; 2007. [[viewed 5 January 2018]]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf . [Google Scholar]
- 54.Heymsfield SB, Mcmanus C, Smith J, Stevens VL, Nixon DW. Anthropometric measurement of muscle mass:revised equations for calculating bone-free arm muscle area. The American journal of clinical nutrition. 1982;36(40):680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 55.Tsiaousi ET, Xatzitolios AI. Anthropometry of malnutrition in end stage Liver disease. In: Preedy VR, editor. Handbook of anthropometry:physical measures of human form in health and disease. New York: Springer; 2012. pp. 2755–2766. [Google Scholar]
- 56.Lean ME, Han TS, Deurenberg P. Predicting body composition by densitometry from simple anthropometric measurements. American Journal of clinical nutrition. 1996;63(1):4–14. doi: 10.1093/ajcn/63.1.4. [DOI] [PubMed] [Google Scholar]
- 57.Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, Cruz-Jentoft A. J. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clinical nutrition. 2016;35(6):1557–1563. doi: 10.1016/j.clnu.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Sousa-Santos AR, Amaral TF. Differences in handgrip strength protocols to identify sarcopenia and frailty - a systematic review. BMC Geriatrics. 2017;17(238) doi: 10.1186/s12877-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohannon RW. Muscle strength:clinical and prognostic value of hand-grip dynamometry. Current Opinion in Clinical Nutrition & Metabolic Care. 2015;18(5):465–470. doi: 10.1097/MCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 60.Yoon J-I, Choi H, Ha YC. Mean Hand Grip Strength and Cut-off Value for Sarcopenia in Korean Adults Using KNHANES VI. Journal of Korean Medical Science. 2017;32(5):868–872. doi: 10.3346/jkms.2017.32.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arvandi M, Strasser B, Meisinger C, Volaklis K, Gothe RM, Siebert U, Ladwig KH, Grill E, Horsch A, Laxy M, Peters Thorand B. Gender differences in the association between grip strength and mortality in older adults:results from the KORA-age study. BMC Geriatrics. 2016;16(201) doi: 10.1186/s12877-016-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middleton A, Fritz SL, Lusardi M. Walking Speed:The Functional Vital Sign. Journal of Aging and Physical Activity. 2015;23(2):314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian Q, Zhang M, Deng Y, Duan J, Tu Q, Cao Y, Zhu Q, Yu W, Lü Y. Does Gait Speed Replace Comprehensive Geriatric Assessment in the Elderly? International Journal of Gerontology. 2016;10(4):232–236. [Google Scholar]
- 64.Jung HW, Jang IY, Lee CK, Yu SS, Hwang JK, Jeon C, Lee YS, Lee E. Usual gait speed is associated with frailty status, institutionalization, and mortality in community-dwelling rural older adults:a longitudinal analysis of the Aging Study of Pyeongchang Rural Area. Clinical interventions in aging. 2018;13:1079–1089. doi: 10.2147/CIA.S166863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohannon RW, Andrews AW. Normal walking speed:a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Mészáros S, Tóth E, Ferencz V, Csupor E, Hosszú E, Horváth C. Calcaneous quantitative ultrasound measurements predicts vertebral fractures in idiopathic male osteoporosis. Joint Bone Spine. 2007;74(1):79–84. doi: 10.1016/j.jbspin.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Miura S, Saavedra OL, Yamamoto S. Osteoporosis in urban post-menopausal women of the Philippines:prevalence and risk factors. Archives of Osteoporosis. 2008;3(1-2):17–24. [Google Scholar]
- 68.Chin KY, Ima-Nirwana S. Calcaneal Quantitative Ultrasound as a Determinant of Bone Health Status:What Properties of Bone Does It Reflect? International Journal of Medical Sciences. 2013;10(12):1778–1783. doi: 10.7150/ijms.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO. World Report on Ageing and Health [online] Geneva: World Health Organization; 2015. [[viewed 9 May 2018]]. Available from: http://apps.who.int/iris/bitstream/handle/10665/1⇆3/s9789240694811_eng.pdf?sequence=1 . [Google Scholar]
- 70.Tadic I, Vujasinovic Stupar N, Tasic L, Stevanovic D, Dimic A, Stamenkovic B, et al. Validation of the osteoporosis quality of life questionnaire QUALEFFO-41 for the Serbian population. Health and Quality of Life Outcomes. 2012;10(74) doi: 10.1186/1477-7525-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lips P, Leplege A. Development and validation of a quality of life questionnaire for patients with vertebral fractures:Qualeffo-41. Quality of Life Research. 2000;9(Sup.1):763–766. [Google Scholar]
- 72.Madureira MM, Ciconelli RM, Pereira RMR. Quality of life measurements in patients with osteoporosis and fractures. Clinics. 2012;67(11):1315–1320. doi: 10.6061/clinics/2012(11)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hita-Contreras F, Martínez-Amat A, Cruz-Díaz D, Pérez-López FR. Osteosarcopenic obesity and fall prevention strategies. Maturitas. 2015;80(2):126–32. doi: 10.1016/j.maturitas.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Ilich JZ, Kelly OJ, Kim Y, Spicer MT. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Archives of Industrial Hygiene and Toxicology. 2014;65(2):139–148. doi: 10.2478/10004-1254-65-2014-2541. [DOI] [PubMed] [Google Scholar]
- 75.Ramírez-Vélez R, Correa-Bautista JE, Sanders-Tordecilla A, Ojeda-Pardo ML, Cobo-Mejía EA, Castellanos-Vega R, Del P, García-Hermoso A, González-Jiménez E, Schmidt-Riovalle J, González-Ruíz K. Percentage of Body Fat and Fat Mass Index as a Screening Tool for Metabolic Syndrome Prediction in Colombian University Students. Nutrients. 2017;9(9):1009. doi: 10.3390/nu9091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P. American Heart Association Obesity Committee Of The Council On Nutrition;Physical Activity And Metabolism;Council On Arteriosclerosis;Thrombosis And Vascular Biology;Council On Cardiovascular Disease In The Young;Council On Cardiovascular Radiology And Intervention;Council On Cardiovascular Nursing Council On Epidemiology And Prevention;Council On The Kidney In Cardiovascular Disease And Stroke Council. Assessing adiposity:a scientific statement from the American Heart Association. Circulation. 2011;124(18):1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 77.Winter JE, Macinnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults:a meta-analysis. The American Journal of Clinical Nutrition. 2014;99(4):875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 78.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic Accuracy of Body Mass Index to Identify Obesity in Older Adults:NHANES 1999-2004. International Journal of Obesity. 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dufour AB, Hannan MT, Murabito JM, Kielk DP, Mclean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations:the Framingham Study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013;68(2):168–174. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bays HE, Seger J, Primack C, Long J, Shah NN, Clark TW, Mccarthy W. Obesity Algorithm presented by the Obesity Medicine Association 2017-2018. Available from: www.obesityalgorithm.org .
- 81.Ilich JZ, Inglis JE, Kelly OJ, McGee DL. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporosis International. 2015;26(11):2587–2595. doi: 10.1007/s00198-015-3186-y. [DOI] [PubMed] [Google Scholar]
- 82.WHO. Prevention and Management of Osteoporosis:Report of a WHO Scientific Group. Geneva: WHO; 2003. pp. 53–85. [Google Scholar]
- 83.Rech CR, Dellagrana RA, Marucci MFN, Petroski EL. Validity of anthropometric equations for the estimation of muscle mass in the elderly. Revista Brasileira de Cineantropometria & Desempenho Humano. 2012;14(Suppl1):23–31. [Google Scholar]
- 84.Kanellakis S, Manios Y. Validation of five simple models estimating body fat in white postmenopausal women:use in clinical practice and research. Obesity. 2012;20(6):1329–1332. doi: 10.1038/oby.2011.403. [DOI] [PubMed] [Google Scholar]
- 85.Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality:evolution of modern measurement concepts in the context of sarcopenia. The Proceedings of the Nutrition Society. 2015;74(4):355–366. doi: 10.1017/S0029665115000129. [DOI] [PubMed] [Google Scholar]
- 86.Pereira PMG, Da Silva GA, Santos GM, Petroski EL, Geraldes AAR. Development and validation of anthropometric equations to estimate appendicular muscle mass in elderly women. Nutrition Journal. 2013;12(92) doi: 10.1186/1475-2891-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Studenski SA, Peters KW, Alley DE, Cawthon PM, Mclean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Thuy-Tien L, Dam T-TL, Vassileva MT. The FNIH Sarcopenia Project:Rationale, Study Description, Conference Recommendations, and Final Estimates. The Journals of Gerontology Series A:Biological Sciences and Medical Sciences. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies:towards a standardised approach. Age and Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 89.González EG, Alonso FJO, Astiz MTV, Felix SS, Cardenas VG, Guzman JO, Abizanda P, Malagon MIV, Sevilla SO, Rexach JAS. Development and validation of a prognostic index for 6-and 12-month mortality in hospitalized older adults. Archives of gerontology and geriatrics. 2017;73:269–278. doi: 10.1016/j.archger.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Russo A, Bernabei R, Onder G. Prevalence and risk factors of sarcopenia among nursing home older residents. The Journals of Gerontology Series A:Biological Sciences and Medical Sciences. 2012;67(1):48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 91.Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK ILAS Research Group. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people:results from the I-Lan longitudinal aging study. Journal of the American Medical Directors Association. 2013;14(7):528.e1–528.e7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, Tay KS, Tan CH, Chong MS. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age. 2015;37(6):121. doi: 10.1007/s11357-015-9860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demura S, Aoki H, Sugiura H. Age differences in hand grip power in the elderly. Archives of Gerontology and Geriatrics. 2011;52(3):176–179. doi: 10.1016/j.archger.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 94.Mattioli RA, Cavalli AS, Ribeiro JAB, Da Silva MC. Association between handgrip strength and physical activity in hypertensive elderly individuals. Revista Brasileira de Geriatria e Gerontologia. 2015;18(4):881–891. [Google Scholar]
- 95.Ko S, Tolea MI, Hausdorff JM, Ferrucci L. Sex-specific differences in gait patterns of healthy older adults:Results from the Baltimore Longitudinal Study of Aging. Journal of Biomechanics. 2011;44(10):1974–1979. doi: 10.1016/j.jbiomech.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guggenbuhl P. Osteoporosis in males and females:Is there really a difference? Joint Bone Spine. 2009;76(6):595–601. doi: 10.1016/j.jbspin.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Alswat KA. Gender Disparities in Osteoporosis. Journal of Clinical Medicine Research. 2017;9(5):382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silva Neto LS, Karnikowiski MG, Tavares AB, Lima RM. Association between sarcopenia, sarcopenic obesity, muscle strength and quality of life variables in elderly women. Revista Brasileira De Fisioterapia. 2012;16(5):360–367. [PubMed] [Google Scholar]
- 99.Beaudart C, Reginster J-Y, Geerinck A, Locquet M, Bruyère O. Current review of the SarQoL®:a health-related quality of life questionnaire specific to sarcopenia. Expert Review of Pharmacoeconomics & Outcomes Research. 2017;17(4):335–341. doi: 10.1080/14737167.2017.1360768. [DOI] [PubMed] [Google Scholar]
- 100.Cheng N, Green ME. Osteoporosis screening for men:Are family physicians following the guidelines? Canadian Family Physician. 2008;54(8):1140–1141. [PMC free article] [PubMed] [Google Scholar]


