Abstract
Objectives:
There is little information about the risk factors for sarcopenia and dynapenia. This study aimed to assess the prevalence of sarcopenia and dynapenia and to verify which risk factors are associated with the elderly population.
Methods:
A total of 387 elderly people were evaluated. We used a questionnaire to identify socio-demographic and behavioral aspects. For physical performance, we used the Short Physical Performance Battery. Using the European Working Group of Sarcopenia in Older People consensus, we defined sarcopenia that includes the occurrence of low muscle mass, added to low muscle strength or low physical performance. Dynapenia was defined using handgrip strength.
Results:
Sarcopenia and dynapenia were identified in 15.3% and 38.2% of the elderly people, respectively; 15.8% of women and 14.2% of men had sarcopenia, and 52.4% of women and 13.5% of men had dynapenia. Sarcopenia was associated with the increase in aging, white race, smoking, and risk of malnutrition. Dynapenia is more likely to occur in women and hospitalized patients.
Conclusion:
Sarcopenia had a greater association with the risk factors evaluated here, mainly with smoking and nutritional status. On the other hand, dynapenia was different, having a greater association with hospital intervention.
Keywords: Prevalence, Strength, Sarcopenia, Muscle, Aged
Introduction
In 1989, Irwin Rosenberg[1] defined the term sarcopenia from Greek (sarco = muscle and penia = loss), which was originally defined as age-related loss of muscle mass[1]. The reduction in muscle mass associated with aging is mainly caused by the loss and atrophy of muscle fibers, notably those of type II2, being more expressive in the lower extremities[3]. This fact can be the main cause of muscle function decline, consequently, increasing the number of elderly individuals with loss of functional mobility, dependence, and fragility[4].
However, sarcopenia cannot be considered only in relation to age, but as a multidimensional geriatric syndrome[4], given its high prevalence in the elderly, since more than 50% of the population over 80 years old suffers from this medical condition[5,6]. In addition to the inherent aspects of aging itself, it is determined by genetic predisposition, life habits, changes in living conditions, and chronic diseases[7], which may contribute to the weakness and loss of independence in daily activities[8], still being an independent predictor of hospitalization, disability, and death[9].
In recent years, some experts have created consensus on a broader diagnosis of sarcopenia: The European Working Group on Sarcopenia in Older People (EWGSOP), which recommends a diagnosis based on decreased muscle mass, which is necessarily associated with the decrease in muscle strength or in physical performance[4].
In addition to this muscle mass decrease, dynapenia is another important factor, currently discussed, which can be related to functional incapacity, “dynapenia” is a Greek term translated as “poverty of strength”; this condition is also associated with advancing age[10]. It is possibly a result of mechanisms responsible for a decrease in muscle strength, attributed to a combination of neural and muscular factors, such as deficiencies in neural activation, thus representing muscle weakness with relatively higher risk for the development of functional disability in the elderly population[3,10,11]. Therefore, it is one of the many factors associated with falls and the high numbers of hospitalizations, and must be taken into account when identifying a risk for falls[12,13].
The FIBRA (Research Network on Fragility in Brazilian Elderly People – Rede de Pesquisa sobre Fragilidade em Idosos Brasileiros) was designed to investigate and survey the prevalence and risk profiles for the biological fragility syndrome, among others existing in elderly Brazilian populations, living in urban areas from localities with different levels of human development. The study considered socio-demographic, anthropometric, physical health, physical functionality, mental and psychological variables[14].
Therefore, this study aimed to evaluate the prevalence of sarcopenia and dynapenia and verify which risk factors are associated with the elderly population living in the city of Cuiabá, Mato Grosso State, Brazil.
Material and methods
Participants
We used the database from the subproject of the FIBRA, which is an exploratory cross-sectional project with a multidisciplinary and multi-centric approach, based on population, conducted between 2009 and 2010 in 17 Brazilian regions, selected by the criterion of quota sampling with different indices of human development.
The FIBRA assessed 513 elderly people living in the urban area of Cuiabá, Mato Grosso State, Brazil, using attendance forms, based on IBGE’s Census, which registered 17,329 elderly people[15]. which registered 17,329 elderly people. As inclusion criteria, we observed some requirements such as, 65 years old or older, have 75% attendance at Senior Centers sign a consent form, and complete all evaluation stages of this study.
In our study, 126 elderly individuals were excluded, who were younger than 65 years, had a severe mental disability, Parkinson’s disease in the severe or unstable stage, amputations and important orthopedic limitations, as well as those in the terminal stage. Thus, only 387 elderly people met all the criteria of this study.
Ethical considerations
The research was approved by the Research Ethics Committee (protocol number 196/96 CNS being approved under number 632/09) of the Júlio Müller University Hospital of the Federal University of Mato Grosso (HUJM-UFMT).
Anthropometric measurements
Body mass was determined using an electronic (Filizola® ID 1500; SP, Brazil) platform scale with a capacity of 200 kg and precision of 0.1 kg. Height was measured by a portable professional stadiometer (Sanny®; SP, Brazil) with a precision of 0.1 cm; then the body mass index (BMI) was calculated (kg/m2).
The calf circumference (CC) was measured with a flexible and inextensible plastic metric tape (Sanny®, SP, Brazil) with a precision of 0.1 cm. The CC was used to verify the nutritional status, considering as well-nourished the elderly people who presented values ≥31 cm for both genders[14].
Diagnosis and classification of sarcopenia
For the diagnosis of sarcopenia, the European Consensus on Sarcopenia in Old People (EWGSOP) was used, which recommends considering the low muscle mass presence along with low muscle function, strength or physical performance (Figure 1)[4].
Figure 1.
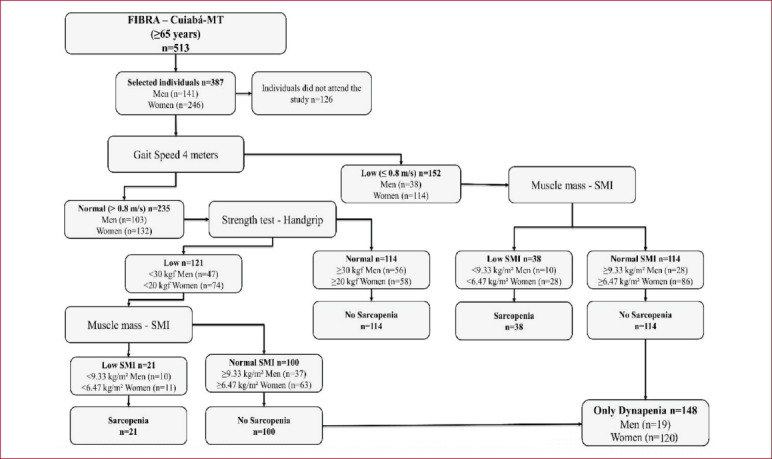
Study profile using the European Consensus on Sarcopenia in the Elderly, suggested for the case of finding sarcopenia in older individuals. [SMI = skeletal muscle index obtained by absolute skeletal muscle mass divided by height in meters squared (kg / m2)].
The skeletal muscle mass (SMM) was estimated using the Lee’s[16] mathematical equation according to the calculation below:
SMM (kg) = 0.244 x body mass + 7.8 x height + 6.6 x gender - 0.098 x age + ethnicity - 3.3
Body mass was determined in kilograms and height was measured in meters. We also used age (years), gender (1 for men and 0 for women), race (-1.2 for Asians, 1.4 for Afro-descendants and 0 for Caucasians).
This equation was validated in the Brazilian population using the dual-energy X-ray (DEXA) method, with a high correlation between the methods (r=0.86 for men and r=0.90 for women, P<0.05), in addition to high specificity (89%) and sensitivity (86%)[17].
The absolute SMM was normalized by height, [muscle mass (kg)/height (m)[2]], denominating the skeletal muscle mass index (SMI)[18]. The low muscle mass was defined by the SMI, with the exclusion based on 20% of the lowest percentile of the population distribution, representing the SMI of ≤6.47 kg/m2 for women and ≤9.33 kg/m2 for men[19].
Measurement of muscle strength
Muscle strength was measured by the handgrip strength (HGS) using a manual hydraulic dynamometer (Saehan Corporation®, Model SH5001, 973, Yangdeok-Dong, Masan 630-728, Korea). Three successive measurements were taken, and the best score out of three trials was recorded for analysis.
Using the EWGSOP consensus, low muscle strength was classified as HGS >30 kgf for men and >20 kgf for women[4], used to diagnose elderly people with dynapenia. We considered elderly people as having dynapenia those who exclusively lost only muscular strength (Figure 1).
The 4-meter walking test
The pace velocity (meters/second) was determined by the Short Physical Performance Battery (SPPB) walking test of 4 meters[20] which evaluates the low physical performance of lower limbs. Used a chronometer, the mean velocity (MV) was estimated by dividing the distance traveled by the time spent in the test. The low performance was classified by the MV (≤0.8 m/s)[4].
Physical activity level
The physical activity level was evaluated through a self-report on the weekly frequency and daily duration of physical exercises, active sports and household activities carried out in the last 14 days before the evaluation, which was based on items from the Minnesota Leisure Time Activity Questionnaire validated for Brazil[21], and adapted for the FIBRA Network’s study, maintaining common activities among Brazilian elderly people and including questions about frequency and duration. The questionnaire consisted of 42 closed yes/no questions, in addition to other questions about the continuity of activities over time, weekly frequency and duration.
The elderly individuals were considered active when they performed at least 150 minutes of weekly physical activity of moderate intensity exercises (from ≥3 MET to ≤6 MET), or 120 minutes of vigorous intensity exercise (>6 MET)[22].
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS for Windows, version 20.0) and the EpiInfo™ version 7.1.3.10. The Kolmogorov-Smirnov test was used to verify the normality of the independent variables. The variables were compared according to the presence or absence of the outcomes of interest, used the Mann-Whitney test for the non-parametric data, and Chi-square to examine the differences in the categorical variables between the two groups.
The prevalence of sarcopenia and dynapenia was estimated with a 95% confidence interval (CI). Multiple logistic regression analysis was used to evaluate the potential clinical and functional risk factors associated with sarcopenia and dynapenia. The multiple logistic regression model was calculated by including all variables that were associated with a P≤0.20 value in the univariate analysis, using the stepwise forward selection method to remove the unnecessary variables. Model 1 includes sarcopenia as a dependent variable and model 2 includes dynapenia. For all final analyses, the value of P≤0.05 and 95% confidence interval were used.
Results
The results were stratified in normal elderly individuals with sarcopenia and dynapenia. Sarcopenia and dynapenia were identified in 15.3% and 38.2% of the elderly participants, respectively. Age was associated with the groups, and the highest values of sarcopenia and dynapenia were observed in older people (older than 75 years and older than 80 years, respectively). The majority of the elderly individuals with dynapenia and sarcopenia were women; they had prevalent medical conditions of arterial hypertension and lower walking speed. Elderly participants with sarcopenia were significantly more likely to have lower BMI, CC, and SMM and SMI. Most of the elderly classified as having sarcopenia were at an increased nutritional risk because the average calf circumference was lower than the reference value (31.0 cm). The elderly with dynapenia had lower handgrip strength; however, in the SPPB score, those with sarcopenia obtained lower scores than the other groups (Table 1).
Table 1.
Descriptive characteristics of participants by group without sarcopenia and dynapenia, with only dynapenia or sarcopenia status in residents in Cuiabá (MT), Brazil, 2010 (n = 387).
| Variables | Without sarcopenia/dynapenia n = 180 (46.5%) | Dynapenia n = 148 (38.2%) | Sarcopenia n = 59 (15.3%) |
|---|---|---|---|
| Age, years | |||
| 65-69 | 77 (43%)* | 60 (41%)* | 10 (17%)* |
| 70-74 | 54 (30%)* | 45 (30%)* | 16 (27%)* |
| 75-79 | 37 (20%)* | 19 (13%)* | 15 (25%)* |
| 80 or more | 12 (7%)* | 24 (16%)* | 18 (31%)* |
| Gender | |||
| Men | 102 (57%)* | 19 (13%)* | 20 (34%)* |
| Women | 78 (43%)* | 129 (87%)* | 39 (66%)* |
| Race (white) | 61 (34%)* | 45 (30%)* | 29 (49%)* |
| Smoking (Yes) | 19 (11%) | 15 (10%) | 11 (19%) |
| Alcoholism (Yes) | 48 (27%) | 29 (20%) | 16 (27%) |
| Body mass, median (min-max), kg | 73.1 (42,3-116,3)a | 61.7 (42.3-106,4)b | 49.7 (34.1-70.7)c |
| Height, median (min-max), m | 1.60 (1.38-1.84)a | 1.51 (1.33-1.73)b | 1.54 (1.29-1.80)c |
| BMI, median (min-max), kg/m2 | 27.8 (15.1-41.0)a | 27.4 (20.9-39.6)a | 21.1 (15.3-26.8)b |
| Calf circumference, median (min-max), cm | 35.0 (25.5-49.5)a | 33.5 (26.0-46.0)b | 30.5 (23.5-37.0)c |
| SMM, median (min-max), kg | 26.0 (13.7-39.2)a | 18.0 (12.7-30.8)b | 14.9 (10.2-26.9)c |
| SMI, median (min-max), kg/m2 | 9.6 (5.2-12.9)a | 7.9 (6.5-13.4)b | 6.2 (4.5-9.3)c |
| Physical active (yes) | 65 (36%) | 60 (47%) | 26 (44%) |
| Cardiovascular disease (yes) | 34 (19%) | 23 (15%) | 12 (20%) |
| Hypertension (yes) | 125 (69%)* | 114 (77%)* | 30 (51%)* |
| Cerebrovascular accident (yes) | 8 (4%) | 6 (4%) | 3 (5%) |
| Diabetes mellitus (yes) | 37 (21%)* | 38 (26%)* | 6 (10%)* |
| Cancer (yes) | 6 (3%) | 5 (3%) | 1 (2%) |
| Arthritis (yes) | 60 (33%) | 60 (41%) | 29 (49%) |
| Pulmonary disease (yes) | 11 (6%) | 14 (10%) | 6 (10%) |
| Osteoporosis (yes) | 41 (23%)* | 64 (43%)* | 26 (44%)* |
| Hospitalization (Yes) | 27 (15%) | 36 (24%) | 13 (22%) |
| Falls (Yes) | 51 (28%)* | 64 (43%)* | 20 (34%)* |
| Hand-grip, median (min-max), kgf | 31.7 (20.0-51.3)a | 15.3 (2.0-29.7)b | 17.6 (4.0-31.4)b |
| Average speed, median (min-max), m/s | 0.97 (0.0-2.41)a | 0.80 (0.4-1.56)b | 0.74 (0.36-1.64)b |
| SPPB score total | 10.0 (1.0-12.0)a | 9.0 (0.0-12.0)b | 8.0 (3.0-12.0)b |
BMI = body mass index; SMM = skeletal muscle mass; SMI = skeletal muscle index; SPPB = short physical performance battery.
Indicates association between groups. Chi-square test with data expressed in number (% for subgroup number).
Different letters indicate difference between groups and same letters indicate similarity between groups (P≤0.05) Mann-Whitney test with data expressed in median (minimum-maximum).
The prevalence of sarcopenia was 15.8% in women and 14.2% in men (Table 2), and for dynapenia, it was 52.4% in women and 13.5% in men (Table 3). The prevalence of sarcopenia increased with advancing age, being higher in individuals aged 80 years or older and significantly different regarding the gender in all age groups, which was different from dynapenia.
Table 2.
Prevalence (%) and Confidence Interval (95%) of sarcopenia by age and gender in Cuiabá, MT, Brazil, 2010 (n = 387).
| Total | 65-69 years | 70-74 years | 75-79 years | 80 or more years | |
|---|---|---|---|---|---|
| Men | 14.2 | 8.0* | 13.3* | 15.2* | 38,5* |
| CI 95% | (8.9-21.1) (n = 20) | (2.2-19.2) (n = 4) | (5.1-26.8) (n = 6) | (5.1-31.9) (n = 5) | (13.9 – 68.4) (n = 5) |
| Women | 15.8 | 6.2* | 14.3* | 26,3* | 31.7* |
| CI 95% | (11.5-21.0) (n = 39) | (2.3-12.9) (n = 6) | (7.1-24.7) (n = 10) | (13.4-43.1) (n = 10) | (18.1-48.1) (n = 13) |
Indicates association between the age group within the gender group (P≤0.05). Chi-square to examine the differences in the categorical variables between the groups. CI = confidence interval.
Table 3.
Prevalence (%) and Confidence Interval (95%) of dynapenia by age and gender in Cuiabá, MT, Brazil, 2010 (n = 387).
| Total | 65-69 years | 70-74 years | 75-79 years | 80 or more years | |
|---|---|---|---|---|---|
| Men | 13.5 | 16.0 | 17.8 | 6.1 | 7.7 |
| CI 95% | (8.3-20.2) (n = 19) | (7.2-29.1) (n = 8) | (8.0-32.1) (n = 8) | (0.7-20.2) (n = 2) | (0.2-36.0) (n = 1) |
| Women | 52.4 | 53.6 | 52.9 | 44.7 | 56.1 |
| CI 95% | (46.0-58.8) (n = 129) | (43.2-63.8) (n = 52) | (40.6- 64.9) (n = 37) | (28.6-61.7) (n = 17) | (39.8-71.5) (n = 23) |
Chi-square to examine the differences in the categorical variables between the groups. CI = confidence interval.
The logistic regression analysis for the sarcopenia and dynapenia models is shown in [Table 4]. The odds ratio (OR) and 95% CI on the final model for the factors statistically associated with sarcopenia were: 2.85 (95% CI= 1.08-7.55) for those aged 75-79 years; 8.08 (95% CI= 3.03-21.58) for those aged 80 years and over; 3.02 (95% CI= 1.53-5.92) for whites; 2.87 (95% CI= 1.15-7.17) for smokers; and 11.14 (95% CI= 5.42-22.88) for those at risk of malnutrition related to the calf circumference (≤31 cm).
Table 4.
Multiple logistic regression models to test the association of sarcopenia and dynapenia with risk factors in 387 elderly residents in Cuiabá (MT), Brazil (2010).
| Variables | Sarcopenia Model | Dynapenia Model | ||||
|---|---|---|---|---|---|---|
| OR | CI 95% | P-value | OR | CI 95% | P-value | |
| Age (years) | ||||||
| 65-69 | 1.00 | 1.00 | ||||
| 70-74 | 1.80 | (0.70-4.61) | 0.216 | 1.17 | (0.66-2.07) | 0.571 |
| 75-79 | 2.85 | (1.08-7.55) | 0.033 | 0.65 | (0.32-1.31) | 0.229 |
| 80 or more | 8.08 | (3.03-21.58) | 0.000 | 0.96 | (0.47-1.96) | 0.913 |
| Men | 1.19 | (0.55-2.57) | 0.715 | 0.13 | (0.07-0.23) | 0.000 |
| Race white (yes) | 3.02 | (1.53-5.92) | 0.001 | 0.66 | (0.41-1.07) | 0.093 |
| Smoking (Yes) | 2.87 | (1.15-7.17) | 0.024 | 1.07 | (0.50-2.27) | 0.853 |
| Alcoholism (Yes) | 1.62 | (0.76-3.47) | 0.214 | 1.04 | (0.58-1.86) | 0.887 |
| Nutritional risk (CC ≤ 31 cm) | 11.14 | (5.42-22.88) | 0.000 | 0.50 | (0.27-0.93) | 0.027 |
| Physically active (yes) | 1.17 | (0.59-2.33) | 0.641 | 1.09 | (0.67-1.75) | 0.714 |
| Falls in the last year (yes) | 0.74 | (0.37-1.50) | 0.413 | 1.49 | (0.92-2.40) | 0.102 |
| Hospitalization (yes) | 0.84 | (0.38-1.88) | 0.678 | 2.10 | (1.15-3.82) | 0.015 |
OR = odds ratio; CI = confidence interval; CC = calf circumference.
However, for creating the dynapenia model, the other factors were maintained. Therefore, the risk factors associated with dynapenia were: 0.13 (95% CI= 0.07-0.23) for men, 0.50 (CI 95%= 0.27-0.93) for those at risk of malnutrition related to calf circumference (≤31 cm) and 2.10 (95% CI= 1.15-3.82) for those who already had been hospitalized. No significant associations were found in the other variables with the dynapenia model.
Discussion
The prevalence of sarcopenia and dynapenia may vary in different populations, age, gender, diagnostic methods and evaluation instruments. These conditions were investigated in a population not studied yet, with different cultural aspects, which had a prevalence of sarcopenia of 15.9% in women and 14.2% in men, associated with advancing age. As for the prevalence of dynapenia, in our study, it was diagnosed in 52.4% of women and in 13.5% of men. Because this is a recent issue, there are few studies about it.
The EWGSOP consensus algorithm to determine the prevalence of sarcopenia has been used in several studies. A study in a cohort in Mexico City[23], including 345 individuals aged 70 years and over, detected a prevalence of sarcopenia of 33.6% (27.4% in men and 48.5% in women). Other study in the Eastern Sydney[24], participants were recruited from a cohort of 792 community-dwelling older people, identified 22% of participants as sarcopenic. Other studies carried out in Denamark of patients recruited from a geriatric out-patient clinic[25], one with 299 community-dwelling older persons in the Netherlands[26], and 5046 subjects of 60 years of age or more in the Mexico[25], also found a high prevalence of sarcopenia (26%, 22.1% and 22%, respectively). These results are higher than those observed in the present study, possibly due to the sample profile, considering an older age and the method used to estimate muscle mass.
Other studies found a prevalence of sarcopenia lower than the one found in our study, as can be observed in the United Kingdom (UK)[27], the prevalence of sarcopenia in the elderly individuals was 7.8% (4.6% in men and 7.9% in women); however, in this case, the muscle mass was estimated by skinfolds. In elderly Italian individuals[28], 7.5% had sarcopenia, in study population included residents from three areas of Tianjin, China[2]9, 9.3% were identified as being affected by sarcopenia, 6.4% males and 11.5% females. Other study was carried out at the south-western area of Nigeria[30], observed the point prevalence of sarcopenia was 5.4%, higher among the females (7.1%) compared with the males (2.8%). Study more recent observed in the UK[31] and less than 3.3%, much lower than our results.
However, results similar to ours were found in a prospective study conducted with Belgian elderly people[32], in which 12.5% of the participants were classified as having sarcopenia, all of them were older than 80 years and used electrical bio-impedance to assess the SMM. Similar results were also found in Brazil, carried out in the city of São Paulo, which identified 15.4% of people with sarcopenia, of which 16.1% were women, and 14.4% were men[33]. Other study in Pelotas, the overall prevalence of sarcopenia was 13.9%[34].
However, these studies, despite having similarities in age average, other factors may explain the higher prevalence sarcopenia in our population. These factors include anthropometric, socioeconomic and life habits differences in the populations, different techniques and instruments used to measure muscle mass, and the adopted exclusion points, as well as the evaluator’s technique, equipment model, anatomical point location, and redistribution of body fat in different ethnicities which can represent considerable errors[35]. Regarding this variation, it is necessary to emphasize the importance of adopting a standardized and operational definition of sarcopenia for multidimensional geriatric assessment. However, a global consensus on the definition of sarcopenia was not achieved yet.
Regarding the prevalence of dynapenia, studies carried out in Mexico[36], and in the United States[37] showed lower values, compared to those found in our research. These studies lasted five years and evaluated the handgrip strength of elderly people; the first one recorded a prevalence of dynapenia in 24.7% of men and 25.1% of women[36] and the second one recorded 37.5% of elderly women with dynapenia[37]. As of another study with elderly Australian men (≥70 years)[38], there was a prevalence in 24.4% of the participants. Similar results were observed in a recent study conducted in the USA[39], which evaluated men and women (≥55 years), and found a prevalence of dynapenia in 24.3% of elderly patients, evaluating the leg extension strength (kgf) with a dynamometer.
Another study on elderly people (≥60 years) carried out in two cities in China[40], in which handgrip strength was measured by a dynamometer, recorded a dynapenia prevalence of 27.3%. A cohort study performed with Brazilian elderly people (≥60 years)[41], using some methods similar to those used in our study to classify dynapenia, found 29.5% of elderly people with dynapenia. Most of the studies recorded a higher predominance of dynapenia in elderly women. Such finding can also be observed in our study.
The prevalence of dynapenia found in previous studies is lower than those recorded in our study. Some reasons may explain this fact such as the absence of enough evidence in the literature to identify specific exclusion points, the lack of a definition and complete assessment of risk factors, and finally, there is no global consensus with methods and instruments used to define the diagnosis of dynapenia.
Nevertheless, a diagnostic algorithm for dynapenia was recently created by Manini & Clark[3] since they believe that the assessment of muscle strength only by handgrip strength is useful only in the screening phase, explaining about 40% of the variation of lower limbs strength. These authors consider the evaluation of muscle strength through knee extension more relevant in the diagnosis of dynapenia, due to its association with walking speed and physical function[3].
The handgrip strength was used in this study to measure dynapenia, due to being strongly related to the body’s overall strength, mortality, the risk of complications and functional disabilities[13,40]. Moreover, it is comparable to the strength of knee extensors, in addition to being easier to apply and has a low cost[9].
Some factors recorded in our study were associated with sarcopenia, corroborating previous findings, demonstrating that with the advancing age, sarcopenia is more likely to be diagnosed[40]–[42]. In addition, we observed an association between smoking and sarcopenia[19,33], possibly due to the compromising of the system’s ability to obtain muscular energy[33]. Smoking may be associated with other diseases, increased muscle fatigue, protein catabolism, and reduced muscle function and mass[2,19]. However, since smoking is a risk factor for sarcopenia, it should be determined by a prospective study. Moreover, it was not associated with dynapenia.
In our study, sarcopenia was associated with a high risk of malnutrition, measured by calf circumference, being highly prevalent in elderly people[33]. In addition to age, other factors may influence nutritional status, such as socioeconomic status, schooling, and diet[33,43]. It is also a consequence of energy and protein deficiencies that cause adverse effects on body composition, reducing muscle mass and, consequently, affecting body function[2,43].
The calf circumference measurement is one of the methods used to evaluate the nutritional status; it correlates with the muscle mass[44], explaining a small part of the variation[45], related to the decreased muscle mass of the legs. However, it is more representative of muscle mass in very sick or debilitated elderly people or in those at the end of life than in healthy or obese individuals[46], and in primary evaluation[44], besides being a poor screening tool for sarcopenia due to the low sensitivity indicated by the DEXA absorptiometry[45]. There was an association of CC with dynapenia; however, it was inversely to sarcopenia, making the dynapenia prediction based on nutritional risk difficult in elderly people; thus making CC difficult to be interpreted in the clinical evaluation of elderly individuals, since the measurements may differ according to the compressibility of the skin and subcutaneous tissue[17].
In our study, in addition to CC, dynapenia differed from sarcopenia in other factors. Its occurrence was higher in women than in men[40], and it was associated with hospitalization[13,47]. Therefore, the decreased upper extremity strength is associated with the risk of hospitalization, being considered a possible risk factor for future adverse health results[13,47].
In the present study, only sarcopenia was associated with advancing age. Some studies have shown there is an important loss of muscle function between 60 and 80 years of age, of which is become more accentuated after 80 years of age[5,6].
Muscle strength decreases faster than muscle mass in older people, and loss of strength cannot be explained only by decreased muscle mass[48]. Moreover, it is suggested that after 80 years of age, muscle strength and physical performance may be more relevant indicators for sarcopenia than using only the muscle mass index[5,38]. The handgrip is a global indicator of muscle strength, which can predict mortality through mechanisms different from those that lead a disease to affect the muscles[37]. Nevertheless, if muscle mass decreases to a critical point, the increased physical function can be compromised[49].
Firstly, this is a cross-sectional study, and therefore, a cause-and-effect relationship could not be established, and this is one of its limitations. Secondly, SMM and SMI were estimated by regression equations validated in American and Brazilian populations, presenting a high correlation with magnetic resonance and DEXA. Therefore, it may underestimate or overestimate the prevalence of sarcopenia. However, few studies have used DEXA in epidemiological and community studies, especially those carried out in Brazil, due to the high cost to estimate SMM. Thus, simple and viable alternatives which have the same function of DEXA without causing risk to population are indicated. At last, comparisons with other studies are difficult because dynapenia has neither a definition nor standardized and established measurement.
In the studied population, there is a high prevalence of sarcopenia and dynapenia, which have different associated factors. Sarcopenia increases with aging and in white people, in addition to smoking and nutritional risk estimated by the calf circumference. Dynapenia was more associated with women who had already been hospitalized. These syndromes are influenced by several factors; therefore, the ethnicity, lifestyle and physical performance should be considered when prescribing an appropriate intervention for the elderly people. Thus, the elderly with these characteristics should be the target of differentiated prevention strategies. However, prospective studies in this population and regions are necessary to explore the natural progression and predisposition of such factors, respecting their specificities.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50(5):1231–1233. [Google Scholar]
- 2.Walrand S, Guillet C, Salles J, Cano N, Boirie Y. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27(3):365–85. doi: 10.1016/j.cger.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Manini TM, Clark BC. Dynapenia and aging:an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia:European consensus on definition and diagnosis:Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31(4):461–7. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 6.Faulkner Ja, Larkin LM, Claflin DR, Brooks S V. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34(11):1091–6. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength;a quantitative review. Front Physiol. 2012 Jul;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcell TJ. Sarcopenia:causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58(10):M911–6. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Clark BC, Manini TM. Sarcopenia ≠dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 11.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–6. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pijnappels M, van der Burg PJCE, Reeves ND, van Dieën JH. Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol. 2008;102(5):585–92. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Silva Rodrigues RA, Martinez Espinosa M, Duarte Melo C, Rodrigues Perracini M, Rezende Fett WC, Fett CA. New values anthropometry for classification of nutritional status in the elderly. J Nutr Health Aging. 2014;18(7):655–61. doi: 10.1007/s12603-014-0451-2. [DOI] [PubMed] [Google Scholar]
- 15.Instituto Brasileiro de Geografiae Estatística - IBGE. Sinopse do Censo Demográfico de 2010. RJ - Brasil: Rio de Janeiro; 2011. Available at: http://www.ibge.gov.br/home/estatistica/populacao/censo2010/sinopse.pdf . [Google Scholar]
- 16.Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total-body skeletal muscle mass:development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72(3):796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 17.Rech CR, Dellagrana RA, Marucci M de FN, Petroski EL. Validade de equações antropométricas para estimar a massa muscular em idosos. Rev Bras Cineantropometria e Desempenho Hum. 2012;14(1):23–31. [Google Scholar]
- 18.Janssen I. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am J Epidemiol. 2004;159(4):413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function:Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Lustosa LP, Pereira DS, Dias RC, Britto R, Parentoni A, Pereira L. Tradução e adaptação transcultural do Minnesota Leisure Time Activities Questionnaire em idosos. Geriatr Gerontol. 2011;5(2):57–65. [Google Scholar]
- 22.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults:recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 23.Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU. Prevalence of sarcopenia in Mexico City. Eur Geriatr Med. 2012;3(3):157–160. [Google Scholar]
- 24.Menant JC, Weber F, Lo J, et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people:time to abandon the term sarcopenia? Osteoporos Int. 2017;28(1):59–70. doi: 10.1007/s00198-016-3691-7. [DOI] [PubMed] [Google Scholar]
- 25.Christensen MG, Piper KS, Dreier R, Suetta C, Andersen HE. Prevalence of sarcopenia in a Danish geriatric out-patient population. Dan Med J. 2018;65(6):3–8. [PubMed] [Google Scholar]
- 26.Reijnierse EM, Trappenburg MC, Blauw GJ, et al. Common Ground? The Concordance of Sarcopenia and Frailty Definitions. J Am Med Dir Assoc. 2016;17(4) doi: 10.1016/j.jamda.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition:findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42(3):378–84. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people:application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2014;69(4):438–46. doi: 10.1093/gerona/glt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han P, Kang L, Guo Q, et al. Prevalence and Factors Associated With Sarcopenia in Suburb-dwelling Older Chinese Using the Asian Working Group for Sarcopenia Definition. Journals Gerontol Ser A Biol Sci Med Sci. 2016;71(4):529–535. doi: 10.1093/gerona/glv108. [DOI] [PubMed] [Google Scholar]
- 30.Adebusoye L, Ogunbode A, Olowookere O, Ajayi S, Ladipo M. Factors associated with sarcopenia among older patients attending a geriatric clinic in Nigeria. Niger J Clin Pract. 2018;21(4):443–450. doi: 10.4103/njcp.njcp_374_17. [DOI] [PubMed] [Google Scholar]
- 31.Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of Sarcopenia:Associations with Previous Falls and Fracture in a Population Sample. Calcif Tissue Int. 2015;97(5) doi: 10.1007/s00223-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legrand D, Vaes B, Matheï C, Swine C, Degryse J-M. The prevalence of sarcopenia in very old individuals according to the European consensus definition:insights from the BELFRAIL study. Age Ageing. 2013;42(6):727–34. doi: 10.1093/ageing/aft128. [DOI] [PubMed] [Google Scholar]
- 33.Alexandre T, da S, Duarte YA, de O, Santos JLF, Wong R, Lebrão ML. Prevalence and associated factors of sarcopenia among elderly in Brazil:findings from the SABE study. J Nutr Health Aging. 2014;18(3):284–90. doi: 10.1007/s12603-013-0413-0. [DOI] [PubMed] [Google Scholar]
- 34.Barbosa-Silva TG, Bielemann RM, Gonzalez MC, Menezes AMB. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city:results of the COMO VAI?study. J Cachexia Sarcopenia Muscle. 2016;7(2):136–143. doi: 10.1002/jcsm.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micozzi MS, Harris TM. Age variations in the relation of body mass indices to estimates of body fat and muscle mass. Am J Phys Anthropol. 1990;81(3):375–9. doi: 10.1002/ajpa.1330810307. [DOI] [PubMed] [Google Scholar]
- 36.Al Snih S, Markides KS, Ray L, Ostir G V, Goodwin JS. Handgrip Strength and Mortality in Older Mexican Americans. J Am Geriatr Soc. 2002;50(7):1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 37.Rantanen T, Volpato S, Luigi Ferrucci M, Eino Heikkinen M, Fried LP, Guralnik JM. Handgrip Strength and Cause-Specific and Total Mortality in Older Disabled Women:Exploring the Mechanism. J Am Geriatr Soc. 2003;51(5):636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 38.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability:the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58(11):2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 39.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(1):71–7. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- 40.Yang M, Ding X, Luo L, Hao Q, Dong B. Disability associated with obesity, dynapenia and dynapenic-obesity in Chinese older adults. J Am Med Dir Assoc. 2014;15(2):150.e11–6. doi: 10.1016/j.jamda.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 41.da Silva Alexandre T, de Oliveira Duarte YA, Ferreira Santos JL, Wong R, Lebrão ML. Sarcopenia According to the European Working Group on Sarcopenia in Older People (EWGSOP) Versus Dynapenia as a Risk Factor for Disability in the Elderly. J Nutr Health Aging. 2014;18(5):547–53. doi: 10.1007/s12603-014-0465-9. [DOI] [PubMed] [Google Scholar]
- 42.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 43.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–23. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 44.Pagotto V, Santos KF, dos Malaquias SG, Bachion MM, Silveira EA. Calf circumference:clinical validation for evaluation of muscle mass in the elderly. Rev Bras Enferm. 2018;71(2):322–328. doi: 10.1590/0034-7167-2017-0121. [DOI] [PubMed] [Google Scholar]
- 45.Rolland Y, Lauwers-Cances V, Cournot M, et al. Sarcopenia, calf circumference, and physical function of elderly women:a cross-sectional study. J Am Geriatr Soc. 2003;51(8):1120–4. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 46.Martin AD, Spenst LF, Drinkwater DT, Clarys JP. Anthropometric estimation of muscle mass in men. Med Sci Sports Exerc. 1990;22(5):729–733. doi: 10.1249/00005768-199010000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–9. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28(5):495–503. doi: 10.1016/j.nut.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]


