Abstract
Objectives:
We conducted a systematic review to identify adverse effects of physical activity and/or exercise for adults with osteoporosis/osteopenia. We synthesised evidence from observational studies, and updated three previously published systematic reviews.
Methods:
We searched MEDLINE, EMBASE, CINAHL, Web of Science, grey literature and reference lists of relevant studies. Selection criteria were: (1) observational studies in patients with osteoporosis/osteopenia; and (2) in accordance with the criteria used in the previous reviews. A narrative synthesis was conducted for the observational data. Random effects meta-analysis was undertaken for the review updates.
Results:
For the observational synthesis 14 studies were included. The majority of studies reported no adverse events, reduced incidence/improvement, or no significant change after physical activity or exercise. Activities that involved spinal flexion (certain yoga moves and sit-ups) were associated with a greater risk of vertebral fractures but these events were rare. For the update of reviews, 57 additional studies were identified. Exercise was generally associated with a greater number of minor adverse events including mild muscle/joint pain. Serious adverse events were rare and could not be attributed to the intervention.
Conclusion:
Patients with osteoporosis/osteopenia can safely participate in structured exercise programmes, whether at home or in supervised facilities. Systematic review registration for observational studies: PROSPERO 2017: CRD42017070551
Keywords: Osteoporosis, Physical activity, Exercise, Adverse events, Systematic review
Introduction
Osteoporosis is one of the most common diseases to affect older people. With the ageing of the population worldwide, the numbers are expected to increase[1], and by the year 2025 it has been estimated that more than 30 million men and women aged 65 and older will be affected in the European Union alone[2]. It has been predicted that 40% of these women and 15-30% of men will develop at least one fragility fracture during their remaining lifetime[3,4]. Osteoporosis is a serious public health burden. Fragility fractures are associated with significant morbidity, mortality, decreased quality of life, and are a burden on health systems[5].
In the management of osteoporosis, pharmacological therapy, as well as advice on healthy living, is commonly provided to patients[6,7]; there is also an emphasis on maintaining or increasing physical activity and exercise to improve bone health and reduce falls. Given the substantially increased risk of falls and fractures in patients with osteoporosis (patients with osteoporosis tend to be women, elderly, frail, and have an increased risk of falls and fractures due to bone fragility), healthcare professionals, as well as the patients themselves, often question the safety of physical activity and exercise. Indeed, exercise programmes for such patients should be safe as well as sustainable. It is well established that physical activity, particularly weight-bearing exercise, is beneficial in preventing bone fractures and falls in individuals with low bone density, as well as in the prevention of osteoporosis[8-11]. However, providing accurate advice on safety and adverse effects of physical activity and exercise in patients with osteoporosis is hampered by limited data on adverse events and inconsistencies in the literature.
Three systematic reviews of interventional studies of exercise for adults with osteoporosis, treatment or prevention of osteoporosis and prevention of falls in older people[12-14] have been undertaken. However, there is lack of a comprehensive synthesis of the observational and non-randomised literature on the safety and adverse effects of physical activity or exercise in adults with osteoporosis, apart from two consensus statements[15,16].
In this context, using systematic review methodology, we aimed to identify evidence on specific adverse effects of physical activity and/or exercise in adults with osteoporosis or osteopenia. To do this we have carried out a systematic review of observational or non-randomised studies. The first objective was to assess the characteristics of people with osteoporosis or osteopenia who experience adverse events after physical activity and/or exercise. A second objective was to ascertain the specific exercises or movements within the activities that led to the adverse events. In addition, we assessed the various types of adverse events experienced after physical activity and/or exercise in people with osteoporosis or osteopenia. We also sought to identify any gaps in the available literature. Finally, given the publication of several interventional studies on the topic since 2011-16 covering exercise in people with osteoporosis, osteopenia or at risk of falls, we have updated the three published systematic reviews with a specific focus on adverse events.
Methods
(a) Observational and non-randomised studies
Data sources and search strategy: observational and non-randomised studies
We conducted the observational review using a predefined protocol, registered in the PROSPERO prospective register of systematic reviews (CRD42017070551). All reviews were carried out in accordance with PRISMA guidelines[17] (Appendix 1). Observational (prospective cohort, nested case-control, or case-control, retrospective cohort) studies, case reports, case studies, and non-RCTs which have reported on adverse events and safety issues associated with physical activity and/or exercise in patients with osteoporosis, were searched in MEDLINE, EMBASE, and CINAHL databases from inception to 27 June 2017. The computer-based searches combined free and MeSH search terms and combination of key words related to the exposures/interventions (e.g., “physical activity”, “exercise”) and outcomes (e.g., “adverse events”, “fractures”, “injuries”) and population (e.g., “osteoporosis”, “fragility fracture”) with no restrictions on language. The MEDLINE search strategy was adapted to the other databases using the appropriate controlled vocabulary. Reference lists of relevant articles such as reviews were manually scanned for additional studies likely to have been missed by the electronic search. Finally, we searched the ISI Web of Science for papers which cited the studies initially included in the review. Details on the MEDLINE search strategy are provided in Appendix 2.
Eligibility criteria
Studies were eligible if: (i) they were conducted in adults with osteoporosis or osteopenia; and (ii) they employed physical activity and/or exercise, either as a healthcare intervention or as a lifestyle choice. Adverse events were defined as any events the authors described as ‘adverse’. To ensure all relevant studies were included, no criteria were included to describe the definition of adverse events.
Data extraction and quality assessment
The initial screening of titles and abstracts to retrieve potentially relevant articles was performed by one reviewer (S.K.K.). Detailed evaluation of the full texts of these relevant articles was conducted to determine whether they met all inclusion criteria and this was conducted independently by two reviewers (S.K.K. and E.C.). If necessary, disagreements and uncertainties over inclusion/exclusion of an article were resolved by discussion with a third reviewer (S.L.). A standardized predesigned data collection form was used for data extraction. Data were extracted, where available, on study publication date, study design, geographical location, baseline age, percentage of females, duration of follow-up, sample size, type of exercise or physical activity, frequency and duration of activity, type of adverse events, and risk estimates where relevant. For non-randomised studies including cohort and case-control studies, methodological quality was assessed based on the nine-point Newcastle–Ottawa Scale (NOS)[18]. It uses three pre-defined domains namely: selection of participants (population representativeness), comparability (adjustment for confounders), and ascertainment of outcomes of interest. The NOS assigns a maximum of four points for selection, two points for comparability, and three points for outcome. Nine points on the NOS reflects the highest study quality. A score of ≥5 indicates adequate methodological quality.
Data synthesis and analysis
It was not possible to calculate summary measures or average effects across studies given the diversity of the intervention designs and study methods and the heterogeneous nature of the outcome measures. Effective comparisons could also not be made across studies because of the heterogeneity of the data. The findings of each relevant included study were summarized in tables that included the main characteristics of the study and the results in natural units as reported by the investigators. A narrative synthesis with separate results from each study was also performed. For the above reasons, it was not possible to conduct subgroup and sensitivity analyses.
(b) Updated systematic review of interventional evidence
We took a pragmatic decision to undertake a systematic review to update these three already published reviews[12-14] rather than carry out a new complete review ourselves. To identify all studies published since the systematic reviews, trials were selected in accordance with the exclusion and inclusion criteria used in the previous reviews. Databases searched were synonymous with those searched in the previous reviews in order to replicate the methodology to the highest degree possible. Two authors independently screened the searches, and independently assessed full-text articles for those with potential eligibility and resolved disagreement through discussion (SK and EC for the observational data; JW and LJ for the updated Sherrington review[12]; JW and MC for the updated Howe review[13]; JW and NG for the updated Giangregorio review[14]). One author (either MC, LJ or NG) extracted data on adverse events from the new eligible trials plus all the trials included in the previous reviews using a self-made data extraction form. Adverse events were reported as the number of participants reporting adverse events. Data were collected for serious adverse events, the total adverse events reported, the adverse events that were attributable to the exercise or control group, and finally the general adverse events reported. In trials that reported data on adverse events for both control and intervention participants, adverse event rates were pooled and analysed using random-effects meta-analysis using the ‘metan’ command in Stata (StataCorp 2011, Release 12). Where possible, all reported adverse events were calculated as incidence per person years using the number of adverse events divided by the number of participants in the study arm and the reported follow up time.
Results: observational and non-randomised data
Study identification and selection
The [Figure 1] shows the flow of studies through the review. The initial search of relevant databases and manual scanning of reference lists of relevant studies identified 1,013 potentially relevant citations. After the initial screening which was based on titles and abstracts, 32 articles remained for full text evaluation. Following detailed evaluation, 18 articles were excluded because: (i) they included populations not relevant to review (n=7); (ii) adverse event outcomes were not reported (n=5); and (iii) study designs were not relevant to review e.g. were randomised controlled trials (n=6). The remaining 14 articles comprising 13 unique studies met the inclusion criteria and were included in the review[19-32].
Figure 1.
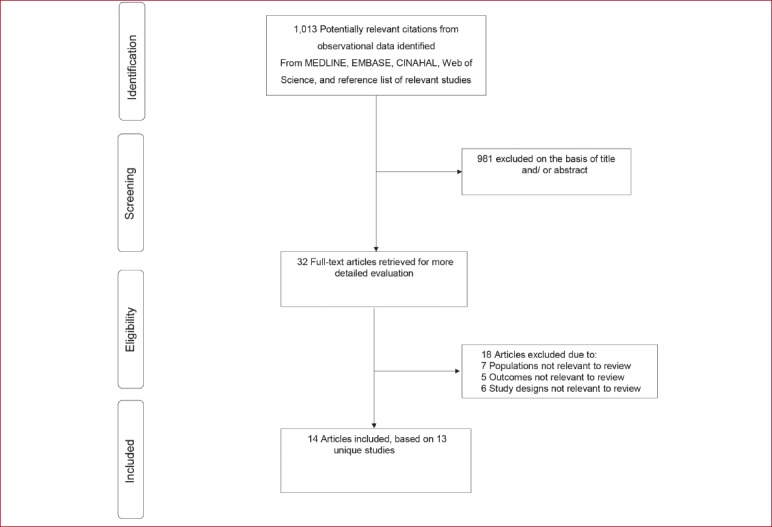
Selection of studies included in the review of observational studies.
Study characteristics and quality
[Table 1] summarizes the characteristics of the studies included in the review. Studies were published between 1984 and 2016. Two articles were based on findings from the same study[20,21], but the recent publication was based on 5-year follow-up findings. The majority of studies were conducted in North America (USA and Canada), with five conducted in Europe (Norway, Sweden, Austria, Turkey, and Croatia), and two conducted in Asia (Japan and Korea). Except for three case reports[27-29], all studies utilised observational cohort designs. The majority of the study designs had multiple intervention groups which were non-randomised, while four used before and after designs[25,26,31,32]. Methodological quality of included observational cohort studies using Newcastle–Ottawa Scale criteria ranged from 3-6 (Table 1).
Table 1.
Summary characteristics of studies included in the review.
| Lead author, publication date | Location | Baseline year | Study design | Further details on study design | Population | Population source | Exclusion criteria | Age range (years) | Females (%) | Sample size | Drop-out rate | Duration of follow-up | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sinaki, 1984 | USA | 1969-1981 | Prospective cohort | Nonrandomised design with multiple intervention groups | Postmenopausal women with spinal osteoporosis and back pain | Healthcare setting | History of cancer, metabolic bone disease, secondary osteoporosis, and major health problems that could cause difficulty with compliance of exercise programmes | 49-60 | 100 | 59 | NR | 1-6 years | 6 |
| Ekin, 1993 | USA | N/A | Case report | N/A | Three postmenopausal women with osteoporosis who were healthy, active, and long-term golfers | Healthcare setting | N/A | 63; 58; and 66 years respectively | 3 women | 3 | N/A | N/A | N/A |
| Harrison, 1993 | Canada | 1983 | Prospective cohort | Nonrandomised design with multiple intervention groups | Postmenopausal women with osteoporosis | Referred from physicians | NR | 64.0* | 100 | 139 | 78 remained at 4-year follow-up | 4 years | 5 |
| Walker, 2000 | Canada | 1983 | Retrospective cohort | Nonrandomised design with multiple intervention groups | Postmenopausal women with osteoporosis | Referred from physicians | NR | NR | 100 | 89 | NR | 5 years | 5 |
| Kerschan-Schindl, 2000 | Austria | NR | Prospective cohort | Nonrandomised design with intervention plus control group | Women with a history of postmenopausal fractures and an age-adjusted low bone mass | Healthcare setting | Smoking, secondary osteoporosis, neurologic disease, and any chronic disease other than osteoporosis | 45-75 | 100 | 33 | 25 with complete data at end of study | 9.7 years | 5 |
| Liu-Ambrose, 2004 | Canada | NR | Prospective cohort | Groups assigned intervention on the basis of ‘sway’ measures | Women with low bone mass | Population databases as well as media (newspaper, radio, poster advertisements) | Women living in care facilities, of non-Caucasian race, regularly exercising twice weekly or more, had a history of illness or a condition for which exercise may cause adverse effects, had a history of illness or a condition that would affect balance (i.e. stroke and Parkinson’s disease), or had a MMSE score of ≤ 23. | 75-85 | 100 | 98 | NR | 13 weeks | 5 |
| Yamazaki, 2004 | Japan | 1999-2000 | Prospective cohort | Nonrandomised design with intervention plus control group | Postmenopausal women with osteopenia/ osteoporosis | Healthcare setting | History of oestrogen replacement therapy or had ever taken medication that affects bone metabolism, past or current smokers, history of cardiopulmonary disease or severe osteoarthritis and osteopathy that might have affected physical activity, participated in a sporting activity with a frequency of one or more times a week for at least the previous 5 years | 49-75 | 100 | 50 | 27 subjects in the exercise group and 15 in the control group completed the study | 12 months | 5 |
| Murphy, 2008 | USA | NR | Prospective cohort | Before and after design | Community-dwelling women with or at risk for developing osteoporosis | Community dwellers | Involved in any Tai Chi within the past 12 months, required use of an assistive device or lower extremity orthosis to ambulate independently, history of neurological impairment or neuromuscular disease, had persistent pain >7/10 on a 0–10 pain scale, scored <20/30 on the MMSE, scored worse than 20/40 on the Snellen Chart with corrective lenses, or had their primary care provider identify a medical condition not identified during the screening process that would increase their fall risk or jeopardize their health by participating in the study. | 55-80 | 100 | 42 | 31 completed the study | 12 months | 4 |
| Tuzun, 2010 | Turkey | NR | Prospective cohort | Observational cohort with a quasi-randomised element (divided into groups) | Postmenopausal women with osteoporosis | Healthcare setting | Systemic or psychiatric disorders and abnormal laboratory values | 55-85 | 100 | 26 | NR | 12 weeks | 6 |
| Sinaki, 2013 | USA | N/A | Case report of 3 cases | N/A | Three cases of women with osteopenia | Healthcare setting | N/A | 87; 61; and 70 years | 3 women | 3 | N/A | N/A | N/A |
| Cesarec, 2014 | Croatia | NR | Prospective cohort | Before and after design | Women with osteopenia or osteoporosis | Healthcare setting | NR | 36-84 | 100 | 39 | NR | 4 weeks | 3 |
| Hakestad, 2015 | Norway | NR | Prospective cohort | Before and after design | Postmenopausal women with osteopenia and a healed forearm fracture | Healthcare setting - were part of an ongoing randomised controlled trial | History of hip or vertebral fracture, more than 3 osteoporotic fractures, medical conditions precluding active rehabilitation, already performing moderate to intense physical activity for more than 4 hours per week, inability to understand written or spoken Norwegian. | > 50 | 100 | 42 | Complete data available for 31 participants | 1 year | 4 |
| Lu, 2016 | USA | 2005 | Prospective cohort | Before and after design | Majority of patients with osteoporosis or osteopenia | Internet recruited volunteers | Abnormal values on tests of circulating biomarkers, metabolic or bone disease | 68.2* | Of 227 compliant patients, 202 were female | 741 | 227 were compliant | 2 years | 4 |
| Oh, 2016 | Korea | N/A | Case report of 2 cases | N/A | Two cases of women with low bone mineral density | Healthcare setting | N/A | 44 and 49 years | 2 women | 2 | N/A | N/A | N/A |
, are mean/median ages; N/A, not applicable; NR, not reported; MSSE, Mini Mental State Exam
Baseline characteristics of study populations evaluated
Sample size of cohorts (excluding case reports) ranged from 26 to 741 participants (Table 1). Overall, the 14 studies involved 1,226 participants. Lu and colleagues[32], included men but the majority of studies included only female participants. Patients were recruited from diverse sources and these included healthcare settings, the community, referral from physicians, population databases, and the media (newspaper, radio, poster advertisements, as well as the internet). Baseline age of study participants ranged from 36 years and above and duration of follow-up, where follow-up occurred, ranged from 4 weeks to 9.7 years. Except for one study (findings reported in two articles[20,21]), all studies (excluding the case reports) had exclusion criteria for recruiting participants and these included history of chronic diseases, health problems or neurological conditions that might impair physical activity or exercise and secondary osteoporosis.
Types of physical activity and/or exercise undertaken
[Table 2] provides types of interventions (physical activity and/or exercise) utilized in eligible studies, frequency and duration of interventions, as well as the population groups evaluated in these studies. There were several diverse types of physical activity or exercise and these included spinal extension and flexion exercises; use of free weights; aerobic exercises (walking, jogging, dance routines, exercise bicycles); balance exercises, stretching; Tai Chi; yoga; golfing; as well as horseback riding. The majority of studies adopted the intervention/exercise activity as a programme which involved a combination of various exercise routines including warmup exercises. Yamazaki and colleagues utilized an exercise programme which only consisted of daily outdoor walking, after each participant had learned to walk at a speed that corresponded to their target heart rate using an exercise treadmill[24]. In the study of Tai Chi, a typical session involved stretching exercises, sequential movements of the limbs, and controlled breathing[25]. Lu and colleagues employed a yoga regimen consisting of 12 common poses specifically chosen for their safety, which concentrated on three common fracture sites – spine, hip, and femur, and avoided forward flexion-type exercises[32]. The exercise programmes were either facility based or home-based and were supervised or unsupervised after initial instructions had been provided. For some unsupervised groups, this was after patients had undergone a period of supervised exercise classes[20,22,24]. One study utilized an exercise programme combined with a patient education programme[26]. The frequency of physical activity or exercise sessions ranged from once a week to about four times a week and total duration ranged from 4 weeks to 12 years. The majority of studies used 2-3 intervention groups.
Table 2.
Key characteristics of physical activity and/or exercises interventions included in review.
| Lead author, publication date | Population | Type of physical activity or exercise | Frequency of physical activity or exercise program | Total duration of physical activity or exercise | Intervention groups | Adverse outcome measures assessed | Baseline status prior to study entry | Reported findings | Study conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Sinaki, 1984 | Postmenopausal women with spinal osteoporosis and back pain | Spinal extension and flexion exercises. Extension exercises consisted of exercises to strengthen erector muscles of the spine. Flexion exercises consisted of exercises that involved abdominal muscles, such as sit ups. All the treatment programmes included infrared heat and massage. Supervised exercise programme | NR | 1-6 years | Extension (n=25); Flexion (n=9); Combined (extension plus flexion) (n=19); No exercise (n=6) | Wedging and compression fractures | NR | Fracture rate: Extension (16%); Flexion (89%); Combined (53%); Occurrence of fractures is significantly higher in flexion group compared with extension group | Spinal extension or isometric exercises more appropriate for patients with postmenopausal osteoporosis |
| Ekin, 1993 | Three postmenopausal women with osteoporosis who were healthy, active, and long-term golfers | Golfing | NR | Were long-term golfers | N/A | Back pain and vertebral compression fractures | Were healthy, active, and long-term golfers | Severe back pain and vertebral compression fractures | The safety of golfing in women with osteoporosis is an issue. In the absence of robust evidence from RCTs, golfers with osteoporosis should wear rigid back support |
| Harrison, 1993 | Postmenopausal women with osteoporosis | The exercise program consisted of a 20-minute low load, strength training session and 30 minutes of aerobic activities. Major muscle groups of the upper and lower extremities were strengthened using free weights. The patients performed 10 repetitions for each muscle group. Aerobic exercises consisted of walking and various aerobic ‘dancercize’ routines, choreographed to music. Supervised and unsupervised exercise programme | Twice a week | 4 years | Supervised exercise group (n=36); Independent exercise group (n=37); Refused follow-up testing (n=5) After the exercise program, patients were grouped by improvement in fitness Group 1 – Least improvement in fitness Group 2 – Greatest improvement in fitness | Back pain and new fractures | There was no significant difference in initial level of fitness between the independent exercise group and the supervised exercise group. 44/78 patients had vertebral fractures and 45 had a total of 86 non-vertebral fractures. About 18 patients had back pain. | There was no significant difference in degree of improvement in fitness between the independent exercise group and the supervised exercise group. Overall, there was no significant improvement in back pain and there was an increase in vertebral and non-vertebral fractures at follow-up after the exercise programme. Compared with the group who experienced the least improvement in fitness, the group who experienced the greatest improvement in fitness had fewer vertebral fractures and less back pain (though not statistically significant). | Majority of patients in exercise programme found it to be of sufficient benefit. With encouragement from other osteoporotic patients, they are able to carry out more vigorous exercises without pain. |
| Walker, 2000 | Postmenopausal women with osteoporosis | The exercise programme consisted of a 20-minute low load, strength training session and 30 minutes of aerobic activities. Major muscle groups of the upper and lower extremities were strengthened using free weights. The patients performed 10 repetitions for each muscle group. Aerobic exercises consisted of walking and various aerobic ‘dancercize’ routines, choreographed to music. Supervised and unsupervised exercise programme | Twice a week | 5 years | Supervised (n=42) and unsupervised groups (n=47) | Incidence of fracture and loss of height | At study entry, 11 subjects in each group reported having had at least one fracture in the previous 12 months. | The incidence of fractures was reduced over the course of the study period. At study entry, 11 subjects in each group reported having had at least one fracture in the previous 12 months, while 2 in each group reported having had a fracture over the 5-year period. | Patients received a significant benefit as a result of their participation in the exercise program, irrespective of whether they exercised in a supervised program or in an unsupervised environment. |
| Kerschan-Schindl, 2000 | Women with a history of postmenopausal fractures and an age-adjusted low bone mass | Home exercise programme. Included a warm-up period (brisk walking, modest jogging, etc), stretching exercises (hamstring, gastrocnemius, iliopsoas, pectoralis, and external rotation muscles of the hips), and movement patterns directed towards improving posture and coordination. Apart of the exercise program consisted of exercises with big gymnastic balls. During the initial phase, the exercises were supervised 20 times by a physical therapist. At half-yearly intervals, the subjects had an opportunity to participate in five supervised training sessions of exercise for reinforcement and correction of poor technique. Home exercise programme (supervised during the initial phase and at half-yearly intervals) | Training on a regular basis at home, at least three times a week for 20 min. | 7 to 12 years | Exercise (n=19) and Control group (n=6) | Fracture rates, episodes of falling, neuromuscular performance |
Persons with non-vertebral fractures: Exercise group – 15 (79%) Control group – 4 (67%) Persons with vertebral fractures: Exercise group – 9 (48%) Control group – 2 (33%) |
No differences between groups in terms of fracture rates, falling episodes, and neuromuscular performance were observed. Persons with at least one non-vertebral fracture: Exercise group – 9 (47%) Control group – 3 (50%) Persons with at least one vertebral fracture: Exercise group – 6 Control group – 0 |
A home exercise program does not affect the outcome of postmenopausal women at high risk of fracture |
| Liu-Ambrose, 2004 | Women with low bone mass |
Resistance training – progressive and high-intensity in nature with the aims of increasing muscle strength in the extremities. Agility training – aims were to challenge hand-eye coordination, foot-eye coordination, dynamic balance, static balance and psychomotor performance (reaction time). Involved ball games, relay races, dance movements, and obstacle courses. Stretching - consisted of stretching exercises, deep breathing and relaxation techniques, and general posture education Supervised exercise programme |
50-minute exercise classes twice weekly | 13 weeks | Resistance training (n=32); Agility training (n=34), and Stretching (sham) exercises (n=32). | Relationship between change in balance confidence, and the changes in fall risk and physical abilities |
Balance Confidence: mean (SD) Resistance – 76.3 (22.7) Agility – 78.3 (14.5) Stretching – 75.6 (23.7) Fall Risk Score: mean (SD) Resistance – 2.22 (0.70) Agility – 2.40 (0.86) Stretching – 1.92 (0.83) |
Improvement in balance confidence which did not significantly correlate with changes in fall risk score, physical activity level, and physical abilities. Baseline balance confidence was not significantly different between the three groups. Balance Confidence: mean (SD) Resistance – 80.9 (17.1) Agility – 83.2 (12.2) Stretching – 76.3 (17.6) Fall Risk Score: mean (SD) Resistance – 1.39 (0.98) Agility – 1.49 (0.97) Stretching – 1.50 (0.95) |
Both resistance training and agility training significantly improved balance confidence in community-dwelling older women with low bone mass after 13 weeks of participation. |
| Yamazaki, 2004 | Postmenopausal women with osteopenia/osteoporosis | Daily outdoor walking at moderate intensity Home exercise programme (supervised during initial phase) | At least 1 hr duration with more than 8000 steps, at a frequency of 4 days per week | 12 months | Exercise programme (n=32) and controls (n=18) | Fractures | None of the subjects revealed any evidence of thoracic or lumbar vertebral fractures | None of the subjects suffered any fractures during study period | Authors noted that the sample size was too small and study period was too short to detect the effect of exercise on the risk of vertebral fracture |
| Murphy, 2008 | Community-dwelling women with or at risk for developing osteoporosis | 5-Form, Yang Style Tai Chi (TC) Supervised exercise programme | TC sessions twice a week, with self-practice at least 1 day per week. A typical session lasted 60– 90 minutes and consisted of 10–20 minutes of warm-up, 45–60 minutes of TC practice, and approximately 7 minutes of cool-down activity | 12 weeks | Before and after design | Balance confidence, balance performance, functional strength, mobility, and incidence of falls | Number of falls that occurred during a 12-month time interval preintervention - 27 | TC significantly improved balance performance, functional strength, and functional mobility immediately postintervention. All improvements, except for balance on the right leg, were sustained at the 6-month follow-up. But only improvements in functional strength and mobility remained evident at the 12-month follow-up. There was no significant change in falls incidence Number of falls recorded during 12-months postintervention - 27 | Five-Form, Yang Style TC appears to be a safe and relatively low-cost exercise intervention that can enhance balance, functional strength, and mobility among an older group of independent, community-dwelling females. |
| Tuzun, 2010 | Postmenopausal women with osteoporosis | Yoga programme (combination of breathing and movement) and classical osteoporosis exercise program (involved strengthening and stretching exercises of the abdominal, back, quadriceps and hamstring muscles, balance and posture exercises Supervised exercise programme | 1-hour sessions twice a week | 12 weeks | Yoga group (n=13) and exercise group (n=13) | Quality of life, balance, physical function, and pain | NR | Both yoga training and classical osteoporosis exercises had beneficial effects on balance and quality of life. Compared with yoga, the exercise programme demonstrated significant improvements in pain and functional activities | Yoga training seems to be more effective than classical exercises |
| Sinaki, 2013 | Three cases of women with osteopenia | Strenuous yoga flexion exercises | NR | Woman C performed for 10 weeks | N/A | Back pain and vertebral compression fractures | Were in good health and pain-free | Severe back pain and vertebral compression fractures | In older persons, flexibility of the spine achieved with yoga can lead to adverse effects rather than benefits |
| Cesarec, 2014 | Women with osteopenia or osteoporosis | Osteoporosis exercise programme Supervised exercise programme. | 30-45 minutes | 4 weeks | Before and after design | Quality of life | NR | Significant improvement in all nine dimensions of the quality of life questionnaire (physical functioning, role-physical, role-emotional, social functioning, mental-health, vitality, bodily pain, general health and general health status) compared to the period a year ago. | Programmed physical activity program for osteoporosis is effective and affects the psychological aspects of patient’s life |
| Hakestad, 2015 | Postmenopausal women with osteopenia and a healed forearm fracture | Exercise programme combined with a patient education program called OsteoINFO. Consisted of 2 group exercise sessions and 1 home exercise session per week. The program included exercises for strength, balance, coordination, and core stability, and included the use of weight vests. Also incorporated into the programme were ergonomic exercises, such as rising from a lying to a standing position and lifting heavy weights while maintaining the spine in a neutral position and keeping the weights close to the body. Supervised and unsupervised exercise programme. | Three 60-minute sessions per week. | 6 months | Before and after design | Joint pain, muscle soreness, and falls | Past history of fracture, median (min-max): 2 (1-3) | No adverse events reported | A 6-month active rehabilitation program, which included an exercise program with the use of a weight vest in addition to a patient education program is feasible, had high adherence with no adverse events. The results however cannot be generalized to patients with severe established osteoporosis or to those with vertebral or hip fracture |
| Lu, 2016 | Majority of patients with osteoporosis or osteopenia | Yoga regimen consisting of 12 poses Web-based program | 12-minute sessions daily | 2 years | Before and after design | Compliance and self-reported and radiological measures of injury | 109 fractures reported on radiographs prior to study entry | After more than 90,000 hours of Yoga, no self-reported or radiographic fractures or serious injuries related to yoga have been reported by any of the participants. | The 12 yoga poses studied here appear to be a safe and effective means to reverse bone loss in the spine |
| Oh, 2016 | Two cases of women with low bone mineral density | Horseback riding | Once a week and 1 hour every week day respectively | 1 and 2 months respectively | N/A | Back pain and vertebral compression fractures |
Woman A History of hypercholesterolaemia and total hysterectomy without adnexectomy due to myoma. Woman B History of hyperlipidaemia, osteoporosis, and regular menstruation |
Woman A felt upper back pain after her fourth riding lesson. Back pain lasted for 4 months. A radiograph confirmed a vertebral compression fracture. Woman B reported back pain that was aggravated by horseback riding. A radiograph confirmed a vertebral compression fracture. |
Horse riding in osteoporosis may cause back pain and vertebral compression fractures |
N/A, not applicable; SD, standard deviation.
There were three case reports of adverse events during unstructured or unsupervised physical activities. One presented clinical findings of two women with low bone mineral density (BMD) after participation in horseback riding[27]. Another reported findings of three patients with osteopenia after they had participated in strenuous yoga flexion exercises[28]. The third case report presents post-golfing clinical findings in three healthy, active, postmenopausal patients with osteoporosis[29].
Adverse events experienced with physical activity and/or exercise
In addition to outcomes such as bone mineral density, circulating blood bone markers, functional strength, and neuromuscular performance; adverse events evaluated by included studies were fractures, back pain, falls, joint pain, balance problems, mobility, quality of life, muscle soreness, and injuries. These were assessed by a variety of measures across studies, but were most often unexplained.
Studies reporting no, reduced or unchanged adverse events
The majority of studies (ten out of fourteen) either reported no adverse events, reduced incidence/improvement in adverse events, or no significant change in adverse events after physical activity or exercise (Table 2). These beneficial effects were not confined to any specific age group of patients, but the majority of participants in these studies were women. All participants in these studies had low bone mass, defined by a variety of methods including DXA T score <-1.0[23], DXA Z score <-1.0[22], DXA T score <-1.5[26], DXA T score <-2.5[21,30], neutron activation analysis[20] or undefined[24,25,31,32]. Some studies included participants with previous low trauma fractures[20,22,26]. Some studies did not mention use of medications to reduce fracture risk by participants[23,25,31,32]; some reported all participants were on calcium supplementation[20,21] or bisphosphonates[30]; and some reported that a proportion of participants were taking calcium supplements[22,26], oestrogen[20-22], sodium fluoride[20,22] or ‘other medications known to affect bone homeostasis’[21]. One study reported no participants were on any medications to reduce fracture risk nor calcium supplements[24]. Many of these studies, with no or reduced rates of adverse events, excluded participants with additional medical problems or illnesses including metabolic bone diseases or those that may be associated with secondary osteoporosis[22-25,30,32], participants with reduced cognition[23,25], smokers[22,24], those who lived in care facilities[23,25], those who used walking aids[25], or those who already participated in regular exercise[23-25]. One study excluded participants with severe osteoporosis as defined by previous hip or vertebral fractures, or a history of more than three low trauma fractures[26].
In a study of daily outdoor walking at moderate intensity for 12 months in postmenopausal women with osteoporosis or osteopenia (exercise group versus controls), none of the participants suffered any fractures during the study period[24]. However, the authors noted in their conclusion that their sample size was too small and study period too short to detect the effect of exercise on the risk of vertebral fracture. Hakestad and colleagues in their before-after design study reported no adverse events (joint pain, muscle soreness, and falls) after 6 months of an exercise programme combined with an education programme[26]. After over 90,000 hours of a yoga regimen, no self-reported or radiographic fractures or serious injuries related to yoga were reported by any of the participants in the study by Lu and colleagues[32]. It is of note that they specifically chose poses that were ‘safe’. Walker and colleagues reported a reduction in incidence of fractures after a 5-year follow-up of an exercise programme which consisted of a 20-minute low load, strength training session and 30 minutes of aerobic activities[21]. After 12 weeks of Tai Chi in community-dwelling women with or at risk for osteoporosis, there was no significant change in the incidence of falls[25]. In a study that compared participants in a home exercise programme with controls, there were no differences between the groups in terms of fracture rates, falling episodes, and neuromuscular performance[22]. In a 12-week comparison of a yoga programme with a classical osteoporosis exercise programme, both yoga training and exercises had beneficial effects on balance and quality of life. Compared with yoga, the exercise programme demonstrated significant improvements in pain and functional activities[30].
Studies associated with adverse events
The findings of one study suggested that a number of vertebral compression fractures occurred in patients with postmenopausal spinal osteoporosis who undertook spinal flexion exercises compared with extension exercises[19]. In this study, participants were 59 women aged 49 to 60 years, with a ‘radiographic diagnosis’ of spinal osteoporosis. Use of medications to reduce fracture risk was not reported. Women older than 60, with reduced cognition, or with other medical problems such as cancer, metabolic bone diseases or potential causes of secondary osteoporosis were excluded. Those participants who carried out spinal flexion exercises (sitting in a chair, leaning forward with arms hanging down and head bent forward, and stretching the erector spinal muscles by forward flexing the spine such as sit-ups) were more likely to develop new vertebral fractures than those who carried out spinal extension exercises. No other differences, apart from exercise type, were reported between those participants who developed vertebral fractures and those who did not.
A report of three cases of women (aged 87, 61 and 70) with osteopenia measured by DXA (T scores less than -1.0), unknown medication usage, but in otherwise good health and pain-free, reported participation in yoga involving spinal flexion exercises resulted in back pain and vertebral compression fractures[28]. The specific yoga poses were the Paschimottasana pose (seated forward bend/fold or the half-hero stretch), the Halasana pose (Plow yoga pose) and the Setu Bandha Sarvangasana (Bridge yoga pose) (Figure 2).
Figure 2.
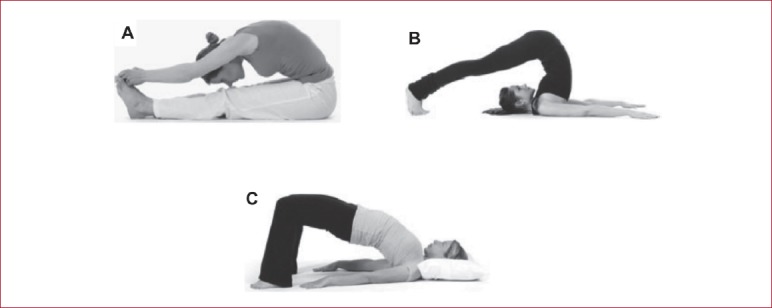
Common yoga poses associated with extreme spinal flexion (adapted from Sinaki MS 2013). (A) Paschimottasana (seated forward bend/fold); (B) Halasana (Plow yoga pose); and (C) Setu Bandha Sarvangasana (Bridge yoga pose).
In a case report of two women (aged 44 and 49), one with a DXA T score -1.9 and one with undefined ‘osteoporosis’, neither on any medications to reduce fracture risk, horseback riding was associated with new onset back pain and vertebral compression fractures[27]. Both fractures occurred whilst the horse was walking, not jumping over obstacles. In an additional case report, three postmenopausal women (aged 54, 58 and 66) with DXA T scores <-1.3, one of whom had previously taken calcium supplementation and medications to reduce fracture risk, who were all experienced golfers suffered acute pain and vertebral compression fractures during a golfing mid-swing stroke[29]. One further study of an ‘exercise programme modified to meet individual requirements’ reported an increased proportion of vertebral fractures in the subgroup who showed the least improvement in fitness[20], but in a 5-year follow-up report of the same study, the incidence of fractures was significantly reduced over the course of the study period[21].
Robustness of findings from observational and non-randomised studies
Though the reported conclusions from the included studies generally suggested physical activity and exercise to be beneficial and safe for patients living with osteoporosis and osteopenia (Table 2); the findings should be interpreted with caution given some limitations in the methodological quality of the eligible studies such as the study designs (observational cohorts and case reports), lack of appropriate controls in some of the studies, self-reports of outcomes by study participants, selective reporting of outcomes, and authors of studies being involved in design of interventions. Few studies recruited women over the age of 60 or with co-morbid conditions.
Excluded studies
The list of excluded studies after full-text evaluation is provided in Appendix 3. Reasons for exclusion were irrelevant populations recruited (participants without osteoporosis or osteopenia), outcomes, and study designs. One was a review of case reports and series evaluating the adverse events associated with yoga[33] but had not identified any additional papers to the yoga papers identified by this review.
Results: update of interventional data
Exercise to prevent and treat osteoporosis in post-menopausal women, by Howe et al.[13]
1471 trials were identified, and following screening, full texts were acquired for 76 trials for consideration (see Appendix 4). After scrutiny, 27 additional trials were included. When added to the 35 previous trials, the update contains information from 62 trials involving a total of 6607 participants. Supervision of exercise intervention varied across studies, with one supervised by a physiotherapist[34], 14 by a combination of research staff or exercise class staff[35-48], and 5 mixed independent at home and supervised gym classes[47,49-52]. Overall, 70% of the interventions were supervised. 16 of the additional trials did not report whether the intervention was supervised.
11 trials reported on numbers of fractures occurring as an adverse event. In these 11 studies, there were 31 fractures occurring in 536 intervention group participants (5.8%) and 43 fractures occurring in 449 control group participants (9.6%). Meta-analysis showed a trend for the intervention to reduce fractures (OR 0.60, 95%CI 0.36 to 1.01, P=0.053). Nine studies reported on falls occurring as an adverse event. In the pooled analysis there was no effect of exercise on falls overall (RR 1.04, 95%CI 0.79 to 1.37, P=0.779). 24 studies reported on minor adverse events including joint pain, muscle soreness, headaches, and itchiness. The overall pooled effect was an increase in these minor adverse events reported by the intervention group participants (RR 1.55, 95%CI 1.05 to 2.28, P=0.029). In most cases reported, these minor adverse events did not stop participation in the study. Two trials reported serious adverse events[53,54] with one myocardial infarction and one asthma attack in the intervention groups, and one myocardial infarction and one cardiac dysrhythmia in the control groups.
Exercise for improving outcomes after osteoporotic vertebral fractures, by Giangregorio et al.[14]
568 trials were identified, and following screening, full texts were acquired for 17 trials for consideration (see Appendix 5). After scrutiny, 3 additional trials were included. When added to the 7 previous trials, the update contains information from 10 trials involving a total of 678 participants. None of the new trials included male participants. The interventions varied across the three additional studies. Two were undertaken twice weekly[50,55] whilst the other was three times per week[56]; low intensity exercise consisting of pelvic, abdominal and spinal control exercise was used by one trial[56], no intensity was specified in the trial that used a structured programme including stretching, strength training and weight-bearing exercises[50], and no intensity was specified in the study that used a circuit training programme involving balance training, agility training and postural exercises[55].
There was no indication that adverse events were systematically monitored in any of the included studies. One study reported no adverse events[55]. One study reported two serious adverse events of breathing difficulties experienced by two participants whilst undertaking functional exercises during activities of daily living[56]. In the study by Evstigneeva et al., four participants in the intervention and two in the control sustained a fracture. Vertebral fractures occurred equally between intervention and control groups with two in each group. There were five non-vertebral fractures in the control and two in the intervention group, a difference which was not significant[50]. There was also one report of worsening knee pain attributable to the intervention[50].
Exercise to prevent falls in older adults by Sherrington et al.[12]
1982 trials were identified, and following screening, full texts were acquired for 68 trials for consideration (see Appendix 6). After scrutiny, 19 additional trials were included. When added to the 88 previous trials, the update contains information from 107 trials involving a total of 23,288 participants. 16 of the additional trials were randomised by individual, three were cluster randomised (one by General Practitioner[57], one by retirement village[58] and one by health centre[59]), and one used a cross-over design[60]. Greater than 50% of participants in the additional trials were female. Four trials recruited on the basis of participants having a clinical diagnosis of Parkinson’s Disease[60-63], one with visual impairment[64] and one with a clinical diagnosis of osteoporosis[60]. Either a history of falls or having at least one risk factor for falls such as frailty or balance impairment were a pre-requisite for participants in eight trials. Severe or significant cognitive impairment was used as an exclusion criterion in >50% of the trials.
55 trials (51.4%), including 8 of the additional trials, did not report on adverse events. 26 trials (24.2%) reported an occurrence of zero adverse events, of which seven were reported as no serious adverse events only[64-70]. Data presentation for adverse events was highly heterogeneous between trials, with some reporting only those events deemed attributable to the intervention. Out of 11 participants randomised to the intervention group in Wesson et al.[71], four reported mild complaints of pain, dizziness and stiffness. Complaints were resolved by exercise modification. Sherrington et al.[72] reported 12 adverse events attributable to the exercise intervention including muscle and joint pain, and exacerbations of pre-existing symptoms. Other trials reported all adverse events that occurred in its participants regardless of study arm. For example, Dean et al.[73] reported three deaths, all control group participants, in addition to one participant suffering from exacerbation of incontinence as a result of the exercise intervention. Sparrow et al.[60] in women with osteoporosis, reported 7 adverse events, of which only two (one knee and one quadriceps pain) were deemed attributable to the intervention. Only one trial reported two adverse serious events attributable to the intervention: Clemson et al.[74] reported a groin strain followed by surgery for inguinal hernia in the structured exercise group, and one pelvic stress fracture in the Lifestyle Integrated Functional Exercise group (participants with known osteoporosis was not an exclusion criteria for this trial). Eight trials of community-dwelling participants reported on the number of adverse events for intervention and control participants. The pooled effect of adverse events associated with the intervention in this group was 1.08 (95%CI 1.04 to 1.13). In a study evaluating the effect of brisk walking on post-menopausal women who had previously sustained a distal forearm fracture, the intervention group had significantly more falls in the first year of follow-up compared to the control group (42 and 26 falls per 100 person years respectively)[75].
The incidence of the commonly reported adverse events was calculated as person-years. Trials in this review reported falls to be the adverse event with the highest incidence (156/1000 person-years). The next most frequently reported adverse events were musculoskeletal complaints such as muscle strain and soreness and joint/unspecified pain (132/10000 person-years). Fractures were reported with the lowest incidence (21/1000 person-years).
Discussion
Key findings
We have systematically reviewed the evidence on the adverse effects of physical activity and/or exercise on adults with osteoporosis or osteopenia. In a comprehensive search we identified 71 studies (14 observational studies comprising of 11 observational cohorts and three case reports; and 57 randomised controlled trials in an update of three previous systematic reviews of interventional evidence) which met our inclusion criteria. The results were consistent for the great majority of observational studies – no reported incidences in adverse events, reduced incidence or improvement in adverse events, or no significant change in adverse events after physical activity or exercise. However, most included supervised initial instruction to check technique. Most of these studies and reports did not include men or older women, and excluded those with multiple co-morbidities or secondary osteoporosis. The updated systematic reviews of interventional data highlighted that none of the trials were powered to look at adverse events including fractures, and these were poorly reported. However, although participants in the exercise groups reported more adverse effects than those in the control groups, these were mainly minor adverse events and did not stop participation. Severe or serious adverse events were rarely reported. Overall, our conclusion is that most physical activities and structured exercise programmes are safe for postmenopausal women with osteoporosis or low bone mass. Care, however, should be taken by those naïve to activities which may increase risk (horse riding, golf and yoga or sit-ups) as technique is obviously important to adverse outcomes.
Implications of our findings: overall conclusion is that physical activity or exercise is safe for patients with osteoporosis or low bone mass
The generally consistent findings reported by the included studies suggest that physical activity or exercise is safe for patients with osteoporosis or low bone mass. No clear difference in serious adverse events was identified between supervised or unsupervised exercise programmes. Furthermore, the updated meta-analysis of interventional studies has provided the first evidence to suggest that exercise interventions for bone health can reduce fractures. The increase in minor adverse events identified in the interventional studies is what would normally be expected with physical exertion such as muscle aches. Given the variation in the frequency, intensity, and duration of the exercise programmes reported by eligible studies, no conclusions could be made as to whether these factors affected the rate of occurrence of adverse events. However, the reports do suggest that the prescribed exercise programmes were tailored or modified to meet the individual requirements of the patients. The current findings are very relevant as they provide several implications for clinical practice. Health professionals have often questioned the safety of recommending physical activity or exercise programmes in addition to pharmacological interventions to patients with osteoporosis. As a result, healthcare professionals can be extremely cautious when recommending physical activity or exercise for these patients. Patients with osteoporosis also tend to restrict physical activity due to the fear of falling or sustaining fractures[76] – so-called ‘kinesiophobia’[77]. However, there is evidence that being involved in exercise programmes can actually reduce both falls and the fear of falling[12,78]. Considering the growing evidence that sedentary behaviour, and in particular prolonged periods of sitting, can reduce bone mass and physical function, hence leading to more falls and frailty, there is a need to reverse this behaviour[79-81]. Taking the overall evidence together, being physically more active and perhaps joining exercise programmes should be recommended for patients with osteoporosis and osteopenia.
Types of exercise or physical activity that may have an increased risk of adverse events in patients with osteoporosis or low bone mass
Our findings from the observational data suggest that repetitive spinal flexion exercises, horseback riding, and golfing may be associated with a greater risk of adverse events such as back pain and vertebral compression fractures, although the evidence on horseback riding and golfing is based on one case series each and must be interpreted with caution. The evidence suggesting that spinal flexion may be associated with vertebral fractures is based on two papers only[19,28]. However, it is mechanically plausible that forward flexion increases the load on the front of the vertebral bodies, and is likely to increase the risk of vertebral fractures[82]. It therefore seems reasonable to recommend that people with osteoporosis avoid specific and repetitive spinal forward flexion exercises, and instead focus on spinal extension. However, it needs to be emphasized that evidence that forward flexion exercise interventions cause vertebral fractures are limited and caution should be used in giving advice to patients about appropriate movements based on their low BMD measurements alone. Pragmatically it makes sense for older people who may be at risk of fractures to avoid brisk walking as there appears to be an increased risk of falls with brisk walking as opposed to walking at a normal speed[12], and also repetitive or loaded forward flexion. However, there is no evidence that single forward flexion movements in daily activities or ‘roll down’ Pilates movements cause vertebral fractures – a common misconception [personal communication from SL based on the National Osteoporosis Society charity’s helpline statistics on common concerns expressed by patients]. There should be a focus on how to lift weight or pick up things from the floor safely so that everyday activities that involve spinal flexion are performed with good technique[15]. In terms of specific yoga poses that appear to increase risk, there are modified versions of all yoga poses that experienced teachers can recommend, rather than avoiding yoga completely. In addition, it is important to highlight that proper instruction on correct spinal movements during general daily life is relevant to everyone, not just those with low bone density.
Equestrian sport is a popular but potentially hazardous recreational activity, with approximately one in five riders suffering a serious injury during their riding career[83]. This makes it difficult to interpret the report of two cases of vertebral fractures occurring in women with low bone density during horseback riding[27]. The authors suggest that the potential reasons that the vertebral fractures occurred were the low bone density, but also the fact that these were less skilled riders who were not absorbing the movements of the horse, instead of sitting stiff and tense. It may, therefore, be sensible for healthcare professionals to discuss the potential increased risk of vertebral fractures in amateur horseback riders with low bone density. However, it is difficult to draw a strong conclusion to suggest avoiding this activity, particularly when there may be many additional associated quality of life benefits including having a keen leisure interest, a positive owner-animal relationship and social interactions. Perhaps those who are experienced horse riders may not have the same potential adverse events. Similarly, golfing is a popular sport amongst older people as it is challenging without undue exertion, is socially fulfilling and is associated with physical health benefits and improved wellness[84]. However, the incidence of golfing injury is moderate, with back injuries the most frequent[84], again making it difficult to draw strong conclusions from the limited evidence of vertebral fractures occurring during a golfing mid-swing in three older women with osteoporosis[29]. The authors of this paper suggest that a rigid back support for golfers with osteoporosis may protect against fractures, but no evidence was provided that such an orthosis is beneficial. Nonetheless, it would be important for healthcare professionals to discuss the potential increased risk of vertebral fractures during golf swings, without making firm conclusions about avoiding this activity. Instead, if golf is important to someone, the risk/benefits of continuing should be discussed.
Gaps in the literature
Our review has also identified gaps in the existing evidence which include: (i) uncertainty around which age groups are more at risk of adverse events; (ii) whether supervised or unsupervised exercise programmes are associated with a higher risk of adverse events; (iii) uncertainty on the relationship between the severity of osteoporosis and occurrence of adverse events and no evidence on the association between people with high fracture risk as assessed by tools such as FRAX and adverse events; (iv) lack of evidence for men with osteoporosis; (v) lack of evidence for the very elderly; (vi) lack of studies including participants with multiple co-morbidities or secondary osteoporosis; and (vii) lack of clear reporting of adverse events in interventional exercise and physical activity studies. In light of these further robust research is needed, particularly as it is unclear whether the many studies identified in this review that did not report any adverse events were doing so because none occurred, or because the data was not recorded.
Strengths and limitations
This review is the first to our knowledge to systematically examine the adverse effects of physical activity and/or exercise on adults with osteoporosis or osteopenia in non-randomised studies. We have also updated the evidence on previous findings from interventional studies. We have attempted to ascertain the baseline characteristics of people who experience adverse events after physical activity and/or exercise, explored the various types of physical activity or exercise being undertaken by these patient populations, and identified gaps in the evidence. We employed a detailed literature search which spanned several databases. The review of observational and non-randomised studies was limited by the potential for biases in the study designs employed. However, these study design criteria were used because recent systematic reviews of exercise and physical activity interventions on bone health report adverse events already. The generalizability of the findings is also limited by: (i) the greater majority of studies focusing on only postmenopausal women; (ii) the exclusion criteria used by the majority of eligible studies and therefore populations recruited did not include patients with additional medical problems or illnesses including metabolic bone diseases or those that may be associated with secondary osteoporosis, or those who lived in care facilities; and (iii) physical activity or exercises covered were mainly group-based exercise programmes containing common physical activities used within the general population and not high impact activities such as tennis, running or aerobics. Finally, we did not carry out a de-novo systematic review and evidence synthesis of all interventional studies for adverse events, instead of updated three previously performed systematic reviews that focussed on exercise and physical activity interventions in people who are likely to have low bone mass or are at high risk of falls. While this is an unorthodox approach, it does allow a wide-ranging summary of the available literature, both randomised and non-randomised, within a single paper.
Conclusions
On the basis of available evidence, people with osteoporosis or osteopenia can be reassured that they can safely participate in common exercise programmes whether at home or in supervised facilities and many unstructured physical activities. However, as expected with participation in physical activity and exercise, minor adverse events such as muscle ache may occur. Specific repetitive forced spinal forward flexion exercises should be undertaken with care as they may be associated with an increased risk of new vertebral fractures. Some types of activities such as brisk walking, horseback riding and golfing need to be discussed on an individual basis, as they may not be appropriate. These findings, however, cannot be generalized to patients with additional medical problems or illnesses, or those who live in care facilities. Further research focussing on efficacy of exercise or physical activity on bone health should focus on reporting adverse events in different age groups or DXA scores, should include men and should consider those that are normally excluded from such studies (see above).
Acknowledgements
Dr. Setor K. Kunutsor is supported by the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The work for this manuscript was commissioned by the National Osteoporosis Society to support the Consensus Statement on Exercise and Physical Activity for Osteoporosis 2017-2018. SL is an Osteoporosis Nurse Consultant at the National Osteoporosis Society and did not take part in the analysis but contributed to the interpretation and discussion.
Supplementary Material
Appendix 1.
PRISMA checklist.
| Section/topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable, background, objectives, data sources, study eligibility criteria, participants, interventions, study appraisal and synthesis methods, results, limitations, conclusions and implications of key findings, systematic review registration number | 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 4-5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (such as web address), and, if available, provide registration information including registration number | 6 |
| Eligibility criteria | 6 | Specify study characteristics (such as PICOS, length of follow-up) and report characteristics (such as years considered, language, publication status) used as criteria for eligibility, giving rationale | 6 |
| Information sources | 7 | Describe all information sources (such as databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 6 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 2 |
| Study selection | 9 | State the process for selecting studies (that is, screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6-7 |
| Data collection process | 10 | Describe method of data extraction from reports (such as piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 6-7 |
| Data items | 11 | List and define all variables for which data were sought (such as PICOS, funding sources) and any assumptions and simplifications made | 6-7 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 6-7 |
| Summary measures | 13 | State the principal summary measures (such as risk ratio, difference in means). | Not applicable |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (such as I2 statistic) for each meta-analysis | Not applicable |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (such as publication bias, selective reporting within studies) | Not applicable |
| Additional analyses | 16 | Describe methods of additional analyses (such as sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | Not applicable |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 8 and Fig. 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (such as study size, PICOS, follow-up period) and provide the citations | 8 and [Table 1] |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessment (see item 12). | [Table 1] |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present for each study (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot | 8-13, [Table 2] |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Not applicable |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | Not applicable |
| Additional analysis | 23 | Give results of additional analyses, if done (such as sensitivity or subgroup analyses, meta-regression) (see item 16) | Not applicable |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (such as health care providers, users, and policy makers) | 14 |
| Limitations | 25 | Discuss limitations at study and outcome level (such as risk of bias), and at review level (such as incomplete retrieval of identified research, reporting bias) | 17-18 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 18 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (such as supply of data) and role of funders for the systematic review | 19 |
Appendix 2.
Literature search strategy.
Relevant studies, published before June 27, 2017 (date last searched), were identified through electronic searches not limited to the English language using MEDLINE, EMBASE, CINAHL, and Web of Science. Electronic searches were supplemented by scanning reference lists of articles identified for all relevant studies (including review articles) and by hand searching of relevant journals. The computer-based searches combined search terms related to physical activity or exercise, adverse events, and osteoporosis.
| 1 | exp Osteoporosis/ (51404) |
| 2 | osteoporo$.mp. (80014) |
| 3 | fragility fracture.mp. (1070) |
| 4 | osteopenia.mp. (8354) |
| 5 | bone loss.mp. (30385) |
| 6 | bone mass.mp. (16652) |
| 7 | exp Bone Density/ (47932) |
| 8 | bone mineral density.mp. (34542) |
| 9 | Demineralised bone.mp. (103) |
| 10 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (131986) |
| 11 | exp Exercise/ (158559) |
| 12 | Physical activity.mp. (82304) |
| 13 | exp Exercise Therapy/ (41437) |
| 14 | exp Physical Exertion/ (56792) |
| 15 | exp Physical Fitness/ (25995) |
| 16 | exp Physical Endurance/ (30348) |
| 17 | exp Sports/ (161452) |
| 18 | exp Pliability/ (4238) |
| 19 | exp Physical Therapy Modalities/ (133497) |
| 20 | exp Resistance Training/ (5979) |
| 21 | exp Weight Lifting/ (4456) |
| 22 | exp Weight Lifting/ (4456) |
| 23 | exp Rehabilitation/ (270150) |
| 24 | Physiotherapy.mp. (15298) |
| 25 | exp Vibration/ (23192) |
| 26 | Vibration therapy.mp. (132) |
| 27 | exp Running/ (17825) |
| 28 | Cycling.mp. (47684) |
| 29 | exp Swimming/ (22288) |
| 30 | exp Skiing/ (3253) |
| 31 | exp Yoga/ (2157) |
| 32 | pilates.mp. (310) |
| 33 | Tai chi.mp. (1281) |
| 34 | exp Hydrotherapy/ (19190) |
| 35 | Bending.mp. (26784) |
| 36 | exp Lifting/ (2367) |
| 37 | Exertion.mp. (65871) |
| 38 | 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 (684603) |
| 39 | Adverse events.mp. (107914) |
| 40 | Side effects.mp. (221346) |
| 41 | exp Fractures, Bone/ (167159) |
| 42 | exp “Wounds and Injuries”/ (827548) |
| 43 | Injuries.mp. (496425) |
| 44 | Broken bones.mp. (182) |
| 45 | exp Accidental Falls/ (19889) |
| 46 | Falls.mp. (47112) |
| 47 | exp Pain/ (356057) |
| 48 | Physical function.mp. (9795) |
| 49 | Muscle function.mp. (9359) |
| 50 | Balance.mp. (212767) |
| 51 | Mobility.mp. (139058) |
| 52 | 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (1886764) |
| 53 | exp Epidemiologic Studies/ (2101541) |
| 54 | exp Case-Control Studies/ (883169) |
| 55 | exp Cohort Studies/ (1701977) |
| 56 | cohort analysis.mp. (5723) |
| 57 | exp Follow-Up Studies/ (590703) |
| 58 | exp Prospective Studies/ (462547) |
| 59 | exp Longitudinal Studies/ (110993) |
| 60 | exp Retrospective Studies/ (658490) |
| 61 | exp Cross-Sectional Studies/ (249728) |
| 62 | Case series.mp. (54067) |
| 63 | exp Case Reports/ (1890524) |
| 64 | 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 (3922083) |
| 65 | 10 and 38 and 52 and 64 (1109) |
| 66 | limit 65 to (humans and “middle aged (45 plus years)”) (854) |
Each part was specifically translated for searching the other databases (EMBASE, CINAHL, and Web of Science databases)
Appendix 3
Reference list of excluded studies.
Cauley JA, Harrison SL, Cawthon PM, Ensrud KE, Danielson ME, Orwoll E, Mackey DC. Objective measures of physical activity, fractures and falls: the osteoporotic fractures in men study. J Am Geriatr Soc 2013;61:1080-1088.
Cramer H, Krucoff C, Dobos G. Adverse events associated with yoga: a systematic review of published case reports and case series. PloS one 2013;8:e75515.
Dohrn IM, Hagstromer M, Hellenius ML, Stahle A. Gait Speed, Quality of Life, and Sedentary Time are Associated with Steps per Day in Community-Dwelling Older Adults with Osteoporosis. J Aging Phys Act 2016;24:22-31.
Gerdhem P, Ringsberg KA, Akesson K, Obrant KJ. Influence of muscle strength, physical activity and weight on bone mass in a population-based sample of 1004 elderly women. Osteoporos Int 2003;14:768-772.
Gregg EW, Cauley JA, Seeley DG, Ensrud KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1998;129:81-88.
Huovinen V, Ivaska KK, Kiviranta R, et al. Bone mineral density is increased after a 16-week resistance training intervention in elderly women with decreased muscle strength. Eur J Endocrinol 2016;175:571-582
Kronhed AC, Moller M. Effects of physical exercise on bone mass, balance skill and aerobic capacity in women and men with low bone mineral density, after one year of training--a prospective study. Scand J Med Sci Sports 1998;8:290-298.
Liu-Ambrose TY, Khan KM, Eng JJ, Gillies GL, Lord SR, McKay HA. The beneficial effects of group-based exercises on fall risk profile and physical activity persist 1 year postintervention in older women with low bone mass: follow-up after withdrawal of exercise. J Am Geriatr Soc 2005;53:1767-1773.
Mackey DC, Hubbard AE, Cawthon PM, Cauley JA, Cummings SR, Tager IB, Osteoporotic Fractures in Men Research G. Usual physical activity and hip fracture in older men: an application of semiparametric methods to observational data. Am J Epidemiol 2011;173:578-586.
Mitchell SL, Grant S, Aitchison T. Physiological Effects of Exercise on Post-menopausal Osteoporotic Women. Physiotherapy 1998;84:157-163.
Pearson JA, Burkhart E, Pifalo WB, Palaggo-Toy T, Krohn K. A lifestyle modification intervention for the treatment of osteoporosis. Am J Health Promot 2005;20:28-33.
Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. The journals of gerontology Series A, Biological sciences and medical sciences 2000;55:M489-491.
Stief F, Schafer A, Vogt L, Kirchner M, Hubscher M, Thiel C, Banzer W, Meurer A. Differences in Gait Performance, Quadriceps Strength, and Physical Activity Between Fallers and Nonfallers in Women with Osteoporosis. J Aging Phys Act 2016;24:430-434.
Sze PC, Cheung WH, Lam PS, Lo HS, Leung KS, Chan T. The efficacy of a multidisciplinary falls prevention clinic with an extended step-down community program. Arch Phys Med Rehabil 2008;89:1329-1334.
Tsutsumimoto K, Doi T, Shimada H, Makizako H, Yoshida D, Uemura K, Anan Y, Park H, Suzuki T. Self-reported exhaustion associated with physical activity among older adults. Geriatr Gerontol Int 2016; 16:625-630.
Vaillant J, Vuillerme N, Martigne P, Caillat-Miousse JL, Parisot J, Nougier V, Juvin R. Balance, aging, and osteoporosis: effects of cognitive exercises combined with physiotherapy. Joint Bone Spine 2006;73:414-418.
Villareal DT, Binder EF, Yarasheski KE, Williams DB, Brown M, Sinacore DR, Kohrt WM. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc 2003;51:985-990.
Grahn Kronhed A-C, Hallberg I, Ödkvist L, Möller M. Effect of training on health-related quality of life, pain and falls in osteoporotic women. Advances in Physiotherapy. 2009;11(3):154-65.
Appendix 4.
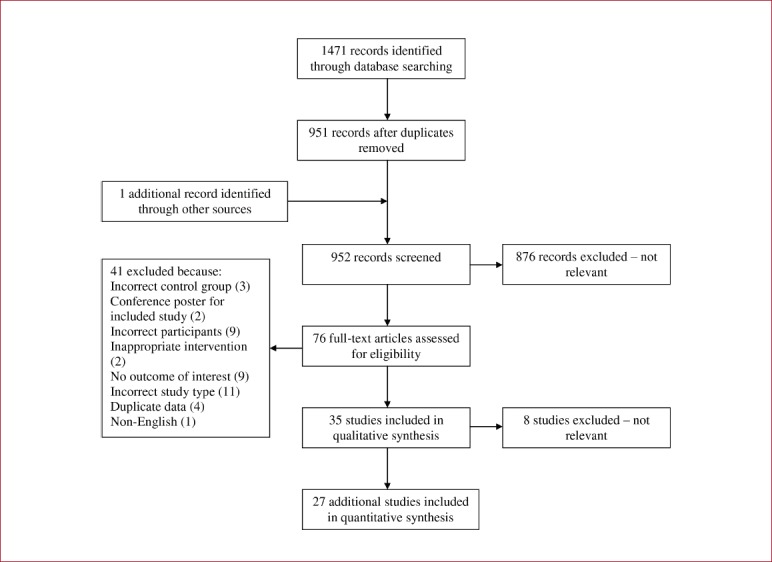
Flow diagram of study selection process for updated Howe review
Appendix 5.
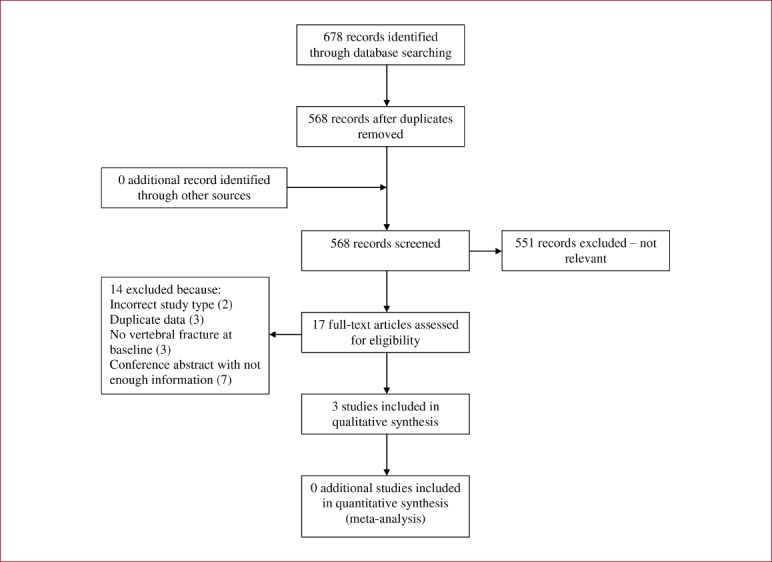
Flow diagram of study selection process for updated Giangregorio review.
Appendix 6.
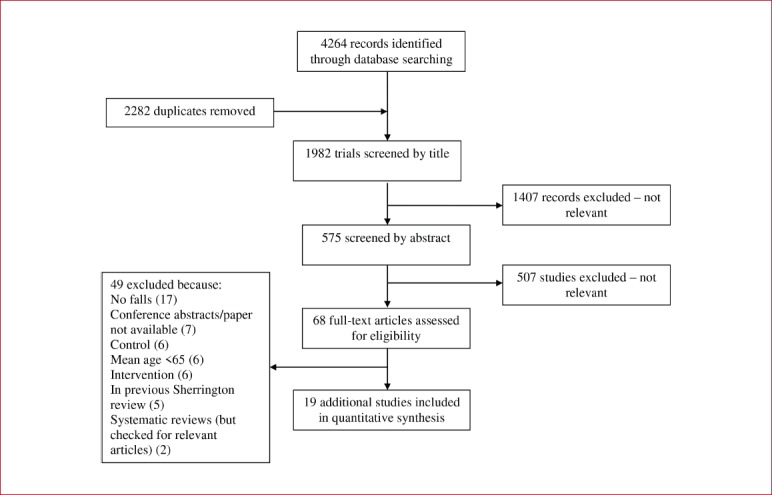
Flow diagram of study selection process for updated Sherrington review.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Reginster JY, Burlet N. Osteoporosis:a still increasing prevalence. Bone. 2006;38(2 Suppl 1):S4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark E, et al. Osteoporosis in the European Union:Medical management, epidemiology and economic burden. A report prepared in collaboration with the Internation Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Archives of osteoporosis. 2013;8 doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7(9):1005–10. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 4.Randell A, Sambrook PN, Nguyen TV, Lapsley H, Jones G, Kelly PJ, et al. Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos Int. 1995;5(6):427–32. doi: 10.1007/BF01626603. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, 3rd, Johnell O, Lau E, Mautalen CA, Seeman E. Osteoporosis and the global competition for health care resources. J Bone Miner Res. 2004;19(7):1055–8. doi: 10.1359/JBMR.040316. [DOI] [PubMed] [Google Scholar]
- 6.Group NOG. Clinical guideline for the prevention and treatment of osteoporosis 2017. Contract No.: www.shef.ac.uk/NOGG .
- 7.SIGN. 142:Management of osteoporosis and the prevention of fragility fractures. A national clinical guideline 2015 [Google Scholar]
- 8.Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 9.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. Jama. 2002;288(18):2300–6. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 10.Kai MC, Anderson M, Lau EM. Exercise interventions:defusing the world's osteoporosis time bomb. Bull World Health Organ. 2003;81(11):827–30. [PMC free article] [PubMed] [Google Scholar]
- 11.de Kam D, Smulders E, Weerdesteyn V, Smits-Engelsman BC. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density:a systematic review of randomized controlled trials. Osteoporos Int. 2009;20(12):2111–25. doi: 10.1007/s00198-009-0938-6. [DOI] [PubMed] [Google Scholar]
- 12.Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults:an updated meta-analysis and best practice recommendations. British Journal of Sports Medicine. 2016;51(24):1749. doi: 10.1136/bjsports-2016-096547. [DOI] [PubMed] [Google Scholar]
- 13.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. The Cochrane Database of Systematic Reviews. 2011;7 doi: 10.1002/14651858.CD000333.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. The Cochrane Database of Systematic Reviews. 2013;1:1–50. doi: 10.1002/14651858.CD008618.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giangregorio LM, McGill S, Wark JD, Laprade J, Heinonen A, Ashe MC, et al. Too Fit To Fracture:outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26:891–910. doi: 10.1007/s00198-014-2881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck BR, Daly RM, Fiatarone Singh MA, Taaffe DR. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. Journal of Science and Medicine in Sport. 2016;20(5):438–45. doi: 10.1016/j.jsams.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp . [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 19.Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis:flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65(10):593–6. [PubMed] [Google Scholar]
- 20.Harrison JE, Chow R, Dornan J, Goodwin S, Strauss A. Evaluation of a program for rehabilitation of osteoporotic patients (PRO):4-year follow-up. The Bone and Mineral Group of the University of Toronto. Osteoporos Int. 1993;3(1):13–7. doi: 10.1007/BF01623171. [DOI] [PubMed] [Google Scholar]
- 21.Walker M, Klentrou P, Chow R, Plyley M. Longitudinal evaluation of supervised versus unsupervised exercise programs for the treatment of osteoporosis. Eur J Appl Physiol. 2000;83(4-5):349–55. doi: 10.1007/s004210000266. [DOI] [PubMed] [Google Scholar]
- 22.Kerschan-Shindl K, Uher E, Kainberger F, Kaider A, Ghanem AH, Preisinger E. Long-term home exercise program:effect in women at high risk of fracture. Arch Phys Med Rehabil. 2000;81(3):319–23. doi: 10.1016/s0003-9993(00)90078-9. [DOI] [PubMed] [Google Scholar]
- 23.Liu-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology. 2004;50(6):373–82. doi: 10.1159/000080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y. Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab. 2004;22(5):500–8. doi: 10.1007/s00774-004-0514-2. [DOI] [PubMed] [Google Scholar]
- 25.Murphy L, Singh BB. Effects of 5-Form, Yang Style Tai Chi on older females who have or are at risk for developing osteoporosis. Physiother Theory Pract. 2008;24(5):311–20. doi: 10.1080/09593980701884790. [DOI] [PubMed] [Google Scholar]
- 26.Hakestad KA, Torstveit MK, Nordsletten L, Axelsson AC, Risberg MA. Exercises including weight vests and a patient education program for women with osteopenia:a feasibility study of the OsteoACTIVE rehabilitation program. J Orthop Sports Phys Ther. 2015;45(2):97–105. doi: 10.2519/jospt.2015.4842. C1-4. [DOI] [PubMed] [Google Scholar]
- 27.Oh J, Oh HM, Lee JI. Horseback Riding-Related Vertebral Compression Fracture from Walking in Women with Low Bone Mineral Density:Reports of Two Cases. Curr Sports Med Rep. 2016;15(1):38–40. doi: 10.1249/JSR.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 28.Sinaki M. Yoga spinal flexion positions and vertebral compression fracture in osteopenia or osteoporosis of spine:case series. Pain Pract. 2013;13(1):68–75. doi: 10.1111/j.1533-2500.2012.00545.x. [DOI] [PubMed] [Google Scholar]
- 29.Ekin JA, Sinaki M. Vertebral compression fractures sustained during golfing:report of three cases. Mayo Clin Proc. 1993;68(6):566–70. doi: 10.1016/s0025-6196(12)60371-1. [DOI] [PubMed] [Google Scholar]
- 30.Tuzun S, Aktas I, Akarirmak U, Sipahi S, Tuzun F. Yoga might be an alternative training for the quality of life and balance in postmenopausal osteoporosis. Eur J Phys Rehabil Med. 2010;46(1):69–72. [PubMed] [Google Scholar]
- 31.Cesarec G, Martinec S, Basic I, Jakopic D. Effect of exercises on quality of life in women with osteoporosis and osteopenia. Coll Antropol. 2014;38(1):247–54. [PubMed] [Google Scholar]
- 32.Lu YH, Rosner B, Chang G, Fishman LM. Twelve-Minute Daily Yoga Regimen Reverses Osteoporotic Bone Loss. Top Geriatr Rehabil. 2016;32(2):81–7. doi: 10.1097/TGR.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer H, Krucoff C, Dobos G. Adverse events associated with yoga:a systematic review of published case reports and case series. PloS one. 2013;8(10):e75515. doi: 10.1371/journal.pone.0075515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angin E, Erden ZFC. The effects of clinical pilates exercises on BMD, physical performance and quality of life of women with postmenopausal osteoporosis. Journal of Back and Musculoskeletal Rehabilitation. 2015;28(4):849–58. doi: 10.3233/BMR-150604. [DOI] [PubMed] [Google Scholar]
- 35.Wen H, Huang T, Li T, Chong P, Ang B. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017;28(2):539–47. doi: 10.1007/s00198-016-3759-4. [DOI] [PubMed] [Google Scholar]
- 36.Marques EA, Wanderley F, Machado L, Sousa F, Viana JL, Moreira-Goncalves D. Effects of resistance and aerobic exercise on physical function, BMD, OPG and RANKL in older women. Experimental Gerontology. 2011;46(7):524–32. doi: 10.1016/j.exger.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Duff W, Kontulainen S, Candow D, Grodon J, Mason R, Taylor-Gjevre R. Effects of low-dose ibuprofen supplementation and resistance training on bone and muscle in postmenopausal women:A randomized controlled trial. Bone Reports. 2016;5:96–103. doi: 10.1016/j.bonr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borba-Pinheiro CJ, Dantas EHM, Vale RGD, Drigo AJ, Carvalho M, Tonini T. Adapted combat sports on bone related variables and functional independence of postmenopausal women in pharmacological treatment:a clinical trial study. Arch Budo. 2016;12:187–99. doi: 10.1016/j.archger.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Borba-Pinheiro CJ, Carvalho M, Drigo AJ, Silva NSL. Combining adapted judo training and pharmacological treatment to improve BMD on postmenopausal women:A two years study. Arch Budo. 2013;9(2):93–9. [Google Scholar]
- 40.Wang H, Yu B, Chen W, Lu Y, Yu D. Simplified Tai Chi resistance training versus traditional Tai Chi in slowing bone loss in postmenopausal women. Evidence-based Complementary and Alternative Medicine. 2015;2015:ID 379451. doi: 10.1155/2015/379451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenaerts L, Verschueren S, Bogaerts A, Boonen S, Delecluse C, Fritscher K. Quantification of training effects on femoral bone quality using patient-specific FE analysis. Journal of Biomechanics. 2012;45:S542. [Google Scholar]
- 42.Karakiriou SK, Douda HT, Smilios IG, Volaklis KA, Tokmakidis SP. Effects of vibration and exercise training on BMD and muscle strength in postmenopausal women. European Journal of Sports Science. 2012;12(1):81–8. [Google Scholar]
- 43.Marques EA, Mota J, Machado L, Sousa F, Coelho M, Moreira P. Multicomponent training programme with weight-bearing exercises elicits favourable bone density, muscle strength and balance adaptations in older women. Calcified Tissue International. 2011;88(2):117–29. doi: 10.1007/s00223-010-9437-1. [DOI] [PubMed] [Google Scholar]
- 44.Ashe MC, Gorman E, Khan K, Brasher P, Cooper D, McKay H. Does frequency of resistance training effect tibial cortical bone density in older women? A randomized controlled trial. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(2):623–32. doi: 10.1007/s00198-012-2000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basat H, Esmaeilzadeh S, Eskiyurt N. The effects of strengthening and high-impact exercises on bone metabolism and quality of life in postmenopausal women:a raondomized controlled trial. Journal of Back and Musculoskeletal Rehabilitation. 2013;26(4):427–35. doi: 10.3233/BMR-130402. [DOI] [PubMed] [Google Scholar]
- 46.Chilibeck P, Vatanparast H, Pierson R, Case A, Olatunbosun O, Whiting S. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women:a randomized clinical trial. Journal of Bone and Mineral Research. 2013;28(4):780–93. doi: 10.1002/jbmr.1815. [DOI] [PubMed] [Google Scholar]
- 47.Bolton K, Egerton T, Wark JD, Wee E, Matthews B, kelly A. Effects of exercise on bone desnity and falls risk factors in postmenopausal women with osteopenia:a randomised controlled trial. Journal of Science and Medicine in Sport. 2012;15(2):102–9. doi: 10.1016/j.jsams.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Nicholson V, McKean M, Slater G, Kerr A, Burkett B. Low-load very high-repetition resistance training attenuates bone loss at the lumbar spine in active postmenopausal women. Calcified Tissue International. 2015;96(6):490–199. doi: 10.1007/s00223-015-9976-6. [DOI] [PubMed] [Google Scholar]
- 49.Brubaker M, Limburg P, Sinaki M. Site specificity of physical activity and its effect on long-term risk of fracture:Spine vs Hip. Journal of Bone and Mineral Research. 2012;27 no pagination. [Google Scholar]
- 50.Evstigneeva L, Lesnyak O, Bultinik I, Lems WF, Kozhemyakina E, Negodaeva E, et al. Effect of 12-month physical exercise program on patients with osteoporotic vertebral fractures:a randomised, controlled trial. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:2515–24. doi: 10.1007/s00198-016-3560-4. [DOI] [PubMed] [Google Scholar]
- 51.Miko I, Szerb I, Szerb A, Poor G. Effectiveness of balance training programme in reducing the frequency of falling in established osteoporotic women:a randomised controlled trial. Clinical Rehabilitation. 2017;31(2):217–24. doi: 10.1177/0269215516628616. [DOI] [PubMed] [Google Scholar]
- 52.Wayne P, Kiel D, Buring J, Bonato P, Yeh G, Cohen C. Impact of Tai Chi exercise on multiple fracture-related risk factors in postmenopausal osteopenic women:A pilot pragmatic randomized trial. BMC Complementary and Alternative Medicine Conference. 2012;12:1–13. doi: 10.1186/1472-6882-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Multanen J, Nieminen MT, Hakkinen A, Kujala UM, Jamsa T, Kautiainen H. Effects of high-impact training on bone and articular cartilage:12-month randomized controlled quantitative MRI study. Journal of Bone and Mineral Research. 2014;29(1):192–201. doi: 10.1002/jbmr.2015. [DOI] [PubMed] [Google Scholar]
- 54.Liu BX, Chen SP, Li YP, Wang J, Zhang B, Lin Y. The effect of the modified eighth section of Eight-Section Brocade on osteoporosis in postmenopausal women:A prospective randomized trial. Medicine. 2015;94(25):e991. doi: 10.1097/MD.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsen CF, Bergland A. The effect of exercise and education on fear of falling in elderly women with osteoporosis and a history of vertebral fracture:results of a randomized controlled trial. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25:2017–25. doi: 10.1007/s00198-014-2724-3. [DOI] [PubMed] [Google Scholar]
- 56.Karakasidou P, Skordilis EK, Dontas I, Papaioannou N, Lyritis GP. Motor control exercise can reduce pain and kyphosis in osteoporotic women with vertebral fractures:A randomised controlled trial. Rev Clin Pharmacol Pharmacokinet Int Ed. 2013;27:95–106. [Google Scholar]
- 57.Siegrist M, Freiberger E, Geilhof B, et al. Fall prevention in a primary care setting - the effects of a targeted complex exercise intervention in a cluster randomised trial [with consumer survey] Deutsches Arzteblatt International. 2016;113(21):365–72. doi: 10.3238/arztebl.2016.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merom D, Mathieu E, Cerin E, et al. Social dancing and incidence of falls in older adults:a cluster randomised controlled trial. PLoS One. 2016;13(8) doi: 10.1371/journal.pmed.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dadgari A, Sizan Hamid T, Hakim MN, et al. Randomized control trials on Otago exercise program (OEP) to reduce falls among elderly community dwellers in Shahroud, Iran. Iranian Red Crescent Medical Journal. 2016;18(5) doi: 10.5812/ircmj.26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sparrow D, DeAngelis TR, Hendron K, et al. Highly challenging balance program reduces fall rate in Parkinson Disease. Journal of Neurologic Physical Therapy. 2016;40(1):24–30. doi: 10.1097/NPT.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin T, Weatherall M, Anderson TJ, et al. A randomized controlled feasibility trial of a specific cueing program for falls management in persons with Parkinson's Disease and freezing of gait. Journal of Neurologic Physical Therapy. 2015;39(3):179–84. doi: 10.1097/NPT.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with Parkinson Disease, Oregon, 2008-2011. Preventing Chronic Disease. 2015;12:E120. doi: 10.5888/pcd12.140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris ME, Taylor NF, Watts JJ. A home programme of strength training, movement strategy training and education did not prevent falls in people with Parkinson's Disease:a randomised trial. Journal of Physiotherapy. 2017;63(2):94–100. doi: 10.1016/j.jphys.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Waterman H, Ballinger C, Brundle C. A feasibility study to prevent falls in older people who are sight impaired:the VIP2UK randomised controlled trial. Trials. 2016;17(1):e464. doi: 10.1186/s13063-016-1565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dangour AD, Albala C, Allen E. Effect of a nutrition supplement and physical activity programme on pneumonia and walking capacity in Chilean older people:a factorial cluster randomized trial. PLoS Med. 2011;8(4):e1001023. doi: 10.1371/journal.pmed.1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freiberger E, Haberle L, Spirduso WW. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults:a randomized controlled trial. Journal of the American Geriatrics Society. 2012;60(3):437–46. doi: 10.1111/j.1532-5415.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- 67.Iwamoto J, Suzuki H, Tanaka K. Preventative effect of exercise against falls in the elderly:a randomized controlled trial. Osteoporosis international:a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(7):1233–40. doi: 10.1007/s00198-008-0794-9. [DOI] [PubMed] [Google Scholar]
- 68.Morris ME, Menz HB, McGinley JL. A randomized controlled trial to reduce falls in people with Parkinson's Disease. Neurorehabilitation and Neural Repair. 2015;29(8):778–85. doi: 10.1177/1545968314565511. [DOI] [PubMed] [Google Scholar]
- 69.Suttanon P, Hill KD, Said CM. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer's disease:a pilot randomized controlled trial. Clinical Rehabilitation. 2013;27(5):427–38. doi: 10.1177/0269215512460877. [DOI] [PubMed] [Google Scholar]
- 70.Uusi-Rasi K, Patil R, Karinkanta S. Exercise and vitamin D in fall prevention among older women:a randomized clinical trial. JAMA Internal Medicine. 2015;175(5):703–11. doi: 10.1001/jamainternmed.2015.0225. [DOI] [PubMed] [Google Scholar]
- 71.Wesson J, Clemson L, Brodaty H. A feasibility study and pilot randomised trial of a tailored prevention programme to reduce falls in older people with mild dementia. BMC Geriatrics. 2013;13(1):89. doi: 10.1186/1471-2318-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherrington C, Lord SR, Volger CM, et al. A post-hospital home exercise programme improved mobility but increased falls in older people:A randomised controlled trial. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dean CM, Rissel C, Sherrington C. Exercise to enhance mobility and prevent falls after stoke:the community stroke club randomized trial. Neurorehabilitation and Neural Repair. 2012;26(9):1046–57. doi: 10.1177/1545968312441711. [DOI] [PubMed] [Google Scholar]
- 74.Clemson L, Singh MAF, Bundy A. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study):randomised parallel trial. BMJ. 2012;345:e4547. doi: 10.1136/bmj.e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebrahim S, Thompson PW, Baskaran V, Evans K. Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age and Ageing. 1997;26(4):253–60. doi: 10.1093/ageing/26.4.253. [DOI] [PubMed] [Google Scholar]
- 76.Howland J, Lachman ME, Peterson EW, Cote J, Kasten L, Jette A. Covariates of fear of falling and associated activity curtailment. Gerontologist. 1998;38(5):549–55. doi: 10.1093/geront/38.5.549. [DOI] [PubMed] [Google Scholar]
- 77.Gunendi Z, Eker D, Tecer D, Karaoglan B. Is the word 'Osteoporosis'a reason for kinesiophobia? Eur J Phys Rehabil Med. 2018 doi: 10.23736/S1973-9087.18.04931-6. doi:10.23736/S1973-9087.18.04931-6. [DOI] [PubMed] [Google Scholar]
- 78.Kendrick D, Kumar A, Carpenter H, Ziilstra GA, Skelton DA, Cook JR, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database of Systematic Reviews. 2014;28(11):CD009848. doi: 10.1002/14651858.CD009848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chastin SF, Mandrichenko O, Helbostadt JL, Skelton DA. Associations between objectively measured sedentary behaviour and physical activity with BMD in adults and older adults:the NHANES study. Bone. 2014;64:254–62. doi: 10.1016/j.bone.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Chastin SF, Mandrichenko O, Skelton DA. The frequency of osteogenic activities and the pattern of intermittence between periods of physical activity and sedentary behaviour affects BMC:the cross-sectional NHANES study. BMC Public Health. 2014;6(14) doi: 10.1186/1471-2458-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Copeland JL, Ashe MC, Biddle SJ, Brown WJ, Buman MP, Chastin SF, et al. Sedentary time in older adults:a critical review of measurement, associations with health, and interventions. Br J Sports Med. 2017;19 doi: 10.1136/bjsports-2016-097210. [DOI] [PubMed] [Google Scholar]
- 82.Yang H, Jekir MG, Riuf SF, Keaveny TM. 20th Annual Symposium on Computational Methods in orthopaedic Biomechanics. Berkeley, C.A: 2012. The influence of disc elasticity on the micromechanics of vertebral fracture for forward bending. [Google Scholar]
- 83.Havlik HS. Equestrian sport-related injuries:a review of current literature. Current Sports Medicine Reports. 2010;9(5):299–302. doi: 10.1249/JSR.0b013e3181f32056. [DOI] [PubMed] [Google Scholar]
- 84.Murray AD, Daines L, Archibald D, Hawkes RA, Schiphorst C, Kelly P, et al. The relationship between golf and health:a scoping review. British Journal of Sports Medicine. 2017;51(1) doi: 10.1136/bjsports-2016-096625. [DOI] [PMC free article] [PubMed] [Google Scholar]


