Abstract
Background:
Patients with STAT5b deficiency have autoimmunity, recurrent infections and combined immune deficiency, which affects T-cell homeostasis and leads to natural killer (NK) cell impairment.
Objective:
In this study, we characterized the NK cell defect in STAT5b-deficient human NK cells as well as Stat5b−/− mice.
Methods:
We used multiparametric flow cytometry, functional NK cells assays, microscopy as well as a Stat5b−/− mouse model to elucidate the effect impaired and/or absent STAT5b has on NK cell development and function.
Results:
This alteration generated a non-functional CD56bright NK cell subset, characterized by low cytokine production. The CD56dim NK cell subset had decreased expression of perforin and CD16 and a higher frequency of cells expressing markers of immature NK cells. We observed low NK cell numbers and impaired NK cell maturation suggesting that STAT5b is involved in terminal NK cell maturation in Stat5b−/− mice. Furthermore, human STAT5b-deficient NK cells had low cytolytic capacity, and fixed-cell microscopy showed poor convergence of lytic granules. This was accompanied by decreased expression of co-stimulatory and activating receptors. Interestingly, granule convergence and cytolytic function was restored after IL-2 stimulation.
Conclusions:
Our results show that, in addition to the impaired terminal maturation of NK cells, human STAT5b mutation leads to impairments in early activation events in NK cell lytic synapse formation. Our data gives further insight into NK cell defects caused by STAT5b deficiency.
Keywords: STAT5b, NK cell deficiency, NK cell maturation, perforin, VEGF, IL-2, IL-15, MTOC, Lytic granule convergence
Capsule summary:
In humans, the absence of STAT5b mimics the abrogated NK cell terminal maturation observed in Stat5b−/− mice. NK cells from STAT5b patients had a decreased cytolytic function and impaired convergence of lytic granules to the microtubule-organizing center.
Graphical Abstract

Introduction
Natural killer (NK) cells are major effectors of the innate immune system and early effector cells against viral infections, pathogens, and malignant cells. They represent 10–15% of lymphocytes in the peripheral blood.1–3 NK cells mature through distinguishable stages (stages 1, 2a, 2b, 3, 4a, 4b-CD56bright, 5-Early CD56dim, and 6-late CD56dim), each of which is characterized by a specific pattern of surface expression, proliferative, and functional capacities, as well as in vivo trafficking.4–8 The ex vivo characterization of NK cell developmental intermediates in human secondary lymphoid tissues revealed a new schema for the stages of NK cell development.5 This classification was proposed according to CD34, CD117, IL-1R, CD94, NKp80, CD16 and CD57 expression.5, 9 The differential NKp80 expression defines stages 4a and 4b. Both stages lack the hallmark phenotype of mature NK cells, but have the capacity to acquire properties of mature NK cells.9 Finally, the up-regulation of CD57 expression defines two functional CD56dim mature (stage 5&6) NK cell subsets; the “early” CD57−CD56dim and the “late” CD57+CD56dim. The CD57+CD56dim subset has been characterized with a more mature phenotype and a potent cytolytic capacity.6, 10
IL-15 can induce NK cell development from human bone marrow-derived hematopoietic progenitor cells and is required for the terminal maturation of fully functional NK cells.11–14 Activation through the intracellular domain of the IL-15 receptor leads to the recruitment and phosphorylation of STAT5 proteins, as a principal downstream effector of this signaling pathway.15, 16 STAT5 proteins are phosphorylated by Janus kinase (JAK) proteins (JAK1 and JAK3). This modification leads to the formation of homo- or heterodimers that translocate to nucleus and bind to consensus sequences in the promoters of genes such as CD25, Bcl-2, and FOXP3.15–17 Most notably, it is known that Stat5b−/− mice have significantly lower levels of perforin expression at baseline and greatly decreased cytolytic function in NK cells.18
The first steps of the interaction between NK cells with their target cells are mediated by a group of receptors which include CD226 (DNAM-1), CD314 (NKG2D), CD244, and lymphocyte function-associated antigen-1 (LFA-1).19–21 The transmission of activation signals through these receptors results in firm adhesion to the target cell, and progression to NK cell cytotoxicity.20 Moreover, human NK cell subsets have different expressions of adhesion receptors that correlates with their distinct trafficking pattern and development.4, 22
In humans, STAT5b deficiency is associated with severe growth hormone (GH)-resistant growth failure and combined primary immunodeficiency.15, 23–25 Affected individuals develop recurrent bacterial and viral infections, and their immunophenotype reveals lymphopenia, decreased accumulation of regulatory T cells, and decreased number of NK cells.17, 24, 26 Here, we focused on the functional defects identified in NK cells of two siblings with a homozygous deletion in STAT5B. These patients have increased susceptibility to viral infections and a significantly low number of NK cells in peripheral blood. We describe a functionally impaired CD56bright subset and an immature CD56dim subset characterized by low levels of perforin. Additionally, we identified NK cell subsets in our patients with low levels of expression of co-stimulatory and activating receptors, which affects granule convergence to the microtubule-organizing center (MTOC). We further extended our study to Stat5b−/− mice and found similarly impaired NK cell terminal maturation. In both mouse and human, STAT5b-deficient NK cells have been characterized with increased levels of VEGF that may trigger their pro-tumorigenic potential. Together, these observations demonstrate that STAT5b plays a critical role in the terminal maturation and function of NK cells in humans and mice.
Methods
Study approval
Blood samples were collected from STAT5b-deficient patients and healthy donors under approved protocols from the Baylor College of Medicine Institutional Review Board. Consent was obtained from all patients and donors and samples were obtained under guidelines established by the Declaration of Helsinki. All peripheral blood samples were collected in sodium heparin collection tubes. PBMCs from healthy donors and patients were purified by Ficoll-Paque Plus density gradient centrifugation (GE Healthcare).
Mouse model
Mice were bred on the C57BL/6 background and maintained at the University of Veterinary Medicine Vienna under pathogen-free conditions. The following mice were studied: Stat5a−/−, Stat5b+/+ (WT), Stat5b+/− and Stat5b−/−.27, 28 All experiments were carried out with age-matched 6-to-12-week old mice and were approved by the institutional animal care committee and review board conforming to Austrian law (licenses: BMWFW-68.205/0093-WF/V/3b/2015 and BMWF-68.205/0218-II/3b/2012).
STAT5b expression
Peripheral blood mononuclear cells (PBMCs), B lymphocyte cell line (BLCL) from healthy donors and STAT5b-deficient patients, were lysed with Pierce RIPA buffer (ThermoFisher Scientific) plus protease inhibitors. Samples were subjected to electrophoresis using NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred onto nitrocellulose membrane (Invitrogen). The following primary antibodies were used: rabbit polyclonal anti-STATb (AF1584, R&D Systems), mouse monoclonal anti-STAT5a (clone 4H1, Cell Signaling Technologies), and mouse anti-beta Actin (BA3R, ThermoFisher Scientific). The secondary goat antimouse IgG conjugated to IRDye 680RD and goat anti-rabbit IgG conjugated to IRDye 680CW antibodies (LI-COR Biosystems) were used. The fluorescence imaging system (LI-COR Biosystems) was used according to the manufacturer’s instructions.
Imaging flow cytometry
BLCLs (106 cells) from healthy donor and STAT5b-deficient patients were stimulated with 50 ng/ml of IL-21 for 30 minutes. The cells were then fixed and permeabilized with Perm buffer III (BD), followed by an incubation with anti-spSTAT5 (pY694) Alexa Fluor 647 conjugated antibody (BD Phosflow) and DAPI (Sigma Aldrich). Samples were acquired on an ImageStream MkII (Luminex) with a 60X objective. Images were processed using IDEAS software v6.1 (Luminex).
Cytotoxicity assays
Antibody-dependent cell-mediated cytotoxicity (ADCC) and NK cell cytotoxicity were measured using the Cr51 release assay.29 ADCC was evaluated with the Raji cell line, which was incubated in the presence or absence of anti-CD20 (Rituximab, 20 μg/mL) and co-cultured with fresh PBMCs for 4 hours at 37°C in 5% CO 2. For natural cytotoxicity, PBMCs from patients and healthy donors were incubated for 4 hours with or without IL-2 (1000 U/mL; Proleukin-aldesleukin, Prometheus) or IL-15 (25 ng/ml Peprotech) and the K562 target cell line. Lytic units were calculated as the number of effectors required to lyse 10% of target cells by using the curves generated with the range of effector to target cell ratios.
Flow cytometry-based NK cell phenotype assay and antibodies
NK cells from STAT5b-deficient patients and healthy donors were studied phenotypically by flow cytometry. To ensure inclusion of age- and gender-matched controls, data from 4 healthy female age-matched donors that were previously reported and published by our group30 were compared to the adult healthy donor control PBMCs evaluated on the same day with the patient samples. Median fluorescence intensity (MFI) and percentage from age/gender matched controls were found to be within the same range for MFI and percentage as HD controls acquired on the same day; these data were therefore pooled for analysis. PBMCs were stained as described previously with the same antibodies and protocol used for the age/gender matched donors.30, 31 Intracellular cytokines were evaluated in cells stimulated with PMA and Ionomycin (Sigma Aldrich) for 6 hours. Brefeldin A (final concentration 10 ug/mL - Sigma Aldrich) was added 4 hours before antibody staining. The cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences). For IFN-γ production by NK cells, PBMCs from healthy donor and patient 1 were stimulated with IL-12 (10 ng/ml), IL-15 (1 ng/ml), and IL-18 (10 ng/ml) by 12 hours.
For detection of LFA-1, cells were stained with CD56 (clone, HCD56; BioLegend) and CD3 (clone, OKT3; BioLegend) antibodies and then stimulated with 10 ng/mL PMA (Sigma Aldrich) for 15 minutes. During this time, the cells were incubated with the CD11a/CD18 antibody (Clone m24, BioLegend). The samples were acquired with an LSR-Fortessa (BD Biosciences) cytometer using FACSDiva version 7.0 and analyzed using FlowJo 10.4.2. The percentage of NK cells that was positive for the receptor of interest was defined using a corresponding fluorescent minus one (FMO) control.32
For analysis of murine NK cells by flow cytometry, the following antibodies were purchased from eBioscience/Thermo Fisher Scientific: CD3ε (17A2, 145-s2C11), CD49b (DX5), NK1.1 (PK136), NKp46 (29A1.4), CD27 (LG.7F9), CD11b (ICRF44), CD122 (TM-b1), CD19 (eBio1D3), Ly6-G (Gr-1, RB6–8C5), and Ter119 (TER-119). Bones were grinded with a mortar and pestle, and filtered through a nylon mesh to obtain single-cell suspensions. Splenocytes were depleted of erythrocytes, and the anti-CD16/CD32 antibody (eBioscience) was added to all samples prior to staining. All mouse samples were recorded on a FACS Canto II flow cytometer (BD Biosciences) and analyzed with BD FACSDiva software version 6.1.2 and 7.0.
VEGF-A expression in human and murine NK cells:
Human NK cells from patient 1 and a healthy donor were isolated from PBMCs using NK cell isolation kit (MACS, Miltenyi Biotec) and the total RNA was isolated using the RNeasy Mini kit (Qiagen). The expression of VEGF-A and the house-keeping gene GAPDH was analysed by qPCR. Following primers were used: hVEGFA-for: AAGGAGGAGGGCAGAATCAT; hVEGFA-rev: ATCTGCATGGTGATGTTGGA; GAPDH-for: TCTCCTCTGACTTCAACAGCG; GAPDH-rev: ACCACCCTGTTGCTGTAGCC. For in vivo tumor model Stat5b+/+ (WT) and Stat5b+/− mice were subcutaneously injected with 106 RMA cells and the tumor growth was monitored. 10 days post injection the mice were sacrificed and the tumors were weighed and snap-frozen. The VEGF-A protein expression was analysed in tumor lysates by ELISA (R&D Systems) according to manufacturer’s protocol and normalized to the total protein amount.
Flow cytometry-based conjugation assay
K562 cells were labeled with CellTrace CFSE (Carboxyfluorescein succinimidyl ester, 0.125 μM, Molecular Probes) for 15 minutes at room temperature. PBMCs were pre-stained with anti-CD3 and anti-CD56 antibodies (Biolegend). PBMCs (106 cells) and 1×105 target cells were incubated at 37°C for 0, 10, 30, or 60 minutes in 200 μl of R10 medium, mixed very gently, and fixed with 1% paraformaldehyde (EMS). Finally, all samples were acquired with the LSR-Fortessa flow cytometer and analyzed using FlowJo.
Fixed-cell microscopy assay
PBMCs (106 cells) were co-cultured with 105 target cells for 15 minutes in presence or absence of IL-2 (1000 UI/ml) or IL-15 (25 ng/ml), and then adhered to silane-coated slides for 20 minutes at 37°C. Fix ation, permeabilization, and staining were performed as described33. The antibodies were used in the following sequence: 1) biotinylated monoclonal mouse anti-tubulin (Invitrogen); 2) streptavidin Alexa Fluor 647 (Invitrogen), Alexa Fluor 488-conjugated mouse anti-perforin clone δG9, and Alexa Fluor 568-conjugated phalloidin (Invitrogen). The slides were mounted with Prolong Gold Antifade reagent (Life Technologies). Cellular conjugates were imaged on a SP8 laser scanning confocal microscope (Leica Microsystems) equipped with 100X 1.4NA objective, a white light laser illumination source and adjustable HyD detectors tuned to the excitation/emission properties of each fluorescent dye. For granule convergence analysis, the lytic granules were detected using the perforin fluorescent immunostaining channel and the MTOC was detected based on the brightest object detected in alpha tubulin immunostaining fluorescent channel. Measurement of the average distance between granules to MTOC as a marker of convergence was performed using Volocity as described previously34, directly on the unprocessed raw data. Measurement of MTOC polarization was performed using Fiji.35 Briefly, the center of the synapse was identified as the centroid of the F-actin positive structure at the interface between the NK cell and the target for each conjugate and the location of the MTOC as the centroid of the brightest dot-shapped element in the alpha-tubulin channel. The Euclidean distance between the two points was measured, plotted and analyzed in Prism version 8.1.1 (GraphPad Software Inc). Measurement of the accumulation of F-actin at the synapse was performed using Fiji on the raw 3D volumes. Identically sized region of interest were then selected: one at the immune synapse encompassing only the NK cell side and the other one on the opposite side of the NK cell, away from any synaptic structure. The ratio was calculated, plotted and analyzed in Prism. Measurement of total perforin content by fluorescence microscopy was also performed using Fiji. An area including all the cytoplasm was drawn and the total fluorescence intensity was measured. The same area was then captured in the target cell to account for background due to the non-specific staining of the perforin antibody. Following background subtraction, data was plotted and analyzed in Prism. Raw images were subjected to signal re-scaling exclusively using linear transformation for display purpose in the figures.
Results
Human STAT5b mutation results in a dramatic reduction of NK cell number in peripheral blood.
In this study, we evaluated two patients with STAT5b deficiency. These patients were originally reported in 2007 by Hwa and co-workers36 (Table I, and see supplementary data and supplementary table E1 for their clinical details and immunological profiles). STAT5 has two isoforms, STAT5b and STAT5a. The STAT5b protein is encoded by the STAT5B gene located approximately 12 kb apart from the STAT5A gene on chromosome 17.37 To asses the effect of the variant in STAT5B (c.1680delG), first, we evaluated the protein expression by Western blot in peripheral blood mononuclear cells (PBMCs) from both patients. This showed a residual expression of STAT5b in primary cells (Fig 1, A) from both STAT5b-deficient patients; we went on to assess the level of STAT5a expression. Interestingly, we observed STAT5a expression in healthy donors and PBMCs from Pt 1 and Pt 2 (Fig 1, A). To determine the effect of c.1680delG variant on STAT5a and STAT5b expression, we generated B lymphocyte cell lines (BLCL) from both patients. STAT5b expression was absent in BLCLs with comparable levels of STAT5a to those found in BLCLs from healthy donors (see Fig E1, A).
Table I: Clinical manifestations of STAT5B-deficient patients.
BAL, Bronchoalveolar lavage; CMV, Cytomegalovirus; EBV, Epstein-Barr virus; UTI; Urinary tract infection.
| Patient 1 | Patient 2 | |
|---|---|---|
| Female 15 years old |
Female 14 years old |
|
| Mutation | c.1680delG | c.1680delG |
| Infections | Rhinovirus, adenovirus and EBV viremia | Rhinovirus, adenovirus, CMV, and EBV viremia UTI by multidrug resistant Escherichia coli BAL sample positive for Aspergillus terreus and Candida albicans |
| Lung | CD4+ lymphocytic infiltration Minimal sub-pleural fibrosis Chronic lymphocytic lung disease |
CD4+ lymphocytic infiltration Minimal sub-pleural fibrosis Pulmonary alveolar proteinosis (PAP) Chronic lymphocytic lung disease |
| Other | Autoimmune hypothyroidism Chronic anemia Type I diabetes mellitus |
Autoimmune hypothyroidism Chronic anemia Juvenile idiopathic arthritis (JIA) |
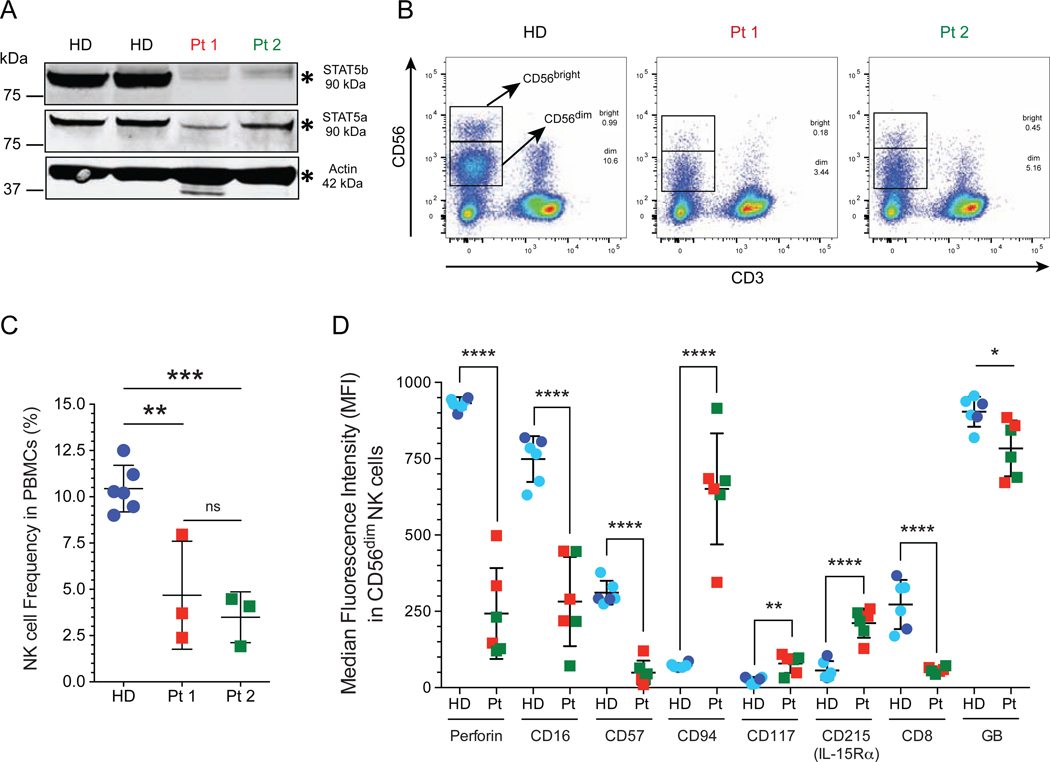
FIG 1: Impaired terminal NK cell maturation in STAT5b-deficient patients.
A, Western blot analysis of STAT5b on total extracts from peripheral blood mononuclear cells (PBMCs) from healthy donors and STAT5b-deficient patients. Polyclonal STAT5a and STAT5b antibodies were used. B, NK cells were identified as CD56brightCD3− and CD56dimCD3− NK cells gated on lymphocytes. C, Frequency of total NK cells in peripheral blood. D, CD56dim NK cells from healthy donors and STAT5b-deficient patients were studied by flow cytometry. The Median fluorescence intensity (MFI) data from age- and sex-matched healthy donor are included (light blue circles)30 in addition to data from unmatched controls acquired at the time of the experiment (dark blue circles). Horizontal bars represent the median, and the vertical bars indicate the standard deviation. Statistical significance was analyzed using the unpaired Student’s t-test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. HD, healthy donors (dark blue circle); previously reported healthy female age and gender matched controls (light blue circle); Pt, STAT5b-deficient patients, Pt 1 (red box); Pt 2 (green box).
Since we observed similar STAT5a protein levels in BLCLs from patients and healthy donors (see Fig E1, A), we evaluated the patient’s B cell lines by imaging flow cytometry using a total phopho-STAT5 antibody to determine the activation and nuclear recruitment of STAT5a dimers. In the absence of stimulation, STAT5a activation was not detected in BLCL from patients and healthy control. Stimulation with IL-21 increased localization of STAT5a in nucleus (see Fig E1, B). This was also observed for healthy donor and patient BLCLs, the colocalization between DAPI and STAT5a as calculated by similarity score of 66.89% for healthy donor, 54.18% for Pt 1, and 62.85% for Pt 2 (median values) (see Fig E1, C).
STAT5b is a principal effector molecule downstream of IL-15, a critical cytokine for NK cell maturation, survival, and proliferation.11, 15 To determine the frequency of NK cells within peripheral blood, we performed flow cytometry analysis of lymphocytes from STAT5b-deficient patients and from six healthy donors (HD) pooled together; two adult HD and 4 matched by age and gender reported previously by our group given MFI and percentage from age/gender matched controls were found to be within the same range for MFI and percentage as same day HD controls.30 NK cells were identified in the lymphocyte gate as CD56+CD3− and further characterized as CD56bright CD3− and CD56dim CD3− NK cell subsets (Fig 1, B, and see Fig E2 for our gating strategy). Gating on CD56+CD3− showed a significant decrease in total NK cell numbers in both siblings (Pt 1, 4.680 ± 1.684%; Pt 2, 3.490 ± 0.7930%; mean ± standard deviation) compared to healthy donors (10.44 ± 0.5138%) (Fig 1, C). Together, these analyses demonstrate that STAT5b expression is absent in STAT5B (c.1680delG) deficient NK cells, and led to reduced NK cell frequency in the peripheral blood. Furthermore, STAT5a expression is retained in both patients and was detectable at similar levels compared to healthy donors, thus showing that STAT5a and STAT5b are not fully redundant.
Human STAT5b mutations result in decreased perforin expression and impaired terminal NK cell maturation.
The CD56dim subset represents the majority of circulating NK cells which have cytotoxic capacity enabled by expression of CD16 (FcγRIII), perforin, and granzyme B.2, 4, 22 To determine if the decreased NK cell frequency seen in peripheral blood from STAT5b-deficient patients is associated with an abnormal NK cell phenotype, we performed extensive flow cytometric analysis. We identified a CD56dim subset with significantly decreased median fluorescence intensity (MFI) for perforin expression in both patients (Fig 1, D, and see Fig E3, A). Interestingly, this difference persists when we analyzed the percentage of CD56dim perforin+ NK cells between healthy donors and the patients (see Fig E3, B). Therefore, human STAT5b mutations affect the frequency of perforin-expressing NK cells in peripheral blood and the median intensity of perforin expression in CD56dim NK cells.
Changes in the expression of cell surface receptors mark the transition from CD56bright to CD56dim NK cells.2 Our analyses identified a seemingly immature CD56dim NK cell subset with low expression of CD8, CD16, CD57, and granzyme B (Fig 1, D). Furthermore, this subset exhibited higher levels of markers classically found on immature CD56bright cells, such as CD94, CD117, and CD215 (IL-15Rα) (Fig 1, D). The same differences were observed between healthy donors and STAT5b-deficient patients when the percentage of positive cells for each receptor was evaluated (Fig E3, B). The impairment in NK cell maturation observed in these patients was accompanied by high expression levels of CD215 (IL-15Rα) (Fig 1, D), which is necessary for human NK cell development.11
Stat5b−/− mice have a block in terminal NK cell maturation.
In murine bone marrow, natural killer precursor cells (NKps, NK1.1− and NKp46−) are characterized as Lin− CD122+ cells.38 The immature NK cells (iNKs) are characterized by acquisition of the NK1.1 receptor, followed by the mature NK cells (mNKs) characterized by an upregulation of NKp46 and CD49b.39 As NK cells mature further, they acquire CD27 expression early and lose CD27 expression as the cells mature.40, 41 Ultimately, cells migrate to the periphery, upregulate CD11b, and acquire their functional capacity. All of these incremental changes represent the critical landmarks of the last stages of mouse NK cell development.39
To correlate the impairment in NK cell maturation observed in humans with the phenotype of Stat5b−/− mice, we analyzed the NK cell development progression from Stat5b−/− mice by flow cytometry. For NK cell development in early stages, we investigated the frequency of precursor and immature NK cells in bone marrow. Interestingly, we observed that Stat5b−/− mice, but not Stat5a−/− mice, had a significantly decreased percentage of NK cell precursors (0.01 ± 0.004%) and immature NK cell (0.02 ± 0.007%) compared to wild-type mice (NKps: 0.03 ± 0.01% and iNKs 0.05 ± 0.01%) (Fig 2, A).
FIG 2: Stat5b−/− mice have impaired terminal NK cell maturation.
A, Bone marrow was stained for lineage (LIN; CD3, CD19, Ly6G, Ter119), CD122, NK1.1, and NKp46. Flow cytometry was used to analyze the percentage of NK precursor cells (NKp), immature NK cells (iNK), and mature NK cells (mNK) gated on lymphocytes. Box plots depict mean ± Min/Max (n≥10 per genotype). One-way ANOVA and Bonferroni’s Multiple Comparison Test were used for statistical analysis of each developing stage. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. B, Splenocytes of mice were stained for CD3, NK1.1, CD49b, and NKp46, and then analyzed by flow cytometry for the percentage of CD3−NK1.1+, CD3−CD49b+ and CD3−NKp46+ NK cells gated on lymphocytes, respectively. Box plots depict mean ± Min/Max (n≥10 per genotype). C, Splenic CD3−NK1.1+ NK cells were analyzed for CD27 and CD11b expression by flow cytometry. Box plots depict mean ± Min/Max (n≥10 per genotype). One-way ANOVA and Bonferroni’s Multiple Comparison Test were applied for statistical analysis of each maturation stage.
In the spleen from Stat5b−/− mice, the percentage of mature NK cells was lower (1.86 ± 0.9371%; mean ± SD) compared to Stat5a−/− mice (3.631 ± 1.15%) and wild-type controls (4.072 ± 0.9889%) (Fig 2, B). Spleens from Stat5b−/− mice, but not Stat5a−/− mice, had a significantly decreased frequency of mature CD27−CD11b+ NK cells (11.85 ± 4.791%) compared with wild-type controls (47.34 ± 13.32%) (Fig 2, C). Moreover, spleens from Stat5b−/− mice had a larger percentage of immature CD27+CD11b− NK cells (27.7 ± 7.789%) compared to wild-type controls (12.81 ± 4.04%) (Fig 2, C). These findings support our results seen in the peripheral blood NK cells of STAT5b-deficient patients with less circulating NK cells compared to healthy donor. These results show that STAT5b is important in early NK cell development. The low number of mature NK cells in spleen is a consequence of the lower numbers of mature NK cells seen in bone marrow (Fig 2, A) and suggests that the paucity of precursor cells in the first stage of NK cell development hinders the transition to mature NK cells.
Cytokine production is largely affected in CD56bright NK cell subset by human STAT5b mutations.
CD56bright NK cells provide early IFN-γ and other cytokines to antigen-presenting cells, thereby promoting control of infections.22, 42 In response to PMA and ionomycin, the CD56bright NK cell subset produces significantly higher levels of cytokines compared with the CD56dim NK cell subset.4, 22, 42 However, in response to these stimulus we observed significantly lower percentages of CD56bright NK cells positive for interferon (IFN)-γ, tumor necrosis factor (TNF)-α and GM-CSF (see Fig E4, A–B), with low median fluorescence intensities (MFI) for each cytokine (Fig 3, A). NK cells are innate lymphocytes with a higher capacity to produce immunoregulatory cytokines, specially IFN-γ in response to IL-12 and IL-18 in the presence of low-dose of IL-15 as a survival factor.43, 44 Further, stimulation with IL-12/15/18 did not show an increase in the percentage of IFN-γ producing CD56bright NK cells in STAT5b-deficient NK cells (see Fig. E4, C). The low levels of IFN-γ produced by this subset may in part explain the persistent viral infections seen in these patients.
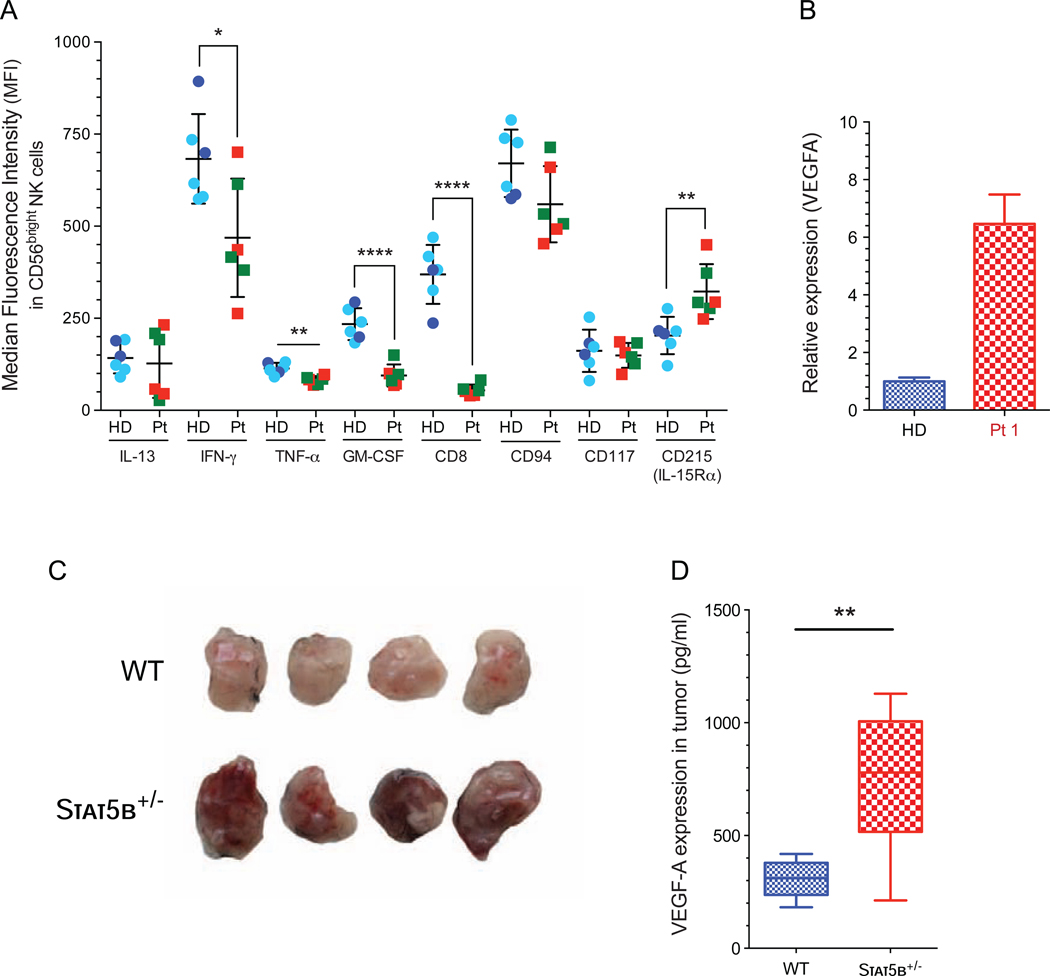
FIG 3: CD56bright NK cell subset with impaired cytokine production and higher VEGF-A expression.
A, Median fluorescence intensity (MFI) for each cytokine and receptor in CD56bright NK cell after stimulation with PMA and ionomycin. Median fluorescence intensity (MFI) data from age- and sex-matched healthy donor are included (light blue circles)30 in addition to data from unmatched controls acquired at the time of the experiment (dark blue circles).Horizontal bars represent the median, and the vertical bars indicate the standard deviation. Statistical significance was analyzed using the unpaired Student’s t-stest. *p<0.05, **p<0.01, ****p<0.0001. HD, healthy donors (dark blue circle); previously reported healthy female age and gender matched controls (light blue circle);30 Pt, STAT5b-deficient patients, Pt 1 (red box); Pt 2 (green box). B, Expression of VEGF-A gene validated by qPCR of total NK cells from patient 1 and a healthy donor. Stat5b+/− and WT mice were challenged with RMA lymphoma cell line subcutaneously for 10 days, **p<0.01. C, Representative pictures of tumors and D, VEGF-A expression in tumor lysates, **p<0.01.
Besides pro-inflammatory cytokines, human CD56+ CD16− NK cells have been shown to produce pro-angiogenic cytokines (e.g. VEGF-A). In murine NK cells, VEGF-A expression is repressed by STAT5b and deletion of total STAT5 changes the NK cell phenotype to pro-tumorigenic.45 We analyzed the expression of VEGFA transcripts in resting NK cells from STAT5b-deficient patient 1 and found a 6 fold-enhanced VEGFA levels (p.<0.006) compared to healthy donor (Fig 3, B). To confirm that STAT5b-regulated VEGF-A expression results in a pro-angiogenic phenotype, we challenged Stat5b+/− mice with the RMA lymphoma cell line. The use of mice heterozygous for Stat5b allowed us to retain sufficient numbers of NK cells (see Fig E4, D), which showed enhanced expression of VEGF-A compared to wild-type NK cells (see Fig E4, E). Indeed tumors, which grew in mice lacking one allele of Stat5b, showed an enhanced angiogenesis (Fig 3, C), as indicated by increased levels of VEGF-A in tumor homogenates (Fig 3, D). In addition, tumors displayed enhanced tumor weight (see Fig E4, F). These data suggest the absence of STAT5b might not only impair the CD56bright NK cell functionality but also render them pro-angiogenic, which may lead to enhanced tumor growth.
The expression of adhesion and co-stimulatory receptors is dramatically affected in both NK cell subsets by STAT5b mutations.
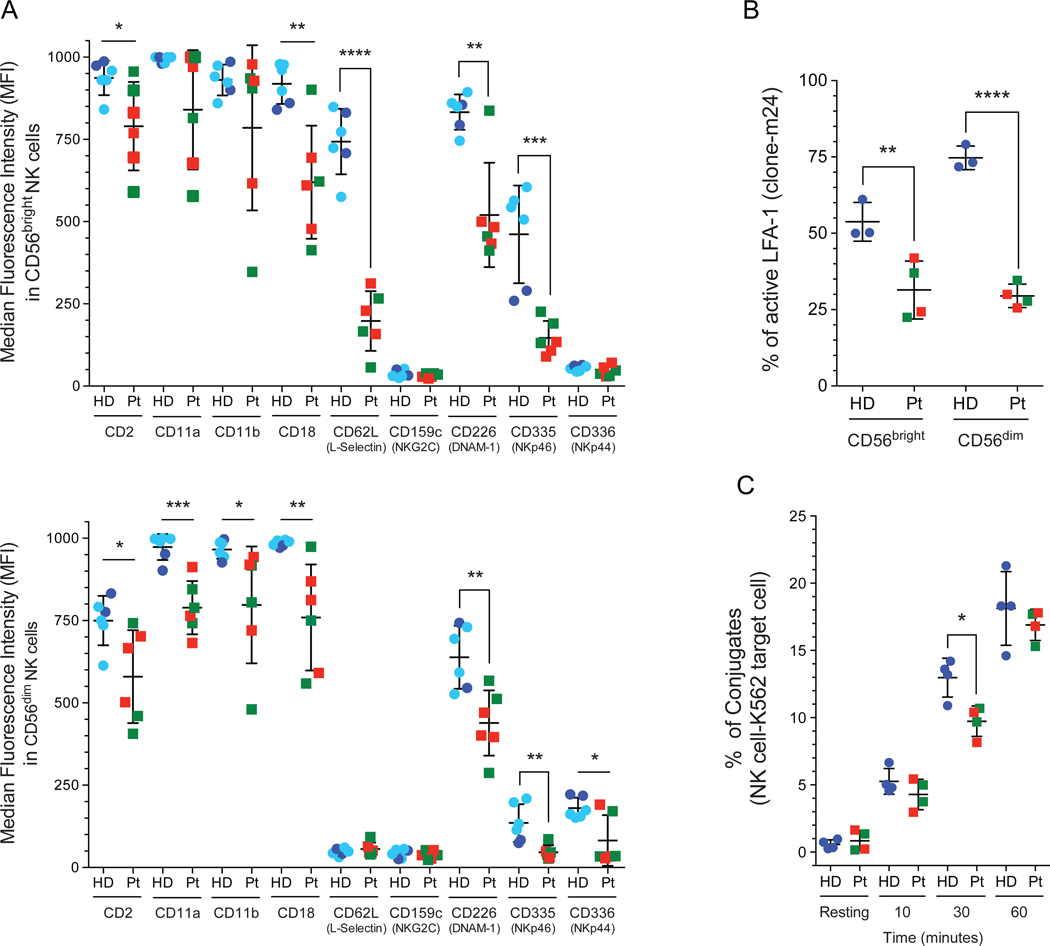
Human NK cell subsets have different expression patterns of adhesion receptors that correlate with their trafficking pattern.4, 22 The interaction between NK cells and their target cells are mediated by a combination of activating receptors, as DNAM-1, NKG2D, and integrin heterodimer such as LFA-1,19–21 resulting in a firm adhesion between NK cells and their target cells.20 Flow cytometric analysis shows that STAT5b-deficient patients had decreased expression of CD62L (L-selectin) in the CD56bright subset (Fig 4, A). This NK cell subset expresses elevated levels of CD62L, which mediates the interaction with the vascular endothelium.46 The down-regulation of CD62L in CD56bright NK cells suggests an abnormal migration through secondary lymphoid organs, which could affect the maturation and transition to the next stage of development. We also observed a significantly decreased expression of CD2, CD11a, CD11b, and CD18 in peripheral blood NK cell subsets (Fig 4, A). Moreover, we observed lower expression of NKp44, NKp46, and DNAM-1 in both NK cell subsets (Fig 4, A) along with significantly low percentage of NK cells positive for DNAM-1, NKp44, and NKp46 compared with healthy donors (see Fig E5).
FIG 4: Decreased level of activated LFA-1 in STAT5b-deficient NK cells.
A, The expression of each activation and adhesion receptors was measured by flow cytometry in CD56bright and CD56dim subsets. Median fluorescence intensity (MFI) data from age- and sex-matched healthy donor are included (light blue circles)30 in addition to data from unmatched controls acquired at the time of the experiment (dark blue circles). HD, healthy donors (dark blue circle); previously reported healthy female age and gender matched controls (light blue circle);30 Pt, STAT5b-deficient patients, Pt 1 (red box); Pt 2 (green box). B, Activated lymphocyte function-associated antigen-1 (LFA-1) levels were evaluated in both NK cell subsets after PMA stimulation. An anti-LFA-1 activation-specific epitope antibody clone m24 was used. C, The conjugation assay was performed with PBMCs and K562 target cells and evaluated by fluorescence-activated cell sorting. Horizontal bars represent the mean, and the vertical bars represent the standard deviation. Statistical significance was analyzed using the unpaired Student’s t-test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. HD, healthy donor; Pt, STAT5b-deficient patients.
Lytic granule convergence to the MTOC is defective in human STAT5b-deficient NK cells.
The integrin LFA-1 (CD11a/CD18) is a key adhesion receptor that participates in the first steps of the formation of the immune synapse in NK cell and is required for optimal cell adhesion and granule convergence.47–50 Using an antibody (clone m24) that binds selectively to the extended conformation of LFA1, we observed significantly decreased levels of activated LFA-1 in both NK cell subsets from STAT5b-deficient patients compared to those from healthy donors after PMA stimulation (Fig 4, B).
Deficiency of the CD18 subunit in leukocyte adhesion disorder type 1 (LAD-1) is characterized by impaired NK cell cytotoxicity with diminished conjugate formation and reduced convergence of lytic granules to the microtubule organizing center (MTOC).47, 51 When measured by flow cytometry, we found that the formation of conjugates by STAT5b-deficient patient cells was significantly delayed at 30 minutes but approached normal levels after 60 minutes (Fig 4, C). Based on this observation, we sought to find out if the crucial features of the immune synapse established by NK cells were also impaired in absence of STAT5b. Formation of the immune synapse is typically sanctioned by 3 majors events easily quantifiable using fluorescence microscopy:52 (1) accumulation of filamentous actin at the interface with the target cell, (2) convergence of the lytic granules around the MTOC and (3), polarization of the granule-loaded MTOC towards the immune synapse.
To assess whether decreased activation of LFA-1 leads to impaired granule convergence in NK cells from patients with STAT5b deficiency, we evaluated the convergence of lytic granules using confocal microscopy images of NK cells conjugated to susceptible target cells. STAT5b-deficient NK cells were incubated with K562 target cells, and then the conjugates were fixed and stained for perforin to highlight lytic granules, and α-tubulin to localize the MTOC.
Filamentous actin was revealed using fluorescently conjugated phalloidin. Measurement of the positioning of lytic granules in STAT5b-deficient NK cells demonstrated that they failed to converge to the MTOC after conjugation with target cells (Fig 5, A and C). However, we observed normal F-actin accumulation and MTOC polarization at the immunological synapse (Fig 5, D). Additionally, normal levels of CD107a in NK cells from both patients indicates normal NK cell degranulation (Fig 5, E). Therefore, low levels of activated LFA-1 observed in STAT5b-deficient NK cells lead to decreased lytic granule convergence to the MTOC but do not affect polarisation of the MTOC to the actin enriched immunological synapse or the capacity for the NK cell to degranulate.
FIG 5: Reduced lytic granule convergence in STAT5b-deficient NK cells.
Images of fixed cells showing representative NK cells from healthy donor and STAT5b-deficient patients conjugated to susceptible K562 target cells (10:1 ratio) in absence A, or presence B, of IL-2 (1000 UI/ml) stimulation. The conjugates were fixed after 30 minutes of incubation and stained with biotinylated monoclonal mouse anti-α-tubulin/Streptavidin Alexa Fluor 647 (green), Alexa Fluor 488-conjugated mouse anti-perforin clone δG9 (red), and Alexa Fluor 568-conjugated Phalloidin (blue). The yellow arrowheads indicate the Microtubule-organizing center (MTOC); TL, transmitted light. C, Lytic granules convergence; D, F-actin accumulation and MTOC polarization in NK cells from healthy donor and each patient. E, CD107a expression in STAT5b-deficient patients. Horizontal bars represent the mean, and the vertical bars indicate the standard deviation. HD, healthy donor; Pt, STAT5b-deficient patients. The statistical differences between healthy donors and patients was determined by Ordinary one-way ANOVA test. *p<0.05. The specific cytotoxicity assay was performed using the Cr51 release protocol. F, PBMCs from helathy donor or STAT5b-deficient patients were incubated with K562 target cells in the absence or presence of 1000 U/mL rhIL-2. Results from Fig 1, A, are shown as lytic units (LU10) require to lyse 10% of target cells. Patients LU10 was normalized to the healthy donor control for each assay. G, Antibody-dependent cell-mediated cytotoxicity (ADCC) was performed against Raji target cells in the presence or absence of 20 μg/ml Rituximab after 4 hours of incubation.
Lytic granule convergence is restored and NK cell cytotoxicity is partially restored after IL-2 stimulation
The canonical IL-2 signaling via STAT5b as the principal downstream effector is a potent NK cell activator and initiator of granule convergence in the first stages of target cell engagement.15, 16 STAT5b-deficient NK cells formed conjugates with K562 target cells after 30 minutes of stimulation with IL-2 (1000 U/mL) and we observed restored lytic granule convergence in response to IL-2 stimulation (Fig 5, B and C). These results confirm previous reports that canonical IL-2 signaling is not necessary for lytic granule convergence,47 and that the non-canonical IL-2 signaling which promotes the activation of MAPK and PI3K pathways to enhance NK cell activation,53, 54 could drive increased killing capacity observed after IL-2 stimulation.
We looked to see if another critical STAT5b dependent cytokine, IL-15, could also restore granule convergence. In support of the hypothesis that IL-2 signaled through an alternative pathway other than STAT5b to restore granule convergence, we show that lytic granule convergence was absent after IL-15 stimulation in NK cells from patient 1 because IL-15 signaling is most dependent on STAT5b (see Fig E6, A and B).
We tested the cytotoxic activity of NK cells in isolated PBMCs. In resting cells, the capacity of NK cells to kill target cells was reduced in both patients compared with a pool of three healthy donors (Fig 5, F). Interestingly, we observed partially restored cytotoxic capacity in presence of IL-2 in both patients (9- and 8-fold, respectively) (Fig 5, F). The cytolytic capacity of STAT5b-deficient patients was evaluated by calculating lytic units required to lyse 10% of K562 target cells. We observed that NK cells from STAT5b-deficient patients had reduced lytic capacity compared with those of the healthy donor after adjustment for the frequency of NK cells within the PBMCs population (Fig 5, F). In addition, we observed partially restored cytotoxic capacity with IL-15 (see Fig E6, C). The lack of lytic granule convergence suggests this cytotoxicity is a result of non-directed degranulation affecting bystander cells34 as the NK cells do have the ability to degranulate with activation as evidenced by normal CD107a expression (Fig 5, E). We additionally measured ADCC using rituximab opsonized Raji target cells for recognition by CD16, and found that ADCC was impaired in both patients (Fig 5, G). The decreased ADCC NK cell cytotoxicity observed further supports a profound functional NK cell defect in immature NK cells which do not express terminal maturation markers like CD16.
Discussion
Autosomal recessive STAT5b deficiency is characterized by severe growth failure, recurrent infections, accompanied by significantly decreased levels of circulating regulatory T cells and NK cells.17, 23 It is important to distinguish from the somatic mutations in STAT5B associated with lymphocytic leukemia55 as well as the recently characterized novel variant N642H gain-of-function mutation shown to be associated with eosinophilia, urticaria, dermatitis, and diarrhea.55, 56 The homozygous mutation identified in our patients was a deletion of a single G at the junction of the exon13–intron1336 which affects the expression of STAT5b in PBMCs and BLCL but not STAT5a. The STAT5b-deficient siblings included in this study had significantly reduced NK cells in the periphery. This likely occurs since STAT5 is the principal downstream effector for the IL-15 signaling pathway that induces development, activation, and proliferation of NK cells.11, 15 The low numbers of NK cells leave these patients susceptible to recurrent viral infections, including rhinovirus, adenovirus, and EBV viremia. The absence of STAT5b also alters the expression of FOXP3 and CD2517 accounting for the low circulating population of regulatory T cells. The impaired homeostasis of Tregs results in autoimmunity and inflammatory disease observed in these patients including chronic lymphocytic lung disease, autoimmune hypothyroidism, juvenile idiopathic arthritis (JIA), and chronic anemia. In addition, the interaction between GH circulating with their receptor leads to STAT5b activation. The absence of STAT5b also explains the severe growth failure observed in these patients.15, 23
During our study we analyzed NK cell numbers in peripheral blood and by using comprehensive flow cytometric analysis, we evaluated the effects of STAT5b deficiency on NK cell development and phenotype. We characterized a CD56dim NK cell subset with decreased levels of perforin which correlates with results from Stat5b−/− mice.18 The expression of CD16 in the CD56dim subset was also significantly decreased, thereby leading to impaired ADCC. In addition, this NK cell subset had high levels of markers associated with immature NK cells, such as CD94, CD117, and low expression of CD57 and CD8 receptors normally found on mature cytotoxic cells. These results suggest an abnormal developmental transition between CD56bright to CD56dim leading to the low cytotoxicity capacity of these cells.
Mice with conditional ablation of total STAT5 in NK cells display many similar characteristics seen in the described human patients. The Stat5fl/flNcr1iCre mice exhibit impaired NK cell development in bone marrow leading to diminished numbers of NK cells in periphery.57 NK cells from Stat5b−/− mice have a defect in their ability to respond to IL-2 and IL-15; consistent with low viability, low proliferative capacity, decreased perforin expression.18 Using this Stat5b−/− mouse model, we observed an impaired terminal maturation of NK cells, which was characterized by an accumulation of immature NK cells with low numbers of mature NK cells in the spleen. Importantly, NK cell development was normal in Stat5a−/− mice, thus confirming that STAT5b has a vital role in NK cell generation. Recent studies from Villarino and colleagues support our findings; using a mouse model where STAT5 is reduced but not ablated, they demonstrated that STAT5b is critical for NK cell maturation and function58. Furthermore, the absence of STAT5 downregulates the expression of multiple lineage-defining transcription factors, such as Tbet and Gata-3 which is associated with impaired development, homeostasis, and function of innate lymphoid cells (ILCs).58 Interestingly, the impact was higher with deletion of Stat5b.58 They also describe depressed cytokine production similar to our results in the human CD56bright cells. CD56bright cells provide cytokines promoting control of viral infections.22, 42, 46 Our patient’s CD56bright NK cell subset was characterized by significantly reduced production of cytokines (IFN-γ, TNF-α, IL-13, and GM-CSF) and an abnormal expression of CD62L (L-selectin). CD62L is a part of the NK cell homing mechanism. It has been shown to play a role in cell migration as well.46 We observe a down-regulation of CD62L which suggests there may be a possible effect on NK cell migration. Along with the defective cytokine production including low levels of IFN-γ, these immature poorly differentiated NK cells heightened the susceptibility to severe viral infections observed in these patients. Furthermore, the patient’s NK cells were characterized by enhanced expression of the pro-angiogenic factor VEGF-A, which may indicate that these poorly cytotoxic NK cells display a pro-tumorigenic phenotype.45 Therefore, tumor surveillance by NK cells might be impaired in STAT5b-deficient patients by more than one mechanism.
The first interaction between NK cells and their target cells is mediated by activating and adhesion receptors that induce commitment to cytotoxicity.19, 59 Our findings show that NK cells from STAT5b-deficient patients had significantly decreased expression of these important receptors: CD2, DNAM-1, NKG2D, NKp46, and CD16. Synergy between NKp46 with DNAM-1, NKG2D or CD2 induces degranulation.60 In NK cells, NKG2D signaling contributes to adhesion, granule convergence, and degranulation through PI3K and CrkL activation.61 Our results suggest a defect in the first steps of NK cell activation leads to impaired cytotoxicity in STAT5b-deficient patients.
The engagement of LFA-1 stimulates convergence of lytic granules to the MTOC47 and MTOC polarization to the immune synapse.62 IL-2 also induces lytic granule convergence independently of JAK3 activation through Src-dependent protein signaling47, suggesting that the canonical IL-2 pathway is not necessary for lytic granule convergence.47 Low levels of activated LFA-1 and decreased lytic granule convergence to the MTOC observed in NK cells from STAT5b-deficient patients, along with low surface expression of CD18 and CD11a, suggests that the absence of STAT5b impairs the early steps of NK cell activation and cytotoxicity. Interestingly, we observed a partial rescue of lytic capacity after IL-2 stimulation. This, combined with our data and others showing that non-canonical IL-2 signaling pathway promotes lytic granule convergence, independently of STAT5b, suggests that restoration of granule convergence by IL-2 is responsible for the improved lytic function in STAT5b-deficient NK cells. Alternatively, STAT5a, which is not affected in our patients, may overcome the STAT5b deficiency under high concentration of IL-2.
In addition, this is supported by our results using IL-15 stimulation. We showed partial restoration of cytolytic capacity but not lytic granule convergence with IL-15 stimulation. Hsu et al. showed NK cells that converge their lytic granules prior to degranulation have more precise and directed killing with less “collateral damage”.34 NK cell cytotoxicity in the absence of granule convergence occurs through degranulation in a non-directed manner affecting bystander cells34. Our data demonstrated that IL-2 induces convergence of the lytic granules around the MTOC and polarization of the granule-loaded MTOC towards the immune synapse in our patients’ NK cells. This is important for directed killing and partial restoration of function seen in our patients. Further, it suggests there is a non-canonical IL-2 signaling pathway in play to restore appropriate convergence of lytic granules for directed killing. However, our data also showed that IL-15 does not induce lytic granule convergence in absence of STAT5b. As disussed, degranulation in a non-directed manner without proper convergence of lytic granules to the MTOC can occur resulting in bystander killing. The concern for bystander killing is morbidity and destruction of surrounding tissues.
Additional experiments were performed to create a model human NK cell lines depleted of STAT5b. However, any efficient reduction of endogenous STAT5b expression led to premature cell death (data not shown), emphasizing the critical role for this regulator of NK cell growth and maturation. In summary, we have defined the NK cell deficiency that occurs as a result of a deleterious STAT5b mutation. This alteration significantly impairs NK cell function and decreases the number of NK cells circulating in peripheral blood, therefore mimicking the abrogated NK cell terminal maturation observed in Stat5b−/− mice. This work demonstrates that STAT5b plays a critical role in NK cell development, maturation, and activation.
Supplementary Material
Key message:
STAT5b is important for natural killer (NK) cell development. NK cell subsets in patients with STAT5b deficiency are immature and display decreased cytolytic function.
Acknowledgment:
The authors would like to thank Sandra A. Salinas for her imaging flow cytometry and IDEAS software analysis.
Funding sources: Chao Physician Scientist Junior Faculty Award (LRF), NIAID R01 AI120989 (JSO), and Jeffrey Modell Foundation Diagnostic and Research Center for Primary Immunodeficiencies (JSO/LRF), VS is supported by the Austrian Science Foundation grants FWF SFB-61-07 and FWF P28571.
Abbreviations:
- STAT5b
Signal Transducer and Activator of Transcription 5b
- MTOC
Microtubule-organizing center
- LFA-1
Lymphocyte function-associated antigen 1
Footnotes
Disclosure of conflicts of interest:
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angelo LS, Banerjee PP, Monaco-Shawver L, Rosen JB, Makedonas G, Forbes LR, et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res 2015; 62:341–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev 2006; 214:56–72. [DOI] [PubMed] [Google Scholar]
- 3.Mace EM, Orange JS. Genetic Causes of Human NK Cell Deficiency and Their Effect on NK Cell Subsets. Front Immunol 2016; 7:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilk E, Kalippke K, Buyny S, Schmidt RE, Jacobs R. New aspects of NK cell subset identification and inference of NK cells’ regulatory capacity by assessing functional and genomic profiles. Immunobiology 2008; 213:271–s83. [DOI] [PubMed] [Google Scholar]
- 5.Scoville SD, Freud AG, Caligiuri MA. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front Immunol 2017; 8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010; 116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas-Hernandez A, Forbes LR. JAK/STAT proteins and their biological impact on NK cell development and function. Mol Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 8.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med 2006; 203:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freud AG, Keller KA, Scoville SD, Mundy-Bosse BL, Cheng S, Youssef Y, et al. NKp80 Defines a Critical Step during Human Natural Killer Cell Development. Cell Rep 2016; 16:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 2009; 206:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood 1996; 87:2632–40. [PubMed] [Google Scholar]
- 13.Boos MD, Ramirez K, Kee BL. Extrinsic and intrinsic regulation of early natural killer cell development. Immunol Res 2008; 40:193–207. [DOI] [PubMed] [Google Scholar]
- 14.Tamzalit F, Barbieux I, Plet A, Heim J, Nedellec S, Morisseau S, et al. IL-15.IL-15Ralpha complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci U S A 2014; 111:8565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai T, Jenks J, Nadeau KC. The STAT5b Pathway Defect and Autoimmunity. Front Immunol 2012; 3:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 2000; 19:2566–76. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol 2006; 177:2770–4. [DOI] [PubMed] [Google Scholar]
- 18.Imada K, Bloom ET, Nakajima H, Horvath-Arcidiacono JA, Udy GB, Davey HW, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med 1998; 188:2067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, et al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol 2014; 92:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009; 114:2657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol 2009; 7:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killercell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 23.Nadeau K, Hwa V, Rosenfeld RG. STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatr 2011; 158:701–8. [DOI] [PubMed] [Google Scholar]
- 24.Bezrodnik L, Di Giovanni D, Caldirola MS, Azcoiti ME, Torgerson T, Gaillard MI. Long-term follow-up of STAT5B deficiency in three argentinian patients: clinical and immunological features. J Clin Immunol 2015; 35:264–72. [DOI] [PubMed] [Google Scholar]
- 25.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, et al. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 2003; 349:1139–47. [DOI] [PubMed] [Google Scholar]
- 26.Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics 2006; 118:e1584–92. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 1997; 11:179–86. [DOI] [PubMed] [Google Scholar]
- 28.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A 1997; 94:7239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Garcia R, Vargas-Hernandez A, Chinn IK, Angelo LS, Cao TN, Coban-Akdemir Z, et al. Mutations in PI3K110delta cause impaired natural killer cell function partially rescued by rapamycin treatment. J Allergy Clin Immunol 2018; 142:605–17 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahapatra S, Mace EM, Minard CG, Forbes LR, Vargas-Hernandez A, Duryea TK, et al. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PLoS One 2017; 12:e0181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas-Hernandez A, Mace EM, Zimmerman O, Zerbe CS, Freeman AF, Rosenzweig S, et al. Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clin Lab Med 2007; 27:469–85, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, Orange JS. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med 2007; 204:2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu HT, Mace EM, Carisey AF, Viswanath DI, Christakou AE, Wiklund M, et al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol 2016; 215:875–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwa V, Camacho-Hubner C, Little BM, David A, Metherell LA, El-Khatib N, et al. Growth hormone insensitivity and severe short stature in siblings: a novel mutation at the exon 13-intron 13 junction of the STAT5b gene. Horm Res 2007; 68:218–24. [DOI] [PubMed] [Google Scholar]
- 37.Crispi S, Sanzari E, Monfregola J, De Felice N, Fimiani G, Ambrosio R, et al. Characterization of the human STAT5A and STAT5B promoters: evidence of a positive and negative mechanism of transcriptional regulation. FEBS Lett 2004; 562:27–34. [DOI] [PubMed] [Google Scholar]
- 38.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol 2001; 31:1900–9. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 2002; 3:523–8. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176:1517–24. [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev 2006; 214:47–55. [DOI] [PubMed] [Google Scholar]
- 42.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001; 97:3146–51. [DOI] [PubMed] [Google Scholar]
- 43.Domaica CI, Fuertes MB, Uriarte I, Girart MV, Sardanons J, Comas DI, et al. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PLoS One 2012; 7:e51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol 2008; 181:1627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotthardt D, Putz EM, Grundschober E, Prchal-Murphy M, Straka E, Kudweis P, et al. STAT5 Is a Key Regulator in NK Cells and Acts as a Molecular Switch from Tumor Surveillance to Tumor Promotion. Cancer Discov 2016; 6:414–29. [DOI] [PubMed] [Google Scholar]
- 46.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol 1998; 161:400–8. [PubMed] [Google Scholar]
- 47.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, et al. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood 2013; 121:2627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood 2010; 116:1272–9. [DOI] [PubMed] [Google Scholar]
- 49.Kohl S, Springer TA, Schmalstieg FC, Loo LS, Anderson DC. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol 1984; 133:2972–8. [PubMed] [Google Scholar]
- 50.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell 2010; 21:2241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med 1987; 38:175–94. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee M, Mace EM, Carisey AF, Ahmed N, Orange JS. Quantitative Imaging Approaches to Study the CAR Immunological Synapse. Mol Ther 2017; 25:1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merida I, Diez E, Gaulton GN. IL-2 binding activates a tyrosine-phosphorylated phosphatidylinositol-3-kinase. J Immunol 1991; 147:2202–7. [PubMed] [Google Scholar]
- 54.Ravichandran KS, Burakoff SJ. The adapter protein Shc interacts with the interleukin-2 (IL-2) receptor upon IL-2 stimulation. J Biol Chem 1994; 269:1599–602. [PubMed] [Google Scholar]
- 55.Rajala HL, Eldfors S, Kuusanmaki H, van Adrichem AJ, Olson T, Lagstrom S, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood 2013; 121:4541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma CA, Xi L, Cauff B, DeZure A, Freeman AF, Hambleton S, et al. Somatic STAT5b gain-of-function mutations in early onset nonclonal eosinophilia, urticaria, dermatitis, and diarrhea. Blood 2017; 129:650–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckelhart E, Warsch W, Zebedin E, Simma O, Stoiber D, Kolbe T, et al. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood 2011; 117:1565–73. [DOI] [PubMed] [Google Scholar]
- 58.Villarino AV, Sciume G, Davis FP, Iwata S, Zitti B, Robinson GW, et al. Subset- and tissue-defined STAT5 thresholds control homeostasis and function of innate lymphoid cells. J Exp Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol 2010; Chapter 11:Unit 11 9B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31:227–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Segovis CM, Schoon RA, Dick CJ, Nacusi LP, Leibson PJ, Billadeau DD. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol 2009; 182:6933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu HT, Orange JS. Distinct integrin-dependent signals define requirements for lytic granule convergence and polarization in natural killer cells. Sci Signal 2014; 7:pe24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.