Abstract
Background:
There is ongoing debate about the best strategy to treat patients with tetralogy of Fallot (TOF) that are symptomatic in the neonatal period.
Objective:
We compared the outcomes of complete vs. staged surgery (i.e., initial palliative procedure for possible later complete repair).
Methods:
We performed a retrospective cohort study using the Pediatric Health Information System (PHIS) database and included patients that underwent complete or staged TOF repair prior to 30 days of age. The primary outcome was death during two-year follow-up after the initial procedure. Inverse probability weighted Cox and logistic regression models were used to examine the association between surgical approach group and mortality, while accounting for patient and hospital level factors. Causal mediation analyses examined the role of intermediate variables.
Results:
A total of 2363 patients were included (1032 complete and 1331 staged group). There were 239 deaths. Complete neonatal repair was associated with a significantly higher risk of mortality during the two-year follow up [hazard ratio (HR) =1.51, 95% confidence interval (CI) 1.05, 2.06], between 7–30 days after initial procedure (HR= 2.29, 95% CI 1.18, 4.41), and during the initial hospital admission (odds ratio= 1.72, 95% CI 1.15, 2.62). Post-operative cardiac complications were more common in the complete repair group and mediated the differences in 30-day and two-year mortality.
Conclusions:
Complete surgical repair for neonates with TOF is associated with a significantly higher risk of early and two-year mortality than the staged approach, after accounting for patient and hospital characteristics. Post-operative cardiac complications mediated these findings.
Keywords: Congenital Heart Disease, Cardiac Surgery, Comparative Effectiveness, Pediatric Health Information System, Inverse Probability Weighting, Causal Mediation Analysis
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease, affecting approximately 1650 children in the United States every year (1,2). This disease is characterized by varying degrees of obstruction to pulmonary blood flow (from pulmonary valve stenosis to atresia). The ventricular septal defect (VSD) allows for shunting of blood between the right and left ventricles, often resulting in cyanosis. Surgical repair for asymptomatic patients with no or acceptable levels of cyanosis occurs electively in the first year of life.
There is a subset of TOF patients that are symptomatic in the neonatal period and require immediate intervention. Symptoms include unacceptable levels of cyanosis or dependency on the ductus arteriosus to maintain pulmonary blood flow. Less commonly, neonates present with “tetralogy” spells, or life-threatening episodes of increased cyanosis, hyperpnea and agitation (3). Symptomatic patients require a surgical or catheter-based intervention in the neonatal period to augment pulmonary blood flow and improve oxygenation. There is ongoing debate about the best treatment strategy for symptomatic neonates with TOF (4–7). Theoretical advantages of palliation followed by later repair (i.e., the staged approach) include a less complex initial procedure with shorter neonatal hospital stay, potential reduction in the need for transannular patch upon future complete repair, and promotion of pulmonary artery growth. Potential advantages of early complete repair include earlier resolution of cyanosis, avoidance of a second hospital admission for reoperation, and potentially less distortion of the pulmonary arteries. The currently reported risk of death after either approach is approximately 6% (4, 8–10).
To date, most studies comparing neonatal interventions in TOF have been single-center studies or multi-center studies that include other diseases or outcomes (5,6,8,11–14). While these studies have elucidated some of the risks and benefits of each option, they have not determined which therapeutic approach yields the best outcomes for symptomatic neonates with TOF. Often, the decision is made by surgeon or center preference (9). We studied the comparative effectiveness of surgical approach in a multi-center cohort study using administrative data to investigate differences in mortality after neonatal TOF intervention.
METHODS
Source Population and Study Sample
The Pediatric Health Information System (PHIS) is an administrative database that contains discharge data from inpatient, emergency department, ambulatory surgery, and observation encounters from over 50 non-profit, tertiary care pediatric children’s hospitals for major metropolitan areas across the United States, maintained by The Children’s Hospital Association (Lenexa, Kansas). Data are de-identified at the time of data submission by participating hospitals and undergo a number of reliability and quality checks before being included in the database. The PHIS contains daily billing data on clinical services, pharmacy, supplies, imaging, laboratory tests and room-and-board charges, as well as patient demographics, discharge disposition, and up to 42 procedures and discharge diagnoses reported in International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (15–17).
The study population consisted of PHIS patients with TOF that underwent an initial procedure (i.e., complete surgical repair or staged approach) in the neonatal period (first 30 days of life) between 01.01.2004 and 03.31.2015. We used an identification and validation algorithm to identify neonates with TOF in the PHIS, which was previously published (18).
This study used de-identified administrative data and was determined to be exempt from Human Subject Protection by our Institutional Review Board. The prior cohort validation study was approved by our Institutional Review Board (18).
Exposures, Covariates and Outcomes
The exposure was the initial surgical treatment: complete or staged. The primary outcome was time from initial procedure to death within a two-year follow up period. All deaths were included: after neonatal procedure (in-hospital and after discharge) for both groups, between initial neonatal procedure and completion of TOF repair in the staged group (interstage death), and deaths that occurred subsequent to completion of TOF repair in the staged group.
Patient-level covariates included sex, age, race, insurance type, prematurity, birth weight, genetic syndromes, and extra-cardiac. Pre-treatment acuity was defined by the use of mechanical ventilation and/or prostaglandin infusion to maintain patency of the ductus arteriosus. Hospital-level covariates included number of hospital beds, number of cardiac surgery cases per year, hospital preference for type of surgery, and length of hospital stay for a commonly performed cardiac surgery (i.e., closure of ventricular septal defect), detailed below. Post-operative complications included need for post-operative circulatory support (ECMO), delayed sternal closure. Pericardial effusion requiring pericardiocentesis, pleural effusion requiring chest tube, need for cardiopulmonary resuscitation (CPR), any shock of the heart, pacemaker insertion, pacing of the heart, and electrical cardioversion.
Statistical Analysis
The goal of the study was to determine whether the surgical approach (complete or staged) in the neonatal period was related to overall survival. To control for differences in patients and hospital level characteristics between the two groups, we used a propensity score model to estimate the probability of undergoing complete or staged repair based on pre-operative characteristics. Since patients were nested within treatment centers, we used a multi-level propensity score model to account for the inter-relatedness of patients at each center.19 We used a mixed-effects logistic regression model with a random intercept for hospital and included patient and hospital-level covariates as fixed effects (19).
Patient-level and hospital-level characteristics were used as predictors of assignment to complete surgery, since both types of characteristics influence the choice of surgical approach received (Online Appendix). Length of hospital stay after a commonly performed cardiothoracic procedure was used as a proxy for hospital quality (20). Since hospital preference could impact the individual likelihood of receiving one treatment over the other, we calculated the percentage of cases that received a complete surgery and assigned each hospital to a quintile (included in the model) associated with that percentage. Hospital-level quintiles for number of beds and number of cardiac patients were included as predictors.
The analysis of surgical approach and mortality was weighted using inverse probability weighting, creating a pseudo-population in which the measured covariates are not confounded with initial procedure type (21–24). We used stabilized weights, taking individual weights which were the inverse probability of receipt of the complete surgery and multiplying them by the marginal probability of surgical approach received in the sample (25). To verify that the distribution of each baseline covariate was balanced across the complete and staged groups, we computed standardized differences for continuous and dichotomous variables weighted by the inverse probability of treatment (25–27). We considered a standard difference of less than 10% to be a negligible imbalance (21).
To estimate the effect of the initial procedure on the hazard of mortality, we used an inverse probability weighted Cox model with the observation period from the date of initial procedure to death within a two-year follow-up period (late mortality). Secondary analyses examined the time until death within 30 days (early mortality). Patients who did not die by the end of follow-up were censored at the last date observed in PHIS within the two-year follow-up time or at the end of the study period (3.31.2015).
If the effect of surgery violated the proportional hazards assumption, we included surgical treatment type by time interaction terms in the Cox regression models, providing hazard ratios for surgery during the time intervals in which the proportional hazards assumption was satisfied.
We used robust standard errors to account for within-hospital correlation in outcome and used a bootstrapped-based method performed within each hospital to account for additional correlation introduced by the use of inverse probability weights (28).
For the analysis of mortality within the first hospitalization, we used logistic regression instead of a survival model since the length of the first hospitalization varied considerably among patients. To odds of mortality within the first hospitalization, we used inverse probability weighted logistic regression with robust standard errors to account for the within-hospital correlation in outcomes.
To address unmeasured confounding and quantify how strong unmeasured confounding would have to be to explain away the associations observed, we calculated E-values (with 95% confidence intervals) for the estimated hazard ratios and odds ratios obtained from the weighted Cox models and weighted generalized estimating equation model (29).
Causal Mediation Analysis
We used causal mediation methods to understand if the effect of surgical approach on mortality was direct (i.e., independent of) or mediated through a post-operative cardiac complication(s) (30). Potential mediators were in-hospital cardiac complications within the first 30 days after the initial procedure (and prior to hospital discharge) (listed above). We used the mediation approach based on counterfactuals in which two sets of weights were created (one for the exposure and one for the mediator) and then used these to generate an overall weight which was applied to the Cox proportional hazards model (or logistic regression model for the initial hospitalization) (30–31). The same set of patient and hospital-level covariates used in the propensity score model was used to create the two sets of weights. We assumed that this set of covariates accounted for the exposure-outcome, mediator-outcome, and exposure-mediator confounding.
Sensitivity Analysis
We obtained data beyond the end of the study period to determine if additional follow-up time for the patients that were censored with less than two years of follow-up time would substantially change the conclusions. We reviewed the data for any activity within PHIS hospitals from 3.31.2015 to 12.31.2017. Those patients with additional PHIS activity after 3.31.2015 were presumed to be alive at two-year follow-up. We ran the primary analysis after imputing survival time to two years with censoring for patients with an unknown status at two years.
Subgroup analyses
We conducted two additional analyses to examine how the effects of BT shunt in the staged group and RV-PA conduit in the complete group might impact results. First, we examined the effect of the initial procedure on the hazard of 2-year mortality by comparing the complete group to only the staged group that received a BT shunt using an inverse probability weighted Cox PH model. Second, we estimated the unadjusted odds of 2-year mortality within the complete group comparing those that received a RV-PA conduit to those that did not. We did not conduct a weighted analysis splitting groups by type of treatment within groups (for example, BT shunt, no BT shunt, RV-PA conduit, no RV-PA conduit) due to difficulty obtaining weighted analyses in four groups.
All descriptive statistics (means, percentages) as well as modeling results for the combined sample of complete and staged patients were weighted. All analyses were conducted using SAS Software version 9.4 (SAS Institute Inc., Cary, North Carolina). All statistical tests were two-sided and alpha was 0.05.
Results
A total of 2363 patients were included in the study sample. Overall there were 1318 (55.8%) males and 1518 (64.2%) whites, 308 (13.0%) African-Americans, and 485 (20.5%) Hispanics. Genetic syndromes were present in 746 (31.6%) patients and 349 (14.8%) were born prematurely (less than 37 weeks gestation). There were 1032 patients (43.7%) in the complete group and 1331 (56.3%) patients in the staged group. Prior to weighting, 892 patients (86.4%) in the complete group and in 1206 patients (90.6%) in the staged group had increased pre-operative acuity (Online Table 1). The unweighted mean ± standard deviation age at first procedure was 11.3 ± 7.8 days (complete) and 9.9 ± 7.4 days (staged). In the weighted sample, the complete and staged groups were well balanced in terms of demographics and clinical patient characteristics, with non-significant standard differences (less than 10%) for all patient characteristics (Table 1).
Table 1:
Weighted Patient Characteristics by Surgical Treatment Group
| Complete Neonatal Repair (N=1032) | Staged Repair (N=1331) | Standard Difference | |
|---|---|---|---|
| Male | 590 (56.6%) | 726 (55.0%) | 3.16% |
| Race | |||
| White or Caucasian | 668 (64.0%) | 854 (64.8%) | −1.44% |
| Black or African American | 147 (14.1%) | 174 (13.2%) | 2.68% |
| Other | 227 (21.8%) | 291 (22.0%) | 0.56% |
| Insurance Payer | |||
| Private | 396 (38.0%) | 527 (39.9%) | −4.04% |
| Public | 547 (52.5%) | 656 (49.7%) | 5.56% |
| Other | 99 (9.5%) | 136 (10.3%) | −2.71% |
| Genetic Syndrome | 340 (32.6%) | 424 (32.1%) | 1.11% |
| Extra-cardiac Anomalies | 155 (14.9%) | 190 (14.4%) | 1.44% |
| Prematurity (< 37 weeks) | 162 (15.6%) | 194 (14.7%) | 2.48% |
| Mean Birth Weight (kg)† | 2.71 ± 0.92 | 2.73 ± 0.76 | −2.37% |
| High Preoperative Acuity | 915 (87.8%) | 1158 (87.8%) | −0.092% |
| Age at First Procedure | |||
| 0–5 days | 343 (32.9%) | 419 (31.7%) | 2.54% |
| 6–8 days | 201 (19.3%) | 270 (20.5%) | −2.88% |
| 9–15 days | 230 (22.1%) | 301 (22.8%) | −1.87% |
| 16–30 days | 268 (25.7%) | 329 (25.0%) | 1.72% |
Values represented as count (percentage) or
mean ± standard deviation.
Patients were treated at 45 different hospitals. Eleven hospitals (24.4%) performed > 65% complete repairs, 15 hospitals (33.3%) performed > 65% staged repairs, and 19 hospitals (42.2%) had a relatively equal proportion of treatment groups. Length of stay for the initial hospitalization was comparable between the two groups with complete surgery patients having a median length of stay of 16 days (interquartile range 10, 31), and staged patients having a median length of stay of 15 days (interquartile range 8, 29). In the weighted sample, the complete and staged groups had balanced distributions of hospital-level characteristics except for a slight imbalance in the percentage of patients in each group in the lowest quartile of hospital preference for complete repair (standard difference 10.6%) (Online Table 2).
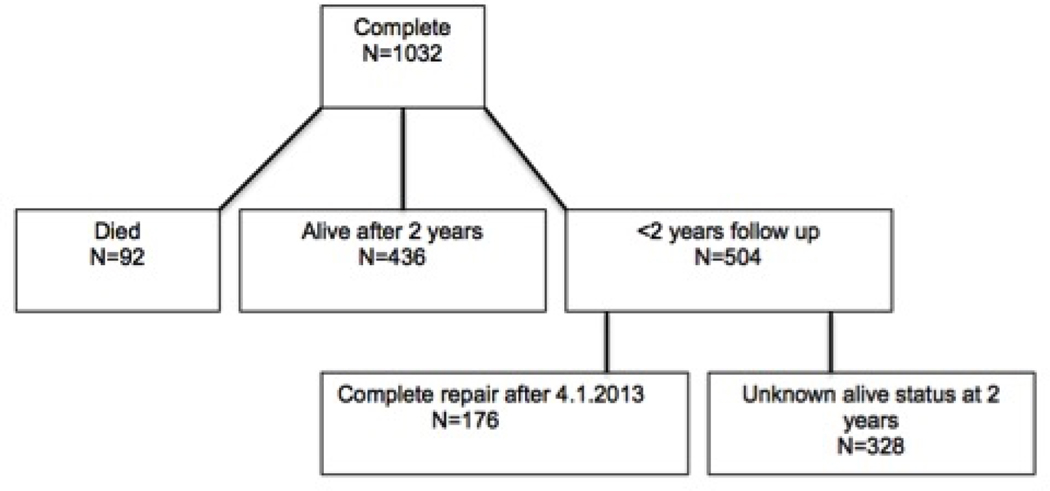
Two hundred and thirty-nine patients died by two years, with 92 (12.5%) deaths in the complete group and 147 (10.8%) deaths in the staged group. In the complete group, 436 patients were alive at two years; 73 of 92 deaths occurred during the initial hospitalization and 19 of 92 deaths occurred between initial hospitalization discharge and two years. There were 328 patients that had less than two years of follow up in PHIS and an unknown mortality status at 2 years, and 176 patients who were operated on after 4.1.2013 and could not be followed for two years (Figure 1).
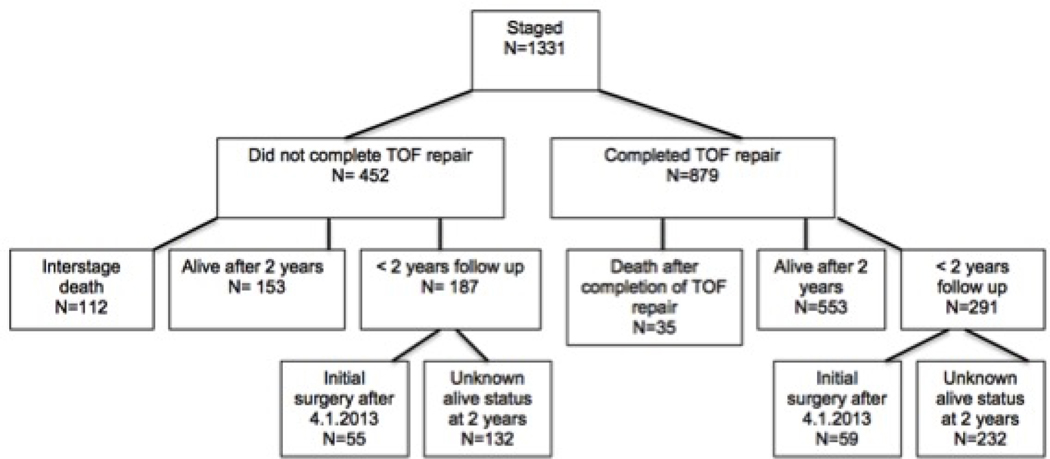
Figure 1: Description of the Complete and Staged Groups.
Detailed flow chart demonstrating the breakdown of the cohort by survivors, deaths, and incomplete follow up for both groups.
Of the 1331 staged patients, 879 patients survived the initial palliation and later received completion of TOF repair. There were 147 deaths in the staged group: 84 occurred during the initial procedure hospitalization, and 63 were interstage deaths that occurred after discharge form the initial procedure hospitalization. Thirty-five of those 63 deaths occurred after completion of TOF surgery. There were 364 patients in the staged group that had less than two years of follow-up and had an unknown mortality status at 2 years, and 114 patients who were operated on after 4.1.2013 and thus could not be followed for two years (Figure 1).
Survival Analysis
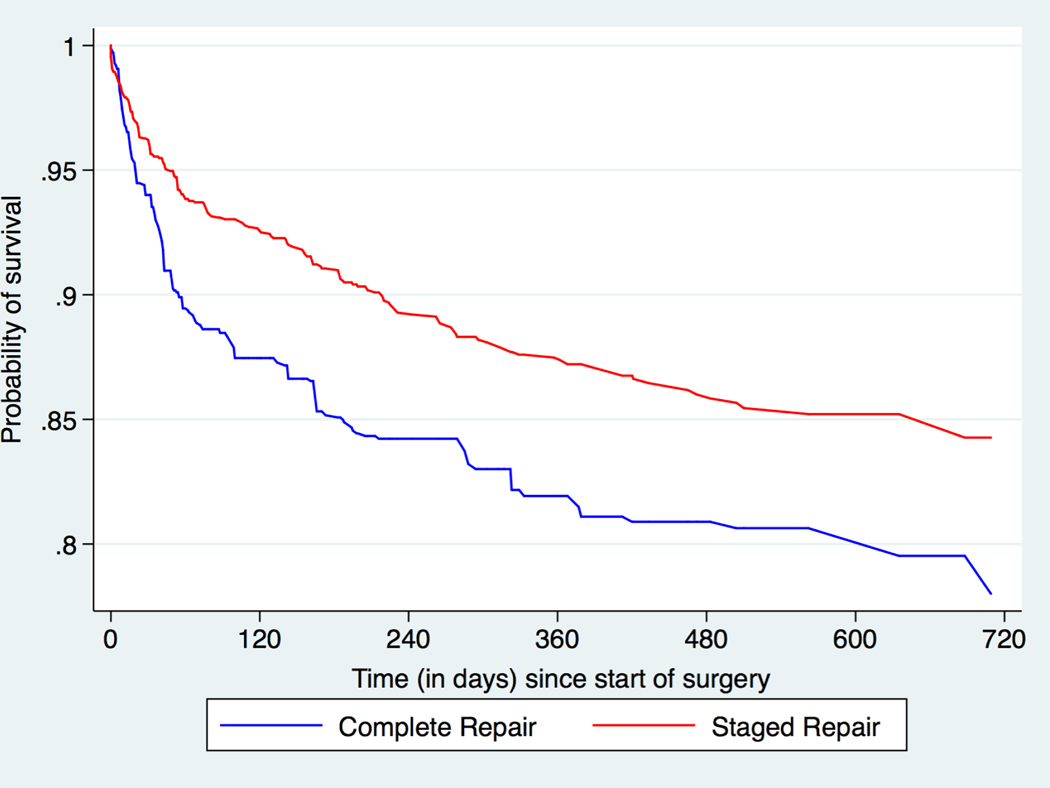
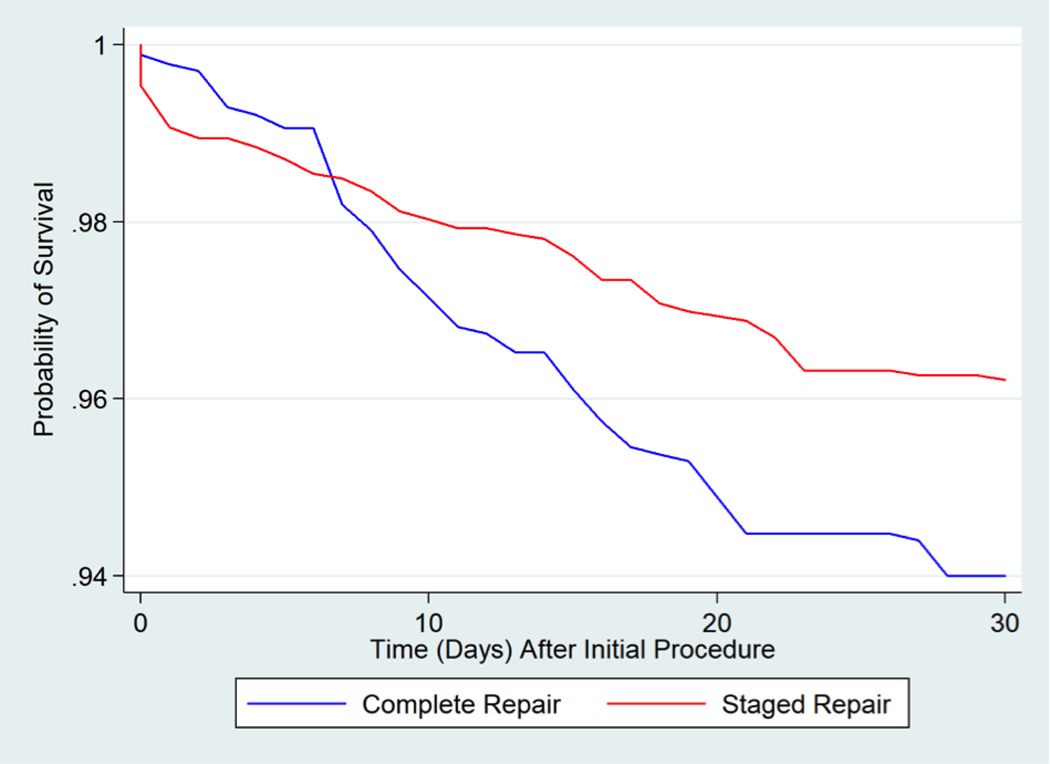
Patients who underwent a complete neonatal repair had a greater risk of mortality within the 2-year follow-up period than patients with a staged repair (HR=1.51, 95% CI: 1.05, 2.06, p=0.024) (Central Illustration; Table 2). For the survival analysis for mortality within the first 30 days, the effect of initial treatment did not satisfy the proportional hazards assumption (i.e., the hazard of complete vs. staged was not constant over time). We separated the estimates into the time intervals in which the proportional hazards assumption was satisfied (0–6 days and 7–30 days). Using the weighted Cox proportional hazards model, there was no difference in the risk of mortality between the two groups in the first week after the initial procedure (HR 0.68, 95% CI: 0.17, 1.48), p=0.49). However, from day 7 until day 30 after the initial procedure, patients in the complete group had a greater risk of mortality than patients in the staged group (HR 2.29, 95% CI: 1.18, 4.41, p=0.011) (Figure 2, Table 2).
Central Illustration: Neonatal Tetralogy of Fallot: Weighted Survival Curves for the First 2 Years after Initial Procedure.
Weighted survival curves of patients that underwent complete or staged TOF repair prior to 30 days of age. The observation period is from the date of the initial TOF procedure to death within a two-year follow-up period. Complete neonatal repair was associated with a significantly higher risk of mortality during the two-year follow up [HR = 1.51, 95% CI 1.05, 2.06].
Table 2:
Effect of Surgical Treatment Group on Mortality after Initial Procedure
| HR or OR (95% CI) | P-Value | E-Value | Lower Limit E-Value* | |
|---|---|---|---|---|
| Mortality Within Two Years From Initial Procedure | ||||
| HR for complete (vs. staged) repair | 1.51 (1.05 – 2.06) | 0.024 | 2.39 | 1.28 |
| Mortality Within 30 Days From Initial Procedure | ||||
| HR for complete (vs. staged) repair within first 6 days | 0.68 (0.17 – 1.48) | 0.49 | N/A | N/A |
| HR for complete (vs. staged) repair between 7–30 days | 2.29 (1.18 – 4.41) | 0.011 | 4.02 | 1.66 |
| Mortality During First Hospitalization | ||||
| OR for complete (vs. staged) repair | 1.72 (1.15 – 2.62) | 0.028 | 2.83 | 1.57 |
Hazard ratio (HR) for risk of death is based on weighted Cox Proportional Hazards model, odds ratio (OR) for odds of death is based on a weighted logistic regression model, 95% confidence interval (CI) is bootstrapped, and E-value is computed for a sensitivity analysis for unmeasured confounding.
The lower confidence bound of the E-value is listed for ease of interpretation of the E-value.
Figure 2: Weighted Survival Curves for the First 30 Days after Initial Procedure.
Weighted survival curves of the complete neonatal repair and staged approach groups examining 30-day mortality. Complete neonatal repair was associated with a significantly higher risk of mortality between 7–30 days after initial procedure [HR = 2.29, 95% CI 1.18, 4.41].
Seventy-three (7.1%) of the complete group and 84 (6.3%) of the staged group died prior to discharge. The weighted logistic regression model revealed that patients in the complete group had a greater risk of death during the initial hospitalization than patients in the staged group (OR 1.72, 95% CI: 1.15, 2.62, p=0.028) (Online Table 4).
It is possible that unmeasured confounding could explain these findings. However, E-value calculations indicated that only the presence of very strong unmeasured confounders associated with both treatment type and mortality with effect estimates of 2.4 to 4.0 each (lower limit of the CI 1.28 and 1.66, respectively) could explain the association of operative approach and survival (Table 2).
Causal Mediation Analysis
At least one in-hospital cardiac complication was observed in 249 (36.1%) patients in the complete group within 30 days of the initial procedure, as compared to 156 (15.4%) in the staged group. Having at least one in-hospital cardiac complication was found to be a mediator of the relationship between initial surgery type and both early (7–30 day) and late (two-year) mortality, so we deconstructed the total effect of complete surgery into its indirect effect through having at least one cardiac complication and its direct effect through other undefined pathways. The complete surgery (vs. staged repair) was associated with an increased risk of mortality through the pathway of having at least one cardiac complication for the 7–30 day, two-year, and in-hospital mortality (Table 3, Online Table 3), The percentage of the total effect of surgical approach on the risk of death that was mediated through cardiac complications was 87% (7–30 days), 80% (two years), and 78.5% (in-hospital) (Table 3). We could not identify a specific cardiac complication that mediated these observed relationships. Having a non-cardiac complication or the number of non-cardiac complications did not mediate of the treatment-mortality relationship (data not shown).
Table 3:
Mediation Analysis
| Total Effect | P-Value | Direct Effect | P-Value | Indirect Effect | P-Value | Proportion Mediated by Cardiovascular Complications | |
|---|---|---|---|---|---|---|---|
| Mortality Within Two Years | |||||||
| Hazard ratio | 1.51 (1.05 – 2.06) | 0.024 | 0.92 (0.75 – 1.12) | 0.38 | 1.21 (1.00 – 1.46) | 0.047 | 80% |
| Mortality Between 7–30 Days | |||||||
| Hazard ratio | 2.29 (1.18 – 4.41) | 0.011 | 0.68 (0.46 – 1.02) | 0.060 | 1.99 (1.34 – 2.95) | 0.0007 | 87% |
| Mortality During First Hospitalization | |||||||
| Odds ratio | 1.72 (1.15 – 2.62) | 0.028 | 0.84 (0.60 – 1.18) | 0.32 | 1.35 (1.25 – 1.46) | < 0.0001 | 78.5% |
The mediator is at least one in-hospital cardiac complication within the first 30 days after the initial procedure (and prior to hospital discharge). The hazard ratios, odds ratios, and 95% confidence intervals represent the effect of complete repair (vs. staged repair).
Sensitivity Analysis
We obtained additional data beyond the study period and imputed follow-up time or those with less than two years follow-up. The HRs for complete vs. staged was 1.33 (95% CI: 1.05, 1.68, p=0.019, weighted effect with standard errors) and 1.33 (95% CI: 0.92, 1.82, p=0.13, weighted effect with robust standard errors and bootstrapped 95% CI), which were similar in magnitude to our main result.
Subgroup analyses
We conducted a further analysis limiting the staged group to patients that underwent a BT shunt and examined the relationship between surgical group and 2-year mortality. There were 1016/1331 patients (76.3%) in the staged group that underwent a BT shunt. The effect estimate was similar in magnitude to that of the main analysis, however there was no statistically significant difference in risk of mortality between the complete repair and those palliated with a BT shunt (HR for complete repair=1.40, 95% CI: (0.94, 2.09) p=0.097). Similarly, within the complete group, there was no difference in the unadjusted odds of 2-year mortality comparing those who received and those who did not receive an RV-PA conduit [OR complete group 1.35, 95% CI: (0.86, 2.13), p=0.20).
Discussion
We found that complete neonatal repair for TOF was associated with a higher risk of early and late mortality compared to staged repair in a comparative effectiveness study (Central Illustration). We accounted for patient, hospital and systematic factors in our analysis, which might have influenced the approach taken for each patient, including a hospital preference for one surgical strategy over the other (21,32,33). To our knowledge, this is the first, large multicenter study of outcomes after neonatal intervention for TOF with long-term mortality data and extensive consideration of confounders. Other studies have not focused on the outcomes of complete and staged approaches, the symptomatic neonate, or center preference and clustering within hospitals (4,6,9,10,34).
Patients with TOF undergoing complete neonatal repair had increased risk of death as compared to those in the staged repair group at both early and later time points (7–30 days and two years after initial procedure, respectively). This increased risk was in large part mediated by more post-operative cardiac complications that occurred in the first week after complete repair with short- and long-term adverse effects (12,35–39). Although we did not identify a single complication associated with higher risk of mortality, we postulated that this finding reiterates the complex nature of complete TOF repair in the newborn and supports the need to identify the ideal candidate for complete neonatal repair. Complete neonatal repair could be associated with more complications and higher mortality through an accentuated inflammatory response following cardiopulmonary bypass or the inability of the neonatal right ventricle to maintain or recover function in the peri-operative period (40). Procedures that occur in the critical transitional neonatal period potentially have a higher mortality, a well-known phenomenon in general pediatric congenital surgery, and likely reflects greater vulnerability of all organ systems in addition to the complexity of procedures performed (41–42). An intervention to prevent complications after neonatal complete repair could theoretically improve the associated outcomes.
Contrary to our findings, another multicenter study using PHIS data reported a similar proportion of deaths for complete repair and staged repair and in TOF (10). However, the analysis was not adjusted for confounders. As such, there was a higher proportion of patients with genetic syndromes and coronary artery anomalies in the staged group which may have biased the study’s results to the null. Furthermore, only patients with surgical procedure codes for Blalock-Taussig shunt and TOF repair were included as opposed to all palliative approaches, and the study did not examine long-term mortality.
A recent meta-analysis compared patients undergoing complete neonatal repair to patients that underwent later repairs preceded by a palliation (43). Although the groups were not exactly comparable to those in our study, it is worth highlighting the fact that neonatal complete repair was associated with greater mortality as compared to patients that underwent repair at a later age (43). A multi-center study reported increased mortality in patients with TOF undergoing either complete or staged repair prior to age three months, and although the study did not compare outcomes by approach, it demonstrated significant institutional variability in the choice of initial approach, emphasizing the importance of multicenter studies such as ours which are generalizable (44). These findings, along with ours, suggest that neonatal repair for TOF may be associated with adverse outcome. Although there are fewer reports on outcomes after initial palliation as the approach of choice for the symptomatic neonate with TOF, centers that favor a staged approach report low mortality and complication rates (37,45–47). When limiting the comparison to patients that received BT shunts in the staged group, there was no statistically significant difference in the risk of mortality, even though the magnitude of the effect estimate was similar to that from our main analysis, and the lower bound of the confidence interval barely crossed 1. It is possible that with a smaller sample size we were underpowered for this analysis.
Limitations
There were several possible limitations in this study. First, there is the risk of misclassification of surgical approach. We conducted a priori cohort validation to reduce this bias. If present, misclassification would unlikely be differential in terms of the outcome, meaning that any bias would be towards the null (attenuating the results), so that the association between operative approach and outcome may be even stronger than shown. Second, if sicker patients were more likely to get a complete repair, this would result in confounding by severity of illness. If higher risk patients were operated on at centers with a preference for complete repairs (or which had worse outcomes), this would result in confounding by center. This is unlikely as 1) if anything, surgeons generally use a staged repair in sicker patients and 2) our weighted analysis accounts for pre-operative acuity, patient factors such as lower weight, prematurity and genetic syndromes that could have impacted the decision surrounding surgical approach, and center factors and apparent center preference for one approach over the other. In addition, an unmeasured confounder would have to have a very strong relationship with the outcome in order to explain our findings. It is possible but unlikely that such a strong determination of surgery and outcome was not included in our analysis. While this study focused on the hospitals in the PHIS, the generalizability of these results to centers that perform surgical procedures for TOF is an important strength. We were reassured by the sensitivity analysis, which demonstrated an effect estimate similar to that from our main analysis, despite a non-significant P-value in the sensitivity analysis using robust errors. An additional limitation was our inability to determine the pulmonary valve anatomy in PHIS in order to take this into account in our analysis. Finally, this is an observational study, which limits causal inferences. A randomized clinical trial of surgical approach to TOF may be warranted to confirm these findings before changing clinical practice.
Conclusion
Complete neonatal repair for TOF is associated with greater early and late adjusted risk of mortality when compared to mortality after staged repair and beyond completion of repair. This risk was mediated by post-operative complications.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care and Procedural Skills:
For neonates with tetralogy of Fallot, complete surgical repair is associated with a higher risk of mortality than a staged approach, mainly because of post-operative cardiac complications.
Translational Outlook:
Randomized trials are needed to compare the short and long-term outcomes of various strategies to correct tetralogy of Fallot in symptomatic neonates.
Acknowledgments
Funding: Dr. Savla receives support from the NIH National Heart, Lung, and Blood Institute (NHLBI), grant T32 HL007915, and the NIH NHLBI LRP Award L40 HL143663.
Dr. Kawut receives support from the NIH NHLBI, K24HL103844
Dr. Mercer-Rosa receives research support from the NIH NHLBI grant K01 HL125521, and the Pulmonary Hypertension Association Supplement to HL125521
Abbreviations:
- CPR
Cardiopulmonary resuscitation
- ECMO
Extra-corporeal membrane oxygenation
- HR
Hazard Ratio
- ICD-9
International Classification of Diseases-9
- PHIS
Pediatric Health Information Systems
- TOF
Tetralogy of Fallot
- VSD
Ventricular septal defect
Footnotes
Tweet: Complete surgical repair for neonates with tetralogy of Fallot confers higher risk of mortality than the staged approach.
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. [DOI] [PubMed] [Google Scholar]
- 3.Behrman RE VVI, McKay RM. Nelson Textbook of Pediatrics. 13th edition Philadelphia, PA:Saunders;1987. [Google Scholar]
- 4.Al Habib HF, Jacobs JP, Mavroudis C, et al. Contemporary patterns of management of tetralogy of Fallot: data from the Society of Thoracic Surgeons Database. The Annals of thoracic surgery. 2010;90:813–820. [DOI] [PubMed] [Google Scholar]
- 5.Di Donato RM, Jonas RA, Lang P, Rome JJ, Mayer JE Jr., Castaneda AR. Neonatal repair of tetralogy of Fallot with and without pulmonary atresia. J Thorac Cardiovasc Surg. 1991;101:126–137. [PubMed] [Google Scholar]
- 6.Dorobantu DM, Pandey R, Sharabiani MT, et al. Indications and results of systemic to pulmonary shunts: results from a national database. Eur J Cardiothorac Surg. 2016;49:1553–1563. [DOI] [PubMed] [Google Scholar]
- 7.Van Arsdell GS, Maharaj GS, Tom J, et al. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000;102:III123–129. [DOI] [PubMed] [Google Scholar]
- 8.Kanter KR, Kogon BE, Kirshbom PM, Carlock PR. Symptomatic neonatal tetralogy of Fallot: repair or shunt? The Annals of thoracic surgery. 2010;89:858–863. [DOI] [PubMed] [Google Scholar]
- 9.Steiner MB, Tang X, Gossett JM, et al. Alternative repair strategies for ductal-dependent tetralogy of fallot and short-term postoperative outcomes, a multicenter analysis. Pediatr Cardiol. 2015;36:177–189. [DOI] [PubMed] [Google Scholar]
- 10.Steiner MB, Tang X, Gossett JM, Malik S, Prodhan P. Timing of complete repair of non-ductal-dependent tetralogy of Fallot and short-term postoperative outcomes, a multicenter analysis. J Thorac Cardiovasc Surg. 2014;147:1299–1305. [DOI] [PubMed] [Google Scholar]
- 11.Dodge-Khatami A, Tulevski II, Hitchcock JF, de Mol BA, Bennink GB. Neonatal complete correction of tetralogy of Fallot versus shunting and deferred repair: is the future of the right ventriculo-arterial junction at stake, and what of it? Cardiol Young. 2001;11:484–490. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch JC, Mosca RS, Bove EL. Complete repair of tetralogy of Fallot in the neonate: results in the modern era. Ann Surg. 2000;232:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail SR, Kabbani MS, Najm HK, Abusuliman RM, Elbarbary M. Early outcome of tetralogy of Fallot repair in the current era of management. J Saudi Heart Assoc. 2010;22:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolcz J, Pizarro C. Neonatal repair of tetralogy of Fallot results in improved pulmonary artery development without increased need for reintervention. Eur J Cardiothorac Surg. 2005;28:394–399. [DOI] [PubMed] [Google Scholar]
- 15.Desai AV, Kavcic M, Huang YS, et al. Establishing a high-risk neuroblastoma cohort using the Pediatric Health Information System Database. Pediatr Blood Cancer. 2014;61:1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savla JJ, Fisher BT, Faerber JA, Huang YV, Mercer-Rosa L. Complete Versus Staged Repair for Neonates With Tetralogy of Fallot: Establishment and Validation of a Cohort of 2235 Patients Using Detailed Surgery Sequence Review of Health Care Administrative Data. Med Care. 2018;56(11):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Zaslavsky AM, Landrum MB. Propensity score weighting with multilevel data. Stat Med. 2013;32:3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silber JH, Rosenbaum PR, Calhoun SR, et al. Outcomes, ICU Use, and Length of Stay in Chronically Ill Black and White Children on Medicaid and Hospitalized for Surgery. J Am Coll Surg. 2017;224:805–814. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc. 1987;82. [Google Scholar]
- 23.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum PR. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 25.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33- [Google Scholar]
- 27.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. The American Statistician. 1986;40:249–251. [Google Scholar]
- 28.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 30.Vanderweele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY:Oxford;2015. [Google Scholar]
- 31.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176:190–195. [DOI] [PubMed] [Google Scholar]
- 32.Bolch CA, Chu H, Jarosek S, Cole SR, Elliott S, Virnig B. Inverse probability of treatment-weighted competing risks analysis: an application on long-term risk of urinary adverse events after prostate cancer treatments. BMC Med Res Methodol. 2017;17(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaney JA, Platt RW, Suissa S. The impact of unmeasured baseline effect modification on estimates from an inverse probability of treatment weighted logistic model. Eur J Epidemiol. 2009;24:343–349. [DOI] [PubMed] [Google Scholar]
- 34.Patel A, Costello JM, Backer CL, et al. Prevalence of Noncardiac and Genetic Abnormalities in Neonates Undergoing Cardiac Operations: Analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. The Annals of thoracic surgery. 2016;102:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennein HA, Mosca RS, Urcelay G, Crowley DC, Bove EL. Intermediate results after complete repair of tetralogy of Fallot in neonates. J Thorac Cardiovasc Surg. 1995;109:332–344. [DOI] [PubMed] [Google Scholar]
- 36.Kirsch RE, Glatz AC, Gaynor JW, et al. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: a 14-year experience at a single center. J Thorac Cardiovasc Surg. 2014;147:713–717. [DOI] [PubMed] [Google Scholar]
- 37.Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. The Annals of thoracic surgery. 2005;80:1431–1439. [DOI] [PubMed] [Google Scholar]
- 38.Pigula FA, Khalil PN, Mayer JE, del Nido PJ, Jonas RA. Repair of tetralogy of Fallot in neonates and young infants. Circulation. 1999;100:II157–161. [DOI] [PubMed] [Google Scholar]
- 39.Boening A, Scheewe J, Paulsen J, et al. Tetralogy of Fallot: influence of surgical technique on survival and reoperation rate. Thorac Cardiovasc Surg. 2001;49:355–360. [DOI] [PubMed] [Google Scholar]
- 40.DiLorenzo MP, Elci OU, Wang Y, et al. Longitudinal Changes in Right Ventricular Function in Tetralogy of Fallot in the Initial Years after Surgical Repair. J Am Soc Echocardiogr. 2018;7:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alsoufi B, Gillespie S, Mahle WT, et al. The Effect of Noncardiac and Genetic Abnormalities on Outcomes Following Neonatal Congenital Heart Surgery. Semin Thorac Cardiovasc Surg. 2016;28:105–114. [DOI] [PubMed] [Google Scholar]
- 42.Pagowska-Klimek I, Pychynska-Pokorska M, Krajewski W, Moll JJ. Predictors of long intensive care unit stay following cardiac surgery in children. Eur J Cardiothorac Surg. 2011;40:179–184. [DOI] [PubMed] [Google Scholar]
- 43.Loomba RS, Buelow MW, Woods RK. Complete Repair of Tetralogy of Fallot in the Neonatal Versus Non-neonatal Period: A Meta-analysis. Pediatr Cardiol. 2017;38:893–901. [DOI] [PubMed] [Google Scholar]
- 44.Mulder TJ, Pyles LA, Stolfi A, Pickoff AS, Moller JH. A multicenter analysis of the choice of initial surgical procedure in tetralogy of Fallot. Pediatr Cardiol. 2002;23:580–586. [DOI] [PubMed] [Google Scholar]
- 45.Fraser CD Jr., McKenzie ED, Cooley DA. Tetralogy of Fallot: surgical management individualized to the patient. The Annals of thoracic surgery. 2001;71:1556–63. [DOI] [PubMed] [Google Scholar]
- 46.Morales DL, Zafar F, Heinle JS, et al. Right ventricular infundibulum sparing (RVIS) tetralogy of fallot repair: a review of over 300 patients. Ann Surg. 2009;250:611–617. [DOI] [PubMed] [Google Scholar]
- 47.Ross ET, Costello JM, Backer CL, Brown LM, Robinson JD. Right ventricular outflow tract growth in infants with palliated tetralogy of fallot. The Annals of thoracic surgery. 2015;99:1367–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.