Abstract
The craniofacial region is anatomically complex and is of critical functional and cosmetic importance, making reconstruction challenging. The limitations of current surgical options highlight the importance of developing new strategies to restore the form, function, and esthetics of missing or damaged soft tissue and skeletal tissue in the face and cranium. Regenerative medicine (RM) is an expanding field which combines the principles of tissue engineering (TE) and self-healing in the regeneration of cells, tissues, and organs, to restore their impaired function. RM offers many advantages over current treatments as tissue can be engineered for specific defects, using an unlimited supply of bioengineered resources, and does not require immunosuppression. In the craniofacial region, TE and RM are being increasingly used in preclinical and clinical studies to reconstruct bone, cartilage, soft tissue, nerves, and blood vessels. This review outlines the current progress that has been made toward the engineering of these tissues for craniofacial reconstruction and facial esthetics.
Keywords: Craniofacial, Facial aesthetics, Regeneration medicine, Tissue engineering
The craniofacial anatomy is highly specialized and individualized and is of critical functional and cosmetic importance but is prone to genetic and environmental insults. The head and face are commonly affected by cancer and trauma,1,2 and of all the live births affected by a minor or major anomaly, one-third involve the head and face.3 Around 85% of the global population is in need of craniofacial tissue at some point during their lifetime,4 and >28,000 head and neck reconstructions are performed every year in the United States (US).5 Craniofacial deformities can have a dramatic impact on quality of life, and adequately reconstructing the complex form, function, and esthetics of facial anatomy is challenging. The criterion standard approach is to replace missing or damaged tissue with autologous grafts. Donor bone and soft tissues, however, are in finite supply and their harvest can result in significant morbidity.6 Allogeneic grafts from cadavers or living donors bring the risk of infection, inflammation, lifelong immunosuppression,7,8 and unpredictable donor-recipient anatomical compatibility.9 Prosthetic alloplastic materials are unable to restore the multiple complex sensory and motor functions of craniofacial structures, do not expand in growing children, and are at risk for failure and infection.10–13 Thus, a clear need exists to develop alternative strategies to reconstruct craniofacial tissues.
Regenerative medicine (RM) is an emerging interdisciplinary field which combines the principles of cellular and molecular biology, material science, and bioengineering, to support endogenous healing and replace or regenerate cells, tissues, or organs, with restoration of impaired function. Tissue engineering (TE) is a related concept centered on the engineering and manufacturing aspects of tissue replacement; however, TE and RM are often combined and treated as a single research pursuit.14,15 TE/RM supports natural tissue regeneration processes by using cells, natural or artificial scaffolding materials, growth factors (GFs), gene manipulation, or combinations of these elements (Fig. 1). The cells of interest, often stem or progenitor cells, are typically isolated, expanded, differentiated ex vivo, seeded onto scaffolds, then reinserted into the defected areas with the scaffolds, often in combination with tissue-specific GFs. Mesenchymal stromal cells (MSCs) are the most common cell type used because of their ethical acceptance, ease of harvesting, robust proliferative capacity, and their ability to give rise to the cells commonly required for craniofacial reconstruction, namely osteoblasts, chondroblasts, adipocytes, tenocytes, myoblasts, and stromal cells.16,17 MSCs are mostly sourced from human bone marrow-derived stem cells (BMSCs) or adipose tissue-derived stem cells (ADSCs). Although BMSCs were described first and have been the focus of TE strategies, ADSCs are in greater abundance and can be harvested with less patient-morbidity from liposuction or fat excision procedures. Scaffold and biomimetic materials can assist cellular growth and differentiation by providing a dynamic three-dimensional [3D] framework for cellular attachment, migration, and protection. Ideally scaffolds are able to withstand the immune response and eventually undergo resorption. Synthetic biomaterials have been created and refined for different tissues. The application of GFs aims to provide the necessary stimuli to promote the activity and differentiation of stem/progenitor cells toward certain cell fates required for tissue healing. Vectors or nonchemical extracellular environmental changes, such as atmospheric pressure, can also be used to alter cellular activity.
FIGURE 1.
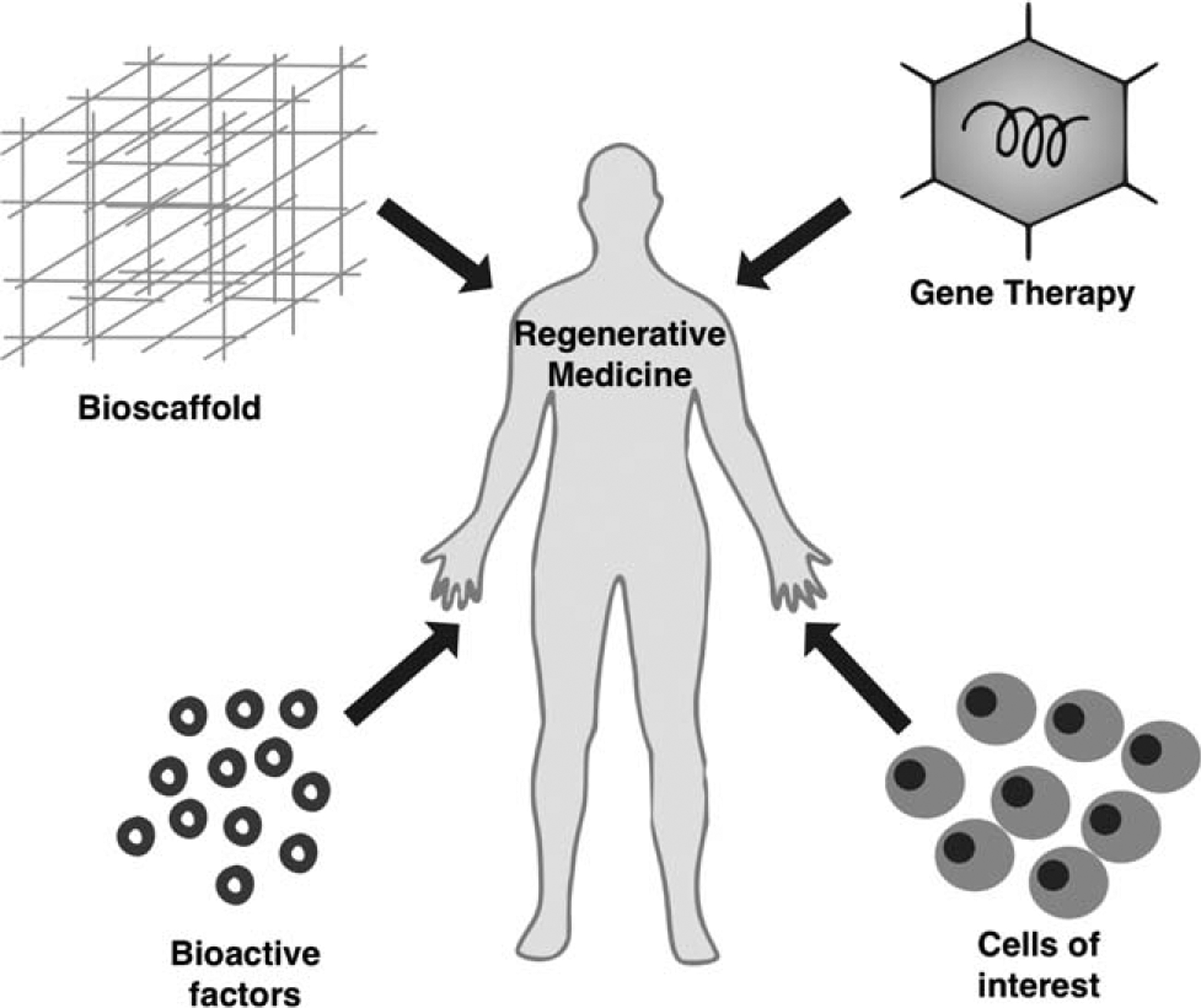
The process of tissue engineering/regenerative medicine.
TE/RM promises many advantages over current standards of treatment for craniofacial reconstruction; customized tissues can be created for specific defects, using an unlimited supply of bioengineered resources, enabling reconstruction without the need for immunosuppression. In craniofacial and facial esthetic surgery, numerous tissue types require repair following congenital or acquired defects. This review provides for the first time a unique synopsis of current concepts of TE/RM in craniofacial reconstruction and facial esthetic surgery. We focus on TE/RM of various types of tissues relevant to the field, including skeleton, soft tissue, adipose tissue, and neurovasculature, and provide an up-to-date report on future perspectives and challenges.
BONE
The craniofacial skeleton provides the stable, rigid, structural framework for facial soft tissue, cartilage, and dental structures, and is a key determinant of facial esthetics.18 Loss of craniofacial bone, owing to congenital, traumatic, or neoplastic causes, can result in significant structural and functional deformities. Bone TE has large applications within craniofacial surgery and has been extensively studied (Fig. 2).19–21 Preclinical studies have demonstrated that both BMSCs and ADSCs are effective osteoblastic precursors and when placed into mandibular or critical-sized cranial defects, they can accelerate bone regeneration.22–25 Additionally, MSCs modified ex vivo using bone morphogenetic protein 2 (BMP-2) and applied to the dog mandible during distraction, accelerated osteogenesis.26 In humans, MSCs have been used to engineer bone in the jaw and cranium in clinical case series and case reports.27–32 One randomized clinical trial (RCT, n = 30) reported that β-trical-cium phosphate (β-TCP) scaffolds seeded with BMSCs, monocytes, and macrophages promoted more bone to form in areas of maxillary sinus deficiency than did scaffolds without cells.33 The most effective bone-forming MSC remains to be identified. Specific subpopulations of ADSCs,23 induced pluripotent stem cells (IPSCs),34 and genetically modified ADSCs,35 all may have enhanced osteogenic potential and are able to promote bone formation in the craniofacial skeleton of animals. Recently, the mouse (mSSC) as well as the human skeletal stem cell (hSSC) have been identified, defined as cells with the ability for self-renewal, which give rise to bone, cartilage, and stromal tissue in vitro and in vivo.36,37 SSCs appear to be a promising cellular candidate for future skeletal tissue engineering therapies with the unique advantage of requiring less exogenous stimulation to drive differentiation of bone and cartilage compared to MSCs and ADSCs. The bone-regenerating ability of ADSCs and BMSCs can be encouraged by GFs. The members of the transforming GF-beta (TGFβ) family, such as BMPs, fibroblast GFs (FGFs), vascular endothelial GF (VEGF), and platelet-derived GF (PDGF), have all been used for bone TE.38 Two recombinant BMPs have been approved by the US Food and Drug Administration (FDA) for clinical use: Infuse Bone Graft, containing rhBMP-2 (Medtronic and Wyeth, Watford, UK), and Osigraft, containing rhBMP-7 (Stryker Biotech, Ontario, Canada). BMP-2 and BMP-7 are able to promote bone regeneration in the alveolar ridge and facilitate the repair of critical-sized craniofacial bone defects in humans.39–41 GFs can be incorporated into seeded cells via molecular or genetic modification,42 or alternatively can be combined with scaffolds by soaking or bonding. Soaking results in the quick release of GFs by passive diffusion or upon degradation of the biomaterial.42 GFs that are encapsulated or covalently bound to scaffolds are released according to cellular demands, which may more accurately recapitulates the natural bone-healing process which occurs over several weeks.43 Multiple GFs added in unison, or in temporal/spatial succession, may have synergistic results and further promote bone formation,44 which remains to be defined in future studies. Platelet-rich plasma (PRP) is rich in GFs45 which are released upon platelet activation and induce cellular differentiation, enhance healing, and promote bone regeneration.46 PRP is gaining interest in bone TE and is being increasingly used in orthopedic surgery.47 In the craniofacial skeleton, a PRP membrane incorporating MSCs promoted the healing of critical-sized cranial defects in both mice and rabbits,48 as well as the healing canine mandibular defects, with evidence of improved vascularization.49
FIGURE 2.
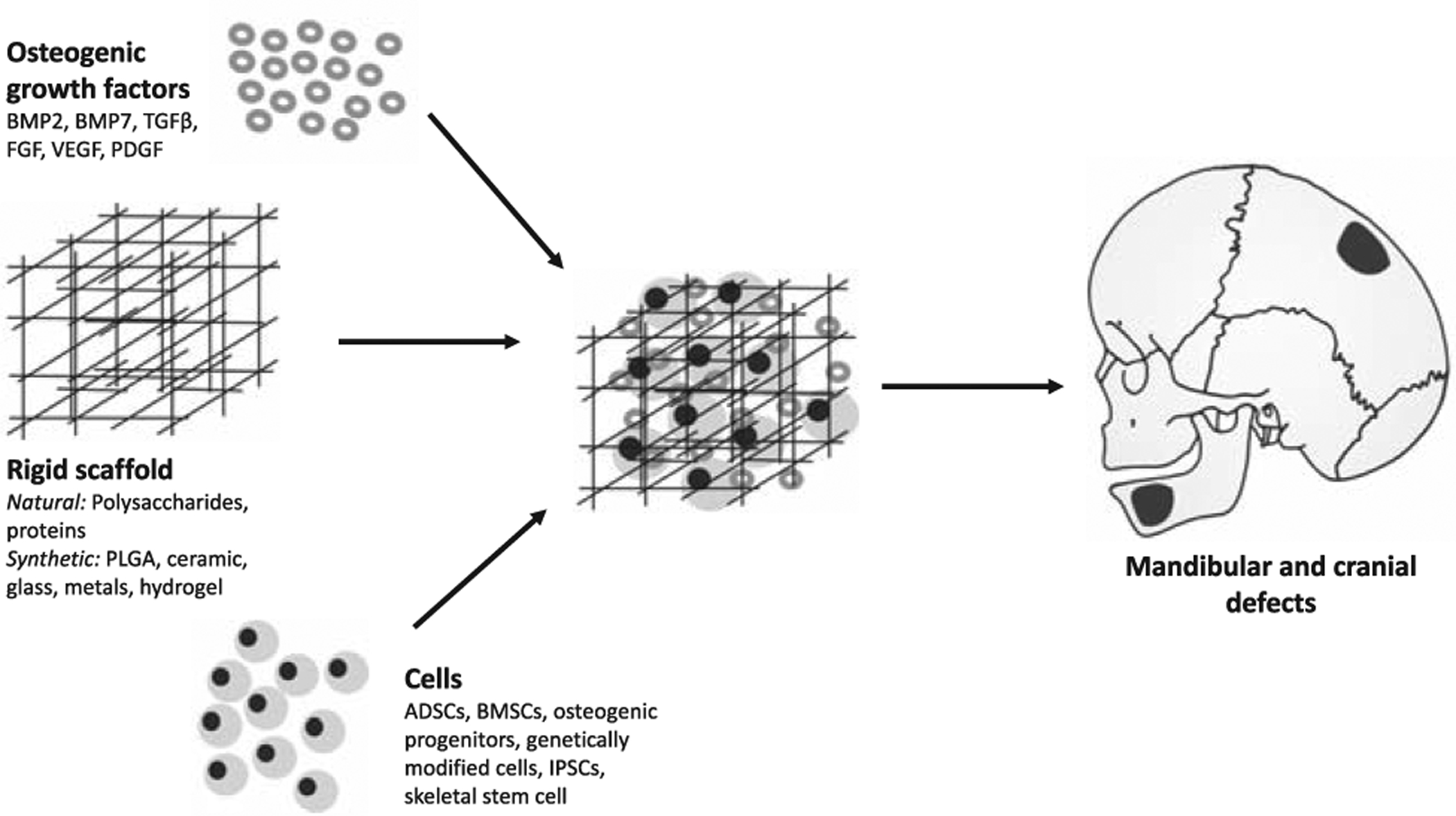
Skeletal tissue engineering.
A variety of scaffolds act as carriers for cells and GFs. Natural biodegradable polymers made of substances, such as polysaccharides (eg, chitosan) or proteins (eg, collagen), are highly biocompatible but may lack significant mechanical strength and may cause an immune response. Synthetic polymers, such as poly(lactic-co-glycolic acid) (PLGA) and polymer of lactic acid (PLA) have easily controllable mechanical and physical properties50 and, once degraded, do not obscure computed tomography (CT) scans51; however, they are expensive and generally have weak cell adhesive ability. Other bone TE scaffold biomaterials include bioactive ceramics, glass, hydrogels, metals, and composite scaffolds.52 Hydrogels closely resemble the extracellular matrix but may lack mechanical strength. Bone is composed of 85% calcium phosphate; therefore, biphasic calcium phosphate bioceramics, such as hydroxyapatite (HA) and TCP, have been widely investigated for their use as bone scaffolds. These bioceramics are highly biocompatible, not immune reactive, and can be easily assessed radio-graphically. Biodegradable metals are absorbed within the physiologic environment and may have superior mechanical properties to biodegradable polymers.50 Composite materials, composed of ≥2 biomaterials, could offer the best qualities from each material.53 PLGA, for example, can be combined with HA to significantly enhance its osteogenic environment.54 Advances in patient imaging and computer-assisted design (CAD) technologies have advanced the field, enabling the fabrication of complex 3D custom-fit scaffolds with optimal pore sizes to improve load-bearing strength, cellular adhesion, and the delivery of biomolecules.55 3D printing and electrospinning, for example, are based on CAD software and allow for the creation of scaffolds with more tightly manipulated internal morphology and gross geometry.56,57 Electrospinning is able to create scaffolds made of nanofibers with architectural, functional, and morphologic similarities to collagen fibrils.58 These nanofibrous scaffolds can be designed to have high porosity and high surface-to-volume ratio which enhances cellular attachment and proliferation.59,60 Incorporation of metallic nanoparticles can increase the mechanical strength, cellular adhesion, long-term osteoblast function, collagen synthesis, alkaline phosphatase activity, and calcium deposition of bioscaffolds.58,61
Bone TE approaches have also been explored in dentistry. The teeth are highly specialized facial structures with important influences on facial esthetics. Recent systematic reviews of preclinical studies have highlighted the success of bone TE strategies to regenerate oral peri-implant bone,62 alveolar bone,19 and periodontal bone.63 In humans, a clinical case series showed the benefits of bone TE for the reconstruction of oral peri-implant defects,64 and a systematic review of clinical trials reported the effectiveness of bone TE for alveolar bone regeneration.20,21 Another question which is important to address is whether bone TE/RM may have clinical efficacy in patients whose regenerative capacity is impaired by infection or irradiation. Delivery of BMP-2 in a collagen sponge helped to reconstruct calvarial bone in irradiated65 or infected wounds in rabbits.66 Dermal fibroblasts transduced ex vivo to express BMP-7 were able to promote the regeneration of calvarial bone in the defects of mice subjected to therapeutic doses of radiation.67 These studies suggest TE/RM strategies have value in these complex clinical scenarios. To summarize, preclinical and early clinical studies demonstrate an enormous potential of TE/RM in craniofacial bone repair. There are, however, large variations in study methodology with regards to the nature of cells, biomaterial scaffolds, and type/dimensions of defects used. Future work must identify the biomaterials, cell, and GFs with most clinical effectiveness for certain clinical situations, in more standardized, preclinical, and clinical studies.68
CARTILAGE
The cartilage of the craniofacial skeleton is found in the nose, ears, pharynx, eyelids, and joints. Cartilaginous tissue has a limited ability for spontaneous repair because of its avascular, aneural, and almyphatic nature, as well as the low mitotic activity of chondrocytes. Cartilaginous deficiencies can therefore result in dramatic deformities. Existing therapies for cartilage repair are limited and surgical reconstruction of the craniofacial cartilage, including the external ear, are arguably some of the most challenging reconstructive operations because of the complex patient-specific 3D architecture. Cartilage-based TE has potentially vast application in craniofacial reconstructive surgery (Fig. 3). Implantation of autologous chondrocytes can regenerate extracranial articular cartilage in humans in extracranial regions,69,70 and the FDA has approved autologous chondrocyte implantation/transplantation (ACI/ACT) for the clinical treatment of joints.71–74 However, the progress made in the orthopedic field will need be duplicated in the craniofacial region, given that its cartilage is mostly subcutaneous, exposed to different physical forces, and is present in a highly antigenic environment with frequent immunological responses, including phagocytosis.75 Initial preclinical and clinical studies have been promising; bovine articular chondrocytes seeded into 3D polyglycolic acid-(PGA)-PLA templates constructed in the form of a human ear and transplanted into the dorsum of 10 athymic mice produced lasting cartilage, both morphologically and histologically, after 12 weeks of implantation.76 Using the same model, poly(l-lactic acid-ε-caprolactone) (PCL) copolymer scaffolds molded into human ear shapes and seeded with articular chondrocytes supported the development and maintenance of cartilage in a human ear shape over 40 weeks.77 Additionally, human septal chondrocytes, expanded ex vivo in culture with TGFβ, FGFs, and PDGF and resuspended in alignate polymer structures maintained the size, shape, and viability, with histological, biochemical, and biomechanical features of nasal septum up to 60 days post implantation in the dorsum of athymic mice.38 In a clinical series of 4 patients with microtia, a 2-stage ACI approach was used. Autologous chondrocytes were harvested from the underdeveloped ear, expanded first in vitro and then in a subcutaneous pocket in the patient’s abdomen for 6 months, before they were explanted and hand-sculpted into an ear framework used for auricular reconstruction. Five years following auricular reconstruction, the ear had retained its form.78 Nasal alar lobe defects of 5 patients were repaired using autologous chondrocytes harvested from nasal septal cartilage biopsies, expanded ex vivo, seeded onto fibrous collagen scaffolds, and cultured with autologous serum onto collagen type I and III membranes for 4 weeks. Engineered cartilage grafts were shaped intraoperatively and successfully implanted in regions where tumors had been excised under paramedian forehead or nasolabial flaps.79 Another clinical trial used autologous nasal chondrocytes to reconstruct the nasal alar of 11 patients with nasal alar valve collapse. Nasal cartilage was harvested in small strips, disaggregated, centrifuged, and resuspended in autologous PRP. This fibrin gel was placed into the external nasal valve collapse defects and, at 12 months following surgery, formed persistent cartilage with the appearance of an augmented nasal dorsum without obvious contraction and deformation.80
FIGURE 3.

Facial cartilage tissue engineering.
Despite these promising studies, ACI/ACT therapies are limited by the paucity of donor chondrocytes, the morbidity associated with their harvesting, and the slow proliferation capacity of harvested chondrocytes. MSCs and perichondrocyte cartilage progenitor cells81 are gaining use in cartilage-engineering strategies. They are multipotent and have high replicative abilities, so fewer cells are required at harvest. Synovium-derived MSCs82,83 may have a superior capacity for chondrogenesis.84,85 MSCs, however, have the potential to form unstable cartilage with reduced mechanical stiffness and are prone to fibroblast dedifferentiation or hypertrophy in vitro and in vivo.70,86 Additionally, chondrogenically induced BMSCs tend to lose their chondrogenic ability with passage, resulting in ectopic ossification upon subcutaneous transplantation.87,88 Co-transplant of chondrocytes and MSCs can overcome the limitations of using each cell individually.89,90 Human microtia chondrocytes and BMSCs seeded onto a human ear-shaped PGA scaffold in a ratio of 1:3, grown for 1 week in vitro, and then transplanted subcutaneously into nude mice, resulted in the formation of de novo cartilage 12 weeks after transplant, and maintained the delicate cartilaginous structure with proper elasticity of the cartilaginous tissue. This suggests the chondrocytes had a stable chondroinductive effect on the BMSCs.91 In a second study, human ADSCs and auricular chondrocytes from microtia specimens and were co-grafted into a nude mouse in a 3:7 ratio, and after 8 weeks formed cartilaginous tissue with translucent appearance and good elasticity with cartilage-specific ECM components, including collagen type II, glycosaminoglycans (GAGs), aggrecan, and elastic fibres.92 An alternative approach in future investigations may be to exploit the capacity of the aforementioned mSSC36 to both self-renew and differentiate into cartilage and bone.
The GFs most intensively studied for their molecular control of chondrogensis of MSCs and proliferation of chondrocytes include the BMPs, Wnts, and FGFs.93,94 Combined BMP-2 and TGFβ1 can induce MSC chondrogenesis in pellet culture.95 Dexamethasone also induces the chondrogenic differentiation of BMSCs.96 GF receptor expression is thought to dynamically change during chondrogenesis and temporal control in administration of chondrogenic factors may be required to improve cell growth, matrix deposition, and the phenotype of the cartilage formation.97,98 Similar natural and synthetic materials used for bone have been explored in cartilage TE. Natural materials include agarose, alginate, hyaluronic acid, gelatin, fibrin glue, collagen derivatives, and acellular cartilage matrix, but these may have inferior mechanical strength, disease transfer, and antigenicity, and be prone to rapid and variable host-related degradation. Of the synthetic polymers polyhydroxyacids, such as PLLA, PGA, and PCL have been well studied and are easily extruded into fibrous or open-lattice sponges. One study comparing these 3 polymers found all promoted cartilage formation, but the PCL template yielded neocartilage with the best gross architecture akin to a human ear.75 PEG-based hydrogels are also able to promote chondrogenesis99–101 and are biomaterials already approved by the US FDA.
The temporomandibular joint (TMJ) is a synovial joint which can be damaged by tumors, trauma, and degenerative joint disorders including rheumatoid arthritis, osteoarthritis, and ankylosis.102 TMJ dysfunction can cause pain, problems with speech, swallowing, and mastication, and facial asymmetry. Unlike the hyaline cartilage of the knee joints, the articular surfaces of the TMJ mandibles are covered by fibrocartilage.103,104 Additionally, regeneration of the TMJ articular surface requires an adequate bone-cartilage interphase.105–107 This can be achieved by seeding both osteoblasts and chondrocytes into scaffolds that are able to fulfill the biological and mechanical requirements for the regeneration of cartilage and bone. The scaffolds can also be shaped fit the unique TMJ environment. In a preclinical study, bovine osteoblasts and chondrocytes were seeded onto a scaffold composed of PGA and PLA formed in the shape of a human mandible condyle. The scaffold was implanted into subcutaneous pockets of athymic mice, and after 12 weeks, there was evidence of trabecular bone and hyaline cartilage on the articular surface.108 Alternatively, scaffolds can be seeded with mesenchymal progenitor cells or differentiated MSCs, as MSCs have the ability to differentiate into both bone and cartilage depending on cues in their cellular microenvironment such as matrix stiffness.109 In one preclinical study, chondrogenic and osteogenic differentiated BMSCs were encapsulated into a bilayered osteochondral PEG-based construct that had been carved into a human mandibular condyle shape. After 8 weeks of transplantation into the dorsum of immunodeficient mice, the construct had formed de novo mandibular condyles with areas of both cartilage and bone.99 Chondrogenic differentiated MSCs have also been directly injected into the intra-articular space of the TMJ in a rabbit model of TMJ osteoarthritis, where they were reported to integrate into the mandible and form both subchondral cancellous bone, cartilage, and synovial membrane 4 weeks post-transplantation. Interestingly, the cartilage repair was better in rabbits transplanted with differentiated, compared to non-differentiated, MSCs.110 Developing an injectable approach for cartilage regeneration of the TMJ could satisfy a patient’s desire for minimally invasive surgery, but this requires substance that is viscous or semisolid to be injected, and once injected to maintain a desired shape or form without diffusion.111 BMSCs have also been modified using NEL-like molecule 1 (NELL-1—a GF thought to target cells toward an osteochondral lineage) seeded onto a PLGA composite and transplanted into large condylar defects in goat mandibles. This rapidly regenerated both bone and cartilage tissue.112 Low-intensity pulsing ultrasound can stimulate stem cell growth and differentiation, and demonstrates enhanced formation of bone and cartilage tissue and their integration in rabbit mandibular condyles.113 In humans, HA/collagen blocks have been successfully used in TMJ ankylosis with PRP to regenerate a new functioning condyles in a case series (n = 19) of children and adolescents.114 This is a promising initial finding but longer-term clinical studies in a larger cohort of patients are required. In conclusion, the use of cartilage TE in the craniofacial region is expanding, but there are unique challenges to overcome before the more widespread use of engineered cartilage is possible for reconstruction of the nose, ears, and TMJ.
SKIN
The skin is an important tissue in the craniofacial region. In addition to its barrier functions it provides the insertion for facial muscle which is important in communication. Given the face is the most exposed part of the body, the facial skin is frequently damaged in traumatic, congenital, and neoplastic processes. Disruption of the epithelial contiguity impairs the skin’s barrier, pigmentary, thermoregulatory, mechanical, and cosmetic functions.115 Wounds that penetrate beyond the epidermis in adult mammals heal by scarring, forming tissue that is of inferior functional quality to that of normal skin.115 Skin was one of the first tissues to be successfully engineered and FDA-approved, and there are currently a number of skin substitutes available for clinical use (Fig. 4).
FIGURE 4.
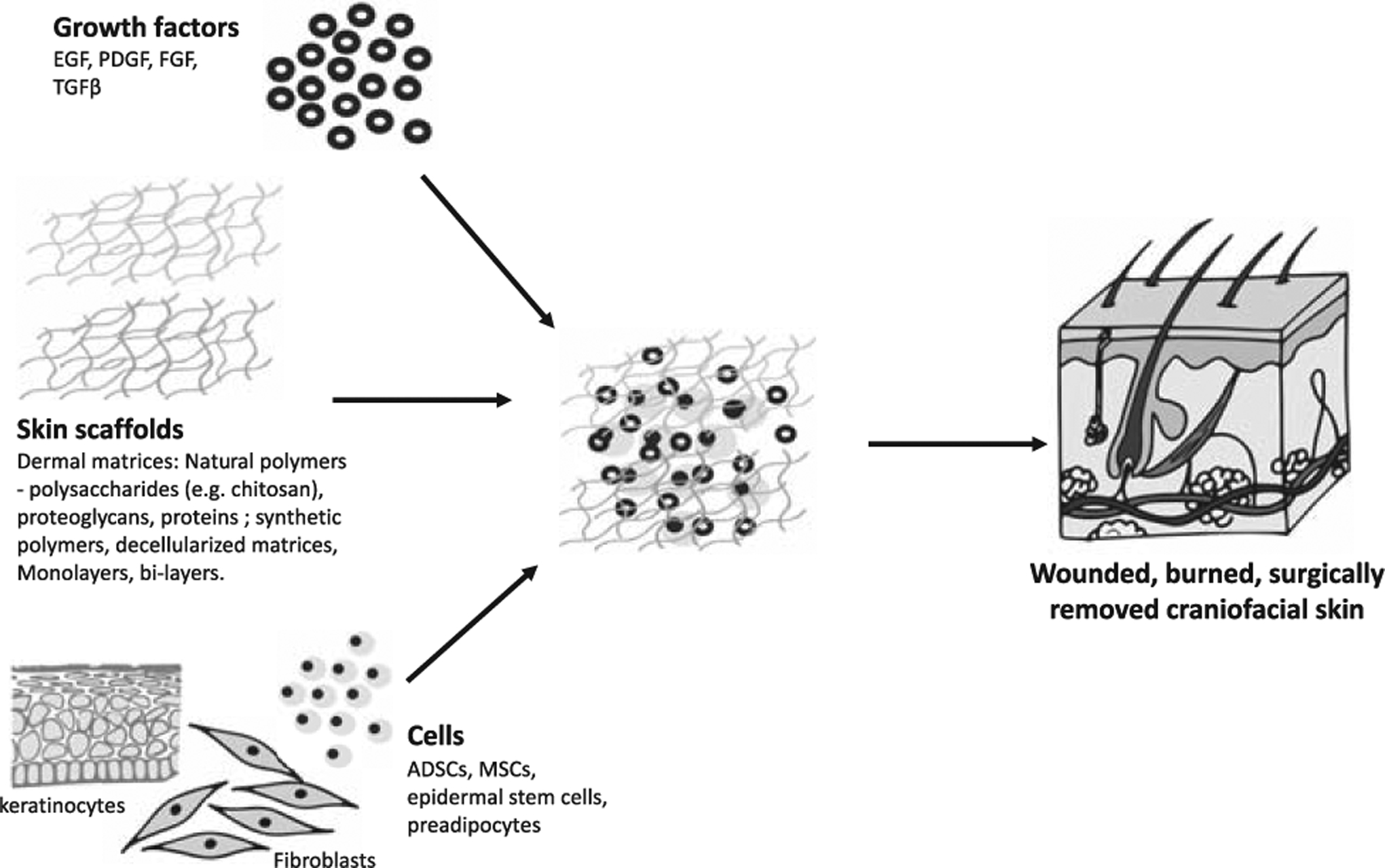
Skin tissue engineering.
The first skin substitutes were biodegradable porous matrices that emulated the dermis and functioned as templates for dermal regeneration. Typically, these matrices are placed on the wound bed, and promote healing by increasing adherence and proliferation of regenerative cells, and by acting as a vehicle for drug delivery. After sufficient integration and vascularization, the matrices are covered with autografts.116 Natural and synthetic biomaterials can be used for dermal matrices. Natural polymers include polysaccharides (eg, chitosan), proteoglycans, proteins (eg, collagen). These materials are biocompatible and biodegradable with similar composition to the ECM.117,118 Integra was the first commercially available skin substitute. It is made of cross-linked collagen and chondroitin-6-sulfate, with a silicon sheet attached to one side which functions as a temporary epidermal layer.119 Integra is primarily used for treating deep burn wounds. It is able to facilitate dermal regeneration while preventing wound contraction, which results in improved wound healing, function, and appearance.120 Natural polymers, however, have variable degradation rates, limited ability to be modified, and can cause immunogenic reactions.121 Decellularized-derived matrices are made by removing cells and keeping the protein component. They preserve native skin architecture, have low immunogenicity, and reduce the risk of disease transmission.119,122 Alloderm is a skin substitute made from decellularized donor skin used both for wound repair and reconstructive surgery. In the craniofacial region, it has been used to reconstruct the eyelid. Alloderm is sufficiently rigid to act as a replacement for the tarsus and also behaves as a scaffold promoting the regrowth of the conjunctiva on its surface.122 Synthetic polymers may be absorbable, such as PLA, PLGA, PGA, PCL, PEG, or nonabsorbable, such as polyurethane, nylon, polytetrafluoroethylene (PTFE), and polyethylene terephthalate. Synthetic polymers are cheaper, more homogeneus, bypass the immunogenic effects, and can easily be manipulated to exhibit controlled GF release or antimicrobial effects.123–126 Synthetic polymers have been shown to accelerate wound closure in diabetic patients.127 Nanomaterials are extremely versatile with regards to fabrication and design methodology permitting the modification and customization of material properties to suit the wound repair environment.128 Of the numerous matrix materials, it is difficult to identify which materials would be best for widespread clinical translation.
Subsequent approaches to skin TE focused on developing keratinocyte culture techniques to produce live cultured skin products. Traditionally, keratinocytes were cultured into epidermal autografts (CEA) or seeded onto natural or synthetic dermal scaffolds.129,130 Epicel is an example of a cultured autologous epidermis first produced in 1988. Without a dermal layer, these scaffolds are thin, fragile, and lack elasticity, suppleness, and tensile strength. Epicel and epidermal constructs are used for major burns wherein very little autologous viable skin remains. Dermoepidermal skin substitutes (DESS), containing both epidermal and dermal layers, were subsequently developed. In addition, the inclusion of fibroblasts in dermal substitutes was found to improve wound healing.131,132 Autologous skin cells are used where possible but several weeks are required before skin biopsy cells are sufficiently expanded for grafting.119 Commercial skin substitutes such as Apligraf and Dermagraft, therefore, use allogenic epidermal and dermal cells and can be immediately applied to injured skin.133 Dermagraft consists of allogeneic neonatal dermal fibroblasts cultured on a polyglactin mesh. As the mesh degrades, the cells produce de novo ECM matrix proteins.119 Apligraf consists of neonatal foreskin fibroblasts and keratinocytes cultured to form dermal and epidermal layers.119 The fibroblasts are first mixed with type 1 collagen to form a strong network of cells and matrix proteins, and the keratinocytes are then seeded onto the construct and form stratified layers. Dermagraft and Apligraf are ultimately rejected but before rejection can help heal the cutaneous layers.134,135 “Minced micrografting” is a new approach where a small full-thickness skin sections are removed from the patient, minced, mixed with hydrogel, and applied onto the wound. It is cheap, simple, and effective in grafting large wounds with little donor skin amounts.136
A number of GF families are integral to endogenous wound healing and may promote healing if applied at specific time points during injury. Topical application of epidermal GF (EGF) in a double-blind clinical trial (n = 12) accelerated the epidermal regeneration of skin graft donor sites.137 A double-blind RCT (n = 118) found that topical application of PDGF safely and effectively stimulated healing of chronic full-thickness diabetic ulcers.138 FGF accelerated the healing of chronic wounds in 2 RCTs (n = 58, n = 50 respectively).139,140 Stromal cell-derived factor 1141 and TGFβ142 have shown beneficial effects in animal studies and results remain to be translated to humans. Topical factors must be able to withstand degradation by the wound’s proteolytic environment.143 The method by which to deliver GF to achieve maximum therapeutic effect remains to be determined.128
The skin substitutes in current clinical use have established milestones in the treatment of skin disorders,116,144 and address the primary therapeutic concern which is rapid recovery of the skin’s barrier function to diminish water loss and prevent infection. However, these substitutes are not routinely used because of their high cost, limited effectiveness, and the inability to fully recreate the functions and aesthetics of skin.145 Long-term success is limited by the absence of self-renewing stem/progenitor cells.146 A number of animal studies have demonstrated the benefit of the topical application of ADSCs147–151 and BMSCs152–155 on healing wounds. A small number of clinical studies have shown that autologous BMSCs help to heal chronic wounds.156–159 Two randomized studies found that intramuscular injection of BMSCs into the limbs containing chronic ulcers, in addition to the topical application of cultured autologous BMSCs on the ulcers, decreased wound size (n = 41, n = 24, respectively).157,160 Autologous ADSCs, topically applied to wounds resulting from radiation injuries, using an artificial dermis and supplemented with FGF, helped to heal the chronic wounds (n = 10).161 ADSCs differentiated into adipocytes and injected into depressed scars resulted in long-term restoration of volume (n = 17).162 ADSCs may also promote skin rejuvenation and the repair of atrophic and photo-damaged skin, perhaps through the secretion of cytokines and GFs that stimulate dermal fibroblasts to synthesize collagen, a process that decreases in skin aging.163 Subcutaneous injection of ADSCs in hairless aged mice increased dermal thickness and collagen density,162 increased angiogenesis, and reduced UVB-irradiated induced wrinkles.164,165 In one clinical study, autologous lipoaspirate composed of around 25% ADSCs was injected intradermally into the photo-aged skin of a patient, and improvement was noted in skin texture and wrinkles at 2 months with increase in dermal thickness detected with ultrasound.163 These initial clinical studies are promising but are few in number and limited by sample size and long-term follow-up.166 They remain to be validated in larger studies conducted with more patients.
Another limitation of currently available skin substitutes is their inability to carry out the functions of normal skin owing to missing dermal appendages.115,116 The sweat and sebaceous glands, hair follicles, adipose tissue, Langerhans cells, and neurovasculature are responsible for thermoregulation, insulation, sensation, ultraviolet protection, and the esthetic appearance of skin. Inclusion of progenitor cells may facilitate generation of dermal components. Hair follicle cells are skin progenitor cells able to reconstitute all components of the cutaneous epithelium,167 and facilitate re-epithelialization as well as the formation of sebaceous glands and hair follicles following cutaneous wounding.168–173 Application of hair follicle cells to wounds in animal studies reduces time for closure and forms skin with cycling hair follicles.169,174,175 A preliminary clinical study (n = 10) demonstrated that inclusion of hair follicles in skin grafts reduces the wound size in chronic leg wounds with evidence of increased re-epithelialization and vascularization on histology at 18 weeks.176 An RCT (n = 12) demonstrated that implantation of skin grafts containing scalp hair follicles reduced wound size of chronic wounds compared to non-hairy skin grafts and formed hair-bearing skin.177 No clinical studies have attempted to reconstitute scalp hair, and this remains a subject of future investigation. Hair follicles are also able to guide nerve migration both in vitro and in vivo and their inclusion in skin grafts enhances the innervation of tissue-engineered skin.178 Full restoration of sensation in engineered skin, however, has not yet been demonstrated. To form pigmented skin, keratinocytes, fibroblasts, and melanocytes were co-transplanted subcutaneously into mice and rats and were demonstrated to successfully form pigmented skin with functioning melanocytes several months after surgery.179–181 Current skin substitutes neglect the subdermal fat layer which results in skin with reduced mobility and more noticeable contour defects. Multilayered skin substitute have been made in vitro by co-culturing preadipocytes and keratinocytes,182 or by simply growing ADSCs, which are able to generate 3-layered skin composites with epidermal, dermal, and hypodermal elements by a self-assembly process.183 Gene therapy is another possible way to augment the regeneration potential of skin cells, and gene-targeted epidermal cells showed long-term regenerative capacity when grafted into immunodeficient mice.184 Many of these preclinical studies, however, remain experimental,185 and although skin TE has undergone substantial development, there remains a number of areas need to be elucidated before it is possible to engineer fully functional adult human skin, and to create skin capable of healing without scarring.
ADIPOSE TISSUE
Fat transplantation is a technique used for a variety of reconstructive procedures in the craniofacial region, including the treatment of contour and soft tissue alterations following trauma, infection, radiation therapy-related, involutional disorders such as hemifacial atrophy, or for cosmetic procedures such as lip augmentation and wrinkle therapy. Fat transplantation, however, is limited by variable resorption rates186 and partial necrosis, which can lead to shape and volume loss with time and unreliable long-term outcomes.187–189 Late enlargement of facial fat grafts can also occur with overall patient weight gain and requires surgical intervention. Adipose TE bypasses these issues (Fig. 5). Preclinical and early clinical series show that ADSCs enhance soft tissue augmentation and survival of fat grafts.190–192 BMSC and ADSC-seeded hydrogel or collagen scaffolds promote adipogenesis and show retention of form for up to four weeks in mice.193,194 In humans, the addition of ADSCs to aspirated fat before lipotransfer (“cell-assisted lipostransfer” or CAL) improves survival and vascularity of the adipose used to augment human breast tissue.192,195,196 This has become a popular technique in plastic surgery. In the craniofacial region, BMSCs (n = 10)197 and ADSCs (n = 5)198 have been successfully used to enhance fat grafting into the face of human patients with Parry-Romberg syndrome, a condition characterized by progressive hemifacial atrophy of skin, dermis, subcutaneous fat, muscle, cartilage, and bone. Additionally, a RCT reported that ADSC grafting was effective, safe, and superior to conventional lipoinjection for facial recontouring in patients with craniofacial microsomia.199 CAL was found to improve volume and symmetry in a patient with Parry-Romberg syndrome.200 CAL has also been used in facial augmentation operations to recontour the faces of patients with lupus erythematosus profundus (n = 3),201 and in face-lift procedures (n = 9).202 CAL is effective, safe, and potentially superior to conventional lipoinjection or fat grafts. Compared to autologous fat grafts, grafts supplemented with ADSCs underwent less resorption when assessed by CT in patients with hemifacial atrophy.198 An RCT (n = 20) comparing autologous fat grafts with CAL found patients receiving CAL required no further treatments, whereas those receiving fat grafts required multiple treatments.203 The micro-RNA21 in ADSCs has been found to be regulated by EGF, and addition of EGF can therefore increase proliferation and inhibit the apoptosis of ADSC.204 These preliminary studies thus suggest that ADSCs improve retention capabilities of transplanted fat in a minimally invasive therapy for facial tissue deformity. The efficacy of ADSC-based adipose TE treatments, however, remains to be evaluated for safety and efficacy in large randomized double-blind controlled trials. The FDA considers autologous ADSCs to be a “drug” because collagenase enzyme is used to separate the ADSCs from lipoaspirates, and CAL-based therapies therefore require complete regulation.205 The 2 major limiting factors in facial CAL include lack of cell survival and vascularization.206
FIGURE 5.
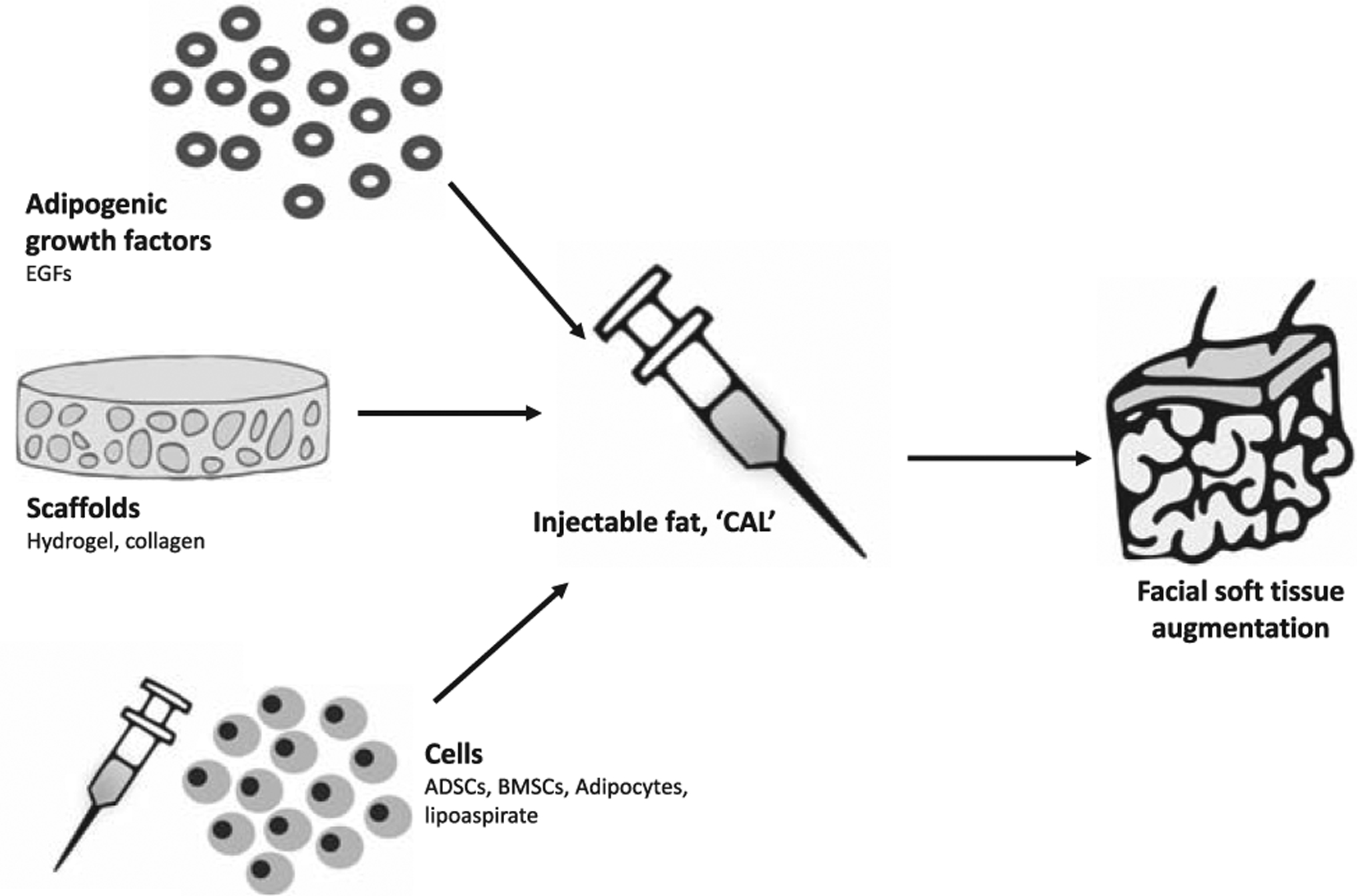
Facial adipose tissue engineering.
PERIPHERAL NERVES
The facial nerve is susceptible to injury during parotid tumor surgery, trauma, and petrous bone surgery, or may be congenitally absent. Facial nerve defects can lead to functional movement deficits and facial asymmetry, which can significantly affect quality of life. When primary nerve end coaptation is not possible, autografting is the criterion standard surgical reconstruction,207 but is limited by availability of donor nerves and the morbidity associated with grafting, such as scarring, infection, and pain. Neuronal TE has been increasingly used in preliminary studies to regenerate the facial nerve (Fig. 6). The cells used in neural TE include neural stem cells (NSCs) which are able to promote nerve regeneration in animals,208 but are difficult to isolate. Instead, BMSCs209 and ADSCs210 can be transdifferentiated into cells similar to Schwann cells which provide trophic, structural, and directional support to regenerating axons in the peripheral nervous system. BMSCs and ADSCs secrete trophic factors and are able to produce myelin-forming which can establish the supportive microenvironment for nerve regeneration.211,212 ADSCs express genes that belong to the glial phenotype and are responsible for neuron metabolism and function,213 and their secretome includes neurotrophic factors such as nerve GF (NGF), glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor.214,215 A number of animal studies have used ADSCs, BMSCs, or dental pulp cells to promote regeneration of the facial nerve.216–224 Uncultured stromal vascular cells seeded onto nerve conduits extending from the facial nerve were able to promote more nerve regeneration in rats than the nerve conduit alone.225 Gene therapy has shown promise as a means of enhancing neural regeneration by promoting overexpression of neurotrophic factors, but durable improvements in functional outcomes and the consequences of vector-mediated gene delivery remain unknown.226 Biomaterial scaffolds can guide axon regeneration.227 The nerve conduits are designed as cylindrical tubes with internal channels or matrices, constituting porous walls to guide regeneration. Different material conduits (glasses, collagen, PGA) have been used,228 and cells or bioactive agents can be incorporated. In studies on facial nerve regeneration, the GF employed basic FGF, delivered using acidic gelatin hydrogel microspheres to enhance its half-life,229 GDNF,216 and NGF.222 Methods to improve delivery and bioavailability are needed.230 Translational studies are required as considerable optimization of these therapies will be required for their potential to realize in their clinical potential.228
FIGURE 6.
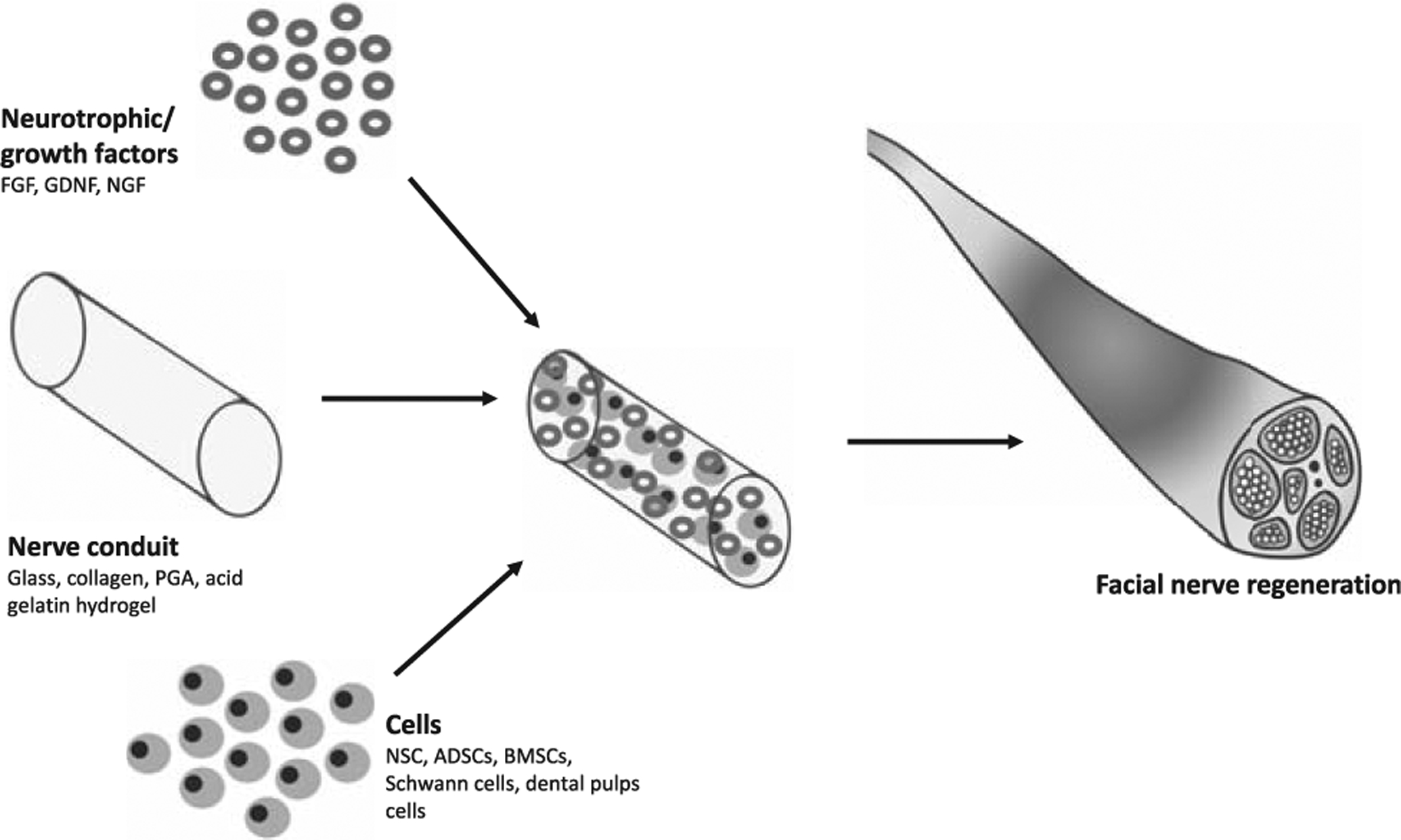
Facial nerve tissue engineering.
BLOOD VESSELS
Sufficient vascularization is a challenge common to all engineered tissues, except cartilage which is avascular, and becomes especially important when using 2D or 3D constructs.231 Scaffold microarchitecture significantly influences the ability of engineered tissue to become vascularized. Scaffold pore sizes of 150 to 500 mm are recommended to support vascularization and blood vessel invasion.232 Another option is co-transplantation of endothelial progenitor cells (EPCs) with MSCs which has shown to improve vascularization of bone and muscle. In critical-sized bone defects in animals, co-transplantation of EPCs and MSCs improved vascularization and was associated with increased release of VEGF.233–235 Alternatively, vasculogenic and stimulatory ligands, such as VEGF and erythropoietin, can be added to synthetic scaffolds, to encourage neovascularization post-implantation.236 In bone scaffolds, controlled release of VEGF from scaffolds can induce a more organized vasculature compared to the vasculature associated with uncontrolled VEGF release.237 Despite these options, vascularization of large TE constructs remains a major limiting factor for organ engineering. The concept of axial vascularization may provide a promising strategy to overcome this challenge in the future. Axial vascularization of TE constructs may be provided by the principles of prelamination238 and prefabrication.239 Prelamination involves the implantation of a nonvascularized TE construct into a highly vascularized territory (eg, a flap) which then serves to create an axially vascularized unit suitable for free transplantation via its pedicle. Warnke et al240 showed that axially vascularized flaps may serve as an “in vivo bioreactor” for ex vivo engineered bone constructs. For mandibular reconstruction after cancer resection, they loaded a computer-designed custom titanium mesh with HA blocks coated with recombinant human bone morphogenetic protein-7 and bone marrow-derived MSCs. Using the principle of prelamination, the construct was implanted into the patient’s latissimus dorsi muscle for a period of 7 weeks to promote heterotopic bone growth and vascularization from the thoracodorsal artery before successful inset into the bony defect.240,241
Prefabrication of TE constructs is achieved by implantation of vascular pedicles, such as arteriovenous (AV) bundles or AV loops, thus enabling the vascularization of bone or soft-tissue constructs, which then can be transplanted into distant defect areas.242 This technique has shown promising results for mandibular reconstruction in large animal models.243
CHALLENGES AND LIMITATIONS
Despite the significant progress in techniques of TE/RM in recent years, there are still considerable challenges, which have to be addressed before successful translation into routine clinical applications can be achieved. Although a combination of different cell types, GFs, and scaffolds builds the foundation of many promising TE approaches, the successful vascularization of TE constructs remains a major limiting factor for TE of large volumes or whole organs. As adequate perfusion is a crucial determining factor for the development and host integration of TE constructs, the translation of TE concepts into clinical applications depends on the success of future strategies to improve vascularization.
A further limitation which remains to be systematically analyzed in future studies is the oncologic safety of the components used for tissue engineering approaches such as GFs, cell types, and scaffolds. Especially for ADSCs, which may be a potential treatment strategy for defect reconstruction following oncologic resection or irradiation, oncologic safety concerns have been raised. ADSCs used in CAL may promote tumor growth and recurrence through stimulatory paracrine actions with in vitro and in vivo animal studies suggesting a prooncologic effect of ADSCs.244 To date, only few studies have investigated the use of ADSCs in craniofacial reconstruction of congenital anomalies200 and long-term data on oncologic safety are not available.
Functional reconstruction of tissues or organs is a further limitation of TE/RM. Owing to missing dermal appendages like sebaceous glands, hair follicles, as well as neurovascular structures, skin substitutes are yet unable to recreate fully functional skin layers. Substantial progress is still needed in all areas of TE, to translate functional TE applications from bench to bench side.
The conclusions that can be drawn from the current literature are limited variations in study methodology regarding the nature of cells, different scaffolds, and defect characteristics. Future more standardized comparative studies are needed to identify cell types, scaffolds, and GFs with high effectiveness for certain clinical scenarios.
FUTURE PERSPECTIVES
Recent technologic advances which have been proven to be particularly valuable to the field of craniofacial reconstruction are 3D printing of biomaterials and nanotechnology.
3D printing is a rapidly emerging technology which enables the organization of a template into an appropriate 3D structure using computer-enabled printers and has the potential to replace more complicated processes of template fabrication in TE. In craniofacial reconstruction, the use of 3D printing for calvarial bone TE can produce porous structures with superior interconnectivity and fabricate custom templates for calvarial bone defects with specific anatomic shapes.245 Published case series in human patients have shown high success rates with a limited number of complications despite being of high methodological bias.246
The use of nanomaterials such as nanoparticles, nanotubes, or nanofibers has been shown to improve mechanical properties of scaffolds, increase cellular attachment, and facilitate tissue regeneration. Several studies have shown improved biomechanical and biochemical properties of nanomaterials highlighting a great potential for TE in various tissues of the oral and maxillofacial region. With the risk of potential accumulation of nanomaterials in different organs, reliable dose-response and toxicity evaluation techniques, however, are urgently needed before routine clinical applications can be conducted.247,248
CONCLUSION
In craniofacial reconstruction and facial esthetic surgery current concepts of TE and RM provide strategies for reconstruction of several tissue types such as bone, cartilage, soft tissue, nerves, and blood vessels to treat congenital or acquired defects. Significant advances have been made and the results of preclinical and clinical studies are encouraging, however, differences in study methodology limit the conclusions that can be drawn and clinical translation. Early clinical success has been demonstrated with TE of bone and cartilage and experimental results for soft tissue reconstruction are promising. Larger and more systematic studies are required to determine the most effective cell types, scaffold characteristics, and delivery methods before TE and RM principles in craniofacial surgery can be brought from bench to bedside. Further advances will be possible through interdisciplinary collaboration between the fields of molecular biology, polymer chemistry, molecular genetics, materials science, robotics, and mechanical engineering.
Acknowledgments
This work was supported by the NIH grant R01 GM116892.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Greenlee RT, Murray T, Bolden S, et al. Cancer statistics, 2000. CA Cancer J Clin 2000;50:7–33 [DOI] [PubMed] [Google Scholar]
- 2.Streubel SO, Mirsky DM. Craniomaxillofacial trauma. Facial Plast Surg Clin North Am 2016;24:605–617 [DOI] [PubMed] [Google Scholar]
- 3.Gorlin RJ, Cohen MM Jr, Hennekam RC. Syndromes of the Head and Neck. Oxford University Press; 2001 [Google Scholar]
- 4.Scheller E, Krebsbach P, Kohn D. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil 2009;36:368–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooney DJ, Mikos AG. Growing new organs. Sci Am 1999;280:60–65 [DOI] [PubMed] [Google Scholar]
- 6.Gordon CR, Zor F, Siemionow M. Skin area quantification in preparation for concomitant upper extremity and face transplantation: a cadaver study and literature review. Transplantation 2011;91:1050–1056 [DOI] [PubMed] [Google Scholar]
- 7.Wiggins OP, Barker JH, Martinez S, et al. On the ethics of facial transplantation research. Am J Bioeth 2004;4:1–12 [DOI] [PubMed] [Google Scholar]
- 8.Vasilic D, Alloway RR, Barker JH, et al. Risk assessment of immunosuppressive therapy in facial transplantation. Plast Reconstr Surg 2007;120:657–668 [DOI] [PubMed] [Google Scholar]
- 9.Gordon CR, Susarla SM, Peacock ZS, et al. Osteocutaneous maxillofacial allotransplantation: lessons learned from a novel cadaver study applying orthognathic principles and practice. Plast Reconstr Surg 2011;128:465e–479e [DOI] [PubMed] [Google Scholar]
- 10.Moore KL, Dalley AF, Agur AM. Clinically Oriented Anatomy.Lippincott Williams & Wilkins; 2013
- 11.Cendales L, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant 2008;8:1396–1400 [DOI] [PubMed] [Google Scholar]
- 12.Gordon CR, Siemionow M, Papay F, et al. The world’s experience with facial transplantation: what have we learned thus far? Ann Plast Surg 2009;63:572–578 [DOI] [PubMed] [Google Scholar]
- 13.Gosain AK, Persing JA. Biomaterials in the face: benefits and risks. J Craniofac Surg 1999;10:404–414 [DOI] [PubMed] [Google Scholar]
- 14.Salgado AJ, Oliveira JM, Martins A, et al. Tissue engineering and regenerative medicine: past, present, and future. Int Rev Neurobiol 2013;108:1–33 [DOI] [PubMed] [Google Scholar]
- 15.Katari R, Peloso A, Orlando G. Tissue engineering and regenerative medicine: semantic considerations for an evolving paradigm. Front Bioeng Biotechnol 2015;2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 17.Horwitz EM. Stem cell plasticity: the growing potential of cellular therapy. Arch Med Res 2003;34:600–606 [DOI] [PubMed] [Google Scholar]
- 18.Mendelson B, Wong C-H. Changes in the facial skeleton with aging: implications and clinical applications in facial rejuvenation. Aesthet Plast Surg 2012;36:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanbhag S, Pandis N, Mustafa K, et al. Alveolar bone tissue engineering in critical-size defects of experimental animal models: a systematic review and meta-analysis. J Tissue Eng Regen Med 2017;11:2935–2949 [DOI] [PubMed] [Google Scholar]
- 20.Shanbhag S, Shanbhag V. Clinical applications of cell-based approaches in alveolar bone augmentation: a systematic review. Clin Implant Dent Relat Res 2015;17(suppl 1):e17–e34 [DOI] [PubMed] [Google Scholar]
- 21.Padial-Molina M, O’Valle F, Lanis A, et al. Clinical application of mesenchymal stem cells and novel supportive therapies for oral bone regeneration. Biomed Res Int 2015;2015:341327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung MT, Liu C, Hyun JS, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 2013;19:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan CM, Shi Y-Y, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004;22:560–567 [DOI] [PubMed] [Google Scholar]
- 25.Khojasteh A, Behnia H, Hosseini FS, et al. The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report. J Biomed Mater Res B 2013;101:848–854 [DOI] [PubMed] [Google Scholar]
- 26.Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, et al. Human Bone Morphogenetic Protein 2–Transduced Mesenchymal Stem Cells Improve Bone Regeneration in a Model of Mandible Distraction Surgery. Journal of Craniofacial Surgery 2012;23:392–396 [DOI] [PubMed] [Google Scholar]
- 27.Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. Journal of Cranio-maxillo-facial Surgery 2004;32:370–373 [DOI] [PubMed] [Google Scholar]
- 28.Sándor GK, Tuovinen VJ, Wolff J, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice–level adipose stem cells for bone regeneration. J Oral Maxillofac Surg 2013;71:938–950 [DOI] [PubMed] [Google Scholar]
- 29.Meijer GJ, de Bruijn JD, Koole R, et al. Cell based bone tissue engineering in jaw defects. Biomaterials 2008;29:3053–3061 [DOI] [PubMed] [Google Scholar]
- 30.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 2009;38:201–209 [DOI] [PubMed] [Google Scholar]
- 31.Kulakov A, Goldshtein D, Grigoryan A, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med 2008;146:522–525 [DOI] [PubMed] [Google Scholar]
- 32.Sándor GK. Tissue engineering of bone: clinical observations with adipose-derived stem cells, resorbable scaffolds, and growth factors. Ann Maxillofac Surg 2012;2:8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaigler D, Avila-Ortiz G, Travan S, et al. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res 2015;30:1206–1216 [DOI] [PubMed] [Google Scholar]
- 34.Levi B, Hyun JS, Montoro DT, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci 2012;109:20379–20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y-H, Chang Y-H, Sung L-Y, et al. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials 2014;35:4901–4910 [DOI] [PubMed] [Google Scholar]
- 36.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell 2015;160:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan CK, Gulati GS, Sinha R, et al. Identification of the human skeletal stem cell. Cell 2018;175:43–56e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cillo JE, Gassner R, Koepsel RR, et al. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol 2000;90:147–154 [DOI] [PubMed] [Google Scholar]
- 39.Dickinson BP, Ashley RK, Wasson KL, et al. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg 2008;121:209–217 [DOI] [PubMed] [Google Scholar]
- 40.Yuan JW, McCarthy M, Holley SR, et al. Physiological down-regulation and positive emotion in marital interaction. Emotion 2010;10:467–474 [DOI] [PubMed] [Google Scholar]
- 41.Hong P, Boyd D, Beyea SD, et al. Enhancement of bone consolidation in mandibular distraction osteogenesis: a contemporary review of experimental studies involving adjuvant therapies. J Plast Reconstr Aesthet Surg 2013;66:883–895 [DOI] [PubMed] [Google Scholar]
- 42.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012;40:363–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zisch AH, Lutolf MP, Ehrbar M, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 2003;17:2260–2262 [DOI] [PubMed] [Google Scholar]
- 44.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol 2008;26:434–441 [DOI] [PubMed] [Google Scholar]
- 45.Yoshida K, Sumita Y, Marukawa E, et al. Effect of platelet-rich plasma on bone engineering with an alloplastic substitute containing BMP2. Biomed Mater Eng 2013;23:163–172 [DOI] [PubMed] [Google Scholar]
- 46.Huang S, Wang Z. Platelet-rich plasma-derived growth factors promote osteogenic differentiation of rat muscle satellite cells: in vitro and in vivo studies. Cell Biol Int 2012;36:1195–1205 [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez IA, Growney Kalaf EA, Bowlin GL, et al. Platelet-rich plasma in bone regeneration: engineering the delivery for improved clinical efficacy. Biomed Res Int 2014;2014:392398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Backly RM, Zaky SH, Muraglia A, et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A 2012;19:152–165 [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y, Ueda M, Naiki T, et al. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng 2004;10:955–964 [DOI] [PubMed] [Google Scholar]
- 50.Yusop A, Bakir A, Shaharom N, et al. Porous biodegradable metals for hard tissue scaffolds: a review. Int J Biomater 2012;2012:641430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad N, Lyles J, Panchal J. Outcomes and complications based on experience with resorbable plates in pediatric craniosynostosis patients. J Craniofac Surg 2008;19:855–860 [DOI] [PubMed] [Google Scholar]
- 52.Tevlin R, McArdle A, Atashroo D, et al. Biomaterials for craniofacial bone engineering. J Dental Res 2014;93:1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyun JS, Montoro DT, Lo DD, et al. The seed and the soil: optimizing stem cells and their environment for tissue regeneration. Ann Plast Surg 2013;70:235–239 [DOI] [PubMed] [Google Scholar]
- 54.Ward B, Brown S, Krebsbach P. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis 2010;16:709–716 [DOI] [PubMed] [Google Scholar]
- 55.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater 2005;4:518. [DOI] [PubMed] [Google Scholar]
- 56.Petrovic V, Zivkovic P, Petrovic D, et al. Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:e1–e9 [DOI] [PubMed] [Google Scholar]
- 57.Li G, Zhang T, Li M, et al. Electrospun fibers for dental and craniofacial applications. Curr Stem Cell Res Ther 2014;9:187–195 [DOI] [PubMed] [Google Scholar]
- 58.Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target 2007;15:241–252 [DOI] [PubMed] [Google Scholar]
- 59.Bhattarai SR, Bhattarai N, Yi HK, et al. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials 2004;25:2595–2602 [DOI] [PubMed] [Google Scholar]
- 60.WU J-C, LORENZ HP. Electrospinning of biomaterials and their applications in tissue engineering. Nano LIFE 2012;2:1230010 [Google Scholar]
- 61.Tran N, Webster TJ. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater 2011;7:1298–1306 [DOI] [PubMed] [Google Scholar]
- 62.Shanbhag S, Pandis N, Mustafa K, et al. Bone tissue engineering in oral peri-implant defects in preclinical in vivo research: a systematic review and meta-analysis. J Tissue Eng Regen Med 2018;12:e336–e349 [DOI] [PubMed] [Google Scholar]
- 63.Yan X-Z, Yang F, Jansen JA, et al. Cell-based approaches in periodontal regeneration: a systematic review and meta-analysis of periodontal defect models in animal experimental work. Tissue Eng Part B Rev 2015;21:411–426 [DOI] [PubMed] [Google Scholar]
- 64.Yamada Y, Nakamura S, Ito K, et al. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells 2013;31:572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinsella CR Jr, MacIsaac ZM, Cray JJ, et al. Novel animal model of calvarial defect: part III. Reconstruction of an irradiated wound with rhBMP-2. Plast Reconstr Surg 2012;130:643e–650e [DOI] [PubMed] [Google Scholar]
- 66.Kinsella CR Jr, Cray JJ, Smith DM, et al. Novel model of calvarial defect in an infected unfavorable wound: reconstruction with rhBMP-2. Part II. J Craniofac Surg 2012;23:410–414 [DOI] [PubMed] [Google Scholar]
- 67.Nussenbaum B, Rutherford RB, Teknos TN, et al. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther 2003;14:1107–1115 [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Chen S-K, Li L, et al. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat 2015;3:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016;98:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nayyer L, Birchall M, Seifalian AM, et al. Design and development of nanocomposite scaffolds for auricular reconstruction. Nanomedicine 2014;10:235–246 [DOI] [PubMed] [Google Scholar]
- 71.Lee KB, Wang VT, Chan YH, et al. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid—a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singapore 2012;41:511–517 [PubMed] [Google Scholar]
- 72.Chow JC, Hantes ME, Houle JB, et al. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2-to 5-year follow-up study. Arthroscopy 2004;20:681–690 [DOI] [PubMed] [Google Scholar]
- 73.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–895 [DOI] [PubMed] [Google Scholar]
- 74.Komarek J, Vališ P, Repko M, et al. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation: long-term results. Acta Chir Orthop Traumatol Cech 2010;77:291–295 [PubMed] [Google Scholar]
- 75.Shieh S-J, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials 2004;25:1545–1557 [DOI] [PubMed] [Google Scholar]
- 76.Cao Y, Vacanti JP, Paige KT, et al. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 1997;100:297–302discussion 3–4 [DOI] [PubMed] [Google Scholar]
- 77.Isogai N, Asamura S, Higashi T, et al. Tissue engineering of an auricular cartilage model utilizing cultured chondrocyte–poly (L-lactide-(-caprolactone) scaffolds. Tissue Eng 2004;10:673–687 [DOI] [PubMed] [Google Scholar]
- 78.Yanaga H, Imai K, Fujimoto T, et al. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg 2009;124:817–825 [DOI] [PubMed] [Google Scholar]
- 79.Fulco I, Miot S, Haug MD, et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet 2014;384:337–346 [DOI] [PubMed] [Google Scholar]
- 80.Gentile P, Scioli MG, Bielli A, et al. Reconstruction of alar nasal cartilage defects using a tissue engineering technique based on a combined use of autologous chondrocyte micrografts and platelet-rich plasma: preliminary clinical and instrumental evaluation. Plast Reconstr Surg Glob Open 2016;4:e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elsaesser A, Schwarz S, Joos H, et al. Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies. Cell Biosci 2016;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327:449–462 [DOI] [PubMed] [Google Scholar]
- 83.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev 2012;18:301–311 [DOI] [PubMed] [Google Scholar]
- 84.Shirasawa S, Sekiya I, Sakaguchi Y, et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem 2006;97:84–97 [DOI] [PubMed] [Google Scholar]
- 85.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 2005;52:2521–2529 [DOI] [PubMed] [Google Scholar]
- 86.Farrell MJ, Fisher MB, Huang AH, et al. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J Biomech 2014;47:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farrell E, Both SK, Odörfer KI, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 2011;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emans PJ, van Rhijn LW, Welting TJ, et al. Autologous engineering of cartilage. Proc Natl Acad Sci 2010;107:3418–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang N, Liu X, Guan Y, et al. Effects of co-culturing BMSCs and auricular chondrocytes on the elastic modulus and hypertrophy of tissue engineered cartilage. Biomaterials 2012;33:4535–4544 [DOI] [PubMed] [Google Scholar]
- 90.Pleumeekers MM, Nimeskern L, Koevoet WL, et al. Cartilage regeneration in the head and neck area: combination of ear or nasal chondrocytes and mesenchymal stem cells improves cartilage production. Plast Reconstr Surg 2015;136:762e–774e [DOI] [PubMed] [Google Scholar]
- 91.Zhang L, He A, Yin Z, et al. Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials 2014;35:4878–4887 [DOI] [PubMed] [Google Scholar]
- 92.Cai Z, Pan B, Jiang H, et al. Chondrogenesis of human adipose-derived stem cells by in vivo co-graft with auricular chondrocytes from microtia. Aesthet Plast Surg 2015;39:431–439 [DOI] [PubMed] [Google Scholar]
- 93.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem 2006;97:33–44 [DOI] [PubMed] [Google Scholar]
- 94.Yanaga H, Imai K, Koga M, et al. Cell-engineered human elastic chondrocytes regenerate natural scaffold in vitro and neocartilage with neoperichondrium in the human body post-transplantation. Tissue Eng Part A 2012;18:2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toh WS, Liu H, Heng BC, et al. Combined effects of TGF(1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors 2005;23:313–321 [DOI] [PubMed] [Google Scholar]
- 96.Johnstone B, Hering TM, Caplan AI, et al. In vitrochondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998;238:265–272 [DOI] [PubMed] [Google Scholar]
- 97.Handorf AM, Li WJ. Induction of mesenchymal stem cell chondrogenesis through sequential administration of growth factors within specific temporal windows. J Cell Physiol 2014;229:162–171 [DOI] [PubMed] [Google Scholar]
- 98.Buxton AN, Bahney CS, Yoo JU, et al. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A 2010;17:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alhadlaq A, Mao J. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dental Res 2003;82:951–956 [DOI] [PubMed] [Google Scholar]
- 100.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. J Biomed Mater Res Part A 2002;59:63–72 [DOI] [PubMed] [Google Scholar]
- 101.Williams CG, Kim TK, Taboas A, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 2003;9:679–688 [DOI] [PubMed] [Google Scholar]
- 102.Stohler CS. Muscle-related temporomandibular disorders. J Orofac Pain 1999;13:273–284 [PubMed] [Google Scholar]
- 103.Xu L, Polur I, Lim C, et al. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthritis cartilage 2009;17:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Man C, Zhu S, Zhang B, et al. Protection of articular cartilage from degeneration by injection of transforming growth factor-beta in temporomandibular joint osteoarthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:335–340 [DOI] [PubMed] [Google Scholar]
- 105.Hunziker E Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2002;10:432–463 [DOI] [PubMed] [Google Scholar]
- 106.Buckwalter J Articular cartilage injuries. Clin Orthop Relat Res 2002;402:21–37 [DOI] [PubMed] [Google Scholar]
- 107.Smith G, Knutsen G, Richardson J. A clinical review of cartilage repair techniques. Bone Joint J 2005;87:445–449 [DOI] [PubMed] [Google Scholar]
- 108.Weng Y, Cao Y, Arevalo C, et al. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg 2001;59:185–190 [DOI] [PubMed] [Google Scholar]
- 109.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–689 [DOI] [PubMed] [Google Scholar]
- 110.Chen K, Man C, Zhang B, et al. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg 2013;42:240–248 [DOI] [PubMed] [Google Scholar]
- 111.Hausamen J-E. The scientific development of maxillofacial surgery in the 20th century and an outlook into the future. J Craniomaxillofac Surg 2001;29:2–21 [DOI] [PubMed] [Google Scholar]
- 112.Zhu S, Zhang B, Man C, et al. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage 2011;19:743–750 [DOI] [PubMed] [Google Scholar]
- 113.El-Bialy T, Uludag H, Jomha N, et al. In vivo ultrasound-assisted tissue-engineered mandibular condyle: a pilot study in rabbits. Tissue Eng Part C Methods 2010;16:1315–1323 [DOI] [PubMed] [Google Scholar]
- 114.Mehrotra D, Kumar S, Dhasmana S. Hydroxyapatite/collagen block with platelet rich plasma in temporomandibular joint ankylosis: a pilot study in children and adolescents. Br J Oral Maxillofac Surg 2012;50:774–778 [DOI] [PubMed] [Google Scholar]
- 115.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol 2005;23:403–412 [DOI] [PubMed] [Google Scholar]
- 117.Rho KS, Jeong L, Lee G, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006;27:1452–1461 [DOI] [PubMed] [Google Scholar]
- 118.Park CJ, Clark SG, Lichtensteiger CA, et al. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater 2009;5:1926–1936 [DOI] [PubMed] [Google Scholar]
- 119.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Am J Clin Dermatol 2001;2:305–313 [DOI] [PubMed] [Google Scholar]
- 120.Stiefel D, Schiestl C, Meuli M. Integra Artificial Skin( for burn scar revision in adolescents and children. Burns 2010;36:114–120 [DOI] [PubMed] [Google Scholar]
- 121.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS One 2008;3:e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pushpoth S, Tambe K, Sandramouli S. The use of AlloDerm in the reconstruction of full-thickness eyelid defects. Orbit 2008;27:337–340 [DOI] [PubMed] [Google Scholar]
- 123.Oh G-W, Ko S-C, Je J-Y, et al. Fabrication, characterization and determination of biological activities of poly ((-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int J Biol Macromol 2016;93:1549–1558 [DOI] [PubMed] [Google Scholar]
- 124.Pawar MD, Rathna G, Agrawal S, et al. Bioactive thermoresponsive polyblend nanofiber formulations for wound healing. Mater Sci Eng C 2015;48:126–137 [DOI] [PubMed] [Google Scholar]
- 125.Pascual A, Tan JP, Yuen A, et al. Broad-spectrum antimicrobial polycarbonate hydrogels with fast degradability. Biomacromolecules 2015;16:1169–1178 [DOI] [PubMed] [Google Scholar]
- 126.Xie Z, Paras CB, Weng H, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013;9:9351–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004;27:S145–S149 [DOI] [PubMed] [Google Scholar]
- 128.Pang C, Ibrahim A, Bulstrode NW, et al. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J 2017;14:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gallico GG III, O’Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 1984;311:448–451 [DOI] [PubMed] [Google Scholar]
- 130.Carver N, Leigh IM. Keratinocyte grafts and skin equivalents. Int J Dermatol 1991;30:540–551 [DOI] [PubMed] [Google Scholar]
- 131.Lamme EN, Van Leeuwen RT, Brandsma K, et al. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol 2000;190:595–603 [DOI] [PubMed] [Google Scholar]
- 132.Coulomb B, Friteau L, Baruch J, et al. Advantage of the presence of living dermal fibroblasts within in vitro reconstructed skin for grafting in humans. Plast Reconstr Surg 1998;101:1891–1903 [DOI] [PubMed] [Google Scholar]
- 133.Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF() accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 1999;7:201–207 [DOI] [PubMed] [Google Scholar]
- 134.Marston WA, Hanft J, Norwood P, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–1705 [DOI] [PubMed] [Google Scholar]
- 135.Hu S, Kirsner RS, Falanga V, et al. Evaluation of Apligraf( persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen 2006;14:427–433 [DOI] [PubMed] [Google Scholar]
- 136.Boggio P, Tiberio R, Gattoni M, et al. Is there an easier way to autograft skin in chronic leg ulcers?.’Minced micrografts’, a new technique. J Eur Acad Dermatol Venereol 2008;22:1168–1172 [DOI] [PubMed] [Google Scholar]
- 137.Brown GL, Nanney LB, Griffen J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–79 [DOI] [PubMed] [Google Scholar]
- 138.Steed DL. Clinical evaluation of recombinant human platelet–derived growth factor for the treatment of lower extremity diabetic ulcers. J Vasc Surg 1995;21:71–81 [DOI] [PubMed] [Google Scholar]
- 139.Yao C, Yao P, Wu H, et al. Acceleration of wound healing in traumatic ulcers by absorbable collagen sponge containing recombinant basic fibroblast growth factor. Biomed Mater 2006;1:33–37 [DOI] [PubMed] [Google Scholar]
- 140.Robson MC, Phillips LG, Lawrence WT, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 1992;216:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo R, Chai L, Chen L, et al. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cell Dev Biol Anim 2015;51:578–585 [DOI] [PubMed] [Google Scholar]
- 142.Pierce GF, Mustoe TA, Lingelbach J, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol 1989;109:429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 144.Ehrenreich M, Ruszczak Z. Update on dermal substitutes. Acta Dermatovenerol Croat 2006;14:172–187 [PubMed] [Google Scholar]
- 145.Burd A, Ahmed K, Lam S, et al. Stem cell strategies in burns care. Burns 2007;33:282–291 [DOI] [PubMed] [Google Scholar]
- 146.Charruyer A, Ghadially R. Stem cells and tissue-engineered skin. Skin Pharmacol Physiol 2009;22:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kato Y, Iwata T, Washio K, et al. Creation and transplantation of an adipose-derived stem cell (ASC) sheet in a diabetic wound-healing model. J Vis Exp 2017. doi: 10.3791/54539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee EY, Xia Y, Kim WS, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 2009;17:540–547 [DOI] [PubMed] [Google Scholar]
- 149.Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009;62:317–321 [DOI] [PubMed] [Google Scholar]
- 150.Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011;20:205–216 [DOI] [PubMed] [Google Scholar]
- 151.Lu F, Mizuno H, Uysal CA, et al. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg 2008;121:50–58 [DOI] [PubMed] [Google Scholar]
- 152.Uysal CA, Tobita M, Hyakusoku H, et al. The effect of bone-marrow-derived stem cells and adipose-derived stem cells on wound contraction and epithelization. Adv Wound Care 2014;3:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 154.Kwon DS, Gao X, Liu YB, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008;5:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 157.Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–366 [DOI] [PubMed] [Google Scholar]
- 158.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow–derived cells. Arch Dermatol 2003;139:510–516 [DOI] [PubMed] [Google Scholar]
- 159.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008;121:860–877 [DOI] [PubMed] [Google Scholar]
- 160.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36 [DOI] [PubMed] [Google Scholar]
- 161.Akita S, Akino K, Hirano A, et al. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int 2010;2010:532704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim JH, Jung M, Kim HS, et al. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol 2011;20:383–387 [DOI] [PubMed] [Google Scholar]
- 163.Park B-s, Jang KA, Sung J-h, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 2008;34:1323–1326 [DOI] [PubMed] [Google Scholar]
- 164.Kim W-S, Park B-S, Park S-H, et al. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 2009;53:96–102 [DOI] [PubMed] [Google Scholar]
- 165.Kim W-S, Park B-S, Sung J-H. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res 2009;301:329–336 [DOI] [PubMed] [Google Scholar]
- 166.Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 2004;22:411–417 [DOI] [PubMed] [Google Scholar]
- 168.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000;102:451–461 [DOI] [PubMed] [Google Scholar]
- 169.Zheng Y, Du X, Wang W, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol 2005;124:867–876 [DOI] [PubMed] [Google Scholar]
- 170.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–1354 [DOI] [PubMed] [Google Scholar]
- 171.Kobayashi K, Nishimura E. Ectopic growth of mouse whiskers from implanted lengths of plucked vibrissa follicles. J Invest Dermatol 1989;92:278–282 [DOI] [PubMed] [Google Scholar]
- 172.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev 2003;17:1189–1200 [DOI] [PubMed] [Google Scholar]
- 173.Stenn KS, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol 2005;16:493–497 [DOI] [PubMed] [Google Scholar]
- 174.Wu J-J, Zhu T-Y, Lu Y-G, et al. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch Dermatol Res 2006;298:183–190 [DOI] [PubMed] [Google Scholar]
- 175.Ma D, Kua JEH, Lim WK, et al. In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy 2015;17:1036–1051 [DOI] [PubMed] [Google Scholar]
- 176.Jiménez F, Garde C, Poblet E, et al. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen 2012;20:806–814 [DOI] [PubMed] [Google Scholar]
- 177.Martínez M-L, Escario E, Poblet E, et al. Hair follicle–containing punch grafts accelerate chronic ulcer healing: a randomized controlled trial. J Am Acad Dermatol 2016;75:1007–1014 [DOI] [PubMed] [Google Scholar]
- 178.Gagnon V, Larouche D, Parenteau-Bareil R, et al. Hair follicles guide nerve migration in vitro and in vivo in tissue-engineered skin. J Invest Dermatol 2011;131:1375–1378 [DOI] [PubMed] [Google Scholar]
- 179.Hachiya A, Sriwiriyanont P, Kaiho E, et al. An in vivo mouse model of human skin substitute containing spontaneously sorted melanocytes demonstrates physiological changes after UVB irradiation. J Invest Med 2005;125:364–372 [DOI] [PubMed] [Google Scholar]
- 180.Böttcher-Haberzeth S, Biedermann T, Klar AS, et al. Characterization of pigmented dermo-epidermal skin substitutes in a long-term in vivo assay. Exp Dermatol 2015;24:16–21 [DOI] [PubMed] [Google Scholar]
- 181.Böttcher-Haberzeth S, Klar AS, Biedermann T, et al. Trooping the color”: restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr Surg Int 2013;29:239–247 [DOI] [PubMed] [Google Scholar]
- 182.Keck M, Haluza D, Lumenta DB, et al. Construction of a multi-layer skin substitute: simultaneous cultivation of keratinocytes and preadipocytes on a dermal template. Burns 2011;37:626–630 [DOI] [PubMed] [Google Scholar]
- 183.Trottier V, Marceau-Fortier G, Germain L, et al. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008;26:2713–2723 [DOI] [PubMed] [Google Scholar]
- 184.Larcher F, Dellambra E, Rico L, et al. Long-term engraftment of single genetically modified human epidermal holoclones enables safety pre-assessment of cutaneous gene therapy. Mol Ther 2007;15:1670–1676 [DOI] [PubMed] [Google Scholar]
- 185.Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet 2001;358:1445–1448 [DOI] [PubMed] [Google Scholar]
- 186.Cherubino M, Marra KG. Adipose-derived stem cells for soft tissue reconstruction. Regen Med 2009;4:109–117 [DOI] [PubMed] [Google Scholar]
- 187.Stosich MS, Moioli EK, Wu JK, et al. Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape. Methods 2009;47:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials 2006;27:6052–6063 [DOI] [PubMed] [Google Scholar]
- 189.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg 2000;26:1159–1166 [PubMed] [Google Scholar]
- 190.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 2006;12:3375–3382 [DOI] [PubMed] [Google Scholar]
- 191.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 2006;118 (3S):121S–128S [DOI] [PubMed] [Google Scholar]
- 192.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg 2008;32:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng 2005;11:556–566 [DOI] [PubMed] [Google Scholar]
- 194.Stosich MS, Mao JJ. Adipose tissue engineering from human adult stem cells: clinical implications in plastic and reconstructive surgery. Plast Reconstr Surg 2007;119:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthet Plast Surg 2011;35:1022–1030 [DOI] [PubMed] [Google Scholar]
- 196.Yoshimura K, Asano Y, Aoi N, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 2010;16:169–175 [DOI] [PubMed] [Google Scholar]
- 197.Jianhui Z, Chenggang Y, Binglun L, et al. Autologous fat graft and bone marrow–derived mesenchymal stem cells assisted fat graft for treatment of Parry-Romberg syndrome. Ann Plast Surg 2014;73:S99–S103 [DOI] [PubMed] [Google Scholar]
- 198.Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue–derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg 2012;69:331–337 [DOI] [PubMed] [Google Scholar]
- 199.Tanikawa DY, Aguena M, Bueno DF, et al. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg 2013;132:141–152 [DOI] [PubMed] [Google Scholar]
- 200.Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, et al. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg 2012;39:659–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 2008;34:1178–1185 [DOI] [PubMed] [Google Scholar]
- 202.Lee SK, Kim D-W, Dhong E-S, et al. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg 2012;39:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sterodimas A, de Faria J, Nicaretta B, et al. Autologous fat transplantation versus adipose-derived stem cell–enriched lipografts: a study. Aesthet Surg J 2011;31:682–693 [DOI] [PubMed] [Google Scholar]
- 204.Liu Y, Chen X, Qiang S, et al. Effects of EGF on apoptosis of adipose derived stem cells by regulating miRNA-21. Wound Med 2016;12:10–14 [Google Scholar]
- 205.Salibian AA, Widgerow AD, Abrouk M, et al. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg 2013;40:666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma L, Wen H, Jian X, et al. Cell-assisted lipotransfer in the clinical treatment of facial soft tissue deformity. Plast Surg 2015;23:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Millesi H Techniques for nerve grafting. Hand Clin 2000;16:73–91viii [PubMed] [Google Scholar]
- 208.Murakami T, Fujimoto Y, Yasunaga Y, et al. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res 2003;974:17–24 [DOI] [PubMed] [Google Scholar]
- 209.Mantovani C, Terenghi G, Shawcross SG. Isolation of adult stem cells and their differentiation to Schwann cells. Methods Mol Biol 2012;916:47–57 [DOI] [PubMed] [Google Scholar]
- 210.di Summa PG, Kalbermatten DF, Pralong E, et al. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011;181:278–291 [DOI] [PubMed] [Google Scholar]
- 211.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 2007;207:267–274 [DOI] [PubMed] [Google Scholar]
- 212.Xu Y, Liu L, Li Y, et al. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res 2008;1239:49–55 [DOI] [PubMed] [Google Scholar]
- 213.Lattanzi W, Geloso MC, Saulnier N, et al. Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. Biomed Res Int 2011;2011:468705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lopatina T, Kalinina N, Karagyaur M, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 2011;6:e17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Marconi S, Castiglione G, Turano E, et al. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A 2012;18:1264–1272 [DOI] [PubMed] [Google Scholar]
- 216.Shi Y, Zhou L, Tian J, et al. Transplantation of neural stem cells overexpressing glia-derived neurotrophic factor promotes facial nerve regeneration. Acta Otolaryngol 2009;129:906–914 [DOI] [PubMed] [Google Scholar]
- 217.Costa HJZR, Bento RF, Salomone R, et al. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed invivo after six weeks enhance facial nerve regeneration. Brain Res 2013;1510:10–21 [DOI] [PubMed] [Google Scholar]
- 218.Zhang H, Wei YT, Tsang KS, et al. Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J Transl Med 2008;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ghoreishian M, Rezaei M, Beni BH, et al. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg 2013;71:577–587 [DOI] [PubMed] [Google Scholar]
- 220.Sasaki R, Aoki S, Yamato M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 2011;5:823–830 [DOI] [PubMed] [Google Scholar]
- 221.Wang X, Luo E, Li Y, et al. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res 2011;1383:71–80 [DOI] [PubMed] [Google Scholar]
- 222.Guo B-F, Dong M-M. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg 2009;140:159–164 [DOI] [PubMed] [Google Scholar]
- 223.Sun F, Zhou K, Mi W-j, et al. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 2011;32:8118–8128 [DOI] [PubMed] [Google Scholar]
- 224.Watanabe Y, Sasaki R, Matsumine H, et al. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med 2017;11:362–374 [DOI] [PubMed] [Google Scholar]
- 225.Matsumine H, Numakura K, Climov M, et al. Facial-nerve regeneration ability of a hybrid artificial nerve conduit containing uncultured adipose-derived stromal vascular fraction: an experimental study. Microsurgery 2017;37:808–818 [DOI] [PubMed] [Google Scholar]
- 226.Tannemaat MR, Verhaagen J, Malessy M. The application of viral vectors to enhance regeneration after peripheral nerve repair. Neurol Res 2008;30:1039–1046 [DOI] [PubMed] [Google Scholar]
- 227.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci 2009;16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.de Souza Lucena EE, Guzen FP, de Paiva Cavalcanti JRL, et al. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg 2014;72:1001–1012 [DOI] [PubMed] [Google Scholar]
- 229.Matsumine H, Sasaki R, Tabata Y, et al. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regen Med 2016;10:E559–E567 [DOI] [PubMed] [Google Scholar]
- 230.Kemp SW, Walsh SK, Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol Res 2008;30:1030–1038 [DOI] [PubMed] [Google Scholar]
- 231.Cassell CO, Hofer OS, Morrison WA, et al. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plast Surg 2002;55:603–610 [DOI] [PubMed] [Google Scholar]
- 232.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. JBJS 2004;86:1541–1558 [DOI] [PubMed] [Google Scholar]
- 233.Seebach C, Henrich D, Wilhelm K, et al. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant 2012;21:1667–1677 [DOI] [PubMed] [Google Scholar]
- 234.He X, Dziak R, Yuan X, et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One 2013;8:e60473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He T Distinct osteogenic activity of BMPs and their orthopaedic applications. J Musculoskelet Neuronal Interact 2005;5:363–366 [PubMed] [Google Scholar]
- 236.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature 2008;453:338–344 [DOI] [PubMed] [Google Scholar]
- 237.Ehrbar M, Djonov VG, Schnell C, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 2004;94:1124–1132 [DOI] [PubMed] [Google Scholar]
- 238.Pribaz JJ, Fine NA. Prelamination: defining the prefabricated flap—a case report and review. Microsurgery 1994;15:618–623 [DOI] [PubMed] [Google Scholar]
- 239.Epple C, Haumer A, Ismail T, et al. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials 2019;192:118–127 [DOI] [PubMed] [Google Scholar]
- 240.Warnke P, Springer I, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. The Lancet 2004;364:766–770 [DOI] [PubMed] [Google Scholar]
- 241.Boos AM, Loew JS, Weigand A, et al. Engineering axially vascularized bone in the sheep arteriovenous-loop model. J Tissue Eng Regen Med 2013;7:654–664 [DOI] [PubMed] [Google Scholar]
- 242.Schmidt VJ, Wietbrock JO, Leibig N, et al. Collagen-elastin and collagen-glycosaminoglycan scaffolds promote distinct patterns of matrix maturation and axial vascularization in arteriovenous loop-based soft tissue flaps. Ann Plast Surg 2017;79:92–100 [DOI] [PubMed] [Google Scholar]
- 243.Eweida AM, Nabawi AS, Abouarab M, et al. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin Oral Invest 2014;18:1671–1678 [DOI] [PubMed] [Google Scholar]
- 244.Koellensperger E, Gramley F, Preisner F, et al. Alterations of gene expression and protein synthesis in co-cultured adipose tissue-derived stem cells and squamous cell-carcinoma cells: consequences for clinical applications. Stem Cell Res Ther 2014;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lee J-Y, Choi B, Wu B, et al. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013;5:045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Maroulakos M, Kamperos G, Tayebi L, et al. Applications of 3D printing on craniofacial bone repair: a systematic review. J Dent 2019;80:1–14 [DOI] [PubMed] [Google Scholar]
- 247.Lee C-M, Jeong H-J, Yun K-N, et al. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int J Nanomed 2012;7:3203–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li G, Zhou T, Lin S, et al. Nanomaterials for craniofacial and dental tissue engineering. J Dent Res 2017;96:725–732 [DOI] [PubMed] [Google Scholar]


