Abstract
The application of cancer gene therapy has heretofore been restricted to local, or locoregional, neoplastic disease contexts. This is owing to the lack of gene transfer vectors which embody the requisite target cell selectivity in vivo required for metastatic disease applications. To this end, we have explored novel vector engineering paradigms to adapt adenovirus for this purpose.
Our novel strategy exploits three distinct targeting modalities that operate in functional synergy. Transcriptional targeting is achieved via the hROBO4 promoter, which restricts transgene expression to proliferative vascular endothelium. Viral binding is modified by incorporation of an RGD4C peptide in the HI loop of the fiber knob for recognition of cellular integrins. Liver sequestration is mitigated by ablation of Factor X binding to the major capsid protein hexon by a serotype swap approach. The combination these technologies into the context of a single vector agent represents a highly original approach. Studies in a murine model of disseminated cancer validated the in vivo target cell selectivity of our vector agent. Of note, clear gains in therapeutic index accrued these vector modifications.
Whereas there is universal recognition of the value of vector targeting, very few reports have validated its direct utility in the context of cancer gene therapy. In this regard, our report validates the direct gains which may accrue these methods in the stringent delivery context of disseminated neoplastic disease. Efforts to improve vector targeting thus represent a critical direction to fully realize the promise of cancer gene therapy.
INTRODUCTION
A wide range of strategies have been developed to apply gene therapy to the context of neoplastic disease (1–4). The central goal embodied in these molecular interventions is the achievement of an improved therapeutic index compared to conventional cancer therapies. Heretofore, such in vivo cancer gene therapies have been applied for local, or locoregional, neoplastic disease. This is owing to the fact that currently available gene transfer vectors lack the in vivo target cell selectivity mandated for the clinical context of disseminated disease.
In this regard, there has been a field-wide recognition of the need for gene transfer vectors which embody the capacity for target cell selectivity (5). Indeed, an NIH report on gene therapy highlighted this goal as the highest mandate for the field. For cancer, such a vector targeting capacity thus represents the sine qua non for practical advancement of these promising strategies to the problematic clinical setting of metastatic disease. Given this consideration, the paucity of reports of the successful application of vector targeting for cancer gene therapy is noteworthy.
To this end, we have endeavored modification of adenoviral vectors (Ad) to address this key gene delivery mandate. Based on the unique molecular promiscuity of the parent virus, we hypothesized that targeting might be achieved exploiting multiple biologic axes. Further, we sought to combine such distinct targeting strategies to realize functional synergy vis-à-vis the achievement of target cell selectivity. On this basis, the requirement for in vivo selectivity, in the context of disseminated neoplastic disease, might be achieved.
MATERIALS AND METHODS
Adenovirus production
The replication incompetent E1-deleted Ad5 vectors used for study were prepared using a two-plasmid cloning method. Untargeted or triple targeted Ad5 encoding the GFP reporter gene or the HSVtk therapeutic gene were produced in accordance with the standard techniques (6). Briefly, adenoviral genome-including plasmids were digested with PacI for releasing the recombinant viral genomes, and transfected into HEK293 cells. Rescued viruses was serially amplified, and then purified by centrifugation on CsCl gradients according to standard protocols. For in vitro and in vivo study, viruses were dialyzed against phosphate-buffered saline (PBS) containing 10% glycerol, and stored at −80°C. The titers of physical viral particles (vp) were determined by methods described by Maizel et al.(7).
In vitro validation of adenovirus
HUVEC (Human primary endothelial), bEnd-3 (Mouse primary endothelial) and NIH/3T3 (mouse embryonic fibroblast) cells were obtained from the ATCC and maintained for assays according to the manufacturer’s instructions. Total expression levels of HSVtk proteins in whole cell lysate were determined with anti-tk antibody (kindly provided by Dr. Summers) by western blot analysis in accordance with the standard protocols. For HSVtk/GCV killing activity assay, cells were infected with virus encoding either GFP or HSVtk gene, ganciclovir (Selleckchem) prodrug was administered to cells via serial diluted drug concentration. To measure the cellular ATP contents (Promega) as a marker for cell viability, assay plates were read in a microplate luminometer (Berthold detection system) and cell viability was analyzed. Dose-response curve (DRC) analysis curves were plotted by Graph Pad Prism v7.0c software.
Murine xenograft models
Triple immunodeficient NOD/SCID/IL2Rγ (NSG) mice were injected subcutaneously with 1×106 786–0 renal carcinoma cells (mCherry expressed cell line). Two weeks later the mice were intravenously injected with 1×1011 vp of un-targeted Ad5.CMV.GFP or triple targeted Ad-GFP viruses. To perform histopathological analysis in tumor or organs, mice were sacrificed under anesthesia (Avertin, Sigma-Aldrich) at three days post-virus injection. The tumor bearing tissues were harvested, followed by post-fixed in 4% paraformaldehyde for 2 hours at room temperature, cryopreserved in 30% sucrose for 16 hours at 4°C, and cryo-embedded in NEG50 (Thermo Fisher Scientific) over 2-methylbutane/liquid nitrogen. Histopathological images were captured by epifluorescence microscopy. To perform histopathological analysis in SPC6 subcutaneous tumor, all assay procedures were conducted in accordance with same protocols.
Tissue harvest and Immunofluorescence staining
Mice were administrated with 1×10 11 VP of triple targeted Ad via tail-vein injection. Three days post virus infection, mice were anesthetized and tissues (Liver, Lung, Pancreas, Spleen, Kidney, Small Bowel, Heart, Muscle and Brain) harvested for immunofluorescence staining. For frozen sections, organ slices were cryo-preserved in 30% sucrose in PBS at 4oC overnight, embedded in NEG50 mounting medium (Thermo Fisher Scientific), and then frozen in a liquid nitrogen pre-chilled 2-methylbutane containing bucket. Sectioning of frozen organs was carried out using the CryoJane taping system (Leica Biosystems Inc). All frozen section slides were subject to immunofluorescence staining analysis in accordance with the standard techniques (8). Primary antibodies used in this study included hamster anti-CD31 (EMD Millipore), rat anti-endomucin (eBioscience), rat anti-PDGFRβ (eBioscience), rabbit anti-HSVtk (Dr. Summers’s lab) and Alexa Fluor 488 or 594-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories).
Animal studies for therapeutic index gains
Triple immunodeficient NSG mice allow for modeling of all metastatic sites as published previously (8,9). For systemic metastases, mice was injected intracardially with 5×105 786–0 renal carcinoma cells (Luciferase gene expressed). Tumor growth was monitored and quantified using bioluminescence imaging (BLI) analysis. Three weeks after tumor implantation, mice were intravenously injected via tail vein with 1×1011 vp of each virus. During the following 7 days, mice received daily intraperitoneal injection of GCV (50mg/kg/7days) according to the different treatment groups ([n=8 mice/group]: un-targeted Ad5-GFP, un-targeted Ad5-HSVtk and triple targeted Ad-HSVtk plus GCV). All mice in each group were monitored for body weight during the treatment time and compared the survival time. For study of tumor progression, final ROI values were monitored and compared (Increased fold=last ROl value/initial ROl value) in the survival mice. Survival data was plotted on a Kaplan-Meier curve in all animal groups by Graph Pad Prism v7.0c software. The studied was terminated at 8 weeks (56 days post-tumor implantation) according to approved protocols and pursuant to NIH guidelines for the care and use of laboratory animals standards. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Washington University in Saint Louis, School of Medicine (protocol #.20160292).
RESULTS
Generated triple targeting vector for gene therapy
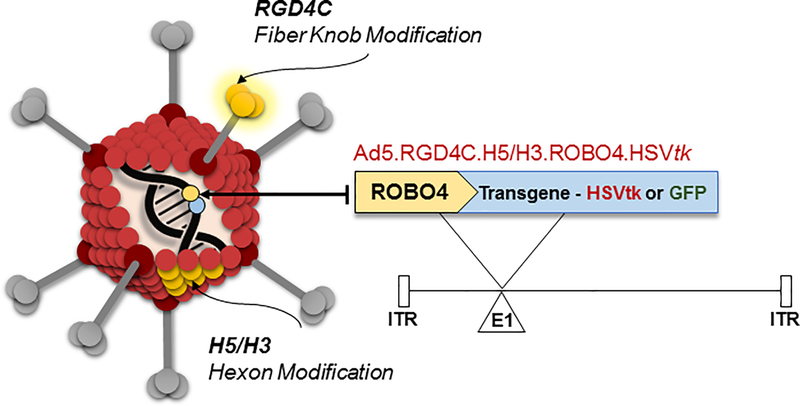
As the main limit to the systemic employment of Ad is liver sequestration (10), we first sought to address this issue. Our studies, and those of others, had linked this phenomenon to vector capsid association with serum factor X (11,12). On this basis, we employed a strategy of capsid chimerism whereby the major capsid protein hexon of the serotype 5 vector base was substituted with a cognate from the alternate human adenovirus serotype 3. We had previously shown that this modification substantially mitigated sequestration in the reticuloendothelial system (RES) in the context of systemic vector administration (12,13). This liver “un-targeting” method was then combined with modifications designed to direct the expression of delivered transgenes to tumor vascular endothelium. Specific vector particle binding was enhanced for the target cells by the incorporation into the Ad capsid fiber knob of the RGD4C peptide (14,15). This ligand exhibits preferentiality for integrins of αvβ3 and αVβ3 class over-expressed in angiogenic endothelium. This approach was also combined with a transcriptional targeting strategy to restrict transgene expression target cells. In this case, the promoter of the roundabout guidance receptor 4 (ROBO4) gene, which is selectively inductive for proliferative endothelium, was employed. We hypothesized that the combination of the liver un-targeting and vector targeting methods (Fig. 1) would provide functional synergy with respect to the achievement of the in vivo selectivity mandated for disseminated neoplastic disease cancer gene therapy.
Figure 1. Schema of ‘triple-targeted’ genetic modifications of adenovirus to accomplish in vivo targeting of tumor endothelium.
Liver un-targeting has previously been accomplished via genetic modification of hexon domains that can associate with serum factor X. Hexon ectodomain hypervariable regions (HVR7) of the vector was replaced base human adenovirus serotype 5 with the corresponding domains from human adenovirus serotype 3 (H5/H3). In addition, for transcriptional targeting, the 5’ upstream region of the therapeutic gene for roundabout 4 (ROBO4; endothelial specific promoter) was configured within the deleted adenovirus E1A/B site. The integrin targeting peptide RGD4C (CDCRGDCFC) was incorporated into HI loop of Ad fiber knob for transductional targeting.
Evaluated vector for cancer gene therapy
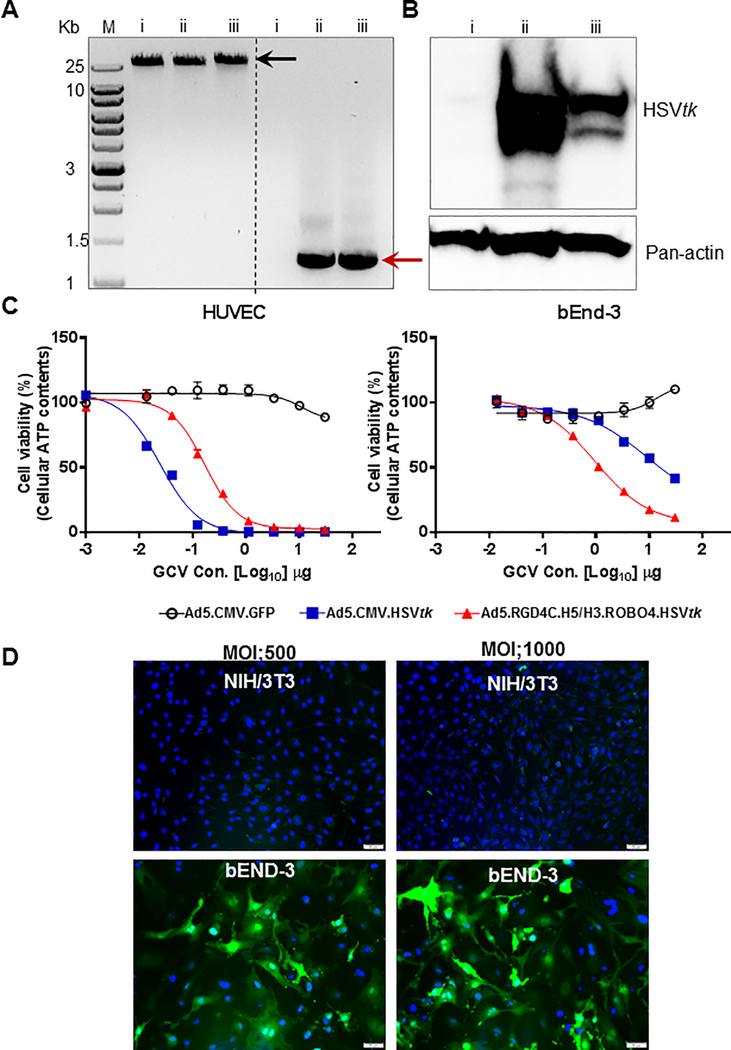
Such a triple targeted Ad was thus constructed to achieve expression of the HSVtk therapeutic gene selectively within proliferative tumor endothelium in vitro. This gene has been applied historically for molecular chemotherapy cancer gene therapy approaches (16,17). Its employment here thus served to reconcile our findings with the extensive literature relating to the use of HSVtk for cancer gene therapy. After rescue and upscale, the virion was subject to genomic analysis (Fig. 2A). This study confirmed that the control un-targeted adenovirus, and the triple targeted adenovirus, both contained the cassette with the HSVtk therapeutic gene. Infection of human endothelial cells (HUVEC), with follow-on Western blot analysis, confirmed expression of HSVtk in cells infected via both vectors (Fig. 2B). Next, analysis of the specificity of expression was carried-out utilizing an in vitro assay whereby HSVtk expression sensitized cells to the cytotoxic effects of GCV. Target cells were human endothelial (HUVEC) or murine endothelial cells (bEnd-3). In this analysis, it could be seen that the target cells were sensitive to vector-mediated cytotoxicity exclusively after infection with the triple targeted Ad encoding HSVtk (Fig. 2C), under GCV treatment. In addition, regarding to the transduction efficacy, we confirmed with NIH/3TC cells (mouse embryonic fibroblast) including demonstration of the target cells specificity (Fig. 2D). These studies thus validated the endothelial selectivity of our vector targeting schema.
Figure 2. In vitro validation of adenoviral vectors.
A, Assessment of inclusion the HSVtk gene into E1 region from purified viral genomes (i Ad5, ii Ad5.CMV.HSVtk and iii triple targeted Ad-HSVtk) by polymerase chain reaction (PCR) using HSVtk specific primers. Viral genomes were shown in black arrow, and amplified HSVtk gene from each of the viral genomes were shown in red arrow. B, Validation of HSVtk gene delivery and expression via Ad vectors in HUVEC cells using western blotting analysis. C, Evaluation of cell killing efficiency by HSVtk/GCV treatment on endothelial cells. Each of HUVEC (human) and bEnd-3 (mouse) were infected with each virus encoding either GFP or HSVtk and then treated with Ganciclovir (GCV) prodrug (serial diluted drug concentration shown in x-axis). Results are presented as the relative percentage of cell viability measured by cellular ATP contents. D, Assessment of transduction efficacy in vitro. Each of NIH/3T3 (mouse embryonic fibroblast) and bEnd-3 (mouse endothelial cell) were infected with triple targeted Ad5-GFP with two different MOI (500 and 1000). Blue: nuclei (stained with DAPI), and green: GFP signal through Ad vectors.
Advanced vector targeting to tumor in vivo
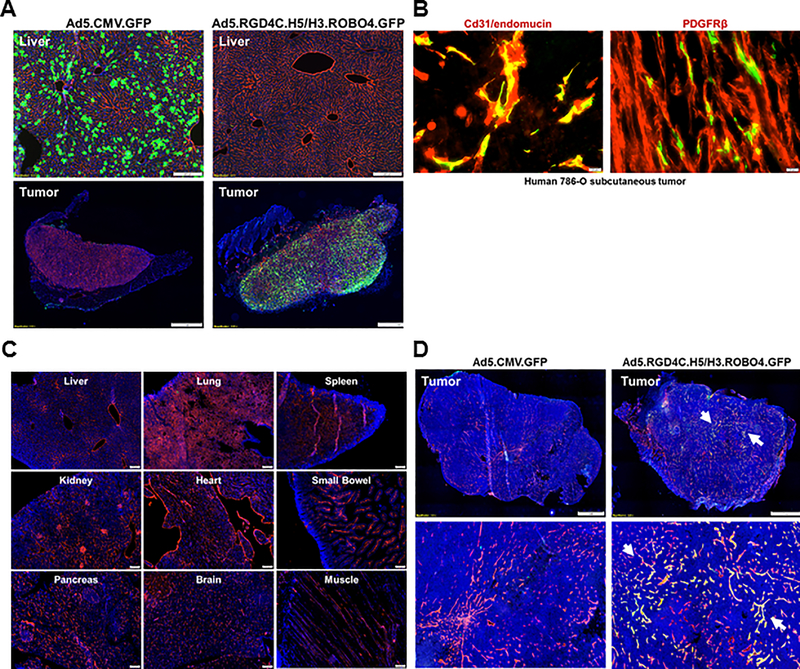
We next sought to validate the in vivo selectivity of our vector methods in a systemic delivery context. For this analysis, we derived versions of the control and triple targeted adenovirus which encoded the GFP reporter gene. Immunodeficient NOD/SCID/IL2Rγ (NSG) mice were xenografted with subcutaneous nodules of the human renal carcinoma cell line 786–0 (8). Animals were then challenged with 1×1011 particles of the control and targeted Ad with analysis of harvested tumor and organs at 3 days later. As the major site for ectopic Ad localization is the liver, analysis was limited to this site and target tumor. In these studies it could be seen that untargeted Ad was largely sequestered in the liver, with no detectable reporter noted within vascular areas of the tumor (Fig. 3A). In marked contrast, the triple targeted Ad avoided ectopic localization within the liver and spleen. Of note, high levels of reporter gene were seen within the harvested subcutaneous tumor nodules via secondary staining analysis confirmed the identity of gene modified cells as tumor endothelium (Fig 3B). Our findings confirm that the hexon modification of our triple targeted Ad achieves the desired goal of liver un-targeting, without the accrual of major new sites of ectopic gene delivery (Fig. 3C). Furthermore, as an even more stringent model (xenografted with subcutaneous nodules of the syrian golden hamster pancreatic carcinoma cell line SHPC6), it could be seen the targeting specificity of our triple targeted adenovirus in the context of other tumors (Fig. 3D). Thus, our triple targeting strategy achieves highly specific in vivo selectivity.
Figure 3. Evaluation of vector targeting via systemic delivery in murine xenograft models (Triple immunodeficient NOD/SCID/IL2Rγ [NSG] mice).
A, Comparison analysis of targeting capacity with GFP reporter maker by histopathological methods. Experimental procedures for this study was described in material and methods parts in detail. The cryosections of the liver (upper images) and tumor (bottom images) were obtained from each of Ad5 or triple targeted Ad5-GFP injected mice at three days later. All assay were conducted and compared in parallel conditions. Blue: nuclei (stained with Hoechst 33258), Red: mouse CD31/endomucin in the liver, or mcherry signal from the tumor and Green: GFP signal through Ad vectors. B, In vivo confirmation of triple targeted Ad5-GFP transduction on tumor endothelium. The cryosection of the tumor (Human 786–0 subcutaneous) was obtained from triple targeted Ad5-GFP infected mice (same as Figure 3A), and were subject to immunofluorescence staining analysis with specific antibodies (anti-Cd31/anti-endomucine or anti-PDGFPβ) as an endothelium marker. In the tumor, endothelium and triple targeted Ad5-GFP are co-localized in Yellow. C, In vivo validation of ectopic gene delivery and transduction with our triple targeted Ad5-GFP. NSG mice were intravenously injected with 1×1011 vp of viruses (each of Ad5-GFP or triple targeted Ad5-GFP) and then harvest of all tissues (Liver, Lung, Spleen, Kidney, Heart, Small Bowel, Pancreas, Brain and Muscle) for histopathological assay by immunofluorescence staining. Blue: nuclei (stained with Hoechst 33258), Red: mouse CD31/endomucin. D, Evaluation of targeting specificity of our triple targeted Ad5-GFP in the context of SGH SHPC6 subcutaneous tumor (SHPC6: Syrian Hamster Pancreatic Carcinoma). Experimental procedures for this study was accordance as like Figure 3A. Blue: nuclei (stained with Hoechst 33258), Red: mouse CD31/endomucin in the tumor, and Green: GFP signal through triple targeted Ad vectors. Tumor endothelium and triple targeted Ad5-GFP are co-localized in Yellow (arrow).
Improved vector to achieve the therapeutic index gains in vivo
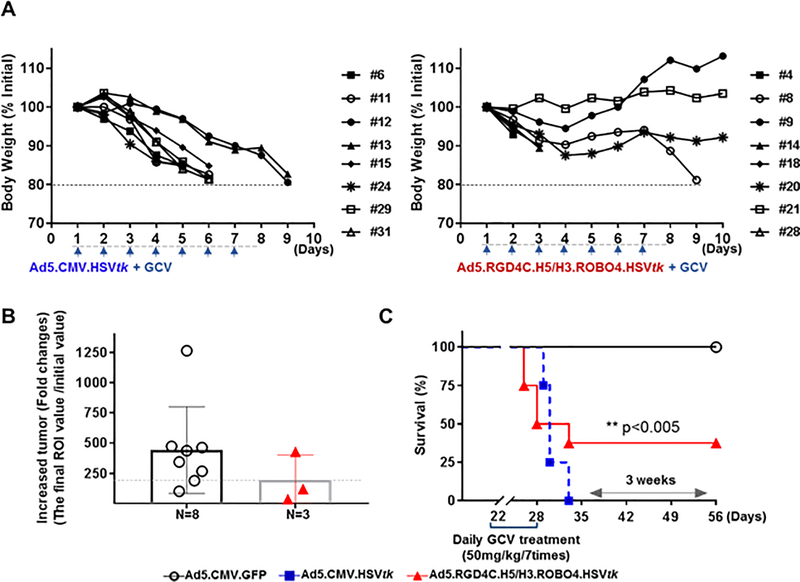
Lastly, we endeavored evaluation of the therapeutic index gains which accrue synergistic targeting. The therapy experiment with targeted adenovirus (vs. non-targeted adenovirus) was employed in a murine model of metastatic disease. The triple targeting specificity mitigated the vector-associated toxicity known to be associated with the systemic application of un-targeted Ad encoding HSVtk (18) (Fig. 4A). In parallel with that, of note, it could be seen that extended survival time accrued to the treatment group that received only the triple targeted Ad encoding HSVtk with significant differences [**p<0.005] (Fig. 4C). Our demonstration of antitumor therapy was accomplished in a context more stringent than that required only for local control, however, it could be seen the potential for improvement the therapeutic effect (Fig. 4B). Also, It could be seen the persistence of the vector in the metastatic tumor as well. These finding demonstrated directly the improved therapeutic index which accrued the employed of vector targeting for this established cancer gene therapy approach.
Figure 4. Assessment of the therapeutic index gains in a metastatic murine models.
A, The immunodeficient NSG mice were intracardiac injected with 5×105 786–0 renal carcinoma cells (Luciferase/mCherry gene expressed cell line). Three weeks after tumor implantation, NSG mice were intravenously injected with 1×1011 vp of viruses [n=8 mice/each of group with Ad5.CMV.GFP, Ad5.CMV.HSVtk and triple targeted Ad-HSVtk], and treated the GCV (50mg/kg/7days by intraperitoneal injection). All mice were monitored the body weight to determine their survival time [>20% loss of initial body weight as a marker for death. The comparative analysis of therapeutic index gains was assessed between the groups by the tumor progression in B, and survival time in C. The statistical significance of differences between data determined as a p-values (**p< 0.05, by Graph Pad Prism v7.0c software). Tumor progression was detected through ROI value (final ROI value divided by initial ROl) by bioluminescence imaging (BLI) analysis, respectively. The final ROl value were only determined with survival mice at the end of study days (at 56 days post-tumor implantation). All mice from Ad5.CMV.HSVtk virus injected group were died at 3 weeks ago due to of hepatotoxicity as shown in A.
DISCUSSION
Our study highlights the direct benefits that may derive from the application of vector targeting methods to the context of cancer gene therapy. In this regard, in vivo cancer gene therapies have been restricted to local diseases contexts owing to the limits of currently available gene transfer vectors. Thus, the problematic clinical context of metastatic cancer has not been addressable to this point via gene therapy methods. Further in this regard, the universal recognition of the potential value of vector targeting has not been translated to the context of cancer gene therapy studies demonstrating therapeutic gains. The advent of our novel vector targeting technology now provides adequate selectivity in vivo to test this hypothesis of field-wide significance. Our early finding reported here thus provide the rationale for future studies designed to apply cancer gene therapy to this problematic clinical context most warranties novel interventions.
Acknowledgements
We thank Dr. Arbeit for providing of critical precedent research. We also appreciated Dr. Summers’s lab by providing HSVtk antibody essential to this study. We also thank Dr. Toth’s lab by providing SHPC6 (Syrian Hamster Pancreatic Carcinoma) cells essential to this study. We especially thank Amanda Baker Wilmsmeyer for extensive advice on the manuscript and the scientific graphics. This study was funded by the National Institutes of Health (NIH; RO1’s CA211096, PI; David T. Curiel) research grants.
List of Abbreviations
- Ad
Adenoviral Vectors
- TI
Therapeutic Index
- HSVtk
Herpes Simplex Virus thymidine kinase
- GCV
Ganciclovir
- ROBO4
Roundabout Guidance Receptor 4
- RCC
Renal Cell Carcinoma
Footnotes
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Hsiao WC, Sung SY, Chung LWK, Hsieh CL. Osteonectin Promoter-Mediated Suicide Gene Therapy of Prostate Cancer. Methods Mol Biol 2019;1895:27–42 doi 10.1007/978-1-4939-8922-5_3. [DOI] [PubMed] [Google Scholar]
- 2.Sun W, Shi Q, Zhang H, Yang K, Ke Y, Wang Y, et al. Advances in the techniques and methodologies of cancer gene therapy. Discov Med 2019;27(146):45–55. [PubMed] [Google Scholar]
- 3.Yamamoto Y, Nagasato M, Yoshida T, Aoki K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci 2017;108(5):831–7 doi 10.1111/cas.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccolo P, Brunetti-Pierri N. Challenges and Prospects for Helper-Dependent Adenoviral Vector-Mediated Gene Therapy. Biomedicines 2014;2(2):132–48 doi 10.3390/biomedicines2020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker AH, Kritz A, Work LM, Nicklin SA. Cell-selective viral gene delivery vectors for the vasculature. Exp Physiol 2005;90(1):27–31 doi 10.1113/expphysiol.2004.028126. [DOI] [PubMed] [Google Scholar]
- 6.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol 1996;70(7):4805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney JA, Hennessey JP Jr. Evaluation of accuracy and precision of adenovirus absorptivity at 260 nm under conditions of complete DNA disruption. Virology 2002;295(2):284–8 doi 10.1006/viro.2002.1406. [DOI] [PubMed] [Google Scholar]
- 8.Lu ZH, Kaliberov S, Sohn RE, Kaliberova L, Du Y, Prior JL, et al. A new model of multi-visceral and bone metastatic prostate cancer with perivascular niche targeting by a novel endothelial specific adenoviral vector. Oncotarget 2017;8(7):12272–89 doi 10.18632/oncotarget.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu ZH, Kaliberov S, Sohn RE, Kaliberova L, Curiel DT, Arbeit JM. Transcriptional targeting of primary and metastatic tumor neovasculature by an adenoviral type 5 roundabout4 vector in mice. PLoS One 2013;8(12):e83933 doi 10.1371/journal.pone.0083933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas LA, Moreno R, Calderon H, Alemany R. Adenovirus coxsackie adenovirus receptor-mediated binding to human erythrocytes does not preclude systemic transduction. Cancer Gene Ther 2016;23(12):411–4 doi 10.1038/cgt.2016.50. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Gordo E, Denby L, Nicklin SA, Baker AH. The importance of coagulation factors binding to adenovirus: historical perspectives and implications for gene delivery. Expert Opin Drug Deliv 2014;11(11):1795–813 doi 10.1517/17425247.2014.938637. [DOI] [PubMed] [Google Scholar]
- 12.Short JJ, Rivera AA, Wu H, Walter MR, Yamamoto M, Mathis JM, et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther 2010;9(9):2536–44 doi 10.1158/1535-7163.Mct-10-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaliberov SA, Kaliberova LN, Hong Lu Z, Preuss MA, Barnes JA, Stockard CR, et al. Retargeting of gene expression using endothelium specific hexon modified adenoviral vector. Virology 2013;447(1–2):312–25 doi 10.1016/j.virol.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilbao G, Contreras JL, Dmitriev I, Smyth CA, Jenkins S, Eckhoff D, et al. Genetically modified adenovirus vector containing an RGD peptide in the HI loop of the fiber knob improves gene transfer to nonhuman primate isolated pancreatic islets. Am J Transplant 2002;2(3):237–43. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Hemminki A, Siegal GP, Barnes MN, Dmitriev I, Krasnykh V, et al. Adenoviruses with an RGD-4C modification of the fiber knob elicit a neutralizing antibody response but continue to allow enhanced gene delivery. Gynecol Oncol 2005;96(2):341–8 doi 10.1016/j.ygyno.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 16.Wildner O, Morris JC, Vahanian NN, Ford H Jr., Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther 1999;6(1):57–62 doi 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- 17.Hodish I, Tal R, Shaish A, Varda-Bloom N, Greenberger S, Rauchwerger A, et al. Systemic administration of radiation-potentiated anti-angiogenic gene therapy against primary and metastatic cancer based on transcriptionally controlled HSV-TK. Cancer Biol Ther 2009;8(5):424–32 doi 10.4161/cbt.8.5.7589. [DOI] [PubMed] [Google Scholar]
- 18.Yi QY, Bai ZS, Cai B, Chen N, Chen LS, Yuan T, et al. HSVTK/GCV can induce cytotoxicity of retinoblastoma cells through autophagy inhibition by activating MAPK/ERK. Oncol Rep 2018;40(2):682–92 doi 10.3892/or.2018.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]