Abstract
Organic matter decomposition plays a major role in the cycling of carbon (C) and nutrients in terrestrial ecosystems across the globe. Climate change accelerates the decomposition rate to potentially increase the release of greenhouse gases and further enhance global warming in the future. However, fractions of organic matter vary in turnover times and parts are stabilized in soils for longer time periods (C sequestration). Overall, a better understanding of the mechanisms underlying C sequestration is needed for the development of effective mitigation policies to reduce land‐based production of greenhouse gases. Known mechanisms of C sequestration include the recalcitrance of C input, interactions with soil minerals, aggregate formation, as well as its regulation via abiotic factors. In this Minireview, we discuss the mechanisms behind C sequestration including the recently emerging significance of biochemical interactions between organic matter inputs that lead to C stabilization.
Keywords: carbon cycling, nitrogen cycling, plant secondary compounds, tannins, chemical ecology
Beyond the C: Predicting soil C stocks for mitigating climate will be a major issue in the coming years. However, the key mechanisms of soil organic matter degradation or stabilization are still not fully known. Drivers of soil C sequestration are linked to interaction with soil minerals, aggregate formation, but also regulated by the environment. Recent evidence highlights the interactions between plant tannins and fungal necromass as an overlooked pathway of soil C stabilization. Future studies should consider all these mechanisms of C stabilization.
1. Introduction
Anthropogenic activities, such as the use of nitrogen (N) fertilizers and the combustion of fossil fuels, accelerate C and nutrient cycles via increased land‐based greenhouse gas (GHG) emissions (e. g. carbon dioxide, methane, nitrous oxide), that enhance global warming.1 As a consequence, humankind needs to mitigate climate change via sequestering more C and N into slowly cycling pools and retaining C in sinks.2 Terrestrial carbon (C) and nutrient cycling is tightly linked with soil organic matter (SOM) as the key driver of both cycles. In this Minireview, we focus on boreal forest ecosystems which store significant amounts of the global C pool, mostly belowground in SOM.3 However, global warming may increase SOM turnover rate, because an increase in temperature will enhance enzymatic activities responsible for decomposition, thereby shifting C sinks to sources and accelerating climate change. The mechanisms controlling the stability of C and N in belowground organic matter are still not entirely identified, thus limiting the current understanding of the global C cycle in a future climate.
Over the past decades, scientists have argued about the main drivers of C sequestration (i. e. long‐term storage of C) in the soil. Although aboveground plant litter has been considered the main origin of stable organic matter in the past, more recent studies suggest plant roots and fungal necromass as major contributors to the stable organic matter pool [e. g. 3–8]. As organic matter enters the soil, some C is lost to the atmosphere as carbon dioxide or methane from decomposition, whereas other C may be sequestered in the soil by stabilization processes. The recent concept of a soil microbial C pump proposes that microorganisms metabolically process plant residues (i. e. via decomposition) to produce microbial biomass. Following the microbes' death, some of this microbial necromass may eventually be stabilized in the soil.9 The process of decomposition may also be supported by easily available C sources from root exudates (i. e. via the rhizosphere priming effect) and/or soil fauna (i. e. degrading organic matter into more available forms).10 However, root exudates may also affect the microbial community composition in favour of fungi which promote the formation of macroaggregates, leading to an increased C stabilization.11 Current theories explain the persistence of organic matter in the soil with chemical, physical, environmental, and/or biological factors.12 Explicit reviews of the role of environmental and biological drivers on soil organic matter decomposition and stabilization, including interactions between microbial guilds, are discussed for example in Fernandez and Kennedy.13 In this Minireview, we focus on the recent novel insights into the chemical ecology underlying C and N stabilization processes in soil. In section 2, we address how soil chemical structure affects organic matter stabilization in the soil. Section 3 adds the interactions between plant and fungal derived organic matter inputs into soil and section 4 adds the effects of climate change.
2. How Chemical Structure Affects C Stabilization – Recalcitrance vs. C Protection
For many decades and even centuries, the process of humification (leading to “humus” formation) explained C stabilization in soil. Following Wershaw14 humus is composed of compounds with unique properties that are altered by the structure comparing to source compounds. Konova defined humic substances as “relatively high‐molecular weight, yellow to black colored substances formed by secondary synthesis reactions in soils”.15, 16, 17 Furthermore, according to the “humus paradigm”, humification” creates “humic substances”, including humic acids, fulvic acids, and humin (described precisely in18). These humic substances were assumed to be complex macromolecules forming the most stable soil organic matter fraction, but direct observations revealed that they form only a small fraction, and their chemical structure is rather simple.12 Moreover, extracted humic acid‐like substances may emerge from fire disturbance, but not humification.12 Recently, Lehmann and Kleber19, 20 proposed that soil consists of pools with different decomposition turn over time and that the term “humus” should not be used anymore.
It has been hypothesized that the chemically recalcitrant compounds, i. e. for plant litter mainly lignin and lipids21 and, for the fungal inputs, the abundant polysaccharide chitin, and polymers of phenolic and indolic monomers – melanin (Figure 1), form the most stable organic matter.22, 23 However, some studies have shown that lignin turnover in the soil is more rapid than that of bulk organic matter and degradability of fungal necromass and chitin is also relatively high.24, 25 In line with this, it has been proposed that both recalcitrant and labile matter build up stable organic matter and these C inputs are stabilized interacting with soil minerals and aggregates.26, 27 Thus, the question arises, how it is possible that both, labile as well as recalcitrant organic matter is stabilized in the soil?
Figure 1.
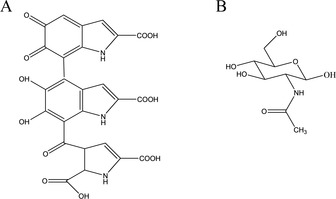
Potentially recalcitrant compounds of fungal necromass: A) melanin (on example of eumelanin) B) N‐acetyl‐D‐glucosamine, monomer of chitin.
Recent evidence suggests not only the influence of biological, but also physicochemical interactions between organic matter and the surrounding environment rather than its humification or recalcitrance as mechanism underlying soil organic matter stabilization.12, 28 From a chemical view, especially the interactions with the mineral phase, particularly iron (Fe) and aluminum (Al) oxides, contribute significantly to the prevention of microbial decomposition and thus stabilize organic C in mineral soils for centuries or millennia.28, 29, 30 However, as the association with minerals does not necessarily confer stability31 and ectomycorrhizal fungi living in symbioses with plants may access mineral bound compounds,32 also this mechanism does not necessary provide long‐term stabilization of organic matter. Overall, potential reactions with minerals do not exclude other stabilization mechanisms, especially not in highly organic soils with low levels of minerals. Moreover, polyphenolics that are abundant in plant roots such as tannins,33, 34 form chelates with Al or Fe ions,35 thereby potentially inhibiting the interaction with organic matter. All in all, although the responses of SOM stocks to land use and climate change are crucial for modelling to develop and evaluate potential strategies for sustainable management of forest ecosystems,36 there is no consensus yet on the main mechanisms underlying soil C stabilization.
3. The influence of the Interactions Between Root Litter and Fungal Necromass on C Stabilization
Root‐ and fungal‐derived litter compounds provide the most stable organic matter4, 22 and may potentially affect the decomposition or stabilization rate.35 For example, decomposition is affected via the quantity and quality of the provided substrate. Plant secondary metabolites (PSMs) can affect both SOM stabilization and decomposition.37 Their potential effect on decomposition and stabilization processes in the soil can be significant, because PSMs can comprise up to 30 % of dry weight of plants and their concentration depends on species, age, organ, as well as environmental conditions including soil nutrient status.34, 38 A quantitatively dominating group of PSMs, tannins (Figure 2), affect C and N mineralization as well as microbial community structure and biomass38, 39, 40 depending on concentration and chemical structure. The underlying mechanism of inhibition by tannins seems to include the formation of recalcitrant complexes with proteins (including enzymes), chelating metal ions, and/or their direct toxicity towards microbes.38 The effects on enzymes are crucial for SOM decomposition rates but depend on the specific interactions between tannin and enzyme, i. e. the chemical structure of both as well as their concentrations, and may lead to either an increase of enzyme activity, a decrease, or no effect at all.33 Moreover, tannin‐bound enzymes that retain their activity33 may act as a reservoir of enzymatic activity ready to react with changing fluxes of substrates.41
Figure 2.
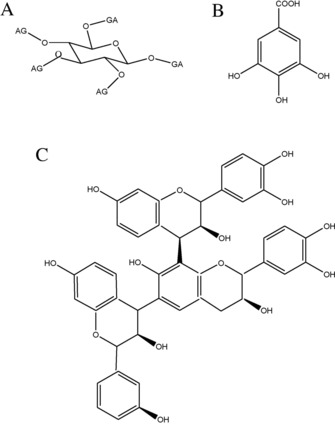
Examples of tannins: A) hydrolysable tannin, here gallotanin, B) gallic acid (GA), C) condensed tannin trimer.
In this Minireview, we highlight the effects of PSMs on C stabilization via their effect on soil N availability through the formation of recalcitrant complexes between tannins and proteins derived from plants, microorganisms, or soil. Northup et al.39, 42 demonstrated that high levels of polyphenols might not only inhibit N mineralization, but also correlate positively with the release of dissolved organic nitrogen (DON) from pine leaf litter suggesting that plants can benefit from increasing the DON:mineral N ratio in strongly N‐limited ecosystems. Furthermore, enhanced N availability may support C sequestration in forest ecosystems because the C : N ratio of litter is an order of magnitude larger than that of SOM.43 In addition, tannin‐protein complexes, though recalcitrant, may be decomposed by basidiomycete fungi via their polyphenol oxidases,35 so formation of tannin‐protein complexes can be beneficial for plants in symbiosis with mycorrhiza which can access N from protein‐tannin complexes.39 However, newest findings expand the role of tannins for C stabilization even further: tannins interact not only with proteins, but also with other N‐containing compounds, such as chitin, nitrogen bases, polyamines, and arginine.44, 45 In boreal forest ecosystems, plants produce astonishing amounts of tannins and their associated ectomycorrhizal fungi are also very abundant – their hyphal biomass reaching up to 600 kg ha−1.46 Formation of complexes between root‐derived tannins and mycorrhizal fungi rich in proteins and chitin become thus very likely and indicate a potentially significant mechanism for C stabilization.37, 47 Given that mycorrhizal fungal compounds react with tannins, it can be expected that first‐order roots and high tannin contents are the primary source for complex formation which was shown in a recent study in which first‐order roots had the lowest decomposition rates among 35 temperate wood species.8 Tannins are overall very abundant in first‐order roots and substantially more than those in leaf litter of the same species in Sun et al.’s8 study. In addition, variation in tannin levels was a main predictor of decomposition which decreased with increasing tannin concentrations.8
Formation of complexes between root‐derived tannins and fungal residues (especially chitin) can be a crucial C sequestration mechanism in ecosystems with tannin‐rich plants and abundant mycorrhization. However, it is also possible that other ecosystems, like tropical forests with high amounts of condensed tannins48 use the same mechanism. All in all, interactions between tannins and fungal necromass potentially provides a novel glimpse into mechanisms of C stabilization originating from fungal mycelium and root litter4 and to the “microbial C pump” concept.9
4. Effects of Climate Change on Plants Secondary Metabolites – Implications for Soil Processes
PSM synthesis and chemistry are affected by climate change stressors, i. e. enhanced atmospheric CO2 levels, rising temperature, and increased periods of drought.34 However, the effects of these predicted changes on PSMs are still not entirely understood. For example, increased CO2 levels lead to an increased synthesis of phenolic compounds, but decreased terpenoid levels.49 On the other hand, warming rather decreases phenolic compound production.49 As predicted for the future, extended drought periods may result in higher levels of polyphenolics.50, 51 Moreover, both elevated temperature and drought affect the concentration and composition of PSMs,52 thereby modifying the consequences of PSMs on SOM stabilization. Furthermore, warming changes plant community composition promoting new plant species with different PSM profiles. For example, in peatlands, warming promotes the growth of ericaceous plants rich in tannins and mycorrhizal fungi rich in melanin due to a lower water table, thereby altering SOM stabilization.22, 53
Overall, the decomposition of plant‐derived organic matter has been the focus of attention lately as shown by the number of recent reviews covering topics from plant‐soil feedbacks,54, 55, 56 abiotic and biotic drivers,57 and the consequences of climate on these drivers,58 but also the impact of plant species on litter degradation.59 Evidence from previous studies suggests that the complexation of proteins by tannins conserves N derived from litter with consequences for plant N acquisition via resulting in organic over mineral N dominated pathways.39, 60 Overall, scientists are only beginning to consider PSMs in these processes,38 and an in‐depth understanding of litter and subsequently SOM degradation as influenced by PSMs on the basis of N and C pools and the resulting consequences for plant N acquisition is also crucial for improving litter decomposition models in climate change scenarios.40
5. Conclusions and Outlook
Predicting soil C stocks for mitigating climate will be a major issue in the coming years. However, the key mechanisms of SOM degradation or stabilization are still not fully understood. Without in‐depth knowledge on the underlying mechanisms on the relationship between soil C and N cycling, the development and evaluation of management strategies for sustainable forest ecosystems will be difficult. The main drivers of soil C sequestration are related to the recalcitrance of C input, interaction with soil minerals, and aggregate formation, but are also regulated by environmental and biological factors. Plant‐derived C cannot be considered only as substrates for microorganisms, as some plant‐derived compounds may control SOM decomposition and stabilization. Recent evidence highlights also the interactions between plant tannins and fungal necromass as yet overlooked pathway of soil C stabilization. Future studies should consider all these mechanisms of C stabilization in different ecosystems, including both forest and agricultural systems.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the manuscript and approved it for publication.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Bartosz Adamczyk was born in Lodz (Poland) in 1979. He received a Master's degree in 2003 (Master of Biology) and PhD degree in 2009 (Doctor of Biology), both from the University of Lodz (Poland). After defending his PhD, he started as a Post‐Doc at the Finnish Forest Research Institute (Finland). In 2013, he obtained the title of docent (habilitation) from the University of Helsinki (Finland) and started to work there in 2015. In 2018, he started to work at the Natural Resources Institute Finland (LUKE). His main interests span plant biochemistry, soil science, carbon sequestration, and climate smart agriculture.

Biographical Information
Jussi Heinonsalo received a Master's degree in 1997 in Environmental Sciences and a PhD degree in 2004 in General Microbiology, both from the University of Helsinki (Finland). During his PhD project, he spent one year as a researcher at INRA in Nancy, France. After defending his PhD, he worked as a Post‐Doc at the University of Helsinki in several projects. Since 2012, he has worked as principal investigator (PI) at the University of Helsinki and he obtained the title of docent in microbiology (habilitation) in 2017. His main interests are soil microbiology, plant‐soil‐interaction, soil science, carbon sequestration, and climate smart forestry and agriculture.

Biographical Information
Judy Simon leads the Plant Interactions Ecophysiology Group at the University of Konstanz (Germany). After her studies in biology (RWTH Aachen, Germany), biogeography, soil science, and geology (Saarland University, Germany), she conducted her PhD research at the University of Melbourne (Australia). She then worked as a Postdoctoral Fellow at the University of Freiburg (Germany), earning her Habilitation (postdoctoral qualification) in 2013. Since 2014, she conducts her research at the University of Konstanz (Germany) on the influence of global change on plant interactions with regard to resource allocation strategies (i. e. different N acquisition strategies, N allocation to growth vs. defence) in woody species in boreal, temperate, and tropical forest ecosystems.

Acknowledgements
J.S. is financially supported by a Heisenberg Fellowship of the German Research Foundation (DFG; grant no. SI 1556/2‐1).
B. Adamczyk, J. Heinonsalo, J. Simon, ChemistryOpen 2020, 9, 464.
References
- 1. Gruber N., Galloway J., Nature 2008, 451, 293–296. [DOI] [PubMed] [Google Scholar]
- 2. Arneth A., Sitch S., Pongratz J., Stocker B. D., Ciais P., Poulter B., Bayer A. D., Bondeau A., Calle L., Chini L. P., Gasser T., Fader M., Friedlingstein P., Kato E., Li W., Lindeskog M., Nabel J. E. M. S., Pugh T. A. M., Robertson E., Viovy N., Yue C., Zaehle S., Nature Geosci 2017, 10, 79–84. [Google Scholar]
- 3. Pan Y., Birdsey R. A., Fang J., Houghton R., Kauppi P. E., Kurz W. A., Phillips O. L., Shvidenko A., Lewis S. L., Canadell J. G., Ciais P., Jackson R. B., Pacala S. W., McGuire A. D., Piao S., Rautiainen A., Sitch S., Hayes D., Science 2011, 333, 988–993. [DOI] [PubMed] [Google Scholar]
- 4. Clemmensen K. E., Bahr A., Ovaskainen O., dahlberg A., Ekblad A., Wallander H., Stenlid J., Finlay R. D., Wardle D. A., Lindahl B. D., Science 2013, 340, 1615–1618. [DOI] [PubMed] [Google Scholar]
- 5. Kyaschenko J., Clemmensen K. E., Karltun E., Lindahl B. D., Ecol Lett 2017, 20, 1546–1555. [DOI] [PubMed] [Google Scholar]
- 6. Kyaschenko J., Ovaskainen O., Ekblad A., Hagenbo A., Karltun E., Clemmensen K. E., Lindahl B. D., New Phytol. 2018, 221, 1492–1502. [DOI] [PubMed] [Google Scholar]
- 7. Sokol N. W., Bradford M. A., Nature Geosci 2019, 12, 46–53. [Google Scholar]
- 8. Sun T., Hobbie S. E., Berg B., Zhang H., Wang Q., Wang Z., Hättenschwiler S., Proc. Natl. Acad. Sci. India 2018, 115, 10392 LP-10310397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang C., Schimel J. P., Jastrow J. D., Nature Microbiol 2017, 2, 17105. [DOI] [PubMed] [Google Scholar]
- 10. Setälä H., Marshall V. G., Trofymow J. A., Soil Biol. Biochem. 1996, 28, 1661–1675. [Google Scholar]
- 11. Baumert V. L., Vasilyeva N. A., Vladimirov A. A., Meier I. C., Kögel-Knabner I., Mueller C. W., Front. Environ. Sci. 2018, 6, 140. [Google Scholar]
- 12. Schmidt M. W. I., Torn M. S., Abiven S., Dittmar T., Guggenberger G., Janssens I. A., Kleber M., Kögel-Knabner I., Lehmann J., Manning D. A. C., Nannipieri P., Rasse D. P., Weiner S., Trumbore S. E., Nature 2011, 478, 49–56. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez C. W., Kennedy P. G., New Phytol. 2016, 209, 1382–1394. [DOI] [PubMed] [Google Scholar]
- 14. Ghabbour E. A., Davies G., Humic substances: Versatile components of plants, soil, and water, Royal Society of Chemistry ; 2000. [Google Scholar]
- 15. Kononova M. M., Die Humusstoffe des Bodens, Deutscher Verlag der Wissenschaften ; 1958. [Google Scholar]
- 16. Kononova M. M., Soil organic matter: its nature, its role in soil formation and in soil fertility, Pergamon Press Ltd. ; 1966. [Google Scholar]
- 17. Stevenson F. J., Humus chemistry. Genesis, composition, reactions, John Wiley and Sons ; 1994. [Google Scholar]
- 18. Stevenson F. J., Olsen R. A., J. Agron. Educ. 1989, 18, 84–88. [Google Scholar]
- 19. Lehmann J., Kleber M., Nature 2015, 528, 60–68. [DOI] [PubMed] [Google Scholar]
- 20. Kleber M., Lehmann J., J. Environ. Qual. 2019, 48, 207–216. [DOI] [PubMed] [Google Scholar]
- 21. Melillo J. M., Aber J. D., Muratore J. F., Ecology 1982, 63, 621–626. [Google Scholar]
- 22. Fernandez C. W., Heckman K., Kolka R., Kennedy P. G., Ecol Lett 2019, 22, 498–505. [DOI] [PubMed] [Google Scholar]
- 23. Clemmensen K. E., Finlay R. D., Dahlberg A., Stenlid J., Wardle D. A., Lindahl B. D., New Phytol. 2015, 205, 1525–1536. [DOI] [PubMed] [Google Scholar]
- 24. Godbold D. L., Hoosbeek M. R., Lukac M., Cotrufo M. F., Janssens I. A., Ceulemans R., Polle A., Velthorst E. J., Scarascia-Mugnozza G., De Angelis P., Miglietta F., Peressotti A., Plant Soil 2006, 281, 15–24. [Google Scholar]
- 25. Fernandez C. W., Koide R. T., Ecology 2012, 93, 24–28. [DOI] [PubMed] [Google Scholar]
- 26. Mikutta R., Kleber M., Torn M. S., Jahn R., Biogeochemistry 2006, 77, 25–56. [Google Scholar]
- 27. Cotrufo M. F., Soong J. L., Horton A. J., Campbell E. E., Haddix M. L., Wall D. H., Parton W. J., Nature Geosci 2015, 8, 776–779. [Google Scholar]
- 28. Torn M., Trumbore S., Chadwick O. A., Vitousek P. M., Hendricks D. M., Nature 1997, 389, 170–173. [Google Scholar]
- 29. Marschner B., Brodowski S., Dreves A., Gleixner G., Gude A., Grootes P. M., Hamer U., Heim A., Jandl G., Ji R., Kaiser K., Kalbitz K., Kramer C., Leinweber P., Rethemeyer J., Schäffer A., Schmidt M. W. I., Schwark L., Wiesenberg G. L. B., Z. Pflanzenernaehr. Bodenkd. 2008, 171, 91–110. [Google Scholar]
- 30. Dungait J. A. J., Hopkins D. W., Gregory A. S., Whitmore A. P., Glob Change Biol 2012, 18, 1781–1796. [Google Scholar]
- 31. Gabriel C. E., Kellman L., Prest D., PLoS One 2018, 13, e0206847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang T., Tian Z., Bengtson P., Tunlid A., Persson P., Environ. Microbiol. 2017, 19, 5117–5129. [DOI] [PubMed] [Google Scholar]
- 33. Adamczyk S., Adamczyk B., Kitunen V., Smolander A., Soil Biol. Biochem. 2015, 87, 59–66. [Google Scholar]
- 34. Kraus T. E. C., Dahlgren R. A., Zasoski RJ R. J., Plant Soil 2003, 256, 41–66. [Google Scholar]
- 35. Hättenschwiler S., Vitousek P. M., Trends Ecol Evol 2000, 15, 238–243. [DOI] [PubMed] [Google Scholar]
- 36. Kallenbach C. M., Frey S. D., Grandy A. S., Nat. Commun. 2016, 7, 13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adamczyk B., Sietiö O.-M., Strakova P., Prommer J., Wild B., Hagner M., Pihlatie M., Fritze H., Richter A., Heinonsalo J., Nat. Commun. 2019, 10, 3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smolander A., Kanerva S., Adamczyk B., Kitunen V., Plant Soil 2011, 350, 1–2. [Google Scholar]
- 39. Northup R. R., Yu Z., Dahlgren R. A., Vogt K. A., Nature 1995, 377, 227–229. [Google Scholar]
- 40. Adamczyk B., Adamczyk S., Smolander A., Kitunen V., Simon J., Soil Syst 2018, 2, 1.31276103 [Google Scholar]
- 41. Burns R. G., DeForest J. L., Marxsen J., Sinsabaugh R. L., Stromberger M. E., Wallenstein M. D., Weintraub M. N., Zoppini A., Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar]
- 42. Northup R. R., Dahlgren R. A., McColl J. G., Biogeochemistry 1998, 42, 189–220. [Google Scholar]
- 43. Melillo J. M., IGBP Book Series (Eds.: B. Walker, W. Steffen), Cambridge University Press, Cambridge, UK: 1996, pp. 431–450. [Google Scholar]
- 44. Adamczyk B., Adamczyk S., Smolander A., Kitunen V., Soil Biol. Biochem. 2011, 43, 628–637. [Google Scholar]
- 45. Adamczyk B., Simon J., Kitunen V., Adamczyk S., Smolander A., Chem Open 2017, 6, 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wallander H., Nilsson L. O., Hagerberg D., Baath E., New Phytol. 2001, 151, 753–760. [DOI] [PubMed] [Google Scholar]
- 47. Adamczyk B., Sietiö O.-M., Biasi C., Heinonsalo J., New Phytol. 2019, 223, 16–21. [DOI] [PubMed] [Google Scholar]
- 48. Coq S., Souquet J. M., Meudec E., Cheynier V., Hättenschwiler S., Ecology 2010, 91, 2080–2091. [DOI] [PubMed] [Google Scholar]
- 49. Holopainen J. K., Virjamo V., Ghimire R. P., Blande J. D., Julkunen-Tiitto R., Kivimäenpää M., Front. Plant Sci. 2018, 9, 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nogues I., Llusia J., Ogaya R., Munne-Bosch S., Sardans J., Peñuelas J., Loreto F., Plant Biosyst 2014, 148, 268–278. [Google Scholar]
- 51. Rivas-Ubach A., Gargallo-Garriga A., Sardans J., Oravec M., Mateu-Castell L., Perez-Trujillo M., Parella T., Ogaya R., Urban O., Penuelas J., New Phytol. 2014, 202, 874–885. [DOI] [PubMed] [Google Scholar]
- 52. Tharayil N., Suseela V., Triebwasser D. J., Preston C. M., Gerard P. D., Dukes J. S., New Phytol. 2011, 191, 132–145. [DOI] [PubMed] [Google Scholar]
- 53. Bragazza L., Parisod J., Buttler A., Bardgett R. D., Nat. Clim. Change 2013, 3, 273–277. [Google Scholar]
- 54. Bani A., Pioli S., Ventura M., Panzacchi P., Borruso L., Tognetti R., Tonon G., Brusetti L., Appl Soil Ecol 2018, 126, 75–84. [Google Scholar]
- 55. Palozzi J. E., Lindo Z., Soil Biol. Biochem. 2018, 124, 189–198. [Google Scholar]
- 56. Poirier V., Roumet C., Munson A. D., Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar]
- 57. Garcia-Palacios P., Shaw E. A., Wall D. H., Hättenschwiler S., Ecol Lett 2016, 19, 554–563. [DOI] [PubMed] [Google Scholar]
- 58. Suseela V., Tharayil N., Global Change Biol 2018, 244, 1428–1451. [DOI] [PubMed] [Google Scholar]
- 59. Hobbie S. E., Trends Ecol Evol 2015, 30, 357–363. [DOI] [PubMed] [Google Scholar]
- 60. Bennett J. N., Prescott C. E., Oecologia 2004, 141, 468–476. [DOI] [PubMed] [Google Scholar]


