Abstract
Objective:
Pulmonary arterial hypertension (PAH) has high morbidity and mortality in connective tissue diseases (CTDs), especially systemic sclerosis (SSc). In this systematic review, we provide an update on screening measures for early detection of PAH in CTD.
Methods:
Manuscripts published between July 2012 and October 2017 which incorporated screening measures to identify patients with PAH by right heart catheterization were identified. Risk of bias was assessed using the QUADAS-2 tool.
Results:
The systematic review resulted in 1,514 unique citations and 22 manuscripts were included for final review; the majority of manuscripts had lower risk of bias based on the QUADAS-2 tool. There were 16 SSc cohort studies and 6 case-control studies (SSc 4, SLE 2). Four SSc cohort studies evaluated transthoracic echocardiography (TTE) only. Eight SSc cohort studies evaluated composite measures including ASIG, DETECT, and a combination of tricuspid regurgitation velocity (TRV) and PFT variables. DETECT and ASIG had greater sensitivity and negative predictive value (NPV) compared to the 2009 ESC/ERS guidelines in different cohorts. The addition of PFT variables, such as DLCO or FVC/ DLCO ratio, to TRV resulted in greater sensitivity and NPV compared to TRV alone.
Conclusion:
Current screening for PAH in CTDs is centered on SSc. Data continues to support the use of TTE and provides additional evidence for use of composite measures.
Keywords: Connective tissue disease, pulmonary arterial hypertension, screening
2.1. INTRODUCTION:
Pulmonary arterial hypertension (PAH) is not uncommon in connective tissue diseases (CTDs). Compared to idiopathic PAH (IPAH), patients with connective tissue disease (CTD) associated-PAH (CTD-PAH) have worse clinical outcomes and survival [1]. Among the CTDs, systemic sclerosis (SSc) has the highest known PAH prevalence [2]. PAH affects approximately 10–12% of patients with SSc, the prevalence of PAH is 19% in SSc patients with diffusing capacity of the lung for carbon monoxide (DLCO) < 60% predicted at time of RHC, and PAH is a leading cause of mortality in SSc [3, 4]. Thus, most of the data known regarding screening for CTD-PAH has been based on SSc.
Current screening guidelines for PAH in asymptomatic patients with SSc recommend annual transthoracic echocardiography (TTE) and other modalities, such as pulmonary function tests (PFTs), N-terminal pro-brain natriuretic peptide (NT-ProBNP) and/or the DETECT algorithm [5–7]. Regular screening and earlier PAH detection in SSc may improve survival [8, 9]. As part of an initiative to update guidelines for screening and diagnosis of CTD-PAH for the 2018 World Symposium on Pulmonary Hypertension (WSPH), we conducted a systematic review to identify the best evidence for screening and diagnosis of PAH in CTD.
2. MATERIAL AND METHODS
2.1. Selection criteria:
Studies were considered for inclusion in this systematic review according to the following inclusion and pre-defined exlusion criteria (table 1)
Table 1:
Stepwise exclusion of manuscripts
| Step 1 exclusion criteria |
|
| Step 2 exclusion criteria |
|
| Step 3 exclusion criteria |
|
RHC = right heart catheterization, PAH = pulmonary arterial hypertension, CTD = connective tissue disease, PH = pulmonary hypertension
2.2. Participants:
Adults aged ≥ 18 years with a diagnosis of CTD (based on American College of Rheumatology classification criteria) and PAH (based on RHC)
2.3. Intervention:
Any intervention used to screen or diagnose CTD-PAH
2.4. Comparison:
A concurrent control group of patients with CTD-no-PAH
2.5. Outcomes:
Diagnosis of PAH confirmed by RHC
2.6. Study design:
Case control or cohort studies with atleast 20 CTD patients
2.7. Structured Search Strategy
We conducted a systematic search across four databases—Medline via PubMed, Embase via Embase.com, the Web of Science Core Collection (including SCI, SSCI, AH&I, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED), and Scopus—to find articles pertinent to pulmonary hypertension and CTDs with the aid of an experienced librarian (WT). We used the same hedge as published by our team in February 2014 [5] as we set out to update our prior initial systematic review. Thus, a search was conducted to find literature published from June 1, 2012 to October 2, 2017, which is the most up to date literature after the previously published systematic review. No language or publication type limits were enforced to guarantee comprehensiveness. The Medline search strategy was conducted by combining relevant keywords, medical subject headings, and the Clinical Queries search hedge for diagnostic clinical studies. The following search details were used: (((“Diagnosis”[mesh] OR “diagnosis”[sh] OR “prevention and control”[sh] OR diagnost* OR diagnosi* OR diagnose* OR screen* OR “transthoracic echocardiogram” OR “pulmonary function test” OR “echocardiogram” OR “EKG” OR “chest radiograph” OR “N-terminal pro-brain natriuretic peptide” OR “NT-Pro-BNP” OR “NT-Pro BNP” OR (Diagnosis/Broad[filter]))) AND (“Hypertension, Pulmonary”[mesh] OR “pulmonary hypertension” OR “pulmonary arterial hypertension” OR (ayerza AND syndrome))) AND (“Connective tissue diseases”[mesh] OR “Collagen disease” OR “Collagen diseases” OR “Collagen vascular disease” OR “Connective tissue disease” OR “Connective tissue diseases” OR Dermatomyositis OR “Inflammatory myositis” OR Lupus OR “Mixed connective tissue disease” OR Polymyositis OR “Rheumatic disease” OR “Rheumatic diseases” OR “Rheumatoid arthritis” OR Scleroderma OR Sclerosis OR “Sjogren syndrome” OR Vasculitis). All search terms were investigated unless otherwise indicated. A unique search strategy was developed for each database in to ensure appropriate utilization of keywords and relevant controlled vocabulary terms (MeSh terms in Medline, and EMTREE headings ins EMBASE). The full search strategies for all databases are available upon request from the corresponding author.
2.8. Selection of Instruments and Creation of Item Library
Under the supervision of the corresponding author, AY, VN, MB, and MH systematically reviewed the citations. The review process was divided into three stages with unique exclusion criteria at each stage: titles, abstracts, and manuscripts (figure 1 and table 1). All titles, abstracts, and manuscripts were assessed by two team members for relevancy and were excluded if they fulfilled exclusion criteria. Any disagreements between reviewers were reviewed and resolved by the corresponding author; if uncertainty persisted, the title, abstract or manuscript was included for comprehensiveness. When there was lack of data clarity in pertaining to exclusion criteria in manuscripts, the corresponding authors were contacted [10–13]. Any manuscripts published in 2012 and included in the initial systematic review by Gladue et al. were excluded [5]. For articles evaluating TTE, we converted the estimated right ventricular systolic pressure (RVSP) to tricuspid regurgitation velocity (TRV), if TRV was not reported, and assumed a right atrial pressure (RAP) of 10 mmHg, if RAP was not reported, to calculate the TRV using the Bernoulli equation as done in our previous systematic review.
Figure 1:
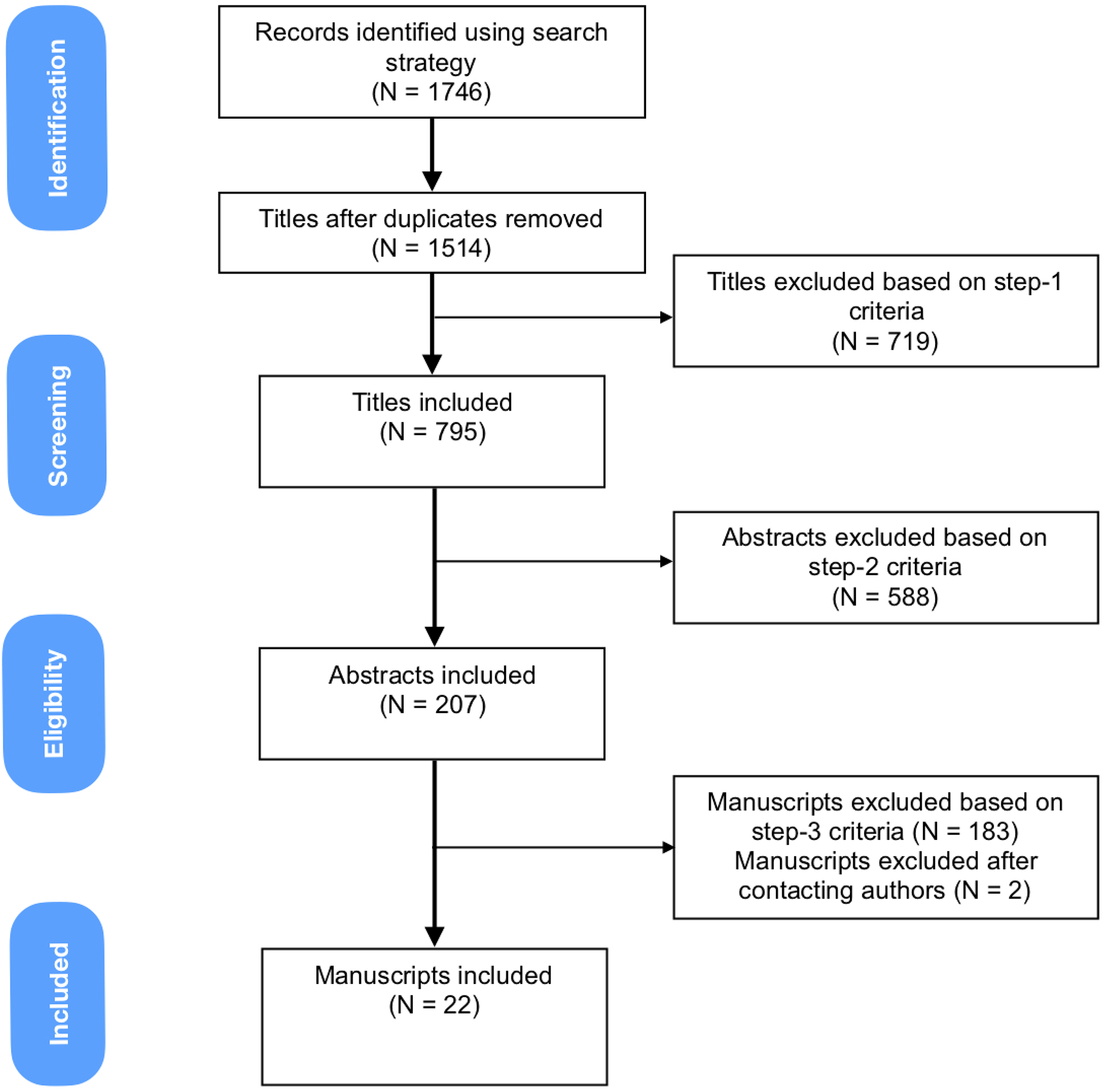
Systematic Review Schematic Flow Diagram
2.9. Quality assessment
Two reviewers (AY and VN) independently assessed the risk of bias and quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) evaluation tool [14]. A third reviewer (DK) was available to resolve any disagreements between the two reviewers. QUADAS-2 comprises 4 domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias; and the patient selection, index test and reference standard domains are also assessed regarding applicability [14]. Studies scored with low risk in all domains are considered to have the highest quality.
3. RESULTS
Using the search strategy, 22 of the 1514 titles fulfilled the criteria for RHC defined PAH. Of these, six were case control and 16 were cohort studies (Table 2 and Supplemental material). Cohort studies had a lower risk of bias or applicability concerns on QUADAS-2 evaluation (Figure 2). The following sections provide a summary of our search.
Table 2.
Baseline Characteristics of Patients with CTD Included in Systematic Review
| Type of Study Author Year (REF) | Total Cohort | PAH | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Age-mean (SD) | % Female | % lcSSc | N | Age-Mean (SD) | % Female | % lcSSc | |
| Cohort SSc | ||||||||
| Biomarker | ||||||||
| Avouac 2015 [15] | 161 | 58 (22–88)# | 84 | 60 | 13 | NA | NA | NA |
| Chung 2016 [16] | 157 | 57.6 (10.4) | 86 | 67 | 16 | NA | NA | NA |
| Iudici 2013 [13]* | 867 | 59 (12–89)# | 89 | 78 | 29 | NA | 90 | 93 |
| Composite | ||||||||
| Gladue 2013 [17]** | 347 | 57.2 (11.6) PHAROS 53.7 (11.8) Cochin | 84 | 60 | 69 | 62.4 (10.3) PHAROS 63.1 (12.9) Cochin | 84 | 75 |
| Guillen de Castillo 2017 [18] | 83 | 62.4 (11.6) | 94 | 84 | 35 | 64.4 (10.8) | 97 | 89 |
| Hao 2015 [19] | 73 | NA | 67 | 82 | 27 | 66.8 (8.3) | 93 | 85 |
| Morrisroe 2016 [20] | 1579 | NA | NA | NA | 132 | 62.7 (10.3) | 85 | 69 |
| Morrisroe 2017 [21] | 1636 | 57.2 (12.8) | 86 | 69 | 209 | 63.1 (10.4) | 87 | 74 |
| Soukup 2016 [22] | 34 | 58.4 (11.7) | 88 | 41 | 7 | 71.3 (5.4) | 71 | 43 |
| Thakkar 2013 [23] | 49 | NA | NR | NA | 17 | 56.4 (13.4) | 100 | 82 |
| Vandecasteele 2017 [24] | 195 | 54 (42–62)# | 80 | 71 | 3 | NA | NA | 100 |
| TTE | ||||||||
| Meune 2016 [25] | 212 | 55.3 (13.2) | 81 | 51 | 27 | 63.3 (14.4) | 74 | NA |
| Kusunose, 2015 [26] | 78 | 58 (12) | 91 | 70† | 16 | 60 (15) | 77 | NA |
| Yoo 2016 [27] | 37 | 54 (9.9) | 76 | 68 | 4 | 58.5 (13.6) | 100 | 25 |
| PFT | ||||||||
| Sivova 2013 [28] | 63 | 55 (NA) | 81 | 79 | 6 | 68 (NA) | 100 | 83 |
| CPET | ||||||||
| Dumitrescu 2016 [29] | 173 | NA | 85 | NA | 48 | 67 (9) | 90 | NA |
| Type of Study Author Year (REF) | Total Cohort | PAH | ||||||
| N | Age-mean (SD) | % Female | % lcSSc | N | Age-Mean (SD) | % Female | % lcSSc | |
| Case-control SSc | ||||||||
| Biomarker | ||||||||
| Kozij 2017 [30] | 16 | 51 (NA)# | 100 | 94 | 7 | 51 (NA)# | 71 | 71 |
| Morrisroe 2014 [31] | 816 | NA | NA | NA | 124 | NA | NA | NA |
| Nailfold capillaroscopy | ||||||||
| Corrado 2017 [11] | 20 | 55.8 (11.4) | NA | NA | 19 | 48.1 (6.8) | NA | NA |
| Case-control CTD | ||||||||
| Biomarker | ||||||||
| Hachulla 2017 (SLE) [32] | 101 | 46.9 (16.2) | 92 | NA | 51 | 47.6 (12.2) | 92 | NA |
| Huang 2016 (SLE) [33] | 444 | 34.6 (8.5) | 97 | NA | 111 | 34.6 (8.6) | 97 | NA |
| Tamura 2012 (CTD) [34] | 34 | 56.3 (2.7) | 91 | 59‡ | 17 | 56.3 (4.6) | 88 | NA |
Iudici 2013 also evaluated TTE and PFT parameters in a multivariate analysis [13].
Gladue 2013 also evaluated TTE and PFT parameters separately in addition to their combination [17].
Huang 2016 also evaluated various clinical manifestations [33].
Median (IQR).
70% had SSc.
59% had SSc
CTD- connective tissue disease, PAH – pulmonary arterial hypertension, SSc- systemic sclerosis, lcSSc – limited cutaneous systemic sclerosis, SLE – systemic lupus erythematosus, REF-reference, TTE – transthoracic echocardiography, PFT- pulmonary function test, CPET – cardiopulmonary exercise test, NA – not available
Figure 2. Quality Assessment of Diagnostic Accuracy Studies (QUADAS) for Articles Included in Systematic Review.
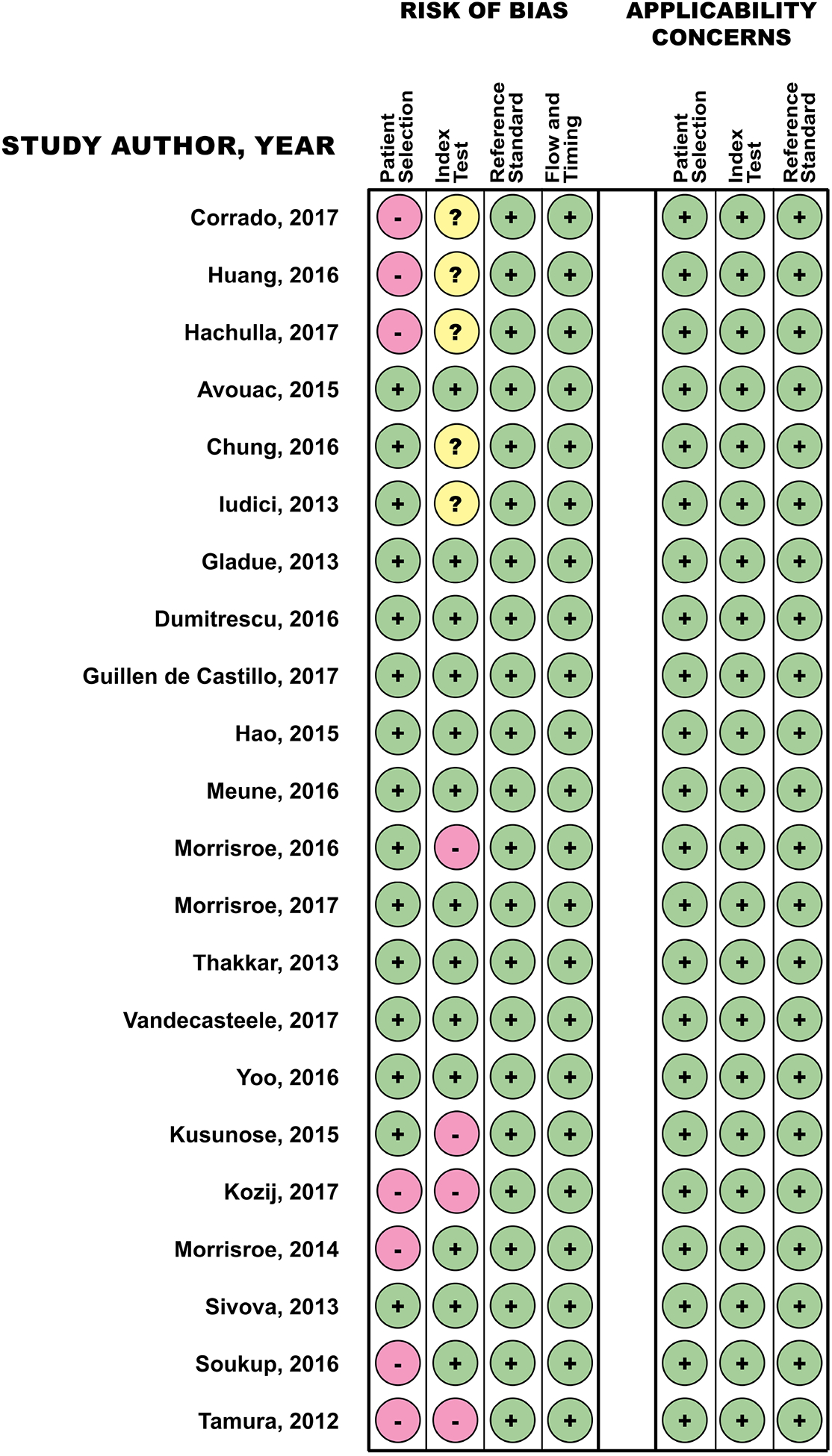
QUADAS-2 comprises 4 domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias; and the patient selection, index test and reference standard domains are also assessed regarding applicability.
3.1. CARDIOLOGY MEASURES
3.1.1. Transthoracic echocardiography (TTE)
Four cohort studies evaluated TTE parameters alone for the diagnosis of SSc-PAH [17, 25–27]. Two studies had TRV cut-offs ranging from 2.5 to 3.16 m/s. The first study involved two large SSc cohorts; the sensitivity (94%) and negative predictive value (NPV) (97%) were higher at a lower TRV cut-off (2.5 m/s), and the specificity (96%) was highest at a higher TRV cut-off (3.16 m/s) [17]. In the second study, a TRV cut-off of > 3.0 m/s suggested PAH diagnosis in 8/37 (22%) SSc patients; six patients underwent RHC and 4/37 (11%) were confirmed to have PAH [27]. In the third study examining TTE, a sub-group analysis of SSc-PAH patients (compared to SSc-no-PAH patients) revealed reduced left ventricular diastolic function (transmitral E/A ratio, p=0.045; and Ea <10 cm/s EA>10 cm/s, p=0.029), reduced overall right ventricular contractility (21.5% versus 4.5%, p=0.03), and reduced right ventricular diastolic function (transtricuspid E/A ratio, p=0.014; 68% versus 29% with impaired function, p=0.001)[25]. The fourth study focused on pressure-flow relationships of the pulmonary circulation obtained by 6-minute walk stress TTE to predict development of PH [26]. Two variables are particularly of interest – 6-minute walk distance (6MWD) and ratio of change in mean pulmonary arterial pressure (mPAP) and change in cardiac output (Q) [ΔmPAP/ΔQ - obtained at baseline and after 6MW]. SSc patients with impaired 6-minute walk distance (HR 0.99, 95% CI 0.98 – 0.99, p=0.010) and increased ΔmPAP/ΔQ (HR1.10, 95%CI 1.04–1.16, p=0.005) had a shorter time course to development of PAH. An additional cohort study assessed multiple variables for prediction of PAH, and on multivariate analysis, TRV > 2.74 was associated with an increased risk for PAH (HR 18.03, 95% CI 9.01 – 36.06, p <0.001)
3.1.2. Cardiopulmonary exercise testing (CPET)
In a multi-centre, prospective cohort study of 173 SSc patients, diagnostic accuracy of CPET in detecting SSc-PAH was evaluated [29]. CPET was performed on a stationary, semi recumbent cycle ergometer and all patients underwent RHC. Peak maximal oxygen consumption (VO2) revealed the highest diagnostic accuracy (sensitivity 87% and specificity 75% at a threshold level of 13.8 mL/min/kg). A peak VO2 of >18.7 mL/min/kg was achieved by 22% of patients and excluded PAH (NPV 100%). Diagnostic accuracy was highest in SSc patients with low pulmonary artery wedge pressure (<12mmHg).
3.2. PULMONARY MEASURES
3.2.1. Pulmonary function tests (PFTs)
Two cohort studies evaluated PFTs only to screen for SSc-PAH [17, 28]. The first study was comprised of two large SSc cohorts and evaluated cut-off ranges for two PFT variables – DLCO% predicted ranging from < 50% to <70% and the ratio of forced vital capacity (FVC)% predicted to DLCO % predicted (FVC/DLCO%) ranging from ≥ 1.6 to ≥ 2.0 [17]. The sensitivity (91%) and NPV (93%) for detecting SSc-PAH were improved at a higher DLCO cut-off (>70%), while the specificity (87%) was improved at a higher FVC%/DLCO% (≥2.0). The second study evaluated the role of DLCO partitioning for detecting SSc-PAH [28]. DLCO, membrane conductance for carbon monoxide (DmCO), alveolar capillary blood volume (Vcap), and the ratio of alveolar capillary blood volume and alveolar volume (Vcap/VA) were reduced in patients with SSc-PAH compared to SSc-no-PAH patients. Both Vcap/VA (93%) and DLCO (93%) had the highest area under the curve to detect PAH. In another cohort study, multivariate analysis of many variables indicated a DLCO ≤ 55% with an FVC > 70% as predictive of PAH (HR 4.45, 95% CI 2.24–8.83, p <0.001) [13].
3.3. LABORATORY VALUES
3.3.1. High-sensitivity cardiac troponin T (HS-cTnT)
Plasma HS-cTnT levels were evaluated as a continuous variable in a large cohort of SSc patients [15]. In a multivariate logistic regression analysis, PAH was independently associated with a HS-cTnT level of >14 ng/L (p=0.039). Normal concentrations of plasma HS-cTnT had 95% NPV for ruling out PAH.
3.3.2. N-terminal pro-brain natriuretic peptide (NT-proBNP)
Three cohort studies evaluated NT-proBNP levels in SSc patients [13, 15, 16]. The first cohort used a NT-proBNP threshold of 97% percentile of normal values stratified by age and gender and had 46% sensitivity, 93% specificity, and 95% NPV for detecting SSc-PAH [15]. The second cohort used a NT-proBNP threshold of >210 pg/mL, which yielded a 73% sensitivity and 78% specificity [16]. In the third cohort, higher than normal levels of NT-proBNP (threshold value not mentioned) had a 44% sensitivity, 84% specificity, and 95% NPV [13].
3.3.3. Brain natriuretic peptide (BNP)
In one cohort study examining BNP in SSc patients, the sensitivity and specificity for SSc-PAH detection using baseline BNP ≥64 pg/mL was 71% and 59%, respectively [16].
3.3.4. Exhaled nitric oxide (NO)
In a case control study, exhaled NO was the biomarker of interest in SSc patients [30]. SSc-PAH patients had lower median (interquartile range) alveolar NO compared to SSc-no-PAH patients [3.3 ppb versus 4.0 ppb; no P value reported].
3.3.5. Blood biomarkers
A case control study assessing anti-cardiolipin antibody (ACLA) levels in SSc patients revealed an association of ACLA-IgG with SSc-PAH and higher titers increased the likelihood [moderate titer (20–39 U/ml) ACLA-IgG OR 1.7, 95% CI 1.01–2.93, p=0.047; high titer (>40 U/ml) ACLA-IgG OR 4.60, 95% CI 1.02–20.8, p=0.047][31]. In a case control study, a novel inflammatory marker which reflects pulmonary vascular degeneration – human pentraxin 3 (PTX3) – was evaluated in CTD patients [34]. In CTD-PAH patients, mean PTX3 concentrations were significantly higher than in CTD patients without PAH (5.02 ± 0.69 vs. 2.40 ± 0.14 ng/mL, respectively; p<0.001). In two independent case control studies, different laboratory and serological tests were compared between SLE-PAH and SLE-no-PAH [32, 33]. In the first study, higher frequency of anti-SSA (p=0.02), anti-SSB (p=0.003), anti-Smith (p=0.04), and anti-double stranded DNA (p=0.0001) antibodies, were noticed in SLE-PAH compared to SLE-no-PAH [32]. In a study by Huang et al., the following variables were independently associated with PAH in SLE patients: anti-RNP antibody (OR=12.4, p<0.001), anti-SSA antibody (OR=4.8, p=0.004), erythrocyte sedimentation rate <20 mm/h (OR=12.0, p<0.001), and urate >6mg/dL (OR= 9.7, p<0.001). Additional clinical variables, in the study by Huang et al., found to be independently associated with PAH in SLE patients were baseline SLE duration (OR= 1.118, p=0.007) interstitial lung disease (OR=17.027, P <0.001), no acute rash (OR= 3.258, P= 0.019), pericardial effusion (OR=21.290, P<0.001), and SLE Activity Index (SLEDAI) ≤ 9 (OR=26.426, P < 0.001) [33].
3.4. NAILFOLD CAPILLAROSCOPY
Only one study included in our systematic review evaluated nailfold capillaroscopy abnormalities in patients with SSc-PAH and those with SSc-no-PAH [11]. A higher number of patients with SSc-PAH patients had more “severe” patterns on nailfold capillaroscopy (considered as active/late patterns) (73.2% vs 50%, p <0.05); additional findings in SSc-PAH included lower capillary density, higher main loop capillary width, higher mean number of megacapillaries, and capillaries with neoangiogenesis (P< 0.05 for all).
3.5. COMPOSITE MEASURES
Eight cohort studies examined the combination of screening tools for detecting SSc-PAH [17, 18, 20–22, 24]. Three screening tools were frequently evaluated– DETECT algorithm, 2009 European Society of Cardiology (ESC)/ European Respiratory Society (ERS) guidelines, and Australian Scleroderma Interest Group (ASIG) algorithm.
Four of the eight studies evaluated the DETECT algorithm. In the first study, DETECT had better sensitivity (100% vs 91%) and NPV (100% vs 89%) compared to 2009 ESC/ERS guidelines and no PAH diagnoses were missed, whereas the 2009 ESC/ERS guidelines missed three PAH patients (8.5%) [18]. In the second study, similar findings were noted with DETECT and ASIG having better sensitivity (100%, 100%, 96%) and NPV (100%, 100%, 91%) in comparison to 2009 ESC/ERS guidelines, respectively; 2009 ESC/ERS guidelines missed one case of PAH [19]. In the third study, PPV estimates were compared between DETECT and 2009 ESC/ERS guidelines in 195 consecutive SSc patients [24]. Three patients were diagnosed with PAH in whom both algorithms recommended RHC referral; DETECT had a 6% PPV (95% CI 2–17%; 3/49 patients), 2009 ESC/ERS guidelines had a 18% PPV (95% CI 6–41%; 3/17 patients), and both DETECT and 2009 ESC/ERS had a 23% PPV (95% CI 8–50%; 3/13 patients). The fourth study modified the DETECT algorithm by substituting right atrial area in step 2 with the variable (1.4 X right ventricle diameter)2 [22]. Using this modified DETECT algorithm, 41% of patients were recommended for RHC compared to 24% when using the 2009 ESC/ERS guidelines; 7 (12%) patients screened using both tools had PAH on RHC.
ASIG was also compared to the 2009 ESC/ERS guidelines in the study by Hao et. al. ASIG had comparable sensitivity to 2009 ESC/ERC guidelines (94% vs 94% respectively), a better specificity (54% vs 32%) and NPV (92% vs 87%). This indicated better performance of the ASIG algorithm mainly due to a higher specificity (54%) (Table 2) [19].
In a consecutively enrolled, large SSc cohort, a six-variable PAH risk prediction model was created using multivariate regression [35]. In this model, ACA was associated with a 1.6-fold increased odds of developing SSc-PAH, esophageal stricture had a 2-fold increased odds, calcinosis had a 1.9-fold increased odds, digital ulcers had a 1.6-fold increased odds, mild ILD had a 2.3-fold increased odds, and sicca symptoms had a 1.6-fold increased odds of PAH. The sensitivity, specificity, PPV and NPV of this model was 100 %, 100%, 100%, and 92%, respectively.
In the analysis of two large SSc cohorts, combination of TRV and PFTs (DLCO% or FVC%/DLCO%) resulted in the detection of 97–100% of patients with RHC confirmed PAH (Table 2) [17]. By combining TTE and PFTs, a clear improvement in sensitivity and NPV was observed. For example, when using TRV >3.16 m/s alone as a screening tool, 36% of PAH cases were missed; however, when using a combination of TRV >3.16 m/s and FVC%/DLCO% ≥1.6, 91% of PAH cases were captured.
In the Australian Scleroderma Cohort Study (ASCS), another clinical decision-making algorithm using a composite of TRV and PFTs was used to screen for SSc-PAH (Supplementary material) [21]. Of the total cohort, 9.7% of patients had PAH identified on sequential screening. Patients with PAH diagnosed at follow-up screening compared to patients in whom PAH was diagnosed at baseline screening were more likely to have diffuse cutaneous SSc (p = 0.03), a better World Health Organization (WHO) Functional Class at PAH diagnosis (p = 0.01), and less advanced PAH based on higher mean 6-minute walk distance (p = 0.03).
4. DISCUSSION
In the current review, we set out to provide an update on the published screening parameters for detection of PAH in CTD over the past 5 years (2012–2017) through another systematic review for the 2018 WSPH. Our systematic review found similar evidence to what was reported in our previous review by Gladue et al. [5]. Despite the updated search, majority of the currently available literature on screening modailities for CTD-PAH are in the context of SSc, with scarce studies focusing on other CTDs. TTE and PFTs continue to play important roles as screening tools and have been increasingly incorporated into composite algorithms, such as ASIG and DETECT. In this systematic review, we are able to find robust data on the incorporation of composite measures like DETECT or ASIG for detection of PAH in SSc. In addition, there is emerging evidence on use of novel echocardiographic variables and CPET as screening tools. The majority of included studies had lower risk of bias and applicability concerns based on QUADAS-2.
Resting TTE remains the most common screening tool used for early detection of CTD-PAH, both as a single measure or part of a composite measure. The 2015 ESC/ERS guidelines for the diagnosis and treatment of PH recommends resting TTE as a screening test in asymptomatic patients with SSc, followed by annual screening with TTE, DLCO and biomarkers, and recommends TTE in symptomatic patients with other CTDs [7]. Beyond TRV, additional TTE parameters may be useful for the detection of PAH [25]. Two-dimensional speckle-tracking TTE appears to be a novel modality to detect subclinical right ventricular involvement in patients with SSc. Speckle-tracking TTE has identified decreased RV systolic strain rate and/or decreased RV strain globally and/or regionally in SSc subjects who have normal ranges of systolic PAP, tricuspid annular plane systolic excursion, and RV fractional area change when compared to healthy controls [36, 37]. Additional data is needed to assess feasibility and validity for use of speckle-tracking TTE for PAH screening.
Composite measures for PAH screening in CTD have been designed, studied, and used primarily in patients with SSc. The two main evidence-based screening algorithms developed for use in SSc are DETECT and ASIG. The original manuscript citing the development and internal validation of the DETECT algorithm was included in the systematic review by Gladue et al [4, 5]. In current systematic review, there were four additional studies evaluating the DETECT algorithm [18, 19, 22, 24]; two of those studies compared DETECT to the 2009 ESC/ERS guidelines and indicated that DETECT had 100% sensitivity and NPV and no missed PAH diagnoses [18, 19] providing external validation for DETECT. We identified two studies evaluating ASIG in comparison to 2009 ESC/ERS guidelines [19, 38]. In one study, ASIG sensitivity and NPV were high at 94% and 87%, respectively, and were similar to the sensitivity and NPV of the 2009 ESC/ERS guidelines [38]. In the second study, ASIG sensitivity and NPV were 100% compared to slightly lower values of 96% and 91%, respectively, for 2009 ESC/ERS guidelines, which missed one patient with PAH [19]. The study by Hao et. al. revealed that DETECT and ASIG worked well as screening tools and did not miss any PAH diagnoses [19]. It is important to highlight that DLCO and NT-ProBNP are part of step 1 of the DETECT algorithm and although TTE is not required before RHC in the ASIG algorithm, TTE is universally performed before RHC to rule out significant left ventricular dysfunction and/or significant aortic and/or mitral valve disease [4, 23].
Our systematic review captured additional studies combining TRV and PFTs as screening tools for PAH. DETECT incorporates use of TTE and PFTs for PAH screening in SSc and performs well as a screening tool [4]. In Gladue et al., combination of TRV and PFTs (DLCO% or FVC%/DLCO%) resulted in improved detection of PAH based on sensitivity and NPV compared to TTE or PFT alone [17].
All cohort studies included in this systematic review had pre-defined inclusion and exclusion criteria that enriched for PAH with increased pre-test probability of PAH based on signs/symptoms, TRV, PFTs, NT-proBNP and DETECT scores (Table 2), likely due to the invasive nature of RHC. This is an important consideration when reviewing the data.
Current guidelines and recommendations for PAH screening in SSc and scleroderma spectrum disorders are evidence or expert opinion based and largely incorporate the combination of screening tests or composite measures to determine if RHC is needed. In the past 5 years, the proposed recommendations largely endorse combination of TTE, PFTs and biomarkers, including DETECT, for PAH screening in SSc. The 2013 recommendations by Khanna et al. incorporate multiple screening tools including the use of TTE based on the 2009 ESC/ERS guidelines and composite measures including DLCO%, FVC%/DLCO%, NT-proBNP, signs/symptoms, and DETECT [6]. The 2013 WSPH recommended annual screening of asymptomatic patients with SSc spectrum of diseases, use of DETECT for asymptomatic SSc spectrum of diseases (but noted DETECT was only validated in patients with a DLCO < 60%), and patients with SSc and other CTDs with clinical signs and symptoms of PH should undergo RHC [39, 40]. Frequently used guidelines for PAH screening in CTD also come from the 2015 ESC/ERS guidelines [7]. The 2015 ESC/ERS guidelines primarily use TTE as a screening tool for PAH with levels of low, intermediate, and high risk for PH based on TRV, and if a patient has additional TTE signs suggesting PH, they can move up to the next level of TTE probability of PH risk [7]. The 2015 ESC/ERS guidelines also provide the following recommendations specific to patients with CTD: asymptomatic SSc patients should undergo resting TTE as a screening test; a combined approach (including biomarkers, PFTs, and TTE) should be considered to predict PH in SSc; initial screening using DETECT should be considered in adult SSc patients with > 3 years of disease duration and DLCO < 60%; annual screening with biomarkers, PFTs and TTE may be considered in patients with SSc, and SSc patients with a mPAP from 21 to 24 mmHg should be monitored closely due to a higher risk of PAH [7].
There continues to be interest in exploring and validating additional non-invasive screening tools for CTD-PAH. Our systematic review identified data describing use of exercise testing for PAH screening in SSc. In the study by Dumitrescu et al., peak VO2 at a threshold level of 13.8 mL/min/kg, had 87% sensitivity, 75% specificity, 57% PPV, and 93% NPV, and when a peak VO2 >18.7 mL/min/kg was used, all patients with PAH were excluded, and when a nadir minute ventilation/carbon dioxide production (VE/VCO2) of > 45.5 was used, all patients had PAH [29]. This is one of the first studies evaluating gas exchange measurements during exercise to screen for PAH non-invasively [29]. Stress TTE is another tool that has been described for detection of PAH in CTD. In our systematic review, a study by Kusunose et al. identified that by using stress TTE with a 6MWT in CTD patients, an exercise associated increase in mPAP relative to cardiac output (with a best cut-off value of >3.3 mm Hg/l/min) was suggestive of development of PAH [26].
SSc remains the primary CTD with data available on screening techniques for PAH according to our systematic review. This is similar to the findings in Gladue et al [11]. Current guidelines and recommendations also recognize patients along the SSc spectrum, who have other CTDs but have features of SSc, as needing to be screened similar to patients with SSc as was previously stated in 2013 recommendations by Khanna et al., 2013 WSPH recommendations, and 2015 ESC/ERS guidelines [6, 7, 39].There are few studies that have evaluated the prevalence of PAH in CTDs other than SSc, and many of those studies in non-SSc CTDs use TTE to diagnose PAH rather than the gold standard of RHC.
5. CONCLUSION
In summary, we know that screening for PAH in patients with CTD, especially SSc, is essential for early diagnosis, and data suggests early treatment can improve patient outcomes [8]. Over the past 5 years, there have been major contributions to the evidence for the various PAH screening methods in SSc. While TTE still plays a key role in PAH screening, there is evidence to support combined use of TTE and PFTs, especially when incorporated into novel screening algorithms (like DETECT or ASIG) to improve screening outcomes. Finding additional non-invasive screening techniques for SSc-PAH is of interest based on the growing data on novel techniques, such as CPET, right ventricular strain and stress TTE. Future studies will need to further evaluate these novel screening algorithms and cardiopulmonary changes during exercise in more longitudinal SSc cohorts. We need more longitudinal observational studies to develop and validate screening algorithms for PAH in non-SSc CTDs.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support and sponsorship: A. Young is supported by T32-AR007080-38. D. Khanna is supported by NIH/NIAMS K24 AR063120 and NIH/NIAMS R01 AR-07047.
Footnotes
CONFLICT OF INTEREST: Dr. Khanna has/had consultancy relationship with Actelion, Bayer, Biogen Idec, Bristol Myers Squibb, Cytori, Eicos, EMD Serono, Genentech/ Roche, Gilead, Glaxo SmithKline, InterMune, Lycera, Medac, Sanofi-Aventis/Genzyme, and Seattle Genetics and has received research funding from Bayer, Bristol Myers Squibb, Pfizer, Scleroderma Foundation and Pulmonary Hypertension Association.
REFERENCES
- [1].Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT, Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype, Chest 138(6) (2010) 1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steen VD, Medsger TA, Changes in causes of death in systemic sclerosis, 1972–2002, Ann Rheum Dis 66(7) (2007) 940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Avouac J, Airo P, Meune C, Beretta L, Dieude P, Caramaschi P, Tiev K, Cappelli S, Diot E, Vacca A, Cracowski JL, Sibilia J, Kahan A, Matucci-Cerinic M, Allanore Y, Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies, J Rheumatol 37(11) (2010) 2290–8. [DOI] [PubMed] [Google Scholar]
- [4].Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, MullerLadner U, Pope JE, Vonk MC, Doelberg M, Chadha-Boreham H, Heinzl H, Rosenberg DM, McLaughlin VV, Seibold JR, D.s. group, Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study, Ann Rheum Dis 73(7) (2014) 1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gladue H, Altorok N, Townsend W, McLaughlin V, Khanna D, Screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension: a systematic review, Seminars in arthritis and rheumatism 43(4) (2014) 536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst DE, Hachulla E, Humbert M, Langleben D, Mathai SC, Saggar R, Visovatti S, Altorok N, Townsend W, FitzGerald J, McLaughlin VV, Scleroderma F, Pulmonary Hypertension A, Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension, Arthritis Rheum 65(12) (2013) 3194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J, 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT), Eur Heart J 37(1) (2016) 67–119. [DOI] [PubMed] [Google Scholar]
- [8].Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, Gressin V, Guillevin L, Clerson P, Simonneau G, Hachulla E, Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival, Arthritis Rheum 63(11) (2011) 3522–30. [DOI] [PubMed] [Google Scholar]
- [9].Chung L, Chen H, Khanna D, Steen VD, Dyspnea assessment and pulmonary hypertension in patients with systemic sclerosis: utility of the University of California, San Diego, Shortness of Breath Questionnaire, Arthritis care & research 65(3) (2013) 454–63. [DOI] [PubMed] [Google Scholar]
- [10].McMahan Z, Schoenhoff F, Van Eyk JE, Wigley FM, Hummers LK, Biomarkers of pulmonary hypertension in patients with scleroderma: a case-control study, Arthritis Res Ther 17 (2015) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corrado A, Correale M, Mansueto N, Monaco I, Carriero A, Mele A, Colia R, Di Biase M, Cantatore FP, Nailfold capillaroscopic changes in patients with idiopathic pulmonary arterial hypertension and systemic sclerosis-related pulmonary arterial hypertension, Microvascular research 114 (2017) 46–51. [DOI] [PubMed] [Google Scholar]
- [12].Codullo V, Caporali R, Cuomo G, Ghio S, D’Alto M, Fusetti C, Borgogno E, Montecucco C, Valentini G, Stress Doppler echocardiography in systemic sclerosis: evidence for a role in the prediction of pulmonary hypertension, Arthritis Rheum 65(9) (2013) 2403–11. [DOI] [PubMed] [Google Scholar]
- [13].Iudici M, Codullo V, Giuggioli D, Riccieri V, Cuomo G, Breda S, Manfredi A, Iannace N, D’Alto M, Ghio S, Rossi R, Vizza CD, Caporali R, Valesini G, Ferri C, Valentini G, Pulmonary hypertension in systemic sclerosis: prevalence, incidence and predictive factors in a large multicentric Italian cohort, Clinical and experimental rheumatology 31(2 Suppl 76) (2013) 31–6. [PubMed] [Google Scholar]
- [14].Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, Q.−. Group, QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies, Ann Intern Med 155(8) (2011) 529–36. [DOI] [PubMed] [Google Scholar]
- [15].Avouac J, Meune C, Chenevier-Gobeaux C, Borderie D, Lefevre G, Kahan A, Allanore Y, Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide, Arthritis care & research 67(7) (2015) 1022–30. [DOI] [PubMed] [Google Scholar]
- [16].Chung L, Fairchild RM, Furst DE, Li S, Alkassab F, Bolster MB, Csuka ME, Derk CT, Domsic RT, Fischer A, Frech T, Gomberg-Maitland M, Gordon JK, Hinchcliff M, Hsu V, Hummers LK, Khanna D, Medsger TA, Molitor JA, Preston IR, Schiopu E, Shapiro L, Hant F, Silver R, Simms R, Varga J, Steen VD, Zamanian RT, Utility of B-type natriuretic peptides in the assessment of patients with systemic sclerosis-associated pulmonary hypertension in the PHAROS registry, Clinical and experimental rheumatology (2016). [PubMed] [Google Scholar]
- [17].Gladue H, Steen V, Allanore Y, Saggar R, Saggar R, Maranian P, Berrocal VJ, Avouac J, Meune C, Trivedi M, Khanna D, Combination of echocardiographic and pulmonary function test measures improves sensitivity for diagnosis of systemic sclerosis-associated pulmonary arterial hypertension: analysis of 2 cohorts, J Rheumatol 40(10) (2013) 1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guillen-Del Castillo A, Callejas-Moraga EL, Garcia G, Rodriguez-Palomares JF, Roman A, Berastegui C, Lopez-Meseguer M, Domingo E, Fonollosa-Pla V, Simeon-Aznar CP, High sensitivity and negative predictive value of the DETECT algorithm for an early diagnosis of pulmonary arterial hypertension in systemic sclerosis: application in a single center, Arthritis Res Ther 19(1) (2017) 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hao Y, Thakkar V, Stevens W, Morrisroe K, Prior D, Rabusa C, Youssef P, Gabbay E, Roddy J, Walker J, Zochling J, Sahhar J, Nash P, Lester S, Rischmueller M, Proudman SM, Nikpour M, A comparison of the predictive accuracy of three screening models for pulmonary arterial hypertension in systemic sclerosis, Arthritis Res Ther 17 (2015) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morrisroe K, Huq M, Stevens W, Rabusa C, Proudman SM, Nikpour M, Risk factors for development of pulmonary arterial hypertension in Australian systemic sclerosis patients: results from a large multicenter cohort study, BMC pulmonary medicine 16(1) (2016) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morrisroe K, Stevens W, Sahhar J, Rabusa C, Nikpour M, Proudman S, Australian Scleroderma Interest G, Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening programme, Arthritis Res Ther 19(1) (2017) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Soukup T, Pudil R, Kubinova K, Hromadkova L, Dusek J, Tosovsky M, Bradna P, Hrncir Z, Bures J, Application of the DETECT algorithm for detection of risk of pulmonary arterial hypertension in systemic sclerosis: data from a Czech tertiary centre, Rheumatology (Oxford) 55(1) (2016) 109–14. [DOI] [PubMed] [Google Scholar]
- [23].Thakkar V, Stevens WM, Prior D, Moore OA, Byron J, Liew D, Patterson K, Hissaria P, Roddy J, Zochling J, Sahhar J, Nash P, Tymms K, Celermajer D, Gabbay E, Youssef P, Proudman SM, Nikpour M, N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study, Arthritis Res Ther 14(3) (2012) R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vandecasteele E, Drieghe B, Melsens K, Thevissen K, De Pauw M, Deschepper E, Decuman S, Bonroy C, Piette Y, De Keyser F, Brusselle G, Smith V, Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort, Eur Respir J 49(5) (2017). [DOI] [PubMed] [Google Scholar]
- [25].Meune C, Khanna D, Aboulhosn J, Avouac J, Kahan A, Furst DE, Allanore Y, A right ventricular diastolic impairment is common in systemic sclerosis and is associated with other target-organ damage, Seminars in arthritis and rheumatism 45(4) (2016) 439–45. [DOI] [PubMed] [Google Scholar]
- [26].Kusunose K, Yamada H, Hotchi J, Bando M, Nishio S, Hirata Y, Ise T, Yamaguchi K, Yagi S, Soeki T, Wakatsuki T, Kishi J, Sata M, Prediction of Future Overt Pulmonary Hypertension by 6-Min Walk Stress Echocardiography in Patients With Connective Tissue Disease, J Am Coll Cardiol 66(4) (2015) 376–84. [DOI] [PubMed] [Google Scholar]
- [27].Yoo SJ, Park JH, Park Y, Lee JH, Sun BJ, Kim J, Yoo IS, Shim SC, Kang SW, Prevalence of Pulmonary Arterial Hypertension in Korean Adult Patients with Systemic Sclerosis: Result of a Pilot Echocardiographic Screening Study, Journal of cardiovascular ultrasound 24(4) (2016) 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sivova N, Launay D, Wemeau-Stervinou L, De Groote P, Remy-Jardin M, Denis G, Lambert M, Lamblin N, Morell-Dubois S, Fertin M, Lefevre G, Sobanski V, Le Rouzic O, Hatron PY, Wallaert B, Hachulla E, Perez T, Relevance of partitioning DLCO to detect pulmonary hypertension in systemic sclerosis, PloS one 8(10) (2013) e78001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dumitrescu D, Nagel C, Kovacs G, Bollmann T, Halank M, Winkler J, Hellmich M, Grunig E, Olschewski H, Ewert R, Rosenkranz S, Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis, Heart 103(10) (2017) 774–782. [DOI] [PubMed] [Google Scholar]
- [30].Kozij NK, Granton JT, Silkoff PE, Thenganatt J, Chakravorty S, Johnson SR, Exhaled Nitric Oxide in Systemic Sclerosis Lung Disease, Can Respir J 2017 (2017) 6736239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Morrisroe KB, Stevens W, Nandurkar H, Prior D, Thakkar V, Roddy J, Zochling J, Sahhar J, Tymms K, Sturgess A, Major G, Kermeen F, Hill C, Walker J, Nash P, Gabbay E, Youssef P, Proudman SM, Nikpour M, The association of antiphospholipid antibodies with cardiopulmonary manifestations of systemic sclerosis, Clinical and experimental rheumatology 32(6 Suppl 86) (2014) S-133–7. [PubMed] [Google Scholar]
- [32].Hachulla E, Jais X, Cinquetti G, Clerson P, Rottat L, Launay D, Cottin V, Habib G, Prevot G, Chabanne C, Fois E, Amoura Z, Mouthon L, Le Guern V, Montani D, Simonneau G, Humbert M, Sobanski V, Sitbon O, Pulmonary Arterial Hypertension Associated With Systemic Lupus Erythematosus: Results From the French Pulmonary Hypertension Registry, Chest (2017). [DOI] [PubMed] [Google Scholar]
- [33].Huang C, Li M, Liu Y, Wang Q, Guo X, Zhao J, Lai J, Tian Z, Zhao Y, Zeng X, Baseline Characteristics and Risk Factors of Pulmonary Arterial Hypertension in Systemic Lupus Erythematosus Patients, Medicine 95(10) (2016) e2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, Kawakami T, Fujita J, Kataoka M, Kimura K, Sano M, Daida H, Satoh T, Fukuda K, Human pentraxin 3 (PTX3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension, PloS one 7(9) (2012) e45834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Morrisroe K, Huq M, Stevens W, Rabusa C, Proudman SM, Nikpour M, Australian Scleroderma I, Risk factors for development of pulmonary arterial hypertension in Australian systemic sclerosis patients: results from a large multicenter cohort study, BMC pulmonary medicine 16 (2016) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Durmus E, Sunbul M, Tigen K, Kivrak T, Ozen G, Sari I, Direskeneli H, Basaran Y, Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension. Speckle-tracking echocardiographic study, Herz 40(4) (2015) 709–15. [DOI] [PubMed] [Google Scholar]
- [37].Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, Abraham TP, Shah AA, Unique Abnormalities in Right Ventricular Longitudinal Strain in Systemic Sclerosis Patients, Circ Cardiovasc Imaging 9(6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thakkar V, Stevens W, Prior D, Youssef P, Liew D, Gabbay E, Roddy J, Walker JG, Zochling J, Sahhar J, Nash P, Lester S, Rischmueller M, Proudman SM, Nikpour M, The inclusion of N-terminal pro-brain natriuretic peptide in a sensitive screening strategy for systemic sclerosis-related pulmonary arterial hypertension: a cohort study, Arthritis Res Ther 15(6) (2013) R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galie N, Simonneau G, The Fifth World Symposium on Pulmonary Hypertension, J Am Coll Cardiol 62(25 Suppl) (2013) D1–3. [DOI] [PubMed] [Google Scholar]
- [40].Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB, Definitions and diagnosis of pulmonary hypertension, J Am Coll Cardiol 62(25 Suppl) (2013) D42–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


