Abstract
Our goal was to establish two new predictive models of prostate cancer to determine whether to require a prostate biopsy when the prostate-specific antigen level is in the diagnostic gray zone. A retrospective analysis of 197 patients undergoing prostate biopsy with prostate-specific antigens between 4 and 10 ng ml−1 was conducted. Of these, 47 patients were confirmed to have cancer, while the remaining 150 patients were diagnosed with benign prostate disease after examining biopsy pathology. Two multivariate logistic regression models were established including age, prostate volumes, free/total prostate-specific antigen ratio, and prostate-specific antigen density using SPSS 19.0 to obtain the predicted probability and Logit P, and then, two receiver operating characteristic (ROC) curves were drawn to obtain the best cutoff value for prostate biopsy: one for the group of all the prostate cancers and one for the group of clinically significant prostate cancers. The best cutoff value for prostate biopsy was 0.25 from the multivariate logistic regression ROC curve model of all the prostate cancers, which gave a sensitivity of 75.4% and a specificity of 75.8%. The best cutoff value for prostate biopsy was 0.20 from the multivariate logistic regression model of clinically significant prostate cancers, which gave a sensitivity of 76.7% and a specificity of 80.1%. We identified the best cutoff values for prostate biopsy (0.25 for all prostate cancers and 0.20 for clinically significant prostate cancers) to determine whether to require prostate biopsy when the PSA level is in the diagnostic gray zone.
Keywords: predictive model, prostate biopsy, prostate cancer, prostate-specific antigen
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy of the male reproductive system.1 The incidence of PCa increases each year, but the mortality rate has not increased.2,3 Prostate-specific antibodies (PSA) are commonly used to screen for PCa, and while their sensitivity is high, their specificity is low.4 The free/total prostate-specific antibody ratio (F/T PSA) and prostate-specific antibody density (PSAD) versus PSA has a better specificity to guide decisions as whether to conduct a prostate biopsy. It is currently widely accepted that a prostate biopsy is required if the F/T is below or equal to 15%5 and the PSAD is above 0.15.6 Prostate biopsy is an invasive procedure that can come with physical and psychological distress. Some low-risk PCas can be dynamically monitored and do not necessarily require active treatment. Prostate biopsy pathology is often negative in many patients when the F/T is below or equal to 15% or the PSAD is above 0.15 in the diagnostic gray zone (4–10 ng ml−1).7 Therefore, we set out to determine if there is a better way to identify which patients will require a prostate biopsy when the diagnostic readings are in the gray zone. The purpose of this study was to establish two logistic regression models of PCa to determine whether to require a prostate biopsy when the PSA level is in the diagnostic gray zone (4–10 ng ml−1).
PARTICIPANTS AND METHODS
Participants
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants. One hundred and ninety-seven patients who underwent prostate biopsy from May 2016 to April 2018 were selected. The age of the patients ranged from 49 to 89 years old, with an average of 66.45 years old (standard deviation, s.d. = 8.04). The PSA of these patients was between 4 and 10 ng ml−1. All patients underwent transperineal prostate biopsy, and a clear pathological diagnosis was obtained. Of these, 47 cases of PCa (17 nonclinically significant prostate cancers N-CSPCa) with Gleason scores ≤6 and 30 clinically significant prostate cancers (CSPCa) with Gleason scores ≥7) and 150 cases of benign prostate disease were confirmed by pathological diagnosis.
Equipment and methods
This study was performed using a sonography scanner incorporating a 5–10 MHz rectal biplanar probe. The biopsy instrument used was an automatic biopsy gun and biopsy needle (18G).
All the patients were requested to take the free PSA (f-PSA) and total serum PSA (t-PSA) test before biopsy and then received prostate examination by transrectal ultrasonography. Prostate volume (PV) (left-right diameter × anteroposterior diameter × vertical diameter × 0.52) was calculated and then the F/T PSA and PSAD (t-PSA/PV) ratios were calculated. Transperineal prostate biopsy was performed according to the systematic 12-point biopsy, which was completed by a urologist, based on the guidance of a sonographer. If suspected malignant nodules were found, these nodules were biopsied again, separately. All specimens were sent for pathological examination.
Pathological diagnosis
All the prostate biopsy specimens required immunohistochemistry analysis and were diagnosed by two experienced pathologists. PCa was graded by the Gleason classification. The main growth mode and secondary growth mode were determined according to the heterogeneity and growth pattern of the tumor. The Gleason score was determined by this method with a minimum of 2 points and a maximum of 10 points.
Statistical analyses
SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. A t-test was used to compare ages, PV, t-PSA, f-PSA, F/T PSA, and PSAD values between PCa and noncancer groups. Two multivariate logistic regression models were established including age, PV, F/T PSA, and PSAD using SPSS 19.0 to obtain the predicted probability (PP) and Logit P, and two receiver operating characteristic (ROC) curves were drawn to obtain the best cutoff value for prostate biopsy.
RESULTS
The P values of age, PV, t-PSA, f-PSA, F/T PSA, and PSAD between the PCa and noncancer groups were 0.044, 0.001, 0.111, 0.191, 0.017, and <0.001, respectively. The P values of age, PV, t-PSA, f-PSA, F/T PSA, and PSAD between the nonclinically significant prostate cancer (N-CSPCa) and noncancer groups were 0.048, 0.003, 0.556, 0.919, 0.024, and 0.002, respectively. The P values of age, PV, t-PSA, f-PSA, F/T PSA, and PSAD between the CSPCa and noncancer groups were 0.037, 0.001, 0.097, 0.056, 0.016, and 0.001, respectively (Table 1).
Table 1.
Characteristics of all patients included in the study
| Total | Cancer | Noncancer | P | N-CSPCaa | P | CSPCab | P | |
|---|---|---|---|---|---|---|---|---|
| Number | 197 | 47 | 150 | 17 | 30 | |||
| Age (year) | 66.45±8.04 | 68.51±7.89 | 65.81±8.00 | 0.044 | 67.12±7.52 | 0.048 | 69.30±8.10 | 0.037 |
| PV (cc) | 44.20±18.44 | 35.82±19.22 | 46.83±17.44 | 0.001 | 37.68±23.99 | 0.003 | 34.76±16.29 | 0.001 |
| t-PSA (ng ml−1) | 7.05±1.64 | 7.38±1.65 | 6.93±1.62 | 0.111 | 7.21±1.81 | 0.556 | 7.48±1.58 | 0.097 |
| f-PSA (ng ml−1) | 1.35±1.08 | 1.20±0.76 | 1.39±1.16 | 0.191 | 1.37±1.06 | 0.919 | 1.15±0.52 | 0.056 |
| F/T PSA | 0.18±0.07 | 0.16±0.07 | 0.19±0.07 | 0.017 | 0.17±0.08 | 0.024 | 0.15±0.07 | 0.016 |
| PSAD (ng ml−1 cc−1) | 0.19±0.09 | 0.25±0.17 | 0.17±0.07 | <0.001 | 0.23±0.10 | 0.002 | 0.26±1.30 | 0.001 |
aN-CSPCa vs noncancer; bCSPCa vs noncancer. PV: prostate volume (cc); t-PSA: total prostate-specific antigen (ng ml−1); f-PSA: free prostate-specific antigen (ng ml−1); F/T PSA: free/total PSA ratio; PSAD: PSA density (ng ml−1 cc−1); N-CSPCa: nonclinically significant prostate cancers; CSPCa: clinically significant prostate cancers
Univariate and multivariate logistic regression analyses demonstrated that all clinical variables predicted PCa (Table 2) and CSPCa (Table 3). The multivariate logistic regression prediction model for all PCas was established including age, PV, F/T PSA, and PSAD as follows: Logit P = 7.217, -0.088 ages, +0.016 PV, +4.064 F/T PSA, –7.430 PSAD. The multivariate logistic regression prediction model for CSPCa was established including age, PV, F/T PSA, and PSAD as follows: Logit P = -8.832, +0.123 age, -0.025 PV, -8.014 F/T PSA, +6.415 PSAD.
Table 2.
Univariate and multivariate binary logistic regression analysis testing the value of clinical variables in predicting prostate cancer (all the prostate cancer)
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | Standard error | P | OR (95% CI) | Standard error | P | |
| Age (year) | 0.960 (0.921–0.999) | 0.021 | 0.046 | 0.915 (0.868–0.965) | 0.027 | 0.001 |
| PV (cc) | 1.044 (1.019–1.071) | 0.013 | 0.001 | 1.016 (0.981–1.051) | 0.018 | 0.038 |
| F/T PSA | 394.708 (2.569–60640.494) | 2.569 | 0.020 | 58.204 (0.079–43133.384) | 3.372 | 0.028 |
| PSAD (ng−1 ml−1 cc−1) | 0.000 (0.000–0.000) | 2.156 | <0.001 | 0.001 (0.000–0.323) | 3.215 | 0.021 |
PV: prostate volume (cc); PSA: prostate-specific antigen; F/T PSA: free/total PSA ratio; PSAD: PSA density (ng ml−1 cc−1); CI: confidence interval; OR: odds ratio
Table 3.
Univariate and multivariate binary logistic regression analysis testing the value of clinical variables in predicting prostate cancer (clinically significant prostate cancers)
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | Standard error | P | OR (95% CI) | Standard error | P | |
| Age (year) | 1.051 (1.004–1.105) | 0.024 | 0.033 | 1.131 (1.058–1.209) | 0.034 | <0.001 |
| PV (cc) | 0.947 (0.917–0.978) | 0.017 | 0.001 | 0.975 (0.931–1.021) | 0.023 | 0.027 |
| F/T PSA | 0.001 (0.000–0.000) | 3.209 | 0.020 | 0.000 (0.000–1.703) | 4.360 | 0.036 |
| PSAD (ng ml−1 cc−1) | 12778.924 (115.009–1419892.645) | 2.403 | <0.001 | 611.185 (0.668–558876.894) | 3.579 | 0.025 |
PSA: prostate-specific antigen; PV: prostate volume (cc); F/T PSA: free/total PSA ratio; PSAD: PSA density (ng ml−1 cc−1); CI: confidence interval; OR: odds ratio
The area under the ROC curve (AUC) of the multivariate logistic regression model, F/T PSA and PSAD for all PCa patients was 0.775 with a 95% CI (0.695, 0.856), 0.609 with a 95% CI (0.510, 0.707) and 0.736 with a 95% CI (0.653, 0.820), and the standard error was 0.041,0.050 and 0.043 respectively. The best cutoff value for PP was 0.25, at which the sensitivity was 75.4% and the specificity was 75.8%, with a positive likelihood ratio of 3.125 and a negative likelihood ratio of 0.329. In the AUC of F/T PSA, when the sensitivity was 75.4%, the specificity was only 46.3%, with a positive likelihood ratio of 1.389 and a negative likelihood ratio of 0.543. In the AUC of PSAD, when the sensitivity was 75.4%, the specificity was only 54.6%, with a positive likelihood ratio of 1.667 and a negative likelihood ratio of 0.455 (Figure 1).
Figure 1.
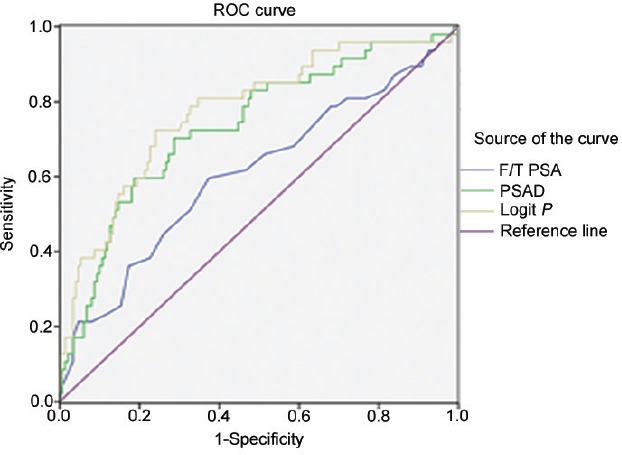
The AUC of F/T PSA, PSAD, and the multivariate logistic regression model for all PCas. AUC: area under the curve; F/T PSA: free/total prostate-specific antibody ratio; PSAD: prostate-specific antibody density; ROC: receiver operating characteristic.
The AUC of a multivariate logistic regression model, F/T PSA and PSAD for CSPCa was 0.819 with a 95% CI (0.732, 0.906), 0.623 with a 95% CI (0.509, 0.737) and 0.750 with a 95% CI (0.648, 0.851), and the standard error was 0.044, 0.058 and 0.052 respectively. The best cutoff value for PP was 0.20, at which the sensitivity was 76.7% and the specificity was 80.1%, with a positive likelihood ratio of 3.850 and a negative likelihood ratio of 0.288. In the AUC of F/T PSA, when the sensitivity was 76.7%, the specificity was only 42.2%, with a positive likelihood ratio of 1.328 and a negative likelihood ratio of 0.548. In the AUC of PSAD, when the sensitivity was 76.7%, the specificity was only 53.3%, with a positive likelihood ratio of 1.638 and a negative likelihood ratio of 0.434 (Figure 2).
Figure 2.
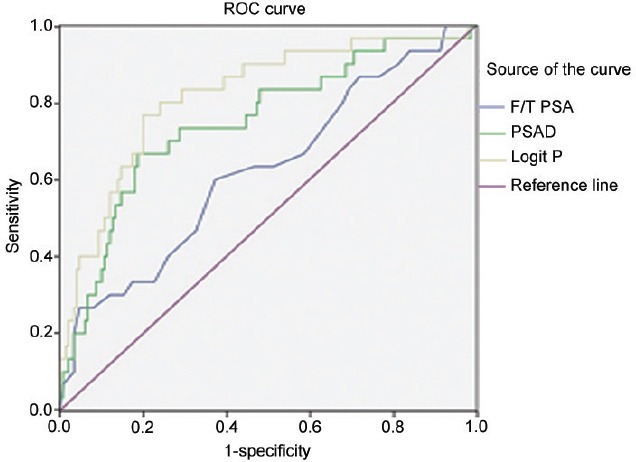
The AUC of F/T PSA, PSAD, and the multivariate logistic regression model for CSPCas. AUC: area under the curve; F/T PSA: free/total prostate-specific antibody ratio; PSAD: prostate-specific antibody density; ROC: receiver operating characteristic.
DISCUSSION
Serum PSA, as a marker of PCa, is a useful tool for the diagnosis of early-stage asymptomatic PCa.4 PSA is a serine protease secreted by prostate epithelial cells and a glycoprotein that is secreted directly into the prostate duct system. Its normal function is to assist in the liquefaction of semen clots, which is important for male fertility. There is a blood–epithelial barrier present around the normal prostate catheter system, which prevents the PSA produced by the prostate epithelium from entering the blood directly, thus maintaining a low concentration of PSA in the blood. PSA is a prostate-specific antigen with high sensitivity but low specificity. PSA increases in PCa, as well as in benign prostate diseases such as prostate hyperplasia and prostatitis.4
The PSA levels in prostate hyperplasia and PCa overlap, in large part, at a range of 4–10 ng ml−1. It is difficult to distinguish PCa from prostate hyperplasia based on PSA levels in this range.4 PSA can be present in the serum in both free and bound states. Free PSA is the fraction of PSA that is not bound in serum, which is denoted as f-PSA, and total serum PSA including free and bound states is represented as t-PSA. The f-PSA concentration is lower in cancer patients than in benign hyperplasia patients. This difference is clinically applied to distinguish early PCa from benign prostate hyperplasia. F/T PSA can assist in the distinction between PCa and benign hyperplasia.8 The reference value is 15%, which means that a ratio below or equal to 15% is likely indicative of PCa. The lower the percentage of f-PSA is, the higher the probability of PCa. Recent studies have shown that f-PSA levels are unstable in serum. The distribution of the F/T is relatively discrete, the correlation is not significant, and it is inaccurate to screen and diagnose PCa in the gray zone based on F/T.9
PSAD refers to the ratio of serum PSA concentration to prostate volume. The volume of the prostate can be measured by ultrasonic instruments. If a patient with a small prostate and moderate serum PSA level is found, there is often the possibility of PCa. The same PSA value for a patient with a large prostate volume is likely indicative of benign prostate hyperplasia. PSAD, therefore, offers better guidance in the decision as to whether to conduct a prostate biopsy when PSA values are in the gray zone.10,11,12 In the past, a PSAD value above 0.15 necessitated prostate biopsy, but the pathological results were often negative.13 Here, in our study, we not only considered F/T PSA and PSAD, but also considered other factors such as age, PV, t-PSA, and f-PSA. There was a statistically significant difference in age (P = 0.044), PV (P = 0.001), F/T PSA (P = 0.017), and PSAD (P < 0.001) and no significant difference in t-PSA (P = 0.111) and f-PSA (P = 0.191) between PCa and non-PCa patients. There was a statistically significant difference in age (P = 0.037), PV (P = 0.001), F/T PSA (P = 0.016), and PSAD (P = 0.001) and no significant difference in t-PSA (P = 0.097) and f-PSA (P = 0.056) between CSPCa and non-PCa patients. The multivariate logistic regression prediction model for all PCaw was established including age, PV, F/T PSA, and PSAD as follows: Logit P = 7.217, -0.088 ages, +0.016 PV, +4.064 F/T PSA, -7.430 PSAD. The multivariate logistic regression prediction model for CSPCa was established including age, PV, F/T PSA, and PSAD as follows: Logit P = -8.832, +0.123 ages, -0.025 PV, -8.014 F/T PSA, +6.415 PSAD. However, we identified two better cutoff values for all PCa and CSPCa. The best cutoff value in this study was 0.25 in the multivariate logistic ROC curve regression model for all PCas, which resulted in an AUC of 0.775, a sensitivity of 75.4%, and a specificity of 75.8%. The best cutoff value in this study was 0.20 in a multivariate logistic ROC curve regression model for all clinically significant PCas, which resulted in an AUC of 0.819, a sensitivity of 76.7%, and a specificity of 80.1%. The sensitivity (75.4%) and the specificity (75.8%) of all the PCas and the sensitivity (76.7%) and the specificity (80.1%) of the CSPCas were no less than and perhaps even better than other studies compared with our study.14,15
PCa is a common cause of malignancies in men.1 The incidence of PCa is increasing, while the mortality rate has not increased.2,3 This suggests that some PCa patients have low malignancy potential. A systematic 12-point biopsy is generally used to diagnose PCa,16 which takes a longer time and requires more specimens, making it quite an invasive procedure. However, without proper tools to distinguish benign from malignant prostate disease, there exists the possibility for overdiagnosis and unnecessary biopsy.17,18
Low-risk PCas usually have a fairly good prognosis and can be closely monitored without immediate surgery.17,18 Even if found in an advanced stage, there are many methods for the treatment of PCa, and the morbidity and mortality is fairly low. High-risk PCa patients need to be actively treated.19 Therefore, we suggest that changing the criteria for biopsy would result in a lower risk of performing an unnecessary invasive biopsy procedures, while not increasing the risk to the patient's health. In our study, the best cutoff value was 0.25 in the ROC curve of a new predictive model for all PCa patients and 0.20 for CSPCa patients, which resulted in an AUC of 0.775 versus 0.609 for F/T PSA, and 0.736 for PSAD for the PCa group. The AUC was 0.819 compared with F/T PSA (AUC = 0.623) and PSAD (AUC = 0.750) for the CSPCa group. When the sensitivity was 75.4%, the specificity of the new predictive model for all the PCas, F/T PSA, and PSAD was 75.8%, 46.3%, and 54.6%, respectively. When the sensitivity was 76.7%, the specificity of the new predictive model for CSPCa, F/T PSA, and PSAD was 80.1%, 42.2%, and 53.3%, respectively. Many of these biopsies will be negative and ultimately put the patient through unnecessary discomfort solely based on F/T PSA or PSAD values. In the PCa group, when the F/T PSA was 15%, the sensitivity was low (55.2%), and the specificity was not high (64.8%). In the CSPCa group, when the F/T PSA was 15%, the sensitivity was also low (51.8%), and the specificity was also not high (58.3%). Therefore, it is easy to escape diagnosis or be misdiagnosed, especially in the CSPCa group, which often leads to a poor prognosis. In the PCa group, when the PSAD was 0.15, the sensitivity was indeed high (85.2%), but its specificity was very low (45.6%). In the CSPCa group, when the PSAD was 0.15, the sensitivity was also high (83.2%), but the specificity was also very low (46.7%). Therefore, it is easy for patients to be misdiagnosed and undergo unnecessary biopsy.
CONCLUSION
We suggest that the best cutoff value is 0.25 in the new predictive model for the PCa group and 0.20 for the CSPCa group, which would be more sensitive and specific for indicating a prostate biopsy when values are in the diagnostic gray zone, all without posing a significant risk to the patient. It could also be used as a measure to assess treatment impact or monitor progression.
AUTHOR CONTRIBUTIONS
JL participated in literature research, clinical studies, manuscript preparation, and manuscript editing. ZQW participated in clinical studies and statistical analysis. ML participated in clinical studies and statistical analysis. MYZ participated in clinical studies data analysis. YFY participated in clinical studies data analysis. WWZ participated in study concepts and design and guaranteed integrity of the entire study.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Alonzo DG, Mure AL, Soloway MS. Prostate cancer and the increasing role of active surveillance. Postgrad Med. 2013;125:109–16. doi: 10.3810/pgm.2013.09.2705. [DOI] [PubMed] [Google Scholar]
- 2.Stattin P, Carlsson S, Holmström B, Vickers A, Hugosson J, et al. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst. 2014;106:dju007. doi: 10.1093/jnci/dju007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt TJ, Dahm P. PSA Screening for prostate cancer: why saying no is a high-value health care choice. J Natl Compr Canc Netw. 2015;13:1566–74. doi: 10.6004/jnccn.2015.0182. [DOI] [PubMed] [Google Scholar]
- 4.Inahara M, Suzuki H, Kojima S, Komiya A, Fukasawa S, et al. Improved prostate cancer detection using systematic 14-core biopsy for large prostate glands with normal digital rectal examination findings. Urol. 2006;68:815–9. doi: 10.1016/j.urology.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Ceylan C, Gazel E, Keleş İ, Doluoǧlu Ö, Yıǧman M. Can the free/total PSA ratio predict the Gleason score before prostate biopsy? Curr Urol. 2016;9:24–7. doi: 10.1159/000442846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha YS, Yu J, Salmasi AH, Patel N, Parihar J, et al. Prostate-specific antigen density toward a better cutoff to identify better candidates for active surveillance. Urol. 2014;84:365–71. doi: 10.1016/j.urology.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Castro HA, Lared W, Santos JE, Solha RS, Shigueoka DC, et al. Impact of PSA density of transition zone as a potential parameter in reducing the number of unnecessary prostate biopsies in patients with PSA levels between 26 and 100 ng/mL. Int Braz J Urol. 2018;44:709–16. doi: 10.1590/S1677-5538.IBJU.2017.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur V, Singh PP, Talwar M, Mukherjee U. Utility of free/total prostate specific antigen (f/t PSA) ratio in diagnosis of prostate carcinoma. Dis Markers. 2004;19:287–92. doi: 10.1155/2004/913870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachour DM, Chahin E, Al-Fahoum S. Human kallikrein-2, prostate specific antigen and free- prostate specific antigen in combination to discriminate prostate cancer from benign diseases in Syrian patients. Asian Pac J Cancer Prev. 2015;16:7085–8. doi: 10.7314/apjcp.2015.16.16.7085. [DOI] [PubMed] [Google Scholar]
- 10.Schoots IG, Osses DF, Drost FH, Verbeek JF, Remmers S, et al. Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease. Transl Androl Urol. 2018;7:132–44. doi: 10.21037/tau.2017.12.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ujike T, Uemura M, Kawashima A, Nagahara A, Fujita K, et al. A novel model to predict positive prostate biopsy based on serum androgen level. Endocr Relat Cancer. 2018;25:59–67. doi: 10.1530/ERC-17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saema A, Kochakarn W, Lertsithichai P. PSA density and prostate cancer detection. J Med Assoc Thai. 2012;95:661–6. [PubMed] [Google Scholar]
- 13.Udeh EI, Nnabugwu II, Ozoemena FO, Ugwumba FO, Aderibigbe AS, et al. Prostate-specific antigen density values among patients with symptomatic prostatic enlargement in Nigeria. World J Surg Oncol. 2016;14:174. doi: 10.1186/s12957-016-0921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Li ZZ, Huang YL, Song HJ, Wang YJ. Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/ml: a meta-analysis. Medicine (Baltimore) 2018;97:e0249. doi: 10.1097/MD.0000000000010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng ZJ, Xue C, Wen JM, Li Y, Wang M, et al. PSAD Test in the diagnosis of prostate cancer: a meta-analysis. Clin Lab. 2017;63:147–55. doi: 10.7754/Clin.Lab.2016.160727. [DOI] [PubMed] [Google Scholar]
- 16.Chun FK, Epstein JI, Ficarra V, Freedland SJ, Montironi R, et al. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol. 2010;58:851–64. doi: 10.1016/j.eururo.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Wang SC, Chen CC, Yang CK, Hung SW, Jan YJ, et al. Pathological outcomes in men with prostate cancer who are eligible for active surveillance. J Chin Med Assoc. 2018;81:348–51. doi: 10.1016/j.jcma.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers L, Peer CJ, Figg WD. Diagnosis, staging, and risk stratification in prostate cancer: utilizing diagnostic tools to avoid unnecessary therapies and side effects. Cancer Biol Ther. 2017;18:470–2. doi: 10.1080/15384047.2017.1323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebesta EM, Anderson CB. The surgical management of prostate cancer. Semin Oncol. 2017;44:347–57. doi: 10.1053/j.seminoncol.2018.01.003. [DOI] [PubMed] [Google Scholar]


