Abstract
This study aimed to further validate the prognostic role of fibrinogen in upper tract urothelial carcinoma (UTUC) in a large Chinese cohort. A total of 703 patients who underwent radical nephroureterectomy were retrospectively identified. Fibrinogen levels of ≥4.025 g l−1 were defined as elevated. Logistic regression analysis was performed to determine the association between fibrinogen and adverse pathological features. Kaplan–Meier analysis and Cox regression models were used to assess the associations of fibrinogen with cancer-specific survival (CSS), disease recurrence-free survival (RFS), and overall survival (OS). Harrell c-index and decision curve analysis were used to assess the clinical utility of multivariate models. The median follow-up duration was 42 (range: 1–168) months. Logistic regression analysis revealed that elevated fibrinogen was associated with higher tumor stage and grade, lymph node involvement, lymphovascular invasion, sessile carcinoma, concomitant variant histology, and positive surgical margins (all P < 0.05). Multivariate Cox regression analysis demonstrated that elevated fibrinogen was independently associated with decreased CSS (hazard ratio [HR]: 2.33; P < 0.001), RFS (HR: 2.09; P < 0.001), and OS (HR: 2.09; P < 0.001). The predictive accuracies of the multivariate models were improved by 3.2%, 2.0%, and 2.8% for CSS, RFS, and OS, respectively, when fibrinogen was added. Decision curve analysis showed an added benefit for CSS prediction when fibrinogen was added to the model. Preoperative fibrinogen may be a strong independent predictor of worse oncologic outcomes in UTUC; therefore, it may be valuable to apply this marker to the current risk stratification in UTUC.
Keywords: fibrinogen, prognosis, radical nephroureterectomy, upper tract urothelial carcinoma
INTRODUCTION
Radical nephroureterectomy (RNU) with bladder cuff excision is the current standard of care for upper tract urothelial carcinoma (UTUC). However, postoperative survival outcomes are still unsatisfactory, and a large portion of patients inevitably experience disease recurrence and possible death after surgery. Therefore, it is essential to identify patients who might experience the greatest benefit from RNU and neoadjuvant therapy.1,2
Thus far, some preoperative and postoperative factors have been used in the risk stratification of UTUC, based on the European Association of Urology (EAU) guidelines.3 Although tumor stage and grade have been commonly adopted by most urologists to assess the prognosis of patients with cancer, many researchers have begun to explore the potential roles of certain blood-based biomarkers in the risk stratification of UTUC.
Increasing evidence suggests that specific homeostatic factors might play a pivotal role in tumor invasion and metastasis.4,5,6 Plasma fibrinogen, one of the major components in the coagulation pathway, is often synthesized in large quantities by cancer cells.7 A growing body of literature has indicated the association of elevated fibrinogen levels with worse survival outcomes in prostate,8 ovarian,9 lung,10 bladder,11 and renal cell cancers.12 Similarly, several studies have investigated the predictive value of fibrinogen in patients with UTUC.13,14,15 Nevertheless, most of these were small sample studies with varying cutoff values for fibrinogen, likely limiting, to some extent, its clinical value for prognostic evaluation.
Therefore, the present study aimed to further validate whether fibrinogen can provide an independent parameter for the assessment of pathological and survival outcomes after RNU in a large cohort with UTUC. In addition, because neutrophil-to-lymphocyte ratio (NLR) is the only serum biomarker recommended in UTUC based on EAU guidelines,3 we also sought to assess the clinical utility of fibrinogen versus NLR in multivariate models.
PATIENTS AND METHODS
Study population
This study was approved by the Committee for Ethics of West China Hospital, Chengdu, China. Between January 2003 and December 2016, 820 consecutive patients were pathologically diagnosed as having UTUC after RNU at the Department of Urology & Institute of Urology, West China Hospital, Chengdu, China. In this study, patients' data were collected from their clinical medical records, and the formal consent is not required due to its respective nature. We excluded patients with coagulation-related diseases or prior anticoagulant therapy (n = 7); inflammatory or autoimmune diseases (n = 17); nonurothelial carcinomas (n = 13); lack of preoperative fibrinogen data (n = 16); and those who were lost to first follow-up (n = 64). Finally, a total of 703 patients with available fibrinogen data within 2 weeks before surgery were qualified and included in our study for further analysis.
All patients had received RNU, which was performed according to the standard procedures (dissection of the kidney with the whole length of the ureter and open bladder cuff excision). Open or laparoscopic RNU was performed in accordance with the urologists' judgment. Lymph node dissection was performed only in patients with suspected enlarged lymph nodes, which were confirmed via preoperative radiology assessment (computed tomography or magnetic resonance imaging) or intraoperative discovery. When lymph node dissection was performed, the following templates were used: for tumors located in the right pelvis and the upper and middle ureter, dissection included the right renal hilar, paracaval, retrocaval, and inter-aortocaval nodes; for tumors located in the left pelvis and the upper and middle ureter, dissection included the left renal hilar and para-aortic nodes; and for tumors located in the lower ureter, dissection included the ipsilateral common iliac, external iliac, obturator, internal iliac, and presacral nodes.
Clinicopathological evaluation
All RNU specimens were independently re-evaluated by two experienced pathologists. Tumor grade and stage were determined based on the World Health Organization/International Society of Urologic Pathology classification of 2004 and the 2002 Union for International Cancer Control tumor node metastasis (TNM) classification system, respectively.4 Information on tumor architecture (sessile or papillary), lymphovascular invasion (LVI), positive surgical margins (PSM), and concomitant variant histology (CVH, urothelial carcinomas with abnormal histological differentiation) was retrieved from related pathological reports. Preoperative laboratory data, including fibrinogen level, platelet count, white blood cell (WBC) count, alkaline phosphatase (ALP) level, lactate dehydrogenase (LDH) level, NLR, and albumin-to-globulin ratio (AGR), were collected within 2 weeks before surgery (if more than one report was available, the most recent one was recorded). NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count; AGR was defined as the value of albumin divided by the value of globulin. The optimal cutoff value for fibrinogen was defined as 4.025 g l−1, based on the receiver-operating characteristic (ROC) curves. The cutoff values for WBC,16 platelet count,16 ALP,17 LDH,18 NLR,19 and AGR17 were determined as previously reported. In addition, other information, including age, sex, body mass index (BMI), smoking status, tumor side and location, bladder cancer status, hydronephrosis, and multifocality, was documented from the medical record of each patient.
Follow-up
Patients were followed up every 3 months in the first year after RNU, every 6 months for the next 2 years, and annually thereafter. Physical examinations, blood laboratory tests, and chest radiography assessments were routinely performed. Computed tomography or magnetic resonance imaging analyses were performed every year or upon suspected recurrence of the disease.
Disease recurrence was defined as recurrence from the operating site, regional or distant lymph nodes, and/or visceral metastasis. Cancer-specific survival (CSS) was defined as the time from RNU to cancer-related death. Disease recurrence-free survival (RFS) was defined as the time from RNU to disease recurrence. Overall survival (OS) was defined as the time from RNU to death from all causes.
Statistical analyses
Student's t-test and the Chi-squared test were used to evaluate continuous variables and dichotomous variables. Associations between fibrinogen and adverse pathological outcomes were assessed using logistic regression analysis, in which odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated. Probabilities of CSS, RFS, and OS were determined using Kaplan–Meier curves. The log-rank test was used to assess the differences between groups. Multivariate Cox proportional hazard models with forward stepwise methods were used to assess the risk factors for CSS, RFS, and OS. C-index was calculated to assess the improvement in discrimination when adding preoperative laboratory factors to the base model, using the R package “survival.” Decision curve analyses were performed to show the benefit of multivariate models that contained preoperative biomarkers. A two-sided probability (P) value of <0.05 was considered statistically significant. Statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) Statistics version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
Of the 703 patients who exhibited UTUC, the median age at RNU surgery was 67 (interquartile range: 59–74) years and the mean fibrinogen level was 3.8 (standard deviation [s.d.]: 1.3) g l−1. Low- and high-grade UTUC were observed in 186 (26.5%) and 517 (73.5%) patients, respectively. Positive lymph nodes were found in 67 (9.5%) patients. Two hundred and eighty-seven (40.8%) patients had received postoperative adjuvant chemotherapy, and none of the patients had received neoadjuvant therapy. Patients were dichotomized into a high fibrinogen group (fibrinogen ≥4.025 g l−1) and a low fibrinogen group (fibrinogen <4.025 g l−1) by ROC curves (Supplementary Figure 1 (905.9KB, tif) ). The area under the ROC curve was 0.689, and the Youden index was 0.316, with a sensitivity of 57.8% and a specificity of 74.7%. There were no significant differences between groups regarding age, BMI, smoking status, tumor side and location, bladder cancer status, hydronephrosis, and adjuvant therapy (each P > 0.05). However, differences were observed in terms of gender, multifocality, surgical approach, tumor grade and stage, lymph node status, tumor size, PSM, tumor architecture, CVH, and laboratory biomarkers (i.e., WBC, platelet count, ALP, LDH, NLR, and AGR) (all P < 0.05; Table 1).
Table 1.
Patients’ characteristics in the present study
| Variables | Total (n=703) | Fibrinogen <4.025 g l−1 (n=454, 64.6%) | Fibrinogen ≥4.025 g l−1 (n=249, 35.4%) | P |
|---|---|---|---|---|
| Age (year), mean±s.d. | 65.8±11.4 | 66.1±11.3 | 65.4±11.4 | 0.680 |
| Gender, n (%) | ||||
| Male | 399 (56.8) | 276 (60.8) | 123 (49.4) | 0.004 |
| Female | 304 (43.2) | 178 (39.2) | 126 (50.6) | |
| BMI (kg m−2), mean±s.d. | 22.6±4.6 | 22.6±4.7 | 22.5±4.4 | 0.470 |
| Smoking status, n (%) | ||||
| No | 502 (71.4) | 313 (68.9) | 189 (75.9) | 0.051 |
| Former/current | 201 (28.6) | 141 (31.1) | 60 (24.1) | |
| Tumor side, n (%) | 0.216 | |||
| Left | 359 (51.1) | 224 (49.3) | 135 (54.2) | |
| Right | 344 (48.9) | 230 (50.7) | 114 (45.8) | |
| Bladder cancer status, n (%) | 0.688 | |||
| No | 602 (85.6) | 386 (85.0) | 216 (86.7) | |
| Previous | 22 (3.1) | 16 (3.5) | 6 (2.4) | |
| Concomitant | 79 (11.2) | 52 (11.5) | 27 (10.8) | |
| Hydronephrosis, n (%) | 0.166 | |||
| No | 264 (37.6) | 179 (39.4) | 85 (34.1) | |
| Yes | 439 (62.4) | 275 (60.6) | 164 (65.9) | |
| Tumor location, n (%) | 0.115 | |||
| Pelvicalyceal | 376 (53.5) | 233 (51.3) | 143 (57.4) | |
| Ureteric | 203 (28.9) | 143 (31.5) | 60 (24.1) | |
| Both | 124 (17.6) | 78 (17.2) | 46 (18.5) | |
| Multifocality, n (%) | 0.032 | |||
| No | 587 (83.5) | 369 (81.3) | 218 (87.6) | |
| Yes | 116 (16.5) | 85 (18.7) | 31 (12.4) | |
| Surgical approach, n (%) | <0.001 | |||
| Open RNU | 473 (67.3) | 284 (62.6) | 189 (75.9) | |
| Laparoscopic RNU | 230 (32.7) | 170 (37.4) | 60 (24.1) | |
| Tumor grade, n (%) | <0.001 | |||
| Low | 186 (26.5) | 156 (34.4) | 30 (12.0) | |
| High | 517 (73.5) | 298 (65.6) | 219 (88.0) | |
| pT stage, n (%) | <0.001 | |||
| pTis, pTa, pT1 | 217 (30.9) | 171 (37.7) | 46 (18.5) | |
| pT2 | 145 (20.6) | 109 (24.0) | 36 (14.5) | |
| pT3 | 241 (34.3) | 141 (31.1) | 100 (40.2) | |
| pT4 | 100 (14.2) | 33 (7.3) | 67 (26.9) | |
| Lymph node status, n (%) | <0.001 | |||
| pN0 | 89 (12.7) | 56 (12.3) | 33 (13.3) | |
| pNx | 547 (77.8) | 373 (82.2) | 174 (69.9) | |
| pN+ | 67 (9.5) | 25 (5.5) | 42 (16.9) | |
| LVI, n (%) | <0.001 | |||
| No | 596 (84.8) | 403 (88.8) | 193 (77.5) | |
| Yes | 107 (15.2) | 51 (11.2) | 56 (22.5) | |
| Tumor size (cm), n (%) | <0.001 | |||
| ≤3 | 225 (32.0) | 166 (36.6) | 59 (23.7) | |
| >3 | 478 (68.0) | 288 (63.4) | 190 (76.3) | |
| PSM, n (%) | 0.001 | |||
| No | 646 (91.9) | 429 (94.5) | 217 (87.1) | |
| Yes | 57 (8.1) | 25 (5.5) | 32 (12.9) | |
| Tumor architecture, n (%) | <0.001 | |||
| Papillary | 221 (31.4) | 179 (39.4) | 42 (16.9) | |
| Sessile | 482 (68.6) | 275 (60.6) | 207 (83.1) | |
| CVH | <0.001 | |||
| No | 543 (77.2) | 375 (82.6) | 168 (67.5) | |
| Yes | 160 (22.8) | 79 (17.4) | 81 (32.5) | |
| Adjuvant chemotherapy, n (%) | 0.455 | |||
| No | 416 (59.2) | 264 (58.1) | 152 (61.0) | |
| Yes | 287 (40.8) | 190 (41.9) | 97 (39.0) | |
| Laboratory tests | ||||
| Fibrinogen (g l−1), mean±s.d. | 3.8±1.3 | 3.1±0.6 | 5.1±1.1 | <0.001 |
| Platelet count (×109 l−1), mean±s.d. | 198.4±85.7 | 177.3±64.0 | 237.0±104.8 | <0.001 |
| WBC count (×109 l−1), mean±s.d. | 6.9±2.6 | 6.4±2.2 | 7.8±3.0 | <0.001 |
| ALP (U l−1), mean±s.d. | 81.5±35.9 | 75.6±22.5 | 92.6±50.8 | <0.001 |
| LDH (U l−1), mean±s.d. | 189.1±69.7 | 179.4±39.4 | 207.4±102.9 | <0.001 |
| NLR, mean±s.d. | 3.4±2.0 | 3.0±1.9 | 4.2±2.0 | <0.001 |
| AGR, mean±s.d. | 1.4±0.3 | 1.5±0.3 | 1.3±0.3 | <0.001 |
| End points | ||||
| Disease recurrence, n (%) | 291 (41.4) | 149 (32.8) | 142 (57.0) | <0.001 |
| Cancerrelated death, n (%) | 204 (29.0) | 86 (18.9) | 118 (47.4) | <0.001 |
| Overall death, n (%) | 253 (36.0) | 118 (26.0) | 135 (54.2) | <0.001 |
RNU: radical nephroureterectomy; LVI: lymphovascular invasion; CVH: concomitant variant histology; PSM: positive surgical margins; pT: pathological tumor; WBC: white blood cell; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; AGR: albumin-to-globulin ratio; HR: hazard ratio; s.d.: standard deviation; BMI: body mass index
Fibrinogen and adverse pathological outcomes
We investigated the associations between fibrinogen and adverse pathological features. After adjusting for pretreatment factors, including age, BMI, smoking status, gender, hydronephrosis, tumor side, tumor location, history of bladder cancer, multifocality, platelet count, WBC count, ALP, LDH, NLR, and AGR, multivariate logistic analysis revealed that elevated fibrinogen (treated as a continuous variable) was independently associated with increased risks of high-grade carcinoma (OR: 1.71, P < 0.001), high pathological tumor stage (OR: 1.69, P < 0.001), lymph node involvement (OR: 1.47, P = 0.001), LVI (OR: 1.30, P = 0.007), sessile carcinoma (OR: 1.45, P < 0.001), CVH (OR: 1.35, P = 0.001), and PSM (OR: 1.38, P = 0.021) (Table 2).
Table 2.
Binary and multivariate logistic regression analysis for fibrinogen level (continuous variable) for pathological outcomes when adjusting for preoperative confounders
| Adverse pathological outcomes | Adjusted ORa | 95% CI | P |
|---|---|---|---|
| High-grade disease | 1.71 | 1.36–2.13 | <0.001 |
| High pT stage (≥ pT3) | 1.69 | 1.40–2.04 | <0.001 |
| Lymph node involvement | 1.47 | 1.18–1.84 | 0.001 |
| LVI | 1.30 | 1.08–1.57 | 0.007 |
| Sessile carcinoma | 1.45 | 1.19–1.78 | <0.001 |
| CVH | 1.35 | 1.13–1.61 | 0.001 |
| PSM | 1.38 | 1.05–1.80 | 0.021 |
aAdjusting for age (continuous), gender, BMI (continuous), smoking status, hydronephrosis, tumor side, tumor location, history of bladder cancer, multifocality, fibrinogen (continuous), platelet count (continuous), WBC count (continuous), ALP (continuous), LDH (continuous), NLR (continuous), and AGR (continuous). LVI: lymphovascular invasion; CVH: concomitant variant histology; PSM: positive surgical margins; pT: pathological tumor; OR: odds ratio; CI: confidence interval; BMI: body mass index; WBC: white blood cell; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; AGR: albumin-to-globulin ratio
Fibrinogen and survival outcomes
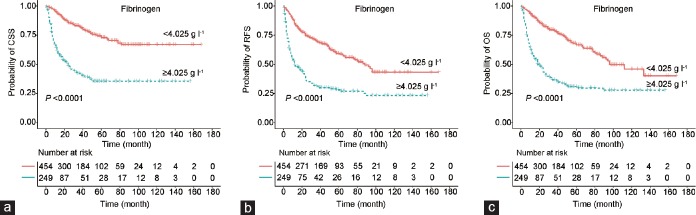
With a median follow-up of 42 (range: 1–168) months, 204 (29.0%) patients died of UTUC, 291 (41.4%) experienced disease recurrence, and 253 (36.0%) died of all causes at the time of the last follow-up. The 5-year CSS, RFS, and OS were 72.9%, 59.3%, and 66.7%, respectively, in the low fibrinogen group, compared with 35.5%, 28.1%, and 30.8%, respectively, in the high fibrinogen group.
Kaplan–Meier curves showed that patients with high fibrinogen levels had lower CSS, RFS, and OS than patients with low fibrinogen levels (log-rank tests, all P < 0.001; Figure 1). Table 3 and Supplementary Table 1 show the results from univariate and multivariate Cox regression analyses. Fibrinogen levels >4.025 g l−1 were significantly associated with worse CSS (hazard ratio [HR]: 3.97; 95% CI: 3.00–5.24), RFS (HR: 2.86; 95% CI: 2.27–3.60), and OS (HR: 3.28, 95% CI: 2.56–4.21) in the univariate Cox regression model. Multivariate analysis showed that pathological tumor stage, lymph node involvement, tumor size, NLR, and fibrinogen were independent predictors of CSS, RFS, and OS; tumor grade and CVH were independent predictors of CSS and OS; and ALP was an independent predictor of CSS. The HR values of fibrinogen were 2.33, 2.09, and 2.09 for CSS, RFS, and OS, respectively (Table 3).
Figure 1.
Kaplan–Meier curves and log-rank tests for survival in UTUC patients according to preoperative fibrinogen level (cutoff value: 4.025 g l−1). (a) Cancer-specific survival, (b) disease recurrence-free survival, and (c) overall survival. UTUC: upper tract urothelial carcinoma; CSS: cancer-specific survival; RFS: disease recurrence-free survival; OS: overall survival.
Table 3.
Forward stepwise multivariate Cox regression analyses of clinicopathological factors predicting survival outcomes in patients with upper tract urothelial carcinoma
| Variables | Cancer-specific survival | Recurrence-free survival | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Tumor grade (high vs low) | 1.92 (1.17–3.15) | 0.010 | – | – | 1.72 (1.15–2.57) | 0.009 |
| pT stage | <0.001 | <0.001 | <0.001 | |||
| pTis, pTa, pT1 | Reference | Reference | Reference | |||
| pT2 | 1.49 (0.85–2.61) | 0.164 | 1.47 (0.97–2.23) | 0.067 | 1.48 (0.92–2.38) | 0.108 |
| pT3 | 2.48 (1.51–4.08) | <0.001 | 2.36 (1.65–3.38) | <0.001 | 2.33 (1.52–3.55) | <0.001 |
| pT4 | 3.64 (2.04–6.49) | <0.001 | 3.81 (2.45–5.92) | <0.001 | 3.58 (2.17–5.89) | <0.001 |
| Lymph node status | <0.001 | <0.001 | 0.001 | |||
| pN0 | Reference | Reference | Reference | |||
| pNx | 2.13 (1.25–3.62) | 0.005 | 1.87 (1.23–2.83) | 0.003 | 1.91 (1.22–3.01) | 0.005 |
| pN+ | 3.16 (1.70–5.86) | <0.001 | 3.27 (1.95–5.47) | < 0.001 | 2.68 (1.54–4.66) | <0.001 |
| Tumor size (>3 cm vs ≤3 cm) | 1.47 (1.02–2.10) | 0.037 | 1.49 (1.12–1.98) | 0.007 | 1.53 (1.11–2.11) | 0.009 |
| CVH (yes vs no) | 1.45 (1.06–1.98) | 0.021 | – | – | 1.36 (1.02–1.80) | 0.037 |
| ALP (≥90 U l−1 vs <90 U l−1) | 1.40 (1.03–1.90) | 0.031 | – | – | – | – |
| NLR (≥2.5 vs <2.5) | 1.83 (1.33–2.52) | <0.001 | 1.56 (1.21–2.01) | 0.001 | 1.67 (1.26–2.20) | <0.001 |
| Fibrinogen (≥4.025 g l−1 vs <4.025 g l−1) | 2.33 (1.69–3.20) | <0.001 | 2.09 (1.62–2.70) | < 0.001 | 2.09 (1.58–2.77) | <0.001 |
CVH: concomitant variant histology; pT: pathological tumor; ALP: alkaline phosphatase; NLR: neutrophil-to-lymphocyte ratio; HR: hazard ratio; CI: confidence interval; –: not included in the analysis; OS: overall survival
Supplementary Table 1.
Univariable Cox regression models predicting survival outcomes in patients with upper tract urothelial carcinoma
| Variables | Cancer-specific survival | Recurrence-free survival | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (≥65 vs <65 years) | 0.848 (0.641–1.122) | 0.249 | 0.881 (0.696–1.115) | 0.293 | 0.966 (0.748–1.246) | 0.788 |
| BMI (>25 vs ≤25, kg/m2) | 0.848 (0.616–1.169) | 0.315 | 0.947 (0.729–1.230) | 0.681 | 0.917 (0.691–1.218) | 0.551 |
| Smoking status (former/current vs no) | 0.844 (0.615–1.159) | 0.294 | 0.864 (0.664–1.124) | 0.275 | 0.878 (0.662–1.164) | 0.364 |
| Gender (male vs female) | 0.824 (0.628–1.081) | 0.163 | 0.857 (0.682–1.076) | 0.184 | 0.876 (0.686–1.019) | 0.290 |
| Tumor side (right vs left) | 1.089 (0.830–1.428) | 0.538 | 1.063 (0.847–1.333) | 0.601 | 1.051 (0.824–1.341) | 0.687 |
| Bladder cancer status | 0.203 | 0.376 | 0.136 | |||
| No | Reference | Reference | Reference | |||
| Previous | 0.345 (0.085–1.391) | 0.134 | 0.903 (0.425–1.920) | 0.792 | 0.297 (0.074–1.198) | 0.088 |
| Concomitant | 1.205 (0.809–1.795) | 0.360 | 1.263 (0.901–1.770) | 0.176 | 1.198 (0.835–1.719) | 0.327 |
| Hydronephrosis (yes vs no) | 1.249 (0.938–1.664) | 0.128 | 1.401 (1.097–1.788) | 0.007 | 1.342 (1.035–1.740) | 0.026 |
| Tumor location | 0.556 | 0.508 | 0.675 | |||
| Pelvicalyceal | Reference | Reference | Reference | |||
| Ureteric | 1.005 (0.729–1.384) | 0.978 | 0.937 (0.715–1.229) | 0.639 | 0.941 (0.704–1.260) | 0.685 |
| Both | 1.217 (0.841–1.762) | 0.298 | 1.152 (0.842–1.577) | 0.377 | 1.118 (0.796–1.569) | 0.521 |
| Multifocality (yes vs no) | 1.059 (0.736–1.524) | 0.758 | 0.993 (0.727–1.358) | 0.967 | 0.971 (0.692–1.361) | 0.864 |
| Surgical approach (Laparoscopic vs Open) | 0.672 (0.485–0.932) | 0.017 | 0.858 (0.662–1.114) | 0.251 | 0.711 (0.529–0.956) | 0.024 |
| Tumor grade (high vs low) | 3.558 (2.305–5.492) | <0.001 | 2.278 (1.675–3.098) | <0.001 | 2.847 (1.992–4.070) | <0.001 |
| pT stage | <0.001 | <0.001 | <0.001 | |||
| pTis, pTa, pT1 | Reference | Reference | Reference | |||
| pT2 | 1.632 (0.966–2.757) | 0.067 | 1.502 (1.011–2.233) | 0.044 | 1.635 (1.045–2.558) | 0.031 |
| pT3 | 3.654 (2.372–5.629) | <0.001 | 2.797 (2.005–3.901) | <0.001 | 3.232 (2.222–4.702) | <0.001 |
| pT4 | 9.339 (5.921–14.729) | <0.001 | 6.936 (4.811–9.998) | <0.001 | 7.955 (5.327–11.881) | <0.001 |
| Lymph node status | <0.001 | <0.001 | <0.001 | |||
| pN0 | Reference | Reference | Reference | |||
| pNx | 1.496 (0.915–2.446) | 0.109 | 1.505 (1.013–2.235) | 0.043 | 1.507 (0.983–2.311) | 0.060 |
| pN+ | 6.124 (3.525–10.638) | <0.001 | 5.546 (3.484–8.831) | <0.001 | 5.361 (3.260–8.814) | <0.001 |
| LVI (yes vs no) | 2.726 (1.991–3.732) | <0.001 | 2.211 (1.676–2.917) | <0.001 | 2.511 (1.884–3.349) | <0.001 |
| Tumor size (>3 vs ≤3), cm | 1.985 (1.439–2.739) | <0.001 | 1.856 (1.425–2.418) | <0.001 | 1.983 (1.486–2.645) | <0.001 |
| PSM (yes vs no) | 2.319 (1.546–3.480) | <0.001 | 1.865 (1.290–2.694) | 0.001 | 2.118 (1.453–3.087) | <0.001 |
| Tumor architecture (Sessile vs Papillary) | 3.675 (2.480–5.447) | <0.001 | 2.500 (1.874–3.335) | <0.001 | 2.928 (2.114–4.055) | <0.001 |
| CVH (yes vs no) | 2.435 (1.825–3.248) | <0.001 | 2.045 (1.595–2.622) | <0.001 | 2.237 (1.722–2.906) | <0.001 |
| Adjuvant chemotherapy (yes vs no) | 0.963 (0.731–1.268) | 0.787 | 1.128 (0.896–1.420) | 0.304 | 0.889 (0.693–1.139) | 0.351 |
| WBC (≥8.3 vs <8.3, ×109 l−1) | 1.772 (1.305–2.407) | <0.001 | 1.455 (1.111–1.904) | 0.006 | 1.577 (1.189–2.092) | 0.002 |
| Platelet Count (≥230 vs <230, ×1099 l−1) | 2.111 (1.592–2.799) | <0.001 | 1.634 (1.276–2.091) | <0.001 | 1.711 (1.317–2.222) | <0.001 |
| ALP (≥90 vs <90, U l−1) | 1.782 (1.338–2.372) | <0.001 | 1.396 (1.092–1.785) | 0.008 | 1.497 (1.153–1.945) | 0.002 |
| LDH (> 220 vs ≤220, U l−1) | 1.613 (1.145–2.272) | 0.006 | 1.485 (1.109–1.989) | 0.008 | 1.553 (1.141–2.113) | 0.005 |
| NLR (≥2.5 vs <2.5) | 2.362 (1.749–3.190) | <0.001 | 1.854 (1.458–2.358) | <0.001 | 2.104 (1.617–2.737) | <0.001 |
| AGR (<1.45 vs ≥1.45) | 2.381 (1.781–3.183) | <0.001 | 1.818 (1.438–2.298) | <0.001 | 2.141 (1.656–2.767) | <0.001 |
| Fibrinogen (≥4.025 vs <4.025, g l−1) | 3.965 (2.998–5.243) | <0.001 | 2.855 (2.265–3.598) | <0.001 | 3.281 (2.559–4.206) | <0.001 |
LVI: lymphovascular invasion; pT: pathological tumor; CVH: concomitant variant histology; PSM: positive surgical margins; WBC: white blood cell; ALP: alkaline phosphatase; LDH: lactate Dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; AGR: albumin-to-globulin ratio; HR: hazard ratio; BMI: body mass index; OS: overall survival
Clinical utility of the prediction models
We calculated the c-index to determine the predictive accuracy of the multivariate models for survival outcomes (Table 4). The base model was built based on tumor grade, stage, lymph node invasion, tumor size, and CVH; the predictive accuracies for CSS, RFS, and OS were 76.2%, 72.4%, and 75.0%, respectively (derived from the multivariate analyses). The predictive accuracy improved upon adding each laboratory biomarker, including ALP, NLR, and fibrinogen (these were significant in the multivariate models), into the base model. The largest improvement was observed when fibrinogen was added to the base model (c-index improvements for CSS, RFS, and OS were 0.032, 0.020, and 0.028, respectively).
Table 4.
Improvement in discrimination when adding preoperative laboratory factors to the base model
| Model | C-index for CSS | Improvement | C-index for RFS | Improvement | C-index for OS | Improvement |
|---|---|---|---|---|---|---|
| Base modelsa | 0.762 | 0.724 | 0.750 | |||
| + ALP | 0.774 | 0.012 | 0.732 | 0.008 | 0.761 | 0.011 |
| + NLR | 0.778 | 0.016 | 0.737 | 0.013 | 0.765 | 0.015 |
| + Fibrinogen | 0.794 | 0.032 | 0.744 | 0.020 | 0.778 | 0.028 |
aThe base models included tumor grade, stage, lymph node invasion, tumor size, and concomitant variant histology. ALP: alkaline phosphatase; NLR: neutrophil-to-lymphocyte ratio; CSS: cancer-specific survival; RFS: disease recurrence-free survival; OS: overall survival
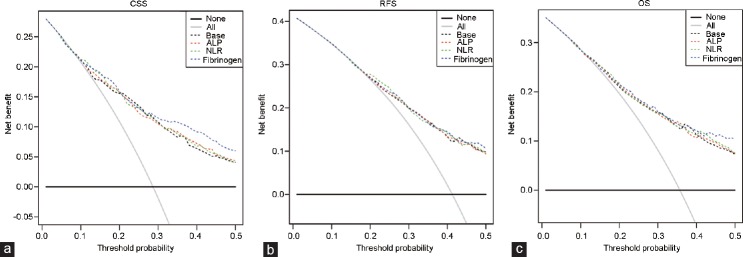
Finally, decision curve analyses were performed to assess the clinical utility of the above findings (Figure 2a). Because these models assist in identifying patients who require more aggressive pre- and postoperative treatments (such as adjuvant therapy, although it remains controversial in the treatment of patients with UTUC), we assumed that a patient would exhibit a relatively high rate of disease recurrence or death before receiving treatment intervention. Therefore, the threshold probability for the decision curve was up to 50%. Our results showed a significant net benefit for CSS gained by adding fibrinogen to the base model when the threshold ability was 0.3–0.5; in contrast, the net benefit gained by adding ALP or NLR to the base model was not obvious (Figure 2).
Figure 2.
Decision curve analyses comparing the added benefit of ALP, NLR, or fibrinogen in addition to standard pathologic characteristics for the outcomes of (a) CSS, (b) RFS, and (c) OS. ALP: alkaline phosphatase; NLR: neutrophil-to-lymphocyte ratio; CSS: cancer-specific survival; RFS: disease recurrence-free survival; OS: overall survival.
DISCUSSION
Our results suggest that elevated fibrinogen is an independent predictor for adverse pathological outcomes and worse survival outcomes in patients with UTUC. In addition, we demonstrate that the addition of fibrinogen may improve the predictive accuracy of the prediction models; most importantly, we reveal an added benefit for CSS prediction when fibrinogen was added to the base model. To the best of our knowledge, this is the largest single-center retrospective study to investigate the prognostic role of fibrinogen among Chinese patients who had received RNU to treat UTUC.
Using blood-based markers (including NLR,20 fibrinogen,13 C-reactive protein [CRP],21 and albumin-to-globulin ratio22) from laboratory examination to predict oncologic outcomes in UTUC is not a novel concept. According to the most recent EAU guidelines, only NLR has been recommended as a preoperative risk factor in UTUC. Therefore, we sought to explore the predictive value of fibrinogen based on several published reports, which showed that an elevated fibrinogen level was an independent risk factor for poor survival in UTUC. Of note, our study showed that both NLR and fibrinogen were independent prognostic factors for UTUC, but that fibrinogen might perform better than NLR. In the multivariate Cox regression model, the HRs of fibrinogen versus NLR were 2.33 versus 1.83 for CSS, 2.09 versus 1.56 for RFS, and 2.09 versus 1.67 for OS. More importantly, the addition of fibrinogen, but not NLR, to the base model achieved a net benefit in the decision curve analysis. Thus, fibrinogen might be a better prognostic predictor than NLR for UTUC.
Plasma fibrinogen, an important factor reflecting an individual's coagulation function, is routinely measured before surgery. Tanaka et al.13 first described the prognostic role of pretreatment fibrinogen level in patients with localized UTUC in a Japanese population. The study enrolled 218 patients, and the results showed that fibrinogen >450 mg dl−1 independently predicted worse pathological features and survival outcomes (CSS and RFS). Subsequently, data from Europe14 and China (East15 and North23) also supported the independent predictive value of fibrinogen in UTUC. Nevertheless, most of these studies incorporated relatively small numbers of cases with short follow-up durations, which made their results relatively inconclusive. In addition, different cutoff values of fibrinogen were reported in these studies, limiting its use for clinical reference. Our study showed that fibrinogen could not only independently predict outcomes in UTUC, but that it increased the discriminative accuracy of predicting survival outcomes in UTUC and achieved an added net benefit for CSS in the decision curve analysis, based on providing additional information for risk stratification in UTUC.
According to our ROC analysis, the optimal cutoff value was determined to be 4.025 g l−1. The area under the curve (AUC) was 0.689, and the Youden index was 0.316, with a sensitivity of 57.8% and a specificity of 74.7%. In the study conducted by Wang et al.8 in prostate cancer, their cutoff value of fibrinogen was 3.225 g l−1, and the AUC was 0.692, with a sensitivity of 68.3% and a specificity of 65.7%. Both studies had comparable AUC values (0.689 vs 0.692), and sensitivity and specificity all exceeded 50%. It should be noted that using fibrinogen alone to predict survival outcomes might be inappropriate in current clinical practice (57.8% sensitivity); we, thus, incorporate this parameter in our multivariate models; and the predictive accuracy could reach approximately 80% when fibrinogen was added.
To date, progress has been made with regard to determining the potential mechanism by which high fibrinogen level contributes to worse oncologic outcomes among cancer patients. Tumor cells and tumor-associated macrophages might induce elevated fibrinogen levels, and fibrinogen is a determinant of metastatic potential.24 In vitro assays have demonstrated that fibrinogen can promote tumor cell proliferation7 and migration25,26 through various signal pathways. An in vivo study conducted by Steinbrecher et al.27 further demonstrated that fibrinogen contributed to tumor progression through interaction with alpha(M)beta(2). Taken together, these data indicate that the elevated fibrinogen level caused by tumor cells promotes tumor cell invasion and metastasis, which might explain its predictive significance in UTUC.
In addition to fibrinogen, our study revealed that postoperative factors such as tumor stage and grade, CVH, and lymph node involvement independently predicted CSS, RFS, and OS; these findings were consistent with published literature.2 Compared with these pathological predictors, fibrinogen is advantageous in that it provides easy preoperative accessibility and a cost-effective approach for determining the necessity of early intervention before surgery (e.g., neoadjuvant therapy), as well as for assisting in identifying the best candidates for such interventions. Nonetheless, we did not find an independent prognostic value for LVI and PSM in this cohort, although previous studies revealed that these parameters were useful in this regard.28,29 Research on UTUC remains limited because of its low morbidity. Our study had the largest sample size for exploration of the prognostic significance of fibrinogen in UTUC and provided useful information regarding UTUC in the West Chinese population.
As with all retrospective studies, this study was limited by its study design, which might lead to selection bias. Although we strictly limited our study population, we were unable to completely exclude those whose condition might affect the plasma fibrinogen level. In addition, we were unable to assess the potential influence of some factors, such as CRP (reportedly valuable in the prognosis of UTUC30) because they were not routinely assessed preoperatively in our center. Moreover, data on postoperative chemotherapeutic regimens were incomplete, hindering analyzing the effects of the types, dosages, and duration of these drugs on prognosis. Furthermore, the potential role of neoadjuvant therapy remains unclear because our study cohort did not receive this treatment. Further prospective multicenter studies are warranted to support our findings.
CONCLUSIONS
Preoperative fibrinogen is a strong independent factor associated with both adverse pathological features and survival outcomes in UTUC. It might be a better indicator than NLR in predicting oncologic outcomes in UTUC. Adding it into the prediction models might be valuable which might aid in clinical decision-making.
AUTHOR CONTRIBUTIONS
HX, JZA and PT participated in project design and performed data collection and statistical analysis. HX drafted the manuscript. HX, PT, THL, and JZA helped with patients' follow-up. XJ, LNG, and HRL participated in data collection and helped analyze data. LY and QW carried out project design and participated in data explanation and manuscript revision. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
The optimal fibrinogen cutoff level (4.025 g l−1) was determined from ROC analysis. The AUC was 0.689, and the Youden index was 0.316, with a sensitivity of 57.8% and a specificity of 74.7%. ROC: receiver-operating characteristic; AUC: area under the curve; CI: confidence interval.
ACKNOWLEDGMENTS
This program was supported by the National key Research and Development program of China (Grant No. SQ2017YFSF090096), the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant No. 81300627, No. 81370855, No. 81702536, and No. 81770756), Programs from Science and Technology Department of Sichuan Province (Grant No. 2018JY0089 and No. 2017HH0063), and Young Investigator Award of Sichuan University 2017 (Grant No. 2017SCU04A17). The funders had no role in patient selection, data extraction, statistical analysis or interpretation, writing of this article, or the decision to publish.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Jeldres C, Sun M, Isbarn H, Lughezzani G, Budäus L, et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. 2010;75:315–20. doi: 10.1016/j.urology.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Lughezzani G, Burger M, Margulis V, Matin S, Novara G, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100–14. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 Update. Eur Urol. 2018;73:111–22. doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Zhang L, Mu Y, Li J, Xu X, et al. Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J Gastroenterol. 2015;21:5303–10. doi: 10.3748/wjg.v21.i17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou YX, Yang ZM, Feng J, Shan YJ, Wang WL, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: a meta-analysis. Tumour Biol. 2013;34:3701–4. doi: 10.1007/s13277-013-0953-2. [DOI] [PubMed] [Google Scholar]
- 7.Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost. 2008;6:176–83. doi: 10.1111/j.1538-7836.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Yin W, Wang Z, Huang J, Pan J, et al. Pretreatment plasma fibrinogen as an independent prognostic indicator of prostate cancer patients treated with androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2016;19:209–15. doi: 10.1038/pcan.2016.6. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Kim HS, Kim M, Lee M, Song YS. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol. 2017;28:e36. doi: 10.3802/jgo.2017.28.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Q, Xue N, Dai D, Xing S, He X, et al. A nomogram based on inflammatory factors C-reactive protein and fibrinogen to predict the prognostic value in patients with resected non-small cell lung cancer. J Cancer. 2017;8:744–53. doi: 10.7150/jca.17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C, Lu B, Diao C, Zhao K, Wang X, et al. Preoperative neutrophil-lymphocyte ratio and fibrinogen level in patients distinguish between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Onco Targets Ther. 2016;9:4917–22. doi: 10.2147/OTT.S107445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obata J, Tanaka N, Mizuno R, Kanao K, Mikami S, et al. Plasma fibrinogen level: an independent prognostic factor for disease-free survival and cancer-specific survival in patients with localised renal cell carcinoma. BJU Int. 2016;118:598–603. doi: 10.1111/bju.13414. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Kikuchi E, Matsumoto K, Hayakawa N, Ide H, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int. 2013;111:857–64. doi: 10.1111/j.1464-410X.2012.11353.x. [DOI] [PubMed] [Google Scholar]
- 14.Pichler M, Dalpiaz O, Ehrlich GC, Stojakovic T, Martin Hernandez JM, et al. Validation of the preoperative plasma fibrinogen level as a prognostic factor in a European cohort of patients with localized upper tract urothelial carcinoma. J Urol. 2014;191:920–5. doi: 10.1016/j.juro.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Yuan Y, Wang Y, Zhang J, Kong W, et al. Prognostic value of preoperative plasma fibrinogen level and platelet-to-lymphocyte ratio (F-PLR) in patients with localized upper tract urothelial carcinoma. Oncotarget. 2017;8:36761–71. doi: 10.18632/oncotarget.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng YC, Huang CN, Wu WJ, Li CC, Ke HL, et al. The prognostic significance of inflammation-associated blood cell markers in patients with upper tract urothelial carcinoma. Ann Surg Oncol. 2016;23:343–51. doi: 10.1245/s10434-015-4781-z. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Tan P, Ai J, Huang Y, Lin T, et al. Prognostic impact of preoperative albumin-globulin ratio on oncologic outcomes in upper tract urothelial carcinoma treated with radical nephroureterectomy. Clin Genitourin Canc. 2018;16:e1059–68. doi: 10.1016/j.clgc.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Tan P, Chen J, Xie N, Xu H, Ai J, et al. Is preoperative serum lactate dehydrogenase useful in predicting the outcomes of patients with upper tract urothelial carcinoma? Cancer Med. 2018;7:5096–106. doi: 10.1002/cam4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan P, Xu H, Liu L, Ai J, Xu H, et al. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in patients with upper tract urothelial carcinoma. Clin Chim Acta. 2018;485:26–32. doi: 10.1016/j.cca.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol. 2018;36:1019–29. doi: 10.1007/s00345-018-2235-5. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka N, Kikuchi E, Shirotake S, Kanao K, Matsumoto K, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol. 2014;65:227–34. doi: 10.1016/j.eururo.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Yu W, Zhou L, He Z, Shen C, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PLoS One. 2015;10:e0144961. doi: 10.1371/journal.pone.0144961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J, Yu M, Zhang N, Wang S, Zhu Y, et al. Prognostic scores based on the preoperative plasma fibrinogen and serum albumin level as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Oncotarget. 2017;8:68964–73. doi: 10.18632/oncotarget.16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–9. [PubMed] [Google Scholar]
- 25.Roche Y, Pasquier D, Rambeaud JJ, Seigneurin D, Duperray A. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb Haemost. 2003;89:1089–97. [PubMed] [Google Scholar]
- 26.Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–43. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612–8. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colin P, Ouzzane A, Yates DR, Audenet F, François A, et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann Surg Oncol. 2012;19:3613–20. doi: 10.1245/s10434-012-2453-9. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa M, Miyake H, Kurahashi T, Fujisawa M. Significance of multiple preoperative laboratory abnormalities as prognostic indicators in patients with urothelial carcinoma of the upper urinary tract following radical nephroureterectomy. Int J Clin Oncol. 2017;23:151–7. doi: 10.1007/s10147-017-1184-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The optimal fibrinogen cutoff level (4.025 g l−1) was determined from ROC analysis. The AUC was 0.689, and the Youden index was 0.316, with a sensitivity of 57.8% and a specificity of 74.7%. ROC: receiver-operating characteristic; AUC: area under the curve; CI: confidence interval.