Abstract
Fusion between the transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG fusion) is a common genetic alteration in prostate cancer among Western populations and has been suggested as playing a role in tumorigenesis and progression of prostate cancer. However, the prevalence of TMPRSS2-ERG fusion differs among different ethnic groups, and contradictory results have been reported in Asian patients. We aim to evaluate the prevalence and significance of TMPRSS2-ERG fusion as a molecular subtyping and prognosis indicator of prostate cancer in Asians. We identified the fusion status in 669 samples from prostate biopsy and radical prostatectomy by fluorescence in situ hybridization and/or immunohistochemistry in China. We examined the association of TMPRSS2-ERG fusion with clinicopathological characteristics and biochemical recurrence by Chi-square test and Kaplan–Meier analysis. Finally, a systematic review was performed to investigate the positive rate of the fusion in Asian prostate cancer patients. McNemar's test was employed to compare the positive rates of TMPRSS2-ERG fusion detected using different methods. The positive rates of TMPRSS2-ERG fusion were 16% in our samples and 27% in Asian patients. In our samples, 9.4% and 19.3% of cases were recognized as fusion positive by fluorescence in situ hybridization and immunohistochemistry, respectively. No significant association between the fusion and clinical parameters was observed. TMPRSS2-ERG fusion is not a frequent genomic alteration among Asian prostate cancer patients and has limited significance in clinical practices in China. Besides ethnic difference, detection methods potentially influence the results showing a positive rate of TMPRSS2-ERG fusion.
Keywords: Asian, Chinese, prostate cancer, systematic review, TMPRSS2-ERG
INTRODUCTION
In the United States, it was estimated that more than 29 000 men would die of prostate cancer (PCa) in 2018.1 The high incidence and cancer-related death rate of PCa make the disease a serious threat for Western men's health.2 However, Asians are several times less likely to develop PCa, although PCa morbidity and mortality have been increasing in Asian countries in the last decades.3,4 These differences may be caused by different lifestyles, environments, medical conditions, and, most importantly, genomic pathogenesis.5
Overexpression of v-ets erythroblastosis virus E26 oncogene homolog(ERG) mRNA in PCa was first reported by Petrovics and his colleagues in 2005,6 following which Tomlins and colleagues7 discovered the mechanism of ERG activation to be the fusion between transmembrane protease serine 2 (TMPRSS2) and ERG. ERG expression was promoted by androgen through TMPRSS2, which finally resulted in the overexpression of proto-oncoprotein ERG.8,9,10 During this period, numerous studies demonstrated that aberrantly expressed ERG combined with phosphatase and tensin homolog (PTEN) loss or other molecular alterations promoted the oncogenesis and metastasis of PCa both in vitro and in vivo.11,12,13,14,15,16 Moreover, according to data from The Cancer Genome Atlas (TCGA), TMPRSS2-ERG fusion is one of the predominant molecular classification factors and promising prognostic markers for localized PCa. TMPRSS2-ERG fusion combined with PCA3 was used in the clinical setting by Tomlins et al.17 to save patients with elevated prostate-specific antigen (PSA) levels referred for biopsy, which potentially decreased the side effects of biopsy and the anxiety associated with waiting for the diagnosis.
However, a series of studies have demonstrated that TMPRSS2-ERG fusion has a strong correlation with ethnicity, and the positive rates of TMPRSS2-ERG fusion differ among different ethnic and geographical groups, at a wide range of 7%–83%.18,19,20 Although more than half of PCa patients in North America and Europe harbor the TMPRSS2-ERG fusion,21,22 it is still controversial whether it is a common gene fusion type in Asian patients.18,23 A rising number of studies have focused on expounding the interaction of TMPRSS2-ERG fusion and PCa in Asia, and with conflicting results. In 2010, Sun and colleagues24 examined TMPRSS2-ERG fusion in 50 Chinese PCa samples by fluorescence in situ hybridization (FISH) and found 39 (78.0%) positive cases. However, another Chinese researcher found that TMPRSS2-ERG fusion was detected by the same method in only 7 (7.5%) of 93 Chinese patients.25
There is evidence that unstandardized detection methods, including FISH, immunohistochemistry (IHC), polymerase chain reaction (PCR), and some other high-throughput methods, may produce different results in detecting TMPRSS2-ERG fusion.23,26 The disparities in the positive rates of TMPRSS2-ERG raise the question of whether there is equal applicability of this genomic alteration in Asian patients.
In the present study, we aimed to evaluate the positive rate of TMPRSS2-ERG fusion in Asian patients by experiment and a systematic review and to assess its clinical significance as a cancer biomarker in Chinese people. We also made efforts to investigate the factors which could influence the measured positive rate of TMPRSS2-ERG fusion.
PATIENTS AND METHODS
Patients and prostate specimens
Paraffin-embedded tissue blocks of 729 consecutive PCa patients who underwent radical prostatectomy or prostate biopsy in Shanghai Changhai Hospital (Shanghai, China), between January 2010 and July 2018, were retrieved from the Hospital's Department of Pathology. Two independent pathologists reviewed corresponding hematoxylin and eosin (H&E)-stained slides of each block (6–15 blocks per patient) to confirm pathological diagnosis, and 669 eligible blocks were selected. For each patient receiving biopsy in the hospital, 12 cores were obtained and the core with the greatest tumor volume was chosen for the experiment. Age at diagnosis, body mass index (BMI), preoperative PSA, Gleason pattern, emission computed tomography (ECT) diagnosis, clinical tumor stage, and perineural and lymphovascular invasion status were retrieved from medical records, and patients' follow-up was conducted in accordance with the Chinese Guidelines for the Diagnosis and Treatment of Urological Diseases.27 Biochemical recurrence (BCR)-free survival is defined by a PSA level ≥0.2 ng ml−1 in two successive follow-ups after surgery. Informed consent was obtained from the patients before surgery, and all procedures performed in this study involving human participants were approved by the Institutional Review Board of Shanghai Changhai Hospital.
Fluorescence in situ hybridization and immunohistochemistry
We detected DNA fusion by ERG break-apart FISH assay, which was demonstrated to be a reliable technique to detect the fusion between two neighboring genes.10,28 Bacterial artificial chromosome (BAC) clones and FISH assay kits (F01015) were obtained from GP Medical Technologies (Beijing, China). Fluorescein (green)-labeled RP11-24A11 and tetramethylrhodamine (red)-labeled RP11137J13 were provided in the FISH assay kit, which spanned the centromeric and telomeric regions of the ERG, respectively. The experiment was performed following the manufacturer's instructions. Briefly, 4-μm sections were deparaffinized and dehydrated followed by Proteinase K digestion (provided in the FISH assay kit). After washing and fixation, the sections were dehydrated and dried. Denaturation was under 85°C for 10 min and hybridization was under 42°C overnight.
Fluorescent images were captured by a ×100 oil lens (Olympus BX51, Tokyo, Japan). A normal cell exhibits a pair of orange signals in nucleus while cells with gene fusion show separated red and green coloring or lack one of these colors. For each case, we counted at least 100 nuclei, and fusion was recorded when there were more than 10% of nuclei exhibited abnormal signals.
Immunohistochemistry
IHC analysis of ERG expression was performed on 4-μm sections using an UltraSensitive TMS-P kit (KIT-9710, MaiXin Biotechnology, Fujian, China). The tissue sections were dewaxed, followed by gradual dehydration. Then, heat-induced antigen retrieval was processed in 0.01 mol l−1 citrate buffer in a microwave for 15 min. Primary antibody incubation for ERG (1/200, ab92513, Abcam, Cambridge, UK) was conducted at 4°C overnight and secondary antibody was included in the kit. DAB staining was performed with a DAB staining kit (DAB-2031, MaiXin Biotechnology) as per the manufacturer's instructions. Slides were scanned using a Nano Zoomer S60 (Hamamatsu Photonics, Iwata City, Japan), and ERG expression status was recorded as negative (no staining or stained area <10%) or positive (weak or strong staining).
Statistical analyses
A Chi-square test was employed for comparing the association between the fusion status and clinical characteristics. BCR-free survival rate was calculated using Kaplan–Meier analysis and a log-rank test. McNemar's test was used to compare the difference between positive rates of gene fusion evaluated by FISH and IHC. Statistical analysis were analyzed using SPSS (IBM SPSS Statistics for Windows, version 19.0, IBM Corp., Armonk, NY, USA), and graphs were drawn using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Statistics were considered statistically significant when two-sided P < 0.05.
Publication search
The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Literature was searched for in the PubMed and Embase databases on December 20, 2017, with no restrictions on publication year. The following search terms were used: “TMPRSS2,” “ERG” OR “ETS related gene,” AND “Prostate cancer,” and both the adjective and noun forms of the name of each Asian country or region. Only abstracts or articles in English were included. Two authors (RC and DPK) independently reviewed the articles, and fusion-related information was extracted.
Meta-analysis
Heterogeneity among studies was measured using the Cochrane Q statistic (P > 0.05 for homogeneity) and the I2 statistic. I2 is calculated using the formula: I2 = (Q − df)/Q, in which df means degree of freedom. I2 < 40% was considered to be that no important heterogeneity existed and I2 > 75% was considered to be that heterogeneity existed.29 The fixed effects model and random effects meta-analysis were applied as being relevant. All statistical analyses for the meta-analysis were performed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) with the Meta libraries.
RESULTS
TMPRSS2-ERG fusion and its clinical association
Table 1 summarizes the clinicopathological characteristics of patients included in the study. In the study, the mean follow-up time of patients was 26.6 (range: 0–96.0) months, and the median was 22.0 months. A total of 669 patients with 179 biopsy samples and 490 prostatectomy samples were evaluated. The mean age of all patients was 67.8 (range: 43–88) years, and biochemical recurrence was observed in 7.5% (22/333) patients during follow-up. Among the 669 samples, 110 (16.4%) showed the TMPRSS2-ERG fusion. Samples from prostatectomy had a higher fusion rate than those from biopsy tissue (17.8% vs 12.9%) though the data were not statistically significant. Similarly, patients with higher BMI seemed more likely to harbor TMPRSS2-ERG fusion. However, a Chi-square test suggested that none of the investigated clinicopathological characteristics were associated with TMPRSS2-ERG fusion. Table 2 shows related details. In addition, Kaplan–Meier survival analysis showed no difference in BCR rates between the fusion-positive and fusion-negative groups (Figure 1).
Table 1.
Clinicopathological characteristics of 669 investigated patients
| Characteristics | Biopsy (n=179) | Prostatectomy (n=490) | All |
|---|---|---|---|
| Age (year), mean±s.d. | 70.0±8.3 | 67.0±7.1 | 67.8±7.6 |
| BMI (kg m−2), mean±s.d. | 24.1±2.9 | 24.6±3.1 | 24.5±3.1 |
| PSA level (ng ml−1), n (%) | |||
| <4 | 38 (21.2) | 62 (12.7) | 100 (15.0) |
| 4–10 | 37 (20.7) | 109 (22.2) | 146 (21.8) |
| >10 | 102 (57.0) | 319 (65.1) | 421 (62.9) |
| Unknown | 2 (1.1) | 0 (0) | 2 (0.3) |
| Major Gleason score, n (%) | |||
| 3 | 63 (35.2) | 219 (44.7) | 282 (42.2) |
| 4 | 79 (44.1) | 218 (44.5) | 279 (41.7) |
| 5 | 37 (20.7) | 53 (10.8) | 90 (13.4) |
| Sum of Gleason score, n (%) | |||
| 6 | 21 (11.7) | 91 (18.6) | 112 (16.7) |
| 3+4 | 41 (23.9) | 119 (24.3) | 160 (23.9) |
| 4+3 | 22 (22.9) | 68 (13.9) | 90 (13.4) |
| >7 | 95 (53.1) | 212 (43.3) | 307 (45.9) |
| Clinical tumor stage, n (%) | |||
| T0/T1 | 28 (15.6) | 112 (22.9) | 140 (20.9) |
| T2 | 65 (36.3) | 275 (56.1) | 340 (50.8) |
| T3 | 36 (20.1) | 81 (16.5) | 117 (17.5) |
| T4 | 28 (15.6) | 2 (0.4) | 30 (4.5) |
| Unknown | 22 (12.3) | 20 (4.1) | 42 (6.3) |
| Aberrant bone scan, n (%) | 54 (30.2) | 70 (14.3) | 124 (18.5) |
| Perineural invasiona, n (%) | NA | 241 (49.7) | NA |
| Lymphovascular invasiona, n (%) | NA | 41 (15.1) | NA |
| Biochemical recurrencea, n (%) | NA | 25 (7.5) | NA |
aMissing data in prostatectomy, 5 for perneural invasion; 218 for lymphovascular invasion; 157 for biochemical recurrence. s.d.: standard deviation; BMI: body mass index; PSA: prostate-specific antigen; NA: not available
Table 2.
Association of transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog fusion status with clinical parameters among 669 prostate cancer samples
| Parameters | Fusion | Positive rate (%) | P | |
|---|---|---|---|---|
| Negative (n) | Positive (n) | |||
| PCa samples | 559 | 110 | 16.4 | |
| Age (year) | 0.57 | |||
| ≤65 | 187 | 40 | 17.6 | |
| >65 | 370 | 70 | 15.9 | |
| BMI (kg m−2) | 0.11 | |||
| <19 | 12 | 2 | 14.3 | |
| 19–27 | 447 | 82 | 15.5 | |
| >27 | 92 | 25 | 21.4 | |
| Sample type | 0.13 | |||
| Biopsy | 156 | 23 | 12.8 | |
| Prostatectomy | 403 | 87 | 17.8 | |
| PSA level (ng ml−1) | 0.31 | |||
| <4 | 87 | 13 | 13.0 | |
| 4–10 | 117 | 29 | 19.9 | |
| >10 | 353 | 68 | 16.2 | |
| Unknown | 2 | 0 | 0 | |
| Major Gleason score | 0.44 | |||
| 3 | 230 | 52 | 18.4 | |
| 4 | 254 | 43 | 14.5 | |
| 5 | 75 | 15 | 16.7 | |
| Sum of Gleason score | 0.93 | |||
| 6 | 94 | 18 | 16.1 | |
| 3+4 | 129 | 31 | 19.4 | |
| 4+3 | 76 | 14 | 15.6 | |
| >7 | 260 | 47 | 15.3 | |
| Clinical tumor stage | 0.79 | |||
| T0/T1 | 115 | 25 | 17.9 | |
| T2 | 287 | 53 | 15.6 | |
| T3 | 100 | 17 | 14.5 | |
| T4 | 24 | 6 | 20.0 | |
| Unknown | 33 | 9 | 21.4 | |
| Perineural invasion | 0.67 | |||
| Positive | 197 | 44 | 18.3 | |
| Negative | 203 | 41 | 16.8 | |
| Unknown | 159 | 25 | 13.6 | |
| Lymphovascular invasion | 0.55 | |||
| Positive | 38 | 7 | 15.6 | |
| Negative | 183 | 44 | 19.4 | |
| Unknown | 182 | 36 | 16.5 | |
| Biochemical recurrence | 0.70 | |||
| Positive | 21 | 4 | 16.0 | |
| Negative | 257 | 51 | 16.6 | |
| Lost | 125 | 32 | 20.4 | |
BMI: body mass index; PSA: prostate-specific antigen; TMPRSS2-ERG: transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog; PCa: prostate cancer
Figure 1.
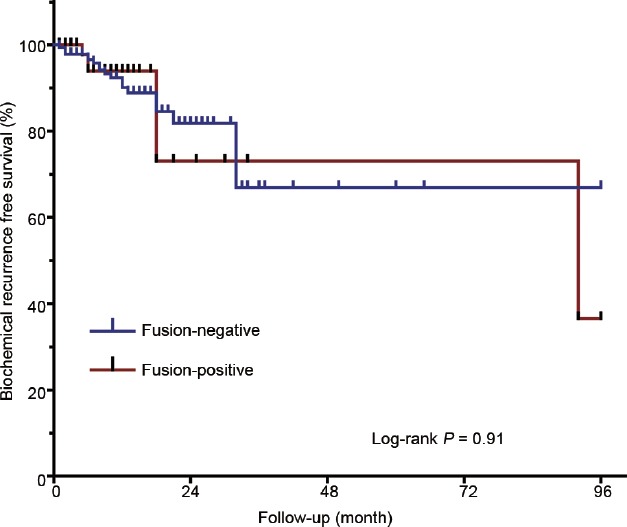
BCR-free survival rates of 490 patients receiving radical prostatectomy. Kaplan–Meier analysis was performed and no significant difference was observed in BCR-free survival rates between the fusion-positive and fusion-negative groups (log-rank P = 0.91). BCR: biochemical recurrence.
TMPRSS2-ERG fusion rates by different detection methods
Samples were randomly assigned into subgroups, in which they were detected by different methods. We evaluated 73 biopsy samples and 194 prostatectomy samples by FISH, and 84 biopsy samples and 196 prostatectomy samples by IHC. In addition, 22 biopsy samples and 100 prostatectomy samples were simultaneously assessed by FISH and IHC. TMPRSS2-ERG fusion was considered positive as long as either of FISH and IHC, or both, detected fusion signals. Supplementary Table 1 and 2 show the number of different sample types and positive rates of samples evaluated by FISH, IHC, or both. Of the 267 samples detected by FISH, 25 (9.4%) were identified as fusion positive while 54/280 (19.3%) fusion was found by IHC. It was surprising that samples were more likely to be defined as fusion positive by IHC than by FISH (P < 0.001). There were 17 cases recognized as fusion positive by IHC and contradictorily negative by FISH. However, no case detecting positive signals by FISH was recognized as fusion negative by IHC. Figure 2 shows representative images of FISH and IHC.
Supplementary Table 1.
Transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog fusion detected in different sample types by fluorescence in situ hybridization and/or immunohistochemistry
| Methods | Fusion positive/total (%) | ||
|---|---|---|---|
| Biopsy | Prostatectomy | Total | |
| FISH | 9/73 (12.3) | 16/194 (8.2) | 25/267 (9.4) |
| IHC | 9/84 (10.7) | 45/196 (23.0) | 54/280 (19.3) |
| FISH and IHC | 5/22 (22.7) | 26/100 (26.0) | 31/122 (25.4) |
FISH: fluorescence in situ hybridization; IHC: immunohistochemistry
Supplementary Table 2.
Comparison of number of fusion positive cases among 122 samples detected by fluorescence in situ hybridization and immunohistochemistry simultaneously
| FISH | IHC | Total | P | |
|---|---|---|---|---|
| Fusion negative | Fusion positive | |||
| Fusion negative | 91 | 0 | 92 | <0.001 |
| Fusion positive | 17 | 14 | 30 | |
| Total | 108 | 14 | 122 | |
FISH: fluorescence in situ hybridization; IHC: immunohistochemistry
Figure 2.
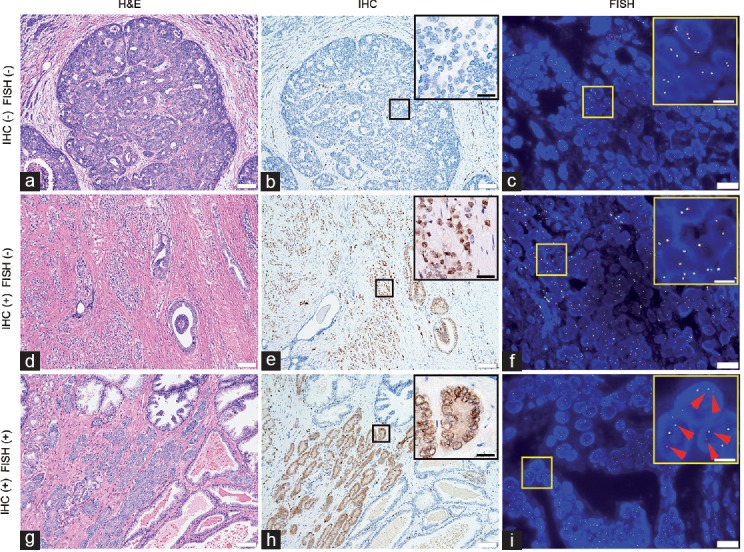
H&E stains, IHC, and FISH images of three cases showing different fusion status. Small boxes indicate areas shown at higher magnification in the larger box. (a) H&E stain of a fusion-negative case of prostate cancer with cribriform glands (Gleason grade 4). (b) IHC shows positive signals in some of the blood vessel endothelium, but no ERG expression in cancerous prostate glands. (c) In FISH images, there was no separation of red and green signals. (d) H&E stain of a case recognized as fusion positive by IHC, but negative by FISH. (e) Strong signals of ERG expression can be seen in the IHC image, but (f) almost all the cells exhibit normal signals in the FISH image. (g) H&E stain of one case of prostate cancer (Gleason grade 3) evaluated as fusion positive by IHC and FISH. (h) IHC shows ERG expression in cancerous prostate glands. (i) In large portion of cell nuclei, one yellow, one red (red arrows), and one green signal (red arrows) indicate TMPRSS2-ERG fusion through translocation. Scale bars = 100 μm in a, b, d, e, g and h; 30 μm in up-right image of b, e and h; 20 μm in c, f, and i; 7.5 μm in up-right image of c, f, and i. H&E: hematoxylin and eosin; IHC: immunohistochemistry; FISH: fluorescence in situ hybridization; TMPRSS2: transmembrane protease serine 2; ERG: v-ets erythroblastosis virus E26 oncogene homolog.
Literature search and study selection
Using the search strategy described above, we identified a list of 184 and 295 studies from PubMed and Embase, respectively. Abstracts of 243 studies were carefully reviewed and studies carried out by Asian authors but investigating non-Asian populations, or studies with experiments performed only in PCa cell lines, were excluded. The abstracts and full articles of the remaining studies were then screened. Consequently, 45 studies with 5371 cases were included in the meta-analysis. Figure 3 shows a flowchart of the literature research.
Figure 3.
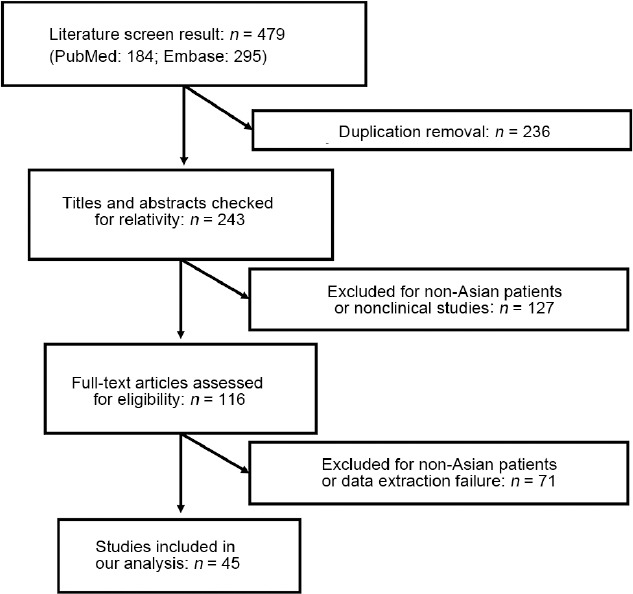
Flowchart of literature screening with inclusion and exclusion criteria.
Overall pooled results of TMPRSS2-ERG fusion in Asian patients
The pooled results indicated that the positive rate of TMPRSS2-ERG fusion was 27% (95% confidence interval [CI]: 24%–32%) in all included studies. Among the 49 selected records, 18 reported a positive rate of 20% or lower and 17 reported a positive rate of 30% or higher. The highest positive rate (78%) was observed in the study by Sun et al.24 and the lowest detection rate (0) was observed in the study by Furusato et al.30 Figure 4 shows a forest plot for the 45 studies.
Figure 4.
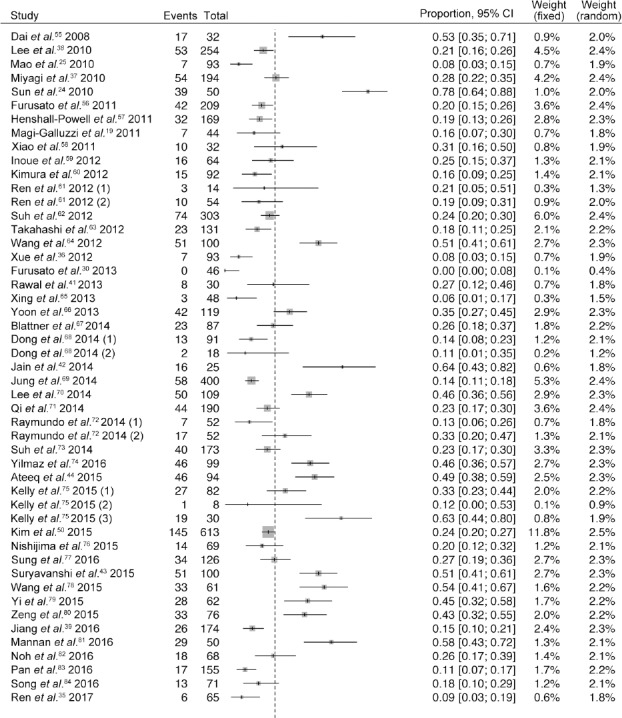
Forest plot of positive rate of TMPRSS2-ERG fusion for 45 included studies. A random effects model is used and the positive rate of TMPRSS2-ERG fusion in Asian PCa patients is 27%. Data were individually analyzed when two or more groups were included in one research and we present these subgroups as (1), (2), (3). CI: confidence interval; TMPRSS2-ERG: transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog; PCa: prostate cancer.
Positive rate of TMPRSS2-ERG fusion by subgroup analysis
Studies were divided into groups to compare the differences between different populations, sample types, detection methods, and study sample size. Most studies were from China (n = 17), South Korea (n = 11), or Japan (n = 8), and patients from these three countries accounted for 86.8% of all patients. The positive rate of TMPRSS2-ERG fusion in the Japanese population was 21% (95% CI: 17%–25%), which was relatively lower than 25% (95% CI: 17%–34%) in Chinese and 26% (95% CI: 20%–32%) in South Koreans. However, Indian and Turkish populations were reported to have higher fusion rates (52% and 46%, respectively). After excluded Aryan and Caucasian population, the positive rate of the fusion in Asians was 24% (95% CI: 20%–29%). Supplementary Table 3 lists the prevalence of the fusion in different countries examined. It is noteworthy that fusion detected in samples from prostate biopsy (34%; 95% CI: 24%–47%) was higher than that in samples from radical prostatectomy (24%; 95% CI: 20%–29%). Samples assessed by PCR showed higher fusion rate (40%; 95% CI: 26%–55%) than samples assessed by IHC (26%; 95% CI: 22%–31%) and FISH (25%; 95% CI: 18%–35%).
Supplementary Table 3.
Prevalence of transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog fusion stratified by examined countries in Asia
| Country | Pooled positive rate (%) | 95% CI | Patient (n) |
|---|---|---|---|
| Korea | 25 | 21–30 | 2323 |
| China | 25 | 17–34 | 1490 |
| Japan | 21 | 17–25 | 849 |
| India | 52 | 43–60 | 329 |
| Philippines | 23 | - | 104 |
| Turkey | 46 | - | 99 |
| Malesia | 13 | - | 8 |
CI: confidence interval
The positive rates of fusion in the smaller sample-sized (n ≤ 30) group and larger sample-sized (n > 30) group was 33% (95% CI: 17%–56%) and 27% (95% CI: 23%–31%), respectively. This implied that a sampling error in some studies may have caused higher prevalence of the fusion.
DISCUSSION
Gene fusions have been recognized as frequent events in diseases including cancer since Peter Nowell and David Hungerford reported BCR-ABL1 fusion in chronic myeloid leukemia (MCL) in the 1960s.31,32 In PCa, TMPRSS2-ERG fusion is one of the most well-known genomic alterations and a large number of studies have been carried out to investigate the function and application of this fusion as an oncogenic factor and a diagnostic or prognostic biomarker.33,34 Nevertheless, these reports were mostly in the Western populations, and the value of TMPRSS2-ERG fusion in Asian patients is quite unclear.
Several studies have reported that Eastern Asian patients are two to five times less likely to harbor the fusion.35,36,37,38 We confirm that 110 (16.4%) of the 669 Chinese PCa patients harbor TMPRSS2-ERG fusion, which coincides with our previous report that ERG protein was overexpressed in 14.9% (26/174) of cases in tissue arrays in our hospital.39 Furthermore, the meta-analysis indicates that 27% (95% CI: 23%–31%) of Asian patients are fusion positive, which is approximately half the rate in the Western populations and is consistent with previous multiracial studies.10,19,40 Exceptions were Indian (Aryan descent) and Turkish (Caucasian descent), of which the fusion rates were 52% (95% CI: 43%–60%) and 46%, respectively, in our meta-analysis. Rawal et al.41 conducted the first investigation into the positivity of TMPRSS2-ERG fusion in an Indian population in 30 evaluable samples, of which they found 8 (27%) fusion-positive cases. They concluded that the positivity of TMPRSS2-ERG fusion in Indian patients was relatively lower, though this was not supported by other following studies. One year later, Jain et al.42 reported a fusion positive rate of 64% in Indian patients. In 2015, Suryavanshi et al.43 and Ateeq et al.44 detected the fusion in 51 of 100 and 46 of 94 samples, respectively. However, this was strong evidence of racial difference in TMPRSS2-ERG fusion.
The clinical utility of TMPRSS2-ERG fusion as a diagnostic and prognostic biomarker of PCa remained under debate both in the Western and Eastern populations. TMPRSS2-ERG fusion has been demonstrated as associated with PSA level, Gleason grade, tumor stage, metastasis, and BCR or tumor-specific death in some studies.10,23,45,46,47 In a multicenter study involving 1312 patients, Tomlins and colleagues17 put forward that urine TMPRSS2-ERG fusion was associated with tumor size, high Gleason score, and upgrading of Gleason score at prostatectomy. However, two prospective studies from Smith and colleagues demonstrated that TMPRSS2-ERG fusion in prostatic secretion could predict neither early BCR among patients receiving prostatectomy48 nor Gleason upstaging among patients receiving active surveillance49 in the US population. It was also observed in Asia that no consensus on the clinical significance of TMPRSS2-ERG fusion was reached. Kim and colleagues reported better BCR-free survival rates among fusion-positive patients50 while Lee found the fusion in Korean patients had no relation with BCR but strong correlation with lower Gleason grade.38 In our study, no significant association between TMPRSS2-ERG fusion and clinical parameters mentioned above was confirmed.
We observed that the positive rate of TMPRSS2-ERG fusion was lower in biopsies (12.9%) than in prostatectomy specimens (17.8%) in our hospital, but with no statistical significance (P = 0.13). This result is supported by Mosquera and other researchers who found that specimens from radical prostatectomy and biopsy had the equal positive rate of TMPRSS2-ERG fusion.10,21,51 Controversially, our meta-analysis reported a higher positive rate in biopsy specimens (35%) than in radical prostatectomy specimens (24%). One reason may be the disparity of the sample distribution; Indian patients accounted for a larger proportion in the biopsy group (26%) than in the prostatectomy group (2%).
We compared FISH with IHC in detecting TMPRSS2-ERG fusion in our samples and found that IHC produced a higher positive rate. In other studies, researchers found that TMPRSS2-ERG fusion was detected in 30% of patients by FISH in the UK46 and the US,52 while van Leenders et al.53 reported that the positive rate of the fusion among US patients was 61% using a specific antibody for fusion generated ERG. Cross-reactivity of antibodies may be responsible for the higher sensitivity, and compared with FISH, which provides direct evidence of gene fusion, IHC is more likely to produce false-positive results. Thus, the actual positive rate of the fusion could be lower than we detected. In our meta-analysis, PCR detected a higher positive rate of fusion than FISH or IHC. However, Hagen and other researchers found that RT-PCR was as reliable as FISH in detecting the fusion.26
To the best of our knowledge, this study has the largest sample size in Asia reporting the TMPRSS2-ERG fusion rate. In addition, a positive rate of fusion was calculated from the latest studies by a meta-analysis among Asian population. However, several limitations should be taken into account in this study. It was a single-center study and most patients were from Eastern China; the long-term prognostic value of TMPRSS2-ERG fusion in our study needs to be updated in follow-up work. The antibody used in the IHC detects both ETS transcription factor ERG (EGR) and Fli-1 proto-oncogene (FLI-1), resulting in the increase of false-positive cases. However, Paulo and colleagues found that FLI-1 protein was expressed in only 1 of 200 PCa patients, indicating that FLI-1 could contribute a limited false-positive rate in detecting ERG by IHC in PCa samples.54
CONCLUSION
The positive rate of TMPRSS2-ERG fusion is much lower in PCa among a Chinese population, and this gene aberration does not correlate with PSA level, Gleason grade, clinical tumor stage, bone metastasis, perineural invasion, lymphovascular invasion, or BCR of patients in the present study. The systematic review confirmed that the positive rate of TMPRSS2-ERG fusion is much lower in Asian PCa patients. Based on our data, we believe that some of the results from Asian studies have been possibly affected by their detection methods and sample size. We assert that TMPRSS2-ERG fusion may be less effective as a diagnostic and prognostic biomarker in Asians because of its low prevalence and insignificant correlation with clinical parameters. Furthermore, it appears important and urgent to find suitable molecular and genomic biomarkers in Asian PCa patients.
AUTHOR CONTRIBUTIONS
XG and YHS contributed to conceptualization and project administration. DPK and GAX performed data curation. WZ and FBW were in charge of formal analysis. RC and YHS contributed to funding acquisition. DPK contributed to investigation and visualization. DPK, CLZ, and NT contributed to methodology. YHS collected the resources. DPK and RC contributed to software, validation and writing the original draft. DPK, RC, and CLZ wrote, reviewed, and edited the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This research was funded by the National Nature Science Foundation Youth Project (Grant No. 81702514), the National Natural Science Foundation of China (Grant No. 81430058), and the Clinical Research Project of Shanghai Municipal Commission of Health and Family Planning (Grant No. 20184Y0130).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel R, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Ren S, Yiu MK, Fai NC, Cheng WS, et al. Prostate cancer in Asia: a collaborative report. Asian J Urol. 2014;1:15–29. doi: 10.1016/j.ajur.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 5.Attard G. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 6.Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–52. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 7.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 8.Furusato B, Tan SH, Young D, Dobi A, Sun C, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13:228–37. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–8. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–41. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 11.Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:2105–10. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–9. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–53. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King JC, Xu J, Wongvipat J, Hieronymus H, Carver BS, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–6. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–24. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, et al. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci U S A. 2009;106:12465–70. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, et al. The TMPRSS2:ERG rearrangement, erg expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489–97. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 20.Sedarsky J, Degon M, Srivastava S, Dobi A. Ethnicity and ERG frequency in prostate cancer. Nat Rev Urol. 2017;15:125–31. doi: 10.1038/nrurol.2017.140. [DOI] [PubMed] [Google Scholar]
- 21.Mosquera JM, Mehra R, Regan MM, Perner S, Genega EM, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15:4706–11. doi: 10.1158/1078-0432.CCR-08-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin MA. ETS rearrangements in prostate cancer. Asian J Androl. 2012;14:393–9. doi: 10.1038/aja.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou CK, Young D, Yeboah ED, Coburn SB, Tettey Y, et al. TMPRSS2:ERG gene fusions in prostate cancer of west African men and a meta-analysis of racial differences. Am J Epidemiol. 2017;186:1352–61. doi: 10.1093/aje/kwx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun QP, Li LY, Chen Z, Pang J, Yang WJ, et al. Detection of TMPRSS2-ETS fusions by a multiprobe fluorescence in situ hybridization assay for the early diagnosis of prostate cancer: a pilot study. J Mol Diagn. 2010;12:718–24. doi: 10.2353/jmoldx.2010.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Yu Y, Boyd LK, Ren G, Lin D, et al. Distinct genomic alterations in prostate cancers in Chinese and Western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res. 2010;70:5207–12. doi: 10.1158/0008-5472.CAN-09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagen RM, Adamo P, Karamat S, Oxley J, Aning JJ, et al. Quantitative analysis of ERG expression and its splice isoforms in formalin-fixed, paraffin-embedded prostate cancer samples. Am J Clin Pathol. 2014;142:533–40. doi: 10.1309/AJCPH88QHXARISUP. [DOI] [PubMed] [Google Scholar]
- 27.Na YQ, Ye ZQ, Sun YH, Sun G. Chinese Guidelines for the Diagnosis and Treatment of Urological Diseases. Beijing: People's Medical Publishing House; 2014. p. 78. [Google Scholar]
- 28.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–8. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furusato B, Takahashi H, Okayasu M, Kido M, Kimura T, et al. Laboratory Investigation. New York: Nature Publishing Group; 2013. Assessment of ERG expression in latent prostate cancer. Abstract; pp. 211A–12A. [Google Scholar]
- 31.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–45. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 32.Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol. 2017;14:735–48. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loeb S, Bruinsma SM, Nicholson J, Briganti A, Pickles T, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol. 2015;67:619–26. doi: 10.1016/j.eururo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren S, Wei GH, Liu D, Wang L, Hou Y, et al. Whole-genome and transcriptome sequencing of prostate cancer identify new genetic alterations driving disease progression. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.08.027. Doi: 101016/jeururo201708027 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Xue L, Mao X, Ren G, Stankiewicz E, Kudahetti SC, et al. Chinese and Western prostate cancers show alternate pathogenetic pathways in association with ERG status. Am J Cancer Res. 2012;2:736–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Miyagi Y, Sasaki T, Fujinami K, Sano J, Senga Y, et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod Pathol. 2010;23:1492–8. doi: 10.1038/modpathol.2010.149. [DOI] [PubMed] [Google Scholar]
- 38.Lee K, Chae JY, Kwak C, Ku JH, Moon KC. TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients. Urology. 2010;76:1267–8. doi: 10.1016/j.urology.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Mao X, Huang X, Zhao J, Wang L, et al. TMPRSS2:ERG fusion gene occurs less frequently in Chinese patients with prostate cancer. Tumour Biol. 2016;37:12397–402. doi: 10.1007/s13277-016-5116-9. [DOI] [PubMed] [Google Scholar]
- 40.Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56:275–86. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 41.Rawal S, Young D, Williams M, Colombo M, Krishnappa R, et al. Low frequency of the ERG oncogene alterations in prostate cancer patients from India. J Cancer. 2013;4:468–72. doi: 10.7150/jca.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain S, Bansal A, Kumar A, Saxena S. Clinical Relevance of TMPRSS2-ERG fusion marker for prostate cancer. FEBS J. 2014;281:446. [Google Scholar]
- 43.Suryavanshi M, Mehta A, Jaipuria J, Sharma AK, Rawal S, et al. Weaker ERG expression in patients with ERG-positive prostate cancer is associated with advanced disease and weaker androgen receptor expression: an Indian outlook. Urol Oncol. 2015;33:331–9. doi: 10.1016/j.urolonc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Ateeq B, Kunju LP, Carskadon SL, Pandey SK, Singh G, et al. Molecular profiling of ETS and non-ETS aberrations in prostate cancer patients from Northern India. Prostate. 2015;75:1051–62. doi: 10.1002/pros.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulda V, Topolcan O, Kucera R, Kripnerova M, Srbecka K, et al. Prognostic significance of TMPRSS2-ERG fusion gene in prostate cancer. Anticancer Res. 2016;36:4787–94. doi: 10.21873/anticanres.11037. [DOI] [PubMed] [Google Scholar]
- 46.Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–63. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Jeske DR, Linehan JA, Wilson TG, Kawachi MH, Wittig K, et al. Two-stage classifiers that minimize PCA3 and the PSA proteolytic activity testing in the prediction of prostate cancer recurrence after radical prostatectomy. Can J Urol. 2017;24:9089–97. [PubMed] [Google Scholar]
- 49.Wittig K, Yamzon JL, Smith DD, Jeske DR, Smith SS. Presurgical biomarker performance in the detection of gleason upgrading in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1643–5. doi: 10.1158/1055-9965.EPI-16-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SH, Joung JY, Lee GK, Hong EK, Kang KM, et al. Overexpression of ERG and wild-type PTEN are associated with favorable clinical prognosis and low biochemical recurrence in prostate cancer. PLoS One. 2015;10:e122498. doi: 10.1371/journal.pone.0122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehra R, Tomlins SA, Shen R, Nadeem O, Wang L, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol. 2007;20:538–44. doi: 10.1038/modpathol.3800769. [DOI] [PubMed] [Google Scholar]
- 52.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–9. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 53.van Leenders GJ, Boormans JL, Vissers CJ, Hoogland AM, Bressers AA, et al. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24:1128–38. doi: 10.1038/modpathol.2011.65. [DOI] [PubMed] [Google Scholar]
- 54.Paulo P, Barros-Silva JD, Ribeiro FR, Ramalho-Carvalho J, Jeronimo C, et al. FLI1 is a novel ETS transcription factor involved in gene fusions in prostate cancer. Genes Chromosomes Cancer. 2012;51:240–9. doi: 10.1002/gcc.20948. [DOI] [PubMed] [Google Scholar]
- 55.Dai MJ, Chen LL, Zheng YB, Chen W, Tao ZH, et al. Frequency and transcript variant analysis of gene fusions between TMPRSS2 and ETS transcription factor genes in prostate cancer. Zhonghua Yi Xue Za Zhi. 2008;88:669–73. Article in Chinese. [PubMed] [Google Scholar]
- 56.Furusato B, van Leenders GJ, Trapman J, Kimura T, Egawa S, et al. Immunohistochemical ETS-related gene detection in a Japanese prostate cancer cohort: diagnostic use in Japanese prostate cancer patients. Pathol Int. 2011;61:409–14. doi: 10.1111/j.1440-1827.2011.02675.x. [DOI] [PubMed] [Google Scholar]
- 57.Henshall-Powell R, Yu C, Bremer R, Sesterhenn I, Tacha D. Laboratory Investigation. New York: Nature Publishing Group; 2011. Evaluation of TMPRSS2-ERG fusion protein in prostate cancer pathogenesis across continents. Abstract; p. 197A. [Google Scholar]
- 58.Xiao L, Zhu XZ, Wang Y, Gong Y, Guo CC. TMPRSS2-ERG gene fusion in metastatic prostate cancers: a study of fine needle aspiration specimens. Zhonghua Bing Li Xue Za Zhi. 2011;40:392–6. Article in Chinese. [PubMed] [Google Scholar]
- 59.Inoue T, Segawa T, Maeno A, Akamatsu S, Yoshikawa T, et al. ERG oncoprotein expression in localized prostate cancer in Japanese population. J Urol. 2012;187:e133–4. [Google Scholar]
- 60.Kimura T, Furusato B, Miki J, Yamamoto T, Hayashi N, et al. Expression of ERG oncoprotein is associated with a less aggressive tumor phenotype in Japanese prostate cancer patients. Pathol Int. 2012;62:742–8. doi: 10.1111/pin.12006. [DOI] [PubMed] [Google Scholar]
- 61.Ren S, Peng Z, Mao JH, Yu Y, Yin C, et al. RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res. 2012;22:806–21. doi: 10.1038/cr.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suh JH, Park JW, Lee C, Moon KC. ERG immunohistochemistry and clinicopathologic characteristics in Korean prostate adenocarcinoma patients. Korean J Pathol. 2012;46:423–8. doi: 10.4132/KoreanJPathol.2012.46.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi H, Furusato B, Kimura T, Okayasu M, Mizukami S, et al. Nature Publishing Group: New York; 2012. In: Laboratory Investigation; p. 44A. [Google Scholar]
- 64.Wang JJ, Liu YX, Wang W, Yan W, Zheng YP, et al. Fusion between TMPRSS2 and ETS family members (ERG, ETV1, ETV4) in prostate cancers from Northern China. Asian Pac J Cancer Prev. 2012;13:4935–8. doi: 10.7314/apjcp.2012.13.10.4935. [DOI] [PubMed] [Google Scholar]
- 65.Xing T, Pei X, Fang W, He H. Laboratory Investigation. New York: Nature Publishing Group; 2013. Erg protein expression and PTEN loss are uncommon in prostate cancer of Chinese population. Abstract; p. 259A. [Google Scholar]
- 66.Yoon G, Park K, MacDonald T, Choi J, Chen Z, et al. Laboratory Investigation. New York: Nature Publishing Group; 2013. Prevalence of ERG rearrangement, SPINK1 overexpression and PTEN deletion in prostate cancer of Korean men. Abstract; p. 260A. [Google Scholar]
- 67.Blattner M, Lee DJ, O'Reilly C, Park K, MacDonald TY, et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia. 2014;16:14–20. doi: 10.1593/neo.131704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong J, Xiao L, Sheng L, Xu J, Sun ZQ. TMPRSS2:ETS fusions and clinicopathologic characteristics of prostate cancer patients from Eastern China. Asian Pac J Cancer Prev. 2014;15:3099–103. doi: 10.7314/apjcp.2014.15.7.3099. [DOI] [PubMed] [Google Scholar]
- 69.Jung WY, Sung CO, Han SH, Kim K, Kim M, et al. AZGP-1 immunohistochemical marker in prostate cancer: potential predictive marker of biochemical recurrence in post radical prostatectomy specimens. Appl Immunohistochem Mol Morphol. 2014;22:652–7. doi: 10.1097/PAI.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 70.Lee B, Yoon N, Choi Y. Laboratory Investigation. New York: Nature Publishing Group; 2014. Analysis of SPOP mutation and its relationship with TMPRSS2-ERG fusion in prostate cancer. Abstract; p. 244A. [Google Scholar]
- 71.Qi M, Yang X, Zhang F, Lin T, Sun X, et al. ERG rearrangement is associated with prostate cancer-related death in Chinese prostate cancer patients. PLoS One. 2014;9:e84959. doi: 10.1371/journal.pone.0084959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raymundo EM, Diwa MH, Lapitan MC, Plaza AB, Sevilleja JE, et al. Increased association of the ERG oncoprotein expression in advanced stages of prostate cancer in Filipinos. Prostate. 2014;74:1079–85. doi: 10.1002/pros.22791. [DOI] [PubMed] [Google Scholar]
- 73.Suh JH, Moon KC. Virchows Archiv. New York: Springer; 2014. The relation between heterogeneity of ERG protein expression and TMPRSS2-ERG gene fusion pattern in prostate cancer. Abstract; p. S162. [Google Scholar]
- 74.Yilmaz O, Berber U, Okcelik S, Soydan H, Ates F, et al. TMPRSS2-ERG gene fusion in Turkish patients with localized prostate cancer: results of radical prostatectomy specimens. Turk J Urol. 2016;42:60–3. doi: 10.5152/tud.2016.94763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly GM, Kong YH, Dobi A, Srivastava S, Sesterhenn IA, et al. ERG oncoprotein expression in prostate carcinoma patients of different ethnicities. Mol Clin Oncol. 2015;3:23–30. doi: 10.3892/mco.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishijima J, Hara T, Ikemoto K, Oga A, Kobayashi K, et al. Clinical significance of ERG rearrangement subtype and its association with increased p53 expression in Japanese and German prostate cancer. Neoplasma. 2015;62:278–87. doi: 10.4149/neo_2015_033. [DOI] [PubMed] [Google Scholar]
- 77.Sung JY, Jeon HG, Jeong BC, Seo SI, Jeon SS, et al. Correlation of ERG immunohistochemistry with molecular detection of TMPRSS2-ERG gene fusion. J Clin Pathol. 2016;69:586–92. doi: 10.1136/jclinpath-2015-203314. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Williamson SR, Zhang S, Huang J, Montironi R, et al. Increased androgen receptor gene copy number is associated with TMPRSS2- ERG rearrangement in prostatic small cell carcinoma. Mol Carcinog. 2015;54:900–7. doi: 10.1002/mc.22162. [DOI] [PubMed] [Google Scholar]
- 79.Yi FX, Li H, Wei Q, Li X, Zeng H. Relationship between TMPRSS2:ERG and the pathological grade of prostate cancer. Zhonghua Nan Ke Xue. 2015;21:887–91. Article in Chinese. [PubMed] [Google Scholar]
- 80.Zeng W, Sun H, Meng F, Liu Z, Xiong J, et al. Nuclear C-MYC expression level is associated with disease progression and potentially predictive of two year overall survival in prostate cancer. Int J Clin Exp Pathol. 2015;8:1878–88. [PMC free article] [PubMed] [Google Scholar]
- 81.Mannan R, Bhasin TS, Manjari M, Singh G, Bhatia PK, et al. Immunohistochemical expression of Ets-related gene-transcriptional factor in adenocarcinoma prostate and its correlation with Gleason score. Indian J Pathol Microbiol. 2016;59:489–95. doi: 10.4103/0377-4929.191794. [DOI] [PubMed] [Google Scholar]
- 82.Noh BJ, Sung JY, Kim YW, Chang SG, Park YK. Prognostic value of ERG, PTEN, CRISP3 and SPINK1 in predicting biochemical recurrence in prostate cancer. Oncol Lett. 2016;11:3621–30. doi: 10.3892/ol.2016.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan X, Zhang X, Gong J, Tan J, Yin X, et al. The expression profile and prognostic value of SPINK1x in initially diagnosed bone metastatic prostate cancer. Prostate. 2016;76:823–33. doi: 10.1002/pros.23173. [DOI] [PubMed] [Google Scholar]
- 84.Song W, Kwon GY, Kim JH, Lim JE, Jeon HG, et al. Immunohistochemical staining of ERG and SOX9 as potential biomarkers of docetaxel response in patients with metastatic castration-resistant prostate cancer. Oncotarget. 2016;7:83735–43. doi: 10.18632/oncotarget.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]


