Abstract
Both thrombocytopenia and microangiopathic hemolytic anemia (TMA) are seen in thrombotic thrombocytopenic purpura (TTP) and HELLP syndrome among other disorders during pregnancy. Although both share backgrounds of endothelial injury and microvascular thrombi and some clinical features, yet, they have different etiologies and courses. In late pregnancy, differentiating between these two pathologies can be extremely difficult due to the immense overlap in clinical and laboratory manifestations and this becomes only possible with the use of specific markers as ADAMTS-13, when available. Hereby, we describe three cases that may exemplify the complex association between PE/HELLP syndrome and TTP. The first case presented with PE/HELLP syndrome and deteriorated postpartum to improve on plasmapheresis. The second case was a known TTP patient who developed superimposed PE/HELLP at 27 weeks gestation which necessitated emergent delivery. The third was a case of preeclampsia that progressed to HELLP syndrome on day 2 postpartum but 3 days later was complicated by TTP. HELLP syndrome and TTP can co-exist, but can also complicate one another. In the absence of instantaneous results of ADAMTS-13 and when diagnosis with clinical judgement alone cannot be done with certainty, a short trial-plasmapheresis could be attempted with close observation of the immediate response. This stepwise approach might prove to be a valuable tool when integrated in the usual workup of clinical and laboratory evaluation both before and after delivery.
Keywords: Thrombotic thrombocytopenic purpura, HELLP syndrome, TMA-associated disorders, Plasmapheresis
Introduction
Both HELLP syndrome and thrombotic thrombocytopenic purpura (TTP) share in common features of thrombotic microangiopathy (TMA) but basically have different pathogenesis [1]. Diagnosis is critically important and should be attained early enough to avoid serious complications and even mortality [2]. Though, ADAMTS-13 can discern between both conditions [3], results usually are not readily available and the differential diagnosis has to be made relying on clinical judgement [1]. The significance of exact antenatal diagnosis is derived from the fact that two divergent management plans are needed. The optimal treatment for TTP is plasmapheresis, and this should be instituted as soon as the diagnosis is entertained [4], while, termination of pregnancy does not ameliorate the condition [5]. On the other hand, delivery remains the definitive treatment in HELLP syndrome [6], whereas, plasmapheresis is not a part of the usual antenatal management plan. In the postpartum period, the condition will continue to deteriorate in TTP unless plasmapheresis is started, while usually resolution, within 2 - 3 days, is anticipated in PE/HELLP syndrome [7]. Here, once more, the clinical condition mandates plasmapheresis irrespective of the identity of the offending disorder [1], pending the results of ADAMTS-13. Hereby, we describe the clinical course and evolution of laboratory values encountered in three pregnancies where both PE/HELLP syndrome and TTP have interplayed in different clinical scenarios. In addition, we present a practical plan for the differential diagnosis and management of HELLP syndrome and TTP.
Case Reports
Case 1: HELLP syndrome co-existing with undiagnosed TTP
A 26-year-old G1P0 pregnant woman at 33 weeks gestation was admitted to our service following several seizures at home. Physical examination revealed blood pressure of 240/120 mm Hg and a new episode of generalized tonic-clonic seizure. MgSO4, diazepam and labetalol were administered followed by intubation. Computed tomography (CT) angiography of the brain excluded intra-cranial hemorrhage. Ultrasound confirmed positive fetal heart activity. Laboratory tests showed hemoglobin 6.8 g/dL, platelets count (PLT) 26 × 109/L, LDH 2,381 IU/mL, uric acid 14 mg/dL, SGOT 130 IU/mL and serum creatinine 1.61 mg/dL. She received two pools of platelets along with 2 units of packed red blood cells then was rushed to cesarean delivery (CD) after stabilization of blood pressure. Intra-operatively, she was found to have 50% placental abruption and features of disseminated intravascular coagulation (DIC). She was then transferred intubated to the ICU. Immediate postpartum PLT was 51 × 109/L. Peripheral blood smear (PBS) showed 15 - 18 schistocytes/HPF which was interpreted as consistent with DIC. On day 2 postpartum, PLT increased to 152 × 109/L, along with improvement of liver enzymes and LDH level. She was then extubated after showing clinical improvement and normalization of blood pressure on nifedipine. On day 6 postpartum, PLT dropped back to 23 × 109/L and LDH increased to 2,775 IU/mL. PBS showed further increase in schistocytes. As TTP was highly suspected, blood for ADAMTS-13 was drawn and plasmapheresis was initiated. After seven sessions, PLT raised to 585 × 109/L. The patient was kept for 15 days in the ICU then discharged in good condition on day 21 postpartum. ADAMTS-13 activity results were available 2 days after discharge and were found to be very low (0.05 IU/mL, normal 0.4 - 1.3 IU/mL) with non-detectable antibody levels. Though, these findings conform to being hereditary-TTP, there is a remote possibility of being also acquired-TTP as 10% of these patients do not demonstrate detectable anti-ADAMTS-13 antibodies. The exact diagnosis of this type of TTP requires the demonstration of persistent absence of the inhibitor during remission or the documentation of ADAMTS-13 mutations [8].
Case 2: TTP complicated by PE/HELLP syndrome
A 36-year-old G3P1011 pregnant woman at 27 weeks gestation was referred to our service for management of severe TTP-related thrombocytopenia discovered during routine follow-up visit to her hematologist. She was diagnosed with acquired-TTP since age 16. Her first pregnancy, 7 years ago, was complicated by severe exacerbation of TTP at 35 weeks for which plasmapheresis and rituximab were administered and CD was done because of twin gestation. One year later, she underwent splenectomy but relapsed after 3 years. On admission, she was asymptomatic and normotensive. Laboratory tests showed PLT 46 × 109/L, LDH 473 IU/L, SGOT 15.6 IU/mL, numerous schistocytes on PBS and 1+ proteinuria. Plasmapheresis (1 session daily) and prednisone (80 mg IV daily) were started. Four days later, she developed headache, epigastric pain, and tachypnea. Physical examination disclosed hypertension of 170/90 mm Hg and bilateral basal crackles. Laboratory tests showed 3+ proteinuria, with SGOT of 36.5 IU/mL. She was transferred to the ICU for bilateral lung congestion. Over the next 2 days, she continued to have hypertension (200/100 mm Hg) despite initiation of labetalol. LDH increased to 1,887 IU/L, PLT dropped to 10 × 109/L. At this stage, she was diagnosed with PE/HELLP syndrome on top of TTP. She underwent secondary CD for fetal distress and was kept in the ICU for treatment of hypertension and acute kidney injury. Six days later, she had acute dyspnea and was diagnosed with antero-apical myocardial infarction. On day 24, she had subarachnoid hemorrhage in the left and right parietal and temporal areas. PLT was 7 × 109/L. Five days later, she had severe vaginal bleeding, hematemesis and cardiac arrest and was declared dead after unsuccessful resuscitations.
Case 3: HELLP syndrome triggering TTP
A 24-year-old G2P1001 woman at 37 weeks with twin gestation was referred to our service with hypertension. Her obstetric history included one uneventful vaginal delivery at term. On admission, physical examination disclosed 1+ lower limbs pitting edema and blood pressure of 160/100 mm Hg. Laboratory tests showed 1+ proteinuria, PLT 204 × 109/L, hemoglobin 11 g/dL and normal liver and renal function tests. The patient was diagnosed to have severe preeclampsia. Antihypertensive medications along with MgSO4 were initiated. The cervix was unfavorable for induction so primary CD was done. Forty-eight h later, she had persistent hypertension, mild headache, thrombocytopenia (PLT 48 × 109/L), hyperuricemia (8.7 mg/dL), SGOT (116 IU/mL), serum creatinine (1.3 mg/dL), LDH (2,500 IU/L) and few schistocytes on PBS. She was then diagnosed with postpartum HELLP syndrome that mandated close monitoring. On day 3 postpartum, dexamethasone (12 mg IV every 12 h) was started. On day 5, further deterioration was observed with PLT 20 × 109/L, LDH 6,000 IU/mL, creatinine 1.8 mg/mL, hemoglobin: 6.4 g/dL, and SGOT 140 IU/mL. TTP was suspected due to non-resolution of the condition coupled with the novel appearance of abundant schistocytosis on PBS. Plasmapheresis was initiated with 40 mL/kg then with 10 mL/kg once daily. This led to normalization of all laboratory parameters. The patient recovered and was discharged in a good condition on day 20 postpartum. She was found to be healthy when examined few months later. ADAMTS-13 testing was done (non-pregnant). The findings of ADAMTS-13 activity below the level of detection, together with detectable antibodies are consistent with acquired immune-mediated TTP in remission (Table 1).
Table 1. Clinical Course and Laboratory Values Evolution of Case Three.
| 5/24 | 5/25 | 5/26 | 5/27 | 5/28 | 5/29 | 5/30 | 5/31 | 6/1 | 6/2 | 6/3 | 6/5 | 6/6 | 6/8 | 6/9 | 6/16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine | 0.5 | 1.2 | 1.3 | 1.6 | 1.7 | 1.4 | 1.3 | 1.2 | 1.3 | 1.3 | 1.2 | 1 | 1.1 | 1.1 | ||
| SGOT | 39 | 20 | 71 | 128 | 135 | 134 | 60 | 47 | 39 | 16 | 24 | 18 | 20 | |||
| LDH | 239 | 230 | 1,214 | 3,800 | 3,628 | 3,168 | 1,520 | 1,450 | 799 | 549 | 357 | 436 | 331 | 286 | 159 | 230 |
| Platelet count (× 100) | 204 | 186 | 93 | 48 | 30 | 24 | 20 | 40 | 80 | 150 | 208 | 247 | 313 | 398 | 424 | 333 |
| Hematocrit | 32 | 33.9 | 31 | 28 | 25 | 24 | 19 | 22 | 22 | 21.4 | 22 | 23 | 24.8 | 24 | 23.1 | 34.1 |
| PBS | × | × | × | × | × | × | ||||||||||
| Plasmapheresis | × | × | × | × | × | ×× | × | × | ||||||||
| Diagnosis | Preeclampsia | HELLP syndrome | TTP | Recovery | ||||||||||||
| Delivery | discharge | |||||||||||||||
PBS: peripheral blood smear.
Discussion
During pregnancy, TMA which is a term used to describe a group of disorders characterized by thrombocytopenia, hemolytic anemia, and widespread thrombosis in the microvasculature, can be applied to patients with HELLP syndrome, TTP, atypical hemolytic uremic syndrome (a-HUS), AFPL, SLE, APLS, and DIC resulting from severe sepsis, abruptio placentae or postpartum hemorrhage [1].
Differential diagnosis between HELLP syndrome and TTP in late pregnancy might prove to be difficult owing to the overlap in clinical presentation and laboratory parameters. When the presentation and the laboratory values are so confusing, it is advocated to resort to circumstantial evidence gathered from judicious interpretation of certain specific laboratory or clinical features that would finally construct a clearer image of the “most-likely working diagnosis of the disease”, pending the decisive results of ADAMTS-13 testing. This is, though, not an easy task and the literature is full of examples of the costly price of doing faulty or misdiagnosis [2].
The association of TTP with HELLP syndrome is complex and can present in different forms. The first form of association is the concurrent existence of both disorders as seen in case number one. The diagnosis of PE/HELLP syndrome could not be disputed with recurrent eclamptic seizures and blood pressure readings of 240/120 mm Hg. Though according to George et al, eclampsia or any other form of severe neurologic dysfunction should alert to the presence of TTP [1], the extreme hypertension was in favor of PE/HELLP syndrome. When the working diagnosis favored PE/HELLP, delivery was done, but later, when the clinical condition did not ameliorate, plasmapheresis was started with instant dramatic improvement. This patient was found to have TTP (probably hereditary) [8] which points to the possibility of co-existence of both disorders at the same instance. Co-existence was estimated by Martin et al to occur in about 17% in his series of 166 patients, and this carried higher maternal mortality rate when compared with cases with pure TTP (44% vs. 21%), possibly due to a delay in diagnosis or in the initiation of plasmapheresis [9]. Here patients present with clinical features common to both disorders and diagnosis could go either way. It seems that this is the most common form of association reported to the literature; however, no clear information was presented on the association type, neither was there documentation of ADAMTS-13 testing in most reports. The second form is when a patient with known pregestational-TTP develops PE/HELLP syndrome, as was exemplified by our second case and has also been reported earlier by others [10]. Making the diagnosis was straight forward where features suggestive of PE/HELLP syndrome as new onset proteinuria with severe hypertension together with fetal distress were distinct and could not be missed. This type of association has also been referred to by Sibai who stated that preeclampsia may be superimposed upon other mimicking disorders thus confounding an already difficult differential diagnosis [11]. George et al also observed an increased rate of PE/HELLP in women who have recovered from TTP [1]. The third and final form is when a case of PE/HELLP triggers TTP. In case 3 the diagnosis of PE/HELLP syndrome was clear during the first 5 days but with the absence of normalization of laboratory tests together with novel appearance of abundant schistocytes on day 6, the working diagnosis shifted in favor of TTP. The laboratory features of TTP were not originally present, but appeared later with the progression of the condition. This might include many of the so-called “non-remittent HELLP syndromes”. This possibility should be kept in mind and detection of this disorder needs vigilant monitoring of the evolution of the condition even in well-established and properly diagnosed cases.
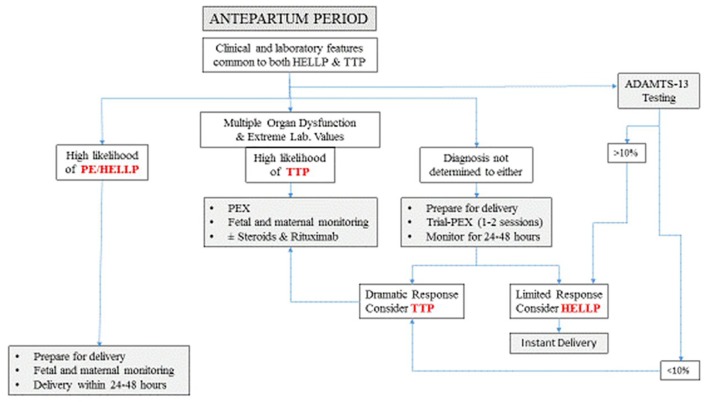
The differential diagnosis in the antepartum period will continue to be difficult depending mostly on clinical and circumstantial evidences due to absence of a readily available laboratory test that can make a decisive and instantaneous distinction between these two disorders. Without a history of pregestational disease, the occurrence of these “in-common” features in early pregnancy (before 20 weeks) speaks for TTP [1]. The difficult diagnosis is limited to cases presenting beyond mid-pregnancy. Here, the odds are usually in favor of being HELLP syndrome which is encountered in around 1% [12] of pregnancies while on the other hand the rate of new-onset TTP is far less frequent with a rate of 1/100,000 pregnancies [11]. In spite of the overlap in clinical and laboratory features common to both disease entities, most of these features tend to be more prominent preferentially in one disease than the other. When HELLP syndrome is highly suspected the curative treatment remains delivery [6]. The antepartum use of plasmapheresis or eculizumab is not a part of the usual management plan, though; both modalities were used by Eckford, yet, with limited benefits [13]. Similarly Martin et al also reported on the poor response of PE/HELLP syndrome to plasmapheresis in the antenatal period [14]. Here, the presence of neurologic or renal dysfunction or extremely abnormal laboratory tests (severe thrombocytopenia, severe anemia, very high LDH and increasing creatinine) are findings that point to TTP as the underlying pathology and plasmapheresis must be started even before laboratory confirmation [4]. When the diagnosis of TTP cannot be ascertained with confidence, trial-plasmapheresis of one or two sessions limited to 24 - 48 h might be started with concomitant monitoring of the response to decide for proceeding with this treatment modality, while preparation for delivery is underway. Although plasmapheresis is not without side-effects [11], no severe complications were reported by studies on recovered TTP cases during pregnancy [1].
Antepartum trial-plasmapheresis might be considered as a part of preparation for delivery, pending the action of corticosteroids and MgSO4 to take effect and ADAMTS-13 results to be available. In addition there is a possible added-value of enhancement of platelet count as reported by Eckford et al [13], which might be needed when cesarean delivery is planned. If this trial-plasmapheresis could induce a dramatic improvement in the clinical and laboratory parameters within this short trial, which is unlikely with HELLP syndrome [13, 14], then we might consider the working diagnosis as TTP, hence plasmapheresis should be continued as indicated with the possibility of adding steroids and rituximab if ADAMTS-13 results showed acquired-TTP. On the other hand, if no improvement was elicited, then delivery must be done as scheduled (within 24 - 48 h) [15]. Trial-plasmapheresis should not be done with the aim of prolonging pregnancy in cases suspected to have HELLP syndrome even if some parameters showed improvement. Awaiting results of ADAMTS-13 in critical situations is precious time wasted, especially when managing two serious disorders, both known to be associated with high maternal and perinatal complication rates and also known for the potential rapid deterioration without notice and without reliable alarming clinical or laboratory signs and symptoms [1, 16] (Fig. 1).
Figure 1.
Antepartum practical management plan. PEX: plasmapheresis.
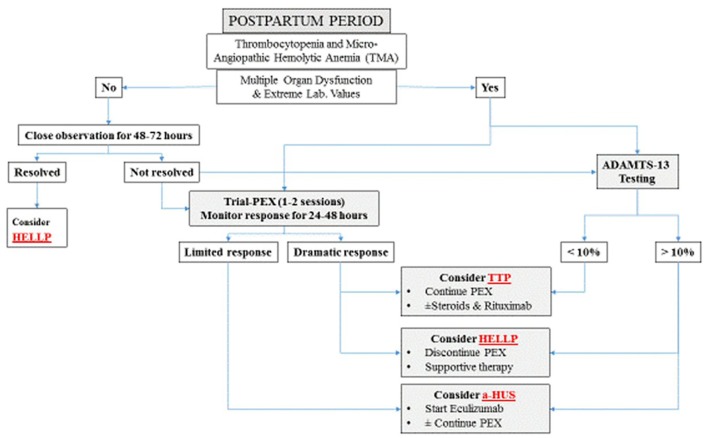
The postpartum management should constitute no major difficulty if a simple, practical and logical stepwise plan was followed (Fig. 2) When the possibility of TTP is entertained, plasmapheresis should be instituted without delay. In the presence of extreme abnormal laboratory findings (PLT, LDH, Hb and creatinine) and multiorgan dysfunction (neurologic and renal) and irrespective of the initial nomenclature of the TMA associated disorder, it is also advisable to start early plasmapheresis [1]. In cases of suspected PE/HELLP syndrome without multi-organ dysfunction, close observation for 48 - 72 h is recommended. Though resolution of platelets and other parameters to normal values in patients with HELLP syndrome was reported to be 2 - 3 days following delivery, it seems that about 10% of such patients show slower rates [17]. For cases of HELLP syndrome with delayed resolution, trial-plasmapheresis of one-two sessions with monitoring of the immediate clinical and laboratory response can be initiated pending the results of ADAMTS-13. The use of plasmapheresis for this specific indication remains debatable. Several authors advocate using this treatment modality [18, 19]. Actually, though these HELLP cases might respond positively and benefit from immediate postpartum plasmapheresis [20, 21], yet, this approach lacks scientific evidence. In fact those cases that benefited from plasmapheresis could have concurrent TTP. The only study to explore this point was by Pourrat et al who reported successful outcome of five patients presenting with non-remittent HELLP, where one patient with very low ADAMTS-13 levels (TTP) was treated with plasmapheresis whereas the rest were managed expectantly [22]. Sibai et al also concluded that HELLP patients with delayed resolution could be safely managed with supportive therapy without the need for plasmapheresis [17]. In non-remitting HELLP a serious search for other disorders needs to be started including determination of ADAMTS-13 level. Since these cases with delayed resolution might harbor TTP as an underlying pathology, though the rate is not exactly known, we believe that trial-plasmapheresis can compensate for the considerable time wasted before results of ADAMTS-13 are available. On the other hand, cases with limited or suboptimal response to plasmapheresis defined as the absence of normalization in platelet count, LDH or improvement in kidney function after 4 - 5 days of daily plasmapheresis [23], could have a-HUS which mandates different management.
Figure 2.
Postpartum practical management plan. PEX: plasmapheresis.
Conclusions
HELLP syndrome and TTP can co-exist masquerading each other, but can also complicate one another. During the antepartum period, the optimal treatment should be guided by determining the ADAMTS-13 level, however, since results are not promptly obtainable, antepartum trial-plasmapheresis with monitoring of its immediate response might be considered as a part of delivery preparation pending corticosteroids to induce effect and the results of ADAMTS-13 testing to be available. In the postpartum period, trial-plasmapheresis has similar supporting value in the differential diagnosis and the added merit of early institution of plasmapheresis which might prove to be invaluable if the underlying pathology proves to be TTP.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.George JN, Nester CM, McIntosh JJ. Syndromes of thrombotic microangiopathy associated with pregnancy. Hematology Am Soc Hematol Educ Program. 2015;2015:644–648. doi: 10.1182/asheducation-2015.1.644. [DOI] [PubMed] [Google Scholar]
- 2.Stella CL, Dacus J, Guzman E, Dhillon P, Coppage K, How H, Sibai B. The diagnostic dilemma of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in the obstetric triage and emergency department: lessons from 4 tertiary hospitals. Am J Obstet Gynecol. 2009;200(4):381. doi: 10.1016/j.ajog.2008.10.037. e381-386. [DOI] [PubMed] [Google Scholar]
- 3.Lattuada A, Rossi E, Calzarossa C, Candolfi R, Mannucci PM. Mild to moderate reduction of a von Willebrand factor cleaving protease (ADAMTS-13) in pregnant women with HELLP microangiopathic syndrome. Haematologica. 2003;88(9):1029–1034. [PubMed] [Google Scholar]
- 4.Coppo P. Management of thrombotic thrombocytopenic purpura. Transfus Clin Biol. 2017;24(3):148–153. doi: 10.1016/j.tracli.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Mokrzycki MH, Rickles FR, Kaplan AA, Kohn OF. Thrombotic thrombocytopenic purpura in pregnancy: successful treatment with plasma exchange. Case report and review of the literature. Blood Purif. 1995;13(5):271–282. doi: 10.1159/000170210. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142(2):159–167. doi: 10.1016/S0002-9378(16)32330-4. [DOI] [PubMed] [Google Scholar]
- 7.Chandran R, Serra-Serra V, Redman CW. Spontaneous resolution of pre-eclampsia-related thrombocytopenia. Br J Obstet Gynaecol. 1992;99(11):887–890. doi: 10.1111/j.1471-0528.1992.tb14435.x. [DOI] [PubMed] [Google Scholar]
- 8.Moatti-Cohen M, Garrec C, Wolf M, Boisseau P, Galicier L, Azoulay E, Stepanian A. et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood. 2012;119(24):5888–5897. doi: 10.1182/blood-2012-02-408914. [DOI] [PubMed] [Google Scholar]
- 9.Martin JN Jr, Bailey AP, Rehberg JF, Owens MT, Keiser SD, May WL. Thrombotic thrombocytopenic purpura in 166 pregnancies: 1955-2006. Am J Obstet Gynecol. 2008;199(2):98–104. doi: 10.1016/j.ajog.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Fyfe-Brown A, Clarke G, Nerenberg K, Chandra S, Jain V. Management of pregnancy-associated thrombotic thrombocytopenia purpura. AJP Rep. 2013;3(1):45–50. doi: 10.1055/s-0032-1331380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibai BM. Imitators of severe preeclampsia. Obstet Gynecol. 2007;109(4):956–966. doi: 10.1097/01.AOG.0000258281.22296.de. [DOI] [PubMed] [Google Scholar]
- 12.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 13.Eckford SD, Macnab JL, Turner ML, Plews D, Liston WA. Plasmapheresis in the management of HELLP syndrome. J Obstet Gynaecol. 1998;18(4):377–379. doi: 10.1080/01443619867182. [DOI] [PubMed] [Google Scholar]
- 14.Martin JN Jr, Perry KG Jr, Roberts WE, Norman PF, Files JC, Blake PG, Morrison JC. et al. Plasma exchange for preeclampsia: II. Unsuccessful antepartum utilization for severe preeclampsia with or without HELLP syndrome. J Clin Apher. 1994;9(3):155–161. doi: 10.1002/jca.2920090302. [DOI] [PubMed] [Google Scholar]
- 15.Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103(5 Pt 1):981–991. doi: 10.1097/01.AOG.0000126245.35811.2a. [DOI] [PubMed] [Google Scholar]
- 16.Haddad B, Barton JR, Livingston JC, Chahine R, Sibai BM. Risk factors for adverse maternal outcomes among women with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183(2):444–448. doi: 10.1067/mob.2000.105915. [DOI] [PubMed] [Google Scholar]
- 17.Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) Am J Obstet Gynecol. 1993;169(4):1000–1006. doi: 10.1016/0002-9378(93)90043-I. [DOI] [PubMed] [Google Scholar]
- 18.Martin JN Jr, Files JC, Blake PG, Perry KG Jr, Morrison JC, Norman PH. Postpartum plasma exchange for atypical preeclampsia-eclampsia as HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1995;172(4 Pt 1):1107–1125. doi: 10.1016/0002-9378(95)91470-6. discussion 1125-1107. [DOI] [PubMed] [Google Scholar]
- 19.Katz VL, Watson WJ, Thorp JM Jr, Hansen W, Bowes WA Jr. Treatment of persistent postpartum HELLP syndrome with plasmapheresis. Am J Perinatol. 1992;9(2):120–122. doi: 10.1055/s-2007-994683. [DOI] [PubMed] [Google Scholar]
- 20.Bayraktaroglu Z, Demirci F, Balat O, Kutlar I, Okan V, Ugur G. Plasma exchange therapy in HELLP syndrome: a single-center experience. Turk J Gastroenterol. 2006;17(2):99–102. [PubMed] [Google Scholar]
- 21.Eser B, Guven M, Unal A, Coskun R, Altuntas F, Sungur M, Serin IS. et al. The role of plasma exchange in HELLP syndrome. Clin Appl Thromb Hemost. 2005;11(2):211–217. doi: 10.1177/107602960501100211. [DOI] [PubMed] [Google Scholar]
- 22.Pourrat O, Coudroy R, Pierre F. ADAMTS13 deficiency in severe postpartum HELLP syndrome. Br J Haematol. 2013;163(3):409–410. doi: 10.1111/bjh.12494. [DOI] [PubMed] [Google Scholar]
- 23.Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol. 2012;157(4):501–503. doi: 10.1111/j.1365-2141.2012.09032.x. [DOI] [PubMed] [Google Scholar]