Abstract
Multiple myeloma is plasma cell neoplasm affecting persons over the age 60. Less than 2% of the cases occur below the age 40. In this case report we discuss a young 20-year-old female presenting with 90% plasma cells on bone marrow biopsy and presence of factor X inhibitor.
Keywords: Multiple myeloma, Factor X inhibitor, AL amyloidosis
Introduction
Multiple myeloma (MM) is a neoplasm of plasma cells producing monoclonal antibodies. It usually presents with one or more of the features described in acronym CRAB (hypercalcemia, renal failure, anemia, bone pain). Myeloma accounts for about 1% of malignancies [1]. Myeloma is generally diagnosed in patients over the age of 40 years. Immunoglobulin light chain amyloidosis (AL amyloidosis) is a deposition of light chain immunoglobulin fragments in different parts of the body. It can present with nephrotic range nephropathy, hepatosplenomegaly and restrictive cardiomyopathy [2]. We will present a rare case of a young female presenting with constellation of signs and symptoms with investigative workup which points towards MM but has features seen in amyloidosis.
Case Report
A 20-year-old female with no significant disease history presented with nausea, vomiting, fatigue and easy bruising to an urgent care. Patient was given antiemetics and subsequently sent home. Routine labs done during the encounter showed acute renal failure (creatinine 2.8mg/dL) with small amount of proteinuria and hematuria, elevated serum protein 11.3gm/dL while albumin was 2.6mg/dL, macrocytic anemia (Hb 8.1 g/dL and MCV 99 fL) with mild thrombocytopenia (platelets count 122 k/uL) and prolonged PTT (65 s). The patient was called back in and further questioning revealed that she had been having increased bleeding and easy bruising for the last 1 month. She would bleed every time when she brushed her teeth. She also stated that she lost about 30 lbs in the past few months. The patient was not in the usual age bracket for MM but workup was sent. Serum protein electrophoresis (SPEP) showed that a large protein spike is noted in the beta region. Urine studies showed elevated excretion of monoclonal lambda light chains 167mg/dL (normal 0.02 - 0.67), kappa/lambda ratio < 0.01 (normal 2.04 - 10.37), urine protein > 3 g. Urine IFE showed a monoclonal IgA heavy chain with associated lambda light chain and excess monoclonal free lambda light chains.
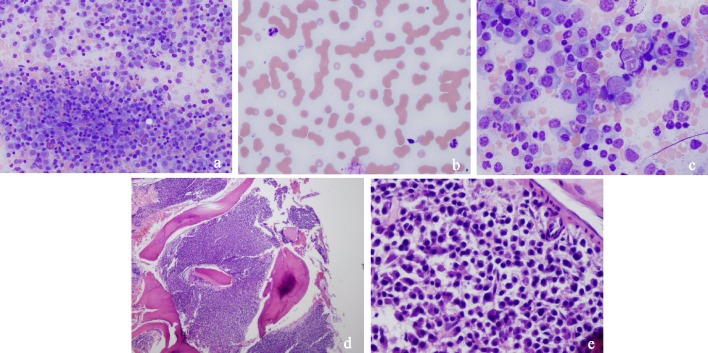
For elevated PTT mixing study was done which didn’t correct the PTT suggesting presence of inhibitor. No lupus anticoagulant was detected. Platelet function ADP and platelet function epinephrine were elevated. Factor X levels were low at 40% (normal 81-157%). Factor VIII and von Willebrand factor were at normal levels. Echo revealed normal EF of 67% and mild pulmonary HTN at about 46 mm Hg. Bone survey was negative for any lytic or blastic lesions. Renal biopsy showed acute tubular injury with atypical lambda chain casts consistent with light chain cast nephropathy. Bone marrow (BM) biopsy (Fig. 1a-e) revealed 90% plasma cells and FISH analysis showed 1q21 (CKS1B) mutation. The diagnosis of MM was made but certain characteristics of the patient suggested amyloidosis. Further workup was sent including Congo red stain of the BM sample which came out as negative. Abdominal fat pad biopsy and cardiac MRI are currently pending as outpatient. The patient was started on cyclophosphamide, bortezomib and dexamethasone during this admission. The patient’s renal function subsequently improved. She was discharged and given follow-up with an oncologist.
Figure 1.
(a) Medium power field of bone marrow smear with numerous plasma cells, hematopoiesis in background. (b) Peripheral blood smear with rouleaux formation. (c) High power field of bone marrow smear showing plasma cells. (d) Low power field of bone marrow biopsy showing packed marrow with plasma cells. (e) Medium power field of bone marrow smear with sheets of plasma cells.
Discussion
MM is a plasma cell dyscrasia usually diagnosed after the age 40. Only less than 1% of the cases are diagnosed below this age bracket and the median age for diagnosis is about 70 years old [3]. The diagnosis of MM requires presence of more than 10 percent plasma cells with signs of end organ damage or more than 60 percent plasma cells without any signs of end organ damage. The signs of organ damage include CRAB (hypercalcemia, renal failure, anemia and lytic bone lesions) seen on imaging [4].
The plasma cells causing MM express CD38, CD56, and CD138. It is believed that overproduction of IL-6 is essential to the survival of the myeloma cells. Other factors believed to be responsible for MM include VGEF, TGF-B and receptor activator of NF-KB [5-6]. Numbers of cytogenic abnormalities have been associated with MM which are detected by florescent in situ hybridization (FISH). Common abnormalities detected include 1q21, 11q13, 14q32, 15q24, 17p3, IGH rearrangement, TP53 deletion or gain on chromosomes 9, 11 or 15. Generally gain of 1q21 (CKS1B), as seen in our patient, is corelated with a poor prognosis [7].
Bone lesions caused in MM are thought to be caused by increased activity of osteoclasts and inhibition of osteoblastic activity. Hence serum ALP levels are usually normal in MM. The increased activity of osteoclasts is thought to be mediated by increased RANKL on osteoblasts and decrease of OPG (osteoprotegerin) [8]. Hypercalcemia ensues due to increased osteoclastic activity. Anemia is caused by overtake of the BM by the myeloma cells and disruption of the natural processes of hematopoiesis. Renal failure occurs due to cast nephropathy secondary to the deposition of the light chains in the renal tubules.
For patients with suspected MM the workup includes complete blood count peripheral smear, coagulation studies, serum vitamin B12 & folate levels, serum ferritin levels, comprehensive metabolic panel, serum and urine protein electrophoresis with Immunofixation, serum free light chains, serum β2-microglobulin, C-reactive protein, and lactate dehydrogenase level. These studies will show anemia, elevated creatinine, increased serum protein-albumin gap, nephrotic range proteinuria, M-spike on SPEP, increased free light chains in UPEP and reduced kappa to lambda ratio. Renal and BM biopsies are usually done to confirm the disease. Renal biopsies would show cast nephropathy and BM biopsies would show plasma cells > 10%.
Factor X inhibition is not seen in MM. Patients with IgA or IgG myeloma can have coagulopathy leading to elevated PTT or PT levels, but it is not seen in light chain myeloma [9]. This is usually a hallmark of AL amyloidosis. It happens because factor X gets entangled in fibrils made in AL amyloidosis [10]. This results in PTT being elevated and it cannot be corrected by mixing studies due to rapid absorption of factor X in fibrils. Other diseases causing elevated PPT levels can include antiphospholipid syndrome, lupus anticoagulant, hemophilia A and B, liver diseases and von Willebrand disease. To confirm the cause of the elevated PTT, studies include mixing studies followed by platelet function studies, factor X levels, activated factor VIII levels, lupus anticoagulant, antiphospholipid antibodies, anticardiolipin antibodies, beta 2-microglobulin levels and dilute Russell viper venom time. Other tests to check for amyloid deposition include Congo red staining of BM aspirate and abdominal fat pad specimen. Cardiac MRI can also show amyloid fibrils. Further invasive tests like cardiac biopsy can also be performed if suspicion is high [11].
Treatment of MM depends on risk stratification based on FISH studies and patient eligibility for autologous hematopoietic cell transplantation (HCT). Induction therapy usually consists of triple regiment for newly diagnosed MM which includes bortezomib, cyclophosphamide, and dexamethasone (CyBorD) [12-13]. Newer agents like daratumumab are second line and reserved for relapse of MM. Early diagnosis and treatment can prevent injury to kidney. Untreated MM can lead to rapid progression of disease leading to early renal failure and increased morbidity and mortality.
Conclusions
MM can present at a younger age than 40 years and any patient with hypercalcemia, acute renal failure, anemia, and serum albumin-protein gap should be worked up for MM. If the coagulation studies show inhibitor on mixing studies, amyloidosis should be considered and Congo red staining should be done on BM aspirate and abdominal fat pad. Cardiac MRI can also be used to look for amyloid fibrils. Early diagnosis and treatment improves the prognosis for the patient leading to increased progression-free survival.
References
- 1.Kariyawasan CC, Hughes DA, Jayatillake MM, Mehta AB. Multiple myeloma: causes and consequences of delay in diagnosis. QJM. 2007;100(10):635–640. doi: 10.1093/qjmed/hcm077. [DOI] [PubMed] [Google Scholar]
- 2.Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol. 2014;7(1):143–156. doi: 10.1586/17474086.2014.858594. [DOI] [PubMed] [Google Scholar]
- 3.Zweegman S, Palumbo A, Bringhen S, Sonneveld P. Age and aging in blood disorders: multiple myeloma. Haematologica. 2014;99(7):1133–1137. doi: 10.3324/haematol.2014.110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 5.Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood. 2010;115(17):3541–3552. doi: 10.1182/blood-2009-09-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacca A, Ria R, Ribatti D, Semeraro F, Djonov V, Di Raimondo F, Dammacco F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88(2):176–185. [PubMed] [Google Scholar]
- 7.Hanamura I, Stewart JP, Huang Y, Zhan F, Santra M, Sawyer JR, Hollmig K. et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108(5):1724–1732. doi: 10.1182/blood-2006-03-009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res. 2002;17(11):1921–1925. doi: 10.1359/jbmr.2002.17.11.1921. [DOI] [PubMed] [Google Scholar]
- 9.Teng HW, Chen PM, Yang YH, Gau JP. The prolonged activated partial thromboplastin time at diagnosis indicates less favorable prognosis in IgA myeloma. Jpn J Clin Oncol. 2007;37(8):609–614. doi: 10.1093/jjco/hym074. [DOI] [PubMed] [Google Scholar]
- 10.Furie B, Voo L, McAdam KP, Furie BC. Mechanism of factor X deficiency in systemic amyloidosis. N Engl J Med. 1981;304(14):827–830. doi: 10.1056/NEJM198104023041407. [DOI] [PubMed] [Google Scholar]
- 11.Prochorec-Sobieszek M, Bilinska ZT, Grzybowski J, Michalak E, Jakubowska E, Sobieszczanska-Malek M, Deptuch T. et al. Cardiac amyloidosis diagnosed by endomyocardial biopsy. Clinical, histopathological, immunohistochemical and ultrastructural studies. Kardiol Pol. 2005;63(7):20–35. [PubMed] [Google Scholar]
- 12.Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, Stewart AK. et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 13.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB, Stewart AK. et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19):4391–4394. doi: 10.1182/blood-2011-11-390930. [DOI] [PMC free article] [PubMed] [Google Scholar]