Introduction
On October 17, 2018 the Cannabis Act came into effect in Canada legalizing activities associated with the production, distribution, sale, and consumption of cannabis. A legal framework exists for physicians to authorize cannabis under the Access to Cannabis for Medical Purposes Regulations (ACMPR). However, this option is currently unavailable to Canadian veterinarians despite calls for revision by the Canadian Veterinary Medical Association (CVMA) (https://www.canadianveterinarians.net/documents/cvma-letter-to-hc-proposed-approach-to-regulation-of-cannabis). Therefore Canadian veterinarians are currently unable to authorize the use of cannabis-derived products for their patients, despite apparent interest from Canadian pet owners (1).
Meanwhile, various cannabis formulations have been marketed directly to pet owners through pet stores or cannabis dispensaries (online or physical storefront). Veterinary Health Products (VHPs) containing hemp may legally be sold, but only if the formulation is derived from approved strains of Cannabis sativa (also known as hemp), dried or extracted from the non-viable seed, and contains ≤ 10 ppm (0.01 mg/mL) of delta-9-tetrahydro-cannabinol (Δ9-THC; https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/veterinary-health-products/list-c.html). In addition, VHPs must refrain from specific claims of safety and efficacy. However, cannabis-derived formulations marketed to pet owners as “CBD (cannabidiol) oil” or “phytocannabinoid tinctures” do not meet the criteria for classification as VHPs, as any product containing appreciable CBD or THC would not be obtained from hemp seed. Furthermore, such unapproved, non-VHP cannabis products may have incomplete labeling and/or marketing materials, making it difficult for veterinarians to provide adequate guidance to clients regarding their validity or appropriate use. Given the unregulated nature of these products, our study objective was to analytically quantify the phytocannabinoid levels of various cannabis-derived formulations marketed to pet owners and compare our results to label information. The authors performed independent assessments using separate analytical methods of products purchased in Ontario (JH, JC, IS) and Saskatchewan (AC, KI, SV). The results were combined to provide a more extensive evaluation of cannabis product composition marketed to pet owners across Canada.
Materials and methods
Acquisition of cannabis-derived products: for the Ontario assessment, 4 cannabis liquid formulations (Liquids A to D) and 2 solid treat products (Treats A and B) marketed for veterinary use were obtained via Canadian online retailers. All products were obtained online between March and May 2019. None required a prescription or proof of veterinary licensure. For the Saskatoon assessment, 9 cannabis-derived oil formulations (Liquids E to L) from 4 separate brands were purchased from 2 local pet stores in July and December, 2019. All products were held at room temperature in the stores, with the exception of 1 product (Liquid H) which was kept refrigerated according to manufacturer instructions. Upon purchase, all products were kept refrigerated until they were analyzed.
Products purchased in Ontario were analyzed at a Health Canada accredited GMP-compliant laboratory. For cannabinoid extraction, products were dissolved in 100% methanol, centrifuged, and filtered. Solid samples were ground to increase the surface area for dissolution. The cannabinoid assay was performed via high performance liquid chromatography (HPLC) with changes to the general method made as needed to improve chromatography and separate overlapping peaks. The calibration curve for CBD analysis consisted of 6 standard points, with lower limits of quantification (LLOQ) and upper limits of quantification (ULOQ) of 500 and 50 000 ng/mL, respectively. Presence or absence of THC in the products was determined via inclusion of single point standards for THC (Δ8-THC at 10 000 ng/mL, Δ9-THC at 50 000 mg/mL).
The pesticides assay was run via gas chromatography — tandem mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). As with the HPLC work, samples were extracted using a combination of grinding, dissolving product in acetonitrile, centrifugation, and filtration. Samples were analyzed on the requisite equipment while comparing to an oil sample spiked with reference pesticides at 10, 50, and 200 ng/mL.
The heavy metal assay was run via inductively coupled plasma mass spectrometry (ICP/MS). The general process for this involved weighing the sample, running it through an acid digestion until it was fully broken down, and then running the samples per United States pharmacopeia (USP). Because the method was not validated, samples were run as-is and compared to samples spiked with known quantities of the elemental impurities. Results were then back-calculated as required.
Liquid products purchased in Saskatoon were analyzed for cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) concentrations using an LC-MS/MS method adapted from a published method in plasma (2). An Agilent 1290 Infinity liquid chromatography system (Agilent Technologies Canada, Mississauga, Ontario) and SCIEX QTrap 6500 triple quadrupole mass spectrometer with electrospray ionization in positive ionization mode (Sciex, Concord, Ontario) were used. Samples were introduced into a Zorbax Eclipse XDB C-18 column (Agilent Technologies Canada) with a flow rate of 700 μL/min. Mobile phase consisted of 0.1 mM ammonium formate in LCMS grade water (mobile phase A) and methanol (mobile phase B), with mobile phase B held at 90% for a sample run time of 12 minutes.
The LC-MS/MS method was validated based on the 2018 FDA Bioanalytical Method Validation guidelines (https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf). A calibration curve consisting of 7 standard points, ranging from 10 to 250 ng/mL and weighted 1/x, displayed linearity (R2 values ≥ 0.98). For both CBD and Δ9-THC, the limit of detection (LOD) and LLOQ were 5 and 10 ng/mL, respectively. The low-quality control (LQC), middle-quality control (MQC), and high-quality control (HQC) concentrations were established as 15 ng/mL, 150 ng/mL, and 225 ng/mL, respectively. Intra- and inter-day accuracy and precision was conducted over 3 days in triplicate, demonstrating all observed LLOQ and QC concentrations were within ± 20% and ± 15% of the nominal concentrations, respectively. Deuterated internal standards were used for accurate quantification of cannabinoids.
Because the stated cannabinoid concentrations of the oil formulations exceeded calibration curve upper limits suitable for the mass spectrometry, all products were diluted 40 000× to enable accurate interpolation of CBD and Δ9-THC concentrations. To mimic use by animal owners, products were vigorously mixed by hand before withdrawing samples for dilution. Liquids G and H were initially outside of the standard curve and were re-analyzed with dilution factors of 500 000× and 5000×, respectively. Dilution integrity was confirmed in triplicate for all dilution factors, remaining within ± 15% of the nominal concentration. Based on the diluted sample LLOQ (10 ng/mL) and a dilution factor of 40 000, the LLOQ of CBD and Δ9-THC in the undiluted liquid products was 0.4 mg/mL (5 and 0.05 mg/mL for Liquids G and H, respectively, due to revised dilution factors). All samples were assayed in triplicate; mean ± standard deviation (SD) results are reported.
Results
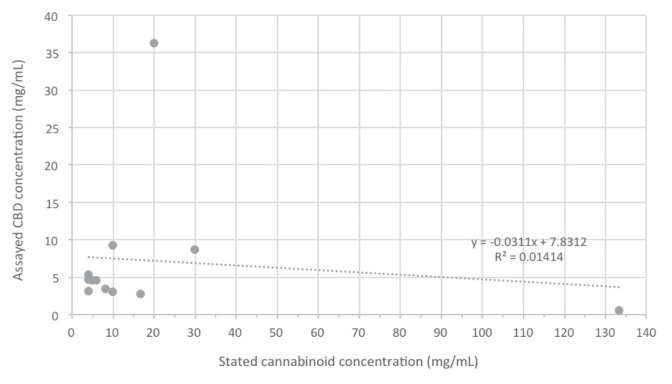
The compositions of cannabis-derived products are reported in Tables 1 (Ontario) and 2 (Saskatchewan). The specific parameters reported vary due to differences in analytical methods used and formulations assessed at each laboratory. Figure 1 illustrates the label-claimed cannabinoid versus assayed CBD concentrations; there is no correlation (R2 = 0.014) between claimed cannabinoid and actual CBD concentration.
Table 1.
Composition of cannabis formulations purchased in Ontario.
| Product | Label description | Assay results (mg/mL) | CBD Potency (as % of claimed cannabinoids) | Heavy metals/Pesticides detected | Label recommendations | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CBD | Δ9-THC | Δ8-THC | |||||
| Liquid A | 30 mg/mL hemp terpenes | 8.7 | ND | Det (1) | 29% | None found | For dogs and cats, but no directions for use. |
| Liquid B | 8.3 mg/mL CBD | 3.4 | ND | ND | 41% | None found | For dogs and cats. Up to 2.27 kg: 10 drops (2.5 mg); 2.27 to 6.8 kg: 20 drops (5 mg); 7 to 11.4 kg: 30 drops (7.5 mg); 11.4 to 15.5 kg: 40 drops (10 mg). |
| Liquid C | none | 0.15 | Det (40*) | Det (0.1) | N/A | Pesticides: Metalaxyl, Myclobutanil, Pyrethrin I, Imidacloprid, Cyfluthrin I–IV, Permethrin-cis, Permethrin-trans | Use as needed orally or topically. |
| Liquid D | 4 mg/mL CBD | 5.3 | Det (0.01) | ND | 132% | Pesticides: Cyfluthrin I–IV, Permethrin-cis, Permethrin-trans | No directions for use. |
| Treat A | none | 4 | ND | ND | N/A | Heavy metals: 1.650 μg/g arsenic** | Apply 1 packet per day with food. |
| Treat B | none | 2.1 | ND | ND | N/A | None found | Administer 2 treats per day. |
ND — Not detected. Actual limit of detection not possible due to single-point standard used.
Det — Cannabinoid detected, but accurate concentration cannot be determined due to single point standard used. Value in parentheses are approximate concentrations only, based on ratio with single point standard.
N/A — Not applicable.
Δ9-THC strength per mL (approximately 40 mg) is roughly 4 times the proposed legal oral serving size for humans (10 mg), and ~4000× times the maximum concentration for a NHP (10 ppm).
Arsenic level is ~160× the maximum permissible level in water, and 8× the maximum permissible amount in food.
Table 2.
Undiluted cannabinoid composition of cannabis products purchased in Saskatoon, Saskatchewan.
| Product | Label descriptiona | CBD conc. (mg/mL) (mean ± SD) | Δ9-THCConc(mg/mL) | CBD Potency (as % of claimed cannabinoids) | Label recommendations |
|---|---|---|---|---|---|
| Liquid E1b | 4 mg/mL hemp terpenes | 4.65 ± 0.10 | < LOD | 116.3 | 0.1 mg per kg BWc |
| Liquid E2b | 4 mg/mL hemp terpenes | 3.11 ± 0.07 | < LOD | 77.7 | |
| Liquid F | 10 mg/mL hemp terpenes | 9.24 ± 0.14 | < LOD | 92.4 | |
| Liquid G | 20 mg/mL hemp terpenes | 36.25 ± 0.49 | < LOD* | 181.3 | |
| Liquid H | 133.3 mg/mL phytocannabinoid tincture in hemp oil | 0.52 ± 0.03 | < LOD** | 0.4 | Chronic = 2 drops per 4.5 kg BW, q12h Acute = 4 drops per 4.5 kg BW, q12h |
| Liquid I | 10 mg/mL hemp oil (unknown) | 3.07 ± 0.18 | < LOD | 30.7 | 1 to 3 dropsd per 2.27 kg BW |
| Liquid J | 16.7 mg/mL hemp oil (unknown) | 2.76 ± 0.12 | < LOD | 16.6 | |
| Liquid K | 5.0 mg/mL hemp isolate in coconut oil | 4.58 ± 0.05 | < LOD | 91.5 | 0.1 mg per kg BW |
| Liquid L | 6.0 mg/mL hemp isolate in coconut oil | 4.59 ± 0.16 | < LOD | 76.5 |
Conc — Concentration; SD — Standard deviation; BW — Body weight; LOD — limit of detection; diluted sample LOD = 5 ng/mL; undiluted sample LOD = 0.2 mg/mL.
Undiluted sample LOD = 2.5 mg/mL.
Undiluted sample LOD = 0.025 mg/mL.
Concentration of specific cannabinoids (CBD &/or THC) not listed on any product label.
Same product purchased from 2 different pet stores.
mg hemp terpenes.
Volume/drop not specified.
Figure 1.
Stated cannabinoid concentration (on label) versus assayed CBD concentration for various cannabis-derived products.
Discussion
The use of cannabis-derived products is an emerging field of veterinary therapeutics. A recent preliminary study demonstrated potential efficacy for CBD treatment of canine osteoarthritis (3), and CBD use for control of seizures in epileptic dogs may demonstrate dose-dependent effects (4). Numerous pharmacokinetic studies using various cannabinoid formulations for dogs and cats have been published (3,5–7). However, no cannabis-derived products are currently approved by Health Canada for use in animals. Therefore, cannabis-derived formulations currently marketed to pet owners have not been independently assessed for product quality, efficacy, or safety; any such claims made by the manufacturer are unsubstantiated.
Incomplete product labelling was noted for all cannabis formulations assessed in this study. No product contained a Veterinary Health Product (VHP) number or labelling consistent with Health Canada requirements. For most of the products assessed, labels lacked a description of specific cannabinoid constituents (such as cannabidiol or Δ9-THC) in the formulations, but rather indicated the mass of “hemp terpenes,” “hemp oil,” “phytocannabinoid tincture,” etc. Such imprecise labelling can mislead consumers, who may incorrectly assume that the “mg” value stated on the label is the CBD and/or THC content. Our results indicate that for some products (e.g., Liquids D to G, K, and L), the specified cannabinoid mass is comprised mainly of CBD. For Liquids D to F, K, and L, the overall CBD potencies (76% to 132%) were in the approximate range of the cannabinoid content stated on the label. However, other products (Liquids A to C, H, and J, Treats A and B) had CBD potencies that were either dramatically lower than the stated cannabinoid content, or simply undetectable. One product (Liquid G) had a CBD potency significantly higher than stated (181%). There was no correlation between claimed cannabinoid concentration and actual CBD potency (Figure 1). Differences between actual CBD/THC content and stated cannabinoid potency could potentially be due to the presence of other cannabinoid constituents (e.g., cannabichromene, cannabigerol, cannabinol, or hemp terpenes) not assayed in this study. The biological effects of such compounds in animals are not known, but in humans are considered to have minimal psychoactive effects (8,9). Wide discrepancies in claimed versus actual cannabinoid concentrations are not limited to formulations intended for use in animals. An evaluation of 21 Dutch cannabis oil formulations intended for human consumption from various unregulated sources found actual CBD potency ranged from 0 to 117% of the stated concentrations (10).
Furthermore, the vague and imprecise nature of labelling on some products makes deriving an accurate dose for a specific cannabinoid impractical (e.g., to replicate the 2 to 2.5 mg/kg CBD dose as used in published efficacy studies) (3,4). Based on the stated concentrations of the products assessed, the volume of cannabinoid liquid required to achieve a 2 mg/kg CBD dose would be 0.07 to 0.5 mL/kg body weight (BW) (0.35 to 2.5 mL per 5 kg animal). The purported label dose recommendations, if present, are substantially lower than published doses from efficacy studies. For example, Liquids D to G come with a recommended dose of 0.1 mg/kg BW. Other products list dose recommendations that are not quantifiable (e.g., number of drops of oil, the volume per drop is not stated or calibrated). No evidence of efficacy or safety is presented to justify the dose recommendations for any product.
The safety of unlicensed cannabinoid products in pets has not been assessed. This is particularly concerning for products with potency that is higher than stated (e.g., Liquid G, CBD content was 181% of stated cannabinoid content), and especially for products high in THC (e.g., Liquid C contained almost no CBD but contained 39.6 mg/mL THC, almost 4000 times the legal limit for a NHP of 10 ppm). Two products (Liquids C and D) contained quantifiable levels of 3 or more pesticides, and 1 (Treat A) contained detectable levels of arsenic. Such contaminants have previously been reported in unregulated cannabinoid oils intended for human consumption (10).
Other deficiencies in product labelling were noted. Most products did not contain directions on storage conditions (e.g., refrigeration, light sensitivity). Only 2 products from 1 company (Liquids K and L) contained information regarding manufacturing identification (batch/lot numbers) or product stability (before use or expiry date). Lack of manufacturing information precluded comparisons of batch-to-batch composition. One potential explanation for the low cannabinoid potency in the majority of products assayed is instability or degradation of cannabinoids in the product over time, particularly due to the unknown storage and handling of the products prior to assay. However, because batch identification and/or in-use dates were not labelled for most products tested, decreased potency due to instability cannot be assessed. Finally, human safety warning statements (e.g., keep out of reach of children, for veterinary use only) were absent on all products except Liquid H.
There is considerable interest in developing ethical cannabis-based drugs for veterinary use. Multiple researchers and pharmaceutical sponsors are working with the Veterinary Drugs Directorate (VDD) to register prescription cannabis products. Companies pursuing regulatory approval are expending considerable resources to ensure the development of properly manufactured, safe, and effective drugs. However, as such products are not currently available for clinical trial or commercial sale, and until veterinarians are allowed to authorize medical cannabis under an amended ACMPR, unapproved cannabinoid formulations are the only options available to pet owners. If clients are intent on purchasing and using these products, veterinarians should counsel them as to legitimate concerns regarding product potency, purity, safety, and efficacy. As well, veterinarians and consumers should communicate specific product concerns to Health Canada using their online product complaint form (http://healthycanadians.gc.ca/apps/radar/MD-IM-0005.08.html). In conclusion, because most cannabinoid products assessed in this study had improper labelling, and/or cannabinoid concentrations that did not meet the label description, “caveat emptor” clearly applies when purchasing unapproved veterinary cannabis products from pet stores or online suppliers.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kogan LR, Hellyer PW, Silcox S, Schoenfeld-Tacher R. Canadian dog owners’ use and perceptions of cannabis products. Can Vet J. 2019;60:749–755. [PMC free article] [PubMed] [Google Scholar]
- 2.Huntsman RJ, Tang-Wai R, Alcorn J, et al. Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: Preliminary results of the CARE-E study. Front Neurol. 2019;10:716. doi: 10.3389/fneur.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamble LJ, Boesch JM, Frye CW, et al. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front Vet Sci. 2018;5:165. doi: 10.3389/fvets.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath S, Bartner LR, Rao S, Packer RA, Gustafson DL. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J Am Vet Med Assoc. 2019;254:1301–1308. doi: 10.2460/javma.254.11.1301. [DOI] [PubMed] [Google Scholar]
- 5.Deabold KA, Schwark WR, Wolf L, Wakshlag JJ. Single-dose pharmacokinetics and preliminary safety assessment with use of CBD-rich hemp nutraceutical in healthy dogs and cats. Animals (Basel) 2019;9(10) doi: 10.3390/ani9100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartner LR, McGrath S, Rao S, Hyatt LK, Wittenburg LA. Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs. Can J Vet Res. 2018;82:178–183. [PMC free article] [PubMed] [Google Scholar]
- 7.Lebkowska-Wieruszewska B, Stefanelli F, Chericoni S, et al. Pharmacokinetics of Bedrocan(R), a cannabis oil extract, in fasting and fed dogs: An explorative study. Res Vet Sci. 2018;123:26–28. doi: 10.1016/j.rvsc.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 8.DeLong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocabinoids. Prog Chem Org Nat Prod. 2017;103:61–101. doi: 10.1007/978-3-319-45541-9_3. [DOI] [PubMed] [Google Scholar]
- 10.Hazekamp A. The trouble with CBD Oil. Med Cannabis Cannabinoids. 2018;1:65–72. doi: 10.1159/000489287. [DOI] [PMC free article] [PubMed] [Google Scholar]