Abstract
As highlighted by Eisenberg and colleagues (1998), parents play a critical role in children’s socioemotional development, in part, by shaping how children and adolescents process, respond to, and regulate their emotions (i.e., emotional reactivity/regulation). Although evidence for associations between parenting behavior and youth’s emotional processing has relied primarily on behavioral measures of emotion, researchers have begun to examine how parenting is related to the neural substrates of youth’s reactivity and regulation. This paper reviews a growing literature linking parental behavior with structural brain development as well as functional activity and connectivity in neural regions supporting emotional reactivity and regulation during infancy, childhood, and adolescence. By focusing on normative parental behaviors, we evaluate the evidence for associations between typical variations in caregiving and neural processes thought to support youth’s emotional reactivity/regulation. The purpose of this review is three-fold: (1) to extend the model put forth by Eisenberg and colleagues to consider the ways that parenting behaviors are related to neural substrates of youth’s emotional reactivity and regulation; (2) to review the empirical evidence for associations between parenting, particularly parental “emotion-related socialization behaviors” (ERSBs), and neural substrates of youth’s emotional reactivity/regulation; and (3) to recommend future directions for this emerging area of research.
Keywords: parenting, emotion regulation, fMRI, EEG, emotion socialization, neurodevelopment
Parents play an important role in children’s socioemotional development, in large part by influencing how children and adolescents process, respond to, and regulate their emotions. As highlighted by Eisenberg and colleagues (1998), individual differences in parental emotion-related socialization behaviors (ERSBs) are concurrently and prospectively linked to individual differences in youth’s emotional reactivity and regulation (Eisenberg, Spinrad, & Eggum, 2010). Furthermore, because problems with emotional reactivity and regulation are widely recognized to play a shared role in risk for psychopathology, an important approach for altering risk trajectories is to identify the mechanisms by which ERSBs promote -- or undermine -- the development of adaptive emotion regulation (ER) in children and adolescents (Cole, Hall, & Hajal, 2013). There has been much behavioral research on this question over the last 20 years (see Morris, Criss, Silk, & Houltberg, 2017), but little is known about how parenting is associated with the neural substrates of youth’s emotional reactivity and regulation. Thus, the purpose of our review is 3-fold: (1) to extend the model put forth by Eisenberg and colleagues to consider associations between parenting and the neural processes that underlie emotional reactivity/regulation in children and adolescents; (2) to review the empirical evidence for such associations, particularly between ERSBs and the structure and function of ER neural networks; and (3) to recommend future research directions for this emerging area of research.
There is a rich base of behavioral and physiological evidence that children’s ability to regulate emotions improves significantly with age (see Beauchaine, 2011; Calkins, Perry, & Dollar, 2016; Kopp, 1989), but studies are just beginning to examine cross-level interactions between the social environment and youth’s developing neural systems. As one of the earliest and most enduring aspects of the social environment, it is critical to understand associations between parenting behaviors and the developmental changes in brain structure and function that support ER-related behaviors. A deeper understanding of how parenting might influence the neurodevelopment of ER is also important for identifying targets for the next generation of parenting interventions. By providing the first review of evidence for associations between parental behavior and brain imaging measures of youth’s emotional reactivity/regulation, we hope to begin to build a foundation for future translational work aimed at incorporating brain-based parenting strategies in programs targeting maladaptive socioemotional developmental outcomes.
Part 1: Neurodevelopment of Emotion Regulation
Child arousal is posited to be an important mediator of associations between parental ERSBs and children’s socioemotional outcomes in Eisenberg and colleagues’ (1998) model. Drawing from research on neural models of ER (e.g., Etkin, Bucehl, & Gross, 2015; Ochsner, Silvers, and Buhle, 2012; Phillips, Ladouceur, and Drevets, 2008), we extend Eisenberg’s emotion socialization model by proposing that normative variations in parental ERSBs are associated with variations in the neurodevelopment of ER processes, which, in turn, influence socioemotional development. As shown in Figure 1, we propose that parental ERSBs are related to individual differences in the structure and functioning of neural networks implicated in the processing and regulation of emotionally-salient information (e.g., Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Surguladze et al., 2003; Vink et al., 2004).
Figure 1. Extension of parental emotion socialization model (Eisenberg and colleagues 1998).
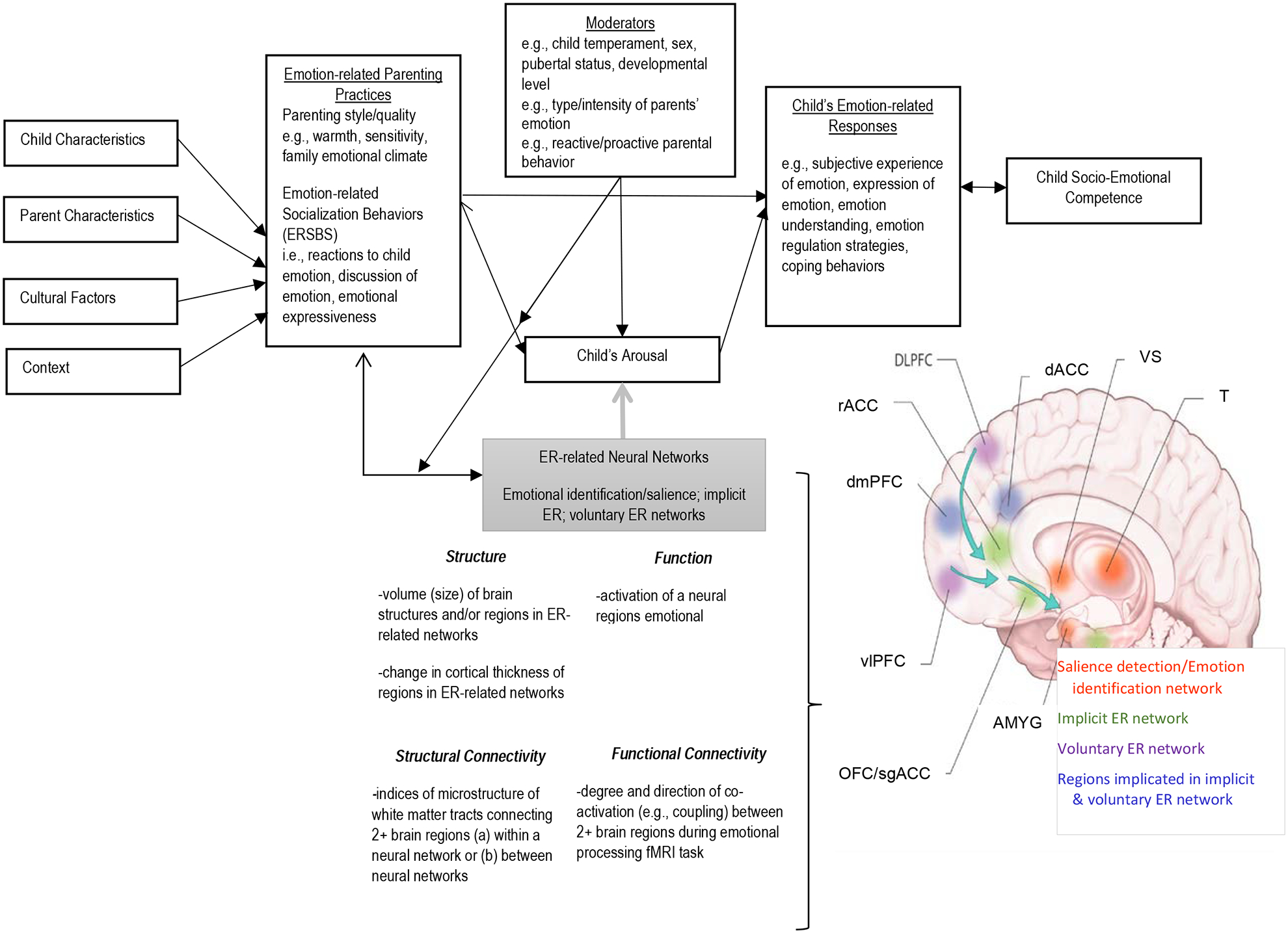
The review examines evidence that brain structure and function during childhood and adolescence varies in association with a normative range of variation in parenting experiences. Eisenberg and colleagues highlighted emotional “overarousal” as a likely consequence of parental ERSBs that were not supportive of youth’s emotion experiences, for example parental suppression/other negative responses to child emotional expression, parental modeling of high negative affect, and discouragement of emotion discussion. We extend their model to suggest that parental ERSBs influence youth’s emotional arousal (i.e., youth’s emotional reactivity/regulation) via ER-related neural networks, specifically the structure and function of salience detection and emotional identification, voluntary emotion regulation (ER), and implicit ER networks. Based on findings from studies examining the neural substrates of emotion regulation (see Phillips et al., 2008), in terms of brain structure, we expect that “negative” or unsupportive parenting behaviors will be associated with reduced gray matter volume in prefrontal cortical regions important for voluntary ER processes (especially before adolescence) and enlarged gray matter volume in neural regions within the salience detection network. In terms of structural connectivity, we hypothesize reduced white matter integrity in tracts linking prefrontal and subcortical regions reflecting alterations to the neural architecture of the voluntary ER network in youth who experience higher levels of negative/unsupportive parenting. We also expect that negative/unsupportive parenting will be related to heightened activation of subcortical regions within the salience network, as well as reduced activation in anterior regions within the voluntary and implicit ER networks (e.g., dlPFC and vlPFC, dACC), particularly when youth are processing negative emotional information. Finally, with regard to functional connectivity, we posit associations between higher levels of negative/unsupportive parenting and weaker inverse coupling between the amygdala and dlPFC/vlPFC after the transition to adolescence (Callaghan & Tottenham, 2016; Silvers et al., 2017). These alterations in neural structure and function would be observed behaviorally as increased negative emotional reactivity, ER difficulties, and increased behavioral problems as well as elevated risk for affective disorders. In contrast, “positive” and supportive parenting behaviors are thought to promote adaptive responses by decreasing youth’s negative emotional arousal and/or enhanced recruitment of PFC regions supporting implicit/voluntary ER processes. At a neural level, this support could occur via the modulation of youth’s neural responses to negative emotional information in regions within the voluntary ER neural network. As such, we hypothesize that positive parenting would be associated with functional activation and connectivity patterns reflecting less activation in the salience detection network when youth encounter negative stimuli. We would also expect greater integrity of white matter tracts between prefrontal and limbic regions (e.g., uncinate fasciculus), which could serve as the neural mechanism for the development of stronger inverse functional connectivity patterns between PFC and amygdala during adolescence.
Notes. AMYG=amygdala; OFC=orbitofrontal cortex; sgACG=subgenual cingulate cortex; vlPFC=ventrolateral prefrontal cortex; dmPFC=dorsolateral prefrontal cortex; rACC=rostral anterior cingulate cortex; dlPFC=dorsolateral prefrontal cortex; dACC=dorsal anterior cingulate cortex; T=thalamus.
Image of brain regions adapted from Ladouceur CD, Versace A., Phillips ML. (2015). Understanding the Neural Circuitry of Emotion Regulation: White Matter Tract Abnormalities and Psychiatric Disorder. In LJ Kirmayer, R Lemelson, & CA Cummings (Eds). Re-visioning Psychiatry: Cultural Phenomenology, Critical Neuroscience, and Global Mental Health (pp. 236–272). New York, NY: Cambridge University Press.
Emotionally-salient information is processed in a set of interconnected subcortical (amygdala, ventral striatum) and cortical (medial prefrontal cortex, dorsal anterior cingulate cortex) brain regions that also include the dorsal anterior cingulate cortex and anterior insula (Menon, 2015). These regions are important in salience detection, or the dynamic detection of personally- or motivationally-salient information, such as stimuli with positive or negative emotional valence. In addition to salience detection, Phillips and colleagues (2008) outlined the neural networks underlying other subprocesses of ER. Voluntary ER refers to explicit regulatory processes, such as cognitive reappraisal, that modulate an emotional response and that are supported by a network of prefrontal cortical regions, including the dorso- and ventrolateral prefrontal cortices (dlPFC, vlPFC), the dorsomedial prefrontal cortex (dmPFC), and dorsal anterior cingulate cortex (dACC). In parallel, there are implicit subprocesses that occur outside the realm of awareness; these include action monitoring and modulation of attention to emotional material as well as reinforcement learning. Animal and human lesion neuroimaging studies indicate that these processes involve a network of brain regions that include the subgenual (sgACC) and rostral anterior cortex (rACC), orbitofrontal cortex (OFC), hippocampus, dACC, and dmPFC. Both the voluntary and implicit ER networks show significant overlap with the salience network, suggesting that salience detection is an integral part of emotion regulation in adults (Kohn et al., 2014) and youth (McRae et al., 2012; Vink et al., 2004).
The brain regions thought to underlie implicit and voluntary ER undergo significant age-related changes in both structure and function that have implications for understanding “expected” age-related differences in youth’s ER (e.g., Perlman & Pelphrey, 2010; Silvers et al., 2016; 2017). Much of the research on the neural basis of emotional processing has found age-related increases prefrontal cortical activation, particularly in regions implicated in voluntary ER processes (e.g., Hare et al., 2008; McCrae et al., 2012; Monk et al., 2003). There is also evidence of age-related differences in white matter tracts connecting prefrontal regions with subcortical structures implicated in salience detection (e.g., Paus et al., 1999). For example, cross-sectional research suggests that relative to younger children, older adolescents show greater structural connectivity within a white matter tract connecting the PFC and amygdala (i.e., the uncinate fasciulus) that is associated with age-related declines in amygdala activation (Swartz et al., 2014). Moreover, recent findings suggest that the down-regulation of amygdala activation by vmPFC to negative emotional material increases with age, and that these age-related changes were mediated by dmPFC activation (Silvers et al., 2017), highlighting development in the dorsal regions of the PFC in the neurodevelopment of ER. These findings are consistent with intriguing cross-sectional findings suggesting a developmental shift from a positive to inverse pattern of co-activation (i.e., functional connectivity) between the PFC and amygdala that occurs during the transition to adolescence (approximately age 10 years); this developmental shift in functional connectivity between the salience and voluntary ER networks is posited to reflect the maturation of projections from the PFC to the amygdala (Gee et al., 2013), an important part of the neural network thought to underlie voluntary ER processes like cognitive reappraisal (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008).
Part 2: Parental Emotion Socialization and ER Neural Networks
Initial studies of caregiver influences on the human brain largely focused on exposure to extreme environments, such as maltreatment and institutionalization (see Belsky & de Haan, 2011). While it is clear that extreme deviations from normative patterns of caregiving influence the development and functioning of ER networks, less is understood about how the structure/function of these networks is sensitive to more subtle differences in parenting behavior. It is therefore important to understand how brain structure and function during childhood and adolescence varies in association with a normative range of variation in parenting experiences. Eisenberg and colleagues (1998) highlighted emotional overarousal as a likely consequence of parental ERSBs that are not supportive of youth’s emotion experiences (for example, parental suppression of child emotional expression, modeling of high negative affect). As shown in Figure 1, we extend their model to suggest that parental ERSBs could impact youth’s emotional arousal via their impact on the salience detection and voluntary/implicit ER neural networks.
Review of Empirical Evidence.
We review child/adolescent neuroimaging studies of emotional reactivity/regulation that incorporated (1) measure(s) of brain volume, morphology, and structural connectivity or functional connectivity and activation in brain regions implicated in ER-related processes and (2) direct measure(s) of parenting behavior that were accepted for publication before January 2019 (see Supplemental Information). Although Eisenberg and colleagues (1998) specifically focused on ERSBs, we include neuroimaging studies that assessed broader indices of parenting behavior and parent-child relationship for two key reasons. First, as an emerging area of research, few studies have specifically assessed ERSBs when investigating associations between parenting and neural indices of youth’s emotional reactivity/regulation. As such, models linking ERSBs and the neurodevelopment of ER networks will need to build from more general studies of parenting behavior. Second, measures of broader parenting constructs often include ERSBs, notably, parental emotional expression and responses to child/adolescent behavior. For example, as discussed by Eisenberg and colleagues (1998), maternal sensitivity is often defined as the degree to which mothers respond to infant emotional cues (e.g., distress). Our search identified 32 empirical studies investigating associations between normative variations in parenting behavior and measures of brain structure (brain volume, structural connectivity) and function (neural activation, functional connectivity) (see Table 1 in Supplemental Information). It is important to note, however, that because the majority (~80%) of studies assessed only mothers, we should be cautious about generalizing the findings to fathers’ parenting.
Broad indices of parenting style/quality.
Following the lead of Eisenberg et al., 1998, we characterize measures of parents’ emotional expressivity and degree of responsiveness to youth’s emotion-related behaviors into “positive” and “negative” dimensions. “Positive” parenting refers to those behaviors and qualities that are traditionally incorporated in measures of sensitivity, warmth, and authoritative parenting style that have been associated with the promotion, or support, of healthy developmental outcomes (Eshel et al., 2006). Conversely, “negative” parenting refers to behaviors/qualities that have been defined as authoritative, controlling, harsh, and/or intrusive – that is, behaviors that do not promote healthy developmental outcomes.
Parenting style/quality: brain volume and structural connectivity.
Much of the research on the associations between parenting behavior and youth’s brain structure has focused on positive parenting style/quality. Findings extend and complement studies linking more extreme, adverse caregiving environments with altered structural development in limbic regions implicated in emotional processing (e.g., Belsky & deHaan, 2011). Specifically, with regards to subcortical brain structures that are part of the salience and implicit ER networks, studies have generally found that high levels of positive parenting style/quality were associated with reduced amygdala volumes (Bernier et al., 2018; Whittle et al., 2014) and larger hippocampal volumes (Engert et al., 2010; Rifkin-Graboi et al., 2015; Schneider et al., 2012; but see Rao et al., 2010). Moreover, findings have been consistent with prospective designs using observational measures that linked positive parenting with the development of larger hippocampal volume in middle childhood/early adolescence (Luby et al., 2012; 2013; 2016).
A smaller body of work has also linked positive parenting with variations in gray matter (GM) volume in regions that are implicated in implicit and voluntary ER processes. Moreover, findings highlight the importance of considering typical, or “expected”, neurodevelopmental trajectories when investigating associations between parenting and developing ER. Specifically, studies have found that higher levels of positive parental behaviors are associated with larger cortical volumes in younger children (Kok et al., 2015) but smaller cortical volumes during adolescence (Avants et al., 2015). Additionally, using a longitudinal design, Whittle et al. (2014) found that observed positive maternal behaviors prospectively predicted greater cortical thinning (i.e., decreases in GM in the cortex) in adolescents’ OFC. In contrast, retrospective reports of positive maternal behavior in childhood were associated with larger volumes in the frontal orbital gyrus (Kim et al., 2010) and the dlPFC in adults (Narita et al., 2010; but see Yang et al., 2018).
Finally, there is a similar pattern of associations with positive parenting style/quality and the structure of another key brain region for implicit and voluntary ER, the ACC. Specifically, a longitudinal investigation found a positive association between observed maternal positive behaviors and greater ACC thinning during adolescence (Whittle et al., 2014). Given that adolescent cortical thinning is associated with superior cognitive and emotional functioning (e.g., Ducharme et al., 2012; Shaw et al., 2006), authors posited that mothers’ positive parenting behavior may support or even promote normative trajectories of cortical development.
Compared to positive parenting style/quality, fewer studies have reported associations between brain structure and negative parenting behaviors. One study by Narita and colleagues (2012) reported no significant (direct) association between parenting and hippocampal volume. However, there were significant associations between retrospective reports of overprotective parenting and hypothalamic-pituitary-adrenal (HPA) axis hypoactivity that were, in turn, associated with GM volume reduction in the hippocampus in adults. A second, longitudinal study by Whittle and colleagues (2016) found that over time, maternal aggression was associated with increases in the cortical thickness of adolescents’ superior frontal gyrus and ER-related neural regions. Overall, findings from this small body of work are consistent with the view that negative parenting alters the expected pattern of neurodevelopment (i.e., cortical thinning during adolescence) in brain structures that are thought to underlie voluntary and implicit ER processes.
Parenting Style/Quality: Neural activation and functional connectivity.
Findings are mixed with regards to the association between positive parenting style/quality and neural activity (i.e., changes in neural activation that occur while participants complete tasks designed to recruit ER-related subprocesses). For example, high levels of positive parenting were related to less activation to negative emotional images in subcortical structures implicated in salience/emotion identification among adolescents (Romund et al., 2016) but elevated amygdala activation in school-aged youth (Pozzi et al., 2019). With regards to the processing and regulation of positive emotional information, high levels of positive parenting were linked to greater activation in the ventral striatum among adolescent girls (Schneider et al., 2012). Other evidence indicated that in early adolescence, low levels of positive parenting style/quality were prospectively linked to greater activity in the dmPFC and ventral striatum during reward anticipation in later adolescence, a pattern of neural response to positive emotional information that mediated relations between low parental warmth and youth depressive symptoms (Casement et al., 2014). Thus, there is preliminary evidence for associations between high levels of positive parenting style/quality and attenuated activation within the salience network when adolescents respond to negative emotional information. On the other hand, low levels of positive parenting are linked to reduced activation in ER neural networks when youth process positive emotional information.
Research on negative parenting style/quality likewise links parenting behavior with the functioning of ER networks but relationships depend on child characteristics. For example, one study showed that negative maternal quality/style is associated with attenuated activation in the vlPFC, a cortical region implicated in voluntary ER, in response to peer rejection among adolescents with behavioral inhibition (Guyer et al., 2015). Negative parenting style/quality has also been associated with elevated activation in the anterior insula, a region important for salience detection, in response to negative emotional images (Marusak et al., 2017). Together, this work is consistent with the view that negative parenting is related to atypical patterns of neural responses to negative emotional information that confer risk for emotional overarousal and ER problems. With regards to youth’s responses to positive emotional information, researchers have found that negative maternal parenting was linked to attenuated responses to positive emotional stimuli in the anterior insula, suggesting that negative parenting might also dampen youth’s responses to positive emotional input (but only among late-pubertal adolescents; Barbosa et al., 2018).
The mixed pattern of findings in associations between parenting style/quality and functional activation in ER networks are, in part, due to differences in sample characteristics (i.e. age at which parenting and neural function were assessed; child temperament). For example, maternal negative parenting was linked to attenuated activity in the vlPFC in response to peer rejection, but only for behaviorally-inhibited adolescents (Guyer et al., 2015). Such findings underscore the need to identify the factors that moderate relations between parenting behavior and ER-related neural function (see discussion on Moderators on p. 18).
Negative parenting has also been posited to disrupt ER processes, in part, by altering the functional connectivity within ER neural networks (see Figure 1). Functional connectivity is a measure of temporal correlation or coupling between activation in brain regions while an individual is performing a task or at rest. Stronger correlations reflect a higher degree of coordinated neural activation, and correlations can reflect either positive neural coupling (e.g., as activation in one region increases, activation in another region also increases) or inverse/negative neural coupling (e.g., as activation in one region increases, activation in another region decreases). Using measures of functional connectivity, Thijssen and colleagues (2017) reported that less parental sensitivity during the preschool years was associated with stronger inverse (or negative) resting-state connectivity between the amygdala and dmPFC in the school-aged years (i.e., when youth were 6 to 10 years-old). Similarly, Kopala-Sibley and colleagues (2018) found that negative maternal parenting style/quality in early childhood was prospectively associated with increased negative connectivity between the salience and implicit ER networks in response to sad faces when youth were approximately 10 years of age. The findings reported by Thjjssen and colleagues (2017) and Kopala-Sibley and colleagues (2018) are strikingly similar to research on adverse caregiving experiences indicating that children who have experienced adverse caregiving develop a seemingly “mature” pattern of negative fronto-limbic connectivity earlier in life (i.e., before puberty). However, investigators have also posited that this stress-accelerated pattern of neurodevelopment may confer later risk for ER capacity, for instance, with respect to youth’s capacity to engage in adaptive emotional self-regulation as adolescents and adults (Callaghan & Tottenham, 2015).
To date, we found only one study examining the association between positive parenting behavior and measures of functional connectivity in youth. This study focused on focused on neural mechanisms underlying maternal regulation of emotion by using a mother/stranger fMRI task. Specifically, Gee and colleagues (2014) found that youth who showed negative amygdala-mPFC connectivity when viewing images of their mothers (vs. stranger) also perceived their mothers to be more sensitive to their emotional needs (i.e., reported higher attachment security) than youth who showed positive connectivity between these brain regions. It is important to note that, unlike prior evidence showing that prolonged maternal separation accelerates amygdala-prefrontal development (Callaghan & Richardson, 2011; Gee et al., 2013) with potential deleterious effects on child functioning, the maternal modulation in this study was phasic in nature. Thus, findings from this study illustrate the potential importance of maternal support in promoting ER through phasic amygdala-prefrontal modulation, a pathway important for the adaptive development of voluntary ER processes and, in turn, socioemotional competency (see Figure 1).
Overall, there is a critical need to understand how negative and positive parenting behaviors shape developmental trajectories of functional connectivity between neural regions within ER networks. That is, given preliminary evidence that negative parenting behaviors might accelerate amygdala-prefrontal development during childhood and that positive parenting supports a similar, “adaptive” neurodevelopmental pattern of connectivity during adolescence, it is important that future studies leverage multi-level longitudinal designs to track how normative variations in parents’ positive and negative behaviors are associated with the individual differences in neural and behavioral indices of ER-related processes.
Parental ERSBs.
A strength of Eisenberg and colleagues (1998) model is the specification of how three types of ERSBs – parental responses to youth’s emotional expressions, expressions of emotion, and emotion coaching – might impact youth’s arousal and, in turn, socioemotional competence. At the time of this review, few neuroimaging studies specifically assessed ESRBs but preliminary findings from this growing area of research are detailed below.
Brain volume and structural connectivity.
We know of only one study relating parental ERSBs (i.e., parental emotion expression) and structural connectivity in youth. This study used diffusion tensor imaging (DTI) to estimate levels of white matter integrity of tracts within fronto-limbic regions. Sheikh and colleagues (2014) found that as compared to girls with low cortisol reactivity, 6-year-old girls with high cortisol reactivity showed lower levels of white matter integrity in the tracts adjacent to the right superior frontal gyrus and rostral ACC when they were approximately 9 years of age. However, among girls with high cortisol reactivity, those whose parents expressed high levels of positive emotion showed fronto-limbic structural connectivity patterns that were similar to girls with low cortisol reactivity. Researchers suggested that these findings support the view of parental positive emotion as a protective moderator of associations between stress reactivity and atypical structural connectivity between fronto-limbic regions–an aspect of neurodevelopment thought to support both implicit and voluntary ER subprocesses.
Likewise, to our knowledge, only one cross-sectional study (Whittle et al., 2009) has examined associations between parental ERSBs and child structural brain development. This study focused on negative parental responses to child emotion and reported relations between higher levels of observed maternal punishing responses to child positive emotion and larger right amygdala volumes in early adolescent boys (but not girls), as well as larger left dorsal ACC and OFC gray matter volume in both boys and girls. This finding may offer a possible neurobiological mechanism by which parental ERSB’s, such as negative parental reactions to child positive emotion, are behaviorally linked to the blunting of youth’s positive affect (e.g., Yap, Allen & Ladouceur, 2008). Given evidence of normative decreases in GM volume in fronto-cortical regions during adolescence, it may be that punishment of youth’s expression of positive emotions is associated with altered patterns of structural development of regions within the salience and/or implicit ER neural networks that were reported by Whittle and colleagues (2009). Although, replications are needed, if this hypothesis is supported, it would have implications for the development of psychopathology such as depression, which has been linked to maladaptive ER and altered neural processing of positive emotion (Forbes & Dahl, 2005).
Indeed, studies examining the link between parenting style/quality and variations in youth’s brain structure and emotional health (e.g., Deane et al., in press; Whittle et al., 2011) have suggested that parental behavior is related to structural neurodevelopment in ways that impact youth’s emotional well-being. Moreover, this view of emotion socialization is consistent with findings from research that investigated parental behavior in relation to neural indices of youth’s emotional reactivity/regulation and rating scales of youth’s symptoms and coping styles (see “Moderators” column in Table 1). Overall, this work supports the hypothesis that the structure/function of ER networks may mediate (or moderate) relations between parenting and affective symptomatology, highlighting neurodevelopment of ER networks as a pathway linking parental emotion socialization with socioemotional competence.
Neural activation and functional connectivity.
A small, but growing number of neuroimaging studies focused on identifying associations between parental emotion expression and activation within neural networks supporting salience detection and other ER subprocesses in children and adolescents. For example, using negative emotional pictures, two fMRI studies of adolescents found that higher levels of maternal negative emotion were related to greater neural activation in brain regions implicated in detecting (e.g., amygdala, anterior insula) and regulating (e.g., ACC, vlPFC) emotions (Chaplin et al., in press; Turpyn et al., 2018). These findings are similar to those from studies of negative parenting; however, some researchers have suggested that associations differ for boys and girls, a potential sex moderator requiring further investigation.
Additionally, although it did not include a direct measure of parenting, a study by Lee and colleagues (Lee, Siegle, Dahl, & Silk, 2015) investigated how a specific type of parental behavior that is particularly relevant to negative parental emotional expression, maternal criticism, might be associated with the functioning of youth’s salience and ER neural networks. When listening to critical comments pre-recorded by mothers, adolescents showed elevated neural activation (relative to neutral comments) in subcortical-limbic regions, but reduced activation in cortical regions (i.e., dlPFC, caudal ACC). The researchers suggested that adolescents show greater neural reactivity to their mother’s criticisms, in part, because they have difficulty recruiting regions from the voluntary ER neural network to regulate their emotional responses to their mothers’ criticisms. These findings advance our understanding of how a specific parenting behavior (i.e., criticism), which characterizes negative parenting style/quality, is processed at the neural level in youth.
Other studies examining associations between parental emotion expression and activation of brain regions within ER neural networks assessed youth’s responses to a specific type of emotional stimuli: feedback to social and non-social information. This research has generally found that higher levels of maternal positive emotion was associated with attenuated responses in the ventral striatum to non-social losses and heightened responses to non-social rewards (Morgan et al., 2014). Moreover, this association was stronger among boys who were exposed to maternal depression, a finding that is consistent with authors’ hypothesis that positive parenting could serve as a protective factor among youth at risk for depression in one aspect of ER, processing of positive emotional (i.e., rewarding) information. Higher levels of observed maternal negative emotion, however, were associated with attenuated neural responses in the dmPFC, dACC, and sgACC to social rewards (i.e., peer acceptance), which were also linked to depressive symptoms (Tan et al., 2014). Taken together, available evidence indicates that mothers’ negative emotional expressions are associated with greater activation in regions within the salience and voluntary ER networks when youth process negative emotional information, a pattern of neural responses that is suggestive of increased emotional reactivity and associated regulatory activity. Positive parental emotion, at least among mothers, has thus far been linked to a presumably adaptive pattern of reduced activity in these networks when youth process negative emotional information, but heightened activity when processing positive emotional information.
A third study targeted an ERSB that is part of an extended model of parental emotion socialization model that addresses family-level factors (Morris et al., 2007). This study found that youth who reported higher levels of family emotional responsiveness exhibited heightened amygdala activation specifically to angry (not sad) facial expressions (Farber et al., 2017), but only for adolescents who reported low levels of recent stress. The authors interpreted this result as a novelty effect: Adolescents raised in a family climate that was emotionally responsive, who also and had low levels of life stress may have had little exposure to interpersonal threats and therefore showed hypersensitivity to less frequently-encountered interpersonal threat stimuli (i.e., angry faces). This interpretation requires further investigation but is suggestive of the possibility that normative exposure to stress, including more negative interactions with family members, provides opportunities for youth to learn and practice ways to regulate of negative emotional information in ways that promote the development of ER neural networks.
Finally, an fMRI study specifically assessed emotion coaching as a parental ERSB. Using a multi-method design that included ecological momentary assessment of adolescent coping behaviors, Butterfield and colleagues (2019) found that anxious adolescents who received higher levels of emotion coaching, specifically encouragement to use engagement-oriented coping behaviors by a parent, exhibited greater activation in neural regions within the salience detection and implicit ER networks. Moreover, structural equation models revealed that more parental coaching of engagement-oriented coping was also associated with less avoidant coping in their daily life. In contrast, non-anxious youth who experienced less emotion coaching exhibited reduced activation in regions within the ER networks, which was not associated with daily avoidant coping. Findings from this study suggest that parents’ (largely mothers’) use of emotion coaching scaffolds the adolescent in ways that shape neural activity when processing and regulating threat in ways that promote adaptive coping in daily life.
Moderators of associations between ERSBs and brain structure/function.
Research on the relations between parenting and structure/function of youth’s ER neural networks has helped researchers identify contextual factors, such as family conflict or the valence of emotional stimuli used in fMRI paradigms, as moderators of the link between ERSBs and brain structure/function. For example, the context in which parental negative emotion is expressed could be especially important to consider. Tan and colleagues (2014) found that maternal negative emotion during parent-adolescent interactions in which adolescents are seeking parents’ support is associated with reduced neural responses to positive emotional stimuli. Using a parent-child interaction task designed to elicit conflict, Turpyn and colleagues (2018) instead found that maternal negative emotion is associated with greater neural responses to negative emotional images among adolescents. In addition to contextual factors, child-level characteristics moderate associations between parental ERSBs and the functioning of youth’s ER networks. For example, one study found that higher levels of maternal negative emotion were associated with greater right rACC activation for girls, but reduced activation for boys. Moreover, for girls only, elevated rACC activation was associated with more depressive symptoms (Chaplin et al., in press). These results highlight the need to consider the sex of both child and parent in future research.
Additionally, both structural and functional imaging studies have indicated that developmental timing may serve as a moderator of associations between measures of parenting and neural measures of emotional reactivity/regulation in youth. For example, Morgan and colleagues (2014) found that for boys whose mothers had experienced depression, lower levels of maternal warmth in early childhood was prospectively associated with less activation in the medial PFC to reward anticipation; however, experiences of decreased maternal warmth during adolescence were associated with greater activation in the caudate to reward anticipation. These findings highlight that the clinical implications of positive parenting during childhood and adolescence are complex – likely moderated by the developmental timing of caregiving experience – and that the behavioral implications of hypo- and hyperactivation of different neural regions need to be carefully investigated when interpreting behavioral function and developmental risk. Finally, much of the extant work on parental ERSBs has examined parental emotion expression and focused almost exclusively on adolescents. Consequently, much work is still needed to determine how different parental ERSBs are associated with specific ER subprocesses. There is a particular need for new methods to measure ESRBs that have been shown to promote the development of adaptive ER behaviors during the childhood years (i.e., emotion coaching, emotion talk/language; Gottman et al., 1996; Roben et al., 2013).
Part 3: Summary and Recommendations for Future Research
Advances in developmental neuroimaging methods have enabled researchers to use multi-level research designs to enrich our conceptual models of emotion socialization. Indeed, since publication of Eisenberg et al.’s review in 1998, studies have provided empirical support for associations between ERSBs and developmental changes in children’s putative ER strategies from infancy into the early school-aged years. Overall, we found mounting evidence that normative variations in parenting style/quality are linked to individual differences in the structure/function of neural networks implicated in emotion processing and regulation of children and adolescents – an extension of Eisenberg and colleagues’ (1998) suggestion that parental ERSBs help to shape emotional development via youth’s emotional arousal (see Figure 1).
First, of the extant research, there appears to be the most support for associations between positive parenting (style/quality and ERSBs) and the structure and function of youth’s ER neural networks, specifically, that supportive parenting is linked with cortical thinning and reduced activation in regions within the salience and implicit ER networks. This line of research is intriguing as it is tentatively consistent with a major tenet of Eisenberg et al.’s 1998 parental emotion socialization model – that supportive ERSBs might protect against emotional (over)arousal – in part by attenuating the activation of neural networks supporting emotional reactivity/regulation. The neurodevelopmental mechanisms underlying this association, however, require further investigation. Multi-modal imaging studies linking neural activation with variations in brain structure (e.g., volume, gray matter thinning, white matter tracts between brain regions) are needed to evaluate the degree to which positive parenting behaviors are associated with reduced recruitment of the salience network, in part, because positive parenting is also associated with smaller amygdala volumes and cortical thinning in ventral striatum and fronto-cortical regions during adolescence.
Available evidence also suggests that positive parenting may promote, perhaps through parental scaffolding, the development of more mature, voluntary ER processes. This possibility requires longitudinal investigations of associations between normative variation in positive parenting behaviors and the development of inverse amygdala-PFC functional connectivity patterns that have been implicated in the development of ER (e.g., Silvers et al, 2017). Consistent with this pattern, youth who experienced low levels of positive parenting typically showed elevated activation in regions within the salience detection network when processing negative emotional information, a pattern of neural activation that might reflect (over)arousal when trying to regulate negative emotions. Thus, parenting styles that are characterized by low levels of support for youth’s emotions may be associated with neurodevelopmental trajectories that potentially confer risk for ER problems.
Second, we also found support for the hypothesis that higher levels of negative (i.e., unsupportive) parenting behaviors would be associated with elevated neural responses to negative emotional information within the salience network. This was observed across fMRI tasks and parenting measures. Third, regarding the structural connectivity of ER neural networks, researchers suggest that negative parenting is linked to alterations in the white matter tracts connecting frontal and subcortical regions that have been implicated in youth’s emotional reactivity and regulation. Taken together, findings from our review highlight how incorporating neuroimaging methods to examine parents’ roles in the neural of networks underlying ER-related processes can deepen our understanding of the neurodevelopmental mechanisms that underlie youth’s socioemotional competence. The potential in leveraging this research to improve translational research for promoting the development of socioemotional competence, however, requires careful consideration of several developmental and methodological factors.
Assessment Considerations.
One important direction for future research is to incorporate the same level of specificity in measures of socialization as those used in behavioral studies of emotional development. Until recently, developmental affective neuroscience studies of normative variations in caregiving experiences have relied on rating scales measuring broad dimensions of parenting or parent-child relationship quality, thereby limiting the scope of our current understanding of how specific ESRBs can account for individual differences in the structure and function of neural networks implicated in youth’s emotional reactivity/regulation. There is also a need for parenting assessments that are suitable for indexing emotion socialization from infancy into late adolescence. For example, many of the observational paradigms for eliciting the specific parenting behaviors that have been identified in behavioral research as important for the early development of ER are mostly applicable to younger children. Recently, however, progress has been made in developing and validating laboratory emotional challenges that can be used with older youth to assess emotion socialization, including behaviors that parents engage in when adolescents are faced with a social stressor in the lab (e.g., speech task; Oppenheimer, Hankin, & Young, 2018). Finally, a large majority of research focuses on maternal behavior, leaving open questions regarding how associations between parenting and neural indices of youth’s ER processes might differ between mothers and fathers.
Another critical measurement issue relates to the need for a systems-level understanding of neurobiological mechanisms supporting child/adolescent ER. Specifically, research on neural structure and function needs to be better integrated to elucidate the functional implications of variations in brain structure. Studies of youth’s ER neural networks also rarely include behavioral indices of youth’s ER skills or difficulties. This limits our ability to evaluate the hypothesis that functional activation of brain regions within neural networks implicated in emotion reactivity/regulation mediates associations between parenting behavior, emotional (over)arousal, and socioemotional adjustment in youth. In addition to the use of multi-level study designs, incorporating new advances in neuroimaging techniques that can integrate temporally-sensitive measures of emotion processing with measures of structural and functional connectivity could specify our knowledge of links between parenting and the neurodevelopment of ER processes.
Improving Ecological Validity.
Methodological constraints, notably the need to reduce movement artifacts, have complicated efforts to improve the ecological validity of computerized tasks used in affective neuroscience research. Recent studies, however, have showcased creative ways of increasing the ecological validity of emotionally-evocative stimuli within the constraints of fMRI/connectivity study designs, such as “chatroom” designs where adolescents believe that they are being evaluated by peers (e.g., Guyer et al., 2012) and incorporation of personalized stimuli from youth’s own family members and friends (e.g., Saxbe et al., 2015), to elicit emotional processing during neuroimaging tasks. Finally, there is a need to characterize parental ERSBs “in the real-world.” Coupled with established methodologies for assessing youth’s social environments (e.g., daily diaries, experience sampling), advances in mobile smartphone/passive sensing technology and machine learning data analytic approaches offer the possibility for researchers to deepen our understanding of ERSBs through specific measures of “in-the-moment” parenting across youth’s natural social environments.
Developmental Considerations: Longitudinal Designs to Inform Developmental Models.
The 1998 parental emotion socialization model not only helped researchers clarify socialization behaviors of interest, but also laid the groundwork for developmental origin and cascade models that consider how early parenting might constrain emotional development. A developmental affective neuroscience lens could further enrich these models, allowing researchers to elucidate mechanisms by which parenting might alter developmental trajectories. However, to date, much of the extant studies linking parenting behaviors to brain development assess parenting and neural structure/function at only one time point, which limits our understanding of how early parental ESRBs predict changes in ER networks. That is, use of cross-sectional designs may be contributing to contradictory findings observed across many studies. There are dynamic, nonlinear changes in neural architecture across childhood and adolescence, and the timing of these changes are typically region-specific. Associations between parenting and indices of brain development at a single time point, therefore, provide a limited understanding of the way in which parenting is associated with neurodevelopmental trajectories of ER-related processes. Overall, there is a clear need for longitudinal studies with repeated measures of brain structure, function, and connectivity. Such designs could also provide further insight into how the effects of various ESRBs might differ across developmental periods, helping to identify potential sensitive periods during which neural systems might be particularly responsive to interventions that target parental ERSBs.
Developmental Complexity.
Consistent with the tenets of developmental psychopathology, it will also be important for future studies to recognize the complex, transactional nature of associations between parenting behaviors and neurodevelopment (Bridgett et al., 2015). For example, analytical tools from dynamic systems perspectives could be particularly helpful in identifying the reciprocal effects of parent and child/adolescent characteristics on the neurodevelopment of ER networks from infancy into adolescence. Second, many of the reviewed studies found evidence that child characteristics moderated associations between parental behavior and the structure/function of ER networks. This pattern of findings underscores the need for considerably larger samples to characterize normative variability in caregiving experiences. Unfortunately, existing large open-science datasets in children and adolescents currently do not include adequate measures of parental ERSBs, highlighting this as a key area for future research.
Another key moderator to consider in future work is parental emotionality. In line with hypotheses from Eisenberg’s (1998) model of parental emotion socialization, findings from a recent study demonstrate the importance of considering parental emotional expression in relation to parental ER. Turpyn and colleagues (2018) showed that when parents reported low levels of ER difficulties, parental negative emotion was unrelated to adolescent ACC or vmPFC activation to negative emotional stimuli, but, when mothers reported high levels of ER difficulties, adolescents showed increased activation in these regions. These intriguing findings suggest that adolescents might exhibit maladaptive, heightened activation of their salience and voluntary ER networks in response to negative emotional information only when high levels of parental negative emotion are experienced in the context of parental ER difficulties. They might also suggest that offspring of parents with ER difficulties may be more vulnerable to maladaptive processing of negative emotions, particularly in a social context of high negative emotion expression − possibilities that require longitudinal investigation.
Finally, consistent with the differential susceptibility hypothesis (Belsky & Pleuss, 2009), there is emerging evidence that some youth characteristics, including the functioning of their ER neural networks, may confer greater neural susceptibility to both maladaptive and adaptive parenting behaviors. For instance, it has been suggested that amygdala volume in girls may be better conceptualized as a marker of susceptibility that contributes to the development of negative outcomes in the context of negative parenting, and positive outcomes in the context of positive parenting (Yap et al., 2008). Other markers of brain development, such as heightened neural activation to negative emotional stimuli, may also represent susceptibility to both positive and negative parenting influences (Rudolph et al., 2018). These markers could be clarified through longitudinal multi-modal neuroimaging studies that incorporate measures of structural and functional connectivity within emotional processing networks.
Considerations of Socio-cultural Context.
The broader social environments with which parents and youth interact also require careful consideration. As noted by others (e.g., Choudhury, 2009), socio-cultural variables have been understudied in affective neuroscience research. This gap in the literature is especially important to address when considering how parental behaviors might shape the neural systems that support children’s ER, as there is substantial evidence that cultural factors influence parental emotion socialization (see Cole & Tan, 2007) and youth’s developing ER capacity (Butler et al., 2007). Research has found that socioeconomic (SES) stress can act as a moderator, specifically that higher levels of maternal “positive” behaviors were linked with decreased growth trajectories in the amygdala for only male adolescents from economically-disadvantaged neighborhoods (Whittle et al, 2007). Studies that include diversity in SES and more nuanced assessments of culture are critically needed in this research area.
Implications for Prevention/Intervention.
Findings from the present review indicate that normative parenting behaviors are associated with patterns of neural activation underlying emotion processing well into adolescence. Psychologists may be able to leverage this information to fine tune parenting interventions for children and adolescents. If, for example, we can provide parents with scientific evidence demonstrating that how they talk to their children about their emotions (e.g., coaching) is directly related to how their child or teen’s brain processes emotional information, parents and can better learn supportive socialization behaviors. This approach has gained some traction in education, where teachers are increasingly attempting to use neuroscience-backed educational strategies to enhance learning (Goswami, 2006). Similarly, psychologists may be able to better pinpoint which parenting strategies, during what optimal developmental windows, are most likely to increase children and adolescents’ adaptive recruitment of salience and ER neural networks and their connectivity. As suggested by Eisenberg and colleagues (1998), we would be poised to improve the effectiveness of our parenting and ER interventions with a more complete understanding of the neurodevelopmental pathways by which parental ERSBs are associated with the structure and function of ER-related networks during the preschool, school-aged, and adolescent years.
Supplementary Material
Acknowledgements
This research was supported in part by grants from K01MH100261 (Tan)
References
- Avants BB, Hackman DA, Betancourt LM, Lawson GM, Hurt H, & Farah MJ (2015). Relation of childhood home environment to cortical thickness in late adolescence: specificity of experience and timing. PloS one, 10(10), e0138217. doi: 10.1371/journal.pone.0138217 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Simmons JG, Vijayakumar N, Dudgeon P, Patton GC, Mundy LK, … & Whittle S (2018). Interaction Between Parenting Styles and Adrenarcheal Timing Associated With Affective Brain Function in Late Childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 57(9), 678–686. doi: 10.1016/j.jaac.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885. doi: 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Belsky J, & de Haan M (2011). Annual research review: Parenting and children’s brain development: The end of the beginning. Journal of Child Psychology and Psychiatry, 52(4), 409–428. doi: 10.1111/j.1469-7610.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Bernier A, Dégeilh F, Leblanc É, Daneault V, Bailey HN, & Beauchamp MH (2019). Mother–infant interaction and child brain morphology: a multidimensional approach to maternal sensitivity. Infancy, 24(2), 120–138. doi: 10.1111/infa.12270 [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, & Pruessner JC (2007). Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. The Journal of neuroscience, 27(10), 2592–2595. doi: 10.1523/jneurosci.3252-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Lee TL, & Gross JJ (2007). Emotion regulation and culture: Are the social consequences of emotion suppression culture-specific?. Emotion, 7(1), 30. doi: 10.1037/1528-3542.7.1.30 [DOI] [PubMed] [Google Scholar]
- Butterfield RD, Siegle GJ, Lee KH, Ladouceur CD, Forbes EE, Dahl RE, … & Silk JS (2019). Parental coping socialization is associated with healthy and anxious early-adolescents’ neural and real-world response to threat. Developmental Science, 22(6). doi: 10.1111/desc.12812 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. doi: 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Perry NE, & Dollar JM (2016). A biopsychosocial model of self-regulation in infancy In Child Psychology: A Handbook of Contemporary Issues: Third Edition (pp. 3–20). Taylor and Francis Inc. 10.4324/9781315764931 [DOI] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, & Forbes EE (2014). Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience, 8, 18–27. doi: 10.1016/j.dcn.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Poon JA, Thompson JC, Hansen A, Dziura SL, Turpyn CC, … Ansell EB (2019). Sex-differentiated associations among negative parenting, emotion-related brain function, and adolescent substance use and psychopathology symptoms. Social Development, 28(3), 637–656. doi: 10.1111/sode.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S (2009). Culturing the adolescent brain: what can neuroscience learn from anthropology?. Social Cognitive and Affective Neuroscience, 5(2–3), 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM (2016). Emotion and the development of psychopathology In Cicchetti D (Ed.), Developmental Psychopathology, Volume 1: Theory and method (pp. 265–324). Hoboken, New Jersey: Wiley and Sons. doi: 10.1093/scan/nsp030 [DOI] [Google Scholar]
- Cole PM, Hall SE & Hajal NJ (2013). Emotion dysregulation as a risk factor for psychopathology In Beauchaine TP & Hinshaw SP (Eds.), Child and adolescent psychopathology (2nd ed., pp. 341–373). Hoboken, NJ: Wiley. [Google Scholar]
- Cole PM, Martin SE, & Dennis TA (2004). Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development, 75(2), 317–333. doi: 10.1111/j.1467-8624.2004.00673.x [DOI] [PubMed] [Google Scholar]
- Cole PM & Tan PZ (2007). Emotional processes in socialization from a cultural perspective In Grusec J & Hastings P (Eds). Handbook of Socialization. New York, NY: Guilford Press. [Google Scholar]
- Cox MJ, Mills-Koonce R, Propper C, & Gariépy J-L (2010). Systems theory and cascades in developmental psychopathology. Development and Psychopathology, 22(3), 497–506. doi: 10.1017/s0954579410000234 [DOI] [PubMed] [Google Scholar]
- Deane C, Vijayakumar N, Allen NB, Schwartz O, Simmons JG, Bousman CA, … & Whittle S (in press). Parenting × Brain Development interactions as predictors of adolescent depressive symptoms and well-being: Differential susceptibility or diathesis stress?. Development and Psychopathology. doi: 10.1017/s0954579418001475 [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen T-V, Karama S, … Group BDC (2012). Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. Journal of the American Academy of Child & Adolescent Psychiatry, 51(1), 18–27. e12. doi: 10.1016/j.jaac.2011.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, & Spinrad TL (1998). Parental socialization of emotion. Psychological Inquiry, 9(4), 241–273. doi: 10.1207/s15327965pli0904_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, & Eggum ND (2010). Emotion-related self-regulation and its relation to children’s maladjustment. Annual Review of Clinical Psychology, 6, 495–525. doi: 10.1146/annurev.clinpsy.121208.131208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert V, Buss C, Khalili-Mahani N, Wadiwalla M, Dedovic K, & Pruessner JC (2010). Investigating the association between early life parental care and stress responsivity in adulthood. Developmental Neuropsychology, 35(5), 570–581. doi: 10.1080/87565641.2010.494752 [DOI] [PubMed] [Google Scholar]
- Etkin A, Büchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Eshel N, Daelmans B, Mello MCD, & Martines J (2006). Responsive parenting: interventions and outcomes. Bulletin of the World Health Organization, 84, 991–998. doi: 10.2471/blt.06.030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber MJ, Romer AL, Kim MJ, Knodt AR, Elsayed NM, Williamson DE, & Hariri AR (2019). Paradoxical associations between familial affective responsiveness, stress, and amygdala reactivity. Emotion, 19(4), 645–654. doi: 10.1037/emo0000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … & Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. doi: 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … & Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. doi: 10.1523/jneurosci.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U (2006). Neuroscience and education: from research to practice? Nature Reviews Neuroscience, 7, 406. doi: 10.1038/nrn1907 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, … & Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, … Ernst M (2012). Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry, 169(2), 205–212. doi: 10.1162/jocn.2008.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Jarcho JM, Pérez-Edgar K, Degnan KA, Pine DS, Fox NA, & Nelson EE (2015). Temperament and parenting styles in early childhood differentially influence neural response to peer evaluation in adolescence. Journal of Abnormal Child Psychology, 43(5), 863–874. doi: 10.1007/s10802-015-9973-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. doi: 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, & Swain JE (2010). Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science, 13(4), 662–673. doi: 10.1111/j.1467-7687.2009.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U (2014). Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage, 87, 345–355. doi: 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok R, Thijssen S, Bakermans-Kranenburg MJ, Jaddoe VW, Verhulst FC, White T, … Tiemeier H (2015). Normal variation in early parental sensitivity predicts child structural brain development. Journal of the American Academy of Child & Adolescent Psychiatry, 54(10), 824–831. e821. doi: 10.1016/j.jaac.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Cyr M, Finsaas MC, Orawe J, Huang A, Tottenham N, & Klein DN (2018). Early Childhood Parenting Predicts Late Childhood Brain Functional Connectivity During Emotion Perception and Reward Processing. Child Development. doi: 10.1111/cdev.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB (1989). Regulation of distress and negative emotions: A developmental view. Developmental Psychology, 25(3), 343. doi: 10.1037/0012-1649.25.3.343 [DOI] [Google Scholar]
- Lee KH, Siegle GJ, Dahl RE, Hooley JM, & Silk JS (2015). Neural responses to maternal criticism in healthy youth. Social and Cognive Affect Neuroscience, 10(7), 902–912. doi: 10.1093/scan/nsu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little K, Olsson CA, Youssef GJ, Whittle S, Simmons JG, Yücel M, … Allen NB (2015). Linking the serotonin transporter gene, family environments, hippocampal volume and depression onset: A prospective imaging gene× environment analysis. Journal of abnormal psychology, 124(4), 834. doi: 10.1037/abn0000101 [DOI] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, … Botteron KN (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences, 109(8), 2854–2859. doi: 10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatrics, 167(12), 1135–1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, & Barch DM (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences, 113(20), 5742–5747. doi: 10.1073/pnas.1601443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2015). Salience Network. Brain Mapping, 597–611. doi: 10.1016/b978-0-12-397025-1.00052-x [DOI] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, … & Ochsner KN (2012). The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. doi: 10.1093/scan/nsr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, … & Pine DS (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. doi: 10.1001/archpsyc.65.5.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Lee GE, Wright AGC, Gilchrist DE, Forbes EE, McMakin DL, … Silk JS (2017). Altered Positive Affect in Clinically Anxious Youth: the Role of Social Context and Anxiety Subtype. Journal Abnormal Child Psychology, 45(7), 1461–1472. doi: 10.1007/s10802-016-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Criss MM, Silk JS, & Houltberg BJ (2017). The Impact of Parenting on Emotion Regulation During Childhood and Adolescence. Child Development Perspectives, 11(4), 233–238. doi: 10.1111/cdep.12238 [DOI] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, & Robinson LR (2007). The role of the family context in the development of emotion regulation. Social Development, 16(2), 361–388. doi: 10.1111/j.1467-9507.2007.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Takei Y, Suda M, Aoyama Y, Uehara T, Kosaka H, … Mikuni M (2010). Relationship of parental bonding styles with Gray matter volume of dorsolateral prefrontal cortex in young adults. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34(4), 624–631. doi: 10.1016/j.pnpbp.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer CW, Hankin BL, & Young J (2018). Effect of Parenting and Peer Stressors on Cognitive Vulnerability and Risk for Depression among Youth. Journal of Abnormal Child Psychology, 46(3), 597–612. doi: 10.1007/s10802-017-0315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, … & Evans AC (1999). Structural maturation of neural pathways in children and adolescents: in vivo study. Science, 283(5409), 1908–1911. doi: 10.1126/science.283.5409.1908 [DOI] [PubMed] [Google Scholar]
- Perlman SB, & Pelphrey KA (2010). Regulatory brain development: balancing emotion and cognition. Social Neuroscience, 5(5–6), 533–542. doi: 10.1080/17470911003683219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833. doi: 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi E, Simmons JG, Bousman CA, Vijayakumar N, Bray KO, Dandash O, … & Yap MB (2019). The influence of maternal parenting style on the neural correlates of emotion processing in children. Journal of the American Academy of Child & Adolescent Psychiatry. doi: 10.1016/j.jaac.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, … Farah MJ (2010). Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage, 49(1), 1144–1150. doi: 10.1016/j.neuroimage.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Kong L, Sim L, Sanmugam S, Broekman B, Chen H, … Chong Y (2015). Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Translational Psychiatry, 5(10), e668. doi: 10.1038/tp.2015.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T, … & Beck A (2016). Maternal parenting behavior and emotion processing in adolescents—An fMRI study. Biological Psychology, 120, 120–125. doi: 10.1016/j.biopsycho.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Rueger SY, Katz RL, Risser HJ, & Lovejoy MC (2011). Relations between parental affect and parenting behaviors: A meta-analytic review. Parenting: Science and Practice, 11(1), 1–33. doi: 10.1080/15295192.2011.539503 [DOI] [Google Scholar]
- Saxbe D, Del Piero L, Immordino-Yang MH, Kaplan J, & Margolin G (2015). Brain correlates of adolescents’ viewing of parents’ and peers’ emotions: Associations with risk-taking behavior and risky peer affiliations. Social Neuroscience, 10(6), 592–604. doi: 10.1080/17470919.2015.1022216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Brassen S, Bromberg U, Banaschewski T, Conrod P, Flor H, … Buchel C (2012). Maternal interpersonal affiliation is associated with adolescents/′ brain structure and reward processing. Translational Psychiatry, 2, e182. doi: 10.1038/tp.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, ′ Giedd J (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440(7084), 676–679. doi: 10.1038/nature04513 [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Joanisse MF, Mackrell SM, Kryski KR, Smith HJ, Singh SM, & Hayden EP (2014). Links between white matter microstructure and cortisol reactivity to stress in early childhood: Evidence for moderation by parenting. NeuroImage: Clinical, 6, 77–85. doi: 10.1016/j.nicl.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Lee KH, Dahl RE, Hooley JM, & Siegle GJ (2014). Neural response to maternal praise and criticism in adolescents with major depressive disord. Paper presented at the Paper presented at the Association for Behavioral and Cognitive Therapy, Philadelphia, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, … & Ochsner KN (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 25, 128–137. doi: 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, … & Ochsner KN (2016). vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514. doi: 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, … & Silk JS (2014). Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Developmental Cognitive Neuroscience, 8, 28–39. doi: 10.1016/j.dcn.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S, Muetzel RL, Bakermans-Kranenburg MJ, Jaddoe VW, Tiemeier H, Verhulst FC, … & Van Ijzendoorn MH (2017). Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Development and Psychopathology, 29(2), 505–518. doi: 10.1017/s0954579417000141 [DOI] [PubMed] [Google Scholar]
- Turpyn CC, Poon JA, Ross CE, Thompson JC, & Chaplin TM (2018). Associations between parent emotional arousal and regulation and adolescents’ affective brain response. Social Development, 27(1), 3–18. doi: 10.1111/sode.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, & Kahn RS (2014). Functional differences in emotion processing during adolescence and early adulthood. Neuroimage, 91, 70–76. doi: 10.1016/j.neuroimage.2014.01.035 [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. doi: 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yücel M, Sheeber L, Simmons JG, Pantelis C, & Allen NB (2009). Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Social Cognitive and Affective Neuroscience, nsp012. doi: 10.1093/scan/nsp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Sheeber L, Dudgeon P, Yucel M, Pantelis C, & Allen NB (2011). Hippocampal volume and sensitivity to maternal aggressive behavior: A prospective study of adolescent depressive symptoms. Development and Psychopathology, 23(1), 115–129. [DOI] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MBH, … Allen NB (2014). Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. doi: 10.1017/s0954579410000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Dennison M, Schwartz O, Simmons JG, Sheeber L, & Allen NB (2016). Observed measures of negative parenting predict brain development during adolescence. PloS one, 11(1), e0147774. doi: 10.1371/journal.pone.0147774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Simmons JG, Dennison M, Schwartz O, Pantelis C, … & Allen NB (2017). Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA: Psychiatry, 74(8), 824–832. doi: 10.1001/jamapsychiatry.2017.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wei D, Wang K, Yi Z, & Qiu J (2018). Regional gray matter volume mediates the relationship between maternal emotional warmth and gratitude. Neuropsychologia, 109, 165–172. doi: 10.1016/j.neuropsychologia.2017.12.017 [DOI] [PubMed] [Google Scholar]
- Yap MB, Allen NB, & Ladouceur CD (2008). Maternal socialization of positive affect: The impact of invalidation on adolescent emotion regulation and depressive symptomatology. Child Development, 79(5), 1415–1431. doi: 10.1111/j.1467-8624.2008.01196.x [DOI] [PubMed] [Google Scholar]
- Yap MBH, Pilkington PD, Ryan SM, & Jorm AF (2014). Parental factors associated with depression and anxiety in young people: A systematic review and meta-analysis. Journal of Affective Disorders, 156, 8–23. doi: 10.1016/j.jad.2015.01.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


