Abstract
Background and Objective: Impaired dermal wound healing represents a major medical issue in today's aging populations. Granulation tissue formation in the dermis and reepithelization of the epidermis are both important and necessary for proper wound healing. Although a number of artificial dermal grafts have been used to treat full-thickness dermal loss in humans, they do not induce reepithelization of the wound, requiring subsequent epithelial transplantation. In the present study, we sought a novel biomaterial that accelerates the wound healing process.
Approach: We prepared a composite biomaterial made of jellyfish and porcine collagens and developed a hybrid-type dermal graft that composed of the upper layer film and the lower layer sponge made of this composite biomaterial. Its effect on dermal wound healing was examined using a full-thickness excisional wound model. Structural properties of the dermal graft and histological features of the regenerating skin tissue were characterized by electron microscopic observation and immunohistological examination, respectively.
Results: The composite biomaterial film stimulated migration of keratinocytes, leading to prompt reepithelization. The regenerating epithelium consisted of two distinct cell populations: keratin 5-positive basal keratinocytes and more differentiated cells expressing tight junction proteins such as claudin-1 and occludin. At the same time, the sponge made of the composite biomaterial possessed a significantly enlarged intrinsic space and enhanced infiltration of inflammatory cells and fibroblasts, accelerating granulation tissue formation.
Innovation: This newly developed composite biomaterial may serve as a dermal graft that accelerates wound healing in various pathological conditions.
Conclusion: We have developed a novel dermal graft composed of jellyfish and porcine collagens that remarkably accelerates the wound healing process.
Keywords: wound healing, jellyfish collagen, dermal graft, composite biomaterial, reepithelization, granulation

Hideaki Sumiyoshi, PhD
Introduction
Impaired dermal wound healing has become a serious medical issue in today's aging society.1 Aging is a major risk factor for insufficient wound healing due to several underlying circumstances such as malnutrition, reduced local blood supply, decreased daily activity, and long-term bed confinement.2 In addition, the presence of obesity and diabetes also causes retarded wound healing. Open dermal wounds are readily susceptible to bleeding and infection, which may lead to life-threatening complications such as sepsis and intravascular coagulopathy. Although a number of attempts have been made to deal with such intractable skin ulcers, none has been successful in treating difficult cases.2
Proper dermal wound healing can be achieved through a well-organized and concerted process of granulation tissue formation in the dermis and reepithelization of the epidermis. While infiltration of inflammatory cells and fibroblasts is important in granulation tissue formation, proliferation and/or migration of keratinocytes play a critical role in the reepithelization process.3,4 We have been studying the cellular and molecular mechanisms responsible for dermal wound healing. Experiments using transgenic mice harboring tissue-specific enhancer/promoter sequences of the type I α2 collagen gene (Col1a2) linked to either a firefly luciferase5 or enhanced green fluorescent protein (EGFP) reporter gene6 indicate that a large number of resident (myo)fibroblasts, and only a limited number of bone marrow-derived cells, participate in granulation tissue formation by producing type I collagen.7 We also showed that a novel small compound that antagonizes TGF-β/Smad signaling accelerated the wound healing process by stimulating migration of both fibroblasts and keratinocytes.8
Collagen represents a family of proteins that is a major component of the extracellular matrix.9 Mammalian collagens are classified, based on their structures and functions, into fibril-forming collagens (fibrillar collagens), basement membrane collagens, and fibril-associated collagens with interrupted triple helices, among others.10,11 Skin is well known as an organ where type I collagen, a representative fibrillar collagen, exists in abundance.10,11 A number of artificial dermal grafts composed of porcine or bovine type I collagen have been developed to treat full-thickness dermal loss caused by deep burns in humans.12–14 Both inflammatory cells and fibroblasts infiltrate the transplanted graft, which is gradually replaced by endogenous granulation tissue.12,13 However, this type of dermal graft transplantation does not induce reepithelization of the epidermis, thus requiring subsequent epithelial transplantation from another part of the patient's body.15 A novel therapeutic approach for proper reepithelization is eagerly sought to treat patients with intractable skin ulcers in a shorter period and in a less invasive manner.
In contrast to the vertebrate collagens described above, the structures and functions of collagenous proteins in invertebrates such as jellyfish have not been fully understood. Jellyfish collagen possesses the common feature of collagen molecules exhibiting a triple helix structure16 and is resistance to pepsin digestion.17 However, it is unknown whether jellyfish collagen is coupled with each other to form thick fibrous bundles. Recent studies have paid attention to marine collagen, including that from jellyfish, as well as its application as biomedical devices.18,19 In addition, collagens extracted from several species of jellyfish exhibit unique functional properties. For example, species of Nemopilema are known to be edible and harmless jellyfish, and their collagen stimulates immune reactions through the TLR4 signaling pathway.20,21 Others have reported that collagen extracted from this species accelerates cartilage differentiation from mesenchymal stem cells.17 On the contrary, collagen extracted from Aurelia species (moon jellyfish) possesses the unique property of high water solubility that collagens from other species of jellyfishes do not.16
In the present study, we propose a novel composite biomaterial made of moon jellyfish collagen and porcine type I collagen that remarkably accelerates the wound healing process. We have developed a hybrid-type dermal graft that is composed of the upper layer film and the lower layer sponge, both of which are made of this composite biomaterial. We demonstrate that the upper layer film induced faster reepithelization of the epidermis, compared with a control film made of porcine type I collagen alone. The regenerating epithelium consisted of two cellular components: keratin 5-positive basal layer keratinocytes and more differentiated upper layer cells expressing tight junction proteins such as claudin-1 and occludin. On the contrary, the lower-layer sponge made of the same composite biomaterial possessed a significantly larger intrinsic space and induced more extensive granulation tissue formation in the dermis than the control sponge. The results of the present study indicate that this newly developed composite biomaterial may serve as a good dermal graft that accelerates wound healing in various pathological conditions and provide novel therapeutic insight into intractable skin ulcers.
Clinical Problem Addressed
Various attempts have been made to treat full-thickness dermal loss in humans. Currently available artificial dermal grafts composed of porcine or bovine collagen require a substantial length of time to allow infiltration of inflammatory cells and fibroblasts into the graft, resulting in delayed granulation tissue formation. Furthermore, these dermal grafts do not induce reepithelization of the wounds, thus requiring subsequent epithelial transplantation. An ideal dermal graft is eagerly sought that accelerates both granulation tissue formation in the dermis and reepithelization of the epidermis without excessive scar formation. Such a novel graft decreases the risk of complications such as bleeding and infection and dramatically improves the quality of life of patients.
Materials and Methods
Preparation of collagens
Moon jellyfish collagen (Aurelia aurita) extracted under neutral pH conditions was purchased from Jellyfish Research Laboratories, Inc. (Kawasaki, Japan).16 Amino acid composition analysis revealed that this product contained high proportions of glycine (32.2%), hydroxyproline (4.3%), and hydroxylysine (3.5%), all of which are characteristic amino acids present abundantly in collagen molucules.16 The water-soluble fraction of jellyfish collagen was separated by centrifugation at 10,000 g for 10 min at 4°C and precipitated with 50% isopropanol. The pellet was washed with isopropanol and finally dissolved in water. The extracted high-molecular-weight (MW) components were purified using a cutoff membrane (>10 kDa) and lyophilized. Porcine type I collagen was prepared by the conventional pepsin extraction method.22,23 In brief, fetal porcine tissues purchased from Tokyo Shibaura Zouki (Tokyo, Japan) were homogenized in phosphate-buffered saline (PBS) with proteinase inhibitors and centrifuged to remove soluble components. The residual insoluble components were extracted in 0.5 M acetic acid with 1 mg/mL pepsin (Sigma-Aldrich, St. Louis, MO) at 4°C for 48 h, and then precipitated with 0.7 M sodium chloride under acidic conditions.
Characterization of jellyfish and porcine collagens
Jellyfish collagen and porcine type I collagen were separated into soluble and insoluble fractions by soaking the specimens in PBS for 12 h at 4°C. The samples were then denatured and subjected to 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) to examine the presence of collagen molecules in each fraction. The purity of jellyfish and porcine collagens used was verified by incubation with 1 mg/mL bacterial collagenase (Fujifilm Wako Pure Chemical, Osaka, Japan) at 22°C for 12 h followed by SDS PAGE. Jellyfish collagen was also tested for its stability at mammalian body temperature by incubation at 37°C for 1 to 3 h. The samples were subsequently digested with 100 μg/mL pepsin in 50 mM acetic acid at 4°C for 12 h and subjected to SDS PAGE. Bands corresponding to collagen molecules were visualized by Coomassie blue staining, and the relative amounts of collagen corresponding to each band were semiquantified using ImageJ software.
Analysis of fibril formation of collagens in vitro
The effects of jellyfish collagen on fibril formation of porcine type I collagen were examined in vitro by measuring the turbidity of mixed solutions.24,25 For this purpose, jellyfish collagen and porcine type I collagen were dissolved separately in 50 mM acetic acid, dialyzed against 5 mM acetic acid, and then mixed in different ratios shown in Fig. 1D. After neutralization with PBS and subsequent incubation at 37°C for the different lengths of time, the turbidity of the mixed solutions was measured at 600 nm using a SpectraMax 250 spectrophotometer (Molecular Devices, San Jose, CA).
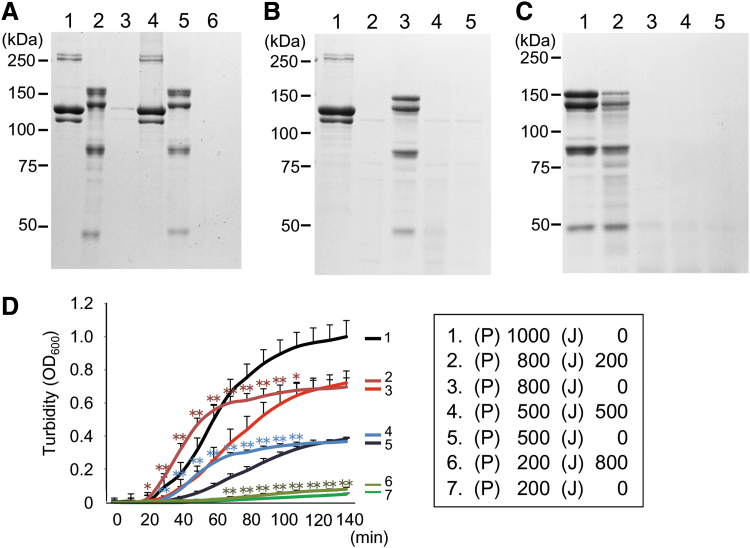
Figure 1.
Biochemical and biological characterization of jellyfish collagen. (A) Porcine type I collagen (lanes 1, 3, and 4) and jellyfish collagen (lanes 2, 5, and 6) were either untreated (lanes 1 and 2) or soaked in PBS for 12 h at 4°C for fractionation into water soluble (lanes 3 and 5) and insoluble fractions (lanes 4 and 6). After being denatured, they were subjected to 7.5% SDS PAGE and stained with Coomassie blue. On the left of the stained gel are shown molecular weight markers. (B) Porcine type I collagen (lanes 1 and 2) and jellyfish collagen (lanes 3 and 4) were either untreated (lanes 1 and 3) or treated with 1 mg/mL bacterial collagenase at 22°C for 12 h (lanes 2 and 4), followed by SDS PAGE and Coomassie blue staining. Note that the faint bands observed with the control sample containing only collagenase represent the enzyme proteins with the approximate sizes of 120 and 75 kDa (lane 5). (C) Jellyfish collagen was digested with 100 μg/mL pepsin at 4°C for 12 h without (lane 2) or with preincubation at 37°C for 1 (lane 3), 2 (lane 4), or 3 h (lane 5). A sample without pepsin digestion was also included as a control (lane 1). Samples were subjected to 7.5% SDS PAGE and subsequent Coomassie blue staining. (D) Porcine type I collagen dissolved in 5 mM acetic acid was left unmixed (lines 1, 3, 5, and 7) or mixed with jellyfish collagen (lines 2, 4, and 6). After neutralization and incubation at 37°C, the relative amounts of collagen fibrils in the mixed solution were measured as OD600 values at the indicated time points. The values represent the means ± SD of eight samples in each group. On the right of the graphs are shown the final concentrations (μg/mL) of porcine (P) and jellyfish (J) collagens in each mixture. The asterisks indicate that the differences were statistically significant (*p < 0.05, **p < 0.01) between the groups (line 2 vs. line 3 from 20 to 110 min, line 4 vs. line 5 from 30 to 100 min, and line 6 vs. line 7 from 70 to 140 min). PBS, phosphate-buffered saline; SDS PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Preparation of collagen films and sponges
Jellyfish and porcine collagens prepared as described above were dissolved in 50 mM acetic acid and then dialyzed against 5 mM acetic acid in PBS for neutralization. Collagen concentration was determined by OD215 ultraviolet absorption using rat tail collagen standard solution (Biocolor Ltd., Carrickfergus, United Kingdom). Collagen films were prepared by the solvent cast method.26 In brief, 125 μg porcine collagen alone or a mixture of jellyfish and porcine type I collagens was dissolved in 250 μL of 5 mM acetic acid in PBS and incubated at 37°C for 1 h to allow fibril formation. The resultant collagen suspension was dripped onto a polyethylene sheet before complete gel formation and then evaporated at room temperature. Dried films were washed three times with 85% ethanol to remove salts. One drip of 125 μg collagen was able to yield an 8 mm diameter collagen film. Collagen sponges were prepared using two different procedures. One was the conventional freeze-dry method26 and the other involved exchanging the solvents to tert-butyl alcohol (t-butyl alcohol) before freeze-drying. Eight hundred micrograms of collagen dissolved in 800 μL of neutralized solution was poured into a mold and incubated at 37°C for 6 h to allow gel formation. The prepared gel was 1 mm thick with the approximately volume of 135 mm3. For preparation of conventional sponges, collagen gels were frozen directly at −30°C and vacuum dried. In the t-butyl alcohol method, collagen gels were dehydrated in ethanol, followed by replacement of ethanol by t-butyl alcohol, and then frozen at −80°C before freeze-drying at 4°C. This modified method is the same as that used to prepare collagen samples for scanning electron microscope (SEM) observation and avoids crystallization of H2O molecule that influences the porosity of sponge. When transplanted onto the wound, collagen sponges prepared using the t-butyl alcohol method were penetrated by water much faster than the sponges prepared by the conventional method (Supplementary Video S1).
Preparation of a hybrid-type collagen film/sponge graft
To prepare a hybrid-type collagen film/sponge graft, we covered the upper surface of a collagen sponge with a 5 μm thick collagen film. For this purpose, sponges were prepared using neutralized solutions of porcine collagen alone or a mixture of 45% jellyfish and 55% porcine type I collagens. This sponge contained 800 μg of collagenous proteins in total. After immersing in 85% ethanol to remove salts, a block of collagen sponge was placed on a polyethylene sheet and smeared with 10 μL of 1 mg/mL collagen (porcine collagen alone or a mixture of 45% jellyfish and 55% porcine collagens) dissolved in 5 mM acetic acid. The formed collagen film was firmly adhered to and partly melted on the sponge under acidic conditions. The hybrid-type collagen film/sponge and a polyethylene sheet were immersed together in 100% ethanol, and subsequently subjected to freeze-drying using t-butyl alcohol. In some experiments, a composite biomaterial made of porcine type I collagen and denatured gelatin was also prepared. For this purpose, gelatin solution was prepared by boiling porcine type I collagen at 100°C for 10 min to allow complete denature. After determining the gelatin concentration, 45% denatured gelatin and 55% intact porcine collagen were mixed to prepare the composite sponge and film in the same way as described above.
Electron microscopic examination
Microstructures of prepared dermal grafts were examined using SEM. For this purpose, lyophilized collagen films and sponges were coated with osmium tetroxide using Neoc-Pro (Meiwafosis, Co., Ltd., Tokyo, Japan). The specimens were visualized and photographed by SEM (JSM-6510LV; JEOL Ltd., Tokyo, Japan). The mean diameter of collagen fibrils and the mean distance between each collagen fibril were measured and compared among the dermal grafts prepared by different methods.
Mice
C57BL/6J female mice, 6-week-old, were purchased from CLEA Japan, Inc. (Tokyo, Japan). In some experiments, a transgenic mouse strain that harbors the −17,000 to −15,500 base pair (bp) tissue-specific enhancer and −350 to +54 bp promoter of the mouse Col1a2 upstream sequence linked to an EGFP reporter gene (COL/EGFP)6 was used. The −17,000 to −15,500 bp Col1a2 sequence has strong enhancer activity that directs tissue-specific gene expression in adult mouse organs such as the liver6,27 and skin7, as well as in the embryonic development period.5 All animals received humane care and were maintained under specific pathogen-free conditions. The experiments were approved by the Animal Experiment Committee of Tokai University, and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Wounding and dermal graft transplantation
Mice were anesthetized and underwent full-thickness excisional wounding on their dorsum using a 6 mm biopsy punch (Kai Medical, Tokyo, Japan).7,8 Immediately after wounding, artificial dermal grafts were transplanted onto the wounds in the form of a film, sponge, or hybrid film/sponge. We then covered the wounds with a dressing tape and sutured the surrounding skin (Supplementary Fig. S1). This procedure prevented the local skin contraction, as well as preventing wound scratching by mice. Mice were housed individually in sterilized cages and given autoclaved food and sterilized water to prevent bacterial infection. Wound healing was estimated macroscopically by measuring the opened wound areas. To recognize the edge of regenerating epithelium easily, we poured mixed solution of 10% May-Grunwald and 10% Giemsa (Merck, Darmstadt, Germany) on the wounds.
Histological and immunohistological examination
Mice were sacrificed under anesthesia on day 6 or 21 after dermal graft transplantation. The obtained graft tissues were fixed with 10% buffered formalin for regular hematoxylin-eosin staining or with 4% paraformaldehyde for immunohistological examination. Immunohistochemical and immunofluorescent staining were performed as previously described28 with the specific primary antibodies listed in Supplementary Table S1, followed by incubation with appropriate horseradish peroxidase-conjugated or fluorescent secondary antibodies. After nuclear staining with 3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide or 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich), they were examined under a conventional light microscope or confocal laser-scanning microscopes (LSM 510META or LSM 880; Carl Zeiss, Oberkochen, Germany). The number of stained cells was counted in whole microscopic fields for each wound section, and the values per unit of area were calculated as previously described.29
Analysis of migration of collagen-producing cells into artificial dermal grafts
A full-thickness wound made on the dorsum of the transgenic COL/EGFP mice was transplanted with an artificial dermal graft composed of either a mixture of jellyfish and porcine collagens or porcine collagen alone as a control. The mice were sacrificed on day 6 after dermal graft transplantation. Obtained graft tissues were visualized under an Axio plan II upright fluorescent microscope (Carl Zeiss) for EGFP fluorescence. The specificity of EGFP signals was verified by the emission fingerprinting method that analyzed the fluorescent spectrum and detected specific EGFP fluorescence using an LSM 510META instrument.28
Statistical analysis
Values are expressed as means ± SD. Mann–Whitney U test was used to evaluate the significance of differences among the groups examined. p Values less than 0.05 were considered statistically significant.
Results
Jellyfish collagen exhibited water solubility and temperature sensitivity
The results of SDS PAGE revealed several bands for jellyfish collagen with approximate MWs of 150, 130, 80, and 45 kDa (Fig. 1A), as previously reported.16 When porcine type I collagen was separated into soluble and insoluble fractions, the majority (more than 99%) was present in the insoluble fraction (Fig. 1A). In contrast, jellyfish collagen was detected exclusively in the soluble fraction (Fig. 1A). All of the bands observed with the jellyfish collagen samples as well as those with the porcine collagen products almost disappeared after preincubation with bacterial collagenase at 22°C for 12 h (Fig. 1B). These results confirmed high purity of jellyfish and porcine collagens used in the present study. In addition, jellyfish collagen was resistant to pepsin digestion due to its triple helix structure (Fig. 1C, lanes 1 and 2), which is a characteristic feature of collagen molecule. Incubation at 37°C for several hours that denatured this triple helix structure resulted in susceptibility to pepsin digestion, indicating its instability at typical mammalian body temperature (Fig. 1C, lanes 3–5).
Jellyfish collagen accelerated fibril formation by porcine type I collagen in vitro
Next, we examined whether the presence of jellyfish collagen affected fibril formation by porcine type I collagen in vitro. Because of its water-soluble property under neutral pH conditions, jellyfish collagen itself did not form fibrils. When porcine type I collagen was mixed with jellyfish collagen in the different ratios shown in Fig. 1D, the presence of jellyfish collagen did not inhibit fibril formation by porcine collagen as estimated by the turbidity of the mixed solution. Instead, it accelerated fibril formation by porcine collagen compared with that of control samples containing the same amounts of porcine collagen alone (Fig. 1D). These findings were in marked contrast to the results of a previous study showing that the presence of type V collagen inhibited fibril formation by type I collagen in vitro30 and denied possible interference between jellyfish and porcine type I collagens before preparing a composite biomaterial made of these two different collagen molecules in the following experiments.
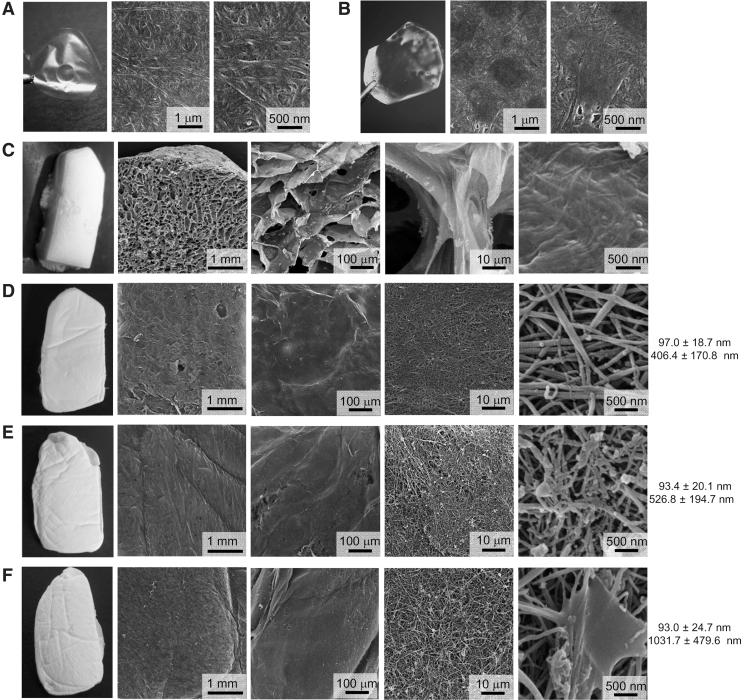
Jellyfish and porcine collagens-containing film and sponge exhibited distinct structural properties
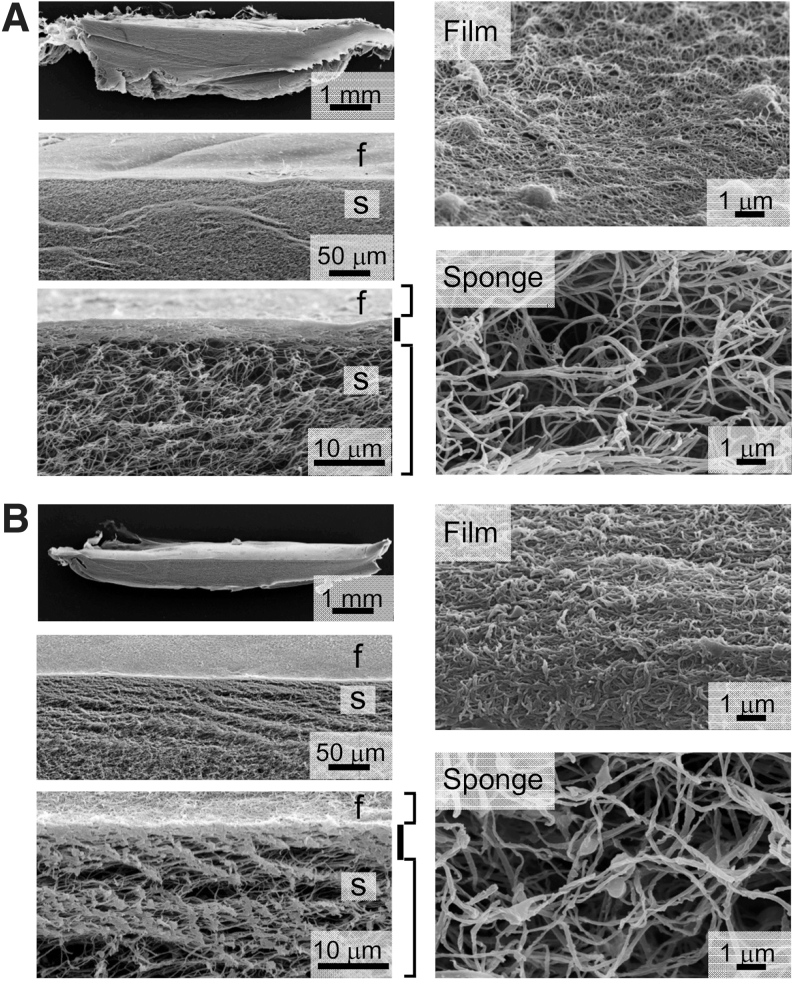
We next prepared a film or sponge graft that contained a mixture of jellyfish and porcine collagens, or porcine collagen alone as a control. Both types of collagen films prepared by evaporation at room temperature appeared highly transparent, and SEM examination revealed similarly smooth surfaces (Fig. 2A, B). We next compared the intrinsic microstructure of porcine collagen sponges prepared by either the conventional method or by a modified freeze-dry method using t-butyl alcohol. The porcine collagen sponges prepared by the conventional method showed a honeycomb structure, and possessed many porous chambers with a mean size of 218 ± 141 μm that had been formed by ice crystals (Fig. 2C). In contrast, porcine collagen sponges prepared by the modified method with t-butyl alcohol showed a fine three-dimensional structure of collagen fibrils, with a mean size of 97.0 ± 18.7 nm diameter (Fig. 2D). Notably, it retained intrinsic vacant spaces, with an approximate distance of 406.4 ± 170.8 nm between each collagen fibril (Fig. 2D). SEM examination also revealed that jellyfish collagen that had been mixed with porcine collagen showed a nonfibrillar structure and was adherent to intact porcine collagen fibrils within the sponge (Fig. 2E, F). The presence of jellyfish collagen did not affect the mean diameter of porcine collagen fibrils: 93.4 ± 20.1 nm in 20% jellyfish collagen-containing sponge, and 93.0 ± 24.7 nm in 50% jellyfish collagen-containing sponge. Rather, the mean distance between each porcine collagen fibril was significantly increased to 526.8 ± 194.7 and 1031.7 ± 479.6 nm by adding 20% (Fig. 2E) and 50% (Fig. 2F) jellyfish collagen, respectively.
Figure 2.
Microstructures of collagen films and sponges. Dermal films were made of porcine type I collagen alone (A) or a mixture (50% each) of porcine and jellyfish collagens (B) and evaporated at room temperature. Artificial dermal sponges were made of porcine type I collagen alone (C, D), or a mixture of porcine and jellyfish collagens (80:20 in E, and 50:50 each in F) using the conventional freeze-dry method with a water-based solvent (C) or a modified freeze-dry method with t-butyl alcohol as a solvent (D–F). The surface of the prepared films and the intrinsic microstructure of both types of sponge were examined by SEM. On the right of each sponge image are shown the mean diameters of collagen fibrils (upper) and the mean distance between each collagen fibril (lower). SEM, scanning electron microscopy; t-butyl alcohol, tert-butyl alcohol.
Composite biomaterial-containing film accelerated reepithelization after wounding
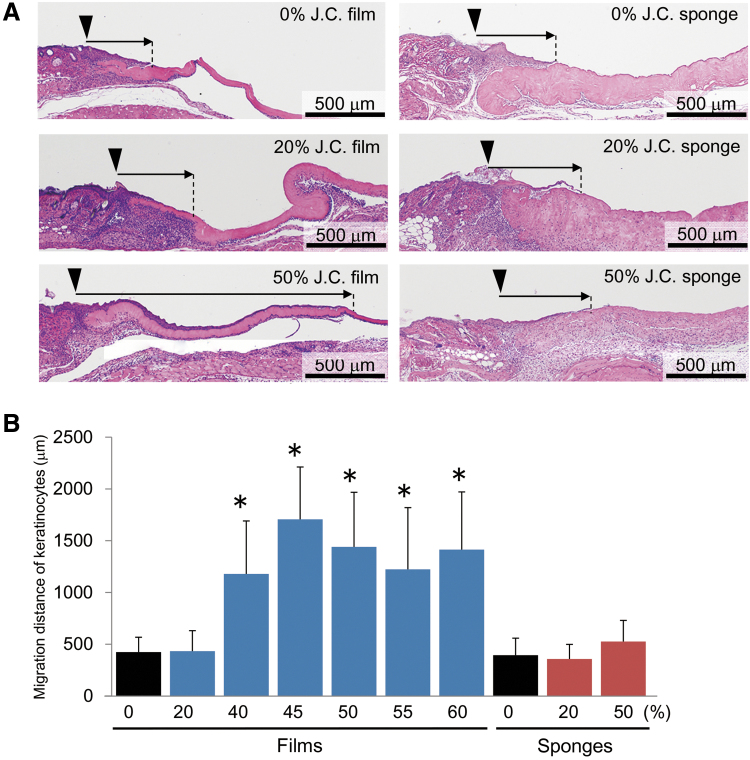
We next examined the effects of jellyfish collagen contained in the composite biomaterial films on the wound healing process in vivo. When the composite biomaterial films were transplanted onto the wounds, regenerating epithelial cells significantly extended toward the center of the wound, depending on the relative amounts of jellyfish collagen mixed with porcine collagen (Fig. 3A, B). A maximal effect was observed with as little as 45% jellyfish collagen content (Fig. 3B). On the contrary, transplantation of collagen sponges did not affect the reepithelization process, irrespective of the relative amounts of jellyfish collagen mixed with porcine collagen (Fig. 3A, B).
Figure 3.
Effects of composite biomaterial-containing films and sponges on reepithelization of epidermis. (A) Dermal specimens were obtained from mice on day 6 after transplantation of jellyfish and porcine collagens-containing films (left) or sponges prepared by the t-butyl alcohol freeze-dry method (right). The dermal grafts contained the indicated ratios of jellyfish collagen (J.C.) mixed with porcine type I collagen. They were subjected to H-E staining to estimate the extent of reepithelization of the epidermis (arrows). The wound margins are indicated by arrowheads. (B) The histograms represent means ± SD of three to four wounds in each group, which were transplanted with the composite biomaterial films (blue) or sponges (red) containing the indicated rations of jellyfish collagen (%). The asterisks indicate that the values were significantly higher (p < 0.01) than those in the control film containing porcine type I collagen alone. H-E, hematoxylin-eosin.
Composite biomaterial-containing sponge stimulated granulation tissue formation after wounding
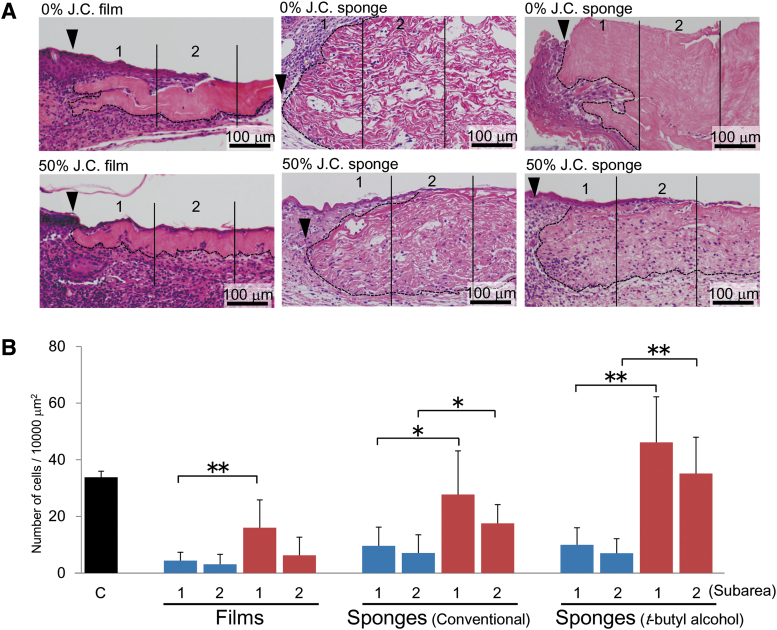
Transplantation of porcine collagen-containing film or sponge caused infiltration of only a small number of cells into the dermal graft compared with the number of cells present in unaffected normal dermis (Fig. 4). The presence of 50% jellyfish collagen in the film significantly increased the number of infiltrating cells compared with the control film containing porcine collagen alone (Fig. 4). However, the effect was modest and insufficient for entire replacement of the transplanted graft film by endogenous granulation tissue (Fig. 4A). On the contrary, transplantation of the composite biomaterial sponge made of 50% each of jellyfish and porcine collagens remarkably increased the number of cells infiltrating the dermal graft, and this effect was further enhanced using the composite biomaterial sponge prepared by the t-butyl alcohol freeze-dry method compared with that by the conventional technique (Fig. 4).
Figure 4.
Effects of composite biomaterial-containing film and sponge on granulation tissue formation. (A) Dermal specimens were obtained as described in the legend for Fig. 3, and subjected to H-E staining. The wound margins and the borders of transplanted dermal graft are indicated by arrowheads and hatched lines, respectively. The extent of cell infiltration into the transplanted dermal grafts was compared between collagen films (left), collagen sponges prepared by the conventional freeze-dry method (middle), and collagen sponges prepared by the t-butyl alcohol freeze-dry method (right). The graft sections were divided into two subareas, depending on the distance from the wound edge: area 1 from 0 to 200 μm, and area 2 from 200 to 400 μm. (B) The numbers of cells infiltrating into the indicated subareas were compared between the graft containing porcine type I collagen alone (blue) and that composed of 50:50 jellyfish and porcine collagens (red). The number of cells present in the control normal dermis (C) from untreated mice was also counted as a control (black). The histograms represent means ± SD of three to four wounds in each group. The asterisks indicate that the differences were statistically significant (*p < 0.05, **p < 0.01) between the groups.
Hybrid-type dermal graft made of the composite biomaterial accelerated wound healing
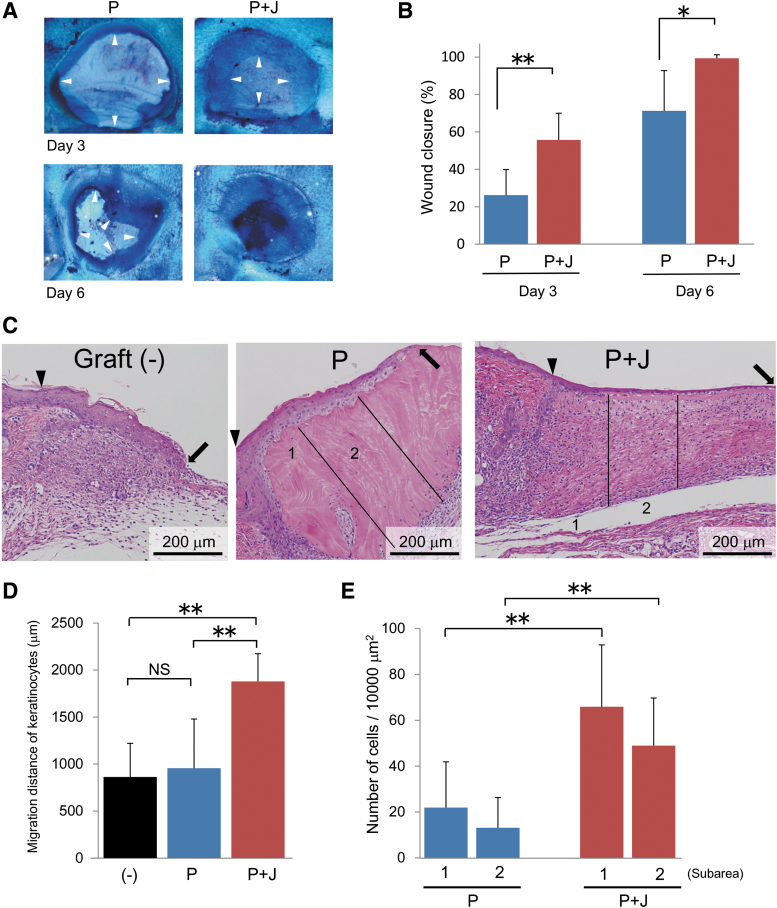
The above findings of accelerated reepithelization and granulation tissue formation using jellyfish and porcine collagens containing film and sponge, respectively, led us to design a hybrid-type dermal graft composed of a collagen film in the upper layer and a collagen sponge in the lower layer. Based on the results of the above mentioned experiments using the different ratios of jellyfish and porcine collagens, we determined the optimal mixing ratio (45% jellyfish collagen and 55% porcine collagen) for both obtaining maximal beneficial effects and maintaining graft integrity, and used this formulation in the following experiments. We prepared two different types of hybrid dermal grafts; one made of 45% jellyfish and 55% porcine collagen in both the upper layer film and the lower layer sponge (Fig. 5B) and the other containing only porcine type I collagen as a control (Fig. 5A). As described above, the presence of jellyfish collagen in the composite biomaterial sponge increased volumes of vacant spaces between each porcine collagen fibril, compared with the control sponge made of porcine collagen alone (Fig. 5A, B). Then, we compared the effects of these two types of hybrid dermal grafts on the wound healing process. The results indicated that transplantation of the composite biomaterial graft significantly accelerated wound closure, as estimated by measuring the opened wound areas, compared with that of the control graft made of porcine collagen alone on both day 3 and 6 after wounding (Fig. 6A, B). These stimulatory effects of the composite biomaterial on wound healing were further confirmed by histological examination. Transplantation of the composite biomaterial graft significantly accelerated keratinocyte migration in the epidermis compared with that of the graft containing porcine collagen alone and the control without graft implantation (Fig. 6C, D) on day 6 after wounding. The dermal graft made of the composite biomaterial also increased the number of infiltrating cells into the sponge (Fig. 6C, E) on day 6 after transplantation.
Figure 5.
Microstructure of hybrid-type dermal grafts. Approximately a 5-μm-thick collagen film (f) was adhered to collagen sponge (S) prepared by the t-butyl alcohol freeze-dry method. Both the collagen film and the sponge were made of either porcine type I collagen alone (A) or a mixture of 55% porcine collagen and 45% jellyfish collagen (B). The surfaces of films and the intrinsic microstructure of sponge composition were examined by SEM.
Figure 6.
Effects of a hybrid-type dermal graft on wound healing. (A) Representative pictures of the wounds on day 3 and 6 after transplantation of hybrid-type dermal grafts made of either porcine type I collagen alone (P) or a mixture of 55% porcine collagen and 45% jellyfish collagen (P + J). May-Grunwald/Giemsa solution was poured on the wounds to recognize the edge of regenerating epithelium (arrowheads). Note that the wound was already closed by day 6 after transplantation of graft made of the composite biomaterial. (B) The wound closure rate was compared between the two groups by measuring the opened wound areas on day 3 and 6 after graft transplantation. (C) Dermal specimens were obtained on day 6 after transplantation of the two different types of hybrid dermal grafts and subjected to H-E staining. Wound healing without graft transplantation (−) was also evaluated as a control. The graft sections were divided into two subareas (1 and 2) as explained in the legend for Figure 4. The wound margins and the frontlines of regenerating epithelium are indicated by arrowheads and arrows, respectively. The extent of epithelial regeneration (D) and the numbers of cells infiltrating into the indicated subareas of dermal graft (E) were compared between the two groups. The histograms represent means ± SD of nine wounds in each group. The asterisks indicate that the differences were statistically significant (*p < 0.05, **p < 0.01) between the groups. NS, nonsignificant.
Histological characterization of regenerating epithelium and granulation tissue induced by transplantation of the composite biomaterial dermal graft
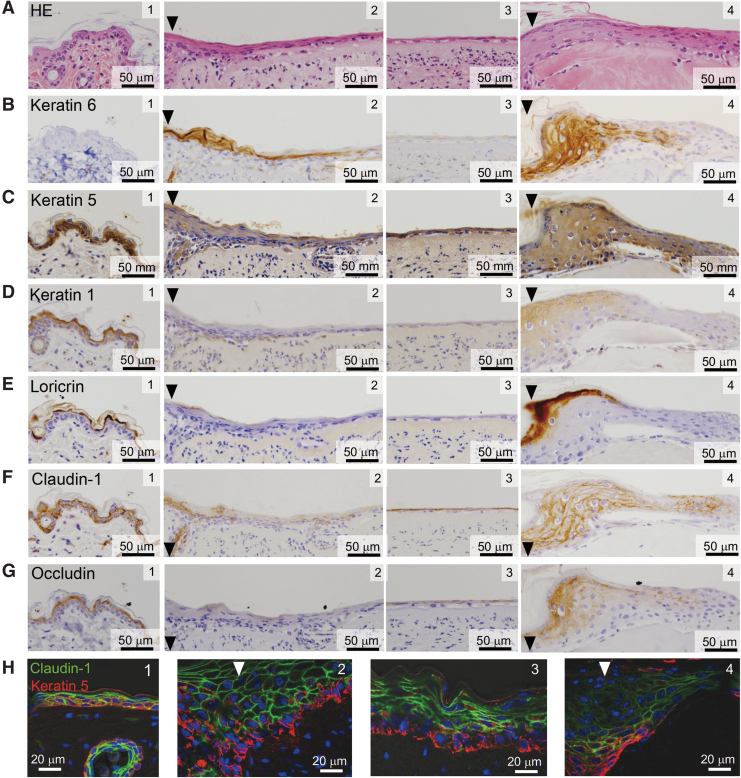
It is well known that the epidermis near the wound edge exhibits characteristic thickening of the keratinocyte layer, termed an epidermal tongue, after full-thickness dermal wounding.31,32 A typical epidermal tongue stained positively for keratin 6 (referred to as stress keratin31) was observed after transplantation of a dermal graft containing porcine collagen alone (Fig. 7A, B, panel 4). In contrast, the epidermal tongue was rather thin (Fig. 7A, B, panel 2), and extensive migration of keratinocytes toward the wound center was observed after transplantation of a hybrid-type dermal graft composed of jellyfish and porcine collagens (Fig. 7A, panel 3). The regenerating epithelium induced by the composite biomaterial essentially consisted of two distinct cell populations. Cells in the lower layer stained positively for keratin 5, a representative marker of basal-layer keratinocytes (Fig. 7C, panels 2 and 3), as observed in normal skin epidermis (Fig. 7C, panel 1). However, none of the cells in either layer stained positively for keratin 1 (Fig. 7D, panels 2 and 3) or loricrin (Fig. 7E, panels 2 and 3), which are markers of prickle and granular cells, respectively. Notably, the upper layer cells expressed tight junction proteins such as claudin-1 (Fig. 7F, panels 2 and 3) and occludin (Fig. 7G, panels 2 and 3). Immunofluorescent double-staining clearly indicated the two-layered regenerating epithelium consisting of claudin-1-expressing upper layer cells and keratin 5-positive basal layer keratinocytes (Fig. 7H, panels 2 and 3).
Figure 7.
Histological characterization of regenerating epithelium induced by the composite biomaterial-containing hybrid-type dermal graft. Dermal specimens transplanted with hybrid-type dermal grafts made of the composite biomaterial were obtained as described in the legend for Fig. 6. They were subjected to either H-E staining (A) or immunohistochemical or immunofluorescent staining with antibodies that recognize the indicated keratinocyte markers and tight junction proteins (B–H). Representative pictures of the proximal site close to the wound edge (panel 2) and the frontline of regenerating epithelium (panel 3), as well as those of normal skin (panel 1) and the wound transplanted with porcine collagen film/sponge (panel 4) as controls, are shown. Nuclei were stained using 4′,6-diamidino-2-phenylindole dihydrochloride (blue) in panels (H). The wound margins are indicated by arrowheads.
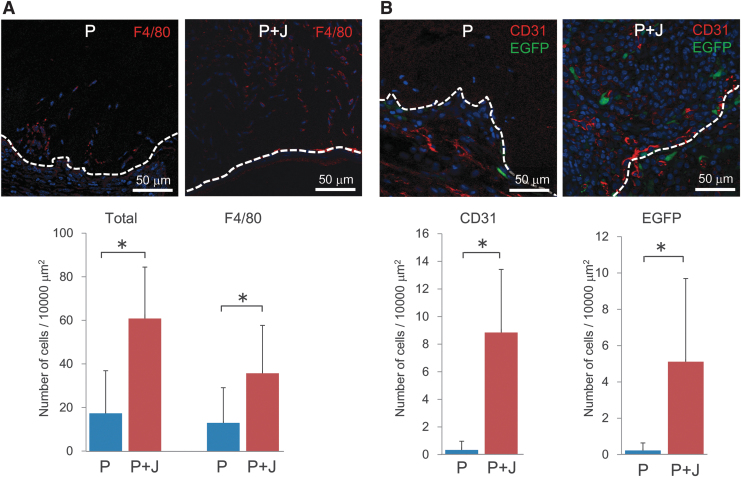
On the contrary, immunofluorescent staining of excised dermal grafts indicated that the numbers of F4/80-positive macrophages (Fig. 8A) and CD31-positive endothelial cells or their progenitors (Fig. 8B) were significantly increased after transplantation of a composite biomaterial graft, compared with that using a control graft containing porcine collagen alone. Furthermore, transplantation experiments using COL/EGFP reporter mice as recipients revealed a significant increase in the number of EGFP-positive collagen-producing cells migrating into the composite biomaterial graft, compared with that into the graft containing porcine type I collagen alone (Fig. 8B).
Figure 8.
Effects of the composite biomaterial-containing hybrid-type dermal graft on cell infiltration. The total numbers of infiltrating cells, and those of F4/80-positive macrophages (A), CD31-positive endothelial cells or their progenitors, and EGFP-positive collagen-producing cells observed in the grafts transplanted into COL/EGFP mice (B) were compared between the two types of hybrid dermal grafts made of porcine collagen alone (P) or a mixture of 55% porcine collagen and 45% jellyfish collagen (P + J). The hatched lines indicate the border of transplanted dermal grafts, which is located in the upper side in each picture). Nuclei were stained using 3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide (blue). Below the representative images are the histograms representing means ± SD of four to five grafts in each group. The asterisks indicate that the differences were statistically significant (p < 0.01) between the groups. EGFP, enhanced green fluorescent protein.
Stimulatory effects of jellyfish collagen on cell infiltration and wound closure were not replicated by using denatured porcine gelatin
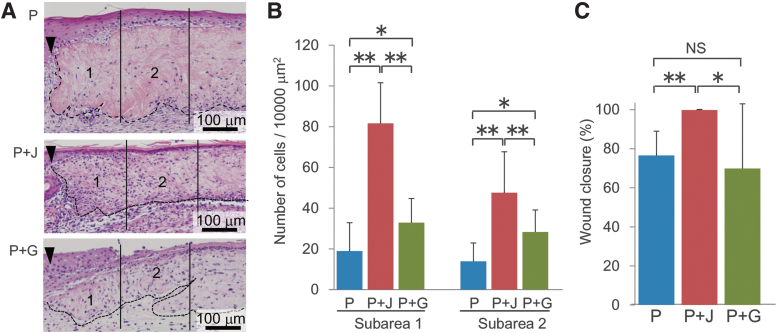
All the above experiments using the jellyfish and porcine collagens-containing graft were performed by setting the total amount of collagen to be the same in the composite biomaterial and the control biomaterial made of porcine collagen alone. Therefore, the effect of composite biomaterial on cell infiltration might simply attribute to lower concentration of porcine collagen and larger intrinsic vacant spaces within the graft after degradation of jellyfish collagen. To deny such possibility, we used another type of composite biomaterial made of porcine type I collagen and water-soluble denatured gelatin, and compared the effects by matching the concentration of intact porcine collagen in the two types of composite biomaterials. The results indicated that, although the graft made of porcine collagen and gelatin stimulated cell infiltration compared with that containing porcine collagen alone, it was not as effective as the composite biomaterial made of jellyfish and porcine collagens (Fig. 9A, B). Furthermore, the stimulatory effect of jellyfish and porcine collagens-containing graft on wound closure was not replicated with a graft made of porcine collagen and denatured gelatin (Fig. 9C).
Figure 9.
Effects of jellyfish collagen and denatured porcine gelatin on cell infiltration into the dermal graft. (A) Two different types of composite biomaterial grafts made of either a mixture of 55% porcine and 45% jellyfish collagens (P + J) or that of 55% porcine collagen and 45% denatured gelatin (P + G), as well as the control graft containing porcine collagen alone (P), were transplanted into the wound. The total amount of collagenous proteins was set to be the same among the groups. Dermal specimens were obtained on day 6 after graft transplantation and subjected to H-E staining. The graft sections were divided into two subareas (1 and 2) as explained in the legend for Figure 4. The wound margins and the borders of transplanted dermal graft are indicated by arrowheads and hatched lines, respectively. The numbers of cells infiltrating into the indicated subareas of dermal graft (B) and the macroscopic wound closure rates (C) measured on day 6 after transplantation were compared among the groups. The histograms represent means ± SD of four to six wounds in each group. The asterisks indicate that the differences were statistically significant (*p < 0.05, **p < 0.01) between the groups.
Transplanted composite biomaterial was replaced by endogenous tissue without excessive scar formation
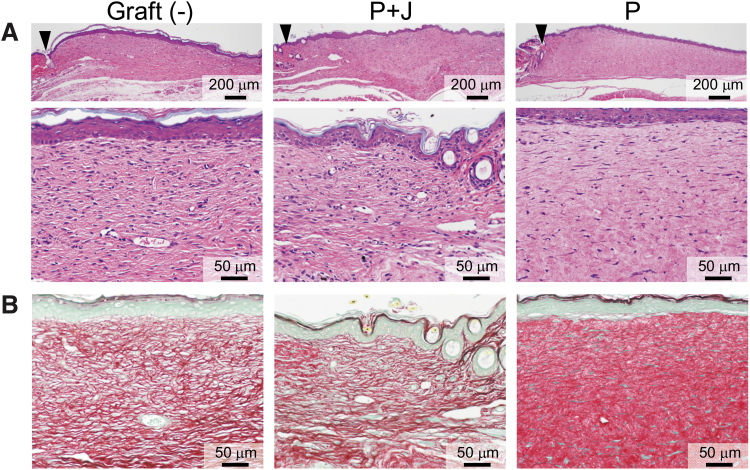
In the last set of experiments, we examined the long-term effect of transplantation of the hybrid-type dermal graft composed of jellyfish and porcine collagens on dermal tissue remodeling. Transplantation of this composite biomaterial graft, as well as that of the control graft made of porcine collagen alone, caused complete wound closure by day 21 after wounding (Fig. 10A). Compared with the wound scar observed without graft implantation, transplantation of the composite biomaterial graft caused no excessive dermal thickness (Fig. 10A). In addition, the transplanted composite biomaterial graft was replaced by endogenous tissue, which exhibited the same fine network of Sirius red-stained collagen fibrils as the wound scar observed without graft implantation (Fig. 10B). In contrast to these findings, the transplanted dermal graft made of porcine collagen alone still remained in the dermis (Fig. 10A) showing dense collagen fibers that was strongly stained with Sirius red (Fig. 10B).
Figure 10.
Long-term effects of the composite biomaterial on dermal tissue remodeling. Dermal specimens were obtained on day 21 after transplantation of dermal grafts made of either porcine type I collagen alone (P) or a mixture of 55% porcine collagen and 45% jellyfish collagen (P + J). Wound scar tissue obtained without graft transplantation was also included as a control. They were subjected to H-E (A) and Sirius red staining (B) to evaluate dermal thickening and accumulation of collagen fibrils, respectively. The wound margins are indicated by arrowheads. Representative pictures are shown from eight wounds in each group.
Discussion
In the present study, we demonstrated unique properties and possible clinical applications of invertebrate collagen derived from moon jellyfish. Jellyfish collagen possesses high water solubility (Fig. 1A) and temperature sensitivity (Fig. 1C), which makes it difficult to use jellyfish collagen alone as a dermal graft material transplanted into the body. Indeed, unlike films made of porcine type I collagen, films composed of jellyfish collagen were rapidly dissolved in water (Supplementary Video S2). We therefore needed to mix jellyfish collagen with porcine type I collagen, which is widely used as an artificial dermal graft in humans, and determined an optimal mixing ratio (45% jellyfish collagen and 55% porcine collagen) in the composite biomaterial for obtaining maximal beneficial effects and for maintaining graft integrity.
Films made of the composite biomaterial exhibited rather smooth surfaces (Fig. 2B) and stimulated migration of keratinocytes compared with the control film made of porcine collagen alone (Fig. 3). On the contrary, the intrinsic vacant spaces observed in porcine collagen sponge prepared using t-butyl alcohol were enlarged by addition of water-soluble jellyfish collagen, possibly allowing infiltration of inflammatory cells and fibroblasts into the graft (Fig. 4). Based on these distinct structural and functional properties of films and sponges made of the composite biomaterial, we propose a hybrid-type dermal graft, composed of the upper layer film and the lower layer sponge that accelerates reepithelization of the epidermis and granulation tissue formation in the dermis, simultaneously (Figs. 5 and 6). Sponges prepared by the t-butyl alcohol method adhered to the wound and was penetrated by water shortly after transplantation (Supplementary Video S1), which is a desirable property as an artificial dermis.12
There are several possible mechanisms by which collagen sponges made of the composite biomaterial stimulate cell infiltration and accelerate granulation tissue formation. One of them is the increased vacant space within the transplanted graft as described above. When mixed with porcine type I collagen, jellyfish collagen did not form fibrillar structures, but was attached to the porcine collagen fibrils within the sponge (Figs. 2 and 5). Such jellyfish collagen was easily dissolved, and only the porcine collagen fibrils remained after being immersed in water (Supplementary Fig. S2). This caused a significant increase in the mean distance between each collagen fibril from 1031.7 ± 479.6 to 2823.0 ± 1032.0 nm when mixing 50% each of jellyfish and porcine collagens in the sponge (Supplementary Fig. S2). In addition, the temperature sensitivity of jellyfish collagen, together with the fact that the collagen sponge used was not crosslinked, suggests that it is immediately denatured at mammalian body temperatures and subsequently degraded by proteases. Because of these unique properties of jellyfish collagen, the intrinsic voids in jellyfish collagen-containing sponge were further enlarged after transplantation. Elasticity and elongation potential of collagen fibrils, together with flexibility in cell morphology, may support infiltration of inflammatory cells and fibroblasts into the graft (Fig. 8).
On the contrary, another possible mechanism is that a short peptide sequence within jellyfish collagen or certain molecule(s) coupled to jellyfish collagen may exert stimulatory effects on cell infiltration. Experiments substituting jellyfish collagen with denatured porcine gelatin clearly indicated that, although degradation of water-soluble gelatin after transplantation increased the size of vacant spaces within the sponge, a mixture of porcine collagen and gelatin was less effective on cell infiltration compared with the composite biomaterial made of jellyfish and porcine collagens (Fig. 9). From these points of view, it is important to examine the immunogenicity of jellyfish collagen. The triple helix structure of mammalian collagens is considered less immunogenic, allowing their application as a medical device. Although jellyfish collagen exhibits a similar triple helix structure, its immunogenicity to humans has not been clarified yet. Further study is needed to determine whether increased infiltration of F4/80-positive macrophages reflects enhanced granulation tissue formation or represents an immune reaction against the implanted jellyfish collagen.
The precise mechanisms, by which films made of the composite biomaterial accelerate reepithelization, are currently unknown. Despite similarly smooth surface structures, transplantation of films composed of porcine collagen alone was not as effective for reepithelization as that of films made of the composite biomaterial (Fig. 3). This is also the case with currently available porcine or bovine collagen-containing grafts used for wound therapy in humans, which require subsequent epidermal transplantation.15,33 Furthermore, experiments using a hybrid-type dermal graft made of porcine collagen and denatured gelatin clearly indicated that it did not exhibit any stimulatory effect on wound closure (Fig. 9C). From these findings, it is plausible to propose that the effects of jellyfish collagen-containing film are not attributable to its intact structure, but are instead exerted by intrinsic functional peptide sequence(s). Alternatively, as already discussed before for the composite material sponge, there may be certain molecules coupled to jellyfish collagen that can easily be released after being dissolved in the mammalian body. Work is in progress in our laboratory to explore the underlying mechanisms involved in, and to determine the functional molecules responsible for, the beneficial effects of jellyfish collagen in the wound healing process. It is also needed to examine the receptor, if present, and the intracellular signaling pathway that mediate the effects of such functional molecules.
From a mechanistic viewpoint, it should be noted that the upper layer of regenerating epithelium stimulated by transplantation of the composite biomaterial grafts expressed tight junction proteins such as claudin-1 and occludin (Fig. 7). Claudin-1 is known to be a critical molecule that is indispensable to maintain the epidermal barrier.34 Furthermore, recent studies have shown that claudin-1 and occludin also play important roles in the wound healing process. For example, expression of claudin-1 is reduced in cutaneous tissues of patients with impaired wound healing.35 In addition, knockdown of these proteins suppresses cell migration.35,36 The presence of claudin-1 and occludin in the regenerating epithelium may indicate their stimulatory effects on keratinocyte migration in addition to their original function as tight junction proteins. It also suggests that prompt wound healing achieved by transplantation of the composite biomaterial graft might be mediated, at least in part, by these molecules.
Jellyfish collagen has a number of advantages over vertebrate collagens derived from porcine and bovine tissues. First, like other species of jellyfish, moon jellyfish (Aurelia species) are widely distributed in the world's oceans. Increase in jellyfish populations has recently become a serious environmental issue.37–39 Jellyfish comprised more than 40% collagenous proteins by dry weight, and can be a good source of biomaterial.18 Second, extensive migration of keratinocytes on surface of artificial dermis, as shown in the present study, has not been observed before using any type of collagen-containing graft. One may argue that early wound closure caused by the composite biomaterial induces excessive scaring and decreases the quality of healing. However, the results of the present study have clearly shown that the novel composite biomaterial accelerates wound healing without excessive scar formation (Fig. 10). If the stimulatory effects can be confirmed in a larger size of wound or under a pathological condition that delays the physiological wound healing process, it may eventually be applicable to humans. Finally, the use of jellyfish collagen may avoid pathogens, such as prions, which cause bovine spongiform encephalopathy. Overall, we propose this newly developed composite biomaterial as a novel therapeutic for regenerative medicine in the fields of dermatology and plastic surgery. Immunogenicity of jellyfish collagen in humans, as well as other possible side effects caused by jellyfish-derived proteins, needs to be carefully examined before its clinical use.
Innovation
Impaired dermal wound healing has become a major medical issue nowadays. Although a number of artificial dermal grafts composed of porcine or bovine collagen are used for treatment of intractable skin ulcers in humans, they do not induce reepithelization, requiring subsequent epithelial transplantation. We propose a novel composite biomaterial made of moon jellyfish collagen and porcine type I collagen, which remarkably accelerates dermal wound healing without excessive scar formation. It stimulates not only granulation tissue formation in the dermis but also reepithelization in the epidermis. This biomaterial may serve as a novel and less invasive therapeutic for intractable skin ulcers.
Key Findings
A novel dermal graft composed of jellyfish and porcine collagens has been developed that remarkably accelerates the wound healing process without excessive scar formation.
The graft composes of two distinct layers: the upper layer film made of the composite biomaterial and the lower layer sponge containing the same biomaterial.
The jellyfish and porcine collagens-containing film stimulated migration of keratinocytes, leading to prompt reepithelization.
The jellyfish and porcine collagens-containing sponge possessed a significantly enlarged intrinsic space and enhanced infiltration of inflammatory cells and fibroblasts, accelerating granulation tissue formation.
Supplementary Material
Acknowledgments and Funding Sources
We are indebted to Dr. Toshiyuki Ikoma of the Tokyo Institute of Technology, Dr. Hironobu Hojo of Osaka University, and Drs. Takayuki Baba and Koji Kihira at Jellyfish Research Laboratories, Inc. for valuable discussions. We also thank Masayoshi Tokunaga, Junko Kato, and other staff of the Support Center for Medical Research and Education at Tokai University School of Medicine for their skillful assistance.
This work was supported, in part, by Grants-in-Aid for Scientific Research (Nos. 15K10071 and 18K08603 to H.S.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, a MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2015–2019) to Tokai University, research grants to H.S. from the Cosmetology Research Foundation, Tokai University School of Medicine Research Aid (2014 to 2017, and 2019), and Tokai University Research Project (2018 to 2020).
Abbreviations and Acronyms
- bp
base pair
- EGFP
enhanced green fluorescent protein
- H-E
hematoxylin-eosin
- PBS
phosphate-buffered saline
- SDS PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM
scanning electron microscopy
- t-butyl alcohol
tert-butyl alcohol
Author Disclosure and Ghostwriting
A part of this study was performed in collaboration with Jellyfish Research Laboratories, Inc., who has taken out a patent (EP2889305B1) for extraction of jellyfish collagen. No other author has a conflict of internet. No ghostwriters were used to prepare this article.
About the Authors
Hideaki Sumiyoshi, PhD, is Senior Assistant Professor at the Center for Matrix Biology and Medicine, Graduate School of Medicine, Tokai University, Japan. He also belongs to the Department of Innovative Medical Science, Tokai University School of Medicine. His major research field is matrix biology, and he is currently focusing on novel biomaterials. Sachie Nakao is an experimental technician working with Dr. Sumiyoshi. Hitoshi Endo, PhD, is Senior Assistant Professor at the Department of Preventive Medicine, Tokai University School of Medicine. Takayo Yanagawa, PhD, is Assistant Professor at the Center for Matrix Biology and Medicine, Graduate School of Medicine, and the Department of Innovative Medical Science, School of Medicine, Tokai University. Yasuhiro Nakano, PhD, is a former postdoctoral fellow at the same institute. Yosuke Okamura, PhD, is Associate Professor at the Course of Industrial Chemistry, Graduate School of Engineering, Tokai University. Akira T. Kawaguchi, MD, PhD, is Adjunct Professor in Tokai University School of Medicine. Yutaka Inagaki, MD, PhD, is the director of the Center for Matrix Biology and Medicine, Graduate School of Medicine, Tokai University, and Professor and Chairman at the Department of Innovative Medical Science, Tokai University School of Medicine. He is the principal investigator of our research group.
Supplementary Material
REFERENCES
- 1. Wicke C, Bachinger A, Coerper S, et al. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Repair Regen 2009;17:25–33 [DOI] [PubMed] [Google Scholar]
- 2. Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 2004;187:65S–70S [DOI] [PubMed] [Google Scholar]
- 3. Martin P. Wound healing-aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 4. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:14–321 [DOI] [PubMed] [Google Scholar]
- 5. Bou-Gharios G, Garrett LA, Rossert J, et al. A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol 1996;134:1333–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higashiyama R, Moro T, Nakao S K. et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 2009;137:1459–1466 [DOI] [PubMed] [Google Scholar]
- 7. Higashiyama R, Nakao S, Shibusawa Y, et al. Differential Contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in mice. J Invest Dermatol 2011;131:529–536 [DOI] [PubMed] [Google Scholar]
- 8. Yamaoka H, Sumiyoshi H, Higashi K, et al. A novel small compound accelerates dermal wound healing by modifying infiltration, proliferation and migration of distinct cellular components in mice. J Dermatol Sci 2014;74:204–213 [DOI] [PubMed] [Google Scholar]
- 9. Sephel GC, Davidson JM. Repair, regeneration and fibrosis. In: Rubin R, Strayer DS, Rubin E, eds. Rubin's Pathology, Clinicopathologic Foundations of Medicine, 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2012:83–114 [Google Scholar]
- 10. Gordon MK, Hahn RA. Collagens. Cell Tissue Res 2010;339:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorushanova A, Delgado LM, Wu Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater 2019;31:e1801651. [DOI] [PubMed] [Google Scholar]
- 12. Yannas IV, Burke JF. Design of an artificial skin. Part I. Basic design principles. J Biomed Mater Res 1980;14:65–81 [DOI] [PubMed] [Google Scholar]
- 13. Koide M, Osaki K, Konishi J, et al. A new type of biomaterial for artificial skin: dehydrothermally cross-linked composites of fibrillar and denatured collagens. J Biomed Mater Res 1993;27:79–87 [DOI] [PubMed] [Google Scholar]
- 14. Schulz III JT, Tompkins RG, Burke JF. Artificial skin. Ann Rev Med 2000;51:231–244 [DOI] [PubMed] [Google Scholar]
- 15. Burke JF, Yannas IV, Quinby WC, Bondoc CC. Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981;194:413–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miki A, Inaba S, Baba T, et al. Structural and physical properties of collagen extracted from moon jellyfish under neutral pH conditions. Biosci Biotechnol Biochem 2015;79:1603–1607 [DOI] [PubMed] [Google Scholar]
- 17. Hoyer B, Bernhardt A, Lode A, et al. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater 2014;10:883–892 [DOI] [PubMed] [Google Scholar]
- 18. Subhan F, Ikram M, Shehzad A, et al. Marine collagen: an emerging player in biomedical applications. J Food Sci Technol 2015;52:4703–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Widdowson PJ, Picton JA, Vinc V, et al. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J Biomed Mater Res B Appl Biomater 2018;106B:1524–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morishige H, Sugahara T, Nishimoto S, et al. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology 2011;63:481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Putra AB, Nishi K, Shiraishi R, Doi M, Sugahara T. Jellyfish collagen stimulates production of TNF-α and IL-6 by J774.1cells through activation of NF-κB and JNK via TLR4 signaling pathway. Mol Immunol 2014;58:32–37 [DOI] [PubMed] [Google Scholar]
- 22. Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol 1982;82:33–64 [DOI] [PubMed] [Google Scholar]
- 23. Schmidt MM, Dornelles RCP, Mello RO, et al. Demiate, Collagen extraction process. Int Food Res J 2016;23:913–912 [Google Scholar]
- 24. Silver FH, Birk DE. Kinetic analysis of collagen fibrillogenesis: I. use of turbidity-time data. Coll Relat Res 1983;3:393–405 [DOI] [PubMed] [Google Scholar]
- 25. Zhu J, Kaufman LJ. Collagen I self-assembly: revealing the developing structures that generate turbidity. Biophys J 2014;106:1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV. Design of an artificial skin. Part III. Control of pore structure. J Biomed Mater Res 1980;14:511–528 [DOI] [PubMed] [Google Scholar]
- 27. Inagaki Y, Truter S, Bou-Gharios G, et al. Activation of proα2(I) collagen promoter during hepatic fibrogenesis in transgenic mice. Biochem Biophys Res Commun 1998;250:606–611 [DOI] [PubMed] [Google Scholar]
- 28. Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology 2007;45:213–222 [DOI] [PubMed] [Google Scholar]
- 29. Yanagawa T, Sumiyoshi H, Higashi K, et al. Identification of a novel bone marrow cell-derived accelerator of fibrotic liver regeneration through mobilization of hepatic progenitor cells in mice. Stem Cells 2019;37:89–101 [DOI] [PubMed] [Google Scholar]
- 30. Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001;32:223–237 [DOI] [PubMed] [Google Scholar]
- 31. Paladini RD, Takahashi K, Bravo NS, Coulombe PA, Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol 1996;132:381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol 2003;121:219–230 [DOI] [PubMed] [Google Scholar]
- 33. Akita S, Tanaka K, Hirano A. Lower extremity reconstruction after necrotizing fasciitis and necrotic skin lesions using a porcine-derived skin substitute. J Plast Reconstr Aesthet Surg 2006;59:759–763 [DOI] [PubMed] [Google Scholar]
- 34. Sugawara T, Iwamoto N, Akashi M, et al. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J Dermatol Sci 2013;70:12–18 [DOI] [PubMed] [Google Scholar]
- 35. Volksdorf T, Heilmann J, Eming SA, et al. Tight junction proteins claudin-1 and occludin are important for cutaneous wound healing. Am J Pathol 2017;187:1301–1312 [DOI] [PubMed] [Google Scholar]
- 36. Du D, Xu F, Yu L, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell 2010;18:52–63 [DOI] [PubMed] [Google Scholar]
- 37. Uye S. Blooms of the giant jellyfish Nemopilema nomurai: a threat to the fisheries sustainability of the East Asian Marginal Seas. Plankton Benthos Res 2008;3:125–131 [Google Scholar]
- 38. Honda N, Watanabe T, Matsushita Y. Swimming depths of the giant jellyfish Nemopilema nomurai investigated using pop-up archival transmitting tags and ultrasonic pingers. Fish Sci 2009;75:947–956 [Google Scholar]
- 39. Malej A, Kogovsek T, Ramsak A, et al. Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cah Biol Mar 2012;53:337–342 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.