Abstract
Because stress tolerance and longevity are mechanistically and phenotypically linked, the sex with higher acute stress tolerance might be expected to also live longer. On the other hand, the association between stress tolerance and lifespan may be complicated by tradeoffs between acute tolerance and long-term survival. Here we use the copepod Tigriopus californicus to test for sex differences in stress resistance, proteolytic activity and longevity. Unlike many model organisms, this species does not have sex chromosomes. However, substantial sex differences were still observed. Females were found to have superior tolerance to a range of acute stressors (high temperature, high salinity, low salinity, copper and bisphenol A (BPA)) across a variety of treatments including different populations, pure vs. hybrid crosses, and different shading environments. Upregulation of proteolytic capacity — one molecular mechanism for responding to acute stress — was also found to be sexually dimorphic. In the combined stress treatment of chronic copper exposure followed by acute heat exposure, proteolytic capacity was suppressed for males. Females, however, maintained a robust proteolytic stress response. While females consistently showed greater tolerance to short-term stress, lifespan was largely equivalent between the two sexes under both benign conditions and mild thermal stress. Our findings indicate that short-term stress tolerance does not predict long-term survival under relatively mild conditions.
Keywords: Aging, Environmental stress, Longevity, Marine, Pollution, Proteolytic activity
1. Introduction
Sex differences were once thought to be important mainly for hormonal and reproductive issues, but we are beginning to learn that sex is an important variable for a range of biological functions including cell physiology, metabolism and gene expression (Mank, 2017; Niveditha et al., 2017; Tower, 2017; Clayton, 2018). In humans, poor understanding of sex differences stems in part from the long-standing practice of excluding women from clinical trials, often in the name of their own protection (Nowogrodzki, 2017). The unintended consequences of this practice are now more understood, leading the U.S. National Institutes of Health (NIH) to develop guidelines for including women in clinical research (e.g. NIH, 1994). Beyond humans, studies across taxa often either fail to mention the sex of tested subjects, combine data for different sexes, or include only one sex. Often, females are disproportionately excluded to avoid complexity associated with female reproduction. In other cases, males are excluded because reproductive effort may be easier to quantify in females than in males.
Among the most widespread sex differences is the tendency for females to live longer than males — a pattern found in many species of mammals, reptiles, amphibians, fishes, arthropods and annelids (but generally not birds, perhaps due to ZW sex determination; Gopalakrishnan et al., 2013). However, sex differences in stress response have not received as much attention. One might expect the sex with longer lifespan to also have higher stress tolerance, since many studies have shown a positive association between lifespan and stress resistance. For example, classic Drosophila studies show that selection for postponed senescence increases tolerance to a range of stressors (desiccation, starvation, ethanol, high temperature; Service et al., 1985), while selection for increased stress tolerance (desiccation, starvation) also increases lifespan (Rose et al., 1992). Further, extended lifespan has been specifically associated with antioxidant capacity in Drosophila (Svensson and Larsson, 2007; Niveditha et al., 2017), Caenorhabditis elegans (Murakami and Johnson, 1996), yeast (Kennedy et al., 1995), mice (Migliaccio et al., 1999) and Arabidopsis (Kurepa et al., 1998). Nevertheless, the association between long life and high stress tolerance is not necessarily universal: strong evidence for the pattern is restricted to a small number of model organisms, and the relationship between oxidative stress and aging has been shown to be far more complicated than was once believed (Salmon et al., 2010; Speakman and Selman, 2011; Lucas et al., 2017). In some cases, selection for increased stress tolerance has failed to increase lifespan (e.g. Harshman and Schmid, 1998; Harshman et al., 1999), and vice versa (e.g. Force et al., 1995; Zwann et al., 1995), possibly due to tradeoffs between short-term stress tolerance and long-term survival. Such tradeoffs can either be genetic, where the same allele has a positive effect on one trait and a negative effect on another (e.g. Blows and Hoffmann, 2005), or physiological, where traits compete for the same energetic resources (e.g. Sokolova, 2013). Because tradeoff architectures may be species or population specific, understanding sex differences in both lifespan and stress tolerance will require studies across diverse taxa, including those with alternative sex-determination systems.
Here we assess sex-specific stress tolerance, stress response and lifespan in Tigriopus californicus, a novel invertebrate model for aging. T. californicus is a marine microcrustacean, specifically a copepod, which inhabits high intertidal rock pools on the west coast of North America. This species has many virtues for experimental work, including short generation time (3–4 weeks), ease of culture, amenability to controlled crosses and abundant genomic and transcriptomic resources (Raisuddin et al., 2007; Barreto et al., 2018). While T. californicus is becoming established as a model for understanding the effects of mitochondria and mitonuclear interactions (Rawson and Burton, 2002; Ellison and Burton, 2008; Barreto et al., 2018), it has not been used for the study of aging. It is a particularly interesting alternative model for understanding sex differences because it does not have sex chromosomes. Instead, sex determination is polygenic with a small environmental component (Ar-Rushdi, 1963; Voordouw and Anholt, 2002a, 2002b; Alexander et al., 2014; Alexander et al., 2015). The absence of sex chromosomes in T. californicus eliminates one of the major mechanisms of sex differences, wherein males in an XY system (or females in a ZW system) suffer from unprotected sex chromosomes (Maklakov and Lummaa, 2013). Further, sexual antagonism in this species cannot be adjusted by moving genes on or off the sex chromosomes, as has been reported in other plants and animals (Spigler et al., 2011; Dean et al., 2014; Hill, 2014). T. californicus thus provides a simplified system for understanding sex-specific stress tolerance and lifespan.
Beyond sex chromosomes, many other explanations — both proximate and ultimate — have been proposed for sexual dimorphism in stress tolerance and lifespan. One potential explanation for male inferiority is the accumulation of male-specific mutation load in mitochondria (e.g. the “mother’s curse”; Gemmell et al., 2004). That is, for organisms in which mitochondria are inherited maternally (including T. californicus: Lee, 1993; Foley et al., 2013), male-harming mitochondrial mutations may accumulate as long as they are neutral or beneficial in females. Another explanation for sex differences is sexual selection. In a female, reproductive success is tied to the number of offspring she can raise, while in a male, reproductive success may be more dependent on the number of times he mates. These scenarios promote male behaviors and physiologies that sacrifice survival in favor of mating success (Trivers, 1985; Maklakov and Lummaa, 2013). Other specific factors that may be involved include sex differences in body size (Matzkin et al., 2007; Matzkin et al., 2009; Tejeda et al., 2014; Baudier et al., 2015), telomere stability (Gopalakrishnan et al., 2013), or activation of stress response pathways (Pomatto et al., 2017; Tower, 2017). Importantly, these potential explanations for sex differences are not mutually exclusive.
The current study used T. californicus to measure sex-specific tolerance to five different stressors: high temperature, high salinity, low salinity, copper (a common marine pollutant due to its use in antifouling paint additives; Brooks and Waldock, 2009) and bisphenol A (a synthetic endocrine disrupting chemical becoming increasingly common in marine habitats; Corrales et al., 2015). These stressors are likely to invoke different response pathways and were tested in different populations across a range of conditions. The current study also assessed the 20S proteasome, a proximate mechanism for responding to acute stress. The proteasome is the central intracellular structure responsible for protein degradation (Raynes et al., 2016), and the 20S confirmation is highly responsive to acute stress (Pickering et al., 2010). In addition to assessments of short-term stress exposure, the current study also measured sex-specific lifespan under benign conditions and under mild thermal stress. In this way, we tested whether sex-specific responses are similar across different stressors, and whether sex differences in acute tolerance predict sex differences in longevity.
2. Materials and methods
2.1. Reproductive biology of the study organism
T. californicus is an obligately sexual, marine copepod whose reproductive biology has been well studied (Egloff, 1967; Burton, 1985). Breeding occurs year-round and both sexes reproduce throughout their lifespan. Males clasp immature, virgin females and guard them until the female reaches maturity. The female is then inseminated and released. Females mate only once and use stored sperm to fertilize multiple egg sacs, with up to 12 clutches of 15–140 eggs produced over her lifetime (Egloff, 1967). In contrast to females, males mate repeatedly over their lifespan.
2.2. Population collection and culture maintenance
T. californicus were collected at four locations (Fig. 1) in the United States: Friday Harbor Labs (=FHL), WA (48.5458°, −123.0107°); Santa Cruz (=SC), CA (36.9495°, −122.0467); Catalina Island (=CAT), CA (33.4466°, −118.4850°); and San Diego (=SD), CA (32.7460°, −117.2551°). Populations were maintained in mass culture in incubators held at 20 °C with a 12 h light, 12 h dark cycle using standard culture medium (0.1 g ground Spirulina (Nutraceutical Science Institute, USA) and 0.1 g ground Tetramin fish food (Tetra Holding Inc., USA) per liter of 37 μm filtered seawater.
Fig. 1.
Map of four collection locations on the west coast of the United States.
2.3. Thermal tolerance
Much previous work on T. californicus has focused on the detrimental effects of interpopulation hybridization (e.g. Burton, 1990; Edmands, 1999; Ellison and Burton, 2006; Hwang et al., 2011), with some evidence that these negative effects are reduced under thermal or salinity stress (Edmands and Deimler, 2004; Willett, 2012; Hwang et al., 2016). To test if any sex differences are robust to the effects of hybridization, we assayed tolerance in both pure populations and F2 interpopulation hybrids. Samples of the three populations used in this study (FHL, SC and SD) were maintained in the laboratory for at least three months (approximately three generations) before experiments began. Five cross treatments were then created to test effects of population and hybridization: two hybrid crosses (FHLf × SDm F2 and SCf × SDm F2) and three intrapopulation crosses (FHLf × FHLm F2, SCf × SCm F2 and SDf × SDm F2). Crosses were established by separating clasped pairs of adult males and virgin females under a dissecting microscope as in Burton et al., 1981, and uniting one male and one female in each 30 mm × 100 mm Petri dish filled with 30 ml standard culture medium. At least sixty replicate dishes were set up for each cross treatment and maintained as before in a 20 °C incubator with a 12 h light, 12 h dark cycle, and weekly feedings of Spirulina and Tetramin. After two weeks, parents were removed from the dishes and F1 offspring were allowed to grow to adulthood and pair freely. When F1 clasped pairs formed, individual pairs were moved to a new Petri dish with standard culture medium. After two weeks, F1 parents were removed and F2 offspring were allowed to grow to maturity and mate freely. F2 adults were then collected for tolerance assays.
Adults were collected from the family-specific groups: females were gravid (bearing egg sacs) while males were either single or separated from clasped pairs. Collected adults were held for 12–24 h in filtered seawater without food before running the assay to minimize stress due to collection. Assays were run in laboratory thermal cyclers. One copepod was placed in each well of a 96-well plate in 200 μl of filtered seawater and the plate was sealed with a plastic cover. Over 2 h, the program increased from 20 °C up to 38 °C, stepwise by 1.8 °C. Temperature was then held for 2 h without a heated lid. After cooling, copepods were assessed as live, knockdown, or dead. Live copepods were defined as actively swimming distances greater than one body length. Knockdown copepods were defined as still or not actively swimming, and may twitch in response to probing. Dead copepods were defined as producing no response from probing and displaying a sharp angle between the urosome and prosome (adapted from Bao et al., 2011). For this study, the live and knockdown categories were combined before comparison to the dead category, because fitness measured after stress challenges was not found to be significantly different between individuals initially scored as live vs. knockdown (Foley, 2017).
2.4. Salinity tolerance
To test effects of population and hybridization, the salinity tolerance assays used the same five cross treatments used in the thermal tolerance assays described above (FHLf × SDm F2, SCf × SDm F2, FHLf × FHLm F2, SCf × SCm F2 and SDf × SDm F2). Twelve to twenty-four hours before running the salinity assays, adult males and gravid, adult females were collected from the family-specific F2 groups and held in artificial seawater at 32 PSU (practical salinity units) and 20 °C without food. Testing solutions were prepared with distilled water and Instant Ocean (Spectrum Brands) to either 32 PSU (control), 75 PSU (hypersalinity) or 5 PSU (hyposalinity). Preliminary testing with osmotic stress in adult copepods provided insufficient levels of mortality in 2–6 h timed exposure, making it incomparable to the thermal challenge. However, acute 10 min exposures provided an opportunity to efficiently address stress tolerance based on activity status. Animals were exposed to testing solutions for 10 min, and their status was recorded as either swimming or not swimming.
2.5. Copper tolerance
Degree of shading may impact copper (Cu) tolerance in Tigriopus, since previous work shows copper tolerance to be sensitive to both temperature and UV radiation (Sun et al., 2018). Therefore, the current study used three shade treatments: no shade, partial shade and full shade (Supplementary Fig. S1). Experiments were done at the Wrigley Marine Science Center on Catalina Island, CA in June and July 2014. Copepods were collected from a single tidepool at the CAT location. Twenty gravid females were used in each of the three shading treatments, with each individual placed in its own 50 ml Falcon tube with approximately 40 ml standard culture medium. The Falcon tubes were placed outdoors in 68 l containers with 40 l of 10 μm-filtered seawater to keep temperature constant and control for evaporation. Each 68 l plastic container housed one of the three shade treatments. Full shading was accomplished by covering the 68 l container with an all-purpose blue poly tarp with UV protection (Sigman, USA). To ensure full aeration and to limit humidity, the tarp was not wrapped around the container, but rather placed as a flat sheet above the container allowing for a roughly 15 cm gap to exist between the lip of the container and tarp. Partial shading was accomplished by covering the 68 l container with a tarp half the size of the full shade treatment. This limited the most intense UV exposure during mid-day, but allowed light to penetrate during dawn and dusk. When offspring hatched, they remained in the same Falcon tube and the mother was removed.
Offspring were used for copper tolerance assays once they reached maturity. Each assay used 10 males or 10 gravid females, with 3 replicates for each combination of sex, copper exposure and shade treatment. Assays were done in Petri dishes (60 mm × 15 mm) filled to a volume of 10 ml with 10 μm-filtered, autoclaved seawater and 2 μg ml−1 copper (CuSO4·5H2O, cupric sulfate, Sigma-Aldrich). Dishes were housed in a 19 °C light-controlled incubator and mortality was evaluated after 96 h. Dead copepods were defined as producing no response from probing and displaying a sharp angle between the urosome and prosome.
2.6. Bisphenol-A tolerance
Copepods were collected from the SD location and tolerance assays were done three months after collection. A stock solution of bisphenol-A (BPA, 2,2-bis(4-hydroxyphenyl) propane; > 99%, Sigma-Aldrich) was prepared using 10% DMSO (dimethyl sulfoxide, Mallinckrodt) as solvent, with 0.100 g BPA, 10 ml DMSO, 90 ml sterile diH2O. The stock BPA was mixed with 37 μm-filtered, autoclaved seawater to produce six different concentrations: 0, 0.1013, 0.2025, 0.405, 0.81 and 1.62 mg BPA/L. DMSO concentrations were adjusted to 0.08% DMSO in all test solutions by using a 10% DMSO stock solution. Assays were done in 60 mm × 15 mm Petri dishes with 11 ml test solution and 10 copepods (either adult males or adult, gravid females) in each dish. Animals were exposed for 96 h in a 20 °C light-controlled incubator, and then scored as live or dead (defined as producing no response from probing and displaying a sharp angle between the urosome and prosome).
2.7. Proteolytic activity during copper and heat exposure
The most commonly-studied confirmation of the proteasome is the ubiquitin-dependent 26S proteasome, which selectively degrades ubiquitin-tagged proteins under normal non-stress conditions. For our assays we targeted a different conformation, the 20S proteasome, which is known to be highly responsive to acute stress (Pickering et al., 2010) and selectively degrades oxidized proteins (Davies, 2001). Loss of protein homeostasis is known as one of the hallmarks of aging (López-Otín et al., 2013) and the age-related loss 20S proteasome capacity has been shown to result in decreased stress resistance (Pomatto et al., 2017; Raynes et al., 2017).
For proteolytic activity assays, T. californicus were collected from the San Diego (SD) location. It should be noted that this is not the same population used for the copper tolerance assays described above. While we did not formally test sex-specific copper tolerance in the SD population, preliminary assays across multiple populations support the general pattern of higher copper tolerance in females (Sun et al., 2015; Foley, 2017).
For the proteolytic assays, control and chronic copper lines were established in triplicate with 100 gravid females several months prior to the experiments (multigenerational copper exposure compared to single generational copper exposure result in similar physiological acclimation responses; P.Y. Sun and B.K. So, unpublished observations/data). Cultures were established and maintained following a previous protocol (see Sun et al., 2014). Briefly, a copper stock was prepared by diluting CuSO4·5H2O (Sigma-Aldrich) in Nanopure water to a concentration of 1 g l−1. The copper stock was added to chronic copper cultures for a final concentration of 13.74 μg l−1 during water changes. These water changes occurred once a week, when the seawater was completely replaced. Cultures were maintained with a 12 h light and 12 h dark cycle at 20 °C in standard culture medium.
Males and females from both the control and copper lines were sorted into 50 ml Falcon tubes, with 10 individuals per tube, in a final volume of 20 ml. For heat stress conditions, Falcon tubes were placed in a 35 °C water bath for 1 h and then placed back into a 20 °C incubator for 1 h recovery before protein extraction. Control treatments were left in the 20 °C incubator for the duration of the experiment. All samples were run in triplicate. Samples were frozen on dry ice for protein extraction. The protocol used follows Pickering et al. (2010). Briefly, protein extraction began with thawing and desiccating the samples followed by homogenization on ice using a tissue grinder in a salt buffer solution (50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol at pH 7.5). Samples were then further lysed by 3× freeze-thaw cycles on dry ice and room temperature water. Next, samples were centrifuged at 10,000 rcf for 10 min after which the supernatant was collected for protein quantification. Protein was quantified using a BCA protein assay kit (Thermo Scientific, USA). 20S proteasome capacity, specifically the enzymatic activity mostly attributable to the β5 subunit that exhibits chymotrypsin-like activity, was measured using a peptide (succinyl-Leu-Leu-Val-Tyr) labeled with a 7-Amino-4-Methylcoumarin (AMC) fluorophore. When AMC is proteolytically cleaved from the peptide substrate, it fluoresces and this is measured as an index of proteolysis. The 20S proteasome is a multiunit complex, with all subunits working in conjunction with each other. These subunits have a similar rate of synthesis (Hayter et al., 2005). As a result, over-expression of one subunit, such as β5, results in an increase in the activity of all other catalytic units as well (Chondrogianni et al., 2005).
Samples were loaded in triplicate at 2.5 μg cell lysate protein per well in a 96-well plate with 2 μM N-succinyl-Leu-Leu-Val-Tyr-AMC (Enzo Life Sciences). The final volume per well was 100 μL. Samples were run alongside AMC standards to create a standard curve of known AMC concentrations. Fluorescence readings (Fluroskan Ascent FL, LabSystems) were taken at 10 min intervals using excitation wavelengths of 355 nm and emission of 444 nm for a total of 4 h. Proteolytic capacity was measured as pmol of AMC released per min of μg of total protein.
2.8. Lifespan and sex ratio vs. temperature
All lifespan assays used copepods collected from the SD location and maintained in standard culture medium. To increase replication, multiple batches from the same location were assayed at both 15 °C and 25 °C. A temperature of 25 °C is a relatively mild stress given that previous work on the same population found median lethality at ~37 °C (Leong et al., 2018). Each batch used a different combination of starting population, starting time and temperature treatment. Due to limitations on the numbers of animals available, offspring of animals used in one batch were used for later batches (Table 1). The same starting populations were used for batches A, D and G, batches B and E, and batches C and F. Each batch was established with 15–40 replicates of a single clasped pair (adult male and virgin female) per 30 mm × 100 mm Petri dish filled with 30 ml culture medium. Replicates were checked for developmental stage and mortality three times a week (either by eye or using a dissection microscope) for the duration of the experiment. When the female parent formed an egg sac, the male parent was removed. The female parent was removed once the eggs hatched and larvae could be seen under the microscope. After all offspring had metamorphosed from nauplii (larvae) to copepodids (juveniles), up to 24 individuals from each family were transferred to a 24-well culture plate, with one individual and 1 ml standard culture medium in each well. Plates were rehydrated with deionized water and fed once per week. Animals were monitored 3× per week, and the date was recorded when sex could be identified for each individual (presence of claspers on antennules for males, presence of an egg sac for females). The date that sex was identified was used as the first day of adulthood, and survival of adult males and females continued until all individuals were dead. Importantly, individuals were isolated before mating could occur, but virgin females in this species still produce egg sacs, which fail to develop (Egloff, 1967).
Table 1.
Batches used for assays of lifespan and sex ratio vs. temperature.
| Batch | Source location | Temperature treatment | Culture maintenance |
|---|---|---|---|
| A | SD | 15 °C | Animals maintained in lab at 20 °C for > 5 years prior to experiment |
| B | SD | 15 °C | Offspring of combined batches A and D |
| C | SD | 15 °C | Animals maintained in lab at 20 °C for 10d prior to experiment |
| D | SD | 25 °C | Animals maintained in lab at 20 °C for > 5 years prior to experiment |
| E | SD | 25 °C | Offspring of combined batches A and D |
| F | SD | 25 °C | Animals maintained in lab at 20 °C for 10d prior to experiment |
| G | SD | 25 °C | Animals maintained in lab at 20 °C for > 5 years prior to experiment. Same as Batch A but experiment began 3 weeks later. |
2.9. Statistics
For assays of temperature and salinity, ANOVA was used to test cohort and sex as fixed effects, as well as their interaction, with family as a random factor (R function lmer, y ~ (cohort ∗ sex) + (1|cohort:family)). Post hoc tests for this mixed effects model were calculated using R function difflsmeans. Similarly, for assays of copper tolerance, ANOVA was used to test environment and sex as fixed effects, as well as their interaction, with replicate as a random factor (R function lmer, y ~ environment ∗ sex) + (1|environment:replicate)), and post hoc tests using difflsmeans. For assays of bisphenol-A tolerance, median lethal concentrations (LC50s) were calculated and compared using the R package drc (Ritz et al., 2015). LC50 values were compared by likelihood ratio tests which have been found to have higher power to detect true differences than the more traditional method of testing for overlap in 95% confidence intervals (Wheeler et al., 2006). For studies of proteolytic activity, sex-specific treatment groups (stress treatment ∗ sex) were compared by one-way ANOVA using R function aov (response~group), to allow comparison to sex-specific control groups, with post hoc tests by Tukey HSD (R function TukeyHSD). For studies of lifespan, initial ANOVA showed a significant effect (p < 0.001) of batch nested within temperature (lmer, lifespan ~ temperature + sex + temperature|batch). The seven batches were therefore analyzed separately. Kaplan-Meier survival curves for each batch were fitted with R function survfit (package survminer) and compared using log rank tests. Within batches, temperature effects on sex-specific lifespan were compared by Welch two sample t-tests (R function t-test), and temperature effects on sex ratio were tested by Pearson’s chi-squared test with Yates’ continuity correction (R function chisq.test). For multiple testing, p-values were adjusted by the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995) using the R function p.adjust. All statistical analyses used R 3.4.2 (R Core Team, 2017).
3. Results
3.1. Thermal tolerance
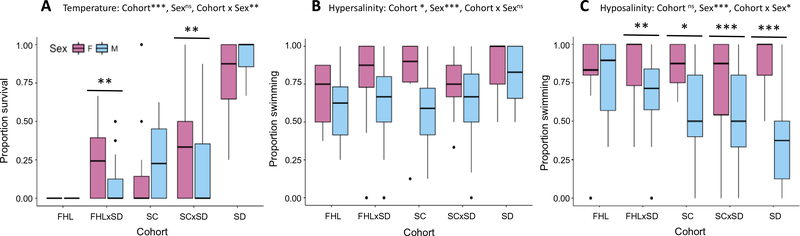
High temperature tolerance (Fig. 2A) was tested in both sexes for five cohorts (FHL, FHL × SD F2 hybrids, SC, SC × SD F2 hybrids and SD). Each thermal tolerance assay used 1–10 adult females (mean = 6.2) or 2–12 adult males (mean = 6.2) from a single family, with 12–35 families assayed for each sex in each cohort (mean = 21.8). ANOVA yielded a significant main effect for cohort (F (4,203) = 70.95, p = 2.2e–16), but not for sex (F (1,203) = 0.34, p = 0.561), and a significant interaction between cohort and sex (F (4,203) = 4.63, p = 0.001). Post hoc analyses for cohort found that southernmost population SD was significantly more tolerant to higher temperature than the four other treatments (p < 0.001 for each comparison). Northernmost population FHL was also significantly less tolerant than FHL × SD F2 hybrids (p < 0.01), SC (p < 0.01) and SC × SD F2 hybrids (p < 0.001). Post hoc analyses showed significant sex differences within two of the five cohorts, with females having an average of 18.3% higher survival in FHL × SD F2 hybrids (p < 0.001) and 14.9% higher survival in SC × SD F2 hybrids (p < 0.001).
Fig. 2.
Sex-specific tolerance of temperature and salinity. ANOVA was used to test effects of cohort, sex, and cohort x sex interaction, with family as a random effect. For significant interactions, significant post hoc sex differences within cohorts are shown. (***p < 0.001, **p < 0.01, *p < 0.05, nsnot significant). (A) Proportion survival after 2 h exposure to high temperature (38 °C). (B) Proportion swimming after 10 min exposure to hypersalinity (75 PSU). (C) Proportion swimming after 10 min exposure to hyposalinity (5 PSU).
3.2. Salinity tolerance
Hypersalinity tolerance (Fig. 2B) was tested in both sexes for five cohorts (FHL, FHL × SD F2 hybrids, SC, SC × SD F2 hybrids and SD). Each hypersalinity assay used 2–10 adult females (mean = 6.5) or 2–15 adult males (mean = 6.3) from a single family, with 9–33 families assayed for each sex in each cohort (mean = 20.1). ANOVA yielded a significant main effect for cohort (F (4,186) = 2.63, p = 0.037), as well as for sex (F (1,186) = 16.31, p = 7.78e–5), but not for the interaction between cohort and sex (F (4,186) = 0.71, p = 0.589). Post hoc analyses for cohort found southernmost population SD to be more tolerant than FHL (p < 0.01), FHL × SD F2 hybrids (p < 0.05), SC (p < 0.05) and SC × SD F2 hybrids (p < 0.05). Post hoc analyses for sex found females to be more tolerant than males (p < 0.001), with an average of 15.0% more individuals swimming.
Hyposalinity tolerance (Fig. 2C) was tested in both sexes for the same five cohorts (FHL, FHL × SD F2 hybrids, SC, SC × SD F2 hybrids and SD). Each hyposalinity assay used 1–8 adult females (mean = 5.2) or 2–12 adult males (mean = 6.2) from a single family, with 9–33 families assayed for each sex in each cohort (mean = 20.6). ANOVA yielded a significant main effect for sex (F (1,191) = 25.93, p = 8.59e–7), but not for cohort (F (1,191) = 2.04, p = 0.093), and the interaction between sex and cohort was significant (F (4,191) = 2.58, p = 0.039). Post hoc analyses for sex found females to be more tolerant than males (p < 0.001), with an average of 23.1% more females swimming. Post hoc analyses showed significant sex differences within four of the five cohorts, with a higher percentage of females swimming in FHL × SD F2 hybrids (16.6%, p < 0.01), SC (28.2%, p < 0.05), SC × SD F2 hybrids (21.0%, p < 0.001), and SD (50.0%, p < 0.001).
3.3. Copper tolerance
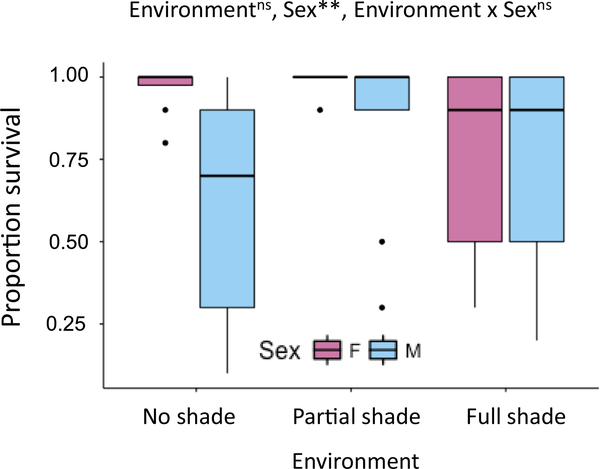
Copper tolerance (Fig. 3) was tested in both sexes from location CAT for individuals born in one of three environments (no shade, partial shade or full shade). ANOVA yielded a significant main effect for sex (F (1,44) = 9.384, p = 0.006), but not for environment (F (2,44) = 1.374, p = 0.273) or for the interaction between environment and sex (F (2,44) = 3.118, p = 0.063). Post hoc analyses for sex found females to be more tolerant than males (p < 0.01), with an average of 14.6% higher survivorship.
Fig. 3.
Sex-specific copper tolerance. Proportion survival after 96 h exposure to copper (2 mg ml−1). ANOVA was used to test effects of environment, sex, and environment x sex interaction, with replicate as a random effect (** p < 0.01, nsnot significant).
3.4. Bisphenol-A tolerance
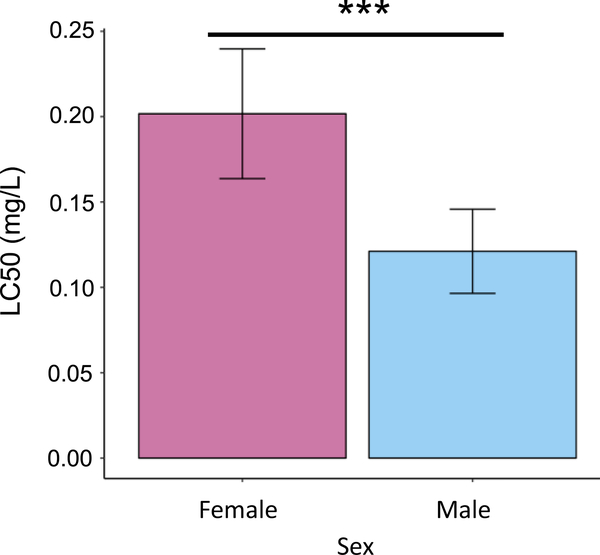
Median lethality after 96 h BPA exposure was estimated in both sexes from location SD (Fig. 4). LC50 in females (0.202, 95%CI [0.164,0.240]) was approximately 67% higher than LC50 in males (0.121, 95% CI [0.097,0.146]), and the difference was highly significant by a likelihood ratio test (LR = 12.288, p = 5e–4).
Fig. 4.
Sex-specific bisphenol A (BPA) tolerance. Median lethality, with 95% confidence intervals, after 96 h exposure. Results for the two sexes were compared by a likelihood ratio test (***p < 0.001).
3.5. Proteolytic activity during copper and heat exposure
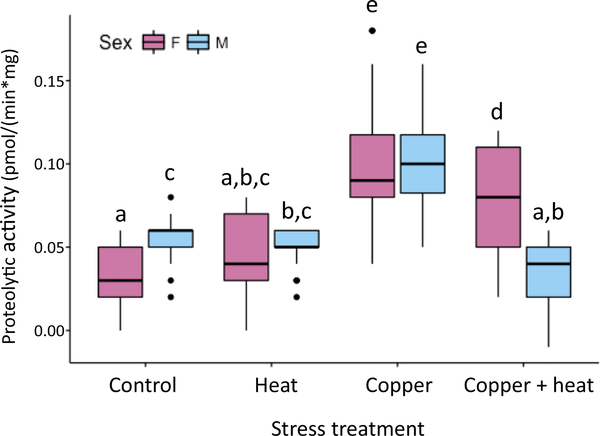
Proteolytic rates were measured under single and combined stress treatments for both sexes from location SD (Fig. 5, Table 2). One-way ANOVA showed a significant effect of group (stress treatment*sex) on proteolytic activity (F(7,328) = 61.54, p = 2e – 16). In post hoc comparisons to controls, acute heat stress did not alter activity in females (p = 0.0851) or males (p = 0.7514). In contrast, chronic copper exposure increased proteolytic activity above control levels for both females (p < 0.0001) and males (p < 0.0001). When chronic copper exposure was followed by acute heat stress, proteolytic activity remained elevated in females (p < 0.0001) but collapsed below control levels in males (p < 0.0001).
Fig. 5.
Sex-specific proteolytic activity under stress. Proteolytic activity in four stress treatments: control (20 °C), acute exposure to heat (1 h at 35 °F), chronic exposure to copper, and chronic exposure to copper followed by acute exposure to heat. Effect of group (stress treatment*sex) was tested by one-way ANOVA. Lower case letters above bars indicate results of post hoc Tukey HSD tests; groups not sharing a letter are significantly different (see Table 2).
Table 2.
ANOVA post hoc tests for differences in proteolytic activity between groups. Mean differences between groups ((groups in top row) - (groups in left column)) are shown below the diagonal. P-values for significance between groups are shown above the diagonal.
| Control, F | Control, M | Heat, F | Heat, M | Cu, F | Cu, M | Cu + heat, F | Cu + heat, M | |
|---|---|---|---|---|---|---|---|---|
| Control, F | – | 0.0001*** | 0.0851ns | 0.0326* | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.9999ns |
| Control, M | −0.0236 | – | 0.5266ns | 0.7514ns | < 0.0001*** | < 0.0001*** | 0.0002*** | 0.0003*** |
| Heat, F | −0.0140 | 0.0095 | – | 0.9999ns | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.1916ns |
| Heat, M | −0.0157 | 0.0079 | −0.0017 | – | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.0851ns |
| Cu, F | −0.0645 | −0.0410 | −0.0505 | −0.0488 | – | 0.9717ns | 0.0046** | < 0.0001*** |
| Cu, M | −0.0695 | −0.0460 | −0.0555 | −0.0538 | −0.0050 | – | 0.0001*** | < 0.0001*** |
| Cu + heat, F | −0.0460 | −0.0224 | −0.0319 | −0.0302 | 0.0186 | 0.0236 | – | < 0.0001*** |
| Cu + heat, M | −0.0017 | 0.0219 | 0.0124 | 0.0140 | 0.0629 | 0.0679 | 0.0443 | – |
not significant. Significant tests shown in bold.
p < 0.05.
p < 0.01.
p < 0.001.
3.6. Lifespan and sex ratio vs. temperature
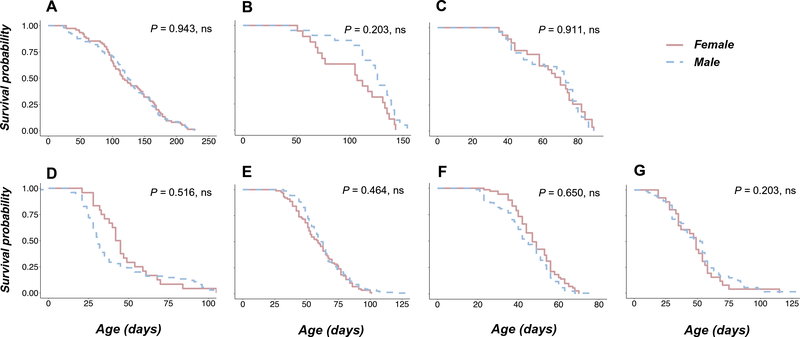
Adult lifespan was measured in SD animals at 15 °C for three batches and 25 °C for four batches (Fig. 6, Table 3). Initial ANOVA showed a highly significant effect of batches nested within temperature treatment, so subsequent analyses were done on individual batches. Compared to an expected sex ratio of 0.5, there was a significant deficit of females for two of three batches at 15 °C, and a highly significant deficit of females for each of four batches at 25 °C (Table 3). Kaplan-Meier survival curves (Fig. 6) for males and females in each batch and temperature were compared by log-rank tests. Survivorship was equivalent between sexes in all seven batches. Mean lifespan was longer in females for batch F at 25 °C (p < 0.05) and equivalent for the remaining six tests (Table 3).
Fig. 6.
Sex-specific adult survival curves at two temperatures. Kaplan-Meier survival curves at 15 °C (batches A-C) and at 25 °C (batches D-G). P-values (FDR-adjusted) are for comparisons of male and female survival curves by a log-rank test. ns not significant.
Table 3.
Sex ratio (proportion females) and sex-specific lifespan (days) at two temperatures.
| Temp | Batch | Sex ratio | Female lifespan |
Male lifespan |
Sex ratio deviation from 0.5? |
Female vs. male lifespan? | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | N | Mean | SE | N | |||||
| 15 °C | A | 0.42 | 125.3 | 5.6 | 75 | 123.0 | 5.1 | 105 | 51* | 0.30166ns |
| 15 °C | B | 0.48 | 99.5 | 7.2 | 19 | 120.4 | 6.1 | 21 | 0.11ns | −1.9036ns |
| 15 °C | C | 0.38 | 65.7 | 3.3 | 27 | 65.1 | 2.7 | 45 | 4.51* | 0.1457ns |
| 25 °C | D | 0.24 | 47.5 | 4.0 | 24 | 40.8 | 3.0 | 75 | 26.31*** | 1.3652ns |
| 25 °C | E | 0.36 | 60.2 | 2.0 | 88 | 63.7 | 1.4 | 158 | 19.91*** | −1.42176ns |
| 25 °C | F | 0.32 | 48.6 | 3.0 | 71 | 44.1 | 1.0 | 154 | 30.61*** | 2.76159* |
| 25 °C | G | 0.26 | 48.2 | 4.0 | 26 | 50.6 | 2.8 | 74 | 23.01*** | −0.4951ns |
Temp, temperature; SE, standard error; N, number of individuals. Sex ratio deviation from 0.5 within each batch and temperature compared by chi-square test (chi-square value, df, significance). Female vs. male lifespan within each batch and temperature compared by t-test (t value, df, significance after FDR-adjustment).
not significant. Significant tests shown in bold.
p < 0.05.
p < 0.001.
4. Discussion
4.1. Main findings
This is the first study to assess acute stress tolerance and lifespan in both sexes of the copepod Tigriopus californicus, a novel invertebrate model for aging. The potential for this study to relate sex-specific stress tolerance to sex-specific lifespan may be complicated by population differences, since the stress assays used multiple populations while the lifespan assays used only a single population. Still, studies of acute tolerance across a range of stressors (temperature, salinity, copper, and bisphenol-A) showed that when significant sex differences were found, females were consistently more tolerant than males, and this pattern was robust to differences in populations, pure vs. hybrid crosses, and different shading environments. One mechanism for dealing with extrinsic stress, upregulation of proteolytic activity, was also found to be sexually dimorphic in the single population tested. In contrast, lifespan in one population exposed to two different temperature treatments was found to be largely equivalent between sexes across replicated tests.
4.2. Effects of population and hybridization on stress tolerance
For acute stressors measured in more than one population (temperature and salinity), geographic patterns were consistent with adaptation to local environmental conditions. Southern populations were found to be more tolerant of high temperature, as has been reported in a series of previous studies (Willett, 2010; Kelly et al., 2012; Leong et al., 2018). For osmotic tolerance, southern populations were found to be more tolerant of high salinity and less tolerant of low salinity, also consistent with previous work (Leong et al., 2018).
A comparison of intra-population and inter-population F2 crosses was done under conditions of high temperature, high salinity and low salinity. In previous studies, inter-population F2 hybrids have repeatedly been shown to exhibit hybrid breakdown, with fitness frequently below both parental populations (e.g. Burton, 1990; Edmands, 1999; Hwang et al., 2011). In contrast, the current study found no cases where F2 hybrid fitness was significantly depressed below both parental populations, and one case where F2 hybrid fitness significantly exceeded one of the parentals (FHL × SD F2 > FHL under high temperature). This is consistent with a reduction in detrimental effects of hybridization in the presence of extrinsic stress, as has been reported in prior investigations of T. californicus and other species (Armbruster et al., 1997; Edmands and Deimler, 2004; Edmands, 2007; Willett, 2012; Hwang et al., 2016). This amelioration of hybrid breakdown under stress may be attributed to increased beneficial effects of within-locus hybridity (e.g. reduced inbreeding depression, possibly due to genetic homeostasis, wherein heterozygotes have greater capacity to buffer against environmental challenges; Lerner, 1954) and/or decreased detrimental effects of between-locus hybridity (e.g. reduced deleterious epistasis; Lynch, 1991).
4.3. Sexual dimorphism in stress tolerance
For temperature, the main effect of sex was not significant, but the interaction between sex and cohort was significant, with sex differences found in two of five cohorts (female tolerance 18% higher in FHL × SD F2 and 15% higher in SC × SD F2). For both high and low salinity, the main effect of sex was significant, with females having an average of 15% higher tolerance to high salinity and an average of 23% higher tolerance to low salinity. For BPA, females again showed superior tolerance, with 67% higher median lethal concentrations. While this is the most comprehensive survey of sex-specific tolerance in this species to date, it is not the first to report superior tolerance in females. For high temperature, both Willett (2010) and Kelly et al. (2012) also found tolerance to be greater in females. For salinity, DeBiasse et al. (2018) found tolerance of low salinity to be greater in males, while tolerance of high salinity was equivalent between sexes. However, DeBiasse et al. (2018) focused on clasped pairs (adult male with juvenile female) so lower female tolerance may be due to their life stage, as juvenile T. californicus have been reported to be less resilient than adults (O’Brien et al., 1988; Forget et al., 1998; Medina et al., 2008). Consistent with our findings of higher female resistance to extrinsic stress, previous work shows higher female tolerance of the intrinsic stress brought on by hybridization. In F2 inter-population hybrids, males showed more hybrid viability QTL (quantitative trait loci) than females, including more single locus QTL, more two-way interactions and more three-way interactions (Foley et al., 2013).
4.4. Sexual dimorphism in proteolytic capacity
The proteolytic response to stress was found to be sexually dimorphic. Chronic copper exposure caused increased capacity in both sexes. However, proteolytic capacity remained elevated in females but dropped below control levels in males when chronic copper exposure was combined with acute heat stress. The loss of the ability to upregulate proteolytic capacity in response to stress negatively impacts fitness (Pomatto et al., 2017; Raynes et al., 2017). Without timely and proper protein degradation, proteins damaged and denatured by oxidative stress can form toxic aggregates. These protein aggregates can lead to further inhibition of proteasome activity (Sitte et al., 2000) because the proteasome becomes sequestered and deactivated as it unsuccessfully attempts to degrade the structures (Grune et al., 2004). Accumulation of these aggregates eventually leads to cytotoxicity (Levine and Stadtman, 2001). Thus, an initial loss of proteasome activity can rapidly result in cytotoxic outcomes if not corrected. The ability of females to maintain proteolytic capacity under extreme conditions may provide a partial explanation for their higher tolerance of a range of stressors.
Male proteasome capacity collapsed under combined stressors (e.g. heat and copper). Other studies have reported similar patterns where increased severity of stress is accompanied by a drop in the ability to upregulate proteolytic capacity (Pickering et al., 2013; Raynes et al., 2017). Additionally, the genetic mechanisms used to respond to copper pollution have been shown to change based on the intensity of the pollution stress (Galletly et al., 2007). For instance, in yeast cells during starvation stress, proteasome activity drops as the cell sequesters proteasome units in storage granules and shifts to non-selective autophagy (Peters et al., 2013), in which a potential trade off occurs from the specificity of proteasome degradation to the rapid removal afforded by autosomal degradation. Though it is uncertain whether a similar mechanism is acting in male copepods under heat and copper stress, the collapse in activity may indicate a shift away from proteasome proteolytic activity to another degradation pathway that is better suited to meet the new intracellular needs. The inability of males to maintain proteasome activity in the presence of intense stress likely contributes to the overall phenotypic observation of higher female tolerance to stress.
4.5. Sex ratio
In this study, sex ratio was measured in adults, since sex in this species cannot be distinguished in early developmental stages (Egloff, 1967). At the benign temperature of 15 °C, significant male bias was found in two out of three batches (mean = 57.3% male), while at the more stressful temperature of 25 °C, highly significant male bias was found in four out of four batches (mean = 70.5% male). Understanding stress effects on adult sex ratio in this system is complicated because environmental factors can alter primary sex ratio (Vacquier, 1962; Vacquier and Belser, 1965; Egloff, 1967; Chalker-Scott, 1995; Voordouw and Anholt, 2002b) and can also cause sex-specific mortality (see above). Since the current study and previous studies (Willett, 2010; Kelly et al., 2012) show males to be less tolerant of high temperature, the increased proportion of males at 25 °C is likely attributable to thermal effects on sex determination. This is consistent with previous work in which a shift from 15 °C to 25 °C caused approximately 5% increase in the percentage of males in the primary sex ratio (Voordouw and Anholt, 2002b). Alternatively, if males develop faster than females, particularly at high temperatures, the protocol of limiting assays to 24 individuals per family may have enriched for males if larger individuals were inadvertently chosen.
4.6. Sex-specific longevity
Adult lifespan was measured in three batches at 15 °C and four batches at 25 °C. Substantial variation was found among batches within temperature treatments, presumably due to differences in the time of sample collection, the conditions of culture maintenance and the time of experimental assays. No significant sex differences were found within batches. These results are in rather dramatic contrast to previous work on adult lifespan in T. californicus by Egloff (1967) who found that when animals had abundant food (as in our study) mean life expectancy was 2.5× longer for males at 15 °C and 2.0× longer for males at 23 °C. However, when animals were starved, the sex bias switched and mean life expectancy was found to be 2.4× longer for females at 15 °C and 3.7× longer for females at 23 °C. Egloff’s finding of a shift toward longer female lifespan under starvation and/or thermal stress is in line with our findings of consistently higher stress tolerance in females and more female biased sex ratios in lifespan assays in the higher temperature treatment. However, their finding of longer male lifespan in fed animals contrasts with our results which show no evidence of longer male lifespan for fed animals in either temperature treatment. One difference is that our study used a population from southern California (San Diego) while Egloff’s study used a population from central California (Pacific Grove), and T. californicus populations are known to be genetically divergent and distinct in life history characters (Edmands and Harrison, 2003). Another major difference is that in our study individuals were raised in isolation once they reached the copepodid (juvenile) stage, while in Egloff’s study groups of males and females were raised together throughout the experiment. These mixed sex groups allow the potential for competition, courtship, mating, and reproduction, all of which can have sex-specific effects on lifespan (e.g. Fowler and Partridge, 1989; Kirkwood and Rose, 1991; Stearns, 1992; Penn and Smith, 2007; Bonduriansky et al., 2008; Leech et al., 2017). Several studies have found the costs of mating and reproduction to be higher for females than males (e.g. Fowler and Partridge, 1989; Penn and Smith, 2007), so the absence of mating and reproduction may have contributed to the equivalent male and female lifespans found in our study. Importantly, our longevity studies incorporated a stressor (a 10 °C increase in temperature) with sex-specific effects in only a subset of populations. Given that females showed higher resistance to all stressors tested, lifespan under multi-stress conditions, such as in the wild, could potentially favor females.
4.7. Potential mechanisms underlying sexually dimorphic patterns
Results showed females to have superior tolerance to a range of acute stresses including high temperature, high salinity, low salinity, copper, and bisphenol-A. Several non-exclusive explanations, both proximate and ultimate, may underlie this female superiority. One molecular mechanism for the pattern may be sex differences in degradation of oxidized proteins, as the most stressful treatment (chronic copper exposure followed by acute heat exposure) caused females to upregulate proteasome activity, while activity collapsed below control levels in males. Proteolytic activity is likely only part of the molecular explanation here, as preliminary work in this system shows ~15% of the transcripts responding to stress (temperature and copper) also show sex-biased expression, while the transcriptome as whole has < 5% sex-biased genes (Foley, 2017). Another contributing factor may simply be body size. Females in this species are generally larger than males (Edmands and Harrison, 2003) and in other taxa larger body size correlates with higher tolerance to stressors such as high temperature (Baudier et al., 2015), desiccation (Matzkin et al., 2007; Matzkin et al., 2009; Tejeda et al., 2014) and starvation (Matzkin et al., 2009). As mentioned previously, the sexual dimorphism in stress response found in this study cannot be attributed to sex chromosomes, since sex determination in T. californicus is known to be polygenic rather than chromosomal (Ar-Rushdi, 1963; Voordouw and Anholt, 2002a; Alexander et al., 2014; Alexander et al., 2015). Instead, another broad genetic explanation for male inferiority is the “mother’s curse”, in which maternal inheritance of mitochondria results in the accumulation of male-harming mitochondrial mutations (Gemmell et al., 2004). To date, this pattern has been demonstrated experimentally only in Drosophila (e.g. Innocenti et al., 2011; Camus et al., 2015; Wolff et al., 2016). Lastly, sexual dimorphism in stress tolerance could result from adaptive sex-specific selection in both sexes (Trivers, 1985; Maklakov and Lummaa, 2013). Higher variance of male reproductive success may favor increased investment in reproductive effort at the expense of somatic maintenance, as well as riskier behaviors and physiologies that increase mating success at the expense of survival. This might be expected in T. californicus where females mate only once while males can mate with multiple females (Egloff, 1967; Burton, 1985). Despite consistent evidence that females have higher tolerance of acute stress, lifespan was largely equivalent between the sexes, suggesting a sex-biased tradeoff. For example, in decorated crickets, early life reproduction is associated with late life oxidative damage in both sexes, but the cost is larger for females (Archer et al., 2013).
5. Conclusions
Studies of a number of model organisms show positive associations between stress resistance and longevity, leading to the simple prediction that the sex with higher acute stress tolerance also has longer lifespan. However, this simple prediction may be confounded by tradeoffs between acute stress tolerance and long term survival. Our results for the alternative model Tigriopus californicus show females to be more stress tolerant than males across a broad range of stressors, and yet their lifespan was found to be equivalent (this study) or even shorter (Egloff, 1967) than males. This implies a sex-biased tradeoff. Our study therefore does not support a simple positive correlation between stress tolerance and lifespan, and instead suggests sex- and age-specific tradeoffs in factors such as reproduction, survival, and the costs and benefits of defense mechanisms. A better understanding of such sex-specific tradeoffs will require assays of both short-term stress tolerance and long-term survival in the same individuals.
Supplementary Material
Acknowledgements
For laboratory assistance, we thank Arkadiy Garber and Tyler Schiffman. For logistical support for experiments conducted at the Wrigley Marine Science Center, we thank Diane Kim and Karla Heidelberg. For thoughtful critiques of various versions of the manuscript we thank Nicole Adams, Ben Flanagan, Ning Li and Eric Watson, as well as two anonymous reviewers.
Funding
H.B.F. was supported by the US National Science Foundation (IOS1154321 to S.E). P.Y.S and K.J.A.D. were supported by the National Institute on Aging of the US National Institutes of Health (AG052374 to K.J.A.D). R.R. was supported by the Genomics and Geology Undergraduate Research Experience Program at the University of Southern California (USC). B.K.S. and E.N. were supported by University Research Associate Program Fellowships at USC. Y.V. was supported by National Science Foundation Research Experience for Undergraduates (OCE1263356 to K. Heidelberg). K.J.A.D. was supported by the National Institute of Environmental Health Sciences of the US National Institutes of Health (ES003598). During the writing of this manuscript, S.E. was partially supported by the National Science Foundation (DEB1656048 to S.E.) and the National Institute on Aging of the US National Institutes of Health (R21AG055873 to S.E.).
Abbreviations
- AMC
7-Amino-4-Methylcoumarin
- BPA
bisphenol A
- Cu
copper
- CuSO4·5H2O
cupric sulfate
- DMSO
dimethyl sulfoxide
- F1
first filial generation
- F2
second filial generation
- LC50
median lethal concentration
- LR
likelihood ratio
- NIH
National Institutes of Health
- PSU
practical salinity unit
- QTL
quantitative trait loci
Footnotes
Conflict of interests
The authors declare no conflict of interests.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2019.02.006.
References
- Alexander HJ, Richardson JML, Anholt BR, 2014. Multigenerational response to artificial selection for biased sex ratios in Tigriopus californicus populations. J. Evol. Biol. 27, 1921–1929. [DOI] [PubMed] [Google Scholar]
- Alexander HJ, Richardson JML, Edmands S, Anholt BR, 2015. Sex without sex chromosomes: genetic architecture of multiple loci independently segregating to determine sex ratios in the copepod Tigriopus californicus. J. Evol. Biol. 28, 2196–2207. [DOI] [PubMed] [Google Scholar]
- Archer CR, Sakaluk SK, Selman C, Royle NJ, Hunt J, 2013. Oxidative stress and the evolution of sex differences in life span and ageing in the decorated cricket, Gryllodes sigillatus. Evolution 67, 620–634. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Bradshaw WE, Holzapfel CM, 1997. Evolution of the genetic architecture underlying fitness in the pitcher-plant mosquito, Wyeomyia smithii. Evolution 51, 451–458. [DOI] [PubMed] [Google Scholar]
- Ar-Rushdi AH, 1963. The cytology of achiasmatic meiosis in the female Tigriopus (Copepoda). Chromosoma 13, 526–539. [Google Scholar]
- Bao VWW, Leung KMY, Qiu JW, Lam MHW, 2011. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species. Mar. Pollut. Bull. 62, 1147–1151. [DOI] [PubMed] [Google Scholar]
- Barreto FS, Watson ET, Lima TG, Willett CS, Edmands S, Li W, Burton RS, 2018. Genomic signatures of mitonuclear coevolution across populations of Tigriopus californicus. Nature Ecol Evol. 10.1038/s41559-018-0588-1. [DOI] [PubMed] [Google Scholar]
- Baudier KM, Mudd AE, Erickson SC, O’Donnell S, 2015. Microhabitat and body size effects on heat tolerance: implications for responses to climate change (army ants: Formicidae, Ecitoninae). J. Anim. Ecol. 84 (5), 1322–1330. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol 57, 289–300. [Google Scholar]
- Blows MW, Hoffmann AA, 2005. A reassessment of genetic limits to evolutionary change. Ecology 86 (6), 1371–1384. [Google Scholar]
- Bonduriansky R, Maklakov A, Zajitschek F, Brooks R, 2008. Sexual selection, sexual conflict and the evolution of ageing and lifespan. Funct. Ecol. 22 (3), 443–453. [Google Scholar]
- Brooks S, Waldock M, 2009. Copper biocides in the marine environment In: Arai T, Harino H, Ohji M, Langston WJ (Eds.), Chapter 24 in Ecotoxicology of Antifouling Biocides. Springer, New York. [Google Scholar]
- Burton RS, 1985. Mating system of the intertidal copepod Tigriopus californicus. Mar. Biol. 86, 247–252. [Google Scholar]
- Burton RS, 1990. Hybrid breakdown in development time in the copepod Tigriopus californicus. Evolution 44, 1814–1822. [DOI] [PubMed] [Google Scholar]
- Burton RS, Feldman MW, Swisher SG, 1981. Linkage relationships among five enzyme-coding gene loci in the copepod Tigriopus californicus: a genetic confirmation of achiasmatic meiosis. Biochem. Genet. 19 (11/120), 1237–1245. [DOI] [PubMed] [Google Scholar]
- Camus MF, Wolf JBC, Morrow EF, Dowling DK, 2015. Single nucleotides in the mtDNA sequence modify mitochondrial molecular functions and are associated with sex-specific effects on fertility and aging. Curr. Biol. 25, 2717–2722. [DOI] [PubMed] [Google Scholar]
- Chalker-Scott L, 1995. Survival and sex ratios of the intertidal copepod, Tigriopus californicus, following ultraviolet-B (290–320 nm) radiation exposure. Mar. Biol. 123, 799–804. [Google Scholar]
- Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES, 2005. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J. Biol. Chem. 280, 11840–11850. [DOI] [PubMed] [Google Scholar]
- Clayton JA, 2018. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 187, 2–5. [DOI] [PubMed] [Google Scholar]
- Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW, 2015. Global assessment of bisphenol A in the environment. Dose-Response 13 (3), 1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, 2001. Degradation of oxidized proteins by the 20S proteasome. Biochimie 83, 301–310. [DOI] [PubMed] [Google Scholar]
- Dean R, Zimmer F, Mank JE, 2014. The potential role of sexual conflict and sexual selection in shaping the genomic distribution or mito-nuclear genes. Genome Biol Evol 6 (5), 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasse MB, Kawji Y, Kelly MW, 2018. Phenotypic and transcriptomic responses to salinity stress across genetically and geographically divergent Tigriopus californicus populations. Mol. Ecol. 27 (7), 1621–1632. [DOI] [PubMed] [Google Scholar]
- Edmands S, 1999. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 53 (6), 1757–1768. [DOI] [PubMed] [Google Scholar]
- Edmands S, 2007. Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol. Ecol. 16, 463–475. [DOI] [PubMed] [Google Scholar]
- Edmands S, Deimler JK, 2004. Local adaptation, intrinsic coadaptation and the effects of environmental stress on interpopulation hybrids in the copepod Tigriopus californicus. J. Exp. Mar. Biol. Ecol. 303, 183–196. [Google Scholar]
- Edmands S, Harrison JS, 2003. Molecular and quantitative trait variation within and among populations of the intertidal copepod Tigriopus californicus. Evolution 57 (10), 2277–2285. [DOI] [PubMed] [Google Scholar]
- Egloff DA, 1967. Ecological Aspects of Sex Ratio and Reproduction in Experimental and Field Populations of the Marine Copepod Tigriopus californicus. PhD thesis. Stanford University, Stanford, CA. [Google Scholar]
- Ellison CK, Burton RS, 2006. Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution 60 (7), 1382–1391. [PubMed] [Google Scholar]
- Ellison CK, Burton RS, 2008. Genotype-dependent variation of mitochondrial transcription profiles in interpopulation hybrids. Proc. Natl. Acad. Sci. U. S. A. 105, 15831–15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley HB, 2017. Tolerance to Thermal, Osmotic, and Copper Stress in the Intertidal Copepod Tigriopus californicus. PhD thesis. University of Southern California, Los Angeles, CA. [Google Scholar]
- Foley BR, Rose CG, Rundle DE, Leong W, Edmands S, 2013. Postzygotic isolation involves strong mitochondrial and sex-specific effects in Tigriopus californicus, a species lacking heteromorphic sex chromosomes. Heredity 111, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force AG, Staples T, Soliman S, Arking R, 1995. Comparative biochemical and stress analysis of genetically selected Drosophila strains with different longevities. Dev. Genet. 17, 340–351. [DOI] [PubMed] [Google Scholar]
- Forget J, Pavillon J, Menasria M, Bocquene G, 1998. Mortality and LC50 values for several stages of the marine copepod Tigriopus brevicornis (Muller) exposed to the metals arsenic and cadmium and the pesticides atrazine, carbofuran, dichlorvos, and malathion. Ecotoxicol. Environ. Saf. 40, 239–244. [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L, 1989. A cost of mating in female fruitflies. Nature 338, 760–761. [Google Scholar]
- Galletly BC, Blows MW, Marshall DJ, 2007. Genetic mechanisms of pollution resistance in a marine invertebrate. Ecol. Appl. 17, 2290–2297. [DOI] [PubMed] [Google Scholar]
- Gemmell NJ, Metcalf VJ, Allendorf FW, 2004. Mother’s curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19 (5), 238–244. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Cheung NKM, Yip BWO, Au DWT, 2013. Medaka fish exhibits longevity gender gap, a natural drop in estrogen and telomere shortening during aging: a unique model for studying sex-specific longevity. Front. Zool. 10, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Jung T, Merker K, Davies KJ, 2004. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 36, 2519–2530. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Schmid JL, 1998. Evolution of starvation resistance in Drosophila melanogaster: aspects of metabolism and counter-impact selection. Evolution 52, 1679–1685. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Moore KM, Sty MA, Magwire MM, 1999. Stress resistance and longevity in selected lines of Drosophila melanogaster. Neurobiol. Aging 20, 521–529. [DOI] [PubMed] [Google Scholar]
- Hayter JR, Doherty MK, Whitehead C, McCormack H, Gaskell SJ, Beynon RJ, 2005. The subunit structure and dynamics of the 20S proteasome in chicken skeletal muscle. Mol. Cell. Proteomics 4, 1370–1381. [DOI] [PubMed] [Google Scholar]
- Hill GE, 2014. Sex linkage of nuclear-encoded mitochondrial genes. Heredity 112, 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang AS, Northrup SL, Alexander JK, Vo KT, Edmands S, 2011. Long-term experimental hybrid swarms between moderately incompatible Tigriopus californicus populations: hybrid inferiority in early generations yields to hybrid superiority in later generations. Conserv. Genet. 12 (4), 895–909. [Google Scholar]
- Hwang AS, Pritchard VL, Edmands S, 2016. Recovery from hybrid breakdown in a marine invertebrate is stronger, faster and more repeatable under environmental stress. J. Evol. Biol. 29 (9), 1793–1803. [DOI] [PubMed] [Google Scholar]
- Innocenti P, Morrow EH, Dowling DK, 2011. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848. [DOI] [PubMed] [Google Scholar]
- Kelly MW, Stanford E, Grosberg RK, 2012. Limited potential for adaptation to climate change in a broadly distributed crustacean. Proc. R. Soc. B 279, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Austriaco NR, Zhang J, Guarente L, 1995. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 80, 485–496. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR, 1991. Evolution of senescence: late survival sacrificed for reproduction. Philos. Trans. R. Soc. B 332, 15–24. [DOI] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Van Montagu M, Inze D, 1998. Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J. 14 (6), 759–764. [DOI] [PubMed] [Google Scholar]
- Lee B-N, 1993. Genetic Structure of Tigriopus californicus Populations Inferred From Mitochondrial Cytochrome Oxidase I DNA Sequences. Ph.D. dissertation. University of Houston. [Google Scholar]
- Leech T, Sait SM, Bretman A, 2017. Sex-specific effects of social isolation on ageing in Drosophila melanogaster. J. Insect Physiol. 102, 12–17. [DOI] [PubMed] [Google Scholar]
- Leong W, Sun PY, Edmands S, 2018. Latitudinal clines in temperature and salinity tolerance in tidepool copepods. J Hered 109, 71–77. [DOI] [PubMed] [Google Scholar]
- Lerner IM, 1954. Genetic Homeostasis Oliver & Boyd, Edinburgh. [Google Scholar]
- Levine RL, Stadtman ER, 2001. Oxidative modification of proteins during aging. Exp. Gerontol. 36 (9), 1495–1502. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ER, Augustniak M, Kedziorski A, Keller L, 2017. Lifespan differences between queens and workers are not explained by rates of molecular damage. Exp. Gerontol. 92, 1–5. [DOI] [PubMed] [Google Scholar]
- Lynch, 1991. The genetic interpretation of inbreeding and outbreeding depression. Evolution 45 (3), 622–629. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Lummaa V, 2013. Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays 35, 717–724. [DOI] [PubMed] [Google Scholar]
- Mank J, 2017. The transcriptional architecture of phenotypic dimorphism. Nature Ecol Evol 1, 0006. [DOI] [PubMed] [Google Scholar]
- Matzkin L, Watts TD, Markow TA, 2007. Desiccation resistance in four Drosophila species: sex and population effects. Fly 1 (5), 268–273. [DOI] [PubMed] [Google Scholar]
- Matzkin L, Watts TD, Markow TA, 2009. Evolution of stress resistance in Drosophila: interspecific variation in tolerance to desiccation and starvation. Funct. Ecol. 23, 521–527. [Google Scholar]
- Medina MH, Morandi B, Correa JA, 2008. Copper effects in the copepod Tigriopus angulatus Lang, 1933: natural broad tolerance allows maintenance of food webs in copper-enriched coastal areas. Mar. Freshw. Res. 59, 1061–1066. [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboidl P, Pandolfi PP, Lanfrancone L, Pelicci PG, 1999. The p66(shc) adaptor protein controls oxidative stress response and life span in mammals. Nature 402 (6759), 309–313. [DOI] [PubMed] [Google Scholar]
- Murakami S, Johnson TE, 1996. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, 1994. NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. 59 FR. pp. 11146–11151.
- Niveditha S, Deepashree S, Ramesh SR, Shivanandappa T, 2017. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B. 187, 899–909. [DOI] [PubMed] [Google Scholar]
- Nowogrodzki A, 2017. Inequality in medicine. Nature 50 (550), S18–S19. [DOI] [PubMed] [Google Scholar]
- O’Brien NP, Feldman H, Grill EV, Lewis AG, 1988. Copper tolerance of the life history stages of the splashpool copepod Tigriopus californicus (Copepoda, Harpacticoida). Mar. Ecol. Prog. Ser. 44, 59–64. [Google Scholar]
- Penn DJ, Smith KR, 2007. Differential fitness costs of reproduction between the sexes. Proc. Natl. Acad. Sci. U. S. A. 104 (2), 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L, Hazan R, Breker M, Schuldiner M, Ben-Aroya S, 2013. Formation and dissociation of proteasome storage granules are regulated by cytosolic pH. J. Cell Biol. 201, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ, 2010. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 432, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Vojtovich L, Tower J, Davies KJA, 2013. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 55, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto LCD, Wong S, Carney C, Shen B, Tower J, Davies KJA, 2017. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaptation and resistance to oxidative stress in Drosophila melanogaster. Aging 9, 1153–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Raisuddin S, Kowk KWH, Leung KMY, Schlenk D, Lee J-S, 2007. The copepod Tigriopus: a promising marine model oranism for ecotoxicology and environmental genomics. Aquat. Toxicol. 83, 161–173. [DOI] [PubMed] [Google Scholar]
- Rawson PD, Burton RS, 2002. Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc. Natl. Acad. Sci. U. S. A. 99, 12955–12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Pomatto LC, Davies KJ, 2016. Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Asp. Med. 50, 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Juarez C, Pomatto LCD, Sieburth D, Davies KJA, 2017. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J. Gerontol. A Biol. Sci. Med. Sci. 72, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz C, Baty F, Streibig JC, Gerhard D, 2015. Dose-response analysis using R. PLoS One 10 (12), e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL, 1992. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp. Gerontol. 27 (241–150). [DOI] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Perez VI, 2010. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 48 (5), 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service PM, Hutchinson EW, Mackinley MD, Rose MR, 1985. Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol. Zool. 58 (4), 380–389. [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ, 2000. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 14, 1490–1498. [DOI] [PubMed] [Google Scholar]
- Sokolova IM, 2013. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 53, 597–608. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Selman C, 2011. The free radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan. BioEssays 33, 255–259. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers KS, Ashman T-L, 2011. Genetic architecture of sexual dimorphism in a subdioecious plant with a proto-sex chromosome. Evolution 65, 1114–1126. [DOI] [PubMed] [Google Scholar]
- Stearns SC, 1992. The Evolution of Life Histories. Oxford University Press, Oxford. [Google Scholar]
- Sun PY, Foley HB, Handschumacher L, Suzuki A, Karamanukyan T, Edmands S, 2014. Acclimation and adaptation to common marine pollutants in the copepod Tigriopus californicus. Chemosphere 112, 465–471. [DOI] [PubMed] [Google Scholar]
- Sun PY, Foley HB, Bao VWW, Leung KMY, Edmands S, 2015. Variation in tolerance to common marine pollutants among different populations in two species of the marine copepod Tigriopus. Environ. Sci. Pollut. Res. 22, 16143–16152. [DOI] [PubMed] [Google Scholar]
- Sun PY, Foley HB, Wu L, Nguyen C, Chaudhry S, Bao VWW, Leung KMY, Edmands S, 2018. Long-term laboratory culture causes contrasting shifts in tolerance to two marine pollutants in copepods of the genus Tigriopus. Environ. Sci. Pollut. Res. 25 (4), 3183–3192. [DOI] [PubMed] [Google Scholar]
- Svensson ML, Larsson J, 2007. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas 144, 25–32. [DOI] [PubMed] [Google Scholar]
- Tejeda MT, Arredondo J, Pérez-Staples D, Ramos-Morales P, Liedo P, Diaz-Fleischer F, 2014. Effects of size, sex and teneral resources on the resistance to hydric stress in the Tephritid fruit fly Anastrepha ludens strains. J. Insect Physiol. 70, 73–80. [DOI] [PubMed] [Google Scholar]
- Tower J, 2017. Sex-specific gene expression and life span regulation. Trends Endocrinol. Metab. 28 (10), 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R, 1985. Social Evolution. Benjamin Cummings, Menlo Park, CA. [Google Scholar]
- Vacquier VD, 1962. Hydrostatic pressure has a selective effect on the copepod Tigriopus. Science 135, 724–725.13870253 [Google Scholar]
- Vacquier VD, Belser WL, 1965. Sex conversion induced by hydrostatic pressure in the marine copepod Tigriopus californicus. Science 150, 1619–1621. [DOI] [PubMed] [Google Scholar]
- Voordouw MJ, Anholt BR, 2002a. Heritability of sex tendency in a harpacticoid copepod, Tigriopus californicus. Evolution 56 (9), 1754–1763. [DOI] [PubMed] [Google Scholar]
- Voordouw MJ, Anholt BR, 2002b. Environmental sex determination a splash pool copepod. Biol. J. Linn. Soc. 76, 511–520. [Google Scholar]
- Wheeler MW, Park RM, Bailer AJ, 2006. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 25 (5), 1441–1444. [DOI] [PubMed] [Google Scholar]
- Willett CS, 2010. Potential fitness trade-offs for thermal tolerance in the intertidal copepod Tigriopus californicus. Evolution 64, 2521–2534. [DOI] [PubMed] [Google Scholar]
- Willett CS, 2012. Hybrid breakdown weakens under thermal stress in population crosses of the copepod Tigriopus californicus. J Hered 103, 103–114. [DOI] [PubMed] [Google Scholar]
- Wolff JN, Pichaud N, Camus MF, Coté G, Blier PU, Dowling DK, 2016. Evolutionary implications of mitochondrial genetic effects on OXPHOS respiration and mitochondrial quantity change with age and sex in fruit flies. J. Evol. Biol. 29, 736–747. [DOI] [PubMed] [Google Scholar]
- Zwann B, Bijlsma R, Hoekstra RF, 1995. Direct selection on life span in Drosophila melanogaster. Evolution 49, 649–659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.