Abstract
Efficacious therapies for measurable metastatic canine osteosarcoma (OSA) are generally lacking. Preliminary retrospective studies suggested that approximately 50% of dogs with measurable metastatic OSA experienced clinical benefit (objective response or clinically meaningful disease stabilisation) following toceranib (TOC) treatment. The purpose of this clinical trial was to prospectively evaluate the clinical outcome following TOC treatment in dogs with measurable pulmonary metastatic OSA. A secondary goal was to identify potential biomarkers of clinical benefit by measuring changes in plasma vascular endothelial growth factor (VEGF) and circulating regulatory T-cell (Treg) percentage.
Twenty-two dogs with pulmonary metastasis from appendicular OSA having undergone previous amputation were treated prospectively with TOC. Adverse events (AEs) were common but predominantly low grade. Nine patients were withdrawn from the study prior to the week 8 assessment of response either due to progressive disease (PD), decreased quality of life or owner perceived unacceptable AEs. Of the patients evaluable for disease progression at week 8 (or earlier), 3/17 (17.6 %) had stable disease with the remainder having PD. The median progression-free survival time for all patients was 57 days (range 7-176 days) with a median overall survival time of 89 days (range 7-574 days). Plasma VEGF concentrations were significantly elevated in patients after 4 weeks of TOC treatment, but no changes were observed in percentage of Treg in peripheral blood. Overall, the results of this clinical trial do not support the use of TOC as single agent therapy for canine metastatic OSA.
Keywords: dog, metastasis, oncology, osteosarcoma, tyrosine kinase
1 |. INTRODUCTION
Appendicular osteosarcoma (OSA) is the most common primary bone tumour in the dog and is both locally aggressive and highly metastatic.1 Standard of care treatment, which consists of local control via amputation followed by adjuvant carboplatin chemotherapy, results in a median survival time range of 277 to 479 days.2–6 While less than 10% of patients have metastatic disease identified on initial presentation (stage III), 90% of patients will ultimately succumb to metastatic disease, most commonly to the lungs. The median survival time (MST) for stage III (those with distant metastasis) patients treated with various combinations of surgery, palliative radiation therapy and chemotherapy was 76 days in 1 study.7 In the aforementioned study, dogs treated with chemotherapy alone had a MST of only 37 days. Another study evaluating cisplatin for gross metastatic OSA reported a MST of 61 days with only 1 partial response to chemotherapy.8 Metastasectomy of pulmonary nodules has been performed, resulting in a MST of 128 days in 1 study, but only a select group of patients qualify for this approach.9 Because appendicular OSA is a common canine tumour and most patients will ultimately develop metastatic disease, effective treatment options for metastatic OSA represent an area of great interest to the veterinary oncology community.
Toceranib phosphate (TOC) (Palladia™, Zoetis, Parsippany, NJ USA) is an orally bioavailable small molecule which inhibits multiple receptor tyrosine kinases (RTK), including vascular endothelial growth factor receptor 2 (VEFGR2), platelet derived growth factor receptor (PDGFR), KIT and fms related tyrosine kinase (FLT-3).10 To demonstrate on-target effects of TOC, previous studies have shown increases in circulating plasma VEGF, which is a surrogate biomarker for VEGFR inhibition.11 In addition to the predicted cytotoxic and anti-angiogenic effects of TOC, a previous study demonstrated that circulating regulatory T lymphocyte (Treg) levels were significantly decreased in dogs treated with TOC.12 Reductions in Treg levels are predicted to improve anti-tumour immunity. The combination of anti-angiogenic effects via VEGFR inhibition, immunomodulatory effects and other unexplored effects of inhibiting RTK signalling, has prompted the empirical use of TOC in multiple different tumour types.11 One survey-based retrospective study, examining the benefits of TOC in multiple solid tumour types, suggested possible clinical benefit in OSA.13 In this study, of the 23 dogs with metastatic OSA treated with TOC, 1 (4.3%) experienced a partial response (at least 30% reduction in the size of lesions) and 10 (43.5%) experienced stable disease with a median duration of treatment of 24 weeks.13 These results prompted more widespread use of TOC for treatment of metastatic OSA. A more recent study prospectively treated patients with standard of care amputation followed by full course adjuvant carboplatin chemotherapy combined with maintenance piroxicam and metronomic cyclophosphamide +/− TOC. This study failed to show benefit for addition of TOC to metronomic cyclophosphamide with piroxicam.14
The purpose of the study presented herein was to prospectively evaluate the clinical benefit of TOC in the setting of gross metastatic pulmonary OSA. Changes in plasma VEGF concentrations and percentage of circulating regulatory T-cells (Treg) were also measured as indicators of drug exposure and potential predictive factors for patient response.
2 |. MATERIALS AND METHODS
2.1 |. Patient selection
Client-owned dogs, which were at least 1-year-old on day 0, having previously undergone amputation with a histologic diagnosis of OSA and having radiographically confirmed pulmonary metastatic disease, were considered for clinical trial enrollment. Eligibility criteria included Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v1.1) constitutional health score of 0 (normal activity) or 1 (mildly decreased from baseline), and life expectancy of > 6 weeks.15 Dogs were required to have adequate hematologic, renal and hepatic function to safely undergo treatment; defined as >2000 neutrophils/μL, >100 000 platelets/μL, hematocrit >25%, serum creatinine concentration <2.5 mg/dL, total serum bilirubin concentration ≤ the upper limit of normal (ULN), transaminase (ALT, AST) activity ≤ 3 times the ULN. If transaminases were >3 times the ULN then serum bile acids were required to be ≤ to the ULN.
Dogs were excluded if they had other serious comorbid diseases, including other malignancies or clinically significant extra-pulmonary metastases. Prior chemotherapy was allowed with a required 2-week washout period. Homoeopathic/alternative therapies were required to be discontinued on day –1. Supplements such as chondroitin sulphate, essential fatty acids and glucosamine were permitted. Patients could remain on non-steroidal anti-inflammatory drugs (NSAIDs) if they were started prior to study enrollment.
Baseline evaluations included medical history, physical examination, CBC, serum biochemistry, urinalysis, urine protein:creatinine ratio (UPC), thoracic radiographs and blood pressure. The Animal Care and Use Committees and/or Clinical Review Boards at each institution approved the clinical protocol, and written informed consent was obtained from the owners before patient enrollment.
2.2 |. Study protocol
This study was designed as a multicentre, single arm, phase II clinical trial. Toceranib was provided by Zoetis, Inc. Once enrolled in the clinical trial, patients received a target dosage of 2.75 mg/kg TOC PO on Monday, Wednesday and Friday. They were rechecked at weeks 2, 3, 4 and 8 following initiation of therapy. At weeks 2 and 3, physical examination and complete blood count were performed. At week 4 and every month thereafter physical examination, CBC, serum biochemistry, urinalysis, urine protein:creatinine ratio (UPC) and blood pressure were performed. Thoracic radiographs were obtained at week 8 and tumour response measurements were determined according to the VCOG Response Evaluation Criteria for Solid Tumours in Dogs (v1.0) (RECIST).16 If patients were deemed to have a response or stable disease they continued therapy and thoracic radiographs were repeated every 8 weeks thereafter.
Adverse events (AEs) were noted and graded according to the VCOG-CTCAE v1.1.15 Dose-limiting toxicities (DLT) were considered to be any grade 3 or higher non-hematologic toxicity or any grade 4 or higher hematologic toxicity. Any DLT was managed at the clinician’s discretion with dose interruptions and dose decreases being permitted. Any dose interruption of more than 14 consecutive days resulted in removal from the clinical trial.
2.3 |. Evaluation of biomarkers
Plasma and peripheral mononuclear blood cells (PBMC) were collected at week 0 (pre-treatment) and week 4 to evaluate for biomarkers of TOC response, including VEGF ELISA and Treg analysis. Both were processed immediately and frozen until analysis.
2.4 |. VEGF ELISA
The concentrations of VEGF were measured in aliquots of plasma using a commercially available human VEGF enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minnesota) that has been successfully used previously to measure canine VEGF,15–18 according to the manufacturer directions. Each plasma sample was assayed in duplicate and the mean value was used for all calculations.
2.5 |. Treg assessment
Briefly, cells were plated at a concentration of approximately 106 cells per well in 96-well plates. PBMCs then were immunostained for surface expression of CD4 with FITC-conjugated anti-canine CD4 monoclonal antibody (clone YKIX302.9) following the method described previously.19 Immunostaining for FoxP3 expression was performed as previously described with a cross-reactive, PE-conjugated murine FoxP3 antibody (clone FJK-16s).20 A directly conjugated rat IgG2A antibody was used as the isotype control.
Flow cytometry was performed with a CyAn ADP-flow cytometer and FlowJo software for data analysis. Analysis gates were set on the live lymphocyte population based on typical forward and side scatter characteristics.21 Treg were identified as based on dual expression of CD4 and FoxP3 following the method described previously.20 The percentage of Treg was calculated by determining the percentage of CD4+FoxP3+ cells within the CD4+ T-cell population.
2.6 |. Statistics
Continuous data were expressed as median and range, and categorical data as frequencies and percentages. Differences in continuous values over time were compared using a paired, 2-tailed t test or Mann-Whitney test depending on data normality. Correlations between continuous values were assessed using χ2 analysis. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of administration of the first dose of TOC to the date of PD or death, respectively. Dogs that died were considered to be dead either secondary to their treatment or their disease. Kaplan-Meier estimation was used to estimate and display PFS and OS. All statistical analyses were performed using a commercial software package (Prism v.7; GraphPad Software, La Jolla, California).
3 |. RESULTS
3.1 |. Patient population
A total of 24 dogs were prospectively enrolled in the clinical trial from November 2015 to September 2015 at the Flint Animal Cancer Center at Colorado State University (n = 9). The Ohio State University (n = 9) and the University of Wisconsin-Madison (n = 6). Two dogs were subsequently removed from statistical analysis as they were found to not meet entry criteria post-enrollment; 1 had a primary lesion that was not appendicular (rib) and the other was found to have metastatic histiocytic sarcoma rather than OSA at necropsy. Patient demographics, tumour location and previous chemotherapy treatments are presented in Table 1.
TABLE 1.
Patient demographics, tumour location and previous therapy
| Median (range) or frequency (%) | |
|---|---|
| Age | 8.5 (4-13) |
| Weight | 32.6 (16.8-61.7) |
| Sex | |
| MC | 14 (63.6) |
| M | 0 |
| FS | 7 (31.8) |
| F | 1 (4.5) |
| Breed | |
| Mixed | 5 (22.7) |
| Labrador retriever | 5 (22.7) |
| Greyhound | 2 (9) |
| Other (1 each) | 10 (45.4) |
| Tumour location | |
| Proximal humerus | 8 (36.3) |
| Distal radius | 5 (22.7) |
| Distal tibia | 3 (13.6) |
| Ulna | 2 (9) |
| Humerus (not specified) | 1 (4.5) |
| Fibula | 1 (4.5) |
| Proximal radius | 1 (4.5) |
| Not indicated | 1 (4.5) |
| Previous chemotherapy | |
| Carboplatin | 19 (86.3%) |
| Median carboplatin doses | 4 (1-6) |
| Carboplatin + Doxorubicin | 1 (4.5) |
| None | 2 (9) |
| Starting toceranib dose (mg/kg) | 2.69 (2.47-2.86) |
Abbreviations: F, female; FS, female spayed; M, male; MC, male castrated.
3.2 |. Patient dosing and toxicity
Patients were started on a target dose of 2.75 mg TOC on Monday, Wednesday and Friday. The actual dosage varied due to tablet sizes with the actual median starting dosage being 2.69 mg/kg on Monday, Wednesday and Friday (range 2.47-2.86 mg/kg). All 22 patients experienced AEs during the study period, with the majority limited to VCOG grade 1 and 2 toxicities (Table 2). The most common AEs included grade 1 diarrhoea (n = 9), grade 1 neutropenia (n = 6), grade 1 anaemia (n = 5) and grade 1 proteinuria (n = 4). Grade 2 hyporexia was the most common grade 2 toxicity (n = 3). Six grade 3 or 4 toxicities were seen, including 2 grade 3 hyporexia and 1 each of grade 3 severe multifocal pain, grade 3 motor neuropathy, grade 4 lethargy and grade 4 pyometra. The patient that developed pyometra was also deemed to have progressive disease and was subsequently euthanized.
TABLE 2.
Adverse events (AE) as defined by the Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events v. 1.1 (2011)
| Category | Term | Grade | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Hematologic | Neutropenia | 6 | 1 | ||
| Anaemia | 5 | ||||
| Thrombocytopenia | 1 | ||||
| Metabolic/Laboratory | ALT elevation | 1 | |||
| ALP elevation | 3 | 1 | |||
| AST elevation | 1 | ||||
| Creatine kinase elevation | 1 | ||||
| Hyperglycaemia | 1 | ||||
| BUN elevation | 1 | ||||
| Albumin, low | 2 | ||||
| Hypernatreamia | 1 | ||||
| Hypokalemia | 1 | ||||
| Hypomagnesemia | 1 | ||||
| Hyperglobulineamia | 1 | ||||
| Gastrointestinal | Vomiting | 2 | |||
| Nausea | 2 | ||||
| Diarrhoea | 9 | ||||
| Anorexia | 3 | 3 | 2 | ||
| GI ulceration | 1 | ||||
| Constitutional | Lethargy | 3 | 1 | 1 | |
| Weight loss | 1 | 1 | |||
| Fever | 1 | ||||
| Renal | Proteinuria | 4 | 1 | ||
| Polyuria | 1 | ||||
| Cardiovascular | Hypertension | 3 | 2 | ||
| Musculoskeletal | Lameness | 2 | |||
| Severe multifocal spinal pain | 1 | ||||
| Muscle weakness | 1 | ||||
| Bilateral tarsal effusion | 1 | ||||
| Soft tissue pain | 1 | ||||
| Neurologic | Seizure | 1 | |||
| Motor neuropathy | 1 | ||||
| Pulmonary/respiratory | Cough | 1 | |||
| Tachypnea | 1 | ||||
| Dyspnea | 1 | ||||
| Pneumothorax | 1 | ||||
| Epistaxis | 1 | ||||
| Reproductive | Pyometra | 1 | |||
| Ocular | Discharge | 2 | |||
Four patients had 1 week dosing delays secondary to AEs (1 each of diarrhoea, hind limb weakness, motor neuropathy and neutropenia). Two patients had delays of a single dose secondary to AEs (hyporexia and diarrhoea). Two patients had dose reductions (14% and 18.8% reductions) secondary to AEs (diarrhoea and neutropenia). Two additional patients had dose reductions based on weight loss and 1 patient had a dose escalation to the full dose after starting on a lower dose.
3.3 |. VEGF ELISA
Eighteen patients had plasma samples evaluable for VEGF measurement prior to starting TOC and 4 weeks post-treatment initiation. There was a significant increase in VEGF when comparing pre-and post-treatment samples (P = .0031, Figure 1A). Plasma VEGF was increased in 15 dogs, decreased in 1 dog, and undetectable at both time points in 2. There was no statistical correlation between basal VEGF or magnitude of change following TOC treatment and clinical outcome (Figure 1B). There was no correlation between magnitude of VEGF change and actual administered TOC dose.
FIGURE 1.

Changes in plasma vascular endothelial growth factor concentrations following toceranib treatment. A, Scatter plot depicting individual and mean +/− SEM plasma VEGF concentrations for 18 dogs prior to and 28 days following toceranib initiation. B, Individual mean (+/− SEM) pre- and post-toceranib values. Arrows indicate dogs experiencing stable disease at the 8-week recheck.
3.4 |. Treg
Seventeen patients had peripheral blood mononuclear cells evaluable for determination of Treg percentage prior to starting TOC and 4 weeks post-treatment initiation. There was no significant difference in Treg percentage when comparing pre-and post-treatment samples (P = .33, Figure 2A). The Treg percentage decreased in 8 patients and increased in 7 patients. There was no statistical correlation between basal Treg percentage or magnitude of change following TOC treatment and clinical outcome (Figure 2B). There was no correlation between magnitude of Treg change and actual administered TOC dose; however, there was a modest but significant negative correlation between change in VEGF and change in Treg percentage (r2 =0.31, P = .048).
FIGURE 2.

Changes in regulatory T-cell percentage following toceranib treatment. A, Scatter plot depicting individual and mean +/− SEM Treg number for 18 dogs prior to and 28 days following toceranib initiation. B, Individual mean (+/− SEM) pre- and post-toceranib values. Arrows indicate dogs experiencing stable disease at the 8-week recheck.
3.5 |. Outcome
Nine patients were removed from the study prior to the week 8 assessment of response. These patients were removed either secondary to owner preference due to perceived AEs of TOC or decreased quality of life (N = 3), progressive disease detected prior to week 8 (N = 4) or some combination of these factors (N = 2). Of these patients, 2 were removed due to progressive hind limb weakness and ataxia of undefined origin. Two were removed for combinations of hyporexia, hypertension and proteinuria where the owner declined dose delays or medical management of these conditions. One patient was removed due to a general decrease in quality of life that was not readily differentiated between disease progression and AEs of TOC. Four patients were removed for progressive disease at weeks 2, 3, 5 and 6. Three of these patients were diagnosed with progressive disease based on multiple new pulmonary nodules, progression of previously noted nodules or a combination of these 2 findings. One patient was diagnosed with pneumothorax in addition to progressive pulmonary nodules.
Of the 13 patients remaining on study to the week 8 recheck thoracic radiographs, 10 had progressive disease noted at that time based on VCOG RECIST criteria. Eight of these patients demonstrated either progression of previous nodules, new nodules or a combination of new nodules and progression of previously identified nodules. One patient had stable pulmonary nodules but progression of a non-target presumed metastatic rib lesion. One patient had progressive pulmonary nodules along with multiple lytic bone lesions in other sites (humerus, scapula, rib), which were suspected to be metastatic in nature. For the 17 patients that were available for repeat thoracic radiographs (including the patients having been diagnosed with progressive disease prior to week 8), 3 patients were stable while 14 were progressive for a clinical benefit rate of 3/17 (17.6%). The median duration of SD in these 3 patients was 112 days (84-176 days).
The median PFS for all patients was 57 days (range 7-176 days) (Figure 3). The 5 patients that were removed from the trial for AEs or decreasing quality of life that did not have repeat thoracic radiographs were censored from PFS at the time of removal from the trial. The median OS time was 89 days (range 7-574 days). Four patients were lost to follow-up after withdrawal from the clinical trial. These patients were censored from survival analysis at the time of last follow-up.
FIGURE 3.
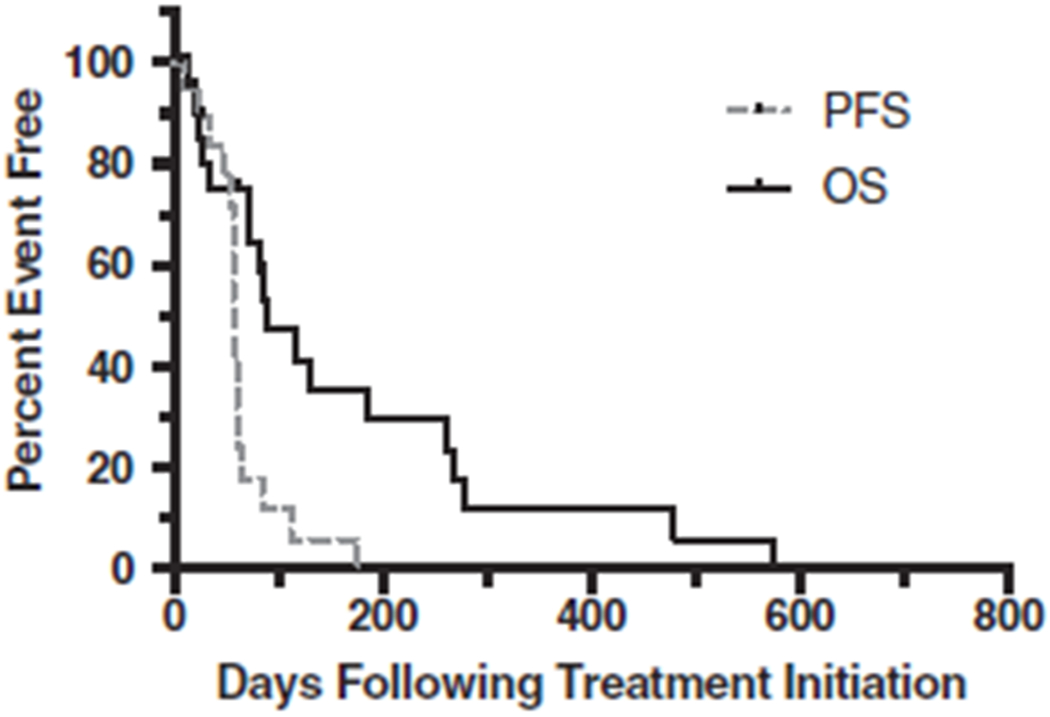
Kaplan-Meier curves showing progression-free and overall survival time from the time of initiation of treatment for metastatic osteosarcoma with toceranib. The median progression-free survival time was 57 days and the median overall survival time was 89 days.
4 |. DISCUSSION
Currently, there are no effective treatment options for metastatic OSA in dogs. Because of the lack of options, TOC is offered to many patients based on the reported 48% clinical benefit rate identified in a previously reported survey-based retrospective study of TOC use for multiple solid tumour types;13 however, prospective data to support the effectiveness of this approach are lacking. In this prospective clinical trial, we sought to characterise the clinical benefit rate in dogs with metastatic OSA treated with TOC. As a secondary measure, we sought to identify changes in circulating Treg and/or VEGF to identify biomarkers that would be useful for prognostication of response in future patients. The observed 17.6% clinical benefit rate and median PFS and OS of 57 days and 89 days, respectively, is not superior to what has been reported historically.7,8 The AEs observed with TOC therapy were similar in type and grade as in previous studies, with the exception of the grade 4 pyometra.14,17 Limitations of the current study include a relatively small patient number of 22 enrolled patients with only 17 patients having repeat imaging to assess response; however, the observed clinical benefit rate in this study was so low that it is unlikely that a significantly higher response/clinical benefit rate would be observed with enrollment of larger numbers of patients.
In the previously discussed retrospective analysis of TOC for solid tumours in which there was an approximately 50% response rate to TOC, there was a subset of patients that received concurrent NSAIDs and/or metronomic cyclophosphamide;13 however, within that study there is no indication of which of the OSA patients received concurrent NSAIDs and/or metronomic cyclophosphamide to identify any differences in benefit rate. The rationale for the combination of TOC with metronomic cyclophosphamide and NSAIDs is to improve anti-angiogenic and immunomodulatory effects. It is known that angiogenesis is an important process in most solid tumours, including canine OSA where pre-treatment VEGF concentrations have been correlated with disease free interval (DFI) and OS.18 Microvessel density of the primary tumour also predicted early metastasis in 1 study.19 Metronomic (low-dose continuous) chemotherapy is proposed to exert anti-angiogenic effects via induction of endothelial cell apoptosis along with decreases in circulating Tregs, which stimulates improved anti-tumour immunosurveillance.20 A combination of TOC and metronomic cyclophosphamide has been shown to reduce circulating Tregs and increase circulating interferon-γ in a canine study.12 With the rationale outlined above, a prospective clinical trial was recently performed and no benefit was detected with combination metronomic cyclophosphamide and TOC in a maintenance protocol following adjuvant carboplatin chemotherapy for canine OSA.14 It must be noted that the cyclophosphamide dose utilised in this study was less than that considered by others to be optimal, and less than that used in the study evaluating changes in Treg.21 Regardless, the failure of this combination in the microscopic disease setting does not necessarily predict failure in the gross disease setting, especially since a similar approach using metronomic chemotherapy has been used in recurrent human glioblastoma multiforme (GBM) with some success.22 Whether such a combinatorial approach would yield better results in measurable metastatic OSA, as opposed to the microscopic disease setting, remains to be seen and would best be tested in a prospective clinical trial.
The lack of Treg decrease with TOC treatment was somewhat surprising. This is especially true considering the effect TOC had on VEGF concentrations, indicating that the drug was active in these patients. There was a significant correlation between VEGF levels and Treg numbers. With very few reports of Treg response to TOC (or other tyrosine kinase inhibitors) in the veterinary literature, this is an area in which more data are needed.
In conclusion, TOC appears to have limited clinical benefit when utilised as a single agent in the setting of gross metastatic OSA, despite indications to the contrary in at least 1 previous retrospective study. This reinforces the fact that while retrospective studies might be useful to identify possible relationships, prospective clinical trials are needed to assess the actual clinical benefit of different treatment strategies.
ACKNOWLEDGEMENTS
The authors acknowledge the clinical trials services at Colorado State University, The Ohio State University and the University of Wisconsin veterinary teaching hospitals for recruitment and care of study patients. The authors also acknowledge Barbara Rose at Colorado State University, who performed the VEGF ELISAs.
Footnotes
Conflict of interest
Financial support for this study was provided by Zoetis, Inc. DMV, CAL and DHT are or have served as paid consultants for Zoetis.
REFERENCES
- 1.Ehrhart NP, Ryan SD, Fan TM. Tumors of the skeletal system In: Withrow SJ, Vail DM, Page RL, eds. Withrow & MacEwen’s Small Animal Clinical Oncology. 5th ed. St. Louis, MO: Elsevier; 2013:463–503. [Google Scholar]
- 2.Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med. 1996;10(2):76–81. [DOI] [PubMed] [Google Scholar]
- 3.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28(2):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skorupski KA, Uhl JM, Szivek A, Allstadt Frazier SD, Rebhun RB, Rodriguez CO Jr. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol. 2016;14(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saam DE, Liptak JM, Stalker MJ, Chun R. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996-2006). J Am Vet Med Assoc. 2011;238(2):195–206. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B, Powers BE, Dernell WS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc. 2009;45(1):33–38. [DOI] [PubMed] [Google Scholar]
- 7.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985-2004). J Am Vet Med Assoc. 2006;228(12):1905–1908. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie GK, Straw RC, Jameson VJ, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987-1991). J Am Vet Med Assoc. 1993;202(2):304–306. [PubMed] [Google Scholar]
- 9.O’Brien MG, Straw RC, Withrow SJ, et al. Resection of pulmonary metastases in canine osteosarcoma: 36 cases (1983–1992). Vet Surg. 1993;22(2):105–109. [DOI] [PubMed] [Google Scholar]
- 10.London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9(7):2755–2768. [PubMed] [Google Scholar]
- 11.Bernabe LF, Portela R, Nguyen S, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res. 2013;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell L, Thamm DH, Biller BJ. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J Vet Intern Med. 2012;26(2):355–362. [DOI] [PubMed] [Google Scholar]
- 13.London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia((R))) in solid tumours. Vet Comp Oncol. 2012;10(3):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London CA, Gardner HL, Mathie T, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: a multi-institutional study. PLoS One. 2015;10(4):e0124889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veterinary Cooperative Oncology Group. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14:417–446. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176–183. [DOI] [PubMed] [Google Scholar]
- 17.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856–3865. [DOI] [PubMed] [Google Scholar]
- 18.Thamm DH, O’Brien MG, Vail DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet Comp Oncol. 2008;6(2):126–132. [DOI] [PubMed] [Google Scholar]
- 19.Coomber BL, Denton J, Sylvestre A, Kruth S. Blood vessel density in canine osteosarcoma. Can J Vet Res. 1998;62(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 20.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 21.Burton JH, Mitchell L, Thamm DH, Dow SW, Biller BJ. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med. 2011;25(4):920–926. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Xu T, Lu Y, Chen J, Wu S. The efficacy of temozolomide for recurrent glioblastoma multiforme. Eur J Neurol. 2013;20(2):223–230. [DOI] [PubMed] [Google Scholar]


