Abstract
The mechanism of resistance in carbapenem-resistant Enterobacteriaceae (CRE) has therapeutic implications. We comprehensively characterized emerging mechanisms of resistance in CRE between 2013 and 2016 at a health system in Northern California. A total of 38.7% (24/62) of CRE isolates were carbapenemase gene-positive, comprising 25.0% (6/24) blaOXA-48 like, 20.8% (5/24) blaKPC, 20.8% (5/24) blaNDM, 20.8% (5/24) blaSME, 8.3% (2/24) blaIMP, and 4.2% (1/24) blaVIM. Between carbapenemases and porin loss, the resistance mechanism was identified in 95.2% (59/62) of CRE isolates. Isolates expressing blaKPC were 100% susceptible to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam; blaOXA-48 like–positive isolates were 100% susceptible to ceftazidime–avibactam; and metallo β-lactamase–positive isolates were nearly all nonsusceptible to above antibiotics. Carbapenemase gene-negative CRE were 100% (38/38), 92.1% (35/38), 89.5% (34/38), and 31.6% (12/38) susceptible to ceftazidime–avibactam, meropenem–vaborbactam, imipenem–relebactam, and ceftolozane–tazobactam, respectively. None of the CRE strains were identical by whole genome sequencing. At this health system, CRE were mediated by diverse mechanisms with predictable susceptibility to newer β-lactamase inhibitors.
Keywords: Carbapenem-resistant Enterobacteriaceae, Carbapenemase, Porin, Avibactam, Relebactam, Vaborbactam
1. Introduction
Carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) have successfully spread worldwide over the recent decades (Munoz-Price et al., 2013; Temkin et al., 2014). In some regions, CP-CRE have become endemic in hospital settings (Ray et al., 2016; Temkin et al., 2014). The proportion of CRE in acute-care hospitals in the United States has increased steadily (CDC, 2013; Gohil et al., 2017; Guh et al., 2014). Infection with CRE is associated with increased morbidity and mortality (Qureshi et al., 2012; Tamma et al., 2017; Tzouvelekis et al., 2014). Successful efforts to decrease rates of CRE infection in the United States are largely attributed to timely initiation of targeted-antimicrobial therapy by early diagnosis and early implementation of infection prevention precautions aimed to prevent nosocomial spread (Woodworth et al., 2018).
The main mechanisms of resistance to carbapenems in CRE include hydrolysis of carbapenems by a plasmid-encoded carbapenemase, impaired outer membrane permeability due to inactivation of particular porins (i.e., OmpC and OmpF in Escherichia coli and their analogs) coupled with high-level expression of cephalosporinases such as AmpC and/or extended-spectrum β-lactamase (ESBL), or a combination of these mechanisms (Temkin et al., 2014; Tzouvelekis et al., 2012). Moreover, CRE commonly encode genetic determinants of resistance to other classes of antibiotics, rendering them pan-resistant (Elemam et al., 2009). The underlying mechanism of resistance in CRE has prognostic and therapeutic implications for the newer β-lactam combination drugs that are approved by the FDA (e.g., ceftazidime–avibactam and meropenem–vaborbactam) and for those in clinical trials (e.g., imipenem–relebactam) (Lapuebla et al., 2015; Lob et al., 2017; Tamma et al., 2017; Vasoo et al., 2015). For example, in vitro studies have shown the ceftazidime–avibactam combination to be effective against isolates harboring serine carbapenemases such as class A β-lactamases Klebsiella pneumoniae carbapenemase (KPC) and class D β-lactamases OXA-48 but not class B metallo β-lactamases [Verona integron encoded Metallo β-lactamse (VIM), IMP, or New Delhi Metallo β-lactamase (NDM)] (Vasoo et al., 2015). However, metallo β-lactamase–producing CRE are susceptible to avibactam when combined with aztreonam (Lapuebla et al., 2015; Lob et al., 2017). Thus, it is important to know both the local prevalence of CRE resistance mechanisms and the in vitro susceptibility to newer β-lactam–β-lactamase inhibitor antibiotics.
Although the incidence of CRE has been lower on the West Coast of the United States compared with Eastern states (CDC, 2013), region-specific data are not available, and comprehensive phenotypic and genotypic characterization of CRE isolates in Northern California has not been performed. We have previously reported sporadic isolation of CP-CRE at our institution (Green et al., 2013; Limbago et al., 2011). The aim of this study was to obtain clinical information and comprehensively characterize the mechanism of carbapenem resistance in CRE isolates at our institution in Northern California over a 4-year period and to correlate the underlying mechanisms of resistance with susceptibility to newer β-lactam–β-lactamase inhibitors.
2. Materials and methods
2.1. Ethics
This study was approved by the Stanford University Internal Review Board.
2.2. CRE isolates
All consecutive CRE isolates from Stanford Health Care or Lucille Packard Children's Health were included between January 2013 and December 2016. CRE from all sources were included. CRE were defined per the pre-2015 Centers for Disease Control and Prevention (CDC) CRE surveillance definition (Chea et al., 2015) as nonsusceptibility to imipenem (i.e., MIC >1 μg/mL), meropenem (i.e., MIC >1 μg/mL), or doripenem (i.e., MIC >1 μg/mL) and resistance to all third-generation cephalosporins tested except for Serratia marcescens–expressing blaSME, which can be susceptible to third-generation cephalosporins. Further details can be found in the Supplementary Material section.
2.3. Chart review
Electronic medical records were reviewed to obtain demographics and clinical characteristics of patients with CRE isolates.
2.4. Antibiotic susceptibility testing
Carbapenem susceptibility testing was performed prospectively on MicroScan WalkAway plus System (Beckman Coulter, San Diego, CA) for nonurinary isolates and Vitek 2 (bioMerieux, Durham, NC) for urinary isolates. Urinary isolates were retrospectively tested using the MicroScan WalkAway plus System (Beckman Coulter) for comparison to nonurine isolates. Imipenem- and meropenem-nonsusceptible (i.e., intermediate or resistant) isolates were confirmed using disk diffusion. Susceptibility testing for ceftolozane–tazobactam and ceftazidime–avibactam was performed by Etest (bioMérieux, Durham, NC), and that for meropenem–vaborbactam was performed by MIC test strip (Liofilchem Diagnostici, Teramo, Italy). Imipenem–relebactam was tested with microbroth dilution method at a fixed concentration of 4 μg/mL for relebactam. Susceptibility testing and interpretation of results were done according to Clinical and Laboratory Standards Institute (CLSI) criteria (CLSI, 2016) except for imipenem–relebactam and meropenem–vaborbactam which were interpreted using imipenem CLSI MIC breakpoints and package insert, respectively (CLSI, 2016). Resistance was defined as ≥16/4 μg/mL for ceftazidime–avibactam and meropenem–vaborbactam and ≥8/4 μg/mL for ceftolozane–tazobactam.
2.5. Genotypic β-lactamase testing
CRE isolates were prospectively tested for blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48 like using a laboratory-developed PCR assay, and results were reported to providers. Isolates were retrospectively screened for plasmid-encoded ESBL and AmpC cephalosporinases using the Check-Points CT 103 XL Check-MDR assay (Wageningen, the Netherlands). Detection of additional carbapenemase genes was carried out with laboratory-developed PCR assays targeting blaSME, blaSIM, blaSPM, blaGES, blaIMI, blaNMC-A, and blaGIM (Supplementary Table 1), as well as Xpert Carba-R cartridge (Cepheid, Sunnyvale, CA) and Check-Points assay. Assay details and control isolates are presented in the Supplementary Material section.
2.6. Carbapenemase activity
CRE isolates were tested for carbapenemase activity using the modified carbapenem inactivation method (mCIM) per the CLSI. Isolates with indeterminate mCIM result were tested for carbapenemase activity (i.e., imipenem degradation) with MALDI-TOF (Bruker Daltonics) as previously described (Lasserre et al., 2015).
2.7. Porin protein expression
Levels of OmpC and OmpF in E. coli and their analogs in other species were measured using mass spectrometry. Fold change in relative porin expression was determined by calculating the ratio of each porin in CRE isolates to averaged expression in four pan-sensitive strains of the same species. Assay details are provided in the Supplementary Material section.
2.8. Porin RNA expression
Quantitative reverse transcription-PCR (qRT-PCR) for porin genes (ompC and ompF in E. coli and their analogs in other species) was performed on the 39 CRE isolates recovered between 2013 and 2015. Expression of porin genes was normalized to rpoB in the same sample. Fold change in porin expression was determined by calculating the ratio of normalized porin expression in CRE isolates to a pan-sensitive control strain of the same species. Assay details are presented in the Supplementary Material section.
2.9. Whole genome sequencing
Sequencing was performed on the Illumina HiSeq 4000 platform to approximately 100× coverage per strain. Sequencing and analysis details are shown in the Supplementary Material section.
2.10. Statistical analysis
Fisher's exact test was used to compare differences in proportions. Statistical analysis was done with the software GraphPad Prism 5.0, San Diego, CA.
3. Results
3.1. CRE rates
Between 2013 and 2016, out of 19,271 nonduplicate Enterobacteriaceae isolates with antibiotic susceptibility results, 62 (0.32%) CRE isolates from 61 patients were identified (Supplementary Table 4). Of the 25 isolates that were tested by both MicroScan and Vitek, 21 were within 1 doubling dilution, and all 25 isolates were nonsusceptible to imipenem and/or meropenem by disk diffusion. Demographic and clinical characteristics of patients with CRE isolates are shown in Table 1.
Table 1.
Demographics and clinical characteristics of patients with CRE isolates.
| Characteristic | No. (%) (n = 61) |
|---|---|
| Female | 25 (41) |
| Age, median (range) | 56 (1–86) |
| Inpatient | 34 (55.7) |
| Comorbidities | |
| Immunosuppression | 33 (54) |
| Solid-tumor malignancy | 13 (21.3) |
| Solid-organ transplant | 11 (18) |
| Hematological malignancy | 8 (13.1) |
| HSCT | 1 (1.6) |
| HIV-AIDS | 0 (0) |
| Diabetes | 12 (19.6) |
| Congestive heart failure | 10 (16.4) |
| Cirrhosis | 4 (6.5) |
| Structural lung disease | 4 (6.5) |
| Major surgery within 30 days | 4 (6.5) |
| Prematurity | 1 (1.6) |
| No comorbidities | 7 (11.5) |
| Specimen source | |
| Urinary tract | 24 (39.3) |
| Blood | 12 (19.7) |
| Intra-abdominal | 8 (13.1) |
| Lower respiratory tract | 7 (11.5) |
| Osteoarticular | 4 (6.5) |
| Biliary tract | 2 (3.3) |
| Skin soft tissue | 2 (3.3) |
| Genital | 1 (1.6) |
| Respiratory sinus | 1 (1.6) |
HSCT = hematopoietic stem cell transplant.
Annual CRE rates between 2013 and 2016 did not vary significantly (0.22%, 0.39%, 0.38%, and 0.32%, respectively) (Supplementary Table 4). When the post-2015 CDC CRE surveillance definition was applied to 2016 cultures, the CRE rate increased from 0.32% to 0.89% (53/5968) (Chea et al., 2015). CRE species included Klebsiella pneumoniae (n = 19), Enterobacter cloacae complex (n = 14), Escherichia coli (n = 11), Enterobacter aerogenes (n = 8), Serratia marcescens (n = 7), and Citrobacter freundii complex (n = 3). Carbapenem MICs for CRE isolates ranged from ≤0.5 to >4 μg/mL (interquartile range [IQR], 4 to >4) for ertapenem, ≤1 to >8 μg/mL (IQR, ≤1 to >8) for imipenem, and ≤1 to >8 μg/mL (IQR, ≤1 to >8) for meropenem.
3.2. Genotypic carbapenemase testing
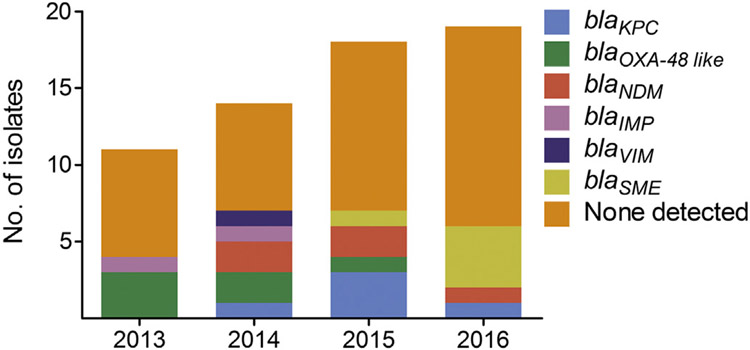
CRE isolates were genotypically tested for previously characterized plasmid-encoded and chromosomally encoded carbapenemases using the Xpert Carba-R cartridge, Check-Points microarray, and lab-developed multiplexed, real-time PCR assays. A single carbapenemase gene was detected in 38.7% (24/62) of CRE isolates which consisted of blaOXA-48 like (n = 6), blaNDM (n = 5), blaKPC (n = 5), blaSME (n = 5), blaIMP (n = 2), and blaVIM (n = 1) Fig. 1 and Supplementary Table 5). The bacterial species encoding carbapenemase genes included E. coli, E. cloacae complex, K. pneumoniae, and S. marcescens. The remaining 61.3% (38/62) of CRE isolates were negative for blaKPC, blaNDM, blaIMP, blaVIM and blaOXA-48 like, blaSME, blaSIM, blaSPM, blaGES, blaIMI, blaNMC-A, and blaGIM. The species in this group included C. freundii complex, E. coli, E. cloacae complex, E. aerogenes, K. pneumoniae, and S. marcescens. Annual non–CP-CRE rates out of total CRE rates between 2013 and 2016 did not vary significantly (63.6%, 50.0%, 61.1%, and 68.4%, respectively). The “SPACE” organisms that are likely to carry chromosomal AmpC such as Serratia, Citrobacter, and Enterobacter made up 63.2% (24/38) of CRE isolates lacking a carbapenemase gene compared with 33.3% (8/24, P = 0.04) of isolates harboring a carbapenemase gene. ESBL and plasmid-encoded AmpC cephalosporinases were detected with the Check-Points assay in 75.0% (18/24) and 20.8% (5/24), respectively, of carbapenemase gene-positive and 34.2% (13/38, P = 0.004) and 28.9% (11/38, P = 0.6), respectively, of carbapenemase gene-negative isolates. Either an ESBL or plasmid-encoded AmpC gene was detected in 79.2% (19/24) of carbapenemase gene-positive and 60.5% (23/38, P = 0.2) of carbapenemase gene-negative isolates. All 5 carbapenemase gene-positive CRE isolates without an ESBL or AmpC gene were blaSME-positive S. marcescens isolates. Compared with carbapenemase gene-negative CRE, a higher proportion of carbapenemase gene-positive CRE showed an elevated imipenem and meropenem MIC >8 (15.8% vs. 45.8%, P < 0.02; 10.5% vs. 58.3%, P < 0.001, respectively) (Supplementary Fig. 1).
Fig. 1.
Carbapenemase genes detected in CRE isolates annually from 2013 to 2016. Carbapenemase genes tested include blaKPC, blaNDM, blaIMP, blaVIM, blaOXA-48 like, blaSME, blaSIM, blaSPM, blaGES, blaIMI, blaNMC-A, and blaGIM.
3.3. Phenotypic carbapenemase testing
To determine whether carbapenemase gene-negative CRE isolates display carbapenemase activity (presumably due to previously uncharacterized carbapenemases), we performed mCIM on all CRE isolates (Pierce et al., 2017). While 100% (24/24) of carbapenemase gene-positive CRE isolates were mCIM positive, 86.8% (33/38) of carbapenemase gene-negative CRE isolates were mCIM negative, and the remaining 13.2% (5/38) were mCIM intermediate. The mCIM-indeterminate isolates were further tested with a MALDI-TOF–based carbapenemase activity assay (Lasserre et al., 2015). All 5 mCIM-indeterminate isolates were confirmed carbapenemase negative, while all carbapenemase gene-positive CRE isolates evaluated tested positive for imipenem hydrolysis.
3.4. Porin expression
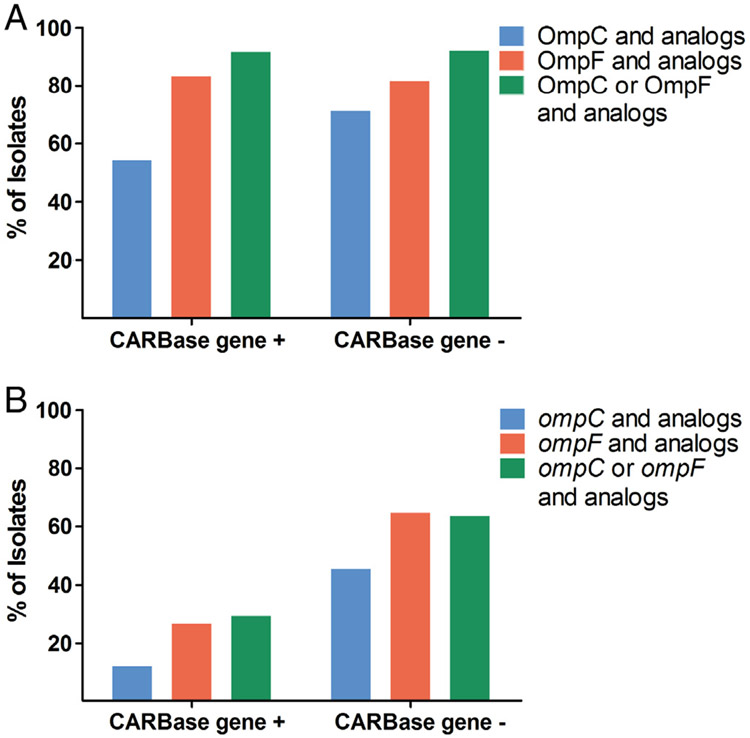
To determine whether porin expression is lower in CRE isolates without a carbapenemase gene, a novel mass spectrometry–based assay was employed to measure porin proteins in all 62 CRE isolates irrespective of their species. Relative porin levels of OmpC and OmpF and their analogs were down 2-fold or greater in 54.2% (13/24) and 83.3% (20/24) of carbapenemase gene-positive and 71.1% (27/38, P = 0.3) and 81.6% (31/38, P = 1.0) of carbapenemase gene-negative CRE isolates, respectively (Fig. 2A and Supplementary Table 5).
Fig. 2.
Porin protein and mRNA levels in CRE isolates with and without a carbapenemase gene. Bars show percentage of carbapenemase gene-positive (CARBase gene +) and gene-negative (CARBase gene−) CRE isolates with relative protein (A) and porin mRNA (B) down 2-fold or more compared with susceptible isolates.
The expression of either OmpC or OmpF and their analogs was decreased in 91.7% (22/24) of carbapenemase gene-positive CRE isolates compared with 92.1% (35/38, P = 1.0) of carbapenemase gene-negative CRE isolates. The two carbapenemase gene-positive isolates with normal porin levels were both blaSME-positive S. marcescens (CRE35 and 49); 3 carbapenemase gene-negative CRE isolates with normal porin levels were 2 strains of E. cloacae complex (CRE71 and 81) and 1 C. freundii complex (CRE21).
We also performed qRT-PCR on 39 CRE isolates recovered between 2013 and 2015 to measure porin mRNA transcripts in CRE isolates. The expression of ompC and ompF and their analogs was downregulated 2-fold or more in 11.8% (2/17) and 26.7% (4/15) of carbapenemase gene-positive CRE isolates compared with 45.5% (10/22, P = 0.04) and 64.7% (11/17, P = 0.04) of carbapenemase gene-negative CRE isolates, respectively. In carbapenemase gene-negative CRE isolates, 63.6% (14/ 22) showed downregulation of either ompC or ompF and their analogs compared with 29.4% (5/17) in carbapenemase gene-positive CRE (P = 0.05) (Fig. 2B and Supplementary Table 5).
3.5. Susceptibility of CRE to newer β-lactam/β-lactamases inhibitors
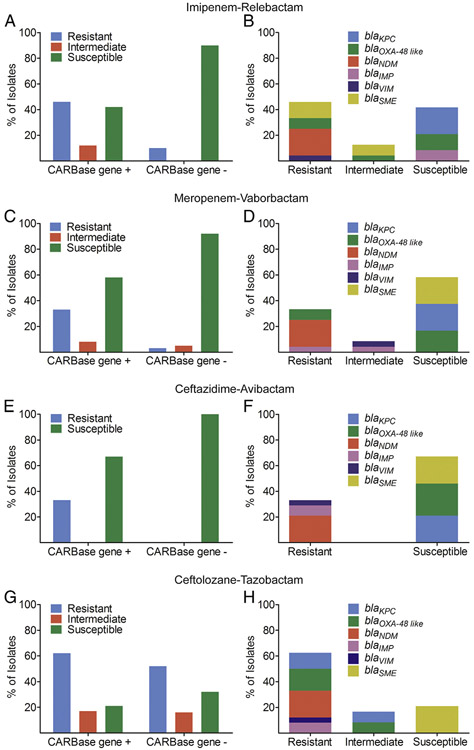
Among carbapenemase-positive CRE isolates, 41.7% (10/24), 58.3% (14/24), and 66.7% (16/24) were susceptible to imipenem–relebactam, meropenem–vaborbactam, and ceftazidime–avibactam, respectively (Fig. 3 and Table 2).
Fig. 3.
Susceptibility of CRE isolates to imipenem–relebactam, meropenem–vaborbactam, ceftazidime–avibactam, and ceftolozane–tazobactam. Bars show percent susceptibility of carbapenemase gene-positive (CARBase gene +) and gene-negative (CARBase gene−) CRE to (A) imipenem–relebactam, (C) meropenem–vaborbactam, (E) ceftazidime–avibactam, and (G) ceftolozane–tazobactam. Graphs on the right (B, D, F, H) show fraction of carbapenemase genes detected in carbapenemase gene-positive isolates that are susceptible, intermediate, or resistant to the respective antibiotic combination.
Table 2.
Antimicrobial susceptibility of CRE isolates.
| Antimicrobial agent | No. (%) of susceptible isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All CRE (n = 62) |
Non–CP-CRE (n = 38) |
CP-CRE (n = 24) | |||||||
| All CP-CRE | blaOXa-48 like (n = 6) |
blaKPC (n = 5) |
blaNDM (n = 5) |
blaIMP (n = 2) |
blaVIM (n = 1) |
blaSME (n = 5) |
|||
| Carbapenems | |||||||||
| Imipenem | 21 (33.9) | 16 (42.1) | 5 (20.8) | 3 (50) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) |
| Meropenem | 21 (33.9) | 17 (44.7) | 4 (16.7) | 4 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ertapenem | 4 (6.5) | 3 (7.9) | 1 (4.2) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Monobactams | |||||||||
| Aztreonam | 8 (12.9) | 3 (7.9) | 5 (20.8) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 1 (100) | 3 (60) |
| Cephems | |||||||||
| Ceftriaxone | 5 (8.1) | 0 (0) | 5 (20.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| Ceftazidime | 7 (11.3) | 2 (5.3) | 5 (20.8) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (80) |
| Cefepime | 25 (40.3) | 18 (47.4) | 7 (29.2) | 1 (16.7) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| β-Lactamase inhibitor combinations | |||||||||
| Piperacillin–tazobactam | 12 (19.4) | 8 (21.1) | 4 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (80) |
| Ceftolozane–tazobactam | 17 (27.4) | 12 (31.6) | 5 (20.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| Ceftazidime–avibactam | 54 (87.1) | 38 (100) | 16 (66.7) | 6 (100) | 5 (100) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| Imipenem–relebactam | 44 (71) | 34 (89.5) | 10 (41.7) | 3 (50) | 5 (100) | 0 (0) | 2 (100) | 0 (0) | 0 (0) |
| Meropenem–vaborbactam | 49 (79) | 35 (92.1) | 14 (60) | 4 (66.7) | 5 (100) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| Fluoroquinolones | |||||||||
| Ciprofloxacin | 33 (53.2) | 24 (63.2) | 9 (37.5) | 0 (0) | 3 (60) | 0 (0) | 0 (0) | 1 (100) | 5 (100) |
| Levofloxacin | 35 (56.5) | 26 (68.4) | 9 (37.5) | 0 (0) | 3 (60) | 0 (0) | 0 (0) | 1 (100) | 5 (100) |
| Aminoglycosides | |||||||||
| Gentamicin | 41 (66.1) | 33 (86.8) | 8 (33.3) | 1 (16.7) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 5 (100) |
| Tobramycin | 36 (58.1) | 27 (71.1) | 9 (37.5) | 2 (33.3) | 1 (20) | 0 (0) | 1 (50) | 0 (0) | 5 (100) |
| Amikacin | 50 (80.6) | 35 (92.1) | 15 (62.5) | 5 (83.3) | 3 (60) | 0 (0) | 1 (50) | 1 (100) | 5 (100) |
| Tigecycline | 54 (87.1) | 34 (89.5) | 20 (83.3) | 4 (66.7) | 5 (100) | 5 (100) | 0 (0) | 1 (100) | 5 (100) |
| Trimethoprim/Sulfamethoxazole | 35 (56.5) | 27 (71.1) | 8 (33.3) | 0 (0) | 1 (20) | 1 (20) | 1 (50) | 0 (0) | 5 (100) |
Non-CP = non–carbapenemase producing; CP = carbapenemase producing.
Isolates that remained resistant to imipenem in the presence of relebactam were positive for class A serine β-lactamase (blaSME), class B metallo β-lactamases B (i.e., blaNDM and blaVIM), and class D serine β-lactamase (i.e, blaOXA-48 like). Isolates that remained nonsusceptible to meropenem in the presence of vaborbactam were positive for class B metallo β-lactamases B (i.e., blaNDM blaIMP, and blaVIM) and class D serine β-lactamase (i.e, blaOXA-48 like). All 8 isolates resistant to ceftazidime–avibactam were positive for class B metallo β-lactamases (i.e., blaNDM, blaIMP, and blaVIM) (Fig. 3 and Table 2). Among carbapenemase gene-negative CRE isolates, 89.5% (34/38), 92.1% (35/38), and 100% (38/38) were susceptible to imipenem–relebactam, meropenem–vaborbactam, and ceftazidime–avibactam, respectively (Fig. 3 and Table 2). Three isolates that were nonsusceptible to both imipenem–relebactam and meropenem–vaborbactam included E. aerogenes (CRE09), E. coli (CRE15), and K. pneumoniae (CRE25) (Supplementary Table 5). Average relative porin levels were lower in these isolates compared with isolates susceptible to imipenem–relebactam and meropenem–vaborbactam that were nonsusceptible to the carbapenem alone (0.01 and 0.002 vs. 1.25 and 0.41 for OmpC and OmpF and their analogs, respectively). A S. marcescens CRE isolate (CRE05) was nonsusceptible to imipenem–relebactam (Supplementary Table 5).
We also investigated the susceptibility of CRE isolates to ceftolozane–tazobactam. Among carbapenemase gene-positive CRE, 20.8% (5/24) were susceptible and 16.7% (4/24) were intermediate to ceftolozane–tazobactam (Fig. 3 and Table 2). All 5 susceptible isolates were blaSME-positive S. marcescens. The 4 intermediate isolates consisted of a blaOXA-48 like–positive K. pneumoniae, a blaOXA-48 like–positive E. coli, a blaKPC-positive K. pneumoniae, and a blaKPC-positive E. cloacae complex. Among carbapenemase gene-negative isolates, 31.6% (12/38) were susceptible and 15.8% (6/38) were intermediate to ceftolozane–tazobactam. There was no evidence of correlation between resistance to ceftolozane–tazobactam and lower porin protein levels (95.0% vs. 88.9%; P = 0.3).
3.6. Molecular epidemiology
Whole genome sequencing was performed to investigate clonality of CRE isolates recovered between 2013 and 2016. The only 2 CRE strains with temporal association were non–CP-CRE K. pneumoniae CRE24 and CRE25 which were isolated on 12/28/2014 and 12/31/2014, respectively, from patients on 2 different medical wards. The number of raw reads per genome and coverage are shown in Supplementary Table 6. Phylogenetic trees constructed based on whole genome sequence analysis are shown in Supplementary Fig. 2. One isolate (CRE30) was excluded due to mixed sequences. The number of single nucleotide variants (SNVs) for closely related strains is shown in Supplementary Table 7. Phylogenetic distance between strains within a species does not indicate any transmission events, as no identical strains were observed in our data set. Even the most closely related CRE strains on the phylogenetic tree (non–CP-CRE K. pneumoniae CRE04 isolated on 4/16/2013 and CRE24 isolated on 12/28/2014) had 5 SNVs. The only 2 CRE strains with temporal association (CRE24 and CRE25) had 10 SNVs. Plasmid-encoded CP-CRE isolates of the same species all had distinct stain types (blaOXA-48 like: CRE07, CRE08, CRE10, CRE14, CRE16, and CRE26; blaNDM: CRE17, CRE18, CRE28, CRE45, and CRE87; blaKPC: CRE13, CRE36, CRE37, CRE43, and CRE73; blaSME: CRE35, CRE49, CRE72, CRE78, and CRE94) (Supplementary Fig. 2 and Supplementary Table 7). Three out of 5 blaSME-positive S. marcescens isolates (CRE35, CRE49, and CRE94) that appeared closely related on the phylogenetic tree had 30 to 40 SNVs between them (Supplementary Fig. 2 and Supplementary Table 7).
4. Discussion
Compared to national CRE rates of 3.7%, based on 2014 data submitted to National Healthcare Safety Network for catheter-associated urinary tract infections, central line–associated bloodstream infections, and surgical site infections (Weiner et al., 2016), the CRE rate of 0.32% between 2013 and 2016 at our health care system serving the Silicon Valley area was about 10-fold lower. Although surveillance data in Northern California are lacking, our CRE rate was also lower than that reported by an academic health system in Los Angeles, CA (0.32% vs. 0.73%) (Pollett et al., 2014). The reason for a lower CRE rate at our institution could be due to differences in patient population (i.e., less health care exposure), differences in infection control and prevention practices (i.e., isolation of patients with CP-CRE), absence of skilled nursing facilities and long-term-acute-care hospitals from our health system (Mathers et al., 2011), and the definition used to define CRE (Chea et al., 2015), although our rates remained lower than national rates even when post-2015 CDC CRE surveillance definition was applied to 2016 cultures. The second difference seems to be critical as CDC reported at least 1 CRE infection in 3.9% of short-stay hospitals compared with 17.8% of long-term acute-care hospitals (CDC, 2013). Furthermore, in this study we applied the pre-2015 CDC CRE surveillance definition, which excludes ertapenem. Inclusion of ertapenem would have increased our CRE rate (Chea et al., 2015). While the rate of health care–associated infection caused by carbapenem-nonsusceptible Enterobacteriaceae has increased in the United States between 2001 and 2011 (CDC, 2013), the annual CRE rates at our institution did not vary significantly over a 4-year study period from 2013 to 2016.
The CP-CRE rate of 38.7% (24/62) among all CRE at our institution is comparable to 47.9% (90/188) reported for metropolitan areas in 7 US states, including Oregon and Colorado, but much lower than 81.7% (94/115) reported by an academic health system in Los Angeles (Guh et al., 2015). Unlike all other North American institutions where KPC predominates as the most common carbapenemase among CP-CRE isolates (Castanheira et al., 2016; Guh et al., 2015; Mataseje et al., 2016; Pollett et al., 2014; Tamma et al., 2017), at our institution, CP-CRE were evenly caused by KPC (20.8%, 5/24), OXA-48 like (25.0%, 6/24), NDM (20.8%, 5/24), and SME (20.8%, 5/24), and less commonly by IMP (8.3%, 2/24) and VIM (4.2%, 1/24). The reason for nonpredominance of KPC at our health system may be in part due to the geographic location of our institution in the Silicon Valley where high-tech industry draws people from major cities around the globe where different plasmid-encoded carbapenemases are endemic. Another important contributor is the fact that nosocomial transmission of KPC did not occur at our institution during the study period. In fact, whole genome sequence-based strain typing showed that all plasmid-encoded CP-CRE were distinct strains and therefore had occurred sporadically. As discussed above, absence of skilled nursing facilities and long-term-acute-care hospitals from our health system may have contributed to lack of CRE transmission (Mathers et al., 2011).
A major strength of study is that we employed comprehensive phenotypic and genotypic analyses to determine the molecular basis of carbapenem resistance in CRE isolates recovered longitudinally at our institution. Importantly, concordance between phenotypic carbapenemase test result and carbapenemase gene detection was 100%, which indicates that genotypic testing would be sufficient to detect all plasmid-mediated (i.e., blaKPC, blaOXA-48 like, blaNDM, blaIMP, and blaVIM) and chromosomally encoded (i.e., blaSME) CP-CRE at our institution. Although FDA-cleared commercial assays currently do not detect blaSME (Findlay et al., 2015), this carbapenemase should be suspected in carbapenem-resistant carbapenemase-producing S. marcescens isolates that are negative for blaKPC, blaOXA-48 like, blaNDM, blaIMP, and blaVIM (Queenan et al., 2000). Resistance in non–CP-CRE is mediated through high-level expression of cephalosporinases such as AmpCs and ESBLs coupled with porin inactivation (Temkin et al., 2014; Tzouvelekis et al., 2012). Using a novel mass spectrometry assay, we showed that porin levels of either OmpC or OmpF and their analogs were down in 92.1% of non–CP-CRE isolates. This approach was superior to qRT-PCR, which showed downregulation of porin RNA in only 63.6% of non–CP-CRE isolates. Although only 60.5% of non–CP-CRE encoded an ESBL or AmpC, respectively, the Check-Points assay does not detect chromosomally encoded AmpCs present in the “SPACE” organisms (i.e., Serratia, Citrobacter, and Enterobacter), which accounted for 63.2% of non–CP-CRE isolates in this study. Overall, between carbapenemase and porin loss detection, we could account for mechanism of resistance in 95.2% (59/62) of CRE isolates in this study. The mechanism of resistance in unaccounted isolates may include efflux pump and target modification (Grobner et al., 2009; Szabo et al., 2006).
The antibiotic susceptibility findings from this study are consistent with prior studies showing the underlying mechanism of carbapenem resistance predicts in vitro susceptibility to newly developed β-lactam–β-lactamase inhibitor combinations, three of which (i.e., ceftazidime–avibactam, meropenem–vaborbactam, and ceftolozane–tazobactam) are FDA cleared and commercially available (Castanheira et al., 2017; Lapuebla et al., 2015; Lob et al., 2017; Vasoo et al., 2015). Susceptibility of CP-CRE isolates to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam was dependent on the molecular class of carbapenemase they encoded such that 100% of isolates encoding blaKPC were susceptible to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam; 100% of isolates encoding blaOXA-48 like were susceptible to ceftazidime–avibactam but not to meropenem–vaborbactam and imipenem–relebactam; and 100% of isolates encoding metallo β-lactamases (i.e., blaNDM, blaIMP, and blaVIM) were nonsusceptible to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam, excluding 1 E. cloacae complex blaIMP-positive isolate, CRE02, that was susceptible to imipenem alone (MIC ≤1 by MicroScan). In non–CP-CRE isolates, 100%, 92.1%, and 89.5% were susceptible to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam, respectively. Susceptibility of non–CP-CRE isolates to imipenem–relebactam is consistent with findings by Livermore and colleagues but not by Lapuebla and colleagues (Lapuebla et al., 2015; Livermore et al., 2013). This discrepancy could be due to extent of porin inactivation among non–CP-CRE isolates in different studies given that we showed that isolates with resistance to imipenem–relebactam had nearly undetectable porins. Overall, our findings indicate that nucleic acid testing for blaKPC, blaOXA-48 like, blaNDM, blaIMP, and blaVIM is sufficient to distinguish between CP-CRE and non–CP-CRE and to provisionally predict susceptibility to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam at our institution. Given the emergence of resistance to ceftazidime–avibactam in blaKPC-positive CP-CRE (Shields et al., 2017), it is essential that phenotypic testing always follows genotypic testing to confirm susceptibility results. Although not developed for treatment of CP-CRE, 100% of S. marcescens encoding blaSME were susceptible to ceftolozane–tazobactam, while the rest of CP-CRE were resistant or intermediate. Further, 31.6% of non–CP-CRE were susceptible to ceftolozane–tazobactam; however, genotypic cephalosporinase testing could not identify this group.
Although our findings are informative for management of patients with CRE infection, this study has several limitations. First, this was a single-center study. Given that patient population, medical management, and infection control practices vary between health systems, our findings might not be generalizable. Thus, a multicenter study in our geographic region is needed to confirm our findings. Second, despite including all CRE isolates at our institution over a 4-year period, the total number of CRE isolates was relatively small. However, this reflects natural epidemiology of CRE at our institution. Studies with large number of CRE isolates are needed to confirm our findings. Third, we compared the susceptibility of CRE isolates to 4 β-lactam combination drugs using 3 different susceptibility testing methods. Although our findings were consistent with prior reports, using a single susceptibility testing method for all 4 drugs might have allowed for more accurate comparison between drugs. Lastly, although phylogenetic analysis showed that plasmid-encoded CP-CRE isolates at our institution all had distinct strain types, we did not perform plasmid typing to rule out the spread of an identical plasmid for each carbapenemase. However, this possibility is unlikely given the lack of temporal association for each plasmid-encoded CP-CRE at our institution.
5. Conclusions
Comprehensive phenotypic and genotypic characterization of CRE isolates longitudinally at a health system in Silicon Valley identified diverse resistance mechanisms including representation of all plasmid-encoded carbapenemases. On-demand nucleic acid testing was able to accurately distinguish between CP-CRE and non–CP-CRE and predict in vitro susceptibility to ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam.
Supplementary Material
Acknowledgment
The study was partially supported by a research grant from Merck Inc. to Niaz Banaei. We thank Robin Patel from the Mayo Clinic, Rochester, MN; Kyungwon Lee from Yonsei University College of Medicine, Seoul, South Korea; Andreas Wendel from Heinrich Heine University Düsseldorf, Germany; and JMI Laboratories, IA, for providing bacterial isolates and Nassim Amiali and Hongtao Xu for technical assistance.
Funding
This work was supported by Merck.
Footnotes
Declarations of interest: none.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diagmicrobio.2018.10.004.
References
- Castanheira M, Deshpande LM, Mills JC, Jones RN, Soave R, Jenkins SG, et al. Klebsiella pneumoniae isolate from a New York City hospital belonging to sequence type 258 and carrying blaKPC-2 and blaVIM-4. Antimicrob Agents Chemother 2016;60:1924–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem–vaborbactam tested against contemporary gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2017;61:e00567–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- Chea N, Bulens SN, Kongphet-Tran T, Lynfield R, Shaw KM, Vagnone PS, et al. improved phenotype-based definition for identifying carbapenemase producers among carbapenem-resistant Enterobacteriaceae. Emerg infect Dis 2015;21:1611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards institute (CLSI). Performance standards for antimicrobial susceptibility testing: twenty-sixth informational supplement. M100–S26; 2016. [Google Scholar]
- Elemam A, Rahimian J, Mandell W. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin Infect Dis 2009;49:271–4. [DOI] [PubMed] [Google Scholar]
- Findlay J, Hopkins KL, Meunier D, Woodford N. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J Antimicrob Chemother 2015;70:1338–42. [DOI] [PubMed] [Google Scholar]
- Gohil SK, Singh R, Chang J, Gombosev A, Tjoa T, Zahn M, et al. Emergence of carbapenem-resistant Enterobacteriaceae in Orange County, California, and support for early regional strategies to limit spread. Am J Infect Control 2017;45:1177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Srinivas N, Watz N, Tenover FC, Amieva M, Banaei N. A pediatric case of New Delhi metallo-beta-lactamase-1–producing Enterobacteriaceae in the United States. Pediatr Infect Dis J 2013;32:1291–4. [DOI] [PubMed] [Google Scholar]
- Grobner S, Linke D, Schutz W, Fladerer C, Madlung J, Autenrieth iB, et al. Emergence of carbapenem-non-susceptible extended-spectrum beta-lactamase–producing Klebsiella pneumoniae isolates at the university hospital of Tubingen, Germany. J Med Microbiol 2009;58:912–22. [DOI] [PubMed] [Google Scholar]
- Guh AY, Bulens SN, Mu Y,Jacob JT, Reno J, Scott J, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 2015;314:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh AY, Limbago BM, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther 2014;12:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuebla A, Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, et al. Activity of imipenem with relebactam against gram-negative pathogens from New York City. Antimicrob Agents Chemother 2015;59:5029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserre C, De Saint Martin L, Cuzon G, Bogaerts P, Lamar E, Glupczynski Y, et al. Efficient detection of carbapenemase activity in Enterobacteriaceae by matrix-assisted laser desorption ionization-time of flight mass spectrometry in less than 30 minutes. J Clin Microbiol 2015;53:2163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbago BM, Rasheed JK, Anderson KF, Zhu W, Kitchel B, Watz N, et al. IMP-producing carbapenem-resistant Klebsiella pneumoniae in the United States. J Clin Microbiol 2011;49:4239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013;68:2286–90. [DOI] [PubMed] [Google Scholar]
- Lob SH, Hackel MA, Kazmierczak KM, Young K, Motyl MR, Karlowsky JA, et al. In vitro activity of imipenem–relebactam against gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (results from the SMART Global Surveillance Program). Antimicrob Agents Chemother 2017;61:e02209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataseje LF, Abdesselam K, Vachon J, Mitchel R, Bryce E, Roscoe D, et al. Results from the Canadian nosocomial infection surveillance program on carbapenemase-producing Enterobacteriaceae, 2010 to 2014. Antimicrob Agents Chemother 2016;60:6787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011;2: e00204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013;13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, et al. The modified carbapenem inactivation method (mCIM) for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 2017;55: 2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollett S, Miller S, Hindler J, Uslan D, Carvalho M, Humphries R. Phenotypic and molecular characteristics of carbapenem-resistant Enterobacteriaceae in a health care system in Los Angeles, California, from 2011 to 2013. J Clin Microbiol 2014;52(11):4003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Torres-Viera C, Gold HS, Carmeli Y, Eliopoulos GM, Moellering RC Jr, et al. SME-type carbapenem-hydrolyzing class A beta-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother 2000;44:3035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012;56: 2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MJ, Lin MY, Weinstein RA, Trick WE. Spread of carbapenem-resistant Enterobacteriaceae among Illinois healthcare facilities: the role of patient sharing. Clin Infect Dis 2016;63:889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, et al. Emergence of ceftazidime–avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017;61:e02097–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo D, Silveira F, Hujer AM, Bonomo RA, Hujer KM, Marsh JW, et al. Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob Agents Chemother 2006;50:2833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, et al. Comparing the outcomes of patients with carbapenemase-producing and non–carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 2017;64:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci 2014;1323:22–42. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20:862–72. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012;25:682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasoo S, Cunningham SA, Cole NC, Kohner PC, Menon SR, Krause KM, et al. In vitro activities of ceftazidime–avibactam, aztreonam–avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing gram-negative bacilli. Antimicrob Agents Chemother 2015;59:7842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016;37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth KR, Walters MS, Weiner LM, Edwards J, Brown AC, Huang JY, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms — United States, 2006–2017. MMWR Morb Mortal Wkly Rep 2018;67:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.