Abstract
Introduction:
Ocular Chlamydia trachomatis infection, the causative agent for trachoma, is responsible for 1.9 million cases of visual loss world-wide. Mass Drug Administration (MDA) with azithromycin to entire trachoma-endemic districts is part of the World Health Organization’s public health strategy for trachoma elimination.
Areas covered:
Background on C. trachomatis and the epidemiology of trachoma are presented, followed by a review of the antibiotics for treatment and the need for a public health approach to trachoma elimination. The effectiveness of mass drug administration is presented, concluding with challenges to trachoma elimination in the future.
Expert opinion:
MDA using azithromycin is a key component of the public health strategy for trachoma elimination. With high coverage in children, there is good evidence that MDA drops the community pool of infection. There are challenges to trachoma elimination by the year 2020, and the drug donation program for country MDAs will be integral to ongoing efforts
Keywords: Chlamydia trachomatis, trachoma, mass drug administration, azithromycin, review
1. Introduction
The diagnosis and treatment of an infection in clinical practice starts with a patient presenting with clinical signs and symptoms, often followed by a diagnostic test to determine the cause of the infection, and (if warranted) the prescription of an antibiotic to eliminate the infection. But what if the “patient“ is a community where infection is widespread, mostly sub-clinical in its acute stage, and characterized by the ability to cause repeated episodes of disease? And worse, the repeated infections can lead to potentially severe debilitating sequelae? Such a scenario would benefit from a public health approach to interrupt transmission and eliminate the community pool of infection. In fact, public health approaches to infectious diseases have a number of success stories, mostly involving the deployment of vaccines to interrupt transmission and reliance on high coverage to provide herd immunity against epidemic outbreaks; the most notable examples are the elimination of smallpox, and the drastic reduction in polio, both viral in origin. Bacterial diseases also have effective vaccines, for example meningitis and typhoid. However, the leading infectious cause of blindness, Chlamydia trachomatis (C. trachomatis), has no vaccine.
Trachoma, an ocular disease caused by repeated infections with C. trachomatis, presents with inflammatory thickening of the conjunctiva and follicles in the acute, active stage. With repeated infection, small stellate scars and lines of fibrous tissue replace the normal conjunctiva. Scarring also causes the contraction of the tarsal conjunctival tissue, resulting in entropion (in-turned lid margin) and trichiasis (in-turned eyelashes). The damage to the tarsal conjunctiva leads to ocular surface disease, and once the lashes touch the cornea, opacification of the cornea ensues leading to irreparable vision loss.
In recognition of this public health problem, the World Health Assembly has targeted the year 2020 to eliminate blinding trachoma (1), and a multifaceted strategy (SAFE) is recommended including antibiotics for treatment of infection. For trachoma-endemic communities, mass treatment with antibiotics is provided annually for years. In this review, we present relevant details on C. trachomatis and the epidemiology of trachoma, followed by a description of the antibiotics typically used to treat ocular C. trachomatis infection. We then discuss the public health approach to the elimination of trachoma, specifically the use of mass drug administration. We describe the effectiveness of this approach, and the challenges of antibiotic treatment of communities, concluding with a discussion of the elimination of trachoma in the future.
2. Chlamydia trachomatis: the pathogen
Chlamydia trachomatis (C. trachomatis) is a gram negative obligate intracellular bacterium. It has a reduced genome, ~ 1 MBp, and thus relies on its host cell for many of its metabolic needs. C. trachomatis has three human biovars: serovars A to C, which infect the ocular conjunctiva, serovars D to K which infect the genital tract, and serovars L1 to L3 which are more invasive and attack the lymph nodes near the genital tract. Serovars D to K can also cause ocular infections in infants and adults exposed to infected genital secretions. Trachoma, the main ocular Chlamydia infection, is caused by repeat bouts of infection with C. trachomatis ocular serovars, A, B, Ba, and C and is the leading cause of infectious blindness worldwide. Humans are the only natural host for these strains (2, 3).
Chlamydia has a highly conserved genome with two-thirds of its genes shared amongst all serovars, however, there is a region with high genomic diversity, the plasticity zone (PZ). The PZ encodes virulence factors that may have a role in the tissue tropism of the differing strains (2–4). Ocular strains of C. trachomatis carry mutations in the tryptophan synthesis alpha-subunit gene (trpA), which resides in the PZ. Tryptophan is an amino acid essential for the development and replication cycle as described below (5).
2.1. Developmental cycle
C. trachomatis has a biphasic developmental cycle. The first phase of development is the extracellular elementary body (EB), the small non-dividing environmentally stable infectious form of C. trachomatis present in infectious discharge. EBs target epithelial cells for entry, which they need in order to access nutrition and begin transformation to the reticulate bodies (RBs). EBs are adept at attaching to the host cell and becoming internalized into endocytic vacuoles. These vacuoles then combine to form intracytoplasmic inclusions. It is within these inclusions that EBs begin to transform into RBs and start replication.). The RBs are large replicative non-infectious form of C. trachomatis. RBs access nutrients within the host cytoplasm required for survival. RBs replicate, by division, within the inclusion. The prevailing theory is that they divide by binary fission (6, 7), however, studies have described polarized cell division similar to budding (8). RBs are converted back to EBs after several rounds of division; this process is delayed and asynchronous. How the RB cell decides to switch from dividing into two RBs to converting into an EB has not yet been fully elucidated. One theory is that the RB size, which steadily decreases after repeatedly dividing, has to reach a certain threshold below which conversion occurs; thus, RB size may control RB to EB conversion (7). Another theory is that the RB transition to an EB might be stimulated by their detachment from the inclusion membrane; the underlying premise is that conversion is inhibited while the RB, through its many projections, is in contact with the inclusion membrane(2, 9). The conversion of RBs to EBs is critical as only EBs are infectious (2). The EBs are then released from the inclusion into the extracellular milieu. The developmental cycle as a whole takes about 48–72 hours and up to 1000 ‘progeny’ can be produced from a single infected cell (2).
2.2. Attachment and invasion
C. trachomatis begins its life cycle extracellularly and needs to engage with and attach to the host cell in order to enter the intracellular space. To facilitate adhesion to the host cell, EBs form low affinity interactions with heparin sulfate proteoglycans, possible cell-surface endocytosis receptors (10). EBs also form high-affinity binding to host cell receptors that help activate actin remodeling which then facilitates inclusion formation and subsequent entry of C. trachomatis into the host cell (2). The inclusion is encased in a dynamic scaffold made up of filamentous structures including F-actin cytoskeleton, intermediate filaments, and microtubules (2); these host cytoskeletal structures act together to stabilize the inclusion(10, 11).
2.3. Intracellular environment modification
C. trachomatis is dependent on host cell nutrients for survival. It remodels the interior of its host cell and alters metabolic pathways to acquire the nutrients necessary for its growth (2). Through the many interactions between the inclusion and the host cell the inclusion creates a safe secluded space for C. trachomatis in which it can establish a protected robust replicative niche while avoiding phagolysosomal fusion.
2.3.1. Inclusion and large organelles
Large organelles including the Golgi apparatus, mitochondria, and the endoplasmic reticulum (ER) remodel around the inclusion (2, 12). The inclusion forms tight associations with the Golgi and centrosomes. C. trachomatis also alters host cell division timing resulting in asymmetric or incomplete cell division, thus, creating multinucleated daughter cells; the connections with the Golgi and centrosomes may help ensure the inclusion localizes to the cell with the most resources (13). The inclusion also interacts with the host cell’s mitochondria. C. trachomatis development depends on the hosts metabolism and thus requires functional host mitochondrial ATP (2).
2.3.2. Inclusion and lipids
Vesicular and non-vesicular trafficking of host cell sphingolipids and cholesterol are essential for C. trachomatis’ intracellular life cycle (14). C. trachomatis infected cells have an increase in lipid droplet content; these lipids may provide the substrates necessary for the production of bacterial phospholipids and membranes as well as provide energy (15, 16). Lipid droplets can translocate directly into the lumen of the inclusion, which allows C. trachomatis to remain hidden from the host’s immune surveillance and prevents the toxic byproducts of glycolysis and lipolysis from activating the host’s active inflammatory response (16).
2.3.3. Metabolic pathway alteration
C. trachomatis infection can alter host cell metabolic pathways and induces aerobic glycolysis (similar to the Warburg effect observed in proliferating cancer cells) (17, 18). This leads to increases in pyruvate, lactate, and glutamate levels; the resultant increased production of additional metabolites in the host cell provides many of the substrates needed for rapid chlamydial proliferation (17, 18).
2.4. Host evasion
The success of C. trachomatis infections depends on the ability of the bacterium to evade the host’s immune system while maintaining host cell viability (2, 19). C. trachomatis counteracts host restriction by altering retromer dependent trafficking and disrupting native protein interactions (4). C. trachomatis effectors also manipulate host surveillance and suicide programs, activating pro-survival signaling pathways while inhibiting apoptotic pathways (2, 20). For example, to avoid host cell stress induced apoptosis during infection, C. trachomatis upregulates host microRNA (miR-30) which helps downregulate apoptotic signaling pathways (21).
C. trachomatis infection affects host macrophages. The bacterium re-directs macrophages to kill phagocytes. There is some evidence that C. trachomatis can also infect macrophages at least transiently and induce apoptosis of T cells, the primary effective immune response to chlamydial infection. C. trachomatis also inactivates transcription regulators for the type 1 interferon and interleukin 10 mediated responses; these responses are essential for the host cell to control C. trachomatis infections (22).
Environmental factors and stresses (i.e. nutrient absence, antibiotic exposure) can result in the developmental cycle becoming arrested, albeit reversibly, where RBs transition to aberrantly enlarged, non-replicative, persistent forms. Persistence may be a method by which C. trachomatis evades the host immune system. Persistence may also contribute to chronic host immune responses which appears to be a driver for the scarring process. However, it is not clear if persistence occurs in vivo (2, 23).
2.5. Host exit
Newly formed EBs are released from the inclusion to continue propagating Chlamydial infection. There are two mechanisms of release: 1) inclusion extrusion, which keeps the host cell intact, or 2) host cell lysis, which results in cell death (2). Extrusion limits any inflammatory response to cell death (2).
An overview of the life cycle provides some of the context for the difficulties in treating diseases caused by C. trachomatis. The fact that chlamydia is an intracellular organism, with a life cycle that is long and includes a non-replicating stage during which antibiotics are not effective, and the possibility that the replicating stage may be pushed, even transiently, to a non-replicative persistent form all create challenges for effective treatment. The next section provides further context for the difficulties in treating trachoma by highlighting the epidemiologic features of the disease as it affects populations.
3. Epidemiology of trachoma
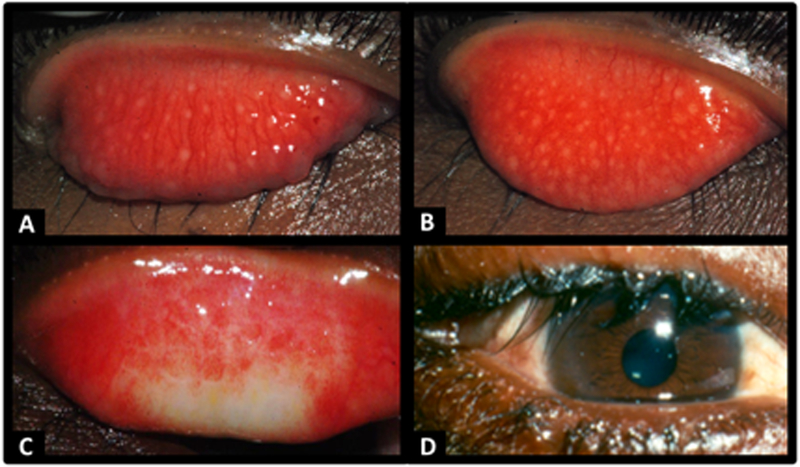
Ocular infections with C. trachomatis are ubiquitous. In developed country settings, chlamydia ocular infections of newborns are due to passage through a birth canal infected with genital strains. These cases of ophthalmia neonatorum are not trachoma, because the risk of re-infection and the potential development of subsequent scarring and entropion is almost non-existent. Trachoma requires both the presence of C. trachomatis infection and an environment that allows the acquisition of repeated infections in children over years of exposure. In low income country settings, trachoma-endemic communities are characterized by poverty and marginalization with limited access to water and sanitation facilities; this environment allows the spread of infected ocular and nasal secretions that enhances the risk of repeated infections, prolonged inflammation, and subsequent development of blinding sequelae (24, 25). There is no obligate intermediate host for trachoma, as there is for onchocerciasis (black flies) or malaria (mosquitos); transmission is strictly from person to person through exposure to infected secretions. In some settings, eye-seeking flies may also transmit ocular secretions but flies are not an obligate vector and trachoma exists in many locations in the absence of flies. In communities endemic for trachoma, ocular chlamydia infection is a chronic conjunctivitis, and the clinical signs of trachoma vary by age (Figure 1). Conjunctival infection, follicles, and inflammation of the conjunctiva are most often found in preschool age children, who are considered the reservoir for infection in the community (26). Stellate scars may be seen in children, but scarring due to trachoma is typically seen in young adults, and more frequently in females (27). Entropion and trichiasis, the result of ongoing scarring, is far more common in women and seen in middle and older aged persons. Corneal blindness due to trachoma is most commonly seen in older adults. There is currently no treatment option for this corneal opacification, or any intervention to halt the scarring process. The most effective method for prevention of these late manifestations of trachoma is to halt the active disease in children.
Figure 1.
Images of A) Trachoma-Follicular (TF), B) Trachoma –Intense (TI), C) Trachomatous scarring (TS) and D) Trachomatous Trichiasis (TT) (images collected by SW and MW during field work).
The World Health Organization (WHO) has developed a simplified grading scheme which is used to report the prevalence of active trachoma and the sequelae in population based surveys (28). Follicular trachoma (TF) is defined as at least five follicles of size 0.5 mm present in the upper tarsal conjunctiva (within a defined area for grading). If there is inflammatory thickening sufficient to obscure at least 50% of the deep tarsal vessels, then trachoma-intense (TI) is present. If there is easily visible scarring, then the conjunctiva is graded as having trachomatous scarring (TS). Trachomatous trichiasis (TT) is defined as at least one lash touching the globe or the presence of epilation (note that evidence of trachomatous scarring should also be present for TT to be truly due to trachoma, although it is not currently part of the definition). Corneal opacity (CO) is present when the opacity is sufficient to obscure the pupillary margin. These clinical signs of trachoma reflect the natural progression of the disease and have meaning for trachoma elimination programs. The first two, TF and TI, are an indication of the extent and severity of “active” trachoma. Currently, the WHO recommends using the prevalence of TF in children ages 1–9 years to monitor the need for MDA, to assess the impact of elimination programs, and to monitor for re-emergence after program cessation. Scarring is an indicator of long-term risk of trichiasis. Data on the prevalence of trichiasis is needed for planning surgical services, and the prevalence of corneal opacity due to trachoma is an indicator of the public health burden of vision loss attributable to trachoma.
The clinical signs of active trachoma take time to resolve after infection has been eliminated. The prevalence of infection in children with active trachoma varies with the severity of inflammation, the level of endemicity of trachoma in the community, and the time elapsed since treatment. In hyperendemic districts, between 30 and 50% of children with TF typically have evidence of infection, and up to 80% of children with TI may also have infection, as measured by nucleic acid amplification tests. If grading for active trachoma is carried out closely following MDA, the association between the clinical signs and infection is weak (29, 30).
In addition, grading the clinical signs of active trachoma becomes more difficult as the disease becomes rarer. For this reason, interest is growing in other potential tools besides clinical assessment that trachoma programs may use to determine the progress towards elimination. One tool being considered is a test of infection. There are highly sensitive and specific nucleic acid amplification tests for C. trachomatis, and one which can be used easily in field settings (31–34). However, even with pooling strategies for specimen testing (35), the cost is high of collecting, preserving and testing ocular specimens. Moreover, there are insufficient data on the prevalence of infection in the population which represents no risk of re-emergent trachoma. Some researchers have reported re-emergence of infection months after communities had achieved no infection, and others have reported the absence of sustained re-emergence of trachoma even when infection was re-introduced into the community (36, 37). One surveillance study conducted in a district 4 years after cessation of program activities found an absence of trachoma even with a prevalence of infection of 1% in children ages 1–9 years (38).
A serologic test for antibodies to C. trachomatis antigens has emerged as a promising tool for surveillance for re-emergent trachoma (39). In particular, the antibody to chlamydial antigen pgp3, a plasmid antigen, seems to be relatively long-lived and serologic testing in children could reflect cumulative exposure to C. trachomatis within communities (38). The absence, or low prevalence, of antibodies in young children following years of cessation of trachoma program activities could indicate the interruption of transmission.. This scenario was observed in Tanzania and Nepal, where clinical disease was below 5% in children in districts where program activities has stopped between 2 to 10 years previously; surveillance surveys revealed a very low prevalence of antibodies (38, 40). Data also suggest that sero-reversion, especially in the absence of ongoing transmission, also occurs (41). Unfortunately, the pgp3 antigen while highly immunogenic is not unique to ocular strains of C. trachomatis. In areas with high rates of genital infections, children exposed at birth may test positive as a result of prolonged respiratory infection. Research is needed to determine the contribution of infection with genital chlamydia to the seropositivity of children in a trachoma endemic area.
Certainly, the global profile of trachoma has changed over time. Once endemic in most countries, trachoma disappeared from Europe and North America well before the advent of antibiotics. The disappearance by the early 20th century is attributed to socioeconomic improvement, especially improved water and sanitation, which decreased transmission of C. trachomatis to the point where transmission throughout the community was unsustainable.
Trachoma continues to be endemic in the poorest and most remote areas of Africa, Asia and the Middle East. Communities with trachoma are often those with the fewest resources to take on health issues, and trachoma preferentially affects the most vulnerable members of those communities, women and children. In 2016, an estimated 190 million people in 37 countries lived in trachoma endemic areas, and 1.9 million suffered visual loss due to trachoma sequelae (42).
In summary, the epidemiology of trachoma is characterized by an active, infectious stage in children, with scarring and trichiasis in older ages, particularly in women. It is a disease of communities, transmitted person to person from infected ocular and nasal secretions. Trachoma elimination requires a multi-faceted public health approach to be successful, which includes mass drug administration of antibiotics.
4. Antibiotics and ocular C. trachomatis
Antibiotics to treat C. trachomatis ocular infection need to be effective, simple for a population to use or community treatment assistants to distribute, and well tolerated with minimal adverse effects. In addition, because the reservoir of infection is in children, the antibiotics need to be safe for pediatric use.. The antibiotics also need to be inexpensive for use, as areas afflicted by trachoma tend to be underdeveloped and resource limited with a low income population. Fortunately, there are multiple antibiotics that have excellent activity against C. trachomatis and meet the criteria for use at the community level in mass drug administration (MDA) programs. We will review these medications below.
4.1. Oral azithromycin
Azithromycin is a macrolide, specifically in the azalide subclass (43). Its mechanism of action includes binding to and interfering with the 50S ribosomal subunit in bacteria which prevents the translocation of peptides and thus inhibits protein synthesis. This process is non-reversible and so results in azithromycin being bactericidal (44, 45). It has good activity against C. trachomatis; minimum inhibitory concentrations (MIC) range from 0.03–0.25 mg/ml (46, 47). Azithromycin has a long half-life in sera of 2 to 4 days, high peak levels, and a short lag in absorption (43, 44). Peak serum concentrations reach 1.53mg/ml and peak tear film concentrations reach 0.15 mg/ml; they both gradually decrease over 6 days (47, 48). This is followed by excellent tissue penetration and concentration, resulting in high intracellular concentrations. This feature is critical for treating intracellular organisms like C. trachomatis (43, 44, 48).
Oral azithromycin has become the drug of choice for treating trachoma (45). It is effective as a single oral dose, 20 mg/kg up to 1gram (43), which avoids the problem of multiple doses over several weeks. The treatment is simple: tablets or reconstituted liquid administered as a single dose, either by weight or by use of height sticks as a proxy for weight (49). This feature greatly improves compliance, compared to other regimes that involve multiple doses over several days, or instillation in the eye (44). Because the medication is systemic, it is active at extraocular sites, including the epithelial layers of the nasopharynx and gut, where C. trachomatis may also reside. Thus, it has a higher chance of achieving clearance of the bacterium (44, 49). It is safe for children, and coverage must be high in this segment of the population to ensure reduction of infection. Ever since the first multi-country study of the effectiveness and safety of azithromycin, it has been the preferred choice for MDA (50).
Minimal side effects include diarrhea, nausea, abdominal pain, and vomiting (44). Pyloric stenosis in infants has been reported with azithromycin, the risk being greatest in those less than 1 year of age (51). Cardiovascular risks, including QT prolongation and possible dysrhythmias, have also been reported with azithromycin, the risk being greater in older individuals (52). However, studies have demonstrated discordant results with some showing that cardiovascular mortality is not increased (53).
Initially, the cost of azithromycin was prohibitive and it was not considered for the community wide treatment of trachoma (49). However, Pfizer began a donation program through the International Trachoma Initiative that eliminates the cost of the drug for countries who apply to use it for trachoma control (54), although there is still the cost of transport from the port of entry to the communities who need it. Currently, for districts where the prevalence of TF is 5% or greater in children ages 1–9 years, the recommendation is annual mass treatment with azithromycin, single dose, to all residents age one year and older. In addition to the use of azithromycin for active trachoma, oral azithromycin following trichiasis surgery has been shown to reduce the risk of recurrent trichiasis (55).
4.2. Topical azithromycin
Topical azithromycin preparations, 1.0 and 1.5% topical solutions, are approved for use in C. trachomatis ocular infections. The typical dosing regimen shown to be effective is one drop twice a day for 3 days. Studies have shown that this regimen of topical azithromycin is non-inferior to a single dose of oral azithromycin (45, 56, 57). Topical azithromycin has been reported to cause occasional ocular discomfort including burning and itching as well as, rarely, blurred vision and foreign body sensation. However, these adverse events were rare with less than 1% of patients reporting treatment-related symptoms.(45). The presence of local symptoms and the need for a slightly prolonged treatment course as compared to oral azithromycin makes topical azithromycin slightly less desirable for mass drug administration, but it could be a useful addition for programs where treatment of infants less than one year of age is needed.
4.3. Topical tetracycline
Topical tetracycline 1% ointment was the standard of care for treatment of trachoma prior to the switch to oral azithromycin. In the early studies of topical treatments, including topical tetracycline, prolonged intermittent treatment was recommended (58–60). In part, the prolonged treatment was chosen in hopes of combating seasonal bacterial conjunctivitis which seemed to worsen the sequelae of trachoma, but the rationale for the actual dosage regimes chosen was never clear. The recommended dosage was initially two times a day 5 days a month for 6 months a year (58, 61). This was eventually simplified to once or twice a day for 4 to 6 weeks (62, 63). Topical tetracycline is effective against trachoma, assuming good compliance, and was the comparator for the initial azithromycin randomized trials (44, 49, 50). Tetracycline acts by binding to the 30S subunit of microbial ribosomes, blocking the attachment of RNA, thus preventing new amino acids from being added to the nascent peptide chain. Although this action is inhibitory, it is also reversible upon withdrawal of tetracycline, making tetracycline bacteriostatic.
Tetracycline ointment has some adverse effects due to its oily properties that result in poor compliance rates, including ocular discomfort and blurred vision, albeit temporary and reversible (63). It is also difficult to apply the ointment in children. Compliance with tetracycline ointment therapy regimens remained poor in program settings for the reasons described and some postulated that this lack of compliance may, in part, have resulted in the failure of control programs to significantly reduce infection rates (57).
4.4. Oral doxycycline
Oral doxycycline is in the tetracycline class of antibiotics; more specifically it is a semi-synthetic derivative of tetracycline thus sharing many of its characteristics. It is given as a single dose once a day, 5 days a week, for a total of 6 weeks. The dose given is 2.5 to 4 mg/kg of body weight (64). An oral form of tetracycline was initially felt to be a more practical and less expensive alternative to tetracycline ointment. It was never implemented for individual treatment or community wide administration on a large scale, however, because it cannot be administered to pregnant or lactating women nor children less than 12; it can permanently damage calcium-rich organs (such as teeth and bone) and cause nasal cavities to erode (65). Other adverse events have also been reported including gastrointestinal upset and photosensitivity as well as allergic reactions. While these are common to the tetracycline antibiotics given orally, they are not seen with topical administration because of the lack of significant systemic absorption.
4.5. Oral and topical erythromycin
Oral erythromycin is a macrolide antibiotic. It has activity against C. trachomatis and has been used in the past, albeit briefly, for the treatment of trachoma. Gastrointestinal upset (diarrhea, nausea, vomiting) are common adverse effects and thus limited the use of this medication. It also has to be taken over 14 days which limited compliance. Topical erythromycin ointment is also available for use, however it is too expensive to use programmatically for trachoma control (62).
4.6. Oral sulfonamides
Oral sulfonamides were the first class of antibiotics used to treat trachoma. Initial reports were positive regarding treatment success, which led to the use of sulfonamides as part of large scale treatment efforts in Australia, Malta, and Ethiopia. However, the serious, potentially lethal, side effect of Stevens-Johnson Syndrome (a rare but serious toxic dermal necrolysis) was observed which led to the discontinuation of the use of sulfonamides and the switch to topical tetracycline ointment (54, 66, 67).
In summary, there is a long history of different antibiotics used for trachoma, but at present azithromycin is the drug of choice. Oral azithromycin also has all the desirable characteristics for a medication to be used for MDA: it is effective, safe, easy to administer and monitor compliance, and (with gratitude to the Pfizer donation program) provided for free. The reasons behind utilizing MDA as part of the multi-pronged strategy for trachoma elimination, and the current progress with MDA and new findings as a result will be covered next.
5. Public health approach to elimination of trachoma
In recognition of the public health burden of trachoma, the WHO has recommended to countries the use of a multi-faceted elimination strategy, the SAFE strategy; SAFE refers to S for surgery (to correct trichiasis), A for antibiotics (to reduce the infection in communities), F for facial cleanliness (to reduce transmission), and E for environmental improvements (to sustain the reduction of transmission). SAFE is implemented in trachoma endemic districts following population-based surveys where the rate of TF in children age 1–9 years is 5% or higher and/or the burden of trichiasis cases who are not already managed by the health care system is at least 1/1,000 population. There is epidemiological evidence to support each component of the SAFE strategy, but in this review, we will focus on the “A” component.
5.1. Mass Drug Administration for trachoma
Case finding and case treatment of trachoma is not cost-effective. Such an approach increases the risk of missing infections that are subclinical, and importantly ignores the need for community-wide programs on hygiene and environmental sanitation to sustainably reduce community transmission.. It is more effective to take a public health approach: determine the prevalence of trachoma at the district level and make decisions on whether MDA is indicated, as part of SAFE implementation. Numerous studies have shown the effectiveness of MDA in lowering infection rates, and these are described in the next section.
The public health approach acknowledges that there will be antibiotic treatment of persons, even communities, that do not have infection. The potential adverse effects must be weighed against the benefit of reduction of trachoma and avoidance of the blinding complications. The decision to implement MDA with a systemic antibiotic was made easier with the advent of azithromycin use because it generally has a good safety profile, especially for children, and it is easy for programs to administer an effective single dose to its populations.
Since 1999 over 766 million doses of azithromycin have been shipped to country trachoma programs. The International Trachoma Initiative reports that as of October 2018, 110 million doses of azithromycin are currently targeted for 782 districts in 26 countries (68). Such a massive effort requires the commitment of countries and partners to get azithromycin from the transportation ports to the districts and distribute it with high coverage to the populations in need. Cadres of Community Drug Distributors (CDDs) (or Community Treatment Assistants, CTAs) have been created and trained to properly provide a weight or height- based dose to children, and a 1 gram dose to adults (69). Ideally, coverage of the population would be 100%, but at least 80% coverage of children has been shown to be as effective as higher coverage in a multi-country study (70–72). Mass treatment occurs over a few days to a few weeks in each district as designed by each program.
Non-participation in MDA is a threat to the success of the program in reducing trachoma, and it does not occur at random in the community (73). In one MDA setting in Tanzania, households with persistent non-participation had a higher burden of familial responsibility and seemed less connected within the community (74). Households who dropped out of MDA after participating in one round were more likely to have a male CTA and also be less well connected within the village (75). The authors concluded that additional days of distribution and lessening CTAs’ travel time to their assigned households may prevent non-participation, and were key to high coverage. Where coverage is less than ideal, multiple years of MDA may yield little impact (76).
Country programs are required to report any serious adverse events in connection with mass drug administration. Most side effects reported are gastrointestinal, and in one study, the rate of diarrhea and vomiting was actually lower in children who took azithromycin than in children who were treated with topical tetracycline (77). An Ethiopian study reported the rate of adverse events immediately after mass treatment, and overall any adverse event was reported in 5% of children aged 1–9 years and in 17% of those 10 years of age and older. The majority of reports were abdominal pain, nausea and vomiting (78). A comprehensive 6 month follow up of children in communities that received MDA compared to communities that did not found reduced incidence rates of acute respiratory disease, diarrheal disease, and malaria parasitemia for children in the one month following MDA; this beneficial effect waned after one month, as expected since tissue levels of azithromycin decrease over time following a single dose (79, 80). No case of pyloric stenosis has been reported to the International Trachoma Initiative, responsible for monitoring adverse events for Trachoma national programs. This is likely due to the fact that azithromycin is not used in children less than a year old, where the risk is greatest (51).
The development of organisms resistant to azithromycin is a serious concern. Ocular C. trachomatis resistance has not been observed, and where tested, azithromycin is still highly efficacious and resistance does not appear to explain treatment failure (81). A recent systematic review suggests that there is very minimal resistance of C. trachomatis to azithromycin (32). However, treatment failure in the context of MDA is more common and associated with a heavy load of infection. In studies immediately pre and post MDA, children with a heavy load of C. trachomatis in eye swabs were more likely to be infected post-treatment (82). In persons with light to moderate loads, 91% had no infection following MDA, whereas in those with the highest loads, 26% had evidence of infection following MDA (76). Treatment failure may be due to an inadequate dose, especially if dosing is based on height sticks in children where the average dose for a given height range may be insufficient for some infections. In addition, there are data to suggest that for some heavy infections, chlamydia may exhibit heterotypic antimicrobial resistance characterized by reduced growth rates (83). In genital isolates from women described as currently infected, decreased susceptibility to azithromycin has been reported (84). However, macrolide genotyping was not performed in that study, so it is uncertain if it represents azithromycin genotype resistance in vivo.
There are concerns for MDA leading to the development of resistance in other organisms, notably Streptococcus pneumoniae. Following a single round of MDA in treatment naïve populations, research has not found persistent or clinically significant resistance (85, 86). However, Coles et al found high rates of resistance in communities 6 months following MDA after several previous annual rounds had been given (87). Another study evaluated evidence of S. pneumoniae resistance following 6 biannual rounds of MDA and found 77% of isolates were resistant but this decreased to 21% after 24 months with no MDA in the interim (88). Macrolide resistance does appear to decrease after antibiotic pressure is removed, although decline may be quite slow (89). Others suggest that resistance can be avoided with use of topical formulations but use of topical tetracycline can induce nasopharyngeal carriage of tetracycline-resistant strains of S. pneumoniae as long as 6 months after treatment cessation (90).
The district target set by the WHO for trachoma is a prevalence of TF less than 5% in children aged 1–9 years. At this level of disease, trachoma is not felt to be a blinding condition. The WHO recommends mass treatment with antibiotics with at least 80% coverage for at least 3 years when the prevalence of TF is more than 10% in children aged 1–9 years at district level, and at least 5 years if the prevalence is 30% or more (91). Re-evaluation, or impact assessments, should then be undertaken to guide further need for MDA. In the next section, we evaluate the effectiveness of MDA in the elimination of trachoma.
6. Effectiveness of Mass Drug Administration for elimination of trachoma
MDA is offered to endemic communities in the context of the full SAFE strategy, so its unique contribution to long term decline in trachoma is difficult to determine. However, immediately following MDA with good coverage, the estimated burden of infection in the community is profoundly decreased (82, 92). In a hyperendemic community, 90% of those with a modest load of infection at baseline had no evidence of infection 2 months after treatment, and 74% of those with a greater load (>20 copies of DNA in an ocular swab) had no infection (82). Trachoma control programs offer mass treatment to communities once per year, and data from Tanzania on speed of re-emergent infection and spread across households suggests annual treatment may be adequate to avoid re-emergence of infection to pretreatment levels (93). A recent Cochrane review of available clinical trials concluded that azithromycin use reduced the risk of active trachoma and C. trachomatis infection in trachoma areas. (94).
How many rounds of annual MDA are needed is a critical question for programs, and there is no simple answer. Clearly, the number of annual rounds needed to achieve a prevalence of TF less than 5% depends on the starting prevalence of trachoma. The initial hopes that 1–2 annual rounds would be sufficient were not realized, especially for highly endemic areas (95), and mathematical models based on Tanzania national program data suggest it could take as long as 7–10 annual rounds of MDA to achieve the goal (96). Data from all country programs where TF prevalence before the start of MDA and after a certain number of rounds were carried out show the heterogeneity of predicting the decline over time according to the number of rounds of MDA administered (97) (Table 1). This is not surprising, given the unpredictability of program coverage, potential gaps in years between treatments, unknown coverage of children and variations in the methods for reporting coverage. Nevertheless, the dependency on starting prevalence was obvious from the data; the predicted probability of 3 rounds of MDA resulting in TF<5% was 71% where the district baseline TF prevalence was 10%, compared to a 30% probability if the baseline TF prevalence was 30%. At a baseline TF prevalence of 50%, the predicted probability of reaching a prevalence of TF<5% with 7 rounds of annual MDA was only 13%.
Table 1.
Baseline Prevalence of Trachomatous –Follicular (TF) and the predicted probability (95% Confidence Interval) of reaching TF prevalence <5% after 3–5 rounds of Mass Drug Administration (adapted from Figure 5 in (97)).

|
MDA distribution incurs costs. A recent estimate of cost per person for MDA across several Neglected Tropical Diseases was a unit cost of US$ 0.50 (2015 USD) if 100,000 people are treated and local volunteers are used (98). This estimate has dependencies on economies of scale, volunteer labor, and number of treatment days needed, but the cost implication is clear: for a district size of 250,000 persons, that estimate for MDA is $125,000. Thus, there is interest in ways to hasten the decline of trachoma and researchers have studied more frequent administration of MDA during a given year. However, studies in Niger and Ethiopia comparing the infection prevalence in communities with more frequent MDA or more frequent drug administration at least in children, have not shown any benefit compared to annual MDA in terms of community prevalence of infection (99, 100). Current research is targeting the timing of more frequent administration, with two administrations within weeks of each other, which may reduce the risk of treatment failure and lead to greater gains.
7. Challenges for trachoma elimination
A major concern is the risk of re-emergence of trachoma once antibiotic pressure is removed. Theoretically, the implementation of facial cleanliness measures and environmental improvements will ensure that even if infection is present or re-introduced, there is minimal transmission and trachoma does not re-emerge. Re-emergence of infection with cessation of mass treatment has been reported at the community level, but this was in a setting where only infection had dropped due to rounds of MDA but clinical TF was not below 5% and the surrounding communities likely still had ongoing transmission (36). In three settings where TF rates were less than 5% at district level and MDA was stopped, surveillance surveys 2 to 10 years later have confirmed absence of re-emergent trachoma (38, 40, 101). It must also be acknowledged that the definition of “re-emergence” is unclear. Country programs are re-treating districts who had trachoma that fell below a TF prevalence of 5% and subsequent surveys found trachoma returned to just over 5%. Clarity on “re-emergence” is critically needed for program use to avoid re-starting programs if there is no real risk of resurgent infection. Consideration could be given to a statistical approach whereby reemergence is a “statistically significant increase” in prevalence of TF from the previous survey. Perhaps use of alternative tools as described previously might be used to decide if ongoing transmission is still an issue, even if the TF rate is fluctuating.
Another challenge is the burden of end-stage trachoma, trichiasis. There is good evidence that incident trichiasis cases will continue to emerge after active trachoma has been eliminated; incident cases of scarring continue to be found, and progression to more severe sequelae is likely (102, 103). The reasons for this progression are not entirely understood, but the implication is that service provision and management will be needed for years after active trachoma is eliminated. Currently, a country cannot apply to WHO for validation of elimination of trachoma unless it not only satisfies the criteria for active trachoma but also provides evidence that in each formerly endemic district, the rate of trichiasis (cases not already identified and managed through the health system) is less than 1/1,000 population. The key will be to develop methods for ongoing trichiasis case finding and management so that any cases found during impact or surveillance surveys are already known to the health system.
As the goal of elimination by the year 2020 approaches, it is heartening that 12 countries have either reported achieving elimination of trachoma or been validated by WHO as having eliminated trachoma as a public health problem. In 2017, 250 districts globally reached the elimination target, no longer requiring MDA (104). Several more countries are close to elimination and await results of the surveillance surveys for confirmation. However, significant challenges remain in understanding the burden of trachoma in conflict areas, like Afghanistan, and in progress towards elimination, notably in Ethiopia where 44% of the global burden of active trachoma reside; 66% of the treatment doses distributed world-wide were in Ethiopia. Southern Sudan is known to have districts with high rates of trachoma endemicity and a lack of infrastructure to deliver the full SAFE strategy. Commitments by countries, Pfizer, and implementation and funding partners will need to continue past 2020 to ensure the global commitment to eliminate trachoma is finally achieved.
8. Expert opinion
MDA with azithromycin has proven to be a critical component of the multifaceted SAFE strategy for trachoma elimination. While trachoma disappeared in some countries prior to the advent of antibiotics, the time frame for the facial hygiene and environmental change components to sustainable interrupt transmission was likely decades if not longer. By judicious use of azithromycin to lower the community pool of infection, the trajectory for reaching a target of TF prevalence <5% in a district can be within a few years. For districts that have proved recalcitrant to rapid declines in trachoma, future research that uses the biology of C. trachomatis and overcomes potential mechanisms for “treatment failure” such as heterotypic antimicrobial resistance to design ideal MDA timing and frequency will be potentially exciting.
The main weakness in country program management of trachoma is the critical need for improved emphasis on facial hygiene and water resources to enable better hygiene behaviors. It is understandable that trachoma programs, nested in Ministry of Health programs, have not taken on the financial burden of embarking on water and sanitation infrastructure development, and there are not sufficient funds from the trachoma funding partners to provide those resources. However, stronger partnerships within the Water, Sanitation, and Hygiene (WASH) sector could be forged, and resources directed towards trachoma endemic districts emphasized as high priority. The concern for re-emergence of trachoma once antibiotic pressure is removed is in part generated by concern that the “F and E” components of SAFE are not sufficiently robust as to sustainably maintain low or no transmission.
Ultimately, a highly efficacious vaccine against C. trachomatis is the ideal control strategy. However, a vaccine that elicits a protective response that is not accompanied by a host response that leads to pathology has proven very elusive. Moreover, it is likely such a vaccine would be targeted towards the prevention of sexually transmitted chlamydia, with the target age range being just before sexual activity commences. For trachoma, the age range would need to be in early childhood, even infancy, to ensure protection against multiple re-infections throughout childhood. With the momentum of the global program towards trachoma elimination, it appears that trachoma may be gone prior to any commercially available vaccine that could be used.
Recognition is growing that trachoma will not be eliminated globally by 2020, and fortunately, Pfizer has extended its donation of azithromycin for the elimination effort through 2025. Mathematical models suggest that elimination is very possible in the next decade, and a significant challenge will be to continue to engage funding partners in the effort into the next decade. However, the goal has substantial importance–eliminating the major infectious cause of blindness worldwide will be an enormous public health success, and worthy of being able to stake a claim that one was part of that effort.
Article highlights.
Repeated infections with ocular C. trachomatis leads to trachoma, the leading infectious cause of blindness in the world.
Trachoma is a disease of communities, where the pool of infection resides in children, and the burden of blinding complications disproportionately affects women.
A public health approach is needed to eliminate trachoma from endemic districts, and this includes mass drug administration using azithromycin
Azithromycin has been shown to dramatically reduce the burden of infection when provided with high coverage
Azithromycin is safe, can be administered as a single dose of tablets/liquid once per year in treatment campaigns, and is currently donated by the manufacturer for country trachoma control programs
The goal of trachoma elimination is to reduce the prevalence of trachoma in 1–9-year-old children to less than 5% at district level.
Major concerns include: The number of rounds of annual MDA that must be provided to districts to achieve the goal; What is the risk of re-emergence of trachoma once antibiotic pressure is removed.
Mass drug administration is a key component of trachoma elimination, but one of four key components, that are part of the strategy to eliminate trachoma within the next decade.
Funding
This paper was funded by the National Eye Institute, U.S. Department of Health and Human Services (5K12EY015025–12).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.World Health Organization. Global Elimination of Blinding Trachoma. In: World Health Assembly, editor. WHA5111 1998. [Google Scholar]
- 2.**.Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nature reviews Microbiology. 2016;14(6):385–400. Epub 2016/04/26. doi: 10.1038/nrmicro.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review of chlamydia cell biology, for further details at the basic science level
- 3.Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM. Chlamydia trachomatis: the Persistent Pathogen. Clinical and vaccine immunology : CVI. 2017;24(10). Epub 2017/08/25. doi: 10.1128/cvi.00203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elwell CA, Czudnochowski N, von Dollen J, Johnson JR, Nakagawa R, Mirrashidi K, et al. Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction. eLife. 2017;6 Epub 2017/03/03. doi: 10.7554/eLife.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.*.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. The Journal of clinical investigation. 2003;111(11):1757–69. Epub 2003/06/05. doi: 10.1172/jci17993. [DOI] [PMC free article] [PubMed] [Google Scholar]; An article that lays out how the biovars may differ, and explains the tissue tropism
- 6.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiological reviews. 1991;55(1):143–90. Epub 1991/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Enciso GA, Boassa D, Chander CN, Lou TH, Pairawan SS, et al. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nature communications. 2018;9(1):45 Epub 2018/01/05. doi: 10.1038/s41467-017-02432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelrahman Y, Ouellette SP, Belland RJ, Cox JV. Polarized Cell Division of Chlamydia trachomatis. PLoS pathogens. 2016;12(8):e1005822 Epub 2016/08/10. doi: 10.1371/journal.ppat.1005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DP, Timms P, McElwain DL, Bavoil PM. Type III secretion, contact-dependent model for the intracellular development of chlamydia. Bulletin of mathematical biology. 2006;68(1):161–78. Epub 2006/06/24. doi: 10.1007/s11538-005-9024-1. [DOI] [PubMed] [Google Scholar]
- 10.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix biology : journal of the International Society for Matrix Biology. 2014;35:51–5. Epub 2013/10/23. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell host & microbe. 2008;4(2):159–69. Epub 2008/08/12. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agaisse H, Derre I. STIM1 Is a Novel Component of ER-Chlamydia trachomatis Inclusion Membrane Contact Sites. PloS one. 2015;10(4):e0125671 Epub 2015/04/29. doi: 10.1371/journal.pone.0125671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun HS, Sin AT, Poirier MB, Harrison RE. Chlamydia trachomatis Inclusion Disrupts Host Cell Cytokinesis to Enhance Its Growth in Multinuclear Cells. Journal of cellular biochemistry. 2016;117(1):132–43. Epub 2015/06/19. doi: 10.1002/jcb.25258. [DOI] [PubMed] [Google Scholar]
- 14.Elwell CA, Engel JN. Lipid acquisition by intracellular Chlamydiae. Cellular microbiology. 2012;14(7):1010–8. Epub 2012/03/29. doi: 10.1111/j.1462-5822.2012.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saka HA, Thompson JW, Chen YS, Dubois LG, Haas JT, Moseley A, et al. Chlamydia trachomatis Infection Leads to Defined Alterations to the Lipid Droplet Proteome in Epithelial Cells. PloS one. 2015;10(4):e0124630 Epub 2015/04/25. doi: 10.1371/journal.pone.0124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(27):9379–84. Epub 2008/07/02. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escoll P, Buchrieser C. Metabolic reprogramming of host cells upon bacterial infection: Why shift to a Warburg-like metabolism? The FEBS journal. 2018;285(12):2146–60. Epub 2018/04/01. doi: 10.1111/febs.14446. [DOI] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–33. Epub 2009/05/23. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jendro MC, Fingerle F, Deutsch T, Liese A, Kohler L, Kuipers JG, et al. Chlamydia trachomatis-infected macrophages induce apoptosis of activated T cells by secretion of tumor necrosis factor-alpha in vitro. Medical microbiology and immunology. 2004;193(1):45–52. Epub 2003/05/17. doi: 10.1007/s00430-003-0182-1. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter V, Chen YS, Dolat L, Valdivia RH. The Effector TepP Mediates Recruitment and Activation of Phosphoinositide 3-Kinase on Early Chlamydia trachomatis Vacuoles. mSphere. 2017;2(4). Epub 2017/07/27. doi: 10.1128/mSphere.00207-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS genetics. 2010;6(1):e1000795 Epub 2010/01/12. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung ATY, Hale C, Lee AH, Gill EE, Bushell W, Parry-Smith D, et al. Exploiting induced pluripotent stem cell-derived macrophages to unravel host factors influencing Chlamydia trachomatis pathogenesis. Nature communications. 2017;8:15013 Epub 2017/04/26. doi: 10.1038/ncomms15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*.Wyrick PB. Chlamydia trachomatis persistence in vitro: an overview. The Journal of infectious diseases. 2010;201 Suppl 2:S88–95. Epub 2010/05/28. doi: 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]; A more in depth discussion of the risk of chlamydia persistence
- 24.Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7(6):717–25. Epub 1985/11/01. [DOI] [PubMed] [Google Scholar]
- 25.Taylor HR, Johnson S, Prendergast R, Schachter J, Dawson C, Silverstein A. An animal model of trachoma: The importance of repeated reinfection. Investigative ophthalmology & visual science. 1982;23:507–15. [PubMed] [Google Scholar]
- 26.West SK BR. Trachoma 3ed. Johnson, Minassian D, Weale R, West SK, editor. London UK: Imperial College Press; 2012. 2012. 455–86 p. [Google Scholar]
- 27.Courtright P, West SK. Contribution of sex-linked biology and gender roles to disparities with trachoma. EmergInfectDis. 2004;10(11):2012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.*.Thylefors B, Dawson C, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull WHO. 1987;65:477–83. [PMC free article] [PubMed] [Google Scholar]; The original article that codified the grading if the clinical signs of trachoma for use by programs.
- 29.Keenan JD, Lakew T, Alemayehu W, Melese M, House JI, Acharya NR, et al. Slow resolution of clinically active trachoma following successful mass antibiotic treatments. Archives of ophthalmology (Chicago, Ill : 1960). 2011;129(4):512–3. Epub 2011/04/13. doi: 10.1001/archophthalmol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JS, Munoz BE, Mkocha H, Gaydos CA, Quinn TC, West SK. The Effect of Multiple Rounds of Mass Drug Administration on the Association between Ocular Chlamydia trachomatis Infection and Follicular Trachoma in Preschool-Aged Children. PLoS neglected tropical diseases. 2014;8(4):e2761 Epub 2014/04/12. doi: 10.1371/journal.pntd.0002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenson A, Dize L, Mkocha H, Munoz B, Lee J, Gaydos C, et al. Field evaluation of the Cepheid GeneXpert Chlamydia trachomatis assay for detection of infection in a trachoma endemic community in Tanzania. PLoS neglected tropical diseases. 2013;7(7):e2265 Epub 2013/07/19. doi: 10.1371/journal.pntd.0002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, Gaydos CA. Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. Journal of clinical microbiology. 2013;51(5):1611–3. Epub 2013/03/15. doi: 10.1128/JCM.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dize L, West SK, Mkocha H, Quinn TC, Gaydos CA. Evaluation of pooled ocular and vaginal swabs by the Cepheid GeneXpert CT/NG assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae compared to the GenProbe Aptima Combo 2 Assay. Diagnostic microbiology and infectious disease. 2015;81(2):102–4. Epub 2014/12/17. doi: 10.1016/j.diagmicrobio.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dize L, Gaydos CA, Quinn TC, West SK. Stability of Chlamydia trachomatis on storage of dry swabs for accurate detection by nucleic acid amplification tests. Journal of clinical microbiology. 2015;53(3):1046–7. Epub 2014/12/30. doi: 10.1128/JCM.03218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dize L, West S, Quinn TC, Gaydos CA. Pooling ocular swab specimens from Tanzania for testing by Roche Amplicor and Aptima Combo 2 assays for the detection of Chlamydia trachomatis: accuracy and cost-savings. Diagnostic microbiology and infectious disease. 2013;77(4):289–91. Epub 2013/10/02. doi: 10.1016/j.diagmicrobio.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakew T, House J, Hong KC, Yi E, Alemayehu W, Melese M, et al. Reduction and return of infectious trachoma in severely affected communities in ethiopia. PLoSNeglTropDis. 2009;3(2):e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton MJ, Holland MJ, Makalo P, Aryee EA, Alexander ND, Sillah A, et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet (London, England). 2005;365(9467):1321–8. [DOI] [PubMed] [Google Scholar]
- 38.*.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, et al. Can We Use Antibodies to Chlamydia trachomatis as a Surveillance Tool for National Trachoma Control Programs? Results from a District Survey. PLoS neglected tropical diseases. 2016;10(1):e0004352 Epub 2016/01/16. doi: 10.1371/journal.pntd.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first use of a serologic test as a surveillance tool for re emergent trachoma at district level.
- 39.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS neglected tropical diseases. 2012;6(11):e1873 Epub 2012/11/08. doi: 10.1371/journal.pntd.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West SK, Zambrano AI, Sharma S, Mishra SK, Munoz BE, Dize L, et al. Surveillance Surveys for Reemergent Trachoma in Formerly Endemic Districts in Nepal From 2 to 10 Years After Mass Drug Administration Cessation. JAMA Ophthalmol. 2017;135(11):1141–6. Epub 2017/10/04. doi: 10.1001/jamaophthalmol.2017.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West SK, Munoz B, Kaur H, Dize L, Mkocha H, Gaydos CA, et al. Longitudinal change in the serology of antibodies to Chlamydia trachomatis pgp3 in children residing in a trachoma area. Scientific reports. 2018;8(1):3520 Epub 2018/02/25. doi: 10.1038/s41598-018-21127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Trachoma 2018. [cited 2018 15 October]. Available from: http://www.who.int/news-room/fact-sheets/detail/trachoma.
- 43.Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrobial agents and chemotherapy. 1987;31(12):1948–54. Epub 1987/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabbara KF, Abu-el-Asrar A, al-Omar O, Choudhury AH, al-Faisal Z. Single-dose azithromycin in the treatment of trachoma. A randomized, controlled study. Ophthalmology. 1996;103(5):842–6. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 45.Garnock-Jones KP. Azithromycin 1.5% ophthalmic solution: in purulent bacterial or trachomatous conjunctivitis. Drugs. 2012;72(3):361–73. Epub 2012/02/10. doi: 10.2165/11208580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Scieux C, Bianchi A, Chappey B, Vassias I, Perol Y. In-vitro activity of azithromycin against Chlamydia trachomatis. The Journal of antimicrobial chemotherapy. 1990;25 Suppl A:7–10. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 47.Karcioglu ZA, El-Yazigi A, Jabak MH, Choudhury AH, Ahmed WS. Pharmacokinetics of azithromycin in trachoma patients: serum and tear levels. Ophthalmology. 1998;105(4):658–61. Epub 1998/04/17. doi: 10.1016/s0161-6420(98)94020-9. [DOI] [PubMed] [Google Scholar]
- 48.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. The Journal of antimicrobial chemotherapy. 1990;25 Suppl A:73–82. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 49.Bailey RL, Arullendran P, Whittle HC, Mabey DC. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet (London, England). 1993;342(8869):453–6. Epub 1993/08/21. [DOI] [PubMed] [Google Scholar]
- 50.**.Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, et al. Azithromycin in control of trachoma. Lancet (London, England). 1999;354(9179):630–5. Epub 1999/08/31. doi: 10.1016/s0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]; The multi-country study that provided the data on the effectiveness of azithromycin used as MDA, and convinced Pfizer to mount a donation program.
- 51.Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics. 2015;135(3):483–8. Epub 2015/02/18. doi: 10.1542/peds.2014-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutton SS. Is cardiovascular risk a concern when prescribing azithromycin? JAAPA : official journal of the American Academy of Physician Assistants. 2017;30(1):11–3. Epub 2016/12/30. doi: 10.1097/01.Jaa.0000511033.34198.95. [DOI] [PubMed] [Google Scholar]
- 53.Sutton SS, Hyche S, Magagnoli J, Hardin JW. Appraisal of the cardiovascular risks of azithromycin: an observational analysis. Journal of comparative effectiveness research. 2017;6(6):509–17. Epub 2017/09/30. doi: 10.2217/cer-2016-0080. [DOI] [PubMed] [Google Scholar]
- 54.West S. Trachoma and antibiotic use: the ‘A’ in SAFE. Expert review of anti-infective therapy. 2012;10(1):75–83. Epub 2011/12/14. doi: 10.1586/eri.11.150. [DOI] [PubMed] [Google Scholar]
- 55.West SK, West ES, Alemayehu W, Melese M, Munoz B, Imeru A, et al. Single-dose azithromycin prevents trichiasis recurrence following surgery: randomized trial in Ethiopia. Archives of ophthalmology (Chicago, Ill : 1960). 2006;124(3):309–14. Epub 2006/03/15. doi: 10.1001/archopht.124.3.309. [DOI] [PubMed] [Google Scholar]
- 56.Cochereau I, Goldschmidt P, Goepogui A, Afghani T, Delval L, Pouliquen P, et al. Efficacy and safety of short duration azithromycin eye drops versus azithromycin single oral dose for the treatment of trachoma in children: a randomised, controlled, double-masked clinical trial. The British journal of ophthalmology. 2007;91(5):667–72. Epub 2006/09/29. doi: 10.1136/bjo.2006.099275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huguet P, Bella L, Einterz EM, Goldschmidt P, Bensaid P. Mass treatment of trachoma with azithromycin 1.5% eye drops in the Republic of Cameroon: feasibility, tolerance and effectiveness. The British journal of ophthalmology. 2010;94(2):157–60. Epub 2009/08/21. doi: 10.1136/bjo.2009.161513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster SO, Powers DK, Thygeson P. Trachoma therapy: a controlled study. American journal of ophthalmology. 1966;61(3):451–5. Epub 1966/03/01. [DOI] [PubMed] [Google Scholar]
- 59.Reinhards J, Weber A, Maxwell-Lyons F. Collective antibiotic treatment of trachoma. Report on comparative trials leading to more economic methods of treatment. Bulletin of the World Health Organization. 1959;21:665–702. Epub 1959/01/01. [PMC free article] [PubMed] [Google Scholar]
- 60.Reinhards J, Weber A, Nizetic B, Kupka K, Maxwell-Lyons F. Studies in the epidemiology and control of seasonal conjunctivitis and trachoma in southern Morocco. Bulletin of the World Health Organization. 1968;39(4):497–545. Epub 1968/01/01. [PMC free article] [PubMed] [Google Scholar]
- 61.Dawson CR, Daghfous T, Whitcher J, Messadi M, Hoshiwara T, Triki F, et al. Intermittent trachoma chemotherapy: a controlled trial of topical tetracycline or erythromycin. Bulletin of the World Health Organization. 1981;59(1):91–7. Epub 1981/01/01. [PMC free article] [PubMed] [Google Scholar]
- 62.Dawson CR, Daghfous T, Hoshiwara I, Ramdhane K, Kamoun M, Yoneda C, et al. Trachoma therapy with topical tetracycline and oral erythromycin: a comparative trial. Bulletin of the World Health Organization. 1982;60(3):347–55. Epub 1982/01/01. [PMC free article] [PubMed] [Google Scholar]
- 63.Bowman RJ, Sillah A, Van Dehn C, Goode VM, Muqit MM, Johnson GJ, et al. Operational comparison of single-dose azithromycin and topical tetracycline for trachoma. Investigative ophthalmology & visual science. 2000;41(13):4074–9. Epub 2000/11/30. [PubMed] [Google Scholar]
- 64.Hoshiwara I, Ostler HB, Hanna L, Cignetti F, Coleman VR, Jawetz E. Doxycycline treatment of chronic trachoma. Jama. 1973;224(2):220–3. Epub 1973/04/09. [PubMed] [Google Scholar]
- 65.Darougar S, Jones BR, Viswalingam N, Poirier RH, Allami J, Houshmand A, et al. Family-based suppressive intermittent therapy of hyperendemic trachoma with topical oxytetracycline or oral doxycycline. The British journal of ophthalmology. 1980;64(4):291–5. Epub 1980/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavery FS. SULPHONAMIDES AND PENICILLIN IN THE TREATMENT OF TRACHOMA. The British journal of ophthalmology. 1946;30(10):591–4. Epub 1946/10/01. [PMC free article] [PubMed] [Google Scholar]
- 67.Freyche MJ. Antibiotics and sulfonamides in the treatment of trachoma. Bulletin of the World Health Organization. 1950;2(4):523–44. Epub 1950/01/01. [PMC free article] [PubMed] [Google Scholar]
- 68.International Trachoma Initiative. 2018. [cited 2018 15 October]. Available from: http://www.trachoma.org/
- 69.Munoz B, Solomon AW, Zingeser J, Barwick R, Burton M, Bailey R, et al. Antibiotic dosage in trachoma control programs: height as a surrogate for weight in children. Investigative ophthalmology & visual science. 2003;44(4):1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.West SK, Bailey R, Munoz B, Edwards T, Mkocha H, Gaydos C, et al. A randomized trial of two coverage targets for mass treatment with azithromycin for trachoma. PLoS neglected tropical diseases. 2013;7(8):e2415 Epub 2013/09/07. doi: 10.1371/journal.pntd.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amza A, Kadri B, Nassirou B, Cotter SY, Stoller NE, West SK, et al. Effectiveness of expanding annual mass azithromycin distribution treatment coverage for trachoma in Niger: a cluster randomised trial. The British journal of ophthalmology. 2018;102(5):680–6. Epub 2017/09/13. doi: 10.1136/bjophthalmol-2017-310916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harding-Esch EM, Sillah A, Edwards T, Burr SE, Hart JD, Joof H, et al. Mass treatment with azithromycin for trachoma: when is one round enough? Results from the PRET Trial in the Gambia. PLoS neglected tropical diseases. 2013;7(6):e2115 Epub 2013/06/21. doi: 10.1371/journal.pntd.0002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ssemanda EN, Munoz B, Harding-Esch EM, Edwards T, Mkocha H, Bailey RL, et al. Mass treatment with azithromycin for trachoma control: participation clusters in households. PLoS neglected tropical diseases. 2010;4(10). Epub 2010/10/20. doi: 10.1371/journal.pntd.0000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ssemanda EN, Levens J, Mkocha H, Munoz B, West SK. Azithromycin mass treatment for trachoma control: risk factors for non-participation of children in two treatment rounds. PLoS neglected tropical diseases. 2012;6(3):e1576 Epub 2012/03/27. doi: 10.1371/journal.pntd.0001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ssemanda EN, Mkocha H, Levens J, Munoz B, West SK. Community mass treatment with azithromycin for trachoma: Factors associated with change in participation of children from the first to the second round. Clinical epidemiology and global health. 2015;3(1):37–43. Epub 2015/10/16. doi: 10.1016/j.cegh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Astale T, Sata E, Zerihun M, Nute AW, Stewart AEP, Gessese D, et al. Population-based coverage survey results following the mass drug administration of azithromycin for the treatment of trachoma in Amhara, Ethiopia. PLoS neglected tropical diseases. 2018;12(2):e0006270 Epub 2018/02/17. doi: 10.1371/journal.pntd.0006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitty CJ, Glasgow KW, Sadiq ST, Mabey DC, Bailey R. Impact of community-based mass treatment for trachoma with oral azithromycin on general morbidity in Gambian children. PediatrInfect Dis J. 1999;18(11):955–8. [DOI] [PubMed] [Google Scholar]
- 78.Ayele B, Gebre T, House JI, Zhou Z, McCulloch CE, Porco TC, et al. Adverse events after mass azithromycin treatments for trachoma in Ethiopia. The American journal of tropical medicine and hygiene. 2011;85(2):291–4. Epub 2011/08/05. doi: 10.4269/ajtmh.2011.11-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coles CL, Levens J, Seidman JC, Mkocha H, Munoz B, West S. Mass distribution of azithromycin for trachoma control is associated with short-term reduction in risk of acute lower respiratory infection in young children. Pediatr Infect Dis J. 2012;31(4):341–6. Epub 2011/12/17. doi: 10.1097/INF.0b013e31824155c9. [DOI] [PubMed] [Google Scholar]
- 80.Schachterle SE, Mtove G, Levens JP, Clemens E, Shi L, Raj A, et al. Short-term malaria reduction by single-dose azithromycin during mass drug administration for trachoma, Tanzania. Emerg Infect Dis. 2014;20(6):941–9. Epub 2014/05/29. doi: 10.3201/eid2006.131302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.West SK, Moncada J, Munoz B, Mkocha H, Storey P, Hardick J, et al. Is there evidence for resistance of ocular Chlamydia trachomatis to azithromycin after mass treatment for trachoma control? The Journal of infectious diseases. 2014;210(1):65–71. Epub 2014/01/22. doi: 10.1093/infdis/jiu046. [DOI] [PubMed] [Google Scholar]
- 82.*.West ES, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW, et al. Mass treatment and the effect on the load of Chlamydia trachomatis infection in a trachoma-hyperendemic community. Investigative ophthalmology & visual science. 2005;46(1):83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first article on the impact of the load of infection on clearance following MDA.
- 83.Horner PJ. Azithromycin antimicrobial resistance and genital Chlamydia trachomatis infection: duration of therapy may be the key to improving efficacy. Sex Transm Infect. 2012;88(3):154–6. Epub 2012/03/15. doi: 10.1136/sextrans-2011-050385. [DOI] [PubMed] [Google Scholar]
- 84.Bhengraj AR, Vardhan H, Srivastava P, Salhan S, Mittal A. Decreased susceptibility to azithromycin and doxycycline in clinical isolates of Chlamydia trachomatis obtained from recurrently infected female patients in India. Chemotherapy. 2010;56(5):371–7. Epub 2010/10/13. doi: 10.1159/000314998. [DOI] [PubMed] [Google Scholar]
- 85.Batt SL, Charalambous BM, Solomon AW, Knirsch C, Massae PA, Safari S, et al. Impact of azithromycin administration for trachoma control on the carriage of antibiotic-resistant Streptococcus pneumoniae. AntimicrobAgents Chemother. 2003;47(9):2765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaynor BD, Holbrook KA, Whitcher JP, Holm SO, Jha HC, Chaudhary JS, et al. Community treatment with azithromycin for trachoma is not associated with antibiotic resistance in Streptococcus pneumoniae at 1 year. The British journal of ophthalmology. 2003;87(2):147–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coles CL, Mabula K, Seidman JC, Levens J, Mkocha H, Munoz B, et al. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistance S. pneumoniae carriage in young children 6 months after treatment. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013. Epub 2013/03/15. doi: 10.1093/cid/cit137. [DOI] [PubMed] [Google Scholar]
- 88.Haug S, Lakew T, Habtemariam G, Alemayehu W, Cevallos V, Zhou Z, et al. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(5):571–4. Epub 2010/07/24. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]
- 89.Bloch EM, West SK, Mabula K, Weaver J, Mrango Z, Munoz B, et al. Antibiotic Resistance in Young Children in Kilosa District, Tanzania 4 Years after Mass Distribution of Azithromycin for Trachoma Control. The American journal of tropical medicine and hygiene. 2017;97(3):815–8. Epub 2017/07/20. doi: 10.4269/ajtmh.17-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaynor BD, Chidambaram JD, Cevallos V, Miao Y, Miller K, Jha HC, et al. Topical ocular antibiotics induce bacterial resistance at extraocular sites. BrJOphthalmol. 2005;89(9):1097–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.World Health Organization. 3rd Global Scientific Meeting on Trachoma Geneva, Switzerland: 2010. [Google Scholar]
- 92.Solomon AW, Holland MJ, Alexander ND, Massae PA, Aguirre A, Natividad-Sancho A, et al. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med. 2004;351(19):1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Broman AT, Shum K, Munoz B, Duncan DD, West SK. Spatial clustering of ocular chlamydial infection over time following treatment, among households in a village in Tanzania. Invest OphthalmolVisSci. 2006;47(1):99–104. [DOI] [PubMed] [Google Scholar]
- 94.Evans JR, Solomon AW. Antibiotics for trachoma. The Cochrane database of systematic reviews. 2011(3):Cd001860 Epub 2011/03/18. doi: 10.1002/14651858.CD001860.pub3. [DOI] [PubMed] [Google Scholar]
- 95.West SK, Munoz B, Mkocha H, Gaydos C, Quinn T. Trachoma and ocular Chlamydia trachomatis were not eliminated three years after two rounds of mass treatment in a trachoma hyperendemic village. Invest OphthalmolVisSci. 2007;48(4):1492–7. [DOI] [PubMed] [Google Scholar]
- 96.West SK, Munoz B, Mkocha H, Gaydos CA, Quinn TC. Number of years of annual mass treatment with azithromycin needed to control trachoma in hyper-endemic communities in Tanzania. The Journal of infectious diseases. 2011;204(2):268–73. Epub 2011/06/16. doi: 10.1093/infdis/jir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.*.Jimenez V, Gelderblom HC, Mann Flueckiger R, Emerson PM, Haddad D. Mass drug administration for trachoma: how long is not long enough? PLoS neglected tropical diseases. 2015;9(3):e0003610 Epub 2015/03/24. doi: 10.1371/journal.pntd.0003610. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article that used district wide data from many districts worldwide to try and determine the number of rounds of MDA needed to reduce the prevalence of trachoma to <5%
- 98.Fitzpatrick C, Fleming FM, Madin-Warburton M, Schneider T, Meheus F, Asiedu K, et al. Benchmarking the Cost per Person of Mass Treatment for Selected Neglected Tropical Diseases: An Approach Based on Literature Review and Meta-regression with Web-Based Software Application. PLoS neglected tropical diseases. 2016;10(12):e0005037 Epub 2016/12/06. doi: 10.1371/journal.pntd.0005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amza A, Kadri B, Nassirou B, Cotter SY, Stoller NE, Zhou Z, et al. A Cluster-Randomized Trial to Assess the Efficacy of Targeting Trachoma Treatment to Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(6):743–50. Epub 2016/12/14. doi: 10.1093/cid/ciw810. [DOI] [PubMed] [Google Scholar]
- 100.Gebre T, Ayele B, Zerihun M, Genet A, Stoller NE, Zhou Z, et al. Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet (London, England). 2012;379(9811):143–51. Epub 2011/12/24. doi: 10.1016/S0140-6736(11)61515-8. [DOI] [PubMed] [Google Scholar]
- 101.Nash SD, Stewart AEP, Astale T, Sata E, Zerihun M, Gessese D, et al. Trachoma prevalence remains below threshold in five districts after stopping mass drug administration: results of five surveillance surveys within a hyperendemic setting in Amhara, Ethiopia. Trans R Soc Trop Med Hyg. 2018. Epub 2018/09/29. doi: 10.1093/trstmh/try096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karani R, Wolle M, Mkocha H, Munoz B, West SK. Risk factors for incidence of trachomatous scarring in a cohort of women in low endemic district. The British journal of ophthalmology. 2018;102(4):419–23. Epub 2018/01/08. doi: 10.1136/bjophthalmol-2017-311301. [DOI] [PubMed] [Google Scholar]
- 103.Ramadhani AM, Derrick T, Holland MJ, Burton MJ. Blinding Trachoma: Systematic Review of Rates and Risk Factors for Progressive Disease. PLoS neglected tropical diseases. 2016;10(8):e0004859 Epub 2016/08/03. doi: 10.1371/journal.pntd.0004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.International Coalition for Trachoma Control. [cited 2018 15 October] Available from: http://www.trachomacoalition.org/trachomastatistics