Abstract
Blood-brain barrier breakdown and associated vascular hyperpermeability leads to vasogenic edema in traumatic brain injury (TBI). Tight junctions maintain blood-brain barrier integrity; their disruption in TBI holds significant promise for diagnosis and treatment. A controlled cortical impactor was used for TBI in mouse studies. Blood was collected 1 h after injury and sent for antibody microarray analysis. Twenty human subjects with radiographic evidence of TBI were enrolled and blood collected within 48 h of admission. Control subjects were individuals with nontrauma diagnoses. The subjects were matched by age and gender. Enzyme-linked immunosorbent assays were performed on each TBI and control sample for tight junction–associated proteins (TJPs), inflammatory markers, and S100β. Plasma was used to conduct in vitro monolayer permeability studies with human brain endothelial cells. S100β and the TJP occludin were significantly elevated in TBI plasma in both the murine and human studies. Monolayer permeability studies showed increased hyperpermeability in TBI groups. Plasma from TBI subjects increases microvascular hyperpermeability in vitro. TJPs in the blood may be a potential biomarker for TBI.
Keywords: Biomarker, blood-brain barrier, microvascular hyperpermeability, tight junction proteins, traumatic brain injury
Traumatic brain injury (TBI) is a widespread and difficult-to-manage public health concern. It is estimated that 10 million people suffer TBIs worldwide each year.1–3 According to the Centers for Disease Control and Prevention, in 2012, the lifetime cost of medical care, lost wages, and productivity for patients with TBI was estimated to be $76.5 billion.4 Currently we rely on physical exam (focused neurological exam and Glasgow Coma Scale [GCS] score) and imaging (computed tomography [CT] and magnetic resonance imaging of the head)5,6 to diagnose and prognosticate the severity of TBI. At this time, point-of-care testing or laboratory tests are not available to diagnose or help in the management of TBI.
The blood-brain barrier (BBB) is a semipermeable membrane that protects the brain from toxins and microbes in the blood and helps to maintain cerebral homeostasis. BBB integrity is determined mainly by tight junctions (TJs) between neighboring endothelial cells that are formed by tight junction–associated proteins (TJPs). These proteins are linked intracellularly to scaffold proteins such as zonula occludens-1 (ZO-1). The indispensable role of TJs in maintaining BBB integrity and their disruption in TBI suggest that components of the TJ complex or its regulatory factors hold significant promise for the diagnosis and possible treatment of TBI.7–13
A serious consequence of brain inflammation after injury is microvascular leakage from BBB dysregulation leading to cerebral edema, neuronal injury, and death.14 A number of studies have attempted to identify a biomarker of TBI in the blood,15–17 but currently no clinically reliable biomarker exists for the diagnosis or prognosis of TBI. We hypothesized that BBB dysfunction and breakdown after TBI results in the degradation of TJPs and that these proteins may be detectable in the blood after injury. The detection of TJPs may offer a novel method to evaluate patients with TBI and estimate injury severity.
METHODS
Male C57BL/6 mice (18–25 g) were purchased from Jackson Laboratories (Bar Harbor, ME) for homogeneity of the population. Animals were maintained at the Texas A&M University Health Science Center College of Medicine and Baylor Scott and White Health animal facility on a 12:12-h dark/light cycle, with free access to food and water, but no food at midnight prior to surgery. The room temperature was maintained at 25° ± 2°C. Surgical and experimental procedures used in this study were conducted after approval from the Baylor Scott and White Health/Texas A&M University Health Science Center College of Medicine institutional animal care and use committee. The facility is approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the National Institutes of Health guidelines. The animals were anesthetized with 30% urethane in phosphate-buffered saline, intraperitoneal injection (2 mL/kg body weight), and were continuously observed by an investigator until the end of the study (up to 1 h following TBI). This was not a survival study and no unexpected animal deaths were observed.
The head of the animal was shaven and the surgical site on the surface of the head was cleaned with an alcohol wipe. Lubricating ointment was applied to the eyes. A midline incision on the scalp exposed the sagittal suture, bregma, and lambda. A circular craniectomy window, 5 mm in diameter, was made over the right hemisphere, between the lambda and bregma using a microdrill. The resulting bone flap was removed. Sham animals (n = 3) received only craniectomy, while TBI injury group animals received brain injury via controlled cortical impactor immediately following the craniectomy procedure. A Benchmark Stereotaxic Impactor (Leica Biosystems Inc., Buffalo Grove, IL) was used to produce TBI in mice. Following craniectomy, the animals were mounted on the stereotaxic frame. An impactor probe of 4 mm diameter was used to impact the exposed part of the brain. The depth of the injury was used to determine the severity of the injury. Settings for mild TBI (n = 4) used in this study were 1 mm depth, 0.50 m/sec velocity, and 100 ms contact time. Moderate TBI (n = 3) settings were 2 mm depth, 0.50 m/sec velocity, and 100 msc contact time. An hour after TBI, all animals were exsanguinated and blood was collected by intracardiac puncture.
Collected blood samples from animals were shipped to RayBiotech, Inc. (Norcross, GA) for antibody microarray analysis to screen for potential biomarkers. The following analytes were studied: ZO-1, junctional adhesion molecule-1 (JAM-1), tricellulin, occludin, vascular endothelial (VE)-cadherin, β-catenin, caspase-3, caspase-8, B-cell lymphoma 2 (Bcl-2)–associated X (BAX), Bcl-2–associated death promoter (BAD), cytochrome C, Bcl-2 homologue-3 interacting-domain death agonist (BID), Bcl-2-like protein 11 (BIM), soluble Fas protein (sFas), Matrix metalloproteinase-9 (MMP-9), interleukin (IL)−1β, and IL-17.
We enrolled 20 subjects with TBI admitted to an American College of Surgeons–verified, state-designated level 1 trauma center in 2016. To be included, patients had to meet trauma activation criteria, have evidence of TBI on radiographic imaging (CT head), and be admitted to the surgical trauma intensive care unit (ICU). We excluded patients with conditions known to adversely influence the BBB: intoxication or substance disorder,18–20 active infection or antibiotic use,21,22 partial- or full-thickness burns to >15% of body surface area,23 penetrating head injury, central nervous system malignancy,24 and spinal cord injury. Information on demographic characteristics, mortality, ICU length of stay, ICU-free days, intracranial pathology, and injury severity score (ISS) was ascertained by chart review. Control patients were individuals presenting to the same center for outpatient care with nontrauma diagnoses. Controls could not have any of the above exclusion criteria. We utilized plasma from each specimen to conduct in vitro analysis of TJ breakdown using monolayer permeability studies. The patients were matched based on age (±3 years) and gender. The study was approved by the Scott and White Medical Center – Temple institutional review board.
The following enzyme-linked immunosorbent assay (ELISA) kits were utilized: human ZO-1, human β-catenin, claudin-5, human NLRP3, and human occluden (LifeSpan Biosciences, Seattle, WA), human S100β (Lifeome, Oceanside, CA), and human MMP-9 platinum and human IL-1β platinum (eBioscience, ThermoFisher Scientific, Waltham, MA). ELISA was performed using a sandwich-based system with antibody-precoated 96-well plates and treated with secondary markers to assess the presence and concentration of the antigen. Assays were performed on each TBI (n = 20) and control sample.
Human brain microvascular endothelial cells (Cell Systems, Kirkland, WA), ECM media (ScienCell, Carlsbad, CA), fluorescein isothiocyanate-dextran (FITC)-dextran (Sigma-Aldrich, St. Louis, MO), and phosphate-buffered saline (GE Healthcare Bio-Sciences, Pittsburgh, PA) were obtained. Phenol red-free media (Dulbecco’s modified Eagle medium–Fluorobrite) was obtained from Life Technologies (Grand Island, NY). Human brain microvascular endothelial cells were grown on fibronectin-coated Transwell inserts as monolayers for 72 h. Monolayers were initially exposed to phenol red-free Dulbecco’s modified Eagle medium for 1 h. The control group was then exposed to normal human plasma (n = 12) for 2 h at specific dilutions in phenol-free media (1:2 dilution). Dilution decreases the chance of confounding factors from other proteins naturally circulating in plasma that could increase permeability.25 The TBI experimental group was then exposed to human TBI plasma (n = 12) for 2 h at a 1:2 dilution. At the end of the treatment, FITC-labeled dextran-10 kDa (5 mg/mL; 30 min) was applied to the luminal (upper) compartment. One hundred microliters of sample was collected from the abluminal (lower) compartment after 30 min and measured fluorometrically at 485/520 nm (excitation/emission) using a Fluoroskan Ascent FL Microplate Fluorometer and Luminometer. This fluorescence quantitates FITC-dextran flux across the monolayer as a marker of permeability. The mean fluorescence in the two study groups was then compared.
In the animal studies, analysis of variance was utilized for statistical comparison between groups, and statistical differences were then compared with post hoc analysis. In the human studies, data are expressed as the mean ± percentage standard error of the mean for the monolayer permeability and mean concentration in ng/mL for the ELISA data. Statistical difference between the groups was determined with Student’s t test using GraphPad Prism 6. Correlation analysis was performed with regard to patient outcomes.
RESULTS
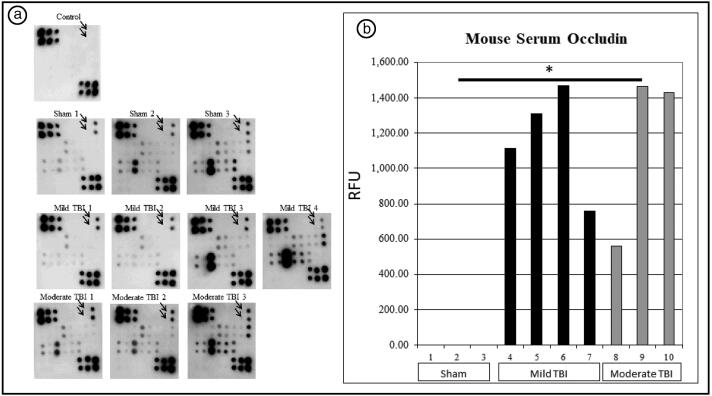
Mean occludin signal was 0 relative fluorescence units (RFU) for sham animals, 1164.01 RFU for mild TBI animals, and 1151.94 RFU for moderate TBI animals (P = 0.01, analysis of variance). Post hoc analysis revealed a significant difference between sham animals and mild TBI (P = 0.01); the difference between sham animals and moderate TBI approached significance (P = 0.06). There was no difference in occludin signal between mild and moderate TBI animals. Figure 1a shows the antibody array for each animal, and Figure 1b shows a graphical representation of the occludin signal in each group. There was no significant difference between sham, mild TBI, and moderate TBI groups in the following biomarkers: ZO-1, JAM-1, tricellulin, VE-cadherin, β-catenin, caspase-3, caspase-8, BAX, BAD, cytochrome C, BID, BIM, sFas, MMP-9, IL-1β, and IL-17.
Figure 1.
(a) Microarray plots from mouse blood samples. Arrows indicate occludin signal. (b) Relative fluorescent units measured in the occludin antibody microarray.
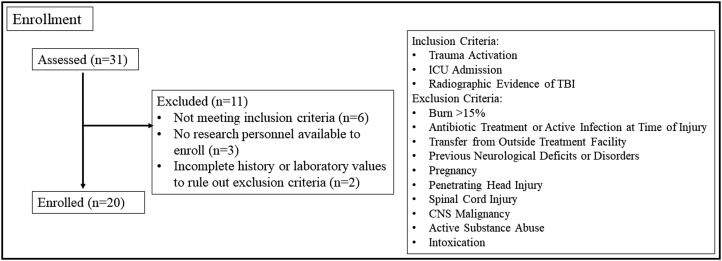
Twenty human subjects were enrolled out of 31 subjects screened in a 2-month period (Figure 2). TBI subjects were mostly female (55%) with a median age of 63.5 (interquartile range [IQR], 49.5, 76) and an overall 25% mortality. Most subjects had isolated head injuries (85%). Median ISS was 22.5 (IQR 14.25, 34.5). Six subjects (30%) had a subarachnoid hemorrhage, six subjects (30%) had a subdural hematoma, and two (10%) had an epidural hematoma. Six subjects (30%) had a combination of subarachnoid hemorrhage, subdural hematoma, epidural hematoma, and intraparenchymal hemorrhage. Median GCS on presentation was 15 (IQR 8.75, 15), with 55% of subjects having no deficits upon presentation; 13 subjects (65%) had mild TBI with a GCS of 13 to 15, two subjects (10%) had moderate TBI with a GCS of 9 to 12, and five subjects (25%) had severe TBI with a GCS <8. Median hospital length of stay was 5 days (IQR 1, 10.5), median ICU length of stay was 2.5 days (IQR 1, 4.75), and median ICU-free days was 1 (IQR 0, 2.25).
Figure 2.
Flowchart for screening and enrollment of study subjects.
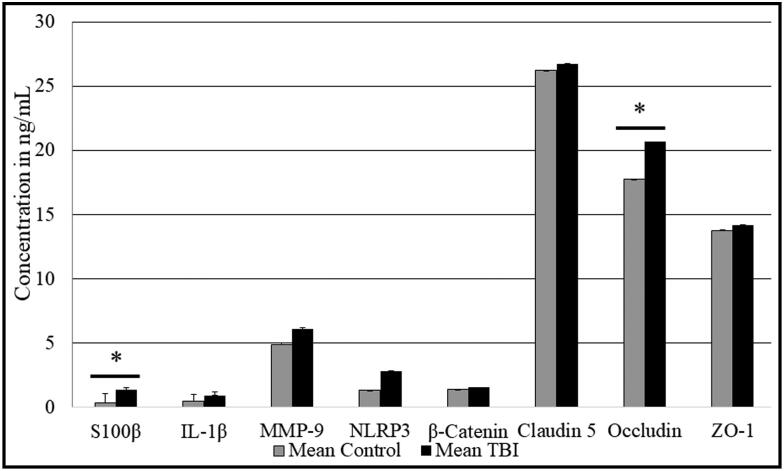
ELISAs were performed on each blood sample (n = 20) in duplicate. All protein concentrations were converted to ng/mL. S100β and occludin levels were significantly elevated in the TBI plasma (Figure 3; P < 0.05). The average S100β level in those with TBI was 0.68 ng/mL vs 0.19 ng/mL in control subjects. The average occludin level in those with TBI was 20.6 ng/mL vs 17.7 ng/mL in controls. There was an increase in MMP-9 and NLRP3 in TBI subjects that did not reach statistical significance. There was no difference in IL-1β, β-catenin, claudin-5, or ZO-1 levels between TBI and control subjects.
Figure 3.
ELISA of plasma samples from control and TBI patients targeting potential TBI biomarkers. ELISAs were performed on each blood sample (n = 20 in each group) in duplicate. *P < 0.05.
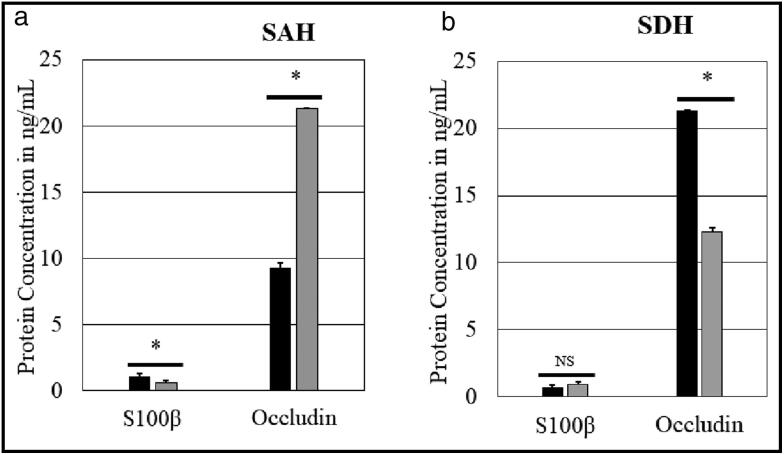
The six subjects with isolated subarachnoid hemorrhage were compared to the eight with no component of subarachnoid hemorrhage on CT scan. Those with subarachnoid hemorrhage had a significantly higher S100β level and a significantly lower occludin level. In the subjects with isolated subdural hematoma (n = 6), there was a statistically significant increase in occludin level compared to those without subdural hematoma (Figure 4).
Figure 4.
Association of S100β and occludin with intracranial pathology: (a) subarachnoid hemorrhage (SAH) and (b) subdural hematoma (SDH). Black bars represent those with SAH (n = 6) or SDH (n = 6) and gray bars represent those without (n = 8). *P < 0.05.
Through chart review, we calculated the TBI group’s ISS and plotted ISS vs S100β or occludin levels. We found no correlation in the level of our positive markers with ISS. For S100β, there was a Pearson r value of −0.339, P value of 0.14, and R2 of 0.115. In occludin, there was a Pearson r value of −0.174, P value of 0.46, and R2 of 0.036.
The S100β and occludin levels were compared between the 25% of subjects who died following their injuries and those who lived. In subjects who died, the mean S100β was 0.71 ng/mL vs 0.68 ng/mL in those who survived; occludin concentrations were 17.61 ng/mL in subjects who died vs 14.61 ng/mL in those who survived (P = NS).
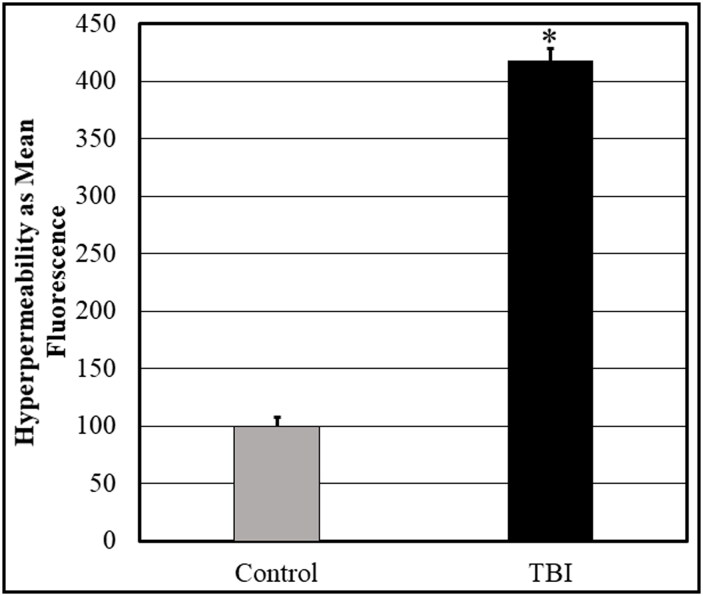
Control subject plasma was compared to that of TBI subjects. Samples were tested in pentaplicate and then the study was repeated. As mentioned, samples were diluted to 1:2. Following exposure of the monolayers to subject plasma (n = 12), the mean fluorescence intensity of each sample was measured. Control samples were considered to be 100% and the fluorescence intensity of the TBI group was compared to the control (Figure 5). TBI plasma alone induced hyperpermeability in human brain endothelial cells after contact exposure.
Figure 5.
Monolayer permeability assay demonstrating the effect of TBI serum on BBB endothelial cell permeability. Samples were diluted to 1:2 and 1:3. The FITC-dextran fluorescence intensity obtained following exposure of the cells to control samples (n = 12) was considered to be 100% and the fluorescence intensity from the TBI group (n = 12) was compared to the control. P < 0.05.
DISCUSSION
Our goal was to better understand the pathophysiology of the BBB in TBI and develop novel diagnostics for the early detection and treatment of TBIs. We were successful in identifying two novel biomarkers found in the circulating bloodstream of TBI patients, S100β and the TJP occludin. In addition, we discovered that these biomarkers were elevated differently based on the underlying pathophysiology of the TBI, specifically subarachnoid hemorrhage vs subdural hematoma. In the process of our work, we demonstrated an in vitro model of induced hyperpermeability in human BBB endothelial cells using plasma from TBI subjects. This had not been previously demonstrated with samples from human subjects. This work potentially sets the foundation for further understanding of TBI, its pathology, new avenues for therapy, and biomarker development.
S100β is an intracellular protein found mostly in astrocytes and has been found to be elevated in TBI.15–17 We found that it was even more elevated with subarachnoid hemorrhage than with other traumatic intracranial pathologies. It may be that injuries leading to subarachnoid hemorrhage are more strongly associated with direct cellular damage in TBI. The TJP occludin was found to be elevated in TBI subjects when compared to control subjects, and this was reinforced in our animal studies. Occludin levels were even higher in those with subdural hematoma compared with subarachnoid hemorrhage. Damage to the intracranial bridging veins may lead to endothelial displacement of the TJP occludin.26 These findings could help in diagnosing and differentiating types of traumatic intracranial hematomas in areas without CT imaging capabilities. Although we did not find an association between these markers and length of stay or mortality, they may have utility in obtaining more information regarding the mechanisms of BBB breakdown in humans after TBI. In addition, this was a short-term study looking at the protein levels upon admission to the ICU, so long-term studies will be needed to assess if these levels can be trended and used diagnostically or prognostically.
In TBI, the primary injury process consists of the rapid acceleration-deceleration producing shear forces and impact with the cranial wall; this can be mitigated only through prevention and improved safety technology. Secondary injury in TBI is frequently mediated by reactive oxygen species produced from ischemia-reperfusion injury, glutamatergic excitotoxicity, or neuroinflammation.27,28 IL1-β, IL-6, IL18, and tumor necrosis factor-α are involved in the inflammatory cascade after TBI that leads to proteolytic enzyme activation, which in turn breaks down TJPs.12,29,30 IL1-β was not significantly elevated in this study, and other interleukins will be the target of future studies. As these cytokines are active in the hours, days, and weeks following injury, trending these markers may serve as a better target for prognosis.
MMPs, caspase-3, the NLRP3 inflammasome, and calpains are increased in the brain following TBI in vitro and in animal models,7–9,31 and their activation leads to damage of TJPs and the BBB. The inhibition of these proteins in cellular and animal models has led to decreased microvascular hyperpermeability.7,8 Our study showed an increase in MMP-9 and NLRP3, though these did not reach statistical significance possibly due to the small sample size or the timing of measurement. Occludin and S100β are elevated early with injury (within the first 48 h), but the inflammatory phase of secondary injury occurs in the days and weeks following injury. Thus, inflammatory markers of TBI may not be elevated until 72 or 96 h after injury. We hope to build on these results by enrolling additional patients with a range of injury severity; acquiring blood samples closer to the time of injury; and then collecting samples 6, 12, 24, 48, 72, and 96 h after injury to capture the temporal nature of these inflammatory and TJP markers.
In our study, TBI plasma alone induced hyperpermeability in human brain endothelial cells. No study of its kind to date has assessed the hyperpermeability that occurs in TBI using plasma from TBI subjects as an inducing agent in an in vitro setting; the only area in which similar findings have been observed in the literature is in subjects undergoing cardiopulmonary bypass.32 This novel finding in TBI subjects can help provide a safe platform for preclinical trials. The hope is that this method, which uses human cells and human blood samples, can serve as an adjunct to testing in small animal models of TBI.
There are recognized limitations in this study. The sample size was small, both in the human study (n = 20 in each group) and in the animal study (n = 3 to 4 in each group). There were restrictions on the timing and quantity of plasma obtained from each subject due to our reliance on clinically directed bedside lab draws. Regarding our in vitro experiments, the monolayer technique does not possess all components of the BBB. Although the endothelium is the most important component of the BBB, the astrocytes, podocytes, and neurons also participate in the barrier function of the BBB.26,33 This study, however, shows some promising novel targets for study.
In conclusion, we found that TBI plasma alone can induce hyperpermeability in the endothelial cells that make up the BBB. S100β and the TJP occludin were significantly elevated in the blood of human and murine subjects with TBI and could be used as biomarkers for TBI. S100β and occludin levels can differentiate subarachnoid hemorrhage from subdural hematoma and may be useful in a situation in which CT imaging is not available.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths, 2002–2006. Atlanta, GA: National Center for Injury Prevention and Control; 2010. doi: 10.15620/cdc.5571. [DOI] [Google Scholar]
- 2.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NRE. 2007;22(5):341–353. doi: 10.3233/NRE-2007-22502. [DOI] [PubMed] [Google Scholar]
- 3.Thurman DJ, Branche CM, Sniezek JE. The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. J Head Trauma Rehabil. 1998;13(2):1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Pearson WS, Sugerman DE, McGuire LC, Coronado VG. Emergency department visits for traumatic brain injury in older adults in the United States: 2006–08. West J Emergency Med. 2012;13(3):289–293. doi: 10.5811/westjem.2012.3.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saatman KE, Duhaime A-C, Bullock R, Maas AI, Valadka A, Manley GT; Workshop scientific team and advisory panel members. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clin N Am. 2010;20(4):527–556. doi: 10.1016/j.nic.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Alluri H, Wilson RL, Anasooya Shaji C, et al. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS One. 2016;11(5):e0154427. doi: 10.1371/journal.pone.0154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alluri H, Grimsley M, Anasooya Shaji C, et al. Attenuation of blood-brain barrier breakdown and hyperpermeability by calpain inhibition. J Biol Chem. 2016;291(53):26958–26969. doi: 10.1074/jbc.M116.735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alluri H, Wiggins-Dohlvik K, Davis ML, Huang JH, Tharakan B. Blood-brain barrier dysfunction following traumatic brain injury. Metab Brain Dis. 2015;30(5):1093–1104. doi: 10.1007/s11011-015-9651-7. [DOI] [PubMed] [Google Scholar]
- 10.Sawant DA, Tharakan B, Wilson RL, Stagg HW, Hunter FA, Childs EW. Regulation of tumor necrosis factor-α-induced microvascular endothelial cell hyperpermeability by recombinant B-cell lymphoma-extra large. J Surg Res. 2013;184(1):628–637. doi: 10.1016/j.jss.2013.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tharakan B, Hellman J, Sawant DA, et al. β-Catenin dynamics in the regulation of microvascular endothelial cell hyperpermeability. Shock. 2012;37(3):306–311. doi: 10.1097/SHK.0b013e318240b564. [DOI] [PubMed] [Google Scholar]
- 12.Nizamutdinov D, Shapiro LA. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017;7(12):11. doi: 10.3390/brainsci7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman LC, Ting J-Y. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136(Suppl 1):29–38. doi: 10.1111/jnc.13217. [DOI] [PubMed] [Google Scholar]
- 14.Rigor RR, Beard RS Jr, Litovka OP, Yuan SY. Interleukin-1β-induced barrier dysfunction is signaled through PKC-θ in human brain microvascular endothelium. Am J Physiol Cell Physiol. 2012;302(10):C1513–C1522. doi: 10.1152/ajpcell.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev. 2016;68:460–473. doi: 10.1016/j.neubiorev.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan R, Szmydynger-Chodobska J, Warren OU, Mohammad F, Zink BJ, Chodobski A. A new panel of blood biomarkers for the diagnosis of mild traumatic brain injury/concussion in adults. J Neurotrauma. 2016;33(1):49–57. doi: 10.1089/neu.2014.3811. [DOI] [PubMed] [Google Scholar]
- 17.Bogoslovsky T, Wilson D, Chen Y, et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid β up to 90 days after traumatic brain injury. J Neurotrauma. 2017;34(1):66–73. doi: 10.1089/neu.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liew H-K, Cheng H-Y, Huang L-C, et al. Acute alcohol intoxication aggravates brain injury caused by intracerebral hemorrhage in rats. J Stroke Cerebrovasc Dis. 2016;25(1):15–25. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Kiyatkin EA, Sharma HS. Breakdown of blood-brain and blood-spinal cord barriers during acute methamphetamine intoxication: role of brain temperature. CNS Neurol Disord Drug Targets. 2016;15(9):1129–1138. doi: 10.2174/1871527315666160920112445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biberthaler P, Mussack T, Wiedemann E, et al. Influence of alcohol exposure on S-100b serum levels. Acta Neurochir Suppl. 2000;76:177–179. doi: 10.1007/978-3-7091-6346-7_35. [DOI] [PubMed] [Google Scholar]
- 21.Danielski LG, Giustina AD, Badawy M, et al. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol. 2018;55(2):1045–1053. doi: 10.1007/s12035-016-0356-7. [DOI] [PubMed] [Google Scholar]
- 22.Sewal RK, Modi M, Saikia UN, Chakrabarti A, Medhi B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 2017;135:176–186. doi: 10.1016/j.eplepsyres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Swann K, Berger J, Sprague SM, et al. Peripheral thermal injury causes blood-brain barrier dysfunction and matrix metalloproteinase (MMP) expression in rat. Brain Res. 2007;1129(1):26–33. doi: 10.1016/j.brainres.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Chang J, Mancuso MR, Maier C, et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat Med. 2017;23(4):450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stagg HW, Whaley JG, Tharakan B, et al. Doxycycline attenuates burn-induced microvascular hyperpermeability. J Trauma Acute Care Surg. 2013;75(6):1040–1046. doi: 10.1097/TA.0b013e3182aa9c79. [DOI] [PubMed] [Google Scholar]
- 26.Luissint A-C, Artus C, Glacial F, Ganeshamoorthy K, Couraud P-O. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9(1):23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017;6(1):20. doi: 10.1186/s40035-017-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng Z, Guo Z, Zhong J, et al. ApoE influences the blood-brain barrier through the NF-κB/MMP-9 pathway after traumatic brain injury. Sci Rep. 2017;7(1):6649. doi: 10.1038/s41598-017-06932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiggins-Dohlvik K, Merriman M, Shaji CA, et al. Tumor necrosis factor-α disruption of brain endothelial cell barrier is mediated through matrix metalloproteinase-9. Am J Surg. 2014;208(6):954–960. doi: 10.1016/j.amjsurg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Quillinan N, Herson PS, Traystman RJ. Neuropathophysiology of brain injury. Anesthesiol Clin. 2016;34(3):453–464. doi: 10.1016/j.anclin.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudra DS, Pal U, Maiti NC, Reiter RJ, Swarnakar S. Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J Pineal Res. 2013;54(4):398–405. doi: 10.1111/jpi.12034. [DOI] [PubMed] [Google Scholar]
- 32.Koning NJ, Overmars MA, van den Brom CE, et al. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br J Anaesth. 2016;116(2):223–232. doi: 10.1093/bja/aev411. [DOI] [PubMed] [Google Scholar]
- 33.Lochhead JJ, Ronaldson PT, Davis TP. Hypoxic stress and inflammatory pain disrupt blood-brain barrier tight junctions: implications for drug delivery to the central nervous system. AAPS J. 2017;19(4):910–920. doi: 10.1208/s12248-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]