Abstract
Ileostomy is a common component of surgical treatments for various gastrointestinal conditions. Loss of the fluid absorptive capacity of the colon results in increased fluid and electrolyte losses, which causes a state of relative fluid depletion. These losses can be offset in part by increased oral intake, but the remaining small intestine also compensates by increasing the efficiency of fluid and electrolyte absorption, a process termed adaptation, which occurs within weeks to months of ileostomy creation. Some patients fail to adapt adequately and have high ileostomy outputs from the time of surgery. Others with a previously well-adapted ileostomy may encounter periods of sustained high output when some additional process causes diarrhea. Many patients experience periods of high output after ileostomy creation and often require hospital readmission for this reason. Any patient with an ileostomy is at great risk of dehydration and electrolyte depletion should output rise dramatically. Prompt attention should be given to rehydration and identification of the underlying cause so that directed therapies may be implemented. This review discusses the alteration of normal intestinal fluid balance from colectomy with ileostomy, proposed mechanisms for adaptation, the differential diagnosis of ileostomy diarrhea, the evaluation of ileostomy diarrhea, and current treatment options.
Keywords: Diarrhea, ileostomy, ostomy, postoperative complications
Ileostomy may be required for permanent or temporary fecal diversion, most often after colectomy. Ileostomies may be fashioned in either an end or a loop configuration. Data are limited on the number of ileostomies performed each year, but it is estimated that approximately 165,000 to 265,000 patients in the US are living with an ileostomy at any given time, with approximately 40,000 new ileostomies created each year.1 Ileostomy diarrhea is a common and potentially dangerous problem. The human colon has a great capacity to absorb over 5 L of excess fluid and electrolytes daily.2 The loss of this absorptive capacity under certain conditions may result in large obligatory fluid losses, and patients with ileostomy are at great risk of dehydration, electrolyte imbalance, and acute kidney injury. Early in the postoperative period, as many as 16% to 50% of patients experience high output, and up to 20% of these patients will require hospital readmission for this reason.3–5 Within 6 months, this number may be as high as 91%.6 Fortunately, the fluid losses seen immediately after ileostomy creation typically decline over several weeks through adaptive changes of the remaining small bowel. However, some patients may fail to adapt adequately, and others may experience intermittent periods of high output when an additional process causes diarrhea. We discuss the physiology of intestinal adaptation after colectomy, the mechanisms and differential diagnosis of ileostomy diarrhea, and the evaluation and treatment of the ileostomy patient with high ileostomy output.
PATHOPHYSIOLOGY
Normal intestinal fluid transport
Under normal circumstances, 9 to 10 L of fluid passes the ligament of Treitz each day, including both oral intake and gastrointestinal secretions from oral, gastric, duodenal, and biliopancreatic sources.7 The jejunum absorbs approximately 6 L, and the ileum another 2.5 L, leaving approximately 1 to 1.5 L of fluid entering the colon per day. Almost all of this fluid and the electrolytes it contains are absorbed in the colon, leaving approximately 100 mL excreted in feces daily. Thus, diversion of the fecal stream at the level of the ileocecal valve would be expected to produce approximately 1 to 1.5 L of stool output per day containing approximately 200 mEq of sodium, 100 mEq of chloride, and 10 mEq of potassium.8
Early postoperative ileostomy output and the role of the ileum
Immediately after recovery from surgery, observed ileostomy output matches what would be expected based on normal fluid transport, approximately 1 to 1.5 L per day.9 However, the output decreases over the following days and weeks, a process termed “adaptation.”10 Kennedy et al found the average daily fecal weight from established (>1 year) ileostomies with minimal ileal resection to be 644 ± 297 g/24 h.11 Another series in established patients found this value to be even lower at 465 ± 219 g/24 h.12 Expected daily output in established ileostomies directly correlates with body mass index.13
The length of ileum resected affects the degree of adaptation significantly.14 The ileum, and specifically the terminal ileum, is believed to have the greatest capacity for adaptive mucosal changes.15 Daily output increases with increasing small bowel resection; resection of 15 to 50 cm of terminal ileum resulted in an increase of >300 g/24 h as compared to controls with <15 cm removed.16 In addition to loss of absorptive surface, loss of the “ileal brake” mechanism likely contributes to poor adaptation with more substantial ileal resections. Under normal circumstances, unabsorbed nutrients reaching the distal ileum and colon stimulate L cells, enteroendocrine cells of the distal small bowel, to secrete peptide YY (PYY), which acts on the upper gastrointestinal tract to decrease gastric emptying, small bowel motility, and pancreatic secretions; the net effect is optimization of proximal gut absorption. This mechanism has been demonstrated by infusion of PYY into healthy volunteers, and its loss after large ileal resections results in rapid transit and decreased absorption of fluid, electrolytes, and nutrients.17 Neal et al found that small bowel transit time was significantly faster in colectomized patients who had greater lengths of ileal resections (50–70 cm), implying an impairment in the “ileal brake” mechanism.18 Additionally, after extensive ileal resection, usually over 100 cm, loss of bile salts may eventually outpace hepatic production, leading to duodenal luminal bile acid deficiency and steatorrhea.19
CONTRIBUTORS TO ADAPTATION
Adaptation is thought to result from a mixture of hormonal, luminal, and mechanical influences that induce structural changes in the mucosa and measurable differences in intestinal motility, permeability, electrolyte transport, and absorptive capacity.20 Human studies regarding small bowel adaptation after colectomy are frequently contradictory, likely due to variability in disease states, operations performed, technical factors, and in the control of external factors such as diet, comorbidities, and medication interactions. Sample sizes are typically small. Animal studies (done in healthy animals) may not always apply to clinical situations in humans but will be cited when they illuminate what may be happening in humans. Additionally, studies examining hormonal alterations are difficult to interpret, as these systems involve complex regulation for which all elements may not be accounted for in a study population. Confounding factors are surely present. The presence of an elevated or decreased hormone level does not necessarily implicate its role in adaptation but may simply be the sequelae of another undetermined process. The combination of these factors contributes to frequently conflicting data.
Changes in mucosal morphology
Mucosal hypertrophy and hyperplasia of remaining intestines is the most cited explanation for adaptation. After ileostomy in rat models, an increased cycling rate and proliferation of intestinal pluripotent stem cells occur in the remaining segments.21 Villous length and cells per villi increase.22 Józsa et al found significant increases in both the mucosal weight (+130% ± 10%) and the thickness (+15% ± 3%) of ileal mucosa within days of ileostomy creation in rats.15 These specimens showed a near doubling of the postoperative ki-67 proliferation index. Studies in humans found that these structural differences may become less prominent by 1 year after ileostomy.23 Thus, these mucosal changes likely play a role in early, but not late, adaptation. The primary driver of these changes is unknown but is likely hormonal. The best evidence in support of this comes from animal studies in which the circulation of a colectomized rat is artificially shared with a noncolectomized rat, termed vascular parabiosis. This process has been shown to induce similar adaptive mucosal changes in the unresected animal.24 Hormones implicated in the adaptive process include mineralocorticoids, growth hormone, insulin-like growth factor, glucagon-like peptide-2, and epidermal growth factor.20
Changes in small bowel electrolyte transport
Intestinal perfusion studies in humans have shown significantly increased potassium excretion and sodium retention early after colectomy.25 Studies in animals suggest that this may be due to the effects of mineralocorticoids. Mineralocorticoids, while primarily inducing renal sodium retention, also cause net sodium resorption in the intestines. Circulating aldosterone levels are elevated after ileostomy and decline again after reversal of a loop ileostomy.26–28 Administration of mineralocorticoid analogs results in an immediate decrease of the sodium/potassium ratio of ileostomy output of up to 30%, and a net increase in the dry weight of output which equates to decreased water content.26,27 Conversely, administration of mineralocorticoid antagonists reverses this effect, and episodes of acute adrenal insufficiency can cause large increases in ileostomy output.29 Retrospective analyses also have associated angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (upstream inhibitors of the RAAS system) with an increased risk of dehydration and hospital readmission after ileostomy (odds ratio = 13.56; 95% confidence interval = 3.54–51.92).30 The effects of mineralocorticoids are likely mediated through direct activation of receptor-mediated electrolyte transport and indirectly mediated through the induction of transcription of adaptive genes. Gene expression maps have been used to compare the ileal tissue of aldosterone-infused rats to that of colectomized rats.31 Aldosterone infusion was shown to induce 11 genes, all of which were also highly expressed after colectomy, and to suppress 10 genes, nine of which were also suppressed after colectomy. In context, in the postcolectomy ileal mucosa, 82 genes were shown to be upregulated and 91 to be suppressed. Thus, aldosterone is not the sole contributor to alterations of gene expression after colectomy but is surely an important mediator.
The epithelial sodium channel (ENaC) is likely an important mediator of aldosterone-induced change. After colectomy in the rat, significant increases in mRNAs encoding ENaC have been found in the ileum compared with unoperated controls, in which there was very little expression.32 Expression peaked 4 weeks after the operation, and the rise coincided with decreasing stool sodium content and increasing electrogenic sodium absorption as measured in vitro. Importantly, the increased electrical current across the ileal mucosa was negated when the ENaC inhibitor amiloride was administered, suggesting that ENaC was a principal mediator for the observed changes. Similar ENaC expression was seen in noncolectomized aldosterone-infused rats, suggesting that aldosterone was the mediator involved.33 Haneda et al attempted to capitalize on this mechanism by administering aldosterone-filled ileal-release microspheres to rats to achieve distal and direct mucosal exposure, thus limiting systemic exposure.34 This produced similar effects on electrogenic sodium resorption without causing elevations in plasma aldosterone. It is clear that aldosterone plays a crucial role in the adaptive process, but it is still to be seen if this process occurs in humans.
An aldosterone-sensitive increase in sodium glucose co-transporter 1 (SGLT1) activity was noted in colectomized rats.33,34 This was not seen in noncolectomized rats given aldosterone infusion, in sodium-depleted rats, or in those rats exposed to luminal aldosterone, suggesting an alternative induction mechanism. If this occurs in humans after colectomy, the increase in SGLT1 could augment the response to oral rehydration solutions.
Alterations in motility
Slowing transit through the bowel increases time available for absorption and is another potential mechanism for adaptation after colectomy. Fasting ileal flow rates measured by intestinal perfusion slow approximately fourfold in patients with established ileostomies as compared to unresected controls; postprandial flow rates decrease by nearly half.25 Gastric emptying of solids, but not liquids, is decreased in established ileostomy patients compared with normal controls.35 A decrease in motility through the upper gastrointestinal tract may be responsible through up-regulation of the “ileal brake,” as previously discussed. However, there are conflicting data on the state of PYY after colonic resection. Adrian et al found significant decreases in fasting and postprandial serum PYY in 16 colectomized patients compared to controls.36 A study of 12 patients with colectomy and ileal pouch anal anastomosis found no difference between basal serum PYY levels compared with controls, and found significantly decreased integrated postprandial PYY.37 These findings argue against this mechanism. However, other studies have found a significant, yet temporary (up to 9 months), increase in the serum and mucosal concentrations of PYY in the prestomal segments of patients with loop ileostomies.23 Animal studies have also shown elevated serum PYY levels after colectomy persisting for 7 days, and others for up to 1 year.38,39 These mixed results are likely the result of significant heterogeneity between patient populations. The well-adapted patient may show increased levels, while a failure to increase the mechanism may contribute to high output. The above studies were mostly done in well-adapted or newly operated patients, limiting generalization. Additionally, efforts to capitalize on this mechanism using PYY analogs in the treatment of high output would likely be hindered by its anorexigenic effects and the potential to induce severe nausea, which was noted when trialed as treatment for obesity.40 Changes in small intestinal smooth muscle may also contribute to decreased motility. Colectomized rats show decreased longitudinal muscle contractile frequency suggestive of decreased propulsive peristalsis.15 This would slow transit time and optimize absorption.
CAUSES OF ILEOSTOMY DIARRHEA
The causes of ileostomy diarrhea should be placed into two contexts: the patient who has failed to adapt and the patient who was previously well adapted presenting with new increase in output (Table 1).
Table 1.
Causes of ileostomy diarrhea
| Context | Causes |
|---|---|
| Failure to adapt | Extensive ileal resection Overlap with short bowel syndrome Proximal/unresected inflammatory bowel disease Enteric fistula Perioperative adrenal insufficiency Idiopathic failure of adaptation |
| Postadaptation | Infectious Clostridioides difficile enteritis Bacterial, viral, protozoal, fungal Small intestinal bacterial overgrowth Medications Structural Stomal dysfunction/stricture Intestinal obstruction Enteric fistula Ileus Lymphoma/lymphatic obstruction Autoimmune Recurrent inflammatory bowel disease Postcolectomy ulcerative colitis–associated enteritis Autoimmune/other enteropathy Endocrine Secretory tumors VIPoma, gastrinoma, carcinoid Hyperthyroidism Adrenal insufficiency Bile acid deficiency Osmotic diarrhea Radiation enteritis Pancreatic insufficiency Factitious disorders |
Failure to adapt
Failure of adaptation most likely relates to the degree of ileal resection, though other factors may contribute. For patients who undergo colectomy for inflammatory bowel disease (IBD), the degree of mucosal inflammation will directly influence the adaptive response. A patient with untreated or subclinical adrenal insufficiency may be unmasked during the perioperative period. Medications that interfere with the renin-angiotensin-aldosterone system may worsen the immediate and delayed adaptive response. Reflex acid hypersecretion causing impaired adaptation has also been reported.41,42
Postadaptation diarrhea
Traditional etiologies of diarrhea remain applicable to the ileostomy patient, including infections, osmotic diarrhea from ingestion of poorly absorbable substrates, celiac disease, and medications. The presentation is simply more dramatic due to the inability to compensate for the fluid loss by colonic absorption. Several additional etiologies must also be considered more frequent in these patients. These include disease recurrence in the case of Crohn’s disease, small intestinal bacterial overgrowth (SIBO), ostomy malfunction or stricture, bile acid deficiency, and hypersecretory pseudo-obstructive states. It should also be noted that colectomy does not negate the potential for Clostridioides difficile to induce an opportunistic enteritis and may carry a higher mortality rate than C. difficile colitis.43–45 This should be considered in any patient with recent antibiotic exposure. Colectomy patients are also at risk for both adhesive disease and abdominal hernias, and bowel obstruction can potentially increase effluent by both a decrease in net fluid absorption in the malfunctioning bowel, as well as a hypersecretory state via excess potassium secretion.46 An enteric fistula due to operative complication or inflammatory bowel disease may result in bypassed absorptive surface and result in diarrhea. Finally, some patients develop reduced motility or have postoperative anatomical variants that may predispose them to small intestinal bacterial overgrowth, which may induce diarrhea.47
Development of small bowel inflammation after colonic resection for established Crohn’s disease is understandable as the natural history of Crohn’s disease; however, inflammatory enteritis after colectomy for ulcerative colitis represents a different challenge. This simply could represent misdiagnosis of colonic Crohn’s disease as ulcerative colitis and may occur in up to 10% of patients who had colectomy for ulcerative colitis. An alternative categorization was proposed by Corporaal et al in a case series of 42 patients presenting with inflammatory enteritis after colectomy for ulcerative colitis.48 These patients demonstrated a spectrum of clinical and pathologic changes consistent with autoimmune gastritis and enteritis and showed response to corticosteroids, calcineurin inhibitors, and immunomodulators. This postcolectomy enteritis is histologically and endoscopically distinct from Crohn’s disease and may represent a unique entity. While colectomy can control ulcerative colitis, it may not be curative in every case.
EVALUATION OF PATIENTS WITH ILEOSTOMY DIARRHEA
History and physical examination
Patients having an ileostomy for the first time have little concept of what represents normal function. Experienced enterostomal therapy nurses play a key role in familiarizing patients with their new situation and can identify excessive ileostomy output and help differentiate it from other potentially distressing aspects of the ostomy such as frequent pouch drainages, leakage, odor, or need for frequent venting. Many of these manifestations may not represent increased output but may be dietary or appliance related. An essential first step is to quantitate output by keeping a record of the frequency and volume of effluent. While no standard definition exists for ileostomy diarrhea, an increase in baseline effluent volume above 1 L per day is considered abnormal. Patients may also report an increase in pouch drainages rather than increased volume. More than six drainages per day suggests an abnormal increase in effluent volume.
Workup for true increased output proceeds similarly to that for any acute or chronic diarrhea patient, beginning with a comprehensive history and physical examination. Every attempt should be made to obtain the patient’s operative reports and any existing imaging studies to ensure accurate knowledge of their anatomy, especially the length of any ileum resected. Similarly, the histological slides and reports of any resected specimens should be sought to ensure correct diagnosis and guide future evaluation. A dietary journal may be useful in identifying culprits that worsen output.
Physical examination should be directed toward evaluation of volume status to determine need for hospital admission and intravenous rehydration. Specifically, vital signs with orthostatic blood pressure measurement, skin turgor, and the state of hydration of mucous membranes should be assessed. Abdominal examination should search for elements suggestive of obstruction, including the presence of hernias (especially stomal and incisional), the character of bowel sounds, tenderness, and distention. The ileostomy itself should be inspected with the collection pouch removed and probed with a gloved finger to assess for stomal prolapse, hernia, or distal stricture.
Laboratory tests, imaging, and endoscopy
The initial evaluation of ileostomy diarrhea mirrors that of acute or chronic diarrhea in patients with intact gastrointestinal tracts. Initial laboratory testing should include a complete blood count, biochemical screening including electrolytes and creatinine, screening for celiac disease with IgA anti-tissue transglutaminase level, thyroid-stimulating hormone, and morning cortisol level. Stool tests should include microbiological tests (stool culture, testing for C. difficile, ova and parasite examination, immunological tests for Giardia and Cryptosporidium, or polymerase chain reaction multiplex testing for a variety of organisms), fecal lactoferrin or calprotectin, and a test for occult blood.
In most individuals, structural evaluation with computed tomography or magnetic resonance enterography or contrasted small bowel radiography should be performed to rule out stricture or obstruction. Retrograde barium enterography for detailed evaluation of the distal ileum and stoma should be considered if evaluation for distal obstruction is equivocal. In cases with a history of inflammatory bowel disease or in those with unclear etiology, upper endoscopy and ileoscopy with mucosal biopsies should be performed.
If the diagnosis remains obscure, timed stool collection can differentiate secretory, osmotic, malabsorptive, and inflammatory causes; this may provide additional clues to the diagnosis. The presence of significant steatorrhea raises the possibility of bile acid deficiency in those with extended ileal resection, pancreatic exocrine insufficiency, mucosal disease, or SIBO.
Lactulose or glucose hydrogen breath testing is considered a standard, if flawed, diagnostic method for SIBO.49 The test relies on detection of an early expired hydrogen peak suggestive of excessive small bowel bacterial metabolism of the ingested carbohydrate. However, traditional diagnostic criteria may be unreliable in patients with surgically altered anatomy or motility disorders. Measuring methane in addition to hydrogen maximizes sensitivity.50 Quantitative culture of intestinal contents is thought to be a better measure of SIBO, but the appropriate threshold in ileostomy patients is undefined. Patients are often given an empiric course of antibiotics for a presumptive diagnosis of SIBO; however, partial response does not necessarily imply that SIBO is the correct diagnosis and may lead to recurrent cycles of inappropriate antibiotic use.
TREATMENT OF HIGH ILEOSTOMY OUTPUT
Fluid repletion
Regardless of the cause of high output, volume status, electrolyte disturbances, and subsequent sequelae must be addressed first. This will most often require intravenous fluids or oral rehydration solutions depending on the severity of the volume depletion. Dehydration prophylaxis immediately after ileostomy creation should be considered. In a randomized controlled trial in patients after diverting ileostomy, patients receiving 1 L daily of a prophylactic oral rehydration solution had zero dehydration-related readmissions compared to 24% in the control group.51 The prophylactic group also showed significant improvements in markers of renal function over 40 days of follow-up. Other studies have suggested that oral rehydration solution actually decreases the output; however, this was not seen in the randomized setting.52 Total parenteral nutrition is generally not required in these patients, as nutrient absorption should not be impaired.
Appliance problems and dietary modifications
Patients with appliance difficulties, changes in gas output, and changes in effluent appearance, texture, or color may report diarrhea. An enterostomal therapist should ensure good device fit and provide the patient with an adequate working knowledge of daily maintenance. Patients with significant peristomal dermatitis or leakage may benefit from agents to increase effluent viscosity.53 The addition of fiber may provide a more manageable viscosity, although the overall output may increase.54 One study showed that the addition of 15 g of sterculia bulk increased ileostomy output viscosity by approximately 100%, and 9 of the 12 patients reported quality of life benefits.53 Another small study found the addition of psyllium wet stool weights to be unchanged, though the dry fraction was significantly higher.55 In a randomized crossover trial involving 28 patients to evaluate the effect of dietary marshmallows on output, 71% of patients reported a reduction in ostomy output, though the difference was modest at 75 mL per day.56 Participants also reported fewer pouch changes and thicker effluent, and 68% reported they would use marshmallows again to control output. A large survey conducted in established ileostomy patients found the foods most known for causing watery output to be rhubarb, alcohol, fried fish, and fruits and vegetables, such as apples, beets, lettuce, and onions.57 The Crohn’s and Colitis Foundation offers a patient-friendly food reference chart that can be utilized to track foods commonly found to alter effluent character.58 Presumably, reducing these foods might alter ileostomy output.
Treatment of adaptation failure
Adaptation failure is a more chronic problem, and the goals of treatment are to manage diarrhea, prevent complications, and improve quality of life. Treatments in this setting include agents to augment the adaptive response, antimotility agents, and antisecretory agents.
Agents to augment adaptive response
Teduglutide is a subcutaneously administered GLP-2 agonist that has been approved for chronic intestinal failure due to short bowel syndrome. It is thought to work via a GLP-2 enterocyte receptor by enhancing mucosal growth, decreasing gastric motility and acid secretion, and increasing mesenteric blood flow.59 GLP-2 receptors have also been identified in the enteric nervous system.60 While no trials exist regarding its use after colectomy with minimal ileal resection, subgroup analysis in short bowel trials suggests greater response rates in patients without a colon in continuity compared to those with a colon.61 Endogenous GLP-2 is released from the colon and distal ileum and may already be contributing to adaptation short bowel patients with an intact colon, thus minimizing the benefit of further supplementation. There is at least one reported case of successful amelioration of high ileostomy output in a colectomized patient without short bowel.62 The cost of this treatment may limit its potential use, though in the severely refractory patient, this must be weighed against the cost of multiple and prolonged hospitalizations and resultant complications of chronic diarrhea.
Antimotility agents
Antimotility agents include loperamide, diphenoxylate/atropine, codeine, morphine, and tincture of opium. Loperamide and diphenoxylate are synthetic mu-opioid agonists with antimotility effects, particularly in the small bowel.63 Atropine is combined with diphenoxylate to discourage abuse; its anticholinergic properties may supplement its antidiarrheal effects slightly. As loperamide has limited ability to cross the blood-brain barrier, it has fewer central and anticholinergic adverse effects than diphenoxylate/atropine or more potent opioids. Loperamide has been shown in several randomized trials to decrease the output of established ileostomies by 22% to 30%.64–67 Studied doses have varied, but the standard dose is 4 mg four times per day before meals and at bedtime.
More potent opioids such as codeine, opium, and morphine may have to be used if more conservative treatment fails. Codeine has been shown to be effective, though less so than loperamide in the dose tested, and carries risk of adverse effects, especially as the dose is escalated.64 Less work has been reported with morphine or tincture of opium in ileostomy diarrhea. Dosing should start at 2 mg of morphine (0.2 mL of deodorized tincture of opium or 0.1 mL of morphine solution) four times a day before meals and at bedtime. The dose can be raised by a similar amount every few days until output comes under control. Most patients require doses <20 mg four times a day. Unlike the analgesic effects of opioids, tolerance to the antimotility effect does not develop. Once an effective dose is found, the need for continually increasing doses should raise suspicion for drug-seeking behavior or diversion. Antimotility agents for chronic diarrhea should be dosed regularly prior to meals and at bedtime, not on an “as-needed” basis. Ileus or obstruction are absolute contraindications for these medications.
Antisecretory agents
Hypersecretion of gastric acid may contribute to initial periods of high output. Animal and human studies have shown that basal and stimulated acid secretion was approximately doubled a year after colectomy.41,42 While an increase in serum gastrin after colectomy has been found in some animal models, other studies in animals and humans have failed to find any correlation.39,68–70 There are reported cases of high ileostomy output that were successfully treated with acid suppression, and an empiric trial of a proton-pump inhibitor is reasonable for otherwise unexplained high output.71,72
The somatostatin analog octreotide has a half-life of 113 min (compared with 2 to 3 min for endogenous somatostatin) and has been shown to be far more potent in many ways.73 In 1984, Williams et al reported the first use of octreotide for management of severe secretory diarrhea in a patient who failed to adapt after ileostomy.74 In this case, output decreased by 82% after a single day, with prolongation of small bowel transit time by 76%. There was little change in gastric emptying. This finding was later replicated in multiple case series.75–78 A small prospective placebo-controlled trial in 12 patients with protective loop ileostomies after ileoanal anastomosis was performed approximately 2 to 3 months postoperatively in patients given standard diets.79 Patients given octreotide 100 mcg subcutaneously three times daily showed a 24.1% decrease in mean daily output over 5 days compared to placebo (970 ± 66 to 736 ± 28 g/day; P < 0.05). Modest reductions in stool sodium and chloride were seen, with no significant change in hormones including C-peptide, insulin, glucagon, renin, or aldosterone. There were no adverse effects during the 5-day period. The long-term adverse effects of somatostatin administration have been explored in other settings and include biliary stasis with cholelithiasis and possibly worsening steatorrhea due to inhibition of exocrine pancreatic function.80 Long-acting depot injection formulations given monthly are available (Octreotide LAR, Lanreotide) for patients who have achieved adequate control and in whom the adequate dose has been determined.
Glucocorticoids may play a significant role in adaptation, may decrease inflammation in IBD, and may also affect ion and water transport through mineralocorticoid activity; as such, they may aid in improving symptoms of high output. The long-term adverse effects of systemic glucocorticoid administration limit application, but budesonide may be an attractive option given significant first-pass hepatic metabolism, which limits systemic exposure. Ecker et al performed two randomized placebo-controlled studies examining the effects of ileal-release budesonide in patients with postcolectomy high output ileostomy due to Crohn’s disease with quiescent inflammatory activity.81,82 Budesonide (3 mg three times daily for 8 days) decreased mean output by 30.2% compared to placebo, with 60% of patients achieving >25% decrease in output. The second study utilized a washout period of 4 weeks followed by a rechallenge in which similar findings were shown. Some of these patients were followed for as long as 155 weeks with continued efficacy. With estimated 10% systemic absorption, the drug does still have potential to induce adrenal suppression and the effects of hypercortisolism. Colonic-release formulations of budesonide should not be used in this population.
Fludrocortisone, a potent mineralocorticoid agonist, has been shown to decrease ileostomy output in subjects without diarrhea.26 Further research is warranted to explore a possible therapeutic benefit in patients with high output.
Bile acid deficiency
Traditional bile acid diarrhea resulting from bile acid malabsorption is not possible in the colectomized patient, as the secretory mechanism resides in the colon. Thus, bile acid binders such as cholestyramine may only serve to worsen fat malabsorption and steatorrhea and should not be prescribed in patients with end ileostomies. In those with larger ileal resections, usually >100 cm, bile acid loss may outpace hepatic production, and steatorrhea due to bile acid deficiency may result. In these cases supplementary bile acid should be considered.19,83 The only bile acid supplement approved by the US Food and Drug Administration (FDA) is cholic acid (Cholbam); however, this drug is approved only for bile acid synthesis disorders and would require off-label use, which would be cost-prohibitive at the current price point. Ox bile supplements can be purchased but are not approved or regulated by the FDA. A reasonable dose would be 1 to 2 g daily taken in divided dose with meals.
Treatment of postadaptation diarrhea
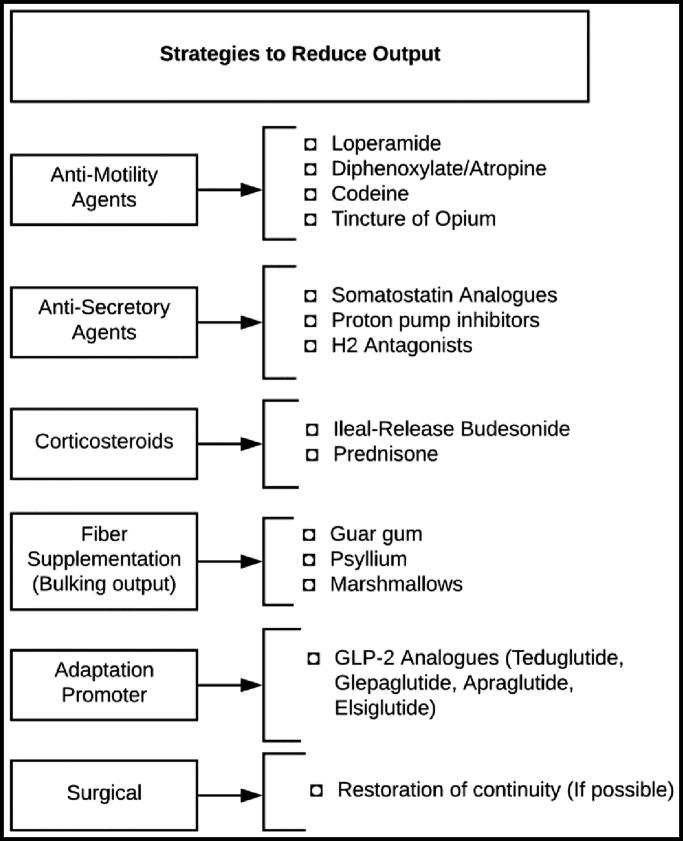
Treatment of postadaptation diarrhea (Figure 1) should be directed at the underlying cause, whether it be supportive care for viral gastroenteritis, directed antibiotic therapy for a bacterial infection, immunosuppression for recurrent IBD, or surgical correction of a dysfunctional stoma, stricture, fistula, or obstructive lesion. During workup and treatment initiation, many patients will also benefit from the symptomatically directed treatments discussed above, provided there are no contraindications.
Figure 1.
Strategies to reduce ileostomy output.
Surgical options
Restoration of continuity should be considered if luminal fluid can be exposed to additional absorptive mucosa. Diverting or loop type ileostomies should be closed if ileostomy diarrhea is problematic and the clinical situation allows. Restoration of continuity may not always be feasible in patients with unfavorable anatomy, distal anastomotic complications, or pouch complications. Multidisciplinary discussion should take place regarding the need for further fecal diversion in this setting.
As many as 11.6% of ileostomy patients may require stomal revision for pain, prolapse, stenosis, or fistula.84 In one prospective series, this number was as high as 75% in patients with Crohn’s disease and 44% in patients with ulcerative colitis over 8 years of follow-up.85 If imaging, physical examination, or endoscopy are suggestive of obstructive processes, surgical correction should be pursued.
CONCLUSION
Ileostomy diarrhea represents complex physiology and can have dramatic consequences for both quality of life and medical complications. Prompt attention should be paid to the ileostomy patient with acute rise in output, with a low threshold for hospital admission to facilitate rehydration and search for the underlying cause. High output should be characterized as a failure of adaptation or a postadaptive process. Common etiologies remain common, but important etiologies to consider specifically in the ileostomy patient include recurrent IBD, bile acid deficiency, SIBO, and anatomic abnormalities requiring surgical correction. Treatment is directed at the underlying cause, with medications used adjunctively to decrease output and improve quality of life.
References
- 1.Turnbull GB. Ostomy statistics: the $64,000 question. Ostomy Wound Manage. 2003;49(6):22–23. [PubMed] [Google Scholar]
- 2.Debongnie JC, Phillips SF.. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698–703. doi: 10.1016/0016-5085(78)90246-9. [DOI] [PubMed] [Google Scholar]
- 3.Arenas Villafranca JJ, López-Rodríguez C, Abilés J, Rivera R, Gándara Adán N, Utrilla Navarro P.. Protocol for the detection and nutritional management of high-output stomas. Nutr J. 2015;14(1):45. doi: 10.1186/s12937-015-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker ML, Williams RN, Nightingale J.. Causes and management of a high-output stoma. Colorectal Dis. 2011;13(2):191–197. doi: 10.1111/j.1463-1318.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 5.Paquette IM, Solan P, Rafferty JF, Ferguson MA, Davis BR.. Readmission for dehydration or renal failure after ileostomy creation. Dis Colon Rectum. 2013;56(8):974–979. doi: 10.1097/DCR.0b013e31828d02ba. [DOI] [PubMed] [Google Scholar]
- 6.Stankiewicz M. Clinical management of ileostomy high-output stomas to prevent electrolyte disturbance, dehydration and acute kidney injury: a quality improvement activity. J Stomal Ther Aust. 2019;39(1):8–10. [Google Scholar]
- 7.Schiller LS. Diarrhea In: Feldman MF, ed. Gastrointestinal and Liver Disease. 10th ed. Philadelphia, PA: Elsevier; 2016:221–241. [Google Scholar]
- 8.Phillips SF, Giller J.. The contribution of the colon to electrolyte and water conservation in man. J Lab Clin Med. 1973;81(5):733–746. [PubMed] [Google Scholar]
- 9.Smiddy FG, Gregory SD, Smith IB, Goligher JC.. Faecal loss of fluid, electrolytes, and nitrogen in colitis before and after ileostomy. Lancet. 1960;1(7114):14–19. doi: 10.1016/S0140-6736(60)92717-3. [DOI] [PubMed] [Google Scholar]
- 10.Crawford N, Brooke BN.. Ileostomy chemistry. Lancet. 1957;272(6974):864–867. doi: 10.1016/S0140-6736(57)91394-6. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy HJ, Al-Dujaili EA, Edwards CR, Truelove SC.. Water and electrolyte balance in subjects with a permanent ileostomy. Gut. 1983;24(8):702–705. doi: 10.1136/gut.24.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaghinis T, Lubran M, Coghill NF.. The composition of ileostomy fluid. Gut. 1963;4(4):322–338. doi: 10.1136/gut.4.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill GL, Millward SF, King RF, Smith RC.. Normal ileostomy output: close relation to body size. BMJ. 1979;2(6194):831–832. doi: 10.1136/bmj.2.6194.831-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez de Segura IA, Aguilera MJ, Codesal J, Codoceo R, De-Miguel E.. Comparative effects of growth hormone in large and small bowel resection in the rat. J Surg Res. 1996;62(1):5–10. doi: 10.1006/jsre.1996.0164. [DOI] [PubMed] [Google Scholar]
- 15.Józsa T, Magyar A, Cserni T, et al. Short-term adaptation of rat intestine to ileostomy: implication for pediatric practice. J Invest Surg. 2009;22(4):292–300. doi: 10.1080/08941930903040106. [DOI] [PubMed] [Google Scholar]
- 16.Gendre JP, Pornin B, Cosnes J, Le Quintrec Y.. Role of the prevalvular terminal ileum in fecal losses in ileostomized patients. Gastroenterol Clin Biol. 1985;9(8-9):578–582. [PubMed] [Google Scholar]
- 17.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR.. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28(2):166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal DE, Williams NS, Barker MC, King RF.. The effect of resection of the distal ileum on gastric emptying, small bowel transit and absorption after proctocolectomy. Br J Surg. 1984;71(9):666–670. doi: 10.1002/bjs.1800710906. [DOI] [PubMed] [Google Scholar]
- 19.Fordtran JS, Bunch F, Davis GR.. Ox bile treatment of severe steatorrhea in an ileectomy-ileostomy patient. Gastroenterology. 1982;82(3):564–568. doi: 10.1016/S0016-5085(82)80408-3. [DOI] [PubMed] [Google Scholar]
- 20.Warner BW. The pathogenesis of resection-associated intestinal adaptation. Cell Mol Gastroenterol Hepatol. 2016;2(4):429–438. doi: 10.1016/j.jcmgh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson WR. Proliferative and morphological adaptation of the intestine to experimental resection. Scand J Gastroenterol Suppl. 1982;74:11–20. [PubMed] [Google Scholar]
- 22.Wright HK, Cleveland JC, Tilson MD, Herskovic T.. Morphology and absorptive capacity of the ileum after ileostomy in man. Am J Surg. 1969;117(2):242–245. doi: 10.1016/0002-9610(69)90310-9. [DOI] [PubMed] [Google Scholar]
- 23.Oh NG, Son GM, Sin JY, Ding XZ, Adrian TE.. Time-course of morphologic changes and peptide YY adaptation in ileal mucosa after loop ileostomy in humans. Dis Colon Rectum. 2005;48(6):1287–1294. doi: 10.1007/s10350-004-0915-2. [DOI] [PubMed] [Google Scholar]
- 24.Williamson RC, Buchholtz TW, Malt RA.. Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology. 1978;75(2):249–254. doi: 10.1016/0016-5085(78)90412-2. [DOI] [PubMed] [Google Scholar]
- 25.Ladas SD, Isaacs PE, Murphy GM, Sladen GE.. Fasting and postprandial ileal function in adapted ileostomates and normal subjects. Gut. 1986;27(8):906–912. doi: 10.1136/gut.27.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulston K, Harrison DD, Skyring AP.. Effect of mineralocorticoids on the sodium/potassium ratio of human ileostomy fluid. Lancet. 1963;2(7307):541–542. doi: 10.1016/S0140-6736(63)92640-0. [DOI] [PubMed] [Google Scholar]
- 27.Huber FX, Lucas M, Stern J, et al. Changes in glucocorticoid and mineralocorticoid hormone levels due to compensation for ileostomy losses. Int J Surg Investig. 2001;2(5):369–375. [PubMed] [Google Scholar]
- 28.Huber FX, Stern J, Hinz U, et al. Effects of restorative proctocolectomy on renal and adrenal function. Dis Colon Rectum. 1999;42(10):1318–1324. doi: 10.1007/BF02234222. [DOI] [PubMed] [Google Scholar]
- 29.Rai S, Hemingway D.. Acute adrenal insufficiency presenting as high output ileostomy. Ann R Coll Surg Engl. 2003;85(2):105–106. doi: 10.1308/003588403321219876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charak G, Kuritzkes BA, Al-Mazrou A, et al. Use of an ACE inhibitor or angiotensin receptor blocker is a major risk factor for dehydration requiring readmission in the setting of a new ileostomy. Int J Colorectal Dis. 2018;33(3):311–316. doi: 10.1007/s00384-017-2961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukushima K, Haneda S, Takahashi K, et al. Molecular analysis of colonic transformation in the ileum after total colectomy in rats. Surgery. 2006;140(1):93–99. doi: 10.1016/j.surg.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Koyama K, Sasaki I, Naito H, et al. Induction of epithelial Na+ channel in rat ileum after proctocolectomy. Am J Physiol. 1999;276(4):G975–G984. doi: 10.1152/ajpgi.1999.276.4.G975. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima K, Sato S, Naito H, et al. Comparative study of epithelial gene expression in the small intestine among total proctocolectomized, dietary sodium-depleted, and aldosterone-infused rats. J Gastrointest Surg. 2005;9(2):236–244. doi: 10.1016/j.gassur.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Haneda S, Fukushima K, Funayama Y, et al. A new drug delivery system targeting ileal epithelial cells induced electrogenic sodium absorption: possible promotion of intestinal adaptation. J Gastrointest Surg. 2007;11(5):568–577. doi: 10.1007/s11605-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 35.Robertson MD, Mathers JC.. Gastric emptying rate of solids is reduced in a group of ileostomy patients. Dig Dis Sci. 2000;45(7):1285–1292. [DOI] [PubMed] [Google Scholar]
- 36.Adrian TE, Savage AP, Fuessl HS, Wolfe K, Besterman HS, Bloom SR.. Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery. 1987;101(6):715–719. [PubMed] [Google Scholar]
- 37.van Battum PLH, Hopman WP, Salemans JM, et al. Impaired release of peptide YY in patients with proctocolectomy and ileal pouch-anal anastomosis. Scand J Gastroenterol. 1999;34(4):404–408. doi: 10.1080/003655299750026425. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong DN, Ballantyne GH, Adrian TE, Bilchik AJ, McMillen MA, Modlin IM.. Adaptive increase in peptide YY and enteroglucagon after proctocolectomy and pelvic ileal reservoir construction. Dis Colon Rectum. 1991;34(2):119–125. doi: 10.1007/BF02049984. [DOI] [PubMed] [Google Scholar]
- 39.Gómez de Segura IA, Marijuan JL, Trillo P, et al.. Changes in plasma levels of intestinal regulatory peptides following colonic resection in the rat. Rev Esp Enferm Dig. 1995;87(1):20–24. [PubMed] [Google Scholar]
- 40.De Silva A, Bloom SR.. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.M’Koma AE, Lindquist K, Liljeqvist L.. Effect of restorative proctocolectomy on gastric acid secretion and serum gastrin levels: a prospective study. Dis Colon Rectum. 1999;42(3):398–402. doi: 10.1007/BF02236361. [DOI] [PubMed] [Google Scholar]
- 42.Osborne MP, Frederick PL, Sizer JS, Blair D, Cole P, Thum W.. Mechanism of gastric hypersecretion following massive intestinal resection. Clinical and experimental observations. Ann Surg. 1966;164(4):622–634. doi: 10.1097/00000658-196610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Causey MW, Spencer MP, Steele SR.. Clostridium difficile enteritis after colectomy. Am Surg. 2009;75(12):1203–1206. [PubMed] [Google Scholar]
- 44.El Muhtaseb MS, Apollos JK, Dreyer JS.. Clostridium difficile enteritis: a cause for high ileostomy output. ANZ J Surg. 2008;78(5):416–416. doi: 10.1111/j.1445-2197.2008.04494.x. [DOI] [PubMed] [Google Scholar]
- 45.Williams RN, Hemingway D, Miller AS.. Enteral Clostridium difficile, an emerging cause for high-output ileostomy. J Clin Pathol. 2009;62(10):951–953. doi: 10.1136/jcp.2008.062901. [DOI] [PubMed] [Google Scholar]
- 46.van Dinter TG, Jr, Fuerst FC, Richardson CT, et al. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology. 2005;129(4):1268–1273. doi: 10.1053/j.gastro.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Cole CR, Frem JC, Schmotzer B, et al. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr. 2010;156(6):941–947.e1. doi: 10.1016/j.jpeds.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corporaal S, Karrenbeld A, van der Linde K, Voskuil JH, Kleibeuker JH, Dijkstra G.. Diffuse enteritis after colectomy for ulcerative colitis: two case reports and review of the literature. Eur J Gastroenterol Hepatol. 2009;21(6):710–715. doi: 10.1097/MEG.0b013e32831bc400. [DOI] [PubMed] [Google Scholar]
- 49.Gasbarrini A, Lauritano EC, Gabrielli M, et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25(3):237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 50.Saad RJ, Chey WD.. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol. 2014;12(12):1964–1972. doi: 10.1016/j.cgh.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 51.Migdanis A, Koukoulis G, Mamaloudis I, et al. Administration of an oral hydration solution prevents electrolyte and fluid disturbances and reduces readmissions in patients with a diverting ileostomy after colorectal surgery: a prospective, randomized, controlled trial. Dis Colon Rectum. 2018;61(7):840–846. doi: 10.1097/DCR.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 52.Chia CLK, Tai YS, Tan KY.. A preliminary study of the use of oral rehydration salts in decreasing ileostomy output. Tech Coloproctol. 2017;21(7):587–588. doi: 10.1007/s10151-017-1646-4. [DOI] [PubMed] [Google Scholar]
- 53.Dalhamn T, Graf W, Nilsson LH.. The effect of sterculia bulk on the viscosity of stomal output from twelve patients with ileostomy. Scand J Gastroenterol. 1978;13(4):485–488. doi: 10.3109/00365527809181926. [DOI] [PubMed] [Google Scholar]
- 54.Steinhart AH, Jenkins DJ, Mitchell S, Cuff D, Prokipchuk EJ.. Effect of dietary fiber on total carbohydrate losses in ileostomy effluent. Am J Gastroenterol. 1992;87(1):48–54. [PubMed] [Google Scholar]
- 55.Gelissen IC, Brodie B, Eastwood MA.. Effect of Plantago ovata (psyllium) husk and seeds on sterol metabolism: studies in normal and ileostomy subjects. Am J Clin Nutr. 1994;59(2):395–400. doi: 10.1093/ajcn/59.2.395. [DOI] [PubMed] [Google Scholar]
- 56.Clarebrough E, Guest G, Stupart D.. Eating marshmallows reduces ileostomy output: a randomized crossover trial. Colorectal Dis. 2015;17(12):1100–1103. doi: 10.1111/codi.12992. [DOI] [PubMed] [Google Scholar]
- 57.Thomson TJ, Runcie J, Khan A.. The effect of diet on ileostomy function. Gut. 1970;11(6):482–485. doi: 10.1136/gut.11.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crohn’s and Colitis Foundation. Ostomates food preference chart. https://www.crohnscolitisfoundation.org/sites/default/files/legacy/assets/pdfs/food_ref_card.pdf.
- 59.Tappenden KA, Edelman J, Joelsson B.. Teduglutide enhances structural adaptation of the small intestinal mucosa in patients with short bowel syndrome. J Clin Gastroenterol. 2013;47(7):602–607. doi: 10.1097/MCG.0b013e3182828f57. [DOI] [PubMed] [Google Scholar]
- 60.Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130(1):150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Jeppesen PB, Gabe SM, Seidner DL, Lee H-M, Olivier C.. Factors associated with response to teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology. 2018;154(4):874–885. doi: 10.1053/j.gastro.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Semmens S, Higgins E, Coyne P.. A treatment for refractory high ileostomy output. J Pain Palliat Care Pharmacother. 2018;32(2-3):155–157. doi: 10.1080/15360288.2018.1529011. [DOI] [PubMed] [Google Scholar]
- 63.Heel RC, Brogden RN, Speight TM, Avery GS.. Loperamide: a review of its pharmacological properties and therapeutic efficacy in diarrhoea. Drugs. 1978;15(1):33–52. doi: 10.2165/00003495-197815010-00003. [DOI] [PubMed] [Google Scholar]
- 64.King RF, Norton T, Hill GL.. A double-blind crossover study of the effect of loperamide hydrochloride and codeine phosphate on ileostomy output. ANZ J Surg. 1982;52(2):121–124. doi: 10.1111/j.1445-2197.1982.tb06083.x. [DOI] [PubMed] [Google Scholar]
- 65.Tytgat GN, Huibregtse K.. Loperamide and ileostomy output—placebo-controled double-blind crossover study. BMJ. 1975;2(5972):667–667. doi: 10.1136/bmj.2.5972.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tytgat GN, Huibregtse K, Meuwissen SG.. Loperamide in chronic diarrhea and after ileostomy: a placebo-controlled double-blind cross-over study. Arch Chir Neerl. 1976;28(1):13–20. [PubMed] [Google Scholar]
- 67.Tytgat GN, Huibregtse K, Dagevos J, van den Ende A.. Effect of loperamide on fecal output and composition in well-established ileostomy and ileorectal anastomosis. Digest Dis Sci. 1977;22(8):669–676. doi: 10.1007/BF01078345. [DOI] [PubMed] [Google Scholar]
- 68.Greenberg GR, Buchan AM, McLeod RS, Preston P, Cohen Z.. Gut hormone responses after reconstructive surgery for ulcerative colitis. Gut. 1989;30(12):1721–1730. doi: 10.1136/gut.30.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Svendsen LB, Bisgård ML, Gustafsen J, Bülow S, Stadil F.. Serum gastrin values in patients with familial adenomatous polyposis. Dis Colon Rectum. 1994;37(1):22–25. doi: 10.1007/BF02047209. [DOI] [PubMed] [Google Scholar]
- 70.Albuquerque JFS, Ferra MA, Portela-Gomes GM.. Adaptive changes of the enterochromaffin and gastrin cells in the rat gastrointestinal tract following subtotal colectomy. Scand J Gastroenterol. 2006;41(8):963–968. doi: 10.1080/00365520500527581. [DOI] [PubMed] [Google Scholar]
- 71.Azzopardi N, Ellul P.. Proton pump inhibitors in the management of tachypnoea following panproctocolectomy: a case of high output ileostomy. Case Rep Gastroenterol. 2011;5(1):212–216. doi: 10.1159/000326928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saunders DR, Saunders MD, Sillery JK.. Beneficial effects of glucose polymer and an H2-receptor blocker in a patient with a proximal ileostomy. Am J Gastroenterol. 1989;84(2):192–194. [PubMed] [Google Scholar]
- 73.Bauer W, Briner U, Doepfner W, et al. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-X. [DOI] [PubMed] [Google Scholar]
- 74.Williams NS, Cooper JC, Axon AT, King RF, Barker M.. Use of a long acting somatostatin analogue in controlling life threatening ileostomy diarrhoea. Br Med J (Clin Res Ed). 1984;289(6451):1027–1028. doi: 10.1136/bmj.289.6451.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper JC, Williams NS, King RF, Barker MC.. Effects of a long-acting somatostatin analogue in patients with severe ileostomy diarrhoea. Br J Surg. 1986;73(2):128–131. doi: 10.1002/bjs.1800730219. [DOI] [PubMed] [Google Scholar]
- 76.Müller MK, Breuer NF, Balzer K, Goebell H.. Treatment of chronic non-secretory diarrhea in ileostomy with the long-acting somatostatin analog SMS 201-995. Z Gastroenterol. 1988;26(3):166–168. [PubMed] [Google Scholar]
- 77.Lamireau T, Galpérine RI, Ohlbaum P, et al. Use of a long acting somatostatin analogue in controlling ileostomy diarrhoea in infants. Acta Paediatr Scand. 1990;79(8-9):871–872. doi: 10.1111/j.1651-2227.1990.tb11572.x. [DOI] [PubMed] [Google Scholar]
- 78.Neef B, Höring E, Gaisberg U. V.. Successful treatment of a life-threatening ileostomy diarrhea with the somatostatin analog octreotide. Dtsch Med Wochenschr. 2008;119(24):869–874. doi: 10.1055/s-2008-1058773. [DOI] [PubMed] [Google Scholar]
- 79.Kusuhara K, Kusunoki M, Okamoto T, Sakanoue Y, Utsunomiya J.. Reduction of the effluent volume in high-output ileostomy patients by a somatostatin analogue, SMS 201-995. Int J Colorect Dis. 1992;7(4):202–205. doi: 10.1007/BF00341221. [DOI] [PubMed] [Google Scholar]
- 80.Peeters M, Van den Brande J, Francque S.. Diarrhea and the rationale to use Sandostatin. Acta Gastroenterol Belg. 2010;73(1):25–36. [PubMed] [Google Scholar]
- 81.Ecker KW, Stallmach A, Löffler J, Greinwald R, Achenbach U.. Long-term treatment of high intestinal output syndrome with budesonide in patients with Crohn’s disease and ileostomy. Dis Colon Rectum. 2005;48(2):237–242. doi: 10.1007/s10350-004-0768-8. [DOI] [PubMed] [Google Scholar]
- 82.Ecker KW, Stallmach A, Seitz G, Gierend M, Greinwald R, Achenbach U.. Oral budesonide significantly improves water absorption in patients with ileostomy for Crohn disease. Scand J Gastroenterol. 2003;38(3):288–293. doi: 10.1080/00365520310000645a. [DOI] [PubMed] [Google Scholar]
- 83.Gruy-Kapral C, Little KH, Fordtran JS, Meziere TL, Hagey LR, Hofmann AF.. Conjugated bile acid replacement therapy for short-bowel syndrome. Gastroenterology. 1999;116(1):15–21. doi: 10.1016/S0016-5085(99)70223-4. [DOI] [PubMed] [Google Scholar]
- 84.Carlsen E, Bergan A.. Technical aspects and complications of end-ileostomies. World J Surg. 1995;19(4):632–636. doi: 10.1007/BF00294742. [DOI] [PubMed] [Google Scholar]
- 85.Carlstedt A, Fasth S, Hultén L, Nordgren S, Palselius I.. Long-term ileostomy complications in patients with ulcerative colitis and Crohn’s disease. Int J Colorect Dis. 1987;2(1):22–25. doi: 10.1007/BF01648993. [DOI] [PubMed] [Google Scholar]