TRAVEL AND ANIMAL CONTACT
The U.S. Department of Commerce estimates that at least 30 to 40 million Americans visit other countries each year.1 Some of these travelers contract diseases while overseas and may return home still symptomatic. Some of these travel-related diseases are zoonoses resulting from contact with domestic or wild animals.
Many international travelers restrict their stays to hotels in urban or other well-developed areas that involve reduced risk of animal contact. Yet the growing popularity of ecotourism, religious pilgrimages, wildlife safaris, and other forms of adventure travel may increase the chances of travelers contracting an animal-related infectious disease during their trips. Animal contacts are possible even in cities and elsewhere on the beaten path; for example, high levels of pet allergens have been found in vacation hotels in some countries.1
Animal travel across borders is increasing as well. In 2006, more than 287,000 dogs were estimated to have entered the United States from foreign countries (including Mexico and Canada), 25% of which were unvaccinated.2 Many of these dogs were imports, accompanying their owners. The increasing popularity of traveling with one's pet may result in a wide range of exposure risks for both the pet and owner. In addition, some individuals may acquire a pet overseas and return home with it.
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
-
•
Consider recent foreign travel in human beings or other animals as a risk factor for unexplained cases or outbreaks of infectious disease in the community.
-
•
Collaborate with agriculture officials on the regulation of animal movement of public health importance.
Human Health Clinicians
-
•
When providing pretravel screening and counseling, inquire about whether the person is traveling with pets and provide counseling about health risks from animal contacts while traveling. See the CDC's “Your Survival Guide to Safe and Healthy Travel,” available online at http://wwwn.cdc.gov/travel/contentSurvival Guide.aspx.
-
•
If a patient is planning to travel with a pet, advise him or her to consult a veterinarian for pretravel risk assessment and preventive care.
-
•
When evaluating the returning traveler with illness, inquire about animal contacts as well as the health of any pets that have accompanied the traveler or that the traveler has acquired overseas.
Veterinary Clinicians
-
•
Provide appropriate pretravel risk assessment and vaccination of pets and documentation of animal health status.
-
•
Counsel owner about signs of illness to monitor in his or her pet and quarantine regulations.
-
•
In the evaluation of an ill animal after travel, consider risks of imported diseases.
-
•
Advise clients regarding the health risks of adopting a pet overseas (e.g., documented cases of rabies, leishmaniasis, and leptospirosis have been reported in imported pets).
TRAVEL MEDICINE
Travel medicine is a medical discipline that deals with prevention of infectious diseases during international travel as well as the personal safety of travelers and the avoidance of environmental risks during travel.3 Medical providers who care for human beings traveling to other countries need to be aware of the principles of travel medicine and be able to perform the functions of pretravel risk assessment and preventive counseling, vaccination, and posttravel evaluation of illness. A complete discussion of the many travel-related health risks is beyond the scope of this book. However, Table 10-1 shows the key elements of travel medicine practice.
Table 10-1.

Elements of Travel Medicine Practice
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Hill DR, Ericsson CD, Pearson RD et al: The practice of travel medicine: guidelines by the Infectious Diseases Society of America, Clin Infect Dis 43:1499, 2006.
© 2010
Veterinary providers who care for animals that are either traveling internationally or arriving from another country also need to be aware of the principles of travel medicine and be able to perform similar functions of pretravel risk assessment and counseling, vaccination, and posttravel assessment on animals.
PRETRAVEL RISK ASSESSMENT AND ANIMAL CONTACT COUNSELING
In a pretravel risk assessment, clinicians consider both the medical status of the traveler as well as the infectious and other environmental risks related to the countries they plan to visit and the activities they plan to undertake (see Table 10-1). Risks of travel-related animal contact may be overlooked during such visits. Table 10-2 summarizes these risks and provides the basics of counseling on risk reduction for particular animal contact situations.
Table 10-2.
Prevention of Human Health Risks Associated With Travel and Animal Contact
| Activity | Types of Contact | Pathogens | Preventive Steps |
|---|---|---|---|
| Bites, scratches from direct contact with animal | Contact with dogs, cats, monkeys | Pasteurella, rabies virus, Bartonella, other | Avoid animal contact in rabies-endemic countries, supervise children around animals |
| Simian immunodeficiency viruses, herpes B |
|
||
| Farm visits, agricultural tourism | Contact with goats, sheep, cattle, poultry, hogs, contaminated dusts | Q fever, brucellosis, E. coli, Nipah, avian influenza, anthrax | Avoid close contact with animals or confined spaces with dust, handwashing after contact |
| Religious pilgrimages, festivals (e.g., Hajj/Eid al-Adha, Lunar New Year) | Slaughter and consumption of animals |
|
|
| Live animal markets | Slaughter of animals; fecal contamination of surfaces, air | Avian influenza, SARS, E. coli, other | Avoid visiting live animal markets |
| Safaris, wilderness travel | Mosquito-borne infections | Malaria, yellow fever, dengue, Chikungunya fever | Use DEET and/or Picaridin insect repellents; other mosquito, tick, and fly precautions |
| Tickborne infections |
|
||
| Fly-borne | Trypanosomiasis, leishmaniasis | ||
| Rodent-infested buildings | Hantavirus | Avoid rodent areas (see Chapter 9) | |
| Infected fresh water | Leptospirosis, E. coli, other pathogens | Avoid swimming in or drinking untreated fresh water | |
| Exposures to bats or bat guano | Rabies, other viral pathogens, histoplasmosis | Use caution visiting caves and sleeping outdoors to avoid bats and bat guano | |
| Local delicacies, bushmeat | Undercooked meat, raw milk | Campylobacter, Salmonella, bovine tuberculosis, brucellosis, listeriosis, anthrax | Avoid uncooked meats, fish, raw/unpasteurized dairy products, soft cheeses |
| Primate meat | Simian viruses, Ebola | Avoid bushmeat consumption | |
| Bear | Trichinella | ||
| Raw fish, shellfish | Cholera, hepatitis, gnathostomiasis, paragonimiasis, rat lungworm | ||
| Souvenirs | Animal skins drums | Anthrax | Avoid purchasing unprocessed animal hide souvenirs |
| Beaches, sandboxes | Walking barefoot, swimming | Hookworm infection, tungiasis, marine envenomations | Do not walk barefoot on beaches, use caution when swimming |
| Travel with pet | Pet can come in contact with local domestic and wildlife species, may also develop vector-borne disease and/or toxic exposures |
|
Use of pet insecticide, periodic deworming of pet, avoid feeding uncooked meat, do not allow pet to roam free outdoors, pretravel and posttravel vet visits (see Boxes 10-2 and 10-3) |
HUMAN HEALTH RISKS OF ANIMAL CONTACT WHILE TRAVELING
Injuries From Animals
Animal bites and other animal-related injuries can be a significant hazard during travel (Color Plate 10-1). Although an attack from a wild animal, including snakes, crocodiles, large felids, and elephants, can cause dramatic injuries, falls from horseback or attacks by bulls or other large domestic animals can also maim and kill (Figures 10-1 and 10-2 ; Color Plates 10-2 and 10-3). A review of animal-associated injuries reported to the GeoSentinel Surveillance Network found that dog bites were the most common animal-associated injury to travelers, followed by bites from monkeys and cats. Three-quarters of the exposures occurred in countries where rabies is endemic, with the majority occurring in Asia. Fifty percent of bitten travelers had a travel duration of 1 month or less, and traveling for tourism was associated with an increased risk of an animal injury. Males were more likely to receive a dog bite, whereas monkey exposures were more common for females. Children were also at increased risk of exposure.4 Despite the risk of rabies from such exposures (fatal cases of human rabies have occurred in U.S. travelers from dog bites received during international travel5), a study of returning travelers with animal injuries found that most had not had pretravel rabies vaccine6 and two thirds of persons with injuries did not receive postexposure prophylaxis (which may not be routinely available in some countries). Rabies vaccine should be considered for all travel to rabies-endemic countries, especially for children, although a minimum travel duration of 6 weeks in an endemic area is recommended by some experts as an indication for vaccine. The strength of that recommendation should be influenced by the availability of medical care and rabies immunization products locally.
Figure 10-1.

Wound from bison goring.
(From Auerbach PS: Wilderness medicine, ed 5, Philadelphia, 2007, Mosby Elsevier. Photo courtesy Karen Hansen.)
Figure 10-2.

African elephant bluff-charges the photographer.
(From Auerbach PS: Wilderness medicine, ed 5, Philadelphia, 2007, Mosby Elsevier. Photo courtesy Cary Breidenthal.)
Other reported diseases related to bite and scratch exposures in travelers include bacterial infection from Pasteurella or other agents, rat-bite fever from rodents (see Animal and Human Bites below), and Bartonella infection from cats, the most common animal-associated infection reported in the GeoSentinel study.
Exposure to nonhuman primates can occur during travel; monkey temples are a popular tourist site in Asia. Simian foamy virus infection has been reported in a visitor to a monkey temple.7 Bites from Old World monkeys also carry a risk of herpes B infection that can cause fatal disease in human beings (see Animal and Human Bites later in this chapter). Herpes B infections in human beings have been mostly observed in occupational settings (see Chapter 12), and to date no known cases have been reported in travelers.8
Travelers should never try to pet, handle, or feed unfamiliar animals, domestic or wild, particularly in areas of endemic rabies. Because children are at greatest risk of animal bites, including severe injuries, and may be less likely to report a bite incident,8 they need to be counseled to avoid petting or handling dogs, cats, or other animals and should be supervised around animals at all times. If an exposure occurs, clean the wound thoroughly and immediately seek medical care for possible rabies postexposure prophylaxis (see Chapter 9). Tetanus prophylaxis is also indicated if the traveler is not up to date with tetanus vaccination. In the case of an Old World monkey bite, medical care should be sought as soon as possible for prophylactic treatment against herpes B infection.
Envenomations from snakes, scorpions, and spiders represent another animal-associated injury risk to travelers; medical care should be sought immediately. The treatment of snakebites and other venomous animal bites usually depends on the species of animal responsible (see Chapter 8).
Farm Visits
Agricultural tourism is the experience of visiting a working farm or related operation for enjoyment and is a growing type of tourism both nationally and internationally (Figure 10-3 ).9 Specialized tour operators offer package tours to farms in a number of countries. During such visits tourists may come in contact with both farm animals and contaminated soils and dusts that may contain infectious pathogens. Q fever is considered to be underdiagnosed in travelers, who can acquire it by exposure to farm animals or contaminated aerosols.10 Travel to a farm in Guyana was associated with a cluster of human cases of Q fever, presumably from contact with a parturient goat and dog on a farm.11 If the farm contains poultry, risks include exposure to Chlamydophila, Campylobacter, Salmonella, and avian influenza virus. Brucellosis is another potential farm-related exposure, either through direct contact with animals or consumption of unpasteurized dairy products. Brucellosis has been termed a “travel-associated foodborne zoonosis” in Germany, where a recent rise in cases has been traced to consumption of unpasteurized cheese from brucellosis-endemic countries.12 In a series of brucellosis cases in San Diego, travel to Mexico was a risk factor for infection.13
Figure 10-3.

Farm contact may expose the traveler to diseases such as Q fever and brucellosis.
(From Centers for Disease Control and Prevention Public Health Image Library, Atlanta, Ga. Photo courtesy Edwin P. Ewing Jr.)
Festivals Involving Animal Contact
An increasing amount of international travel is related to religious and cultural festivals. Each year several million Muslims participate in the Hajj pilgrimage to Mecca. Although local agricultural authorities have taken steps to reduce animal contact risks to pilgrims,14 the end of the Hajj is marked by the festival of Eid al-Adha, which may involve increased exposure to animal slaughtering activities. For example, a suspected outbreak of anthrax was linked to slaughter and distribution of infected meat from a camel during the festival.15
Similarly, the Lunar New Year festivals in Asia often involve increased slaughtering and consumption of poultry, with an attendant increased risk of avian influenza. The CDC has issued travel advisories for U.S. travelers visiting Asia during the Lunar New Year festival. These recommendations include handwashing; avoidance of bird farms or live bird markets; not touching live or dead birds, including chickens, ducks, and wild birds, even if they do not seem sick; and not touching surfaces contaminated with bird feces, blood, or other body fluids.16
Live Animal Markets
At any time of year visits to live animal markets, present in many cities and villages in a large number of countries, carry a risk of infection with zoonotic agents. In such markets numerous species of wildlife and domestic animals may be housed in close proximity, creating an increased opportunity for disease transmission. Color Plate 10-4 shows a typical scene from a live animal market in Asia. Human infection with the H5N1 avian influenza virus has been linked to visits to live bird markets, even for individuals who denied direct contact with sick or healthy-appearing poultry.17 Avian influenza has been found on surfaces and dusts in such markets, and exposure may occur by touching contaminated surfaces as well as breathing contaminated dusts. Severe acute respiratory syndrome (SARS) is another infection linked to live animal markets in Asia. Travelers wishing to reduce risk of zoonotic diseases while traveling should avoid visiting live animal markets, especially if a country is experiencing an outbreak of highly pathogenic avian influenza.18 A current list of countries is maintained at http://www.cdc.gov/flu/avian/outbreaks/current.htm.
Safaris and Wilderness Travel
Wilderness travel overseas increases the risk of exposure to a number of pathogens as well as injuries from snakes, crocodiles, and other wildlife. Visitors on safari to game parks in Africa have become infected with African tick fever and other rickettsial infections that may have a reservoir in the local wildlife populations.19 Although most forms of malaria, one of the most common causes of fever in returning travelers, are not zoonotic, certain monkey malaria species such as Plasmodium knowlesi have been described in human beings and may be underdiagnosed.20 Other zoonotic arthropod-borne infections such as trypanosomiasis, yellow fever, and Chikungunya fever have been reported in safari and wilderness travelers.21, 22 Sleeping in huts and shelters that may also be home to local rodent populations can increase risk of infection with hantaviruses and various hemorrhagic fevers, including Lassa fever.23 Swimming in fresh water in areas frequented by wildlife has led to outbreaks of leptospirosis in adventure travelers.24 Wilderness travelers may be exposed to bats when visiting caves or sleeping outside in areas frequented by bats, where they could come in contact with bat droppings or sustain bat bites. In addition to rabies risk, bats are reservoirs for a wide range of other viral pathogens, including other lyssa viruses, Nipah virus, SARS-like coronavirus, and Marburg virus.25 Exposure to aerosolized bat guano has also been associated with the development of acute pulmonary histoplasmosis in travelers.26
Local Delicacies Involving Animal Products and Bushmeat Consumption
Many local delicacies that travelers encounter may involve animal products from domestic or wild animals. Meat and dairy products may be served raw or undercooked and may be a source for zoonotic infections such as brucellosis, trichinosis, and salmonellosis in returning travelers.27 Raw chicken sashimi from an island in Japan is known to be contaminated with Campylobacter. However, local residents do not appear to become ill, suggesting a role for acquired protective immunity that a traveler would presumably not enjoy.28 Travelers to Southeast Asia and Africa have become infected with parasites, including Paragonimus, trematodes Clonorchis sinensis or Haplorchis pumilio, and gnathostomes. Paragonimiasis is acquired by eating raw freshwater crabs or crayfish. Gnathostomiasis is a nematode infection acquired by ingestion of various intermediate hosts in addition to fish.29, 30 Eating unwashed produce and undercooked foods such as mollusks in some countries poses risks of numerous infectious diseases, including viral hepatitis, bacterial enteritis, and eosinophilic meningitis from rat lungworm.31 There is increasing awareness of the infectious risks of improperly cooked poultry products. Such practices have been linked to cases of avian influenza.32 Bovine tuberculosis transmission to human beings can involve consumption of uncooked meat as well as raw milk (see Chapter 9).33
Bushmeat (wild game killed for food) is an important source of animal protein for communities in many parts of the world. It has been estimated that in Central Africa alone more than 1 billion kilograms of meat from wild animals are consumed each year (Figure 10-4 ).34 Table 10-3 shows the diversity of species that may end up being sold for human consumption, especially during times of civil unrest.35 Emerging pathogens linked to bushmeat consumption include simian immunodeficiency viruses, anthrax, and hemorrhagic fever viruses.36 Although the risk of infection from bushmeat exposure may be greatest to persons who butcher carcasses, travelers to areas where bushmeat is an important part of local diets may be at risk if they consume improperly cooked bushmeat.
Figure 10-4.

South African market with bushmeat for sale.
(From Fowler ME: Zoo and wild animal medicine: current therapy, ed 6, St Louis, 2008, Saunders Elsevier. Photo courtesy R. A. Cook.)
Table 10-3.
Protected and Unprotected Species Sold in Village and Urban Bushmeat Markets in Northeastern Democratic Republic of Congo During Peacetime and Wartime*
| Village (Kiliwa) Market Day Sales | Urban (Dungu) Market Day Sales | |||||
|---|---|---|---|---|---|---|
| Taxon† | Peace (kg) | War (kg) | P | Peace (kg) | War (kg) | P |
| Protected Species | ||||||
| Elephant, Loxodonta africana | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 23.5 ± 1.5 | 120.3 ± 10.3 | <.05 |
| Hippo, Hippopotamus amphibius | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 9.3 ± 0.6 | 48.0 ± 4.2 | <.05 |
| Buffalo, Syncerus caffer | 0.1 ± 0.1 | 0.0 ± 0.0 | NS | 19.6 ± 1.2 | 98.2 ± 8.4 | <.05 |
| Bongo, Tragelaphus euryceros | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.5 ± 0.1 | 2.2 ± 0.5 | <.05 |
| Large antelope, multiple species‡ | 0.2 ± 0.1 | 0.3 ± 0.3 | NS | 3.7 ± 0.3 | 23.7 ± 2.1 | <.05 |
| Pigs, multiple species§ | 0.4 ± 0.1 | 0.4 ± 0.2 | NS | 5.6 ± 0.3 | 9.5 ± 1.1 | <.05 |
| Chimpanzee, Pan troglodytes | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.6 ± 0.1 | 0.5 ± 0.2 | NS |
| Aardvark, Orycteropus afer | 0.1 ± 0.1 | 0.0 ± 0.0 | NS | 0.7 ± 0.1 | 0.8 ± 0.2 | NS |
| All protected species | 0.8 ± 0.2 | 0.8 ± 0.4 | NS | 63.6 ± 3.7 | 303.1 ± 25.5 | <.05 |
| Unprotected Species | ||||||
| Duikers, multiple species|| | 1.0 ± 0.2 | 1.7 ± 0.7 | NS | 16.7 ± 0.5 | 15.7 ± 0.9 | NS |
| Monkeys, multiple species¶ | 1.5 ± 0.3 | 1.8 ± 0.6 | NS | 8.7 ± 0.3 | 8.4 ± 0.5 | NS |
| Crested porcupine, Hystrix cristata | 0.2 ± 0.1 | 0.2 ± 0.1 | NS | 1.0 ± 0.0 | 1.0 ± 0.1 | NS |
| Uganda grass hare, Poelagus marjorita | 0.3 ± 0.1 | 0.7 ± 0.3 | NS | 2.5 ± 0.1 | 2.6 ± 0.1 | NS |
| Cane rat, Thryonomys swinderianus | 0.2 ± 0.1 | 0.1 ± 0.1 | NS | 0.7 ± 0.0 | 0.7 ± 0.0 | NS |
| All unprotected species | 3.1 ± 0.6 | 4.5 ± 1.5 | NS | 29.7 ± 0.8 | 28.3 ± 1.4 | NS |
Mean and standard error of kilograms of fresh meat sold per market day. Tests for statistical significance (P) indicate whether the difference between peacetime and wartime is significant (P < .05) or not significant (NS). Sample sizes (market days) in peacetime and wartime are 96 and 341 for the rural markets and 336 and 120 for the urban markets, respectively. To calculate daily sales (incorporating both market days and nonmarket days), multiply all rural figures by 0.29 (i.e., 2 market days/week) and multiply all urban figures by 2.71 (i.e., 19 market days/weeks).
Taxa are grouped according to protected and unprotected status, although all species are protected within the boundaries of Garamba National Park and ordered by decreasing body size within these groups. Where several species are incorporated into a single taxon, the median species weight is used, with all body size data taken from Rowcliffe JM, de Merode E, Cowlishaw G: Do wildlife laws work? Species protection and the application of a prey choice model to poaching decisions, Proc Biol Sci 271:2631-2636, 2004. Where a taxon is composed of multiple species, allocation of that taxon to either one of these groups depends on the relative proportion of protected and unprotected species; if at least half of the species is protected, the taxon is placed in the protected group. Taxonomy follows Kingdon J: The Kingdon field guide to African mammals, San Francisco, 1997, Academic Press.
Hartebeest (Alcelaphus buselaphus), kob (Kobus kob), waterbuck (Kobus ellipsiprymnus), bohor reedbuck (Redunca redunca), bushbuck (Tragelaphus scriptus), and sitatunga (Tragelaphus spekii). Hartebeest, waterbuck, and sitatunga are protected.
Giant forest hog (Hylochoerus meinertzhageni), common warthog (Phacochoerus africanus), and red river hog (Potamochoerus porcus). Giant forest hog and red river hog are protected.
Bay duiker (Cephalophus dorsalis), red-flanked duiker (Cephalophus rufilatus), blue duiker (Cephalophus monticola), and bush duiker (Sylvicapra grimmia). None of these species is protected.
Agile mangabey (Cercocebus agilis), tantalus monkey (Cercopithecus [aethiops] tantalus), red-tailed monkey (Cercopithecus [cephus] ascanius), patas monkey (Cercopithecus [erythrocebus] patas), Dent's monkey (Cercopithecus [mona] denti), de Brazza's monkey (Cercopithecus neglectus), guereza colobus (Colobus guereza), and olive baboon (Papio anubis). None of these species is protected.
From De Merode E, Cowlishaw G: Species protection, the changing informal economy, and the politics of access to the bushmeat trade in the Democratic Republic of Congo, Conserv Biol 20:1262, 2006.
Beaches and Sandboxes
Travelers should avoid going barefoot on beaches frequented by animals. Many beaches that travelers visit during international vacations are frequented by dogs and cats. Walking barefoot on beaches contaminated with their feces is a risk factor for acquiring zoonotic hookworm infection (cutaneous larva migrans, also known as creeping eruption (see Chapter 9 and Color Plate 9-33).37 Another beach-related zoonosis is tungiasis, a skin infestation caused by the sand flea Tunga penetrans (jiggers). Walking barefoot on beaches also carries risks of stings from jellyfish and other marine organisms (see Chapter 8). Sandboxes and play areas are another source of contact for hookworms, roundworms (Toxocara), and Toxoplasma.
Souvenirs
Persons purchasing souvenirs while traveling should be aware that improperly processed hides used for drums and other souvenirs may be a source of exposure for cutaneous anthrax (see Chapter 9).38 However, this risk is considered to be low.
POSTTRAVEL ASSESSMENT
In the returning traveler with illness, a careful history of animal-related exposures should be obtained in addition to the standard questions (Box 10-1 ). If the response to any of these questions is affirmative, further evaluation is warranted.
BOX 10-1. Evaluation of the Returning Traveler Regarding Animal-Related Risks.
-
•
Were you in the vicinity of live animals, either on a farm, in a house, or in a market? If so, what types of animals and environments? Did you touch any animals or notice any sickness in the animals?
-
•
Were you bitten, scratched, or licked by dogs, cats, monkeys, or other animals?
-
•
Did you eat uncooked or undercooked meat, fish, or shellfish?
-
•
Did you consume bushmeat?
-
•
Did you eat or drink unpasteurized dairy products?
-
•
Did you walk barefoot on beaches or swim in fresh water?
-
•
Did you travel in the wilderness?
-
•
Did you visit caves or sleep outdoors in areas frequented by bats?
-
•
Did you receive bites from ticks, flies, or mosquitoes?
TRAVELING WITH A PET AND OTHER ANIMAL TRAVEL MEDICINE ISSUES
Traveling with pets is becoming increasingly popular. There are a number of reasons why people travel with a pet. These include emotional, economic, gene transfer, and seeking specialized veterinary care.39 Emotional reasons include companionship, treating an animal as a member of the family, and reluctance to leave an animal behind. Economic factors can include the cost of arranging care for the animal while traveling. Gene transfer involves activities such as taking a pedigreed animal to another location to breed with another animal. Finally, owners may take an ill animal to another location for specialized veterinary care.39
Veterinarians should go through similar steps as their human health counterparts in evaluating animals that are traveling. These include pretravel risk assessment and owner counseling, vaccines and other preventive care as necessary, and evaluation of returning and newly imported animals after travel.
Infectious Disease Risks and Pets That Travel
Many of the infectious disease risks to traveling companion animals are shared with human beings. Even travel to different regions in the same country can expose an animal to new health risks. Pets that travel also face potential exposure to animal diseases that are not zoonotic in nature, such as canine parvovirus, canine viral hepatitis, and feline panleukopenia virus.
Particular zoonoses associated with dog travel include rabies, leishmaniasis, and roundworm infection.40., 41., 42., 43. A case of a rabid puppy imported illegally from Morocco to France by a traveling couple resulted in the prophylactic treatment of 21 persons, the euthanasia of a contact animal, and legal action against the travelers.44 In 2007, a puppy rescued from an animal shelter in India by a veterinarian who brought the animal into the United States at 11 weeks of age was found to be positive for rabies by the Alaska Department of Health and Social Services.45 Such introduction of rabies from animal travel can involve foreign rabies virus variants. As a result, the CDC is considering strengthening federal regulations regarding the importation of companion animals.2
Vector-borne diseases are a documented risk to traveling dogs. For example, leishmaniasis has been reported in dogs traveling in high-incidence countries, leading to the introduction of disease upon returning home.
Other Risks Associated With Traveling Animals
Animals may cause allergic reactions if they come into contact with sensitized travelers or airline, train, or other transportation personnel. Animals with allergy may also be exposed to a variety of allergens during travel.
Toxic risks to traveling animals include contaminated pet food,46 water, pesticides, and accidental ingestion of rodenticides (see Chapter 8).
Animals risk physical injury from being shipped in containers or otherwise restrained, heat stress and cold exposures, and fights with other animals. Other potential stressors include unfamiliar surroundings, disruption of circadian schedules, noise, and other noxious stimuli.
Logistics
Travelers wishing to travel with pets will need to identify the logistic requirements for transporting and housing the animal during travel. Airlines may vary in their rules regarding whether an animal can be carried in a pet container that can be placed under a seat, or whether all animals need to be placed in cargo. There have been instances of companion animals dying from heat and cold stress and other trauma while being shipped in cargo, and owners should be aware of the risks to animals undergoing air travel.47 There may be restrictions on the number of animals allowed. Rental car companies, trains, buses, and hotels may also have restrictive policies about pets. These policies should be clarified in advance of travel.
Documentation and Regulation of Animal Travel and Importation
Animals that travel often need documentation of vaccination status, tick or other prophylactic treatments, and a statement from a veterinarian that they are free of communicable disease and able to travel safely. Such documentation may be necessary for the animal to enter another country. Owners should contact the embassies of countries they plan to visit to go through the required entry processes as well as determine the U.S. clearance processes upon return.48 Policies of some countries may require an animal to be quarantined or destroyed without proper documentation. Additional information is available at http://www.aphis.usda.gov/import_export/animals/animal_exports.shtml.
Many countries limit movement of dogs and other animals based on rabies status. Dogs and cats generally cannot pass from a rabies-endemic country to a rabies-free country without a process of quarantine and/or vaccination documentation. Table 10-4 lists countries reporting no indigenous rabies in 2005.
Table 10-4.
Countries and Political Units Reporting No Indigenous Cases of Rabies During 2005*
| Region | Countries |
|---|---|
| Africa | Cape Verde, Libya, Mauritius, Réunion, São Tome and Principe, Seychelles |
| Americas |
North: Bermuda, St. Pierre et Miquelon Caribbean: Antigua and Barbuda, Aruba, Bahamas, Barbados, Cayman Islands, Dominica, Guadeloupe, Jamaica, Martinique, Montserrat, Netherlands Antilles, Saint Kitts (Saint Christopher) and Nevis, Saint Lucia, Saint Martin, Saint Vincent and Grenadines, Turks and Caicos, Virgin Islands (U.K. and U.S.) South: Uruguay |
| Asia | Hong Kong, Japan, Kuwait, Lebanon, Malaysia (Sabah), Qatar, Singapore, United Arab Emirates |
| Europe | Austria, Belgium, Cyprus, Czech Republic†, Denmark†, Finland, France†, Gibraltar, Greece, Iceland, Ireland, Isle of Man, Italy, Luxemburg, Netherlands†, Norway, Portugal, Spain† (except Ceuta/Melilla), Sweden, Switzerland, United Kingdom† |
| Oceania‡ | Australia†, Northern Mariana Islands, Cook Islands, Fiji, French Polynesia, Guam, Hawaii, Kiribati, Micronesia, New Caledonia, New Zealand, Palau, Papua New Guinea, Samoa, Vanuatu |
Bat rabies may exist in some areas that are reportedly free of rabies in other animals.
Bat lyssa viruses are known to exist in these areas that are reportedly free of rabies in other animals.
Most of Pacific Oceania is reportedly rabies free.
From Centers for Disease Control and Prevention: CDC health information for international travel 2008: prevention of specific infectious diseases: rabies.http://wwwn.cdc.gov/travel/yellowBookCh4-Rabies.aspx#653.
A number of federal agencies are involved with regulation of animals entering the United States. The CDC regulates the importation into the United States of dogs, cats, turtles, bats, monkeys, and other animals as well as animal products capable of causing human disease (see http://wwwn.cdc.gov/travel/yellowBookCh7-AnimalImport.aspx).48 Pets taken out of the United States are subject to the same regulations when returning to the country as are newly imported animals. The U.S. Department of Agriculture (USDA) regulates a number of species being imported based on their disease risk to plants and animals of agricultural concern.
No single agency regulates entry of dogs into the United States. Although the CDC does not require a general certificate of health for dogs, dogs with evidence of illness may not be allowed entry. Documentation of rabies vaccination at least 30 days before entry is required, although dogs younger than 3 months and dogs without proof of rabies vaccination may be allowed to enter if the owner completes a confinement agreement and certifies the animal will be vaccinated and kept confined at least 30 days after vaccination. Unvaccinated dogs from countries considered rabies free (see Table 10-4) may also be allowed entry.49 In addition to CDC regulations, the USDA regulates the importation of dogs potentially carrying diseases of agricultural importance, including screwworms or some Taenia species of tapeworm.2
For cats, a general certificate of health is not required to enter the United States, but some states and air carriers may require such documentation. Rabies vaccination is also not required, but some states may require proof of rabies vaccine documentation. All pet cats entering Hawaii and Guam are subject to local quarantine requirements.49
Importation of birds is regulated by the USDA Animal and Plant Health Inspection Service.50 The USDA currently restricts importation of pet birds from countries where highly pathogenic avian influenza H5N1 is present in poultry. To import a pet bird of non-U.S. origin, the importer or owner must obtain a USDA import permit,51 have a certificate of health from a veterinarian in the exporting country, and allow the bird to be quarantined for 30 days in a USDA animal import center at the owner's expense. Pet birds arriving from Canada are not required to be quarantined.52 Entry into the United States of birds that are covered by endangered species conventions is regulated by the U.S. Fish and Wildlife Service.
The CDC periodically issues embargoes on specific animals associated with disease risk, including civets (SARS), birds from specific countries (avian influenza), and African rodents (monkeypox).
CDC regulations do not apply to horses not known to be carrying diseases infectious to human beings, but the USDA may regulate horse import due to risks of diseases of agricultural importance such as screwworm.
Importation of fish into the United States is not regulated by the CDC, but U.S. Fish and Wildlife Service regulations may apply.
Finally, importation of certain animals and animal products is regulated by other federal agencies such as the U.S. Customs Service.53
If animals are traveling, they should have a veterinary evaluation for pretravel risk assessment and administration of necessary vaccines and other preventive treatments. Box 10-2 outlines the elements of such a visit.
BOX 10-2. PRETRAVEL RISK ASSESSMENT CHECKLIST FOR PET.
Health and Appropriateness of Animal
-
•
Determine whether travel is medically appropriate for pet (consider species, medical conditions, age).
-
•
If the pet has an ongoing medical condition, ensure adequate supply of medication.
Vaccination and Preventive Medications
-
•
Ensure current rabies and other core vaccinations are up to date and that owner has documentation.
-
•Consider the following preventable diseases that could be acquired during travel39:
-
•Dogs
- Canine distemper
- Canine parvovirus
- Canine hepatitis
- Canine parainfluenza
- Rabies
- Bordetella
-
•Cats
- Feline panleukopenia virus
- Feline calicivirus
- Feline herpesvirus
- Rabies
-
•
-
•
Perform routine deworming, provide heartworm prophylaxis
-
•
Provide flea and tick treatment and prevention (topical or systemic acaricides)
The veterinarian should determine whether travel is medically advisable for the animal. Travel is riskier for immunocompromised animals, including young animals and animals with underlying illness. Certain species, such as snakes, may not be allowed entry by other countries. Animals with behavioral problems may attack other animals or people or present other travel risks.
The veterinarian should ensure that animals with ongoing illnesses have an adequate supply of medications and that plans for emergency veterinary medical care while traveling are discussed with the owner.
Evaluation of Illness in an Animal After Travel
Animals imported into the United States are required to be healthy. However, if illness develops, evaluation should involve the steps outlined in Box 10-3 . If infectious conditions are identified, the possibility of zoonotic transmission to the animal's owner should be considered, and there should be rapid communication to either a human health care provider, public health department, or both.
BOX 10-3. EVALUATION OF AN ILL PET AFTER TRAVEL.
-
•
Did the animal go through a quarantine process before reentering the country?
-
•
Is there documentation of rabies vaccination status, and did the animal travel to or originate from a rabies-endemic country?
-
•
Is the animal otherwise up to date on necessary vaccinations and preventive treatment (see Box 10-2)?
-
•
Were there other endemic disease risks in the countries visited, including leishmaniasis and other parasitic infections?
-
•
Has illness been noticed in any other animals that were in contact with this one?
-
•
Did the animal visit farms or wilderness areas or have other contact with animals, including sick animals?
-
•
What kind of diet was the animal on during travel? Did it consume raw meat?
-
•
Is there any evidence of infection with endoparasites and/or ectoparasites?
-
•
Are there reports of illness in the owner or other human beings that accompanied the pet that could be related to travel? (This could provide clues regarding shared exposure risks.)
Further steps should be based on the results of this preliminary evaluation.
References
- 1.Office of Travel & Tourism Industries . U.S. citizen air traffic to overseas regions, Canada and Mexico. 2007. http://tinet.ita.doc.gov/view/m-2007-O-001/index.html. Accessed February 28, 2008. [Google Scholar]
- 2.McQuiston J.H., Wilson T., Harris S. Importation of dogs into the United States: risks from rabies and other zoonotic diseases. Zoonoses and Public Health. 2008;55(8–10):421–426. doi: 10.1111/j.1863-2378.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill D.R., Ericsson C.D., Pearson R.D. Infectious Diseases Society of America: The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(12):1499–1539. doi: 10.1086/508782. [DOI] [PubMed] [Google Scholar]
- 4.Gautret P., Schwartz E., Shaw M. GeoSentinel Surveillance Network Animal-associated injuries and related diseases among returned travelers: a review of the GeoSentinel Surveillance Network. Vaccine. 2007;25(14):2656–2663. doi: 10.1016/j.vaccine.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Schmiedel S., Panning M., Lohse A. Case report on fatal human rabies infection in Hamburg, Germany, March 2007. Euro Surveill. 2007;12(5):E070531.5. doi: 10.2807/esw.12.22.03210-en. [DOI] [PubMed] [Google Scholar]
- 6.Gautret P., Shaw M., Gazin P. Rabies postexposure prophylaxis in returned injured travelers from France, Australia, and New Zealand: a retrospective study. Travel Med. 2008;15(1):25–30. doi: 10.1111/j.1708-8305.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones-Engel L., Engel G.A., Schillaci M.A. Primate-to-human retroviral transmission in Asia. Emerg Infect Dis. 2005;11(7):1028–1035. doi: 10.3201/eid1107.040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. CDC health information for international travel 2008: animal associated hazardshttp://wwwn.cdc.gov/travel/yellowBookCh6-Animal.aspx. Accessed October 16, 2008.
- 9.Lobo R, Small Farm Center, University of California-Davis. Helpful agricultural tourism (agritourism) definitionshttp://www.sfc.ucdavis.edu/agritourism/definition.html. Accessed May 29, 2008.
- 10.Terheggen U., Leggat P.A. Clinical manifestations of Q fever in adults and children. Travel Med Infect Dis. 2007;5(3):159–164. doi: 10.1016/j.tmaid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Baret M., Klement E., Dos Santos G. Coxiella burnetii pneumopathy on return from French Guiana [in French] Bulletin de la Societe de Pathologie Exotique. 2000;93(5):325–327,. [PubMed] [Google Scholar]
- 12.Dahouk S.A., Neubauer H., Hensel A. Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerg Infect Dis. 2007;13(12):1895–1900. doi: 10.3201/eid1312.070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troy S.B., Rickman L.S., Davis C.E. Brucellosis in San Diego: epidemiology and species-related differences in acute clinical presentations. Medicine. 2005;84(3):174–187. doi: 10.1097/01.md.0000165659.20988.25. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed Q.A., Arabi Y.M., Memish Z.A. Health risks at the Hajj. Lancet. 2006;367(9515):1008–1015. doi: 10.1016/S0140-6736(06)68429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Society for Infectious Diseases: Dengue/DHF update 2008. http://promedmail.org.
- 16.Centers for Disease Control and Prevention . In the news: keeping yourself safe from bird flu: an important message for people traveling to Asia to celebrate the Lunar New Year. 2008. http://wwwn.cdc.gov/travel/content AvianFluLunarNewYear08.aspx. Accessed February 28, 2008. [Google Scholar]
- 17.Yu H., Feng Z., Zhang X. Avian influenza H5N1 study group. Human influenza A (H5N1) cases, urban areas of People's Republic of China, 2005–2006. Emerg Infect Dis. 2007;13(7):1061–1064. doi: 10.3201/eid1307.061557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtado T.R. Human influenza A (H5N1): a brief review and recommendations for travelers. Wilderness Environ Med. 2006;17(4):276–281. doi: 10.1580/06-weme-ra-007r.1. [DOI] [PubMed] [Google Scholar]
- 19.Buchau A.S., Wurthner J.U., Reifenberger J. Fever, episcleritis, epistaxis, and rash after safari holiday in Swaziland. Arch. Dermatol. 2006;142(10):1365–1366. doi: 10.1001/archderm.142.10.1365. [DOI] [PubMed] [Google Scholar]
- 20.Ng O.T., Ooi E.E., Lee C.C. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14(5):814–816. doi: 10.3201/eid1405.070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelinek T., Bisoffi Z., Bonazzi L. European Network on Imported Infectious Disease Surveillance. Cluster of African trypanosomiasis in travelers to Tanzanian national parks. Emerg Infect Dis. 2002;8(6):634–635. doi: 10.3201/eid0806.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Chikungunya fever diagnosed among international travelers—United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55(38):1040–1042. [PubMed] [Google Scholar]
- 23.Castillo C., Nicklas C., Mardones J. Andes hantavirus as possible cause of disease in travellers to South America. Travel Med Infect Dis. 2007;5(1):30–34. doi: 10.1016/j.tmaid.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Outbreak of acute febrile illness among participants in EcoChallenge Sabah 2000-Malaysia, 2000. JAMA. 2000;284(13):1646. [PubMed] [Google Scholar]
- 25.Wong S., Lau S., Woo P. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007;17(2):67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries P.J., Koolen M.G., Mulder M.M. Acute pulmonary histoplasmosis from Ghana. Travel Med Infect Dis. 2006;4(5):286–289. doi: 10.1016/j.tmaid.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Dore K., Buxton J., Henry B. Multi-provincial Salmonella Typhimurium case-control study steering committee. Risk factors for Salmonella typhimurium DT104 and non-DT104 infection: a Canadian multi-provincial case-control study. Epidemiol Infect. 2004;132(3):485–493. doi: 10.1017/s0950268803001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore J.E., Matsuda M. Consumption of raw chicken sashimi, Kyushu Island, Japan—risk of campylobacteriosis or not. Travel Med Infect Dis. 2007;5(1):64–65. doi: 10.1016/j.tmaid.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Del Giudice P., Cua E., Le Fichoux Y. Gnathostomiasis: an emerging parasitic disease [in French]? Ann Dermatol Venereol. 2005;132(12 Pt 1):983–985. doi: 10.1016/s0151-9638(05)79561-2. [DOI] [PubMed] [Google Scholar]
- 30.Hale D.C., Blumberg L., Frean J. Case report: gnathostomiasis in two travelers to Zambia. Am J Trop Med Hyg. 2003;68(6):707–709. [PubMed] [Google Scholar]
- 31.Weir E. Travel warning: eosinophilic meningitis caused by rat lungworm. CMAJ. 2002;166(9):1184. [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Guidelines and recommendations: interim guidance about avian influenza (H5N1) for U.S. citizens living abroadhttp://wwwn.cdc.gov/travel/contentAvianFluAmericans Abroad.aspx. Accessed February 28, 2008.
- 33.Etter E., Donado P., Jori F. Risk analysis and bovine tuberculosis, a re-emerging zoonosis. Ann N Y Acad Sci. 2006;1081:61–73. doi: 10.1196/annals.1373.006. [DOI] [PubMed] [Google Scholar]
- 34.Karesh W.B., Cook R.A. The human-animal link. Foreign Affairs. 2005;84:38–50. [Google Scholar]
- 35.De Merode E., Cowlishaw G. Species protection, the changing informal economy, and the politics of access to the bushmeat trade in the Democratic Republic of Congo. Conserv Biol. 2006;20(4):1262–1271. doi: 10.1111/j.1523-1739.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe N.D., Daszak P., Kilpatrick A.M. Bushmeat hunting, deforestation, and prediction of zoonoses emergence. Emerg Infect Dis. 2005;11(12):1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Nispen tot Pannerden C., van Gompel F., Rijnders BJ. An itchy holiday. Neth J Med. 2007;65(5):188–190. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Anthrax Q&A: anthrax and animal hideshttp://www.bt.cdc.gov/agent/anthrax/faq/pelt.asp. Accessed March 29, 2008.
- 39.Leggat PA., Speare R. Travel with pets. J Travel Med. 2000;7:325–329. doi: 10.2310/7060.2000.00087. [DOI] [PubMed] [Google Scholar]
- 40.Deplazes P., Staebler S., Gottstein B. Travel medicine of parasitic diseases in the dog [in German] Schweiz Arch Tierheilkd. 2006;148(9):447–461. doi: 10.1024/0036-7281.148.9.447. [DOI] [PubMed] [Google Scholar]
- 41.Barr F., British Small Animal Veterinary Association Scientific Committee Checklist of infections that may be imported into the UK by the travelling pet. J Small Anim Pract. 2001;42(2):95–97. doi: 10.1111/j.1748-5827.2001.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 42.Teske E., van Knapen F., Beijer EG. Risk of infection with Leishmania spp. in the canine population in The Netherlands. Acta Vet Scand. 2002;43(4):195–201. doi: 10.1186/1751-0147-43-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Impact of pet travel on animal and public health. Vet Rec. 2008;162(14):429–430. doi: 10.1136/vr.162.14.429. [DOI] [PubMed] [Google Scholar]
- 44.Galperine T., Neau D., Moiton MP. The risk of rabies in France and the illegal importation of animals from rabid endemic countries [in French] Presse Med. 2004;33(12 Pt 1):791–792. doi: 10.1016/s0755-4982(04)98745-3. [DOI] [PubMed] [Google Scholar]
- 45.Castrodale L., Walker V., Baldwin J. Rabies in a puppy imported from India to the USA March 2007. Zoonoses and Public Health. 2008;55(8–10):427–430. doi: 10.1111/j.1863-2378.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 46.Brown CA., Jeong KS., Poppenga RH. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J Vet Diagn Invest. 2007;19(5):525–531. doi: 10.1177/104063870701900510. [DOI] [PubMed] [Google Scholar]
- 47.Humane Society of the United States. Tips for safe pet air travelhttp://www.hsus.org/pets/pet_care/caring_for_pets_when_you_travel/traveling_by_air_with_pets/. Accessed October 21, 2008.
- 48.Centers for Disease Control and Prevention. Importation of pets, other animals, and animal products into the United Stateshttp://www.cdc.gov/ncidod/dq/animal/index.htm. Accessed February 28, 2008.
- 49.Centers for Disease Control and Prevention. Bringing an animal into the United Stateshttp://www.cdc.gov/ncidod/dq/animal/dogs.htm. Accessed October 21, 2008.
- 50.U.S. Department of Agriculture. Pet travel: tips, facts, and scam information—for you and your pethttp://www.aphis.usda.gov/animal_welfare/pet_travel/content/wp_c_pet_travel_tips.shtml. Accessed May 29, 2008.
- 51.U.S. Department of Agriculture. Animal health permitshttp://www.aphis.usda.gov/permits/index.shtml. Accessed October 15, 2008.
- 52.U.S. Department of Agriculture. Animal and animal product import: non-US origin pet birdshttp://www.aphis.usda.gov/import_export/animals/nonus_pet_bird.shtml. Accessed October 10, 2008.
- 53.U.S. Customs Service. Pets and wildlifehttp://www.cbp.gov/linkhandler/cgov/newsroom/publications/travel/pets_wild.ctt/pets.pdf. Accessed May 29, 2008.
EXOTIC AND WILDLIFE PETS
Animal and human health clinicians need to understand the scope of the exotic and wildlife pet trade, the related health risks, and ways to reduce such risks. Driven by popular demand, trade in live animals for pets has been increasing both in the United States and worldwide.1 More than 200 million animals, representing thousands of individual species, are estimated to be legally imported into the United States from more than 160 countries. The majority of these imports were for the pet and aquarium trade.2 The number of animals illegally imported each year is unknown but is also believed to be considerable.3 As a result, the United States is the world's largest importer of live animals.2 At the same time, many potential pet owners are unaware of the health risks of such nontraditional pets.4
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
-
•Support efforts of the National Association of State Public Health Veterinarians (NASPHV) and Council of State and Territorial Epidemiologists (CSTE) to do the following:
-
○Develop comprehensive federal regulations with enforcement that provide oversight to the private ownership of exotic and wild animals.
-
○Develop and maintain a list of approved species for interstate distribution and importation.
-
○Limit ports of entry.
-
○Improve regulation and inspection of exotic animal breeders, dealers, auctions, swap meets, Internet sales, pet outlets, and animal imports.
-
○Develop a system to track imported and captive-bred exotic animals in the pet trade.
-
○
-
•
Support the USDA and local agencies to ensure that individuals and events selling or bartering exotic animals, including pet stores, are properly licensed and that exotic, wild, and imported animals are screened for known zoonoses, quarantined and observed for signs of disease, and have limited opportunities for ecological release into the nonnative environment.
-
•
Educate the public and health care providers about the risks of ownership and contact with wildlife and exotic pets, including zoonotic infections, injury, and (depending on species) envenomation. Discourage exotic or wild pet ownership among immunocompromised persons and families with children younger than 5 years. Discourage contact with mammals at high risk of transmitting rabies (e.g., bats, raccoons, skunks, foxes, and coyotes).
-
•
Ensure that local pet stores, pet swap meets, petting zoos, schools, and other venues with human-animal contact are aware of the recommendations of the NASPHV for reducing risk of transmission in such settings, including adequate handwashing facilities, separation of animal and nonanimal areas, and avoidance of high-risk species.5
-
•
If a human health clinician reports a zoonotic disease associated with an exotic or wildlife pet, coordinate a response with agricultural and wildlife veterinarians.
-
•
Form interdisciplinary networks and working groups to address exotic and wild animal pet issues that arise locally and regionally. Groups should consist of veterinarians, public health professionals, wildlife agencies and rehabilitators, zoologic park staff, university representatives, physicians, and environmental health professionals.
Human Health Clinicians
-
•
Ask patients about ownership and contact with exotic or wild animals. If they report such contact, list the species, origin, and types of interactions. Consider consulting the patient's veterinarian about the health risks (zoonotic and injury potential) of particular species.
-
•
Discourage acquisition, contact with, and maintenance of exotic and wildlife pets, particularly for families with children or immunocompromised persons.
-
•
If patients report ownership of venomous animals, ensure they are aware of the steps to take if envenomation occurs and that local emergency treatment (such as appropriate antivenin) is available locally (see Chapter 8).
-
•
If zoonotic disease is suspected or identified in a person in contact with exotic or wild animals (pets, recreational or occupational exposure) or works in a pet shop or other animal handling facility, notify the local and state public health department.
Veterinary Clinicians
-
•
Support efforts of state and national agencies to regulate or license ownership of exotic or wild pets.
-
•
Counsel clients to refrain from owning wild-caught animals, about disease transmission routes, and the risks of ownership and contact with wildlife and exotic animals as pets.6
-
•
Assist prospective pet owners in appropriate pet selection.
-
•
Counsel owners on techniques to avoid high-risk contact with exotic or wild animals.
SCOPE OF THE PROBLEM
Much of the pet trade involves traditional pets, species that have been bred and maintained over multiple generations for human companionship and have acknowledged popularity as pets. Examples include dogs, cats, and horses. Caged birds such as parakeets and canaries and pocket pets such as gerbils and hamsters are also popular and are bred for the pet trade. Another large segment of the pet trade involves tropical fish, which have generally been associated with fewer zoonotic diseases than other animals.
Much of the remainder of the pet trade, however, is in nontraditional pets, or species that are not domesticated and are captured from the wild or captive-bred to meet an increasing demand for unusual pets. Nontraditional pets include exotic species that are nonnative to the local ecosystem and native wildlife, which are local wildlife species. The number of wild animals, including reptiles and amphibians, captured in the United States for the pet trade each year is not precisely known but appears to be in the range of millions of animals.7 This segment of the pet trade is extremely lucrative and mainly uses the Internet, specialty newsletters, swap meets, auctions, and private sales rather than pet shops. Such settings are largely unregulated and lack disease prevention and control methods such as quarantine or veterinary care and may contribute to species reduction. Currently, 22 states ban or regulate certain exotic pets.
Reptiles such as turtles and iguanas are popular pets (Figure 10-5 ). However, as subclinical carriers of Salmonella, they have been the source of many human illnesses.8 A 1975 FDA ban on the sale of turtles with a carapace less than 4 inches is estimated to have prevented more than 100,000 infections.
Figure 10-5.

Green iguana.
(From Mitchell M, Tully TN Jr: Manual of exotic pet practice, St Louis, 2008, Saunders Elsevier.)
The importation, trade, and ownership of wild-caught and exotic animals for pets enhances the risk of dissemination and transmission of novel, rare (hantavirus, rabies, lymphocytic choriomeningitis virus), emerging, and exotic (Ebola, monkeypox, Nipah virus) pathogens. Human beings, domestic animals, and native wildlife are at risk.9 Serious injuries are also a risk when handling wild or exotic animals because captivity and human companionship do not change natural behaviors or tame these animals. Many nonhuman primate pets have all their teeth removed but can still injure handlers.10
In addition to the zoonotic disease risk, some exotic and native wildlife species kept as pets are venomous and pose envenomation risks to other animals and pet owners (see Chapter 8).11 Exotic venomous reptiles may produce venom for which antivenin may be not widely available.
INFECTIONS LINKED TO EXOTIC AND WILDLIFE PETS
Not surprisingly, the international trade in animals and the use of wildlife as pets have been linked to a number of zoonoses and other animal diseases. Table 10-5 shows documented examples of exotic and wildlife pets and diseases associated with them. Clearly the potential exists for injuries (trauma, envenomation), infections (viruses, bacteria, parasites, fungi) and allergies, although not specifically documented in the literature as a pet encounter.
Table 10-5.
Reported Infections Related to Nontraditional Pets
| Nontraditional Pet Species | Associated Disease | Affected Species |
|---|---|---|
| Hedgehogs (Atelerix albiventris)12 | Salmonellosis, dermatophytes, herpesvirus, yersiniosis, mycobacteriosis | Human beings |
| Leopard tortoises (Geochelone pardalis)13 | Heartwater (found in Amblyomma ticks on tortoises) | Cattle |
| Various reptiles and amphibians14., 15., 16. | Salmonellosis | Human beings |
| Gambian rat (Cricetomys spp.) | Monkeypox | Human beings, prairie dogs |
| Prairie dogs (Cynomys spp.)17,18 | Tularemia, monkeypox | Human beings |
| Southern flying squirrel (Glaucomys volans)19 | Leptospirosis | Human beings |
| Marmosets (Callithrix jacchus)20 | Rabies | Human beings |
| Macaque monkeys (Macaca spp.)21 | Herpes B | Human beings |
| Egyptian rousette bat (Rousettus egyptiacus)22 | Lagos bat lyssa virus | Human beings |
These cases of reported disease illustrate the complexity of the issues surrounding nontraditional pets. The 1994 outbreak of Salmonella associated with African pygmy hedgehogs (Figure 10-6 ) involved captive-bred animals. Importation of hedgehogs from Africa had been banned since 1991 because of concern about possible importation of foot and mouth disease.23 Other exotic animals linked to salmonellosis include a komodo dragon at a zoo that infected 65 persons, mainly children, with contact to a temporary barrier around the exhibit.24
Figure 10-6.

African hedgehog (Atelerix albiventris).
(From Mitchell M, Tully TN Jr: Manual of exotic pet practice, St Louis, 2008, Saunders Elsevier.)
Spurred and leopard tortoises imported to Florida from Africa have been found to be infested with ticks of the genus Amblyomma, which can be a vector for heartwater, a serious livestock disease caused by the bacterium Cowdria ruminantium.25 As a result, these African tortoises are currently banned in the United States. More recently, African vipers have been diagnosed with severe and even fatal tickborne disease related to a Cowdria-like organism.26
The 2003 multistate outbreak of monkeypox has received significant scientific and media attention and has a number of instructive points about imported zoonotic pathogens of wildlife. The outbreak was traced to the importation of wild-caught giant Gambian pouched rats (Cricetomys gambianus; Figure 10-7 ) and other rodents that had been recently imported from Africa for the pet trade.
Figure 10-7.

Gambian pouched rat.
(From Wikipedia, http://en.wikipedia.org/wiki/Gambian_Pouch_Rat.)
Some of these exotic rodents were carriers of the monkeypox virus. When housed in dealer facilities, they were caged close to a susceptible native wildlife animal species, black-tailed prairie dogs (Cynomys ludovicianus). These prairie dogs then were sold as pets, and in the process 71 human beings, including pet owners, pet store workers, distributors, and veterinarians, contracted infection through contact with sick animals or their secretions (Figure 10-8 ).27., 28., 29. Figure 10-9 shows the complex web of contact and distribution that resulted in human cases. The outbreak did not produce human fatalities but clearly showed the potential for a novel pathogen to cause outbreaks in the United States as a result of the global pet trade.
Figure 10-8.

Monkeypox.
(From the Centers for Disease Control and Prevention, Atlanta, Ga.)
Figure 10-9.
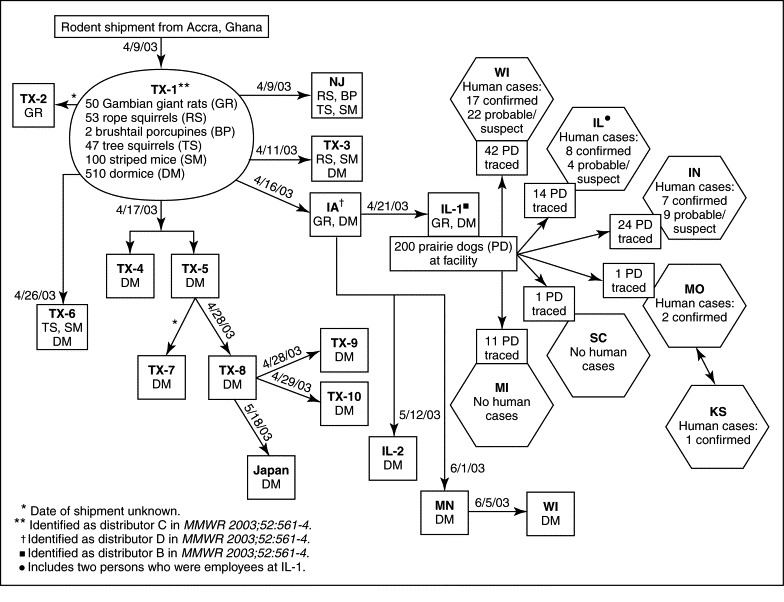
Movement of imported African rodents to animal distributors and distribution of prairie dogs from an animal distributor associated with human cases of monkeypox in 11 states (Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, New Jersey, South Carolina, Texas, and Wisconsin) as of July 8, 2003. Japan is included among the locations having received shipment of rodents implicated in this outbreak. (This does not include one probable human case from Ohio.)
(From Centers for Disease Control and Prevention: Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003, MMWR Morb Mortal Wkly Rep 52:642, 2003.)
Examples of native wildlife used as pets and infecting their owners include a wild-caught prairie dog transmitting tularemia to an owner.18 In Brazil, a new rabies virus variant was identified in human cases associated with pet marmosets (Callithrix jacchus).20
CONTROL EFFORTS
The magnitude and diversity of animal species being maintained as pets and the ongoing potential for novel and rare zoonoses present challenges to health care providers and public health professionals. It appears that reduction of the rate of introduced pathogens can only be achieved by everyone at every level working on some aspect of the prevention and control—from local awareness of the problem to policy changes on a national or international level that restrict the trade in live animals.2 NASPHV has joined with CSTE in developing position statements requesting a federal interagency work group to address the risks posed by the exotic animal trade (see Developing Importation and Importation Restrictions on Exotic and Native Wildlife with Potential Adverse Impact on Public Health, available online at http://www.cste.org/PS/2003pdfs/03-ID-13%20-%20FINAL.pdf).30 In particular, NASPHV has called for the creation of an “approved species” list to reduce the threat of disease importation, more extensive inspection and quarantine of imported animals, and tracking of animals that have been imported. The AVMA is also on record discouraging exotic animals and wildlife as pets.31 Although CDC regulations govern the importation of dogs, cats, turtles with a carapace of less than 4 inches, monkeys, bats, civets, birds from countries with H5N1 influenza, several species of African rodents, and animal products capable of causing human disease,32 only a small number of species (including the African rodents linked to the importation of monkeypox) are currently banned. In addition, most of the animals that are imported, with the exception of avian species, are neither quarantined nor tested for infectious disease agents before entering the country.
NASPHV has developed guidelines titled “Compendium of Measures to Prevent Disease Associated with Animals in Public Settings” to prevent spread of infections at public settings where animal contact could take place, including animal displays, petting zoos, animal swap meets, pet stores, zoological institutions, nature parks, circuses, carnivals, farm tours, livestock-birthing exhibits, county or state fairs, schools, and wildlife photo opportunities.5 Key recommendations of these guidelines, as well as other recently published recommendations to reduce the risk of diseases related to nontraditional pets, are shown in Box 10-4 .
BOX 10-4.

GUIDELINES FOR PREVENTION OF HUMAN DISEASES FROM NONTRADITIONAL PETS AT HOME AND EXPOSURE TO ANIMALS IN PUBLIC SETTINGS
Rights were not granted to include this box in electronic media. Please refer to the printed book.
Adapted from Pickering LK, Marano N, Bocchini JA et al: Committee on infectious diseases: exposure to nontraditional pets at home and to animals in public settings: risks to children, Pediatrics 122:876, 2008.
© 2010
References
- 1.Rosen T., Jablon J. Infectious threats from exotic pets: dermatological implications. Dermatol Clin. 2003;21(2):229–236. doi: 10.1016/s0733-8635(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins PT., Genovese K., Ruffler H. Broken screens: the regulation of live animal importation in the United States. Defenders of Wildlife; Washington DC: 2007. [Google Scholar]
- 3.Karesh WB., Cook RA., Bennett EL. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005;11(7):1000–1002. doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering LK., Marano N., Bocchini JA. Committee on Infectious Diseases: Exposure to nontraditional pets at home and to animals in public settings: risks to children. Pediatrics. 2008;122(4):876–886. doi: 10.1542/peds.2008-1942. [DOI] [PubMed] [Google Scholar]
- 5.National Association of State Public Health Veterinarians Compendium of measures to prevent disease associated with animals in public settings, 2007. MMWR Recomm Rep. 2007;56(RR-5):1–14. [PubMed] [Google Scholar]
- 6.Kuehn BM. Wildlife pets create ethical, practical challenges for veterinarians. J Am Vet Med Assoc. 2004;225(2):171–173. [PubMed] [Google Scholar]
- 7.Reaser JK., Clark EE., Jr, Meyers NM. All creatures great and minute: a public policy primer for companion animal zoonoses. Zoonoses Public Health. 2008;55(8–10):385–401. doi: 10.1111/j.1863-2378.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Reptile-associated salmonellosis—selected states, 1998–2002. MMWR Morb Mortal Wkly Rep. 2003;52(49):1206–1209. [PubMed] [Google Scholar]
- 9.Marano N., Arguin PM., Pappaioanou M. Impact of globalization and animal trade on infectious disease ecology. Emerg Infect Dis. 2007;13(12):1807–1809. doi: 10.3201/eid1312.071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson-Delaney CA. Safety issues in the exotic pet practice. Vet Clin North Am: Exot Anim Pract. 2005;8(3):515–524. doi: 10.1016/j.cvex.2005.05.001. vii. [DOI] [PubMed] [Google Scholar]
- 11.Peterson ME. Toxic exotics. Vet Clin North Am: Exot Anim Pract. 2008;11(2):375–387. doi: 10.1016/j.cvex.2007.12.003. vii–viii. [DOI] [PubMed] [Google Scholar]
- 12.Riley PY., Chomel BB. Hedgehog zoonoses. Emerg Infect Dis. 2005;11(1):1–5. doi: 10.3201/eid1101.040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burridge MJ., Simmons LA., Simbi BH. Evidence of Cowdria ruminantium infection (heartwater) in Amblyomma sparsum ticks found on tortoises imported into Florida. J Parasitol. 2000;86(5):1135–1136. doi: 10.1645/0022-3395(2000)086[1135:EOCRIH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Nagano N., Oana S., Nagano Y. A severe Salmonella enterica serotype Paratyphi B infection in a child related to a pet turtle, Trachemys scripta elegans. Jpn J Infect Dis. 2006;9(2):132–134. [PubMed] [Google Scholar]
- 15.Schroter M., Roggentin P., Hofmann J. Pet snakes as a reservoir for Salmonella enterica subsp. diarizonae (serogroup IIIb): a prospective study. Appl Environ Microbiol. 2004;70(1):613–615. doi: 10.1128/AEM.70.1.613-615.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene S., Yartel A., Moriarty K. Salmonella kingabwa infections and lizard contact, United States, 2005. Emerg Infect Dis. 2007;13(4):661–662. doi: 10.3201/eid1304.060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarner J., Johnson BJ., Paddock CD. Veterinary Monkeypox Virus Working Group: Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10(3):426–431. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avashia SB., Petersen JM., Lindley CM. First reported prairie dog-to-human tularemia transmission, Texas, 2002. Emerg Infect Dis. 2004;10(3):483–486. doi: 10.3201/eid1003.030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomel BB. Wildlife zoonoses. Michigan Veterinary Medical Association. http://www.michvma.org/documents/MVC%20Proceedings/Chomel.pdf
- 20.Favoretto SR., de Mattos CC., Morais NB. Rabies in marmosets (Callithrix jacchus), Ceara, Brazil. Emerg Infect Dis. 2001;7(6):1062–1065. doi: 10.3201/eid0706.010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski SR., Leslie MJ., Parrott T. B-virus from pet macaque monkeys: an emerging threat in the United States? Emerg Infect Dis. 1998;4(1):117–121. doi: 10.3201/eid0401.980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chomel BB., Belotto A., Meslin FX. Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis. 2007;13(1):6–11. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention African pygmy hedgehog–associated salmonellosis—Washington, 1994. MMWR. 1995;44(24):462–463. [PubMed] [Google Scholar]
- 24.Friedman CR., Torigian C., Shillam PJ. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. J Pediatr. 1998;132:802–807. doi: 10.1016/s0022-3476(98)70307-5. [DOI] [PubMed] [Google Scholar]
- 25.Burridge MJ., Simmons LA., Allan SA. Introduction of potential heartwater vectors and other exotic ticks into Florida on imported reptiles. J Parasitol. 2000;86(4):700–704. doi: 10.1645/0022-3395(2000)086[0700:IOPHVA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Kiel JL., Alarcon RM., Parker JE. Emerging tick-borne disease in African vipers caused by a Cowdria-like organism. Ann N Y Acad Sci. 2006;1081:434–442. doi: 10.1196/annals.1373.062. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds MG., Davidson WB., Curns AT. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13(9):1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croft DR., Sotir MJ., Williams CJ. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg Infect Dis. 2007;13(8):1150–1157. doi: 10.3201/eid1308.061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Update: multistate outbreak of monkeypox-Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(27):642–646. [PubMed] [Google Scholar]
- 30.National Association of State Public Health Veterinarians. Council of State and Territorial Epidemiologists: Joint statement: developing importation and exportation restrictions on exotic and native wildlife with potential adverse impact on public health, 2003http://www.cste.org/PS/2003pdfs/03-ID-13%20-%20FINAL.pdf. Accessed August 30, 2008.
- 31.American Veterinary Medical Association. Birds, exotics and wild animalshttp://www.avma.org/careforanimals/animatedjourneys/petselection/birds.asp.
- 32.Centers for Disease Control and Prevention. Global migration and quarantinehttp://www.cdc.gov/ncidod/dq/animal/index.htm. Accessed October 23, 2009.
IMMUNOCOMPROMISED INDIVIDUALS
Available evidence continues to suggest that the psychosocial support value of companion animals, particularly for the elderly or infirm,1., 2., 3. outweighs the risk of acquiring a serious infection from such animals. Nevertheless, issues regarding hygiene and common sense practices must be addressed to support a healthy human-animal bond, especially among immunocompromised people or pets.
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
-
•
Provide public health guidance to reduce opportunistic infections among immunocompromised persons.
-
•
Stress general hygiene principles, including handwashing, and ensure proper food preparation and safe water supplies.
Human Health Clinicians
-
•
Understand the risks and considerable benefits of companion animal ownership among immunocompromised persons.
-
•
Ensure that a thorough history is taken to best manage the patient's potential exposure to zoonotic disease.
-
•
Be aware of the Guidelines for Preventing Opportunistic Infections among HIV-Infected Persons: Recommendations of the US Public Health Service and the Infectious Diseases Society of America (http://www.annals.org/cgi/content/full/137/5_Part_2/435).
-
•
With an immunocompromised patient's permission, consider coordinating preventive interventions with the patient's veterinarian.
-
•
Counsel immunocompromised patients with occupational exposure to animals about zoonotic infection risk and risk reduction measures.
Veterinary Clinicians
-
•
Provide consultation about zoonotic disease risk reduction.
-
•
Provide guidance on maintaining the health of immunocompromised animal patients, including a healthy environment.
-
•
Maintain confidentiality of information regarding immunocompromised persons.
ETIOLOGY OF IMMUNOSUPPRESSION
Millions of people and companion animals in this country are living with less than a robust immune system. Immunosuppression can result from a number of etiologies, either from a primary or genetic malfunction (rare) or, more commonly, as a result of a secondary or acquired factor such as debilitation, immunosuppressive chemotherapy (Figure 10-10 ), human immunodeficiency virus (HIV) in human beings, or feline leukemia virus (FeLV) in cats (Table 10-6 ). In general, defects in humoral immunity (B-cell lines) can lead to increased susceptibility to bacterial infections; cell-mediated immunity (T-cell lines) defects to viral, fungal, or protozoal infections; and defects of phagocytosis or the complement system to disseminated infections.
Figure 10-10.

Alopecia in a 7-year-old Schnauzer undergoing doxorubicin and dacarbazine chemotherapy.
(From Couto CG: Complications of cancer chemotherapy. In Nelson RW, Couto CG (eds): Small animal internal medicine, ed 4, St Louis, 2009, Mosby Elsevier.)
Table 10-6.
Etiologies of Immunocompromise in Human Beings and Other Animals
| Examples (Breed Predilection) | |
|---|---|
| Common to All Species | |
| Chemicals | Immunosuppressive medication (e.g., corticosteroids, cyclosporin, 6-mercaptopurine, methotrexate, azathioprine), mercury, PCBs |
| Radiation | Radiation therapy, excess radiation exposure |
| Neoplasia | Leukemia, other cancers |
| Other causes | Severe malnutrition, chronic diabetes, renal/hepatic/splenic failure, prematurity, advancing age |
| Human Beings | |
| Primary (genetic) | X-linked agammaglobulinemia, X-linked hyper-IgM syndrome, Wiskott-Aldrich syndrome, Ataxia telangiectasia, chronic granulomatous disease, SCID |
| Infections | HIV, other severe infections |
| Dogs | |
| Primary (genetic)4 |
|
| Chemicals | Organophosphate toxicity6 |
| Cats | |
| Primary (genetic) | Chediak-Higashi syndrome (Persian cats) |
| Infectious | FIV/FeLV |
| Horses | |
| Primary (genetic) | SCID (Arabians) |
PCBs, Polychlorinated biphenyls; Ig, immunoglobulin; SCID, severe combined immune deficiency.
IMPACT OF IMMUNODEFICIENCY STATES ON ANIMAL-HUMAN DISEASE TRANSMISSION
A large proportion of American households include pets,7 and pet ownership among immunocompromised persons is common. For example, studies of patients with HIV infection have reported rates of pet ownership similar to that of the general population, with approximately half owning or living with pets.8 In addition, on a global level, the HIV/AIDS pandemic has created large populations of individuals with compromised immune systems, many of whom may also be exposed to zoonotic diseases.9, 10 Human and veterinary clinicians are quite likely to encounter situations in which they may provide appropriate guidance for reducing animal sources of infectious diseases for human beings and vice versa by educating themselves and staff to provide the best available information.
There are at least three possible effects of human immunodeficiency on animal-human disease transmission:
-
1.
An immunocompromised host is more susceptible to infection with opportunistic disease.
-
2.
An immunocompromised host may transmit opportunistic disease to others.
-
3.
A disease may be more severe in an immunocompromised host (e.g., toxoplasmosis, which causes asymptomatic or mild disease in most immunocompetent patients but can cause severe and even fatal systemic disease in immunocompromised individuals).
The knowledge level among immunocompromised persons and their health care providers about the risk of acquiring infections from pets could be increased with an appropriate educational strategy. In one study in which more than 400 patients with AIDS were interviewed—half of whom owned pets—only about 10% who were living with pets were given any information about zoonotic diseases from their health care provider, and one quarter of this information was incorrect or misunderstood (e.g., “fleas can give you rabies” or “cats can give you AIDS”).8 Because pet ownership is as common (and understanding of zoonoses as uncommon) among persons living with HIV as in the general population, human health care providers must be prepared to discuss with immunocompromised patients the risks of living with pets. Overly conservative approaches, including physician recommendations for their patients to relinquish pets, have largely been unheeded as owners often have strong bonds with their animals.11 Armed with complete and accurate information, patients and their care providers can weigh these risks against the often substantial benefits of love, touch, social support, and companionship that accrue to pet owners. Zoonotic disease prevention is a shared responsibility among human, veterinary, and public health professionals. Improving communication among these persons will enhance zoonotic disease prevention.12
HUMAN HEALTH CARE PROVIDERS
Pet-owning human health care providers must be vigilant regarding nosocomial zoonoses, particularly if they are working with immunocompromised patients. In one situation, a common yeast pathogen of canine otitis externa (Figure 10-11 ) was introduced into a neonatal intensive care unit by a dog-owning health care worker, causing colonization in infants, some resulting in serious infections.13, 14 In another case, a cat on a geriatric ward was the likely suspect of staphylococcal infections (see Chapter 5).15
Figure 10-11.

Otitis externa in a dog. Brown, waxy exudate with a secondary yeast infection associated with an underlying allergy.
(From Medleau L, Hnilica KA: Small animal dermatology: a color atlas and therapeutic guide, ed 2, St Louis, 2006, Saunders Elsevier.)
GENERAL GUIDELINES FOR THE PREVENTION OF ZOONOTIC DISEASE IN IMMUNOCOMPROMISED PATIENTS
Guidance From Both Human Health and Veterinary Health Care Providers
Hand and food hygiene is vital (Figures 10-12 and 10-13 ). Pets are not likely to be the most common source of zoonotic disease infection. More likely, contact with raw or undercooked meat or from an environmental infectious source is implicated in transmission. Unequivocally, immunocompromised individuals should avoid raw meat and eggs and unpasteurized dairy products. For example, although cats are the definitive host for Toxoplasma gondii, undercooked meat (less than 165 ° F) and inadequately washed, contaminated fruits and vegetables are likely the source for infection. See USDA food safety fact sheets: http://www.fsis.usda.gov/Factsheets/Keep_Food_Safe_Food_Safety_Basics/index.asp and http://www.fsis.usda.gov/Factsheets/At_Risk_&_Underserved_Fact_Sheets/index.asp.
Figure 10-12.

Handwashing is critical to disease prevention.
(From Centers for Disease Control and Prevention Public Health Image Library, Atlanta, Ga. Photo courtesy Kelly Thomas.)
Figure 10-13.

Pregnant women and immunosuppressed patients can be at greater risk in acquiring foodborne illness and need to take additional precautions when handling raw food products. Cook meat, poultry, and eggs thoroughly. Using a thermometer to measure the internal temperature of meat is a good way to be sure that it is cooked sufficiently to kill bacteria. For example, ground beef should be cooked to an internal temperature of 160 ° F. Eggs should be cooked until the yolk is firm.
(From Centers for Disease Control and Prevention Public Health Image Library. Photo courtesy James Gathany.)
Serologic or fecal evaluation of healthy cats for Toxoplasma infection is not recommended. Oocysts are shed transiently and are easily missed, and serologic evaluation does not predict cats shedding oocysts. Instead, prevention of transmission of Toxoplasma directly from cats should focus on the following husbandry recommendations:
-
•
Avoid contact with feces (have an immunocompetent person clean the litterbox or fecal accidents, or wear gloves followed by washing hands) (Figure 10-14 ).
-
•
Pets should not be given prophylactic antibiotics without clinical signs of infection. For example, Salmonella carriage in reptiles cannot be eliminated. Use of antibiotics for this purpose has been unsuccessful and may favor development of antibiotic-resistant bacteria.16
-
•
Any bite or wound from an animal should be flushed with copious amounts of soap and water and a health care provider should be contacted for wound management, including assessment for appropriate antibiotics, tetanus, and possible rabies postexposure prophylaxis (see Animal and Human Bites later in this chapter).
-
•
Review guidance to reducing exposure to selected opportunistic diseases among persons with HIV (see http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5108a1.htm).
-
•
Encourage questions about healthy living with companion animals and discourage kissing or face-to-face contact.
-
•
Pet vaccines, including live attenuated strains, should be recommended and given as needed; they are not believed to present a human health hazard.17
Figure 10-14.

Have an immunocompetent person clean the litterbox.
(From Bunch SE: The exocrine pancreas. In Nelson RW, Couto CG (eds): Small animal internal medicine, ed 3, St Louis, 2003, Mosby Elsevier.)
Occupational or Recreational Risk Reduction
-
•
Avoid contact with wild animals to reduce the risk of enteric disease, such as acquiring Cryptosporidium from wild birds.
-
•
NEVER touch the feces of any animal.
-
•
Avoid farm animals and petting zoos to decrease exposure to Escherichia coli in ruminants, Bordetella bronchiseptica in swine, and Salmonella in chicks and ducks.
-
•
Occupational health care workers working with high-risk personnel such as veterinarians, veterinary staff, zookeepers, and pet shop workers should counsel immunocompromised workers about the risk of occupational of zoonotic disease infection and possible work restrictions to reduce risk for those with significant impairment of immunity.
Animal Selection Recommendations
-
•
Select healthy, well-mannered dogs or cats 6 months or older to decrease the likelihood of exposure to enteric diseases and Bartonella from kittens.
-
•
Avoid petting or handling free-roaming animals; when selecting a pet, choose one with a documented veterinary health history and current vaccinations.
-
•
Avoid exotic or wild animals to reduce the likelihood of exposure to emerging infections (i.e., monkeypox in rodents) and known diseases such as herpes B infection from macaque monkeys and Salmonella from reptiles (Figure 10-15 ).
Figure 10-15.

Immunocompromised persons should avoid selecting reptiles as pets, such as this frilled dragon (Chlamydosaurus kingii).
(From Mader DR: Reptile medicine and surgery, ed 2, St Louis, 2006, Saunders Elsevier.)
Cockatoos, like pigeons, may shed Cryptococcus in their feces. Transmission of this infection from a cockatoo to its owner who was chronically immunocompromised because of a renal transplant has occurred.18 Therefore some authors have recommended that immunocompromised patients not own cockatoos (Figure 10-16 ).19
Figure 10-16.

Nodular cutaneous cryptococcosis in an HIV-positive cockatoo owner.
(From Rosen T, Jablon J: Infectious threats from exotic pets: dermatological implications, Derm Clin 21:229, 2003.)
A fatal outbreak of lymphocytic choriomeningitis virus (LCMV) in solid-organ transplant recipients was traced back to a pet hamster acquired by the organ donor 17 days before donation (Figure 10-17 ). The prevalence of this virus in rodent populations has led to recommendations that immunocompromised individuals (as well as pregnant women) should avoid owning pet rodents or having contact with wild or pet rodents.20
Figure 10-17.

Pet rodents have been implicated in fatal LCMV infection in human beings.
(From Sheldon CC, Sonsthagen T, Topel JA: Animal restraint for veterinary professionals, St Louis, 2006, Mosby Elsevier.)
Have a veterinarian conduct a physical examination and fecal analysis on a new pet.
ANIMAL HUSBANDRY GUIDANCE
-
•
Seek veterinary care early in the course of clinical disease of pets to limit chances for zoonotic disease exposure.
-
•
Keep pets indoors or leashed on walks to decrease the likelihood of engagement with other animals.
-
•
Because of occasional cases of Bordetella bronchiseptica among immunocompromised persons,21, 22 avoid exposing dogs or owners to situations in which dogs are congregated, such as boarding kennels, grooming parlors, off-leash dog parks, or dog shows.
-
•
Do not allow pets to hunt, scavenge, or eat feces to reduce the likelihood of exposure to enteric infections.
-
•
Do not feed pets raw meat or egg diets or provide unpasteurized dairy products to limit exposure to enteric infections.
-
•
Do not allow your pet to drink from the toilet.
-
•
Keep animals and litterboxes out of food preparation areas.
-
•
Avoid exposure to pet urine, feces, and saliva (don't allow your pet to lick your face or open lesions).
-
•
Have a nonimmunocompromised household member remove a pet's solid waste and dispose daily by flushing down a toilet or discarding in the garbage or in a compost area (not to be spread on fruits or vegetables).
-
•
Avoid animals with diarrhea. Have an immunocompetent household member clean soiled areas in the house of organic debris, followed by a 1:10 household bleach solution.
-
•
Avoid rough play with the pet that could result in being bitten or scratched; keep pet's nails trimmed short.
-
•
Remove and dispose of bird cage linings daily and use “wet” cleaning for the cage and utensils on a weekly basis. Wear gloves when handling items that are contaminated with bird droppings.
-
•
Have a helper clean the fish tank (Figure 10-18 ) or wear disposable gloves during such activities, followed by washing hands thoroughly by rubbing hands together vigorously for 15 to 20 seconds with running water and soap.
-
•
If assistance is required to care for your pet, contact local volunteer groups who may be willing to provide exercise, food, or foster care (e.g., during hospitalization).
Figure 10-18.

Types of fish tanks.
(From Mitchell M, Tully TN Jr: Manual of exotic pet practice, St Louis, 2008, Saunders.)
U.S. PUBLIC HEALTH SERVICE GUIDELINES FOR PERSONS WHO ARE HIV POSITIVE
Although any zoonotic disease that occurs in immunocompetent individuals can also affect immunocompromised patients, the U.S. Public Health Service has highlighted a number of animal agents that pose a significant risk to HIV-infected persons. These include causes of enteritis (especially Campylobacter, Salmonella, and Cryptosporidium), and Bartonella, Toxoplasma, Histoplasma, and Mycobacterium marinum.
The evidence-based recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America are listed in Table 10-7 .
Table 10-7.
Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons
| Topic | Recommendation | Strength of Recommendation* |
|---|---|---|
| General Pet-Related Recommendations | ||
| Health care providers should advise HIV-infected persons of the potential risk of pet ownership but should not routinely advise HIV patients to part with their pets. | DIII | |
| Seek veterinary care when pet develops diarrhea. | BIII | |
| Animals with diarrhea (and animals <6 months old) should be examined and stool tested for Cryptosporidium, Salmonella, and Campylobacter. | BIII | |
| When obtaining a new pet, avoid animals <6 months (1 year for cats) and animals with diarrhea. Avoid stray animals. | CIII | |
| Wash hands after handling pet, including before eating, and avoid contact with pet feces. Supervise handwashing in HIV-positive children. | BIII | |
| Avoid contact with reptiles (e.g., snakes, lizards, iguanas, turtles) as well as chicks and ducklings to reduce salmonellosis risk. | BIII | |
| Use gloves when cleaning aquarium to reduce risk of Mycobacterium marinum infection. | CIII | |
| Avoid contact with exotic pets (e.g., nonhuman primates). | BII | |
| Avoid exposure to calves and lambs and premises where calves and lambs are raised. | BII | |
| Cat ownership | Discuss with HIV patient and caretakers that cat ownership increases risk of Bartonella and enteric infections. | CIII |
| Patients acquiring a cat should adopt or purchase cat >1 year old and in good health; avoid acquiring a stray cat. | BII | |
| Toxoplasmosis | Wash hands after working in a garden; wash all fruits and vegetables and ensure that raw meats are handled separately from raw food. Clean cat litterbox daily, preferably by HIV-negative, nonpregnant person (use gloves and wash hands afterwards), keep cat indoors, do not allow cat to hunt, do not feed cat undercooked meat. | BIII |
| Testing cats for toxoplasmosis | EII (not recommended) | |
| Bartonella infection | Declawing of cat not usually advised. Avoid activities resulting in bites or scratches. | BII |
| Wash bites or scratches promptly. | CIII | |
| Do not allow cats to lick wounds. | BIII | |
| Flea control for cats. | CIII | |
| Testing cat for Bartonella. | DII (not recommended) | |
| Occupational exposures | Occupations with animal contact may pose risk for cryptosporidiosis, toxoplasmosis, salmonellosis, campylobacteriosis, or Bartonella, but data are insufficient to recommend against patients with HIV working in such settings. | |
| Follow recommendations and protocols for protective equipment and clothing. | BII | |
| Avoid contact with young farm animals, specifically those with diarrhea, to reduce cryptosporidiosis infection. | BIII | |
| Handwashing after gardening or soil contact to reduce risk of cryptosporidiosis and toxoplasmosis. In areas endemic for histoplasmosis, avoid risk activities including soil contact, cleaning chicken coops, disturbing soil beneath bird roosts, demolishing buildings, or cave exploring. | CIII | |
| Birds | Screening healthy birds for Cryptococcus neoformans, Mycobacterium avium, or Histoplasma capsulatum is not recommended. | DIII |
Rating strength of recommendation
A, Both strong evidence for efficacy and substantial clinical benefit support recommendation for use; should always be offered.
B, Moderate evidence for efficacy or strong evidence for efficacy but only limited clinical benefit; supports recommendation for use; should usually be offered.
C, Evidence for efficacy is insufficient to support a recommendation for or against use, or evidence for efficacy might not outweigh adverse consequences (e.g., drug toxicity, drug interactions) or cost of the chemoprophylaxis or alternative approaches; use is optional.
D, Moderate evidence for lack of efficacy or for adverse outcome supports a recommendation against use; should usually not be offered.
E, Good evidence for lack of efficacy or for adverse outcome supports a recommendation against use; should never be offered.
Rating quality of evidence supporting the recommendation
I, Evidence from ≥1 correctly randomized, controlled trials.
II, Evidence from ≥1 well-designed clinical trials without randomization, from cohort or case-controlled analytic studies (preferably from more than one center), or from multiple time-series studies, or dramatic results from uncontrolled experiments.
III, Evidence from opinions of respected authorities based on clinical experience, descriptive studies, or reports of consulting committees.
System used to rate the strength of recommendations and quality of supporting evidence is as follows:
Adapted from Kaplan JE, Masur H, Holmes KK: Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America, MMWR Recomm Rep 51(RR-8):1, 2002.
Although these guidelines recommend that human health care providers counsel their immunocompromised patients about zoonotic disease risk reduction, surveys of physicians have indicated that many believe that veterinarians are best equipped to provide such counseling and should therefore be involved in patient education of immunocompromised individuals.12 Some authorities have stated that veterinarians are more qualified than physicians to advise pet owners and persons in high-risk professions about zoonotic risks.23
PUBLIC HEALTH ROLE OF THE VETERINARIAN: SAFER PET OWNERSHIP FOR IMMUNOCOMPROMISED PERSONS AND CARE OF IMMUNOCOMPROMISED PETS
In the veterinary setting, pet owners may be more willing to request information regarding safer pet ownership for immunocompromised persons if there are, for example, posters and handouts encouraging such client education or information in practice newsletters. Veterinarians can address high-risk persons during general discussions about zoonotic disease diagnosis, control, and prevention. Veterinarians should also emphasize among their staff the need for strict confidentiality regarding any personal information an animal owner happens to disclose about his or her own medical status (it is not recommended, for example, to document human medical information in the veterinary record).
Care for Pets of Immunocompromised Owners
-
•
Neuter pets.
-
•
Provide strict adherence to strategic deworming protocols and maintenance of appropriate vaccinations.
-
•
Be prepared to discuss end-of-life planning for the pet's continued care (http://www.hsus.org/pets/pet_care/guidelines_ for_finding_a_responsible_home_for_a_pet.html).
Care for Immunodeficient Animals
-
•
Do not administer modified live virus vaccines.
-
•
Manage secondary and opportunistic infections (Color Plate 10-5).
-
•
Provide supportive care.
Guidance for Owners of Immunodeficient Animals
-
•
Animals with primary immunodeficiency disorders should not be bred.
-
•
Cats with FIV or FeLV can spread the viruses to other cats, typically by bite wounds or close contact (FeLV) (Color Plate 10-6). Therefore separate household contacts that are FIV/FeLV negative. (All cats with unknown FIV/FeLV status with a bite wound should be tested for these viruses at the time of presentation and again 60 days later.)
-
•
Keep these animals indoors; do not allow pets to hunt, scavenge, or consume raw meat or egg diets or unpasteurized dairy products.
-
•
Wash hands before and after handling the pet.
-
•
Provide appropriate endoparasite and ectoparasite control.
-
•
Avoid exposure to other ill animals.
CASE STUDIES
Case Study 1
A public health veterinarian working as an AIDS surveillance coordinator received a number of questions for which the human-animal bond was never more poignant. Indeed, one was from a gentleman who was recently diagnosed with AIDS and who owned a 4-year-old neutered male Golden Retriever. His family practitioner, concerned about any increased risk for opportunistic infections, advised him to find another home for his beloved pet. This man was heartbroken, trying to gather as much information as possible about what specifically he would face if he ignored his doctor's advice. Fortunately, with his permission, the veterinarian was able to contact his physician to discuss the relatively low risk as well as the benefits of owning a healthy pet (one that was receiving regular veterinary care) and encouraged the doctor to continue to contact public health practitioners for information as well as this pet owner's veterinarian (with permission) about the animal's preventive health care. Additionally, this pet owner happened to live in an area with an active volunteer organization that assisted with walking and feeding pets of persons living with AIDS. Armed with these resources, the patient was able to maintain a healthy pet.
Case Study 2
A public health veterinarian who was also a relief clinical veterinarian was presented with a 7-year-old neutered female cat for toxoplasmosis serologic testing and (reluctant) possible euthanasia of the cat. The cat's owner was the spouse of a cancer patient and was concerned that the spouse was on immunosuppressive chemotherapy and therefore at great risk for toxoplasmosis from their pet. The veterinarian explained the life cycle of toxoplasmosis, the epidemiology in people and cats, and that if the serology was positive it could actually be protective. The owner declined both serologic testing and euthanasia because the cat had never hunted in its lifetime, was indoors only, and was fed an exclusively commercial diet. There was no evidence of mice or rats in the house, decreasing the cat's likelihood of exposure to the parasite. However, the spouse enjoyed gardening—a much greater potential for exposure to toxoplasmosis. Fortunately the patient always wore gloves when doing so and thoroughly washed afterwards. The veterinarian and the cat owner discussed additional toxoplasmosis exposure reduction, including proper handling of raw meats and washing raw foods. The veterinarian and cat owner agreed that the owner would be the person cleaning the litterbox, schedule overall wellness examinations of the cat every 6 months, and would bring the cat in for evaluation and zoonotic disease prevention if diarrhea or signs of respiratory disease developed.
References
- 1.Raina P., Waltner-Toews D., Bonnett B. Influence of companion animals on the physical and psychological health of older people: an analysis of a one-year longitudinal study. J Am Geriatr Soc. 1999;47(3):323–329. doi: 10.1111/j.1532-5415.1999.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 2.Castelli P., Hart LA., Zasloff RL. Companion cats and the social support systems of men with AIDS. Psychol Rep. 2001;89(1):177–187. doi: 10.2466/pr0.2001.89.1.177. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JM., Angulo FJ., Detels R. AIDS diagnosis and depression in the Multicenter AIDS Cohort Study: the ameliorating impact of pet ownership. AIDS Care. 1999;11:157–170. doi: 10.1080/09540129948054. [DOI] [PubMed] [Google Scholar]
- 4.Tilley LP., Smith FWK. Blackwell's five-minute veterinary consult: canine and feline. 3rd ed. Blackwell; Ames, IA: 2004. [Google Scholar]
- 5.Day MJ. World Small Animal Veterinary Association World Congress Proceedings. 2004. Immunodeficiency disease in the dog.http://www.vin.com/proceedings/Proceedings.plx?CID=WSAVA2004&PID=8598&O=Generic Accessed May 3, 2009. [Google Scholar]
- 6.Sykora B., Tomsikova A. Kaposi's sarcoma in dog with acquired immunodeficiency after phosphate poisoning. Folia Microbiol (Praha) 1998;43(5):543–544. doi: 10.1007/BF02820816. [DOI] [PubMed] [Google Scholar]
- 7.American Veterinary Medical Association. Market research statistics: U.S. pet ownership—2007http://www.avma.org/reference/marketstats/ownership.asp
- 8.Conti L., Lieb S., Liberti T. Pet ownership among persons with AIDS in three Florida counties. Am J Public Health. 1995;85(11):1559–1561. doi: 10.2105/ajph.85.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etter E., Donado P., Jori F. Risk analysis and bovine tuberculosis, a re-emerging zoonosis. Ann N Y Acad Sci. 2006;1081:61–73. doi: 10.1196/annals.1373.006. [DOI] [PubMed] [Google Scholar]
- 10.Feldman RL., Nickell K. Avian influenza: potential impact on sub-Saharan military populations with high rates of human immunodeficiency virus/acquired immunodeficiency syndrome. Mil Med. 2007;172(7):753–758. doi: 10.7205/milmed.172.7.753. [DOI] [PubMed] [Google Scholar]
- 11.Angulo FJ., Glaser CA., Juranek DD. Caring for pets of immunocompromised persons. J Am Vet Med Assoc. 1994;205(12):1711–1718. [PubMed] [Google Scholar]
- 12.Grant S., Olsen CW. Preventing zoonotic diseases in immunocompromised persons: the role of physicians and veterinarians. Emerg Infect Dis. 1999;5(1):159–163. doi: 10.3201/eid0501.990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HJ., Miller HL., Watkins N. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706–711. doi: 10.1056/NEJM199803123381102. [DOI] [PubMed] [Google Scholar]
- 14.Morris DO. Malassezia pachydermatis carriage in dog owners. Emerg Infect Dis. 2005;11(1):83–88. doi: 10.3201/eid1101.040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus LC., Marcus E. Nosocomial zoonoses. N Engl J Med. 1998;338(11):757–759. doi: 10.1056/NEJM199803123381110. [DOI] [PubMed] [Google Scholar]
- 16.Bradley T., Angulo FJ., Raiti P. Association of Reptilian and Amphibian Veterinarians guidelines for reducing risk of transmission of Salmonella spp. from reptiles to humans. J Am Vet Med Assoc. 1998;213:51–52. [PubMed] [Google Scholar]
- 17.Hemsworth S., Pizer B. Pet ownership in immunocompromised children—a review of the literature and survey of existing guidelines. Eur J Oncol Nurs. 2006;10(2):117–127. doi: 10.1016/j.ejon.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Nosanchuk JD., Shoham S., Fries BC. Evidence of zoonotic transmission of Cryptococcus neoformans from a pet cockatoo to an immunocompromised patient. Ann Intern Med. 2000;132(3):205–208. doi: 10.7326/0003-4819-132-3-200002010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Rosen T., Jablon J. Infectious threats from exotic pets: dermatological implications. Dermatol Clin. 2003;21(2):229–236. doi: 10.1016/s0733-8635(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 20.Amman BR., Pavlin BI., Albarino CG. Pet rodents and fatal lymphocytic choriomeningitis in transplant patients. Emerg Infect Dis. 2007;13(5):719–725. doi: 10.3201/eid1305.061269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz DM., Bechara RI., Wolfenden LL. An unusual cause of cough and dyspnea in an immunocompromised patient. Chest. 2007;131(5):1599–1602. doi: 10.1378/chest.06-1541. [DOI] [PubMed] [Google Scholar]
- 22.Trevejo RT., Barr MC., Robinson RA. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet Res. 2005;36:493–506. doi: 10.1051/vetres:2005011. [DOI] [PubMed] [Google Scholar]
- 23.Nowotny N., Deutz A. Preventing zoonotic diseases in immunocompromised persons: the role of physicians and veterinarians. Emerg Infect Dis. 2000;6(2):208. doi: 10.3201/eid0602.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
ANIMAL AND HUMAN BITES
Each year in the United States several million persons are bitten by animals. Although up to 80% of bites may never be reported, more than 300,000 emergency department visits occur each year for dog bites alone.1 Approximately half of reported animal bite victims are children. Dogs are responsible for the majority of reported animal bite injuries to human beings in the United States, followed by cats and rodents.2 Bites from human beings are rarer. With the increasing popularity of exotic pets, patients may present to emergency departments with bites from a wide range of species. In addition to trauma, infection (Color Plate 10-7) and envenomation (see Chapter 8) can be major concerns with animal bites. Animal bites can also result in allergy and anaphylaxis, which might be immediate or delayed (e.g., anaphylaxis has been reported after rodent bites and horse bites, even when the patient had a history of only mild allergic symptoms in the past3, 4). In addition, the victim and/or animal owner might experience psychological trauma associated with either the bite incident itself or the consequent decisions that must be made regarding the biting animal. Animal (especially dog) bite fatalities in human beings are uncommon but occur at the rate of 1 to 2 dozen each year in the United States.5
Animal bites are common in companion animals and livestock as well (Figure 10-19 ), and fatalities in animals can occur if they are not systematically reported. The initial care and treatment of animals bitten by other animals is similar to that for animal bites in human beings.
Figure 10-19.

A, This goat was attacked by dogs. Note bite wound on the ventral neck area. B, At postmortem, the skin has been removed to show the extensiveness of the injury; note tracheal defects and muscle lacerations.
(From Fubini SL, Ducharme N: Farm animal surgery, St Louis, 2004, Saunders Elsevier. Courtesy John King, Cornell University.)
The optimal prevention and treatment of animal bites require good communication among veterinary, human health, and public health professionals. Often the veterinarian (and sometimes a zoologist) must provide critical information about the animal source of the bite, such as rabies risk status, whereas the public health professional may become involved in issues such as rabies prophylaxis and animal quarantine. As discussed in Chapter 12, animal bites are a major occupational risk of veterinarians, their staff, and other animal workers. This section covers key aspects of management and prevention of animal bites as well as bites by human beings.
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
-
•
Be aware of rabies risk in the community and be available for consultation with clinicians on postexposure prophylaxis.
-
•
Provide public education to avoid feeding or handling wild or stray animals.
-
•
Support community animal control efforts.
-
•
Educate families to never leave a child alone with an animal.
-
•Be aware of educational resources for animal bite prevention, such as:
-
•Humane Society of the U.S. (HSUS): http://www.hsus.org/pets/pet_care/dog_care/stay_dog_bite_free/index.html
- •
- •
-
•US Postal Service: http://www.usps.com/communications/community/dogbite.htm.
-
•
BOX 10-5. CDC RECOMMENDATIONS FOR DOG-BITE PREVENTION.
Things to Consider Before You Get a Dog
-
•
Consult a professional (e.g., veterinarian, animal behaviorist, or responsible breeder) to learn about suitable breeds of dogs for your household.
-
•
Dogs with histories of aggression are inappropriate in households with children.
-
•
Be sensitive to cues that a child is fearful or apprehensive about a dog and, if so, delay acquiring a dog.
-
•
Spend time with a dog before buying or adopting it. Use caution when bringing a dog into the home with an infant or toddler.
-
•
Spay or neuter virtually all dogs (this frequently reduces aggressive tendencies).
-
•
Never leave infants or young children alone with any dog.
-
•
Do not play aggressive games with your dog (e.g., wrestling).
-
•
Properly socialize and train any dog entering the household. Teach the dog submissive behaviors (e.g., rolling over to expose abdomen and relinquishing food without growling).
-
•
Immediately seek professional advice (e.g., from veterinarians, animal behaviorists, or responsible breeders) if the dog develops aggressive or undesirable behaviors.
Preventing Dog Bites
-
•
Teach children basic safety around dogs and review regularly.
-
•
Do not approach an unfamiliar dog.
-
•
Do not run from a dog and scream.
-
•
Remain motionless (e.g., “be still like a tree”) when approached by an unfamiliar dog.
-
•
If knocked over by a dog, roll into a ball and lie still (e.g., “be still like a log”).
-
•
Do not play with a dog unless supervised by an adult.
-
•
Immediately report stray dogs or dogs displaying unusual behavior to an adult.
-
•
Avoid direct eye contact with a dog.
-
•
Do not disturb a dog who is sleeping, eating, or caring for puppies.
-
•
Do not pet a dog without allowing it to see and sniff you first.
-
•
If bitten, immediately report the bite to an adult.
From Centers for Disease Control and Prevention: Dog bite prevention. http://www.cdc.gov/ncipc/duip/biteprevention.htm.
Human Health Clinicians
-
•
Provide information on bite prevention to patients with animals and families with children.
-
•
Counsel pregnant women and immunocompromised persons to avoid contact with rodents.
-
•
Counsel patients on first-aid procedures to be taken in the event of a bite injury and to seek medical care for all bites.
-
•
In caring for animal bite injuries, take a complete history regarding the animal, circumstances of the bite, history of allergies, and rabies and tetanus risk. Documentation that includes a drawing or photograph of the wound may be helpful because of legal implications.
-
•
Systematically evaluate the need for antibiotic, rabies, and tetanus prophylaxis, consulting with veterinary and public health professionals as necessary.
-
•
Consider allergic, envenomation, and posttraumatic stress complications of an animal bite.
-
•
Consider whether other family members or other human beings are at risk.
-
•
Consult public health officials regarding the need for rabies postexposure prophylaxis in particular bite situations.
Veterinary Clinicians
-
•
Counsel pet owners to appropriately socialize and train pets to be well mannered.
-
•
Counsel clients and staff on proper handling precautions to minimize the risk of animal bites to human beings (see Box 10-5).
-
•
Counsel clients and staff on proper first-aid to minimize the risk of animal bite infection.
-
•
When dealing with animal behavioral problems such as aggression, fears, and phobias, assess risk of bites to human beings and whether modifications to the animal environment or handling practices are indicated or whether animal should be euthanized.
-
•
Assist human health care providers in indentifying specific zoonotic disease risks from particular animal species.
-
•
Neuter pets and practice preventative medicine (e.g., ensure all dogs, cats, and ferrets are current with rabies vaccination).
-
•
Counsel cat owners to keep their pets indoors and dog owners to disallow their pets to hunt or garbage feed.
-
•
Provide booster rabies vaccine to pets with animal bite wounds from known or suspected rabid animals and work with local public health officials concerning management for potential rabies exposure.
-
•
Test pet cats with bite wounds for FeLV/FIV at time of presentation and again 6 months later.6
MANAGEMENT OF ANIMAL BITE INJURY IN HUMAN BEINGS
Prevention
Many animal bites can be prevented through avoidance of high-risk situations. Table 10-8 lists some risk factors for human animal bites from dogs and cats. These include factors affecting animal aggressiveness, characteristics of bitten human beings, and characteristics of injury events. Risk factors for animal aggressiveness include female gender in cats and male gender (especially unneutered) in dogs as well as particular dog breeds. Human victims of dog bites tend to be younger than victims of cat bites. Bite injuries are often associated with particular types of aggressive behavior in the biting animal. These include dominance aggression, in which an animal assert its social dominance over another animal (such as a child) that it perceives as being weaker (e.g., members of a wolf pack) and possessive aggression, in which an animal attacks to prevent an object such as a toy or food being taken away.
Table 10-8.
Epidemiologic Characteristics Associated With Dog- and Cat-Related Bites or Scratches
| Characteristics of Cats and Dogs Exhibiting Aggression | ||
|---|---|---|
| Cats | Dogs | |
| Age | Insufficient data | <5 years (49%) |
| Sex | Female (67%) | Male (70%-79%) |
| Ownership status | Stray (57%) | Owned (a significant number of these aggressive dogs live in a household with at least one child) |
| Reproductive status | Insufficient data | Intact (not neutered) |
| Size | Insufficient data | Large dogs (>50 lb) |
| Breed | Insufficient data | Total annual number of bites: mixed breed and German Shepherds |
| Bite rates: German Shepherds and Chow-Chows | ||
| Highest rate of severe or fatal bites: Staffordshire Terriers (pit bulls), German Shepherds, Chow-Chows | ||
| Characteristics of People Injured by Cats and Dogs | ||
| Age | 25-34 years | <20 years, with significant occurrence in those 5-9 years |
| Sex | Females (59%) | Males (62%) |
| Relationship to animal | Victim does not own cat | Victim is family member or acquaintance to the owners. Dog owners are most frequently bitten by dogs but not necessarily their own dogs (family's dog, 30%; neighbor's dog, 50%) |
| Characteristic of the Injury Event (Common Scenario) | ||
| Kinds of aggression | Fear-related aggression, play aggression, redirected aggression, “biting and petting syndrome” | Dominance aggression, possessive aggression, fear-related aggression, protective/territorial aggression, punishment-induced aggression, pain-elicited aggression |
| Time of year | May through August (warm weather) | May through August (warm weather) |
| Time of day | 9:00 am to noon | Late afternoon |
| Other factors | If the cat is owned, 50% of the victims are the owners | Unusually high incidence of bites by chained dogs who are restrained on their own property |
| Typical wound characteristics | 80% of all bites require medical attention | 20%-60% of all bites require medical attention |
| 50% of all wounds become infected | Insufficient data on number of wounds that become infected | |
| 29% of all cat-bite victims return to doctor after initial visit because of complications | 5% of all dog-bite victims return to doctor after initial visit because of complications | |
| Wounds consist of scratches (70%); punctures (27%) and tears (3%) to finger (21%), arm (18%), foot or leg (8%), face or neck (7%); and multiple body locations (3%) | Wounds consist primarily of puncture and tears to extremities (76%) and to face (15%). 70% fatal injuries to children <9 years. Highest death rate to neonates (<1 month old). Death rates of neonates 295/100 million; for children 1-11 months, 47/100 million. | |
| Location of Bite Wounds (% Bites) | ||
| Face, scalp, or neck | 2 | 16 |
| Trunk | 0 | 2 |
| Shoulder, arm, or forearm | 23 | 12 |
| Hand | 63 | 50 |
| Thigh or leg | 9 | 16 |
| Feet | 3 | 4 |
From Greene CE: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders Elsevier.
Education about reduction of animal bite risk can be incorporated into preventive health care counseling by both human health clinicians and veterinarians. Dog bite prevention recommendations by the CDC are summarized in Box 10-5. Other preventive steps include avoiding feeding or handling wild or stray animals (see Exotic and Wildlife Pets in this chapter), and keeping companion animals indoors except for daily, accompanied exercise.
History
The clinician caring for a human victim of an animal bite should determine and document in the chart how, where, and when the bite occurred; the species and distinguishing characteristics of the animal; whether the bite was provoked or unprovoked; whether the animal is available for observation; its current location; and relevant veterinary information about the animal, including rabies vaccination status or any other known illnesses.
It is also necessary to determine the date of the human patient's most recent tetanus vaccination and any history of medical conditions placing them at increased risk of infection, such as immunocompromised conditions (e.g., HIV), cancer, diabetes, splenectomy, or immunosuppressive drugs. A history of prosthetic joints or heart valves is also an important risk factor for complications in human beings.1 The human patient should be asked about any allergies to animals or medications. Finally, for both human beings and companion animals, if considering rabies postexposure prophylaxis, determine whether the patient has previously received rabies vaccination, including where and when.
Table 10-9 lists risk factors for animal bite infections. If these risk factors are present, antibiotic prophylaxis appears reasonable. If none of these risk factors is present, antibiotics may be withheld in many cases and the wound rechecked in 48 hours.
Table 10-9.
Risk Factors for Animal Bite Infection
| High-risk species |
|
| Severity and location of bite |
|
| Host factors |
|
| Other |
|
Physical Examination
A careful head-to-toe physical examination should be performed looking for other signs of trauma. The wound should be carefully explored to assess damage to deeper structures, including joints and bones. A detailed neurovascular examination should be performed to assess damage to nerves and blood vessels.
For human beings bitten by an animal, it may be worthwhile to draw a diagram or take a photograph of the wound because many animal bites result in litigation. Children bitten on the neck or head should have cervical immobilization until cervical fractures can be ruled out by radiological studies.7
Wound Culture
For fresh bite wounds, routine cultures are not necessary. However, if a wound later shows signs of infection such as redness, swelling, or discharge, cultures for both aerobic and anaerobic bacteria should be obtained (Figure 10-20 ).
Figure 10-20.

Cat-bite abscess of the wrist with presentation for care delayed 1 month after injury. Pasteurella multocida was isolated in pure culture.
(From Long SS, Pickering LK, Prober CG: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia, 2008, Saunders Elsevier.)
Initial Wound Care, Irrigation, and Debridement
First-aid for an animal bite should involve control of bleeding and initial cleansing. Bleeding should be controlled with local pressure. Adequate cleaning and irrigation of a bite wound is critical to its management. Initial cleaning can be done immediately after the bite with soap and water. As soon as possible, copious irrigation of the wound with saline solution with an 18- or 19-gauge needle or catheter tip and large syringe should be performed. Many bite wounds involve damage to tissue at the wound edges. Therefore wound tissue that appears devitalized or necrotic should be carefully debrided.1 In human patients, significant bites to the hand may require reconstructive surgery, and a detailed examination for possible injury to tendons, nerves, and other deeper tissues is warranted. A hand surgeon may need to be consulted, especially if there is any evidence of infection. If there are significant facial lacerations, a plastic surgeon consultation is appropriate.
Radiographs
If there is concern about involvement of joints and other bony structures, including facial bones and the skull, take radiographs (Figure 10-21 ) and obtain orthopedic consultation for possible surgical intervention.
Figure 10-21.

Radiograph of finger after a bite by a captive subadult Gila monster (Heloderma suspectum) after touching the lizard's tail. It took approximately 40 seconds to pry the reptile off with tongs. Immediately after, the patient complained of “10 out of 10” pain. Shortly after, he developed swelling and redness extending to his mid-forearm, as well as nausea and vomiting. A tooth was retained in the wound for more than 2 months before it was spontaneously expelled.
(From Auerbach PS: Wilderness medicine, ed 5, Philadelphia, 2007, Mosby Elsevier. Courtesy Sean Bush.)
Wound Closure
Whether to close an animal bite wound with sutures or other technique is controversial. Considerations include risk factors for infection and cosmetic impact of the injury. Dog bites to the face rarely become infected and are often closed to reduce the possibility of disfiguring scars. Wounds that are at high risk of infection (e.g., from a high-risk species, in a high-risk area such as the hand, or in an immunocompromised patient), are more than 24 hours old, or that are already infected generally should not be closed. Low-risk wounds can be closed, after thorough irrigation, with sutures, staples, or other techniques and observed closely for signs of infection.8
Antibiotic Prophylaxis and Therapy
Whether prophylactic antibiotics are indicated for an animal bite is also controversial, and data regarding the bites of many species are limited. A Cochrane review of antibiotic prophylaxis in uncomplicated dog and cat bites found no clear evidence of benefit.9 Clinical decision making regarding antibiotic prophylaxis involves assessing the risk of infection. Risk factors include the species responsible for the bite, any information about the health of the animal, the severity and location of the bite, and whether the patient is immunocompromised or has other conditions that increase the risk of infection. High-risk bites include crush injuries that involve devascularized tissue and puncture wounds, hand involvement, significant edema, possible involvement of joints or bones, and proximity to genitalia or prosthetic joints nearby. Patients with very high-risk bites or evidence of tenosynovitis or deep infection should be admitted for parenteral antibiotics and hospital observation. Immunocompromised individuals and those with asplenia or compromising medical conditions should receive prophylactic antibiotics for most animal bites. Table 10-10 provides recommendations for empiric antibiotic dosing for animal bites to human beings.
Table 10-10.
Pathogens Complicating Animal Bites to Human Beings and Recommended Empiric Therapy
| Empiric Antibiotic Therapy | |||
|---|---|---|---|
| Species | Reported Pathogens | Primary | Alternative |
| Dogs |
|
Amoxicillin-clavulanate 875/125 mg bid or 500/125 mg PO tid30 | Clindamycin 300 mg PO qid + fluoroquinolone (adults) or Clinda + Trimethoprim sulfa (children)30 |
| Cats |
|
Amoxicillin-clavulanate 875/125 mg bid or 500/125 mg PO tid30; itraconazole | Cefuroxime axetil 0.5 gm PO q12 h or doxycycline 100 mg PO bid; do not use cephalexin30 |
| Human beings | Streptococcus viridans, Staphylococcus epidermidis, Corynebacterium, Staphylococcus aureus, Eikenella, Bacteroides, Peptostreptococcus |
|
Cefoxitin 2 g IV q8 h, or ticarcillin-clavulanate 3.1 g IV q6 h or piperacillin-tazobactam 3.375 g IV q6 h or 4-hour infusion 3.375 g q8 h (x-rays for clenched fist injuries)30 |
| Ferrets | Not well documented; consider rabies risk | As for cat and dog bites32 | |
| Horses | Pasteurella caballi38, S. aureus, Neisseria, and other anaerobic gram-negative bacilli33 | As for dog | |
| Sheep | Actinobacillus, others33 | As for dog | |
| Rats |
|
Amoxicillin-clavulanate 875/125 mg bid30 | Doxycycline 100 mg PO bid |
| Pigs | Polymicrobial: gram-positive cocci, gram-negative bacilli, anaerobes, Pasteurella spp.30 | Amoxicillin-clavulanate 875/125 mg bid30; some recommend adding ciprofloxacin34 | Third-generation cephalosporin or ticarcillin-clavulanate or ampicillin-sulbactam or imipenem30 |
| Rabbits | Pasturella multocida35 (rabies has been reported29) | As for dog and cat | |
| Nonhuman primates (e.g., macaque) | Consider risk for herpes B virus (Herpes simiae) | Valacyclovir | Acyclovir26 |
| Hamsters | Francisella tularensis,36Pasteurella spp. | See rat | |
| Reptiles (e.g., iguanas, snakes, alligators) | Pseudomonas aeruginosa, Proteus, Clostridium, Bacteroides fragilis, Salmonella groups IIIa and IIIb,13,37Serratia marescens17 | Ceftriaxone (infected wounds)30 | |
| Bats, raccoons, skunks, foxes | Staphylococcus and Streptococcus spp. (skin flora) | Amoxicillin-clavulanate 875/125 mg bid or 500/125 mg PO tid30 | Doxycycline 100 mg PO bid30 |
| Rabies virus | Assess for rabies postexposure prophylaxis | ||
| Seal | Marine Mycoplasma (sealpox; see Chapter 12) | Tetracycline ×4 weeks30 | |
Dog bites usually do not become infected. Reported infection rates range from 5% to 15%.10 Cat bites often involve deep puncture wounds that are prone to infection. Infection rates from cat bites may be as high as 80%.11 The choice of antimicrobial prophylaxis for cat bites and high-risk dog bites should cover Pasteurella, Streptococcus, and Staphylococcus. A 5- to 7-day course of antibiotics should be administered. Ferret bites are increasing in frequency with their popularity as pets and their tendency to bite. Little is known about their microbial flora. Pig bites are considered to carry a high risk for infection.12
Alligators and related species tend to inflict severe bites and have polymicrobial flora that can lead to infection.13 Many snake bites do not become infected.7 Two controlled trials of prophylactic antibiotics for pit viper envenomation did not show a clinical benefit.14, 15 Nevertheless, snake bites should be monitored for potential infection.16 Iguana bites can be severe and infection has been reported.17
Human bites to another human are considered high-risk bites for infection. In addition to bacterial pathogens (see Table 10-10), risk of infection with bloodborne pathogens such as HIV and hepatitis B should be considered.
Vaccinations
Rabies Postexposure Prophylaxis
The risk of rabies should be assessed in every animal bite situation in a human or pet, with rabies postexposure prophylaxis (PEP) for high-risk bites provided.18 However, studies have shown that rabies PEP is often given inappropriately by clinicians treating bites in emergency care settings, such as when the biting animal is low risk or available for observation or testing (see Chapter 9).19 The biting animal should be captured and monitored for 10 days for signs of rabies if it is a dog, cat, or ferret; for 14 days if livestock; or euthanized and tested if a rabies vector species (e.g., bat, raccoon, skunk, fox) or other wild carnivore such as a river otter (Figure 10-22 ). If animal rabies testing is positive or if the animal is not available for observation or testing and is a high-risk rabies species, PEP should be provided to the patient.
Figure 10-22.
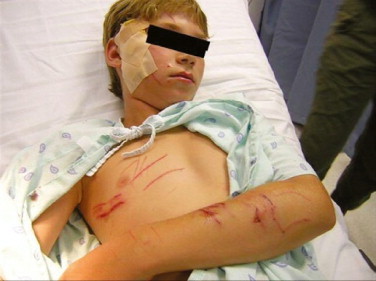
Victim of river otter attack.
(From Auerbach PS: Wilderness medicine, ed 5, Philadelphia, 2007, Mosby Elsevier. Photo courtesy Jill Hanna.)
Tetanus
Tetanus status should be determined in all human bite patients. A booster should be provided for a low-risk bite if the patient was not vaccinated within the past 10 years and for a high-risk bite if the patient was not vaccinated within the past 5 years. If there is not a history of at least 3 tetanus vaccinations in the past (for an adult), tetanus immune globulin should also be given. Table 10-11 details these recommendations.
Table 10-11.
Indications for Tetanus Prophylaxis in Wounds
| Clean, Minor Wound | All Other Wounds* | |||
|---|---|---|---|---|
| History of Absorbed Tetanus Toxoid (Doses) | Tdap or Td† | TIG | Tdap or Td† | TIG |
| Unknown or <3 | Yes | No | Yes | Yes |
| ≥3 | No‡ | No | No§ | No |
Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
Tdap is preferred to Td for adults who have never received Tdap. Td is preferred to TT for adults who received Tdap previously or when Tdap is not available. If TT and TIG are used, tetanus toxoid absorbed rather than tetanus toxoid for booster use only (fluid vaccine) should be used.
Yes, if ≥10 years since the last tetanus toxoid–containing vaccine dose.
Yes, if ≥5 years since the last tetanus toxoid–containing vaccine dose.
From Kretsinger K, Broder KR, Cortese MM et al: Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel, MMWR Recomm Rep 55(RR-17):1, 2006.
SPECIFIC BITE INFECTIONS
Clinicians should be familiar with a number of specific bite-associated infections.
Pasteurella Infection
Pasteurella multocida is the most common agent isolated from infected dog and cat wounds (Figure 10-23 ). It causes cellulitis and can cause bacteremia with sepsis, meningitis, and hemorrhagic complications. Pasteurella wound infections tend to develop rapidly, producing swelling, redness, tenderness, and discharge at the wound area within 12 to 24 hours after the bite.20 Tenosynovitis and osteomyelitis can develop and should be aggressively treated with parenteral antibiotics.
Figure 10-23.

A toddler with facial cellulitis from Pasteurella multocida after a dog bite.
(From Long SS, Pickering LK, Prober CG: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia, 2008, Saunders Elsevier. Courtesy J. H. Brien.)
Rat-Bite Fever
Rat-bite fever from Streptobacillus moniliformis in human beings was traditionally a disease of inner-city children bitten by peridomestic rodents. With the popularity of rats as pets and the use of rats in laboratories, cases now occur in pet store employees, pet owners, and laboratory animal workers. The disease causes a rash (Color Plate 10-8) that may be petechial, hemorrhagic, or purpuric, as well as fever, and systemic symptoms including arthralgias. Without treatment, the case fatality rate can be 10%. Most domestic and wild rats carry the causative agent, S. moniliformis, in their oral flora. Up to an estimated 10% of rat bites can become infected.21 Patients with rat bites need to be counseled about the signs and seriousness of this disease, and prophylactic antibiotics should be considered for infection-prone bites.
S. moniliformis in guinea pigs causes cervical lymphadenitis or granulomatous pneumonia; in mice, purulent lesions and acute septicemia; and endocarditis or septic arthritis in nonhuman primates.
Capnocytophaga Infection
Capnocytophaga canimorsus is found in dog and cat saliva (especially the former). In asplenic and immunocompromised human beings, it can produce septicemia with meningitis, endocarditis, and eye involvement.22 Serious infections have also been reported in persons with no apparent risk factors.23 The first known animal with a Capnocytophaga-infected dog bite wound, a pet rabbit, was successfully treated.24
Herpes B Infection
Herpes B infection causes an often fatal viral meningoencephalitis that can be transmitted to human beings from Old World monkeys of the genus Macaca (Figure 10-24 ; see Chapter 12).25 Although there have been no controlled trials of prophylaxis, the B Virus Working Group recommends antiviral prophylaxis for high-risk bites (or other exposures) from these monkeys. The recommended prophylactic regimen is 1 g valacyclovir three times a day for adults, excluding pregnant women. An alternative regimen is 800 mg acyclovir five times a day. Prophylaxis should begin as soon as possible after exposure. If signs of herpes B infection appear, systemic treatment with antivirals and hospitalization are necessary.26
Figure 10-24.

A lower lip ulceration in a rhesus monkey caused by herpes B virus infection.
(From Mandell GL, Bennett JE, Dolin R: Principles and practice of infectious diseases, ed 6, New York, 2005, Churchill Livingstone Elsevier.)
Lymphocytic Choriomeningitis
LCMV can be present in the saliva of rodents, especially mice, and can pose a risk after a bite (see Chapter 9). Fetal complications can occur in pregnant women and severe disease in immunocompromised persons. Therefore pregnant women and immunocompromised individuals should avoid rodent exposure.
Sporotrichosis
Sporotrichosis is a fungal disease (Color Plate 10-9) that can produce ulcerating skin nodules and systemic infection (rare). It is usually acquired from contact with plants or soil but has been reported in association with cat bites and scratches (with an outbreak of more than 1000 cats and 500 persons in Brazil27) and after a squirrel bite.28 Veterinarians are at increased risk of occupational infection. Sporothrix schenckii has also been reported in horse and dog lesions.
Cat-Scratch Fever
Bartonella infection can be transmitted by cat bites and scratches (see Chapter 9).
MANAGEMENT OF BITES IN ANIMALS
As in human beings, immunocompromised conditions (such as FIV/FeLV in feline patients) predispose an animal to bite-related infection. Pet dogs, cats, and ferrets that are bitten by potentially or known rabid animals should be managed according to their rabies vaccination status. If they are currently vaccinated, a booster vaccine should be immediately administered and the pet should be observed for 45 days for signs of rabies. If the pet is unvaccinated, it should be euthanized or held in strict isolation for 180 days and provided a rabies vaccine 1 month before release from quarantine. Certain domestic animals such as rabbits are at risk of wildlife rabies if caged outside or allowed to roam outside.29
Table 10-12 provides recommended antibiotic therapy for animal bite infections in dogs and cats. Depending on the severity of the bite, other treatment modalities, including physical therapy, may be required for rehabilitation of the animal (Figure 10-25 ).
Table 10-12.
Recommended Therapy for Bite Infections in Cats and Dogs
| Drug | Species | Dose (mg/kg) | Route | Interval (hr) | Antibacterial Spectrum |
|---|---|---|---|---|---|
| Amoxicillin-clavulanate | D | 10-20 | PO | 8 | Most gram-positive and gram-negative aerobes and anaerobes; first-choice drug for most bite wounds |
| D | 13.75 | PO | 12 | ||
| C | 10-20 | PO | 12 | ||
| Ampicillin (amoxicillin) | B | 22 | PO | 8 | Some gram-positive and gram-negative aerobes |
| B | 11-22 | SC, IV | 6–8 | ||
| Ticarcillin | D | 20-50 | IV | 6–8 | Gram-positive and gram-negative aerobes and anaerobes |
| Cefotaxime | B | 15-30 | IV, IM, SC | 6–8 | Sepsis from bite wounds caused by gram-negative aerobes or anaerobes |
| Doxycycline | D | 5 | PO, IV | 12 | Some aerobes and anaerobes; mycoplasmas |
| Clindamycin | B | 5-11 | PO | 8–12 | Gram-positive aerobes and anaerobes |
| Enrofloxacin | D | 5 | PO, SC | 12–24 | Gram-negative aerobes |
| Difloxacin | D | 5-10 | PO | 24 | Gram-negative aerobes |
| Orbifloxacin | D | 2.5-7.5 | PO | 24 | Gram-negative aerobes |
| Marbofloxacin | D | 2-4 | PO | 24 | Gram-negative aerobes |
| Azithromycin | D | 20 | PO | 24 | Gram-positive aerobes; mycoplasmas and mycobacteria |
| Chloramphenicol | D | 25-50 | PO, IV, IM, SC | 8 | Some anaerobes; variable with gram-positive and gram-negative aerobes |
| Metronidazole | B | 10 | PO, IV | 8 | Anaerobes |
D, Dog; C, cat; B, dog and cat; PO, by mouth; SC, subcutaneous; IV, intravenous; IM, intramuscular.
From Greene CE: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders Elsevier.
Figure 10-25.

Cat relaxing on ball after surgery to repair dog bite wound.
(From Gaynor JS, Muir WW 3d: Handbook of veterinary pain management, ed 2, St Louis, 2008, Mosby Elsevier.)
References
- 1.Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116(2):49–52. doi: 10.3810/pgm.2004.08.1572. 55–56, 59. [DOI] [PubMed] [Google Scholar]
- 2.Langley RL. Animal bites and stings reported by United States poison control centers, 2001–2005. Wilderness Environ Med. 2008;19(1):7–14. doi: 10.1580/07-WEME-OR-111.1. [DOI] [PubMed] [Google Scholar]
- 3.Guida G., Nebiolo F., Heffler E. Anaphylaxis after a horse bite. Allergy. 2005;60(8):1088–1089. doi: 10.1111/j.1398-9995.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 4.Lim DL., Chan RM., Wen H. Anaphylaxis after hamster bites—identification of a novel allergen. Clin Exp Allergy. 2004;34(7):1122–1123. doi: 10.1111/j.1365-2222.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- 5.Garth AP, Harris NS. Bites, animalhttp://www.emedicine.com/emerg/topic60.htm. Accessed April 16, 2009
- 6.Journal of the American Veterinary Medical Association . Feline practitioners recommend new FIV and FeLV testing guidelines, initiate public awareness campaign. 2001. http://www.avma.org/onlnews/javma/apr01/s041501g.asp. Accessed April 16, 2009. [PubMed] [Google Scholar]
- 7.Morgan M. Hospital management of animal and human bites. J Hosp Infect. 2005;61(1):1–10. doi: 10.1016/j.jhin.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach PS. Wilderness medicine. 5th ed. Mosby Elsevier; Philadelphia: 2007. [Google Scholar]
- 9.Medeiros IM., Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2) doi: 10.1002/14651858.CD001738. CD001738. [DOI] [PubMed] [Google Scholar]
- 10.Moran GJ., Talan DA., Abrahamian FM. Antimicrobial prophylaxis for wounds and procedures in the emergency department. Infect Dis Clin North Am. 2008;22(1):117–143. doi: 10.1016/j.idc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Louisiana State University School of Veterinary Medicine. What you should know about animal biteshttp://www.vetmed.lsu.edu/animal_bites.htm
- 12.Barnham M. Pig bite injuries and infection: report of seven human cases. Epidemiol Infect. 1988;101(3):641–645. doi: 10.1017/s0950268800029514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertner G. Caiman bite. Wilderness Environ Med. 2006;17(4):267–270. doi: 10.1580/pr40-05.1. [DOI] [PubMed] [Google Scholar]
- 14.Kerrigan KR., Mertz BL., Nelson SJ. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21(4):369–372.. doi: 10.1007/pl00012255. [DOI] [PubMed] [Google Scholar]
- 15.LoVecchio F., Klemens J., Welch S. Antibiotics after rattlesnake envenomation. J Emerg Med. 2002;23(4):327–328. doi: 10.1016/s0736-4679(02)00563-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu CH., Hu WH., Hung DZ. Snakebite complicated with Vibrio vulnificus infection. Vet Hum Toxicol. 2001;43(5):283–285. [PubMed] [Google Scholar]
- 17.Hsieh S., Babl FE. Serratia marcescens cellulitis following an iguana bite. Clin Infect Dis. 1999;28(5):1181–1182. doi: 10.1086/517778. [DOI] [PubMed] [Google Scholar]
- 18.Manning SE., Rupprecht CE., Fishbein D. Advisory Committee on Immunization Practices Centers for Disease Control and Prevention: Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1–28. [PubMed] [Google Scholar]
- 19.Conti LA., Wiersma S., Hopkins R. Evaluation of state-provided rabies post-exposure prophylaxis (PEP), Florida, July-September 1997 and July-September 1998. Southern Med J. 2002;95(2):225–230. [PubMed] [Google Scholar]
- 20.Kristinsson G. Pasteurella multocida infections. Pediatr Rev. 2007;28(12):472–473. doi: 10.1542/pir.28-12-472. [DOI] [PubMed] [Google Scholar]
- 21.Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20(1):13–22. doi: 10.1128/CMR.00016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolivet-Gougeon A., Sixou J-L., Tamanai-Shacoori Z. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents. 2007;29(4):367–373. doi: 10.1016/j.ijantimicag.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ball V., Younggren BN. Emergency management of difficult wounds: part I. Emerg Med Clin North Am. 2007;25(1):101–121. doi: 10.1016/j.emc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.van Duijkeren E., van Mourik C., Broekhuizen M. First documented Capnocytophaga canimorsus infection in a species other than humans. Vet Microbiol. 2006;118(1–2):148–150. doi: 10.1016/j.vetmic.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Andersen E. B virus—the risks in monkey business. AAOHN Journal. 2005;53(9):385–387. [PubMed] [Google Scholar]
- 26.Cohen JI., Davenport DS., Stewart JA. the B Virus Working Group. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine Herpesvirus 1) Clin Infect Dis. 2002;35(10):1191–1203. doi: 10.1086/344754. [DOI] [PubMed] [Google Scholar]
- 27.Schubach A., Schubach TM., Barros MB. Cat-transmitted sporotrichosis, Rio de Janeiro, Brazil. Emerg Infect Dis. 2005;11(12):1952–1954. doi: 10.3201/eid1112.040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saravanakumar PS., Eslami P., Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case report and review. Clin Infect Dis. 1996;23(3):647–648. doi: 10.1093/clinids/23.3.647. [DOI] [PubMed] [Google Scholar]
- 29.Eidson M., Matthews SD., Willsey AL. Rabies virus infection in a pet guinea pig and seven pet rabbits. J Am Vet Med Assoc. 2005;227(6):932–935,. doi: 10.2460/javma.2005.227.932. 918. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert DN., Moellering RC., Eliopoulos GM. Sanford guide to antimicrobial therapy 2009. 39th ed. Antimicrobial Therapy Inc; Sperryville, VA: 2009. [Google Scholar]
- 31.Talan DA., Citron DM., Abrahamian FM. Bacteriologic analysis of infected dog and cat bites. New Engl J Med. 1999;340(2):85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- 32.Applegate JA., Walhout MF. Childhood risks from the ferret. J Emerg Med. 1998;16(3):425–427. doi: 10.1016/s0736-4679(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 33.Angoules AG., Lindner T., Vrentzos G. Prevalence and current concepts of management of farmyard injuries. Injury. 2007;38(suppl 5):S27–S34. doi: 10.1016/j.injury.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Morgan MS. Treatment of pig bites. Lancet. 1996;348(9036):1246. doi: 10.1016/S0140-6736(05)65525-0. [DOI] [PubMed] [Google Scholar]
- 35.Silberfein EJ., Lin PH., Bush RL. Aortic endograft infection due to Pasteurella multocida following a rabbit bite. J Vasc Surg. 2006;43(2):393–395. doi: 10.1016/j.jvs.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) Tularemia associated with a hamster bite—Colorado, 2004. MMWR. 2005;53(51):1202–1203. [PubMed] [Google Scholar]
- 37.Quirk EK. Human and animal bites. In: Starlin R., editor. Infectious diseases subspecialty consult (the Washington Manual subspecialty consult) Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 38.Escande F., Vallee E., Aubart F. Pasteurella caballi infection following a horse bite. Zentralbl Bakteriol. 1997;285(3):440–444. doi: 10.1016/s0934-8840(97)80010-2. [DOI] [PubMed] [Google Scholar]


