SUMMARY
The precise mechanism of general anesthesia remains unclear. In the last two decades, there has been considerable focus on the hypothesis that anesthetics co-opt the neural mechanisms regulating sleep. This hypothesis is supported by ample correlative evidence at the level of sleep-promoting nuclei but causal investigations of potent inhaled anesthetics have not been conducted. Here we tested the hypothesis that chemogenetic activation of discrete neuronal subpopulations within the median preoptic nucleus (MnPO) and ventrolateral preoptic nucleus (VLPO) of the hypothalamus would modulate sleep/wake states and alter the time to loss and resumption of consciousness associated with isoflurane, a potent halogenated ether in common clinical use. We show that activating MnPO/VLPO GABAergic or glutamatergic neurons does not alter anesthetic induction or recovery time. However, activation of these neuronal subpopulations did alter sleep-wake architecture. Notably, we report the novel finding that stimulation of VLPO glutamatergic neurons causes a strong increase in wakefulness. We conclude that activation of preoptic GABAergic or glutamatergic neurons that increase sleep or wakefulness does not substantively influence anesthetic state transitions. These data indicate that the correlative evidence for a mechanistic overlap of sleep and anesthesia at the level of an individual nucleus might not necessarily have strong causal significance.
Keywords: Sleep, wakefulness, general anesthesia, isoflurane, arousal, consciousness, DREADD
eTOC Blurb:
Vanini et al. show that activation of preoptic GABAergic or glutamatergic neurons that regulate sleep and wakefulness does not have a substantive influence on anesthetic state transitions. The authors conclude that current correlative evidence for a mechanistic overlap of sleep and anesthesia might not have a strong causal significance.
INTRODUCTION
General anesthetics have been in continuous clinical use for more than 170 years but the precise mechanisms by which these drugs reversibly suppress consciousness remain unknown. Within the past two decades, there has been increased attention on the relationship between sleep and general anesthesia, with ample correlative evidence suggesting that anesthetics act by co-opting subcortical nuclei that generate sleep and wakefulness [1-4]. To date, research on the mechanisms underlying the sleep-anesthesia connection has focused predominantly on the preoptic region of the hypothalamus, particularly the medial preoptic area [5] and ventrolateral preoptic nucleus (VLPO) [2, 4, 6-8]. Consistent with what has been termed the shared circuits hypothesis, putative sleep-promoting GABAergic neurons in the VLPO express cFos during the loss of consciousness induced by isoflurane, which directly activates these neurons [2]. However, despite biological plausibility and compelling correlative data, there have been no causal investigations to date that demonstrate a role for discrete neuronal subpopulations in the preoptic area of the hypothalamus in the mechanism of general anesthesia. Additionally, the median preoptic nucleus (MnPO) of the hypothalamus is critical for both sleep generation and the regulation of sleep homeostasis [9-12] but its influence on the anesthetized state remains virtually unexplored. In this study we tested the hypothesis that activation of neuronal subpopulations within the preoptic area of the hypothalamus that promote sleep or wakefulness would modulate the entry to or exit from general anesthesia induced by isoflurane, a potent halogenated ether in common clinical use. To test this hypothesis, we used a chemogenetic approach to examine the role of GABAergic and glutamatergic neurons of both the MnPO and VLPO in anesthetic state transitions. We report that, despite altering sleep-wake states, activation of preoptic GABAergic or glutamatergic neurons had no substantive effect on the entry to or emergence from isoflurane anesthesia. These findings prompt a reconsideration of the hypothesis that general anesthesia is produced by activating nuclei in the preoptic hypothalamus known to promote or regulate sleep.
RESULTS
Activation of Neuronal Subpopulations in the Median Preoptic Nucleus Did Not Alter Anesthetic-State Transitions
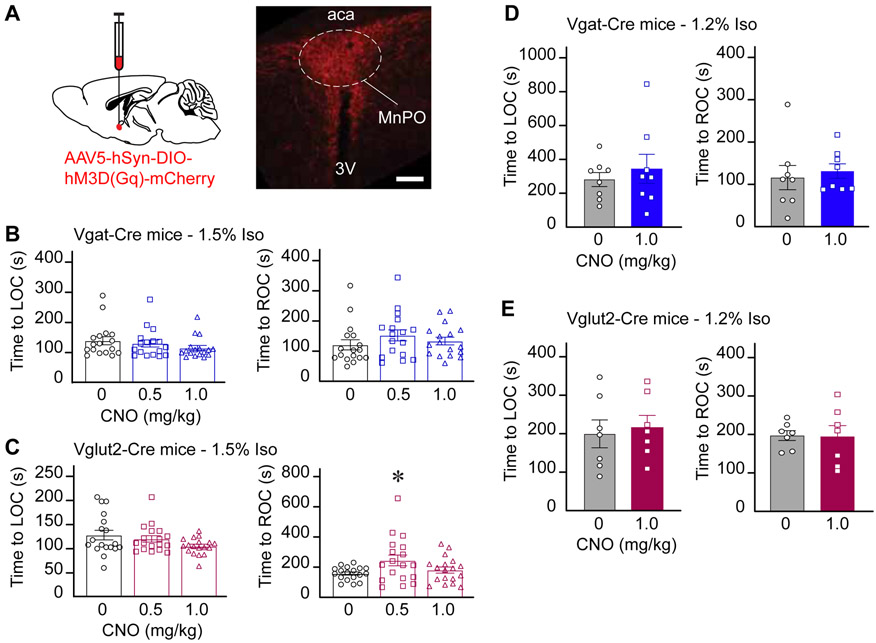
To determine whether activation of GABAergic or glutamatergic neurons in the MnPO (Figure S4) modulates anesthetic induction and recovery time, mice were exposed to a clinically relevant (1.5%) or a sub-anesthetic (1.2%) concentration of isoflurane for 30 minutes. Relative to vehicle, activation of GABAergic neurons in the MnPO (n = 17 mice) prior to exposure to 1.5% isoflurane did not alter anesthetic induction (P = 0.1256) or recovery time (P = 0.2198) (Figure 1B). Activation of glutamatergic neurons (n=18 mice) did not change induction time (P = 0.3577); recovery time was increased (P = 0.0071) after injection of 0.5 mg/kg CNO only (P value for saline versus 1.0 mg/kg CNO > 0.9999) (Figure 1C). The statistical significance between 0.5 mg/kg CNO and vehicle was caused by a single outlier (P value without outlier = 0.2919). The estrous cycle stage was identified in all female mice after each anesthesia experiment by histological assessment of vaginal smears. Female mice were used in 78 experiments, 60% of which were conducted during diestrus, 25% during metestrus, and 15% during estrous or proestrus. Changes in the cycle stage between control versus 0.5 mg/kg CNO, and control versus 1.0 mg/kg CNO were observed, respectively, in 18% and 19% of the experiments. Relative to control, there were no significant differences in induction and recovery time as a function of sex or estrous cycle stage. Similarly, activation of MnPO GABAergic and glutamatergic neurons prior to exposure to 1.2% isoflurane (a sub-anesthetic concentration) did not change anesthetic induction and recovery time (n = 8 Vgat-Cre mice, P = 0.5469 and 0.6406; n = 7 Vglut2-Cre mice, P = 0.6875 and 0.7813) (Figures 1D and 1E). Consistent with these behavioral findings, there was no difference in breathing rates between treatment conditions (P > 0.1) at 1.5% or 1.2% isoflurane. Mice exposed to 1.2% exhibited a few transient wake-like episodes during anesthesia ranging from limb movements to the resumption of righting. Post hoc analysis revealed no differences in the amount of wake-like episodes between vehicle and CNO (P > 0.9999). To rule out any non-specific effect of CNO, an adverse effect from the vector injection (i.e., mechanical and/or inflammatory tissue damage), or Cre-associated toxicity [13] on anesthetic induction/recovery time, we conducted additional anesthesia experiments using the following groups of Vgat-Cre and Vglut2-Cre mice: (1) mice injected with the vector but without expression of hM3Dq receptors (Figure S1; Vgat-Cre, n = 7 and Vglut2-Cre, n = 6), (2) sham operated Vgat-Cre, n = 6 and Vglut2-Cre, n = 5), and (3) naïve mice (n = 5 in each mouse line; the results from both groups are shown in Figure S2). Relative to vehicle, 1.0 mg/kg CNO administration did not alter anesthetic state transitions in any of the three control groups.
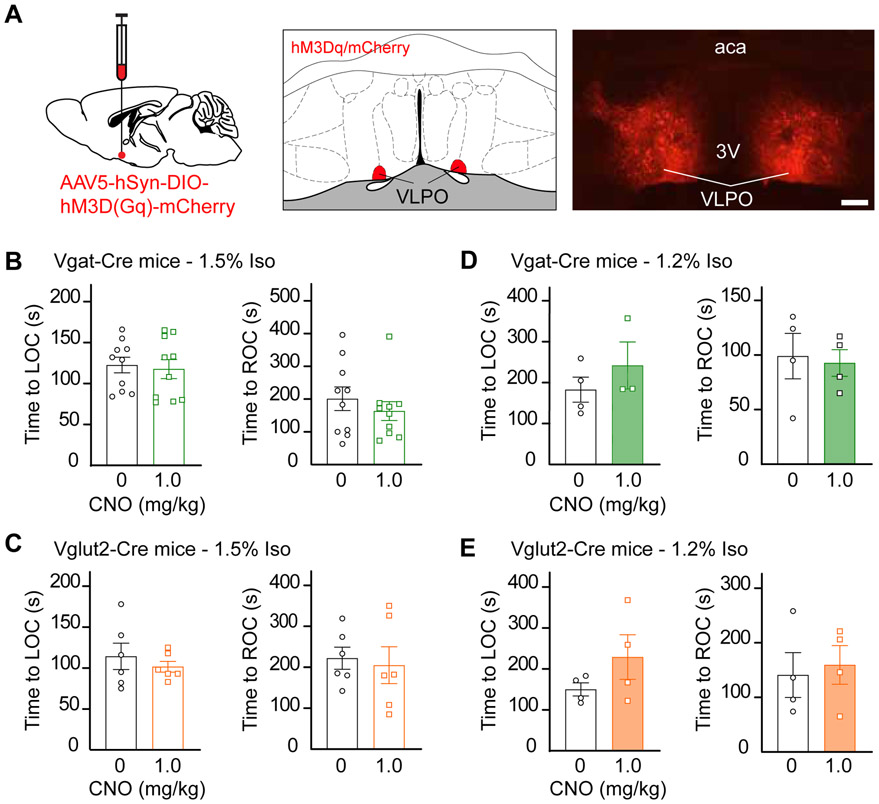
Figure 1. Chemogenetic stimulation of GABAergic and glutamatergic neurons in the MnPO did not alter anesthetic state transitions.
A. Schematic representation of AAV injection into the median preoptic nucleus (MnPO). mCherry (red) indicates the expression of the excitatory designer receptor hM3Dq within the MnPO of a Vglut2-Cre mouse. B. Time to loss and resumption of consciousness (left and right panels, respectively) in Vgat-Cre mice exposed to 1.5% isoflurane. C. Time to loss and resumption of consciousness (left and right panels, respectively) in Vglut2-Cre mice exposed to 1.5% isoflurane. D and E show the time to loss and resumption of consciousness (left and right panels, respectively) in Vgat-Cre and Vglut2-Cre mice exposed to 1.2% isoflurane. The asterisk in C indicates a significant difference (P < 0.05) from vehicle control (0 mg/kg CNO). Statistical comparisons were conducted using a Friedman test followed -when applicable- by a post hoc Dunn’s (B and C) or a two-tailed Wilcoxon test (D and E). Abbreviations: aca, anterior commissure; CNO, clozapine-N-oxide; LOC, loss of consciousness; ROC, resumption of consciousness; 3V, third ventricle. Calibration bar in A, 50 μm. Data in B-E are shown as mean ± standard error of the mean. See also Figures S1, S2 and S4.
Activation of Neuronal Subpopulations in the Median Preoptic Nucleus Altered Sleep-Wake Architecture and Reduced Core Body Temperature
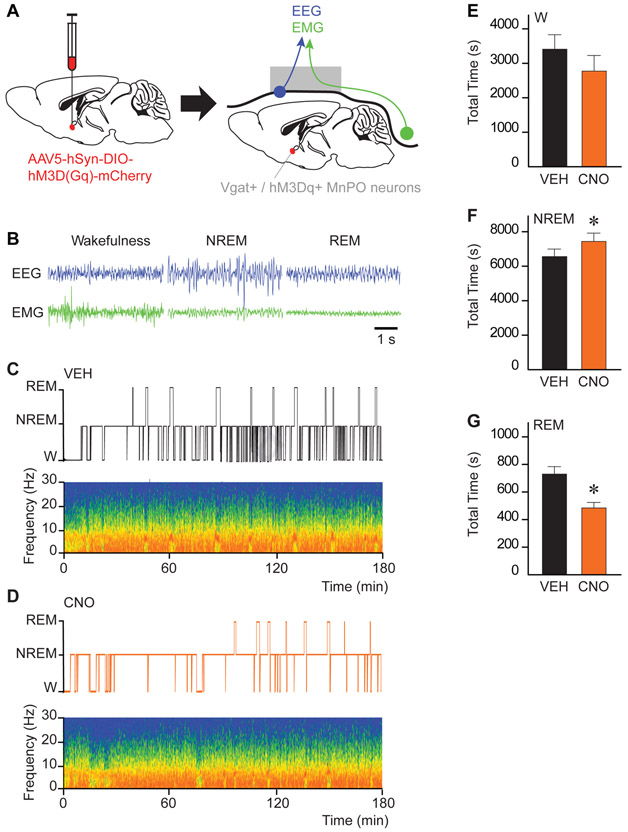
In a second set of experiments, we tested whether selective activation of MnPO GABAergic and glutamatergic neurons (during the same time period during which the anesthesia experiments were conducted) modulates sleep-wake architecture (n = 6 Vgat-Cre and n = 7 Vglut2-Cre mice). Figure 2 shows representative examples of electrophysiological recordings during wakefulness, NREM sleep and REM sleep (2B), and sleep-wake patterns with their respective spectrograms after injection of vehicle (2C) or 1.0 mg/kg CNO (2D) in Vgat-Cre mice. Group data plotted in Figures 2E-G revealed that activation of GABAergic neurons in the MnPO increased the time spent in NREM sleep (P = 0.0313) and decreased REM sleep (P = 0.0156); total time in wakefulness was not significantly different (P = 0.0781). CNO caused a significant reduction in the number of wakefulness (P =0.0156), NREM sleep (P = 0.0156), and REM sleep (P = 0.0313) bouts, and an increase in NREM bout duration (P = 0.0156) (e.g., more consolidated sleep). Activation of MnPO glutamatergic neurons decreased REM sleep (P = 0.0391) but did not alter wakefulness and NREM sleep (P = 0.3438 and 0.5000, respectively). The decrease in REM sleep (695.70 s ± 71.95 versus 382.12 s ± 72.21) is at the expense of non-significant increases in the time spent in wakefulness (3956 s ± 584.70 versus 4199 s ± 434; P = 0.3804) and NREM sleep (6148 s ± 534.50 versus 6219 s ± 423.50; P = 0.4596). To control for potential adverse effects of the vector injection and non-specific effects of 1.0 mg/kg CNO on sleep-wake architecture, additional studies were conducted in sham Vgat-Cre (n = 5) and Vglut2-Cre (n = 5) mice. Duration of wakefulness, NREM sleep, and REM sleep were not different in sham versus transfected mice, and CNO administration to sham mice did not alter the duration of sleep and wakefulness states (Figure S3).
Figure 2. Chemogenetic stimulation of GABAergic neurons in the MnPO increased NREM sleep and decreased REM sleep.
A. Schematic representation of AAV injection into the median preoptic nucleus (MnPO) for expression of the excitatory designer receptor hM3Dq. Following the expression of hM3Dq receptors in the MnPO, mice were implanted with electrodes for recording the electroencephalogram (EEG; blue) and electromyogram (EMG; green). B. Representative EEG and EMG traces recorded from the same Vgat-Cre mouse during wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. C. and D. Hypnograms displaying the time course of sleep and wakefulness during the 3-h recording period. The spectrograms below each hypnogram show state-specific changes in frontal power density (red = maximum, blue = minimum) across the respective recording session. Time 0 on the abscissa indicates the time at which vehicle (VEH) or clozapine-N-oxide (CNO; 1.0 mg/kg) was injected. E, F and G show the effect of CNO administration on the duration (in s) of wakefulness (W), NREM sleep and REM sleep over the 3-h recording period. Asterisks indicate a significant difference (P < 0.05) from vehicle control using a one-tailed Wilcoxon test. Data in E-G are shown as mean ± standard error of the mean. See also Figures S3 and S4
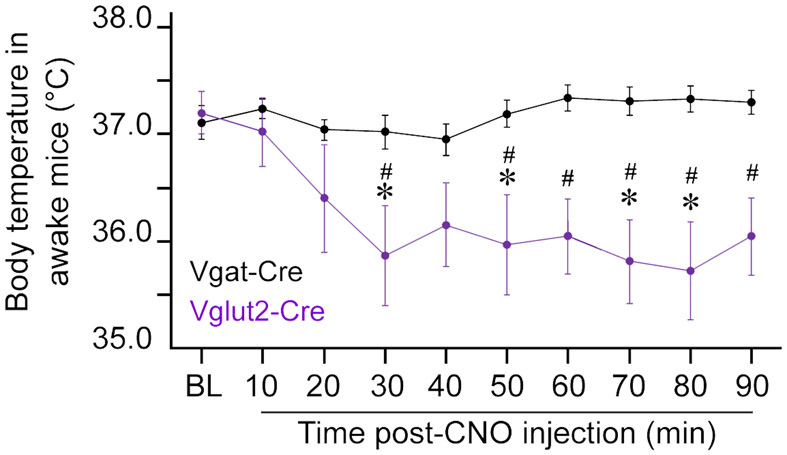
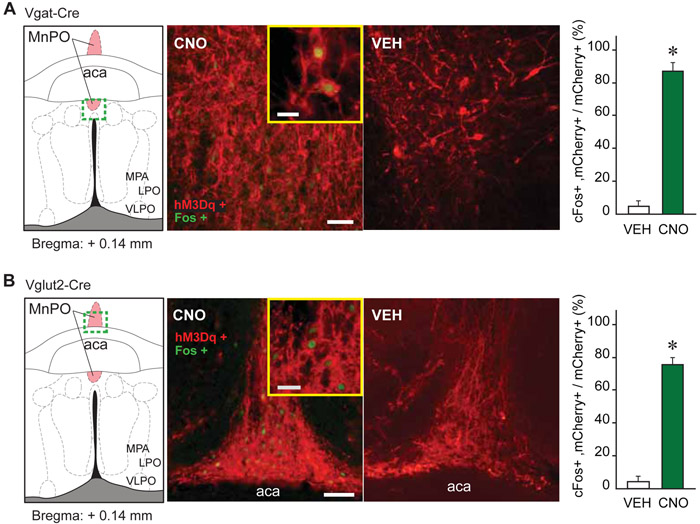
Given the recently identified role of MnPO glutamatergic neurons in regulating both temperature and sleep [9, 10], changes in body temperature were assessed in a subset of awake mice before and during 90 minutes after 1.0 mg/kg CNO administration. As expected [10], CNO administration to Vglut2-Cre mice (n = 9) induced a sustained reduction in body temperature (treatment effect; F(1, 170) = 65.41, P < 0.0001), while there was no temperature change in Vgat-Cre mice (n = 10) (Figure 3). There was no significant correlation between the number of mCherry-positive glutamatergic neurons and the magnitude of temperature change. In both mouse lines, CNO administration (1.0 mg/kg) significantly increased cFos expression in MnPO hM3Dq-positive neurons (P < 0.0001 in Vgat-Cre and P = 0.0002 in Vglut2-Cre mice) (Figure 4). Together, these data indicate that activation of MnPO GABAergic and glutamatergic neurons results in the expected changes of promoting sleep and reducing temperature but does not alter anesthetic state transitions.
Figure 3. Stimulation of glutamatergic (but not GABAergic) neurons in the MnPO produced hypothermia in awake mice.
The figure plots the change in core body temperature in awake mice before and after an intraperitoneal injection of clozapine-N-oxide (CNO; 1.0 mg/kg). Asterisks indicate significant differences (P < 0.05) from baseline and hashtag symbols indicate significant differences between mouse lines. Statistical comparisons were conducted using a two-way ANOVA with Bonferroni’s correction for multiple comparisons. The gray area indicates the time period (60 to 90 minutes) during which the time to loss and resumption of consciousness was assessed in anesthesia experiments. Data are shown as mean ± standard error of the mean. See also Figure S4.
Figure 4. Verification of MnPO Vgat+ and Vglut2+ cell activation by CNO.
A and B. cFos expression (green nuclei) in mCherry expressing (red) neurons within the MnPO of Vgat-Cre and Vglut2-Cre mice, 90 minutes after an intraperitoneal injection of clozapine-N-oxide (CNO; 1.0 mg/kg) or vehicle solution (VEH). The bar graphs plot the percentage (mean + standard error of the mean) of mCherry expressing neurons that also express cFos, over the total number of mCherry positive cells after vehicle or CNO administration. Asterisks indicate a significant difference (P < 0.05) from vehicle control using a one-tailed Mann Whitney test. The strong increase in cFos expression in GABAergic (Vgat+) and glutamatergic (Vglut2+) neurons provides further validation of hM3Dq receptors in the MnPO. Abbreviations: aca, anterior commissure; LPO, lateral preoptic area; MnPO, median preoptic nucleus; MPA, medial preoptic area; VLPO, ventrolateral preoptic nucleus. Calibration bars in A and B, 50 μm and 20 μm (inset).
Activation of Neuronal Subpopulations in the Ventrolateral Preoptic Nucleus Did Not Alter Anesthetic-State Transitions
The expression pattern of hM3Dq designer receptors in this study (Figure S5) is consistent with what has been termed the extended VLPO [14, 15] but we will hereafter refer to it as VLPO. Given the unexpected finding that selective manipulation of two neuronal subpopulations within the MnPO did not modulate the entry to and exit from the anesthetized state, we conducted the same experiments in VLPO, a hypothalamic nucleus that is critical for the generation of NREM sleep, as a control site [15]. Figure 5 shows that activation of GABAergic or glutamatergic neurons in the VLPO, prior to exposure to 1.5% (n = 10 Vgat-Cre and n = 6 Vglut2-Cre mice) or 1.2% (n = 4 Vgat-Cre and n = 4 Vglut2-Cre mice) isoflurane, did not alter anesthetic induction or recovery time.
Figure 5. Chemogenetic stimulation of GABAergic and glutamatergic neurons in the VLPO did not alter anesthetic state transitions.
A. Schematic representation of bilateral AAV injections into the ventrolateral preoptic nucleus (VLPO). mCherry (red) indicates the expression of the excitatory designer receptor hM3Dq within the VLPO of a Vglut2-Cre mouse. B. Time to loss and resumption of consciousness (left and right panels, respectively) in Vgat-Cre mice exposed to 1.5% isoflurane. C. Time to loss and resumption of consciousness (left and right panels, respectively) in Vglut2-Cre mice exposed to 1.5% isoflurane. D and E show the time to loss and resumption of consciousness (left and right panels, respectively) in Vgat-Cre and Vglut2-Cre mice exposed to 1.2% isoflurane. Statistical comparisons were conducted using two-tailed Wilcoxon test. Abbreviations: aca, anterior commissure; CNO, clozapine-N-oxide; LOC, loss of consciousness; VLPO, ventrolateral preoptic nucleus; ROC, resumption of consciousness; 3V, third ventricle. Calibration bar in A, 50 μm. Data in B-E are shown as mean ± standard error of the mean. See also Figures S2 and S5.
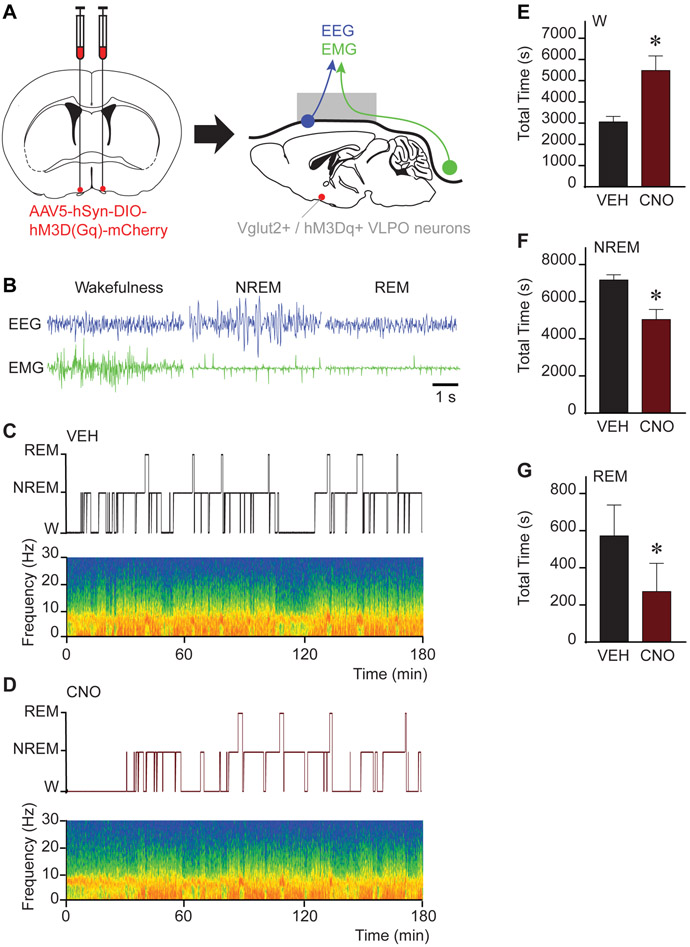
Activation of Neuronal Subpopulations in the Ventrolateral Preoptic Nucleus Altered Sleep-Wake Architecture
Following anesthesia experiments, we conducted sleep studies to determine whether similar conditions of activated GABAergic and glutamatergic VLPO neurons play a role in sleep-wake control. These sleep studies were conducted during the same time period (i.e., 3 h spanning CNO/vehicle injection to emergence) as the anesthesia experiments. Representative examples of the electrophysiological signatures of wake, NREM sleep, and REM sleep in Vglut2-Cre mice are shown in Figure 6B. The temporal organization of sleep-wake states and changes in power spectral density after vehicle or 1.0 mg/kg CNO administration are depicted in Figures 6C and 6D, respectively. Group data plotted in Figures 6E-G demonstrate that activation of glutamatergic neurons in the VLPO (n = 4 mice) increases the time in wakefulness (P = 0.0233; 79% increase) and reduces NREM sleep (P = 0.0351) and REM sleep (P = 0.0176). There were no significant changes in the number of bouts or bout duration in any of the three states. Chemogenetic stimulation of GABAergic neurons in the VLPO (n = 4 mice) did not alter sleep-wake architecture (P > 0.1000 for wakefulness, NREM and REM sleep). Collectively, activation of GABAergic or glutamatergic neurons in the VLPO does not alter state transitions induced by isoflurane, despite a robust alteration of sleep-wake architecture induced by activation of glutamatergic subpopulations.
Figure 6. Chemogenetic stimulation of glutamatergic neurons in the VLPO increased wakefulness and decreased sleep.
A. Schematic representation of bilateral AAV injections into the ventrolateral preoptic nucleus (VLPO) for expression of the excitatory designer receptor hM3Dq. Following the expression of hM3Dq receptors in the VLPO, mice were implanted with electrodes for recording the electroencephalogram (EEG; blue) and electromyogram (EMG; green). B. Representative EEG and EMG signals recorded from the same Vglut2-Cre mouse during wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. C. and D. Hypnograms displaying the time course of sleep and wakefulness during the 3-h recording period. The spectrograms below each hypnogram show state-specific changes in frontal power density (red = maximum, blue = minimum) across the respective recording session. Time 0 on the abscissa indicates the time at which vehicle (VEH) or clozapine-N-oxide (CNO; 1.0 mg/kg) was injected. E, F and G show the effect of CNO administration on the duration (in s) of wakefulness (W), NREM sleep and REM sleep over the 3-h recording period. Asterisks indicate a significant difference (P < 0.05) from vehicle control using a two-tailed Wilcoxon test. Data in E-G are shown as mean ± standard error of the mean. See also Figures S3 and S5.
DISCUSSION
These data show that chemogenetic stimulation of GABAergic or glutamatergic neurons in the MnPO and VLPO does not substantially alter state transitions related to isoflurane anesthesia. To our knowledge, this is the first direct assessment of a causal role for two major sleep-promoting nuclei [9, 10, 15], to the resolution of a single-cell subtype, in the mechanisms of inhaled general anesthesia. Our results are highly relevant –and rather unexpected– because a prevailing hypothesis in the field posits that general anesthetics produce the loss of consciousness by co-opting nuclei that regulate sleep-wake states [1, 2, 16]. This hypothesis has been supported by abundant correlative evidence suggesting that the preoptic region of the hypothalamus, particularly the medial preoptic area [5] and VLPO [2, 4, 6-8], plays a key role in the relationship between sleep and anesthesia. Consistent with the shared circuits hypothesis, previous work using cFos-dependent-tagging and selective stimulation of previously active (tagged) neurons showed that sedation with dexmedetomidine and non-rapid eye movement sleep are produced, in part, by the same neuronal array within the preoptic area [17]. Preoptic galanin neurons mediate, in part, sleep homeostasis as well as the sedative and hypothermic effect of dexmedetomidine [18]. However, dexmedetomidine is clinically used in humans as a sedative and its hypnotic, NREM-sleep-like effect is readily reversible upon stimulation, unlike general anesthesia with isoflurane, a halogenated ether used at clinically relevant concentrations in the current study.
Importantly, our study shows that activation of GABAergic and glutamatergic neurons in the MnPO and VLPO altered sleep-wake architecture during the same time period in which anesthesia experiments were conducted. Consistent with previously published work [9, 10], activation of GABAergic neurons in the MnPO increased the amount of NREM sleep. Another remarkable finding was that activation of glutamatergic neurons in the VLPO caused a strong increase in wakefulness and a reduction in both NREM and REM sleep. These data are novel because no previous studies have examined the role of VLPO glutamatergic neurons in the regulation of sleep and wakefulness. Neurons in the MnPO and VLPO play a dual role in sleep generation and sleep homeostasis [11, 17, 19]. The modulation of sleep and wakefulness caused by stimulation of preoptic neurons is of relevance to our study because of the well-known relationship between sleep homeostasis and anesthesia. For example, sleep deprivation or sleep loss caused by chronic VLPO lesions potentiates the hypnotic effect of propofol and the inhalational anesthetic sevoflurane [6, 20, 21]. Additionally, general anesthesia has been shown to satisfy the homeostatic sleep drive in a drug-specific manner [21-23]. Thus, the dual role of these preoptic neurons in sleep generation and the regulation of sleep homeostasis strengthened our prediction that stimulation of preoptic sleep- or wake-promoting neurons would alter anesthetic induction and recovery time. Contrary to our prediction, activation of preoptic neurons that increase sleep or wakefulness did not affect anesthetic state transitions. An interesting possibility that remains to be explored is whether increasing the time between CNO injection and behavioral testing, allowing more time for the sleep/wake drive to accrue, or varying the circadian time of the experiment (i.e., early morning versus evening), would yield a different outcome. It is also important to consider other subcortical nodes that are not classically considered critical for sleep regulation but might play a more relevant role in general anesthetic mechanisms. For example, recent and noteworthy studies showed that glutamatergic neurons in the lateral habenula facilitate the hypnotic effect of the intravenous anesthetic propofol [24], and neuroendocrine cells within the supraoptic nucleus promote NREM sleep and facilitate general anesthesia [25]. These studies, in conjunction with our complementary findings, should prompt the field to consider exploring additional brain regions and nuclei beyond those classically known to regulate sleep and wakefulness. Furthermore, it is becoming increasingly evident that widespread neural networks encompassing multiple nuclei within the rostral hypothalamus orchestrate the features of complex behaviors and states such as sleep, sedation, and anesthesia [10, 11, 17, 18, 26, 27].
An unexpected finding was that activation of glutamatergic neurons in the MnPO reduced REM sleep and did not alter NREM sleep. Harding et al. [10] recently demonstrated that sleep generation and the reduction of body temperature are controlled by the same subset of glutamatergic neurons (Vglut2, nNOS1) localized within the MnPO and the medial preoptic area. This is important because we show that activation of glutamatergic neurons in the MnPO caused a sustained decrease in body temperature, indicating that we activated at least a proportion of the glutamatergic cells that also promote sleep. Contrasting our results with those obtained in control mice, it seems unlikely that tissue damage caused by the injection procedure or the vector itself [13] selectively lesioned the sleep-promoting cells sparing a neuronal group that causes hypothermia. The fact that VLPO GABAergic neurons did not increase sleep during the light period is not inconsistent with prior studies. GABAergic neurons in the VLPO that co-express galanin project to (among other major wake-promoting centers) the tuberomammillary nucleus of the hypothalamus [28]. Optogenetic and chemogenetic stimulation of VLPO GABAergic/galaninergic neurons increases NREM sleep but chemogenetic activation during the light period only produces a modest increase [15]. An evident limitation of our study is that our approach did not allow us to assess different subpopulations of VLPO GABAergic cells by targeting, for example, projection-specific or galanin-positive neurons. However, the discrepancy between our findings and the evidence reviewed above may also be attributable to the vast neurochemical and functional diversity of both GABAergic and glutamatergic neurons in the preoptic area [9, 10, 29]. It is important to note that the reduction of body temperature in Vglut2-Cre mice, as well as the changes in sleep architecture and Fos expression in both Vgat-Cre and Vglut2-Cre mice, provided functional validation of hM3Dq receptors in the MnPO and VLPO.
Collectively, our data suggest that activation of preoptic GABAergic or glutamatergic neurons that increase sleep or wakefulness does not substantially influence anesthetic state transitions. These data also suggest that the correlative evidence for a mechanistic overlap of sleep and anesthesia at the level of an individual nucleus, or even individual cell subtypes, might not necessarily have strong causal significance. The present study is limited in its restriction to one anesthetic and only used neuronal stimulation techniques, but the findings bring into question the long-standing hypothesis in the field that activation of individual nuclei associated with the promotion of sleep is a major contributor to the mechanism of general anesthesia. Furthermore, given the strong biological plausibility and correlative evidence supporting the shared circuits hypothesis, these data also suggest that the systems neuroscience of general anesthesia requires causal investigations and cannot rely upon a priori or theoretical bases regarding the circuits mediating anesthetic-induced unconsciousness.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
This study did not generate any new unique materials. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Giancarlo Vanini (gvanini@umich.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All procedures using animals were approved by the Institutional Animal Care and Use Committee, the Institutional Biosafety Committee, and were conducted following recommendations from the Guide for the Care and Use of Laboratory Animals. We studied adult Vgat-IRES-Cre: Slc32a1tm2(cre)Lowl/J (JAX® mice, Stock # 016962) [9, 10, 31-34], and Vglut2-IRES-Cre: Slc17a6tm2(cre)Low/J (JAX® mice, Stock # 016963) [32-36] mice (16-20 weeks old, weighing 18 – 25g at the time of surgery; 27 male, 26 female Vgat-Cre and 32 male, 22 female Vglut2-Cre). Mice were bred at the institutional breeding colony facility and housed in a 12-h light: dark cycle with unrestricted access to food and water. All mice were genotyped (Transnetyx) before use.
METHODS DETAILS
Viral Vector and Chemicals
For selective expression of excitatory hM3Dq designer receptors in Vgat+ (GABAergic) and Vglut2+ (glutamatergic) neurons of the MnPO and VLPO we used the Cre-inducible adeno-associated viral vector AAV5-hSyn-DIO-hM3D(Gq)-mCherry [37]. The vector was purchased from the University of North Carolina Vector Core (Chapel Hill, NC, USA) and Addgene (Cat.# 50459-AAV5) and had a titer concentration of 3.7 to 7.8 X 1012 genome copies per ml.
Clozapine-N-oxide (CNO) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). CNO was dissolved in sterile saline containing DMSO. A stock solution of CNO (0.1 mg/ml in saline and 0.5% DMSO) was made, divided in aliquots, and stored at −20°C for subsequent use. For each experiment, one aliquot of the CNO stock solution was allowed to thaw at room temperature protected from the light until use; for the 0.5 mg/kg dose, the CNO solution was diluted in sterile saline to achieve the desired final concentration. The injection volume was 0.1 ml per 10 g of mouse weight.
Surgical Procedures for Stereotaxic Viral Vector Injection and Sleep Studies
The procedure for vector injection into the MnPO and VLPO was conducted under sterile conditions and general anesthesia. Briefly, mice were anesthetized in an acrylic induction chamber with 5.0% isoflurane (Hospira, Inc., Lake Forest, IL, USA) in 100% O2. The delivered concentration of isoflurane was monitored continuously by spectrometry (Cardiocap™/5; Datex-Ohmeda, Louisville, CO, USA). When anesthetized, mice received an injection of carprofen (5 mg/kg, subcutaneous) for preemptive analgesia and were positioned within a Kopf Model 962 stereotaxic frame fitted with a mouse adaptor (Model 922) and a mouse anesthesia mask (Model 907) (David Kopf Instruments, Tujunga, CA, USA). The concentration of isoflurane was then reduced to 1.6 to 2.0% for the remainder of the surgical procedure. Core body temperature was maintained at 37-38° C using a water-filled pad connected to a heat pump (Gaymar Industries, Orchard Park, NY, USA). Based on pilot microinjection studies, we determined that microinjecting 50 nL of the undiluted viral vector was optimal for achieving a robust and sustained receptor expression localized to the MnPO. For VLPO experiments, mice received microinjections of 36 nL of AAV5-hSyn-DIO-hM3D(Gq)-mCherry [15]. For MnPO studies, the vector was microinjected at stereotaxic coordinates 0.40 mm anterior to bregma, 0.0 mm relative to the midline, and 4.75 mm ventral to bregma (Figure 1A and 2A). For VLPO studies, the vector was microinjected bilaterally at stereotaxic coordinates 0.15 mm anterior to bregma, ±0.5 mm relative to the midline, and 5 mm below the dura (Figure 5A and 6A). The virus injection (5 nl/min) was performed using a Hamilton Neuros Syringe 7000 (5 μL; Hamilton Company, Reno, NV, USA) mounted on a microinjection syringe pump connected to a digital Micro2T controller (Model UMP3T-2; World Precision Instruments, Sarasota, FL, USA). Following the injection procedure, the syringe was kept in position for an additional 5 minutes to avoid vector reflux. Three weeks after the vector injection, a subset of mice used for sleep studies was implanted with screw electrodes (8IE3632016XE, E363/20/1.6/SPC; Plastics One, Roanoke, VA, USA) to record the electroencephalogram (EEG) from frontal (1.5 mm anterior to bregma and ±2.0 mm relative to the midline), and occipital (3.2 mm posterior to bregma and ±3.0 mm relative to the midline) cortices (Figures 2A and 6A). A screw electrode implanted over the cerebellum was used as reference for monopolar EEG recordings, and two electrodes (8IE36376XXXE, E363/76/SPC; Plastics One) implanted bilaterally in the dorsal neck muscles were used to record the electromyogram (EMG). Thereafter, all electrodes were inserted into a six-pin electrode pedestal (MS363; Plastics One) and cemented to the skull using dental acrylic (Fast Cure Powder/Liquid, Product# 335201; GC America, Inc., Alsip, IL, USA). Isoflurane delivery was discontinued, and mice were kept warm and monitored until fully ambulatory. Analgesia was maintained with carprofen for a minimum of 48 h post-surgery. All mice were housed with their littermates and allowed to recover for at least two weeks before conditioning began.
Experimental Protocols
Quantification of Anesthetic State Transitions.
Mice were conditioned to being handled and to the gas-tight testing chamber for 5 days, and were given at least 4 weeks between vector injection and experimentation. All studies were conducted between 11:30 AM and 3:30 PM (lights on at 6:00 AM). We used a within-subject study design so each mouse received all treatments and served as its own control in paired-wise comparisons. Experiments in the same mouse were separated by a wash out period of at least 3 days. On the day of the experiment, mice received randomized intraperitoneal injections of vehicle solution or CNO (0.5 or 1.0 mg/kg) and were placed back in their home cage until testing. Thereafter (60 min post-injection, MnPO experiments; 30 min post-injection, VLPO experiments), mice were anesthetized in a gas-tight acrylic chamber pre-filled with 1.5% or 1.2% isoflurane in oxygen, and anesthesia was maintained for 30 minutes. The concentration of isoflurane was measured continuously using a B40v3 monitor (GE Healthcare, Datex-Ohmeda, Inc., Pittsburgh, PA, USA). The temperature at the bottom of the chamber was maintained between 37 – 38° C during the experiment. Anesthetic induction and recovery were quantified, respectively, as the time to loss and resumption of righting response; both are widely used surrogate measures of loss and resumption of consciousness in rodent experiments [6, 16, 17, 38-45]. Additionally, all the experiments were video-recorded for subsequent analysis by an observer who was blinded to the treatment condition and mouse line. Agreement in the quantification of loss and resumption of consciousness between experimenters (real time assessment) and blinded investigator (offline assessment) was greater than 95%. Times to loss and resumption of consciousness from MnPO experiments are illustrated in Figures 1B-E and VLPO experiments in Figures 5B-E (Cre mice expressing hM3Dq receptors) and Figure S1 and S2 (control Cre mice that did not express hM3Dq receptors, sham-injected and naïve).
Measurements of Body Temperature After Activation of GABAergic or Glutamatergic Neurons in the MnPO.
Temperature experiments were conducted after MnPO anesthesia and sleep experiments. Vgat-Cre and Vglut2-Cre mice were briefly and gently restrained for measurements of body temperature using a lubricated mouse rectal probe (Model# 600-1000, Barnant Company, Barrington, IL, USA). Temperature was measured before and after an intraperitoneal injection of CNO (1 mg/kg) in 10-min intervals for 90 minutes [46]. Hypothermic mice were placed back in their cages under a heating lamp between testing intervals. Changes in core body temperature after CNO administration are shown in Figure 3.
Electroencephalographic - Electromyographic Recordings and Analysis of Sleep-Wakefulness States
Three weeks after vector injection, mice were conditioned to tethering in the recording setup for 5 days before sleep experiments began. Thereafter, mice received an intraperitoneal injection (10:00 AM) of vehicle or CNO (1.0 mg/kg), and EEG/EMG signals were recorded continuously for 3 hours –the duration of anesthesia experiments– post-injection. Monopolar EEG signals and EMG signals were recorded and digitized using a Model 1700 AC amplifier (A-M Systems, Sequim, WA, USA), a Micro3 1401 acquisition unit and Spike2 software (Cambridge Electronic Design, Cambridge, UK). Electrophysiologic signals were bandpass filtered between 0.1 – 500 Hz (EEG) and 10 – 500 Hz (EMG). A video recording time-synchronized with our EEG-EMG recordings was used for behavioral assessments during sleep/wake analysis. States of wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep were manually scored off-line in 5-s epochs using standard criteria (Figures 2B and 6B). Wakefulness was recognized by the presence of low-amplitude, high-frequency EEG activity, with high muscle tone and active movements. NREM sleep was identified by the presence of high-amplitude, low-frequency EEG activity with reduced muscle tone. REM sleep was recognized by low-amplitude, high-frequency EEG activity with prominent theta waveforms, along with sustained muscle atonia. The total time spent in wakefulness, NREM sleep and REM sleep was compared between treatment conditions in Cre mice expressing hM3Dq receptors (Figures 2 and 6) and sham-injected controls (Figure S3).
Identification of Reproductive Cycle Stage
After each anesthesia experiment, female mice were briefly restrained and vaginal smear samples were collected following a published protocol [47, 48]. The unstained material was immediately observed using low-illumination light microscopy under a 10x objective (Primo Star, Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). The cycle stage was identified as proestrus, estrus, metestrus and diestrus, according to standard histologic criteria [47].
Immunohistochemistry for Localization of Vector Injection Sites (mCherry) and Assessment of CNO-induced Neuronal Activation (cFos)
For cFos/mCherry immunohistochemistry (Figure 4), mice received an intraperitoneal injection of vehicle or CNO (1 mg/kg) 90 minutes before euthanasia. Mice were deeply anesthetized with isoflurane, perfused transcardially with 0.1 M phosphate buffered saline pH 7.4 (PBS) followed by 5% formalin in PBS using a MasterFlex perfusion pump (Cole Palmer, Vernon Hills, IL, USA). The brains were removed and post-fixed in 5% formalin overnight at 4° C. Thereafter, brains were cryoprotected with 20% sucrose in PBS for 3 to 5 days, frozen in Tissue–Plus (Fisher Healthcare, Houston, TX, USA), and sectioned coronally at 40 μm using a cryostat (CM3050S, Leica Microsystems, Nussloch, Germany). A series of brain sections containing the MnPO were blocked in PBS containing 0.25% Triton X-100 and 3% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 60 min at room temperature. Sections were then processed for mCherry immunolabeling, or sequentially processed for double immunohistochemistry for mCherry and cFos. For mCherry, sections were incubated in primary antiserum overnight at room temperature. We used a rat monoclonal anti-mCherry (1:30000; Thermo Fisher Scientific, Cat.#: M11217). The next morning, sections were washed in PBS and incubated in a donkey anti-rat secondary antiserum (1:500; Alexa Fluor 594, Thermo Fisher Scientific, Cat.#: A-21209) for 2 h at room temperature. For cFos, sections were incubated in a rabbit polyclonal anti-cFos (1:5000; Millipore Sigma, Cat.#: ABE457) overnight at room temperature. The next morning, slices were washed in PBS and incubated in a donkey anti-rabbit secondary antiserum (1:500; Alexa Fluor 488, Thermo Fisher Scientific, Cat.#: A-21206) for 2 h at room temperature. Following incubation in the respective secondary antisera, tissues were washed in PBS, float-mounted on glass slides and coverslipped with SlowFade Diamond (S36972 or S36973; Thermo Fisher Scientific). All brain sections were examined using fluorescence microscopy (BX43, Olympus America Inc., Waltham, MA, USA). Only brains in which the expression of the designer receptor hM3Dq was localized to the MnPO or VLPO were included in the analysis (Figures S4 and S5). A mouse brain atlas [30] was used for reference. The number mCherry-positive neurons that expressed cFos was quantified as the percentage of total neurons expressing mCherry in the MnPO (Figures 4A and 4B).
QUANTIFICATION AND STATISTICAL ANALYSIS
Data analyses were performed using PRISM v7.0 (GraphPad Software Inc., La Jolla, CA). All data were tested for normality and reported as mean ± standard error of the mean. A P value < 0.05 was considered statistically significant. The time to loss and resumption of consciousness is reported as the time in seconds. Differences in the time to loss and resumption of consciousness in MnPO-anesthesia experiments in which 3 concentrations of CNO were used (data shown in Figures 1B, 1C and S1) were assessed by a Friedman test followed, when applicable, by a Dunn’s multiple comparison test. All pairwise comparisons (i.e., vehicle versus 1.0 mg/kg CNO) of time to loss and resumption of consciousness were evaluated by a two-tailed Wilcoxon test. Changes in the time spent in wakefulness, NREM sleep and REM sleep were assessed by a one-tailed (MnPO Vgat and Vglut2, and VLPO Vgat) or two-tailed (VLPO Vglut2) Wilcoxon test. Differences in body temperature as a function of treatment, time and mouse line were evaluated by a two-way analysis of variance (ANOVA) followed by a Bonferroni’s multiple comparisons test. Last, the percentage of active cells (cFos+) after vehicle and CNO administration was compared by a one-tailed Mann Whitney test.
DATA AND CODE AVAILABILITY
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request. This study did not generate any code.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat monoclonal anti-mCherry | Fisher Scientific | M11217 |
| Donkey anti-rat, Alexa Fluor 594 | Fisher Scientific | A-21209 |
| Rabbit polyclonal anti-cFos | Milipore Sigma | ABE457 |
| Donkey anti-rabbit, Alexa Fluor 488 | Fisher Scientific | A-21206 |
| Bacterial and Virus Strains | ||
| AAV5-hSyn-DIO-hM3D(Gq)-mCherry | Addgene | 50459-AAV5 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Clozapine-N-oxide | Sigma-Aldrich | C0832-5MG |
| Dimethyl sulfoxide | Sigma-Aldrich | 276855 |
| Isoflurane | Hospira, Inc., Lake Forest, IL, USA | N/A |
| Carprofen (Rimadyl®) | Pfizer UK | N/A |
| Dental Acrylic | GC America, Inc., Alsip, IL, USA | 335201 |
| Phosphate Buffered Saline | Fisher Scientific | BP3994 |
| Formalin (Buffered, 10%) | Fisher Scientific | SF100-4 |
| Tissue-Plus™ O.C.T. Compound | Fisher Scientific | 23-730-571 |
| Sucrose | Fisher Scientific | S25590 |
| Triton™ X-100 | Sigma-Aldrich | X100 |
| Normal goat serum | Vector Laboratories | S-1000 |
| SlowFade™ Diamond Antifade Mountant | Fisher Scientific | S36972 |
| SlowFade™ Diamond Antifade Mountant with DAPI | Fisher Scientific | S36973 |
| Experimental Models: Organisms/Strains | ||
| Vgat-IRES-Cre: Slc32a1tm2(cre)Lowl/J | Jackson Laboratories | Stock # 016962 |
| Vglut2-IRES-Cre: Slc17a6tm2(cre)Lowl/J | Jackson Laboratories | Stock # 016963 |
| Software and Algorithms | ||
| Prism | GraphPad | Version 7 |
| Spike2 | Cambridge Electronic Design Ltd. | Version 7 |
| Other | ||
| EEG screw electrodes | Plastics One | 8IE3632016XE, E363/20/1.6/SPC |
| EMG electrodes | Plastics One | 8IE36376XXXE, E363/76/SPC |
| Six-pin electrode pedestal | Plastics One | MS363 |
Highlights:
Activation of MnPO GABAergic neurons increased NREM sleep
Activation of VLPO glutamatergic neurons caused a robust increase in wakefulness
Activating these same preoptic neurons did not alter anesthetic state transitions
Neurons controlling sleep-wake states do not necessarily mediate general anesthesia
ACKNOWLEDGEMENTS
The authors thank Mary A. Norat from the Department of Anesthesiology, University of Michigan for expert assistance. We are grateful to Drs. Carol F. Elias and David Garcia-Galiano from the Department of Molecular & Integrative Physiology, University of Michigan, for their guidance to identify the reproductive cycle stage in female mice. This work was funded by the National Institutes of Health (R01 GM124248 to GV and GAM), Bethesda, MD, USA, and the Department of Anesthesiology, University of Michigan Medical School, Ann Arbor.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lydic R, and Biebuyck JF (1994). Sleep neurobiology: relevance for mechanistic studies of anaesthesia. Br J Anaesth 72, 506–508. [DOI] [PubMed] [Google Scholar]
- 2.Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, and Kelz MB (2012). Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol 22, 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, and Maze M (2002). The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5, 979–984. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, and Saper CB (2008). Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 508, 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung A, Bluhm B, and Mendelson WB (2001). The hypnotic effect of propofol in the medial preoptic area of the rat. Life Sci 69, 855–862. [DOI] [PubMed] [Google Scholar]
- 6.Eikermann M, Vetrivelan R, Grosse-Sundrup M, Henry ME, Hoffmann U, Yokota S, Saper CB, and Chamberlin NL (2011). The ventrolateral preoptic nucleus is not required for isoflurane general anesthesia. Brain Res 1426, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarren HS, Chalifoux MR, Han B, Moore JT, Meng QC, Baron-Hionis N, Sedigh-Sarvestani M, Contreras D, Beck SG, and Kelz MB (2014). alpha2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J Neurosci 34, 16385–16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han B, McCarren HS, O'Neill D, and Kelz MB (2014). Distinctive recruitment of endogenous sleep-promoting neurons by volatile anesthetics and a nonimmobilizer. Anesthesiology 121, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT, Hormann N, Chang WC, Zhang Z, Do JP, et al. (2017). Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding EC, Yu X, Miao A, Andrews N, Ma Y, Ye Z, Lignos L, Miracca G, Ba W, Yustos R, et al. (2018). A neuronal hub binding sleep initiation and body cooling in response to a warm external stimulus. Curr Biol 28, 2263–2273 e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam MA, Kumar S, McGinty D, Alam MN, and Szymusiak R (2014). Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol 111, 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, and McGinty D (2002). Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol 543, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezai Amin S, Gruszczynski C, Guiard BP, Callebert J, Launay JM, Louis F, Betancur C, Vialou V, and Gautron S (2019). Viral vector-mediated Cre recombinase expression in substantia nigra induces lesions of the nigrostriatal pathway associated with perturbations of dopamine-related behaviors and hallmarks of programmed cell death. J Neurochem 150, 330–340. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, and Saper CB (2002). Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci 22, 4568–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroeger D, Absi G, Gagliardi C, Bandaru SS, Madara JC, Ferrari LL, Arrigoni E, Munzberg H, Scammell TE, Saper CB, et al. (2018). Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun 9, 4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanini G, Watson CJ, Lydic R, and Baghdoyan HA (2008). Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology 109, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Ferretti V, Guntan I, Moro A, Steinberg EA, Ye Z, Zecharia AY, Yu X, Vyssotski AL, Brickley SG, et al. (2015). Neuronal ensembles sufficient for recovery sleep and the sedative actions of alpha2 adrenergic agonists. Nat Neurosci 18, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Miracca G, Yu X, Harding EC, Miao A, Yustos R, Vyssotski AL, Franks NP, and Wisden W (2019). Galanin neurons unite sleep homeostasis and alpha2-adrenergic sedation. Curr Biol 29, 3315–3322 e3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gvilia I, Xu F, McGinty D, and Szymusiak R (2006). Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26, 9426–9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tung A, Szafran MJ, Bluhm B, and Mendelson WB (2002). Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology 97, 906–911. [DOI] [PubMed] [Google Scholar]
- 21.Pal D, Lipinski WJ, Walker AJ, Turner AM, and Mashour GA (2011). State-specific effects of sevoflurane anesthesia on sleep homeostasis: selective recovery of slow wave but not rapid eye movement sleep. Anesthesiology 114, 302–310. [DOI] [PubMed] [Google Scholar]
- 22.Tung A, Lynch JP, and Mendelson WB (2001). Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg 92, 1232–1236. [DOI] [PubMed] [Google Scholar]
- 23.Pick J, Chen Y, Moore JT, Sun Y, Wyner AJ, Friedman EB, and Kelz MB (2011). Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology 115, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelegen C, Miracca G, Ran MZ, Harding EC, Ye Z, Yu X, Tossell K, Houston CM, Yustos R, Hawkins ED, et al. (2018). Excitatory pathways from the lateral habenula enable propofol-induced sedation. Curr Biol 28, 580–587 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang-Xie LF, Yin L, Zhao S, Prevosto V, Han BX, Dzirasa K, and Wang F (2019). A common neuroendocrine substrate for diverse general anesthetics and sleep. Neuron 102, 1053–1065 e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Lin JS, and Sakai K (2009). Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161, 269–292. [DOI] [PubMed] [Google Scholar]
- 27.Sakai K (2011). Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience 182, 144–161. [DOI] [PubMed] [Google Scholar]
- 28.Sherin JE, Elmquist JK, Torrealba F, and Saper CB (1998). Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18, 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C, et al. (2018). Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G and Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates, Second Edition (San Diego: Academic; ). [Google Scholar]
- 31.Vong L, Ye C, Yang Z, Choi B, Chua S Jr., and Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, and Scammell TE (2017). Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci 37, 1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, and Fuller PM (2015). Basal forebrain control of wakefulness and cortical rhythms. Nat Commun 6, 8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott SB, Machado NL, Geerling JC, and Saper CB (2016). Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci 36, 8228–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. (2014). An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geerling JC, Yokota S, Rukhadze I, Roe D, and Chamberlin NL (2017). Kolliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol 525, 1844–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121, 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkire MT, McReynolds JR, Hahn EL, and Trivedi AN (2007). Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 107, 264–272. [DOI] [PubMed] [Google Scholar]
- 39.Tung A, Herrera S, Szafran MJ, Kasza K, and Mendelson WB (2005). Effect of sleep deprivation on righting reflex in the rat is partially reversed by administration of adenosine A1 and A2 receptor antagonists. Anesthesiology 102, 1158–1164. [DOI] [PubMed] [Google Scholar]
- 40.Pal D, Silverstein BH, Lee H, and Mashour GA (2016). Neural correlates of wakefulness, sleep, and general anesthesia: An experimental study in rat. Anesthesiology 125, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudetz AG, Vizuete JA, and Pillay S (2011). Differential effects of isoflurane on high-frequency and low-frequency gamma oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology 114, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, and Yanagisawa M (2008). An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A 105, 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanini G, Nemanis K, Baghdoyan HA, and Lydic R (2014). GABAergic transmission in rat pontine reticular formation regulates the induction phase of anesthesia and modulates hyperalgesia caused by sleep deprivation. Eur J Neurosci 40, 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidera JA, Taylor NE, Lee JT, Vlasov KY, Pei J, Stephen EP, Mayo JP, Brown EN, and Solt K (2017). Sevoflurane induces coherent slow-delta oscillations in rats. Front Neural Circuits 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor NE, Van Dort CJ, Kenny JD, Pei J, Guidera JA, Vlasov KY, Lee JT, Boyden ES, Brown EN, and Solt K (2016). Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A 113, 12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, and Jensen P (2016). Recombinase-dependent mouse lines for chemogenetic activation of genetically defined cell types. Cell Rep 15, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caligioni CS (2009). Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez G (2016). Determining the stage of the estrous cycle in female mice by vaginal smear. Cold Spring Harb Protoc 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the current study have not been deposited in a public repository but are available from the corresponding author on request. This study did not generate any code.