Abstract
Salt stress is one of the major environmental constraints for plant growth. Although the ways in which mycorrhizal plants deal with salt stress have been well documented, it still is blank for Euonymus maackii, an important local ecological restoration tree, to arbuscular mycorrhizal fungi (AMF) inoculation and salt stress. In this study, we tested the effect of different salt levels (0, 50, 100,150 and 200 mM) and AMF inoculation on E. maackii growth rate, photosynthesis, antioxidant enzymes, nutrient absorption and salt ion distribution. The results indicated negative effect of salt on height, photosynthesis capacity, nutrition accumulation, while salt stimulated the antioxidant defense system and salt ions accumulation. The toxic symptom by excessive accumulation of salt ions worsen with salt level increased gradually (except for the 50 mM NaCl treatment). AMF inoculation alleviated the toxic symptom under moderate salt levels (100 and 150 mM) by increasing photosynthesis capacity, accelerating nutrient absorption and activating antioxidant enzyme activities under salt stress. Meanwhile, effect of AMF was not detected on seedlings under slight (0 and 50 mM) and high (200 mM) NaCl concentration. Our study indicated AMF had positive impact on E. maackii subjected to salt, which suggested potential application of AMF- E. maackii on restoration of salt ecosystems.
1. Introduction
Salt stress is one of the major environmental constraints for plant growth and large amounts of lands in the world are affected by excessive salt ions [1]. Datong Basin in Shanxi Province is a region with distinctive topographical and geological features. This area is characterized by salinity, with total salinity of 4–10 g/kg soil, partly explained by extreme weather and coal industrial pollution [2]. Excessive salt ions in soil will cause native plants to exhibit decreased growth height, weaker photosynthetic capacity, a disordered oxidation defense system, lower nutrient absorption and even death, eventually resulting in ecological environment deterioration [3–5]. Many techniques are employed to restore saline soils, among which the application of soil microorganisms in saline soils is considered a bio-amelioration method for plants [6].
As a native plant that is widespread in Datong Basin, Euonymus maackii Rupr., is important for the restoration of ecosystems [7, 8]. E. maackii, a small shrub is a species in the genus Euonymus, which contains approximately 145 to 183 species, and belongs to the family Celastraceae, order Celastrales [9]. It is an ideal plant for afforestation and landscaping and, has a strong ability to adapt to abiotic stress [7]. Previous studies have mainly focused on its breeding techniques, bioactive components, physiological characteristics and use in biodiesel energy [7–11]. However, the occurrence of microorganisms and E. maackii has not been reported before.
Plants exposed to soil salinity, in addition to employing some intrinsic mechanisms of adaptation, can improve their tolerance by establishing a mutualistic relationship with rhizosphere microorganisms [3,12,13]. Among soil microorganisms, arbuscular mycorrhizal fungi (AMF) have symbiotic relationships with most terrestrial plant species [14]. Previous studies have indicated that AMF can confer salt tolerance to host plants by stimulating enzyme activity protection systems, increasing photosynthesis capacity and enhancing nutrients uptake [15–16]. However, not all AMF species function equally well in improving the salt tolerance of host plants. Some researchers have reported that Funneliformis mosseae was the most efficient fungus for tackling salt stress [17], while others showed that for Acacia nilotica, herbs, woody and perennial plants, G. fasciculatus was a better choice for reducing the negative effects of salt stress [18]. The inconsistent effects of AMF inoculation could result from the salt tolerance of either the fungal species or host species [16].
For the first time, to the best of our knowledge, we attempted to investigate the effects of AMF inoculation on E. maackii exposed to salt stress and its potential ecological restoration function in the Datong Basin in this work. There were two hypothesis in our study: (1) salt stress will limit plant growth, weaken photosynthesis capacity, stimulate the antioxidant defense system and decrease nutrient contents, especially under higher salinity conditions; and (2) arbuscular mycorrhizal (AM)inoculation will enhance the salt tolerance of E. maackii by increasing photosynthesis capacity, accelerating nutrient absorption and activating antioxidant enzyme activities. With few studies about the rhizosphere microorganisms of E. maackii, especially mycorrhizas, we aimed to determine the potential function of AMF in the alleviation mechanisms that are active under salt stress and thus eventually promote acclimatization to the local ecosystem.
2. Materials and methods
2.1 Plant and soil treatments
Seeds of E. maackii, provided by the Shanxi Forestry Technology Promotion Station, were disinfected with 0.5% KMnO4 solution for 20 min, washed three times with distilled water and soaked in sterile water for 24 h. After that, the seeds were put on a tray covered with gauze and rapidly germinated in a 25°C light incubator. During this period, the seedlings were illuminated for 12 h every day, and the water was changed once a day. Once the seedlings had grown to 0.1 cm, they were put into a bowl (47 cm × 33 cm, 66 holes) filled with sterilized vermiculite (3 seeds/hole) and placed in a 25°C light incubator for further cultivation. The seedlings were watered every morning (20 ml/hole) for 1 month, and fully grown seedlings were transplanted to the pots descripted as following.
Topsoil (5–20 cm) was collected from an E. maackii plantation in Shanxi Province, China. The soil was sieved through a 2 mm sieve, mixed with fine washed sand at 1:1.2 (W:W) and then autoclaved at 0.11 MPa and 121°C for 2 h to supply a soil substrate for E. maackii seedlings. The soil physicochemical properties were as follows: pH value 7.9 (the ratio of soil and water was 1:5 by PHS-3B pH device), soil organic carbon (SOC, by the K2Cr2O7 oxidation method) 16.44 g·kg-1, available N (by the AA3 high resolution digital colorimeter) 33.25 mg·kg-1, available P (by the sodium bicarbonate extraction method and the molybdenum antimony colorimetry) 10.37 mg·kg-1 and available K (by the sodium bicarbonate extraction method and flame photometry) 112.17 g·kg-1. Compared with soil, nutrition of sand used was very few and could be ignored.
2.2 AMF inoculation
The inoculum AMF was Rhizophagus intraradices (Schenck & Smith) (BGC BJ09, Beijing Academy of Agriculture and Forestry Science), which contained spores (50 spores per gram of inoculum), mycelia, root fragments and soil.
2.3 Experimental design
Our experiment consisted of a randomized complete block design with two factors: AMF inoculation and salt stress (0, 50, 100, 150, and 200 mM). Salinity level were set by our preliminary experiment, and seedlings could not survive more than 1 month when subjected to 250 mM. We used 15 replications for each treatment, totaling 2 × 5 × 15 = 150 pots. The 150 seedlings were transplanted in 15 cm × 13 cm pots filled with soil substrate (2 kg) and grown in an artificial climatic greenhouse at 25°C with 12 h light per day and stable humidity of 50%. Half of the pots, were inoculated with AMF (30 g of inoculum per pot), while the remaining pots were inoculated with sterile inoculum as nonmycorrhizal controls. For the inoculation group, the inoculum was put 2 cm below the surface of the substrate, and 1 seedling was transplanted into each pot. For the control group, to kill the live inoculum, the inoculum was autoclaved with 15 ml washing solution and filtered through a 1 μm nylon mesh. After being attended to for 3 months, all pots were divided into five groups, and each group contained 15 individuals. For the salt stress groups, each group was treated with different concentrations of NaCl solution (10 mM, 20 mM, 30 mM, or 40 mM) every 2 days until reaching the final concentration, with 5 replicates. The control group was treated with sterilized water. Salt stress lasted for 1 month. According to our preliminary experiment, treatments of 100 mM and 150 mM salt level were set as moderate concentration in this study.
2.4 AMF colonization rate
After 1-month salt treatment, the seedlings were harvested, and the fresh roots were collected, washed, cut into 1 cm segments and fixed with FAA solution. According to the method described by Phillips and Hayman [19], 10% KOH and 0.05% trypan blue in lactophenol were used to clear and stain roots. The AM colonization rate was determined by the gridline intersection method [20]. Data were recorded as the proportion of root length colonized. AMF presence in the roots was confirmed by nested polymerase chain reaction (PCR) with SSUmAf/LSUmAr and SSUmCf/LSUmBr primers [21].
2.5 Growth height
To quantify the growth response, the growth in height (GH) was calculated to represent the average amount of growth over one month, determined from measurements at the beginning and end of the last 30-day NaCl treatment.
2.6 Gas exchange
The gas exchange parameters of the fourth and fifth fully expanded leaves, including the net photosynthesis (Pn), transpiration rate (E), stomatal conductance (Gs) and intercellular CO2 concentration (Ci), were determined between 8.00 and 11:30 am by a Li-Cor 6400XT portable photosynthesis measuring system (Lincoln, USA) within a week before harvest. Instrument parameters: temperature 25°C, light intensity 1000 μmol·m-2·s-1, relative humidity 50% and ambient CO2 400 μmol·mol-1.
2.7 Chlorophyll fluorescence
The fourth and fifth fully expanded leaves of selected plants were placed in dark conditions (25°C, 30 min) before detecting the minimum fluorescence (Fo) and the maximal fluorescence (Fm) yields by a MINIPAM chlorophyll fluorometer (Walz, Germany). Fv/Fm represented the maximum quantum yield of photosystem II (PSII), and ΦPSII indicated the actual quantum yield of PSII. We also calculated nonphotochemical quenching (qN) and photochemical quenching (qP) by using the following formulas:
2.8 Antioxidant enzyme activities
Superoxide dismutase (SOD, EC1.15.1.1) activity was assayed by the superoxide radicals generated photochemically at 560 nm, as described by Dhindsa et al. [22]. Catalase (CAT, EC1.11.1.6) activity was assayed by measuring the absorbance of the reaction mixture containing K-phosphate and H2O2 at 240 nm and peroxidase (POD, EC1.11.1.7) activity was expressed in 0.001 per minute at 470 nm following the procedure described by Mallick and Mohn [23].
2.9 Nutrient distribution measurement
After being dried at 80°C to a constant weight, plants were separately ground and passed through a 20 μm mesh screen. N content was measured by the semi micro-Kjeldahl method with a KjeltecTM 8400 Analyzer Unit (FOSS-Tecator, Hoganas, Sweden) [24]. Phosphorus (P) content was measured by the vanadomolybdate method [25]. K content was measured using flame atomic absorption spectrophotometry [26].
After drying at 80°C for 48 h, the plants were separately ground to a homogeneous powder and passed through a 20 μm mesh screen. Na+ content was detected by atomic absorption spectroscopy and Cl- content was detected by a modified silver ion titration method [27].
2.10 Statistical analysis
The data were analyzed with SPSS 17.0 software, and two-way ANOVA was adopted to examine the effects of soil salinity, R. irregularis inoculation and their interactions in seedlings. The means were compared by Duncan’s multiple range test and HSD test (P < 0.05). PCA was implemented to reduce all physiological parameters to the fewest dimensions (eigenvalue > 1). For PCA, highly correlated variables were removed (only one remained) and all remaining data were standardized and computed. Two-dimensional figures were made by using SigmaPlot version 10.0 [15].
3. Results
3.1 AMF colonization rate
Soil salinity decreased the AMF colonization rate. The AMF colonization rates decreased from 79.44% and 79.47% (in the 0 and 50 mM treatments respectively) to 68.22% (in the 200 mM treatment) (Fig 1). And there was no significant difference between the 0 and 50 mM NaCl treatments. ANOVA results suggested the significant effects of salt levels on AMF colonization rates. Besides, no AMF inoculation was detected in samples from nonmycorrhizal treatments.
Fig 1. AMF colonization rate in E. maackii seedlings.
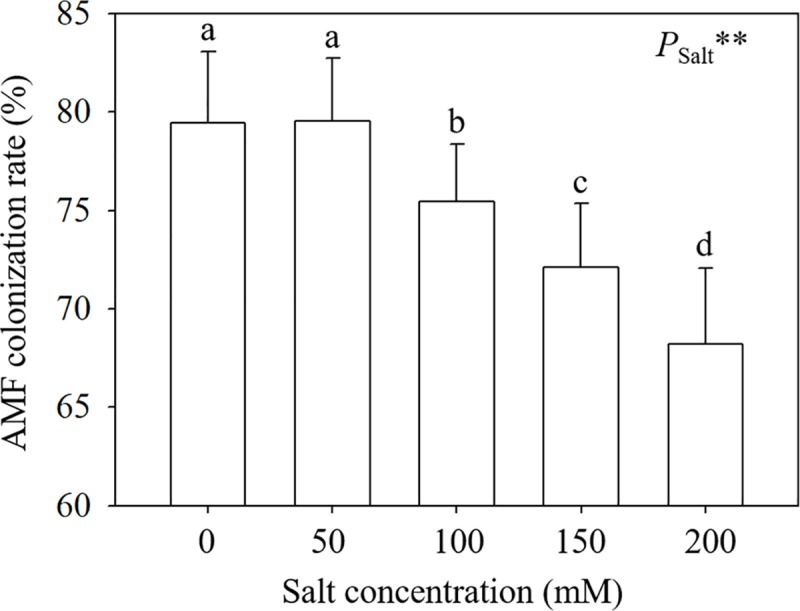
**: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
3.2 Height growth
Salt stress caused significant declines in GH in E. maackii seedlings under NaCl stress from 50 to 200 mM. and AMF inoculation alleviated this symptom to some extend (Fig 2). The GHs of seedlings inoculated with AMF were 10.44% and 13.40% significantly higher than those of uninoculated seedlings at salinity of 100 and 150 mM. ANOVA results revealed the obvious effects by salt, AMF and their interaction.
Fig 2. Effects of AMF inoculation on growth height of E. maackii at different NaCl levels.
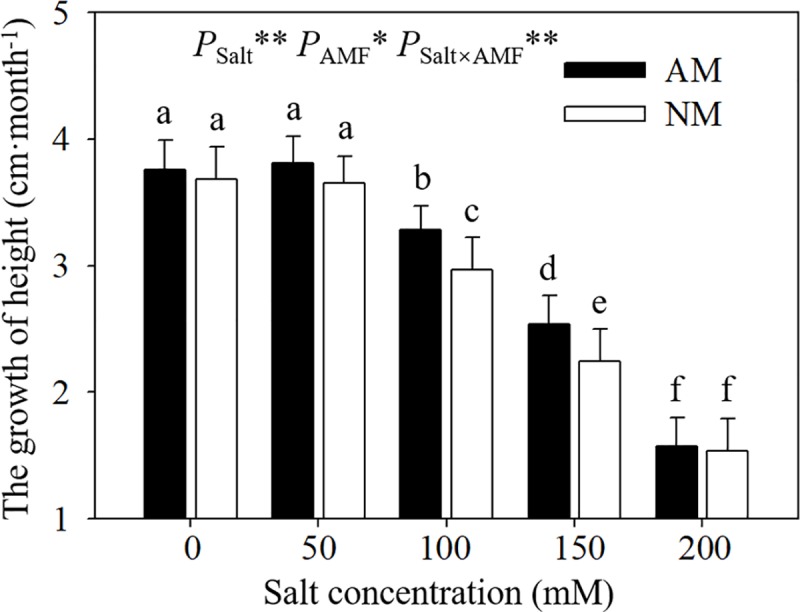
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
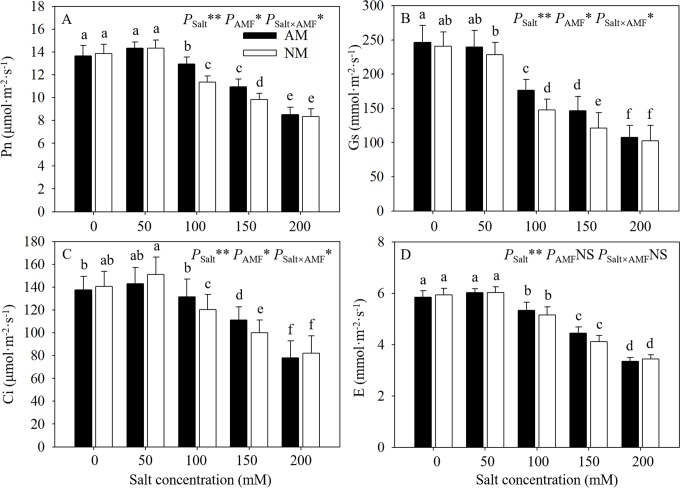
3.3 Gas exchange
Gas exchange indexes showed similar trends as descripted on GH values. Salt stress had a negative effect on gas exchange parameters in seedlings reflected by obvious reduction at 100, 150, and 200 mM NaCl of mycorrhizal and nonmycorrhizal seedlings, and this reduction increased with the salt stress increased gradually (Fig 3). However, in mycorrhizal seedlings subjected to salt stress, the reductions of Pn, Gs and Ci were significantly lower at 100 and 200 mM salt levels compared with nonmycorrhizal seedlings. In addition, there were no significant differences in gas exchange parameters at 0, 50 and 20 mM NaCl between mycorrhizal and nonmycorrhizal seedlings. ANOVA results suggested the effect of salt levels was significant on all the gas exchange parameters. Apart from E, the other 3 indexes were significant impacted by AMF inoculation. And the interaction of AMF and salt level significantly affected Pn, Gs and Ci.
Fig 3. Effects of AMF inoculation on gas exchange parameters of E. maackii at different NaCl levels.
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. NS: no significant effect; *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
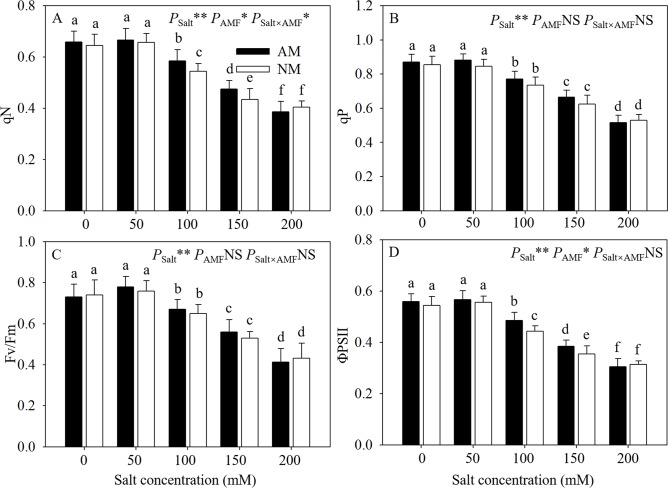
3.4 Chlorophyll fluorescence
Salt stress significantly decreased the chlorophyll fluorescence parameters in seedlings with salt level increased gradually from 50 to 200 mM (Fig 4). Under moderate salt stress, AMF inoculation showed a positive effect on chlorophyll fluorescence parameters. Compared with nonmycorrhizal seedlings, mycorrhizal seedlings had a significantly higher qN and ΦPSII at 100 and 150 mM NaCl. Among qP and Fv/Fm, no obvious differences were detected between mycorrhizal and nonmycorrhizal seedlings under same salt level. Among qN and ΦPSII, effect of AMF inoculation was not obvious between mycorrhizal and nonmycorrhizal seedlings under 0, 50 and 200 mM respectively. ANOVA results indicated that the effect of salt levels was significant on all the chlorophyll fluorescence parameters, and the effect of AMF inoculation only significantly impacted on qN and ΦPSII. Besides, the interaction of AMF inoculation and salt level only remarkably affected qN.
Fig 4. Effects of AMF inoculation on chlorophyll fluorescence parameters of E. maackii at different NaCl levels.
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. NS: no significant effect; *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
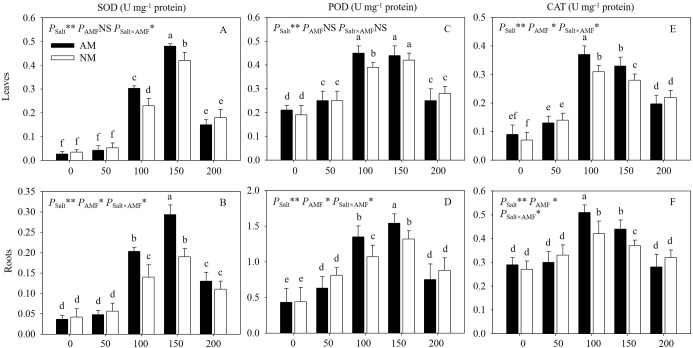
3.5 Antioxidant enzyme activities
With increasing salinity, the antioxidant activities first increased and then decreased, illustrating that the antioxidant system was activated by stress and was damaged under higher salt stress (Fig 5). The inoculated seedlings subjected to 100 and 150 mM NaCl showed higher SOD activity, POD and CAT activity in leaves and roots and POD activity in roots than uninoculated seedlings. However, there was no obvious difference between inoculated seedlings subjected to 0, 50 and 200 mM. Meanwhile, uninoculated plants under 50 mM showed remarkably higher enzymatic activities than those under 0 mM. Besides, no difference was detected between seedlings under 200 mM. Two-way ANOVA results showed that salt levels had significant effects on all these enzymatic activities measured, but AMF inoculation had obvious effects mainly on those of roots, and only CAT activity of three enzymes of leaves was significantly affected by AMF. This suggested the effect of AMF inoculation on the antioxidant enzyme activities were more obvious in roots than in leaves.
Fig 5. Effects of AMF inoculation on SOD, POD and CAT activities of leaves and roots of E. maackii at different NaCl levels.
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. NS: no significant effect; *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
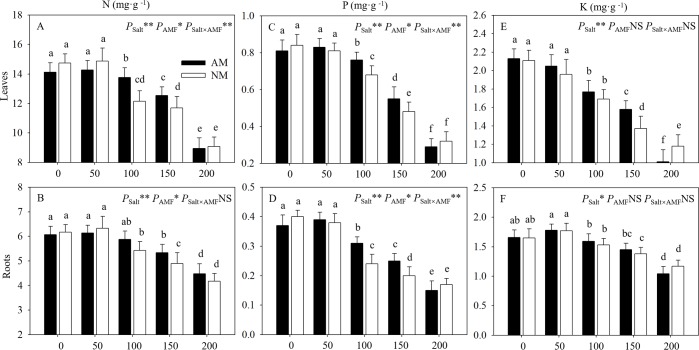
3.6 Nutrient distribution
Gradually increasing salt levels of 50 to 200 mM lead to gradually decrease in N, P and K contents of both AMF inoculated and uninoculated seedlings (Fig 6). Compared with roots, leaves accumulated obviously more N and P, and slightly more K. N and P of leaves of inoculated seedlings were significantly higher than those of uninoculated ones at 100 and 150 mM, which was not detected under other salt levels. And among K contents this trend was only detected under 150 mM. Meanwhile, no significant differences were shown between N, P and K contents of mycorrhizal and nonmycorrhizal seedlings under 0 and 50 mM salt stress. Under 200 mM salt level, significant difference only existed in K contents of leaves between mycorrhizal and nonmycorrhizal seedlings. ANOVA results suggested that salt levels significantly affected N, P and K contents of leaves and roots, and AMF significantly affected N and P contents of leaves and roots.
Fig 6. Effects of AMF inoculation on N, P and K contents of leaves and roots of E. maackii at different NaCl levels.
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. NS: no significant effect; *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
3.7 Na+ and Cl- determination
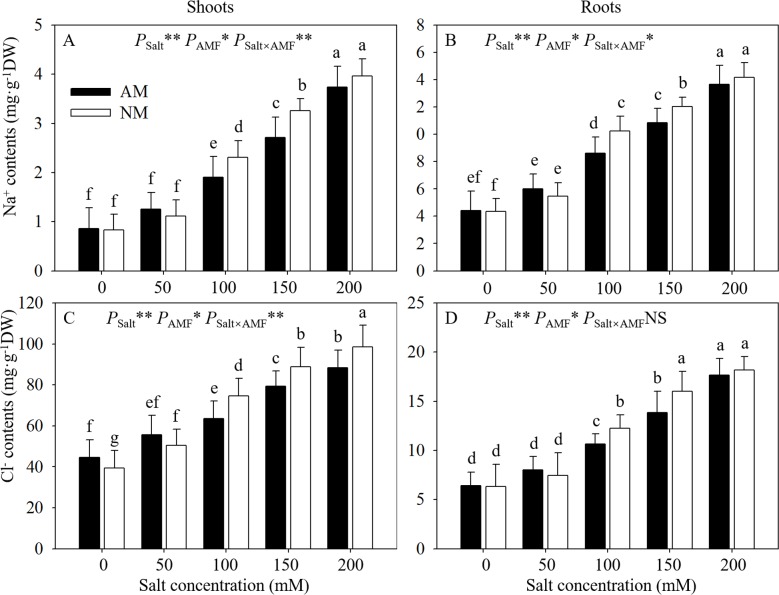
With salt level increasing gradually, salt ion contents in seedlings increased (Fig 7). Under salt stress, AMF inoculation significantly decreased salt ion accumulation by17.46% and 16.59% at 100 and 150 mM NaCl in leaves, while it decreased by 15.75% and 9.65% at 100 and 150 mM NaCl in roots. In addition, compared with nonmycorrhizal seedlings, mycorrhizal seedlings had significantly lower Cl- contents in leaves (15.01% and 10.62% at 100 and 150 mM NaCl, respectively), and lower Cl- contents in roots (13.18% and 13.50% at 100 and 150 mM NaCl, respectively).ANOVA results indicated that salt levels and AMF inoculation had significant impact on Na+ and Cl- contents of leaves and roots, and apart from Cl- contents in roots, the interactions of these 2 factors also obviously affected other parameters.
Fig 7. Effects of AMF inoculation on Na+ and Cl- contents of dry matter of shoots and roots at different NaCl levels.
AM: AMF inoculation; NM: non-inoculation; AMF: AMF formation. NS: no significant effect; *: significant effect at 0.01 ≤ P ≤ 0.05; **: P ≤ 0.01. Means with a letter in common within each variable can’t be considered different at P ≤ 0.05.
3.8 PCA analysis
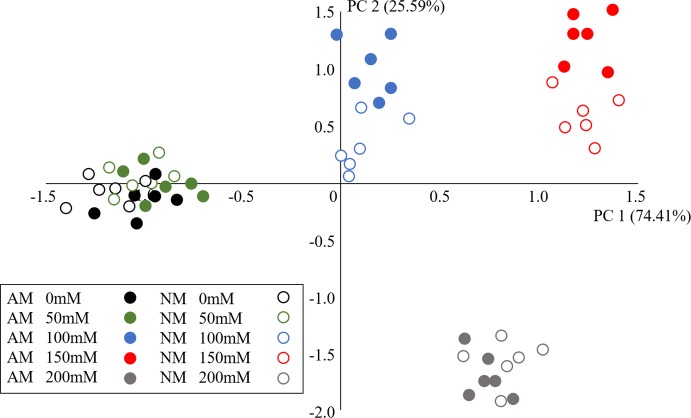
To better reveal physiological response patterns to salt stress and AMF inoculation, PCA analysis was performed by using experimental data sets. We did correlation analysis, highly correlated variables were removed: GH, Pn, Gs, Ci, E, qN, qP, Fv/Fm, ΦPSII, N content, P content, K content, Na content and Cl content were significantly correlated, SOD activities, POD activities, CAT activities were significantly correlated. We selected GH and SOD activity of leaves as presentation for following PCA process. All the physiological parameters detected in this experiment were reduced to the fewest and most representative dimensions. The distance between two treatments in the PCA figure represents the similarity of their overall status. Among all the seedlings, PC1 and PC2 accounted for 74.41% and 25.59% of the variance, respectively (Fig 8). PC1 tended to separate the salinity effects and PC2 tended to separate the AMF inoculation effect. In addition, the distances between mycorrhizal seedlings at different salt levels were closer than those of nonmycorrhizal seedlings at different salt levels (Fig 8), showing that AMF inoculation could enhance the salt tolerance of E. maackii, especially at 100 and 150 mM NaCl.
Fig 8. PCA plots of the two principal components of E. maackii.
AM: AMF inoculation; NM: non-inoculation; PC1: principal component 1; PC2: principal component 2.
4. Discussion
Salt stress is a major environmental stress that drastically constrains plant growth [28]. Plants growing in saline areas are often subject to ion toxicity [1]. The toxic effects of excessive salt ions can cause great damage to the structure of enzymes, disrupt photosynthesis and induce nutrient deficiencies [3,5,15]. In this study, we tested the rate of growth in height, antioxidant enzymes, photosynthesis capacity and nutrient uptake of E. maackii exposed to salt stress and AMF inoculation to explore the potential function of AMF within E. maackii seedlings under abiotic stress.
The AMF colonization rate in E. maackii seedlings was still high (more than 68.22%) even under severe salt stress, suggesting that E. maackii is a suitable host plant for AMF. Talaat and Shawky [29] suggested that slight salt stress is beneficial to AMF colonization but excessive salt ions could weaken the AMF colonization capacity, which supported our findings that the AMF colonization of E. maackii seedlings was suppressed by salt stress from 100 to 200 mM NaCl. The early response of E. maackii seedlings to salt stress manifests as regulation of the growth rate. A severe reduction in height was observed in E. maackii when salinity stress was imposed at 150 and 200 mM NaCl. Beneficial influences of AMF on growth responses in plants under salt stress have also been found in tomato [30], poplar [15] and maize [31].
The reduction in plant growth under salt stress could be attributed to the reduction of photosynthesis capacity caused by excessive salt ions [32]. In this study, although salt stress reduced Pn in all seedlings, the Pn of mycorrhizal seedlings was still higher than that of nonmycorrhizal ones under moderate salt stress, which could result from the improved growth status of AM seedlings under salt stress. Chaves et al. [33] found that salt stress could affect CO2 diffusion through a decrease in mesophyll and stomatal conductance; thus, the Pn enhancement in mycorrhizal seedlings maybe related to the higher Gs than that found in nonmycorrhizal seedlings. In our study, we also observed that the same results under moderate salt stress (100 and 150 mM) and similar Gs values between different treatments at 200 mM salt. The lower reduction of Gs in mycorrhizal seedlings than nonmycorrhizal seedlings under moderate salt stress suggested that the growth status may be better in mycorrhizal seedlings, resulting from less of a decrease in CO2 diffusion through the stomata [34]. The Ci values were higher than those under unstressed conditions, which may indicate that enzymes in the photosynthesis apparatus were destroyed, resulting in a decrease in CO2 assimilation and the accumulation of CO2 in intercellular areas [35]. In our research, mycorrhizal seedlings had a significant higher Ci than nonmycorrhizal seedlings at 100 and 150 mM NaCl. Compared with nonmycorrhizal seedlings, mycorrhizal seedlings had a lower metabolic limit of CO2 assimilation [36].
Photochemical reactions represent the photosynthetic capacity of plants and are sensitive to soil salinity [37]. Many researchers have found that salt-tolerant plants are better able to maintain PSII activity than salt-sensitive plants [1]. We found that the ΦPSII of mycorrhizal seedlings were significantly higher than those of nonmycorrhizal seedlings under salt stress at 100 and 150 mM NaCl. This result may imply that mycorrhizal seedlings suffered less disorder in the electron transport chain than nonmycorrhizal seedlings [38]. In addition, AMF inoculation also increased qN significantly under moderate salt stress, and we thought AMF inoculation might increase the utilization of absorbed light and minimize the dissipation of light energy [39]. Thus, due to their higher PSII efficiency than that of nonmycorrhizal seedlings, mycorrhizal seedlings were more tolerant of moderate salt stress (100 and 150 mM NaCl).
When plants exposed to salt stress, reactive oxygen species, such as H2O2 and O2- accumulated, which created the cytotoxic condition. To minimize the oxidative damage, the plants exposed to salt stress is always associated with the induction of antioxidant defense enzymes [40]. On general, AM formation contributed to scavenging peroxyl radials production, buffering cellular radical potential, and a more powerful reactive oxygen species scavenging system [41]. Seedlings formed AM symbiosis have been shown to have lower lipid peroxidation and higher antioxidant enzymatic activities, which supports our findings that under moderate salt stress (100 and 150 mM), the SOD and CAT activities were significantly higher in mycorrhizal leaves and roots than those of nonmycorrhizal ones. This result also illustrated the potential role of AM symbiosis in activating antioxidant enzyme activities as a protective strategy as described by Wu et al. [15]. However, there was no difference between inoculated seedlings subjected to 0 and 50 mM, which suggested the role of AMF performed better when host plants were suffering moderate stress. Besides, according to ANOVA results, we detected that AM fungi had more significant impact on the roots than the leaves. Given that the ecological niche of AMF is in the root and rhizosphere, we hypothesized that the main effects of AM symbiosis is on the roots rather than leaves.
AM symbiosis improved the absorption of nutrients, which made AMF an essential tool for plants exposed to abiotic stress [16]. Our research showed that the enhanced nutrient absorption observed with AMF inoculation was manifested in the N, P and K contents. Many researchers hold the opinion that the improvement of plant P acquisition is the most important mechanism in mycorrhizal plants under salt stress [42], such as Frosi et al. [43] found that P can be transported into the root via protoplasmic circulation, greatly reducing the transport resistance and increasing the transport speed for the continuous mycelium of AMF. However, some other studies found that even if mycorrhizal and nonmycorrhizal plants have a similar P status under unstressed conditions, mycorrhizal plants still grow better than nonmycorrhizal plants under salt stress [44]. In addition, the AMF inoculation can also promote N assimilation and absorption to reduce the toxic effects [45], which was in line with our findings on N. Different from N and P, K was an important osmotic adjustment element. Previous studies have shown that excessive accumulation of salt ions affects the selective absorption of K by interfering with related proteins, and AMF has a buffering effect on the content of Na, with a certain limit, to promote the absorption of K [34,46]. Our results showed that the nutrient contents increased significantly in mycorrhizal plants under moderate salt stress compared with those of nonmycorrhizal plants, suggesting that AMF was necessary for the plants to reach their optimal mineral nutrition under abiotic stress [47]. The enhanced nutrient absorption capacity observed may result from the fact that AMF hyphae usually penetrate deeper into the soil beyond the rhizosphere, where they can absorb nutrients under more various kinds of conditions than just at the root surface [6]. Thus, mycorrhizal seedlings could maintain photosynthesis and activate the antioxidant defense system by regulating nutrient uptake to relieve toxic effects, which supported our findings [48]. Most terrestrial plans can form exchange associations with AMF, and this exchange is a key factor in the cycling of nutrients during ecological, evolutionary and physiological processes for plants, eventually forming an important component of sustainable soil-plant systems [6,48,49].
The primary impact of excessive Na+ and Cl- concentrations is the disturbance of photosynthesis, defense systems and nutrient uptake, leading to metabolic activity attenuation. To better survive under salt stress, it is essential for most plants to maintain lower Na+ and Cl- contents [1,15,34]. In our study, the Na+ contents in seedling shoots were significantly lower than those in roots, indicating the efficient inhibition of Na+ transport. However, the variation in Cl− content differed from that in Na+ content because of the opposite uptake patterns: seedlings accumulated more Cl- and less Na+ in leaves than roots. In addition to the intrinsic mechanisms of host plants, AM symbiosis can provide external assistance under abiotic stresses [50].
Salt stress can inhibit plant growth through several mechanisms including inhibition of photosynthesis, reduced in nutrient uptake, and damage to enzymes [50,51]. In this experiment, the deleterious effects of NaCl stress could be mediated by mycorrhizal colonization at 100 and 150 mM NaCl levels. Combined with above results on colonization rate, height, photosynthesis capacity and nutrient content, AMF performed under moderate salt stress. However, as we showed, salt stress at 50 and 200 mM had no significant effect on plants, and AM inoculation had no effect. These statements above suggested that AMF performed well when host plants felt stress and the stress level wasn’t fatal. Besides, under moderate salt stress, AMF colonization rates were lower than those under 0 and 50 mM NaCl, but more efficient AM effect was detected under moderate salt stress, which suggested AM effect was not in line with its inoculation rate.
Consistent with previous reports, AMF could enhance the salt tolerance of E. maackii seedlings, but the beneficial effect was finite within the range of salt stress conditions considered. Higher salt stress can cause severe damage to both the host plant and AMF, so AMF inoculation was not effective at 200 mM NaCl. Because of the enhanced nutrient status and photosynthesis capacity, the mycorrhizal seedlings could maintain detoxifying activity by activating antioxidant enzymes under moderate salt stress.
5. Conclusion
Highly consistent with our hypothesis, after germinated and grew under unstressed condition, salt stress caused negative physiological effects, including limited growth, decreased photosynthesis, stimulation of the antioxidant enzyme system and lessened nutrient uptake. In addition, mycorrhizal association improved the salt tolerance of E. maackii by increasing photosynthesis, activating the antioxidant enzyme system and enhancing nutrient uptake, particularly at 100 and 150 mM NaCl. With the help of AMF, E. maackii grown under salt stress could acquire tolerance, which suggested a potential possibility of application of AMF for ecological stability in saline areas.
Supporting information
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received funding by the National Science Foundation for Young Scientists of China (31901227, Zhen Li), Doctoral Research Project of Shanxi Datong University (2018-B-47 Na Wu and 2018-B-45 Zhen Li). Both of them played roles in study design, data collection and analysis, decision to publish, and preparation of the manuscript.
References
- 1.Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza. 2016;26(7):673–684. 10.1007/s00572-016-0704-5 [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Wang YX, Xie XJ. Spatial occurrence and geochemistry of soil salinity in Datong basin, northern China. J Soil Sediment. 2014;14(8):1445–1455. 10.1007/s11368-014-0874-8 [DOI] [Google Scholar]
- 3.Evelin H, Giri B, Kapoor R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza. 2012;22:203–217. 10.1007/s00572-011-0392-0 [DOI] [PubMed] [Google Scholar]
- 4.Bless AE, Colin F, Crabit A, Devaux N, Phioippon O, Follain S. Landscape evolution and agricultural land salinization in coastal area: A conceptual model. Sci Total Environ. 2018;625:647–656. 10.1016/j.scitotenv.2017.12.083 [DOI] [PubMed] [Google Scholar]
- 5.Patykowski J, Kolodziejek J, Wala M. Biochemical and growth responses of silver maple (Acer saccharinum L.) to sodium chloride and calcium chloride. PEERJ. 2018;6:35958 10.7717/peerj.5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. 2019;10:1068 10.3389/fpls.2019.01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JZ, Cui Q, Kang YF, Meng Y, Gao MZ, Efferth T, et al. Euonymus maackii Rupr. Seed oil as a new potential non-edible feedstock for biodiesel. Renew Energy. 2019;133:261–267. 10.1016/j.renene.2018.10.035 [DOI] [Google Scholar]
- 8.Chinese academy of sciences, Flora Republicae Popularis Sinicae, The third volume, Science Press, 1999, p 45. [Google Scholar]
- 9.Zhang A, Zhang T, Kang X, Guo C. Changes of spectral characteristics of plant leaves before and after dust-retention under hyperspectral imaging. Trans Chin Soc Agri Eng. 2018;34(19):170–176. 10.11975/j.issn.1002-6819.2018.19.022 [DOI] [Google Scholar]
- 10.Fung SY. Alkaloids and cardenolides in sixteen Euonymus taxa. Biochem System Ecol. 1986;14(4):371–373. 10.1016/0305-1978(86)90021-9 [DOI] [Google Scholar]
- 11.Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, Wei ZJ, et al. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Tec. 2011;60(3):251–257. 10.1016/j.postharvbio.2011.01.006 [DOI] [Google Scholar]
- 12.Li Z, Wu N, Liu T, Chen H, Tang M. Effect of arbuscular mycorrhizal inoculation on water status and photosynthesis of Populus cathayana males and females under water stress. Physiol Plantarum. 2015;155:192–204. 10.1111/ppl.12336 [DOI] [PubMed] [Google Scholar]
- 13.Cordero I, Balaguer L, Rincon A, Pueyo JJ. Inoculation of tomato plants with selected PGPR represents a feasible alternative to chemical fertilization under salt stress. J Plant Nutr Soil Sci. 2018;181(5):694–703. 10.1002/jpln.201700480 [DOI] [Google Scholar]
- 14.Smith SE, Read DJ. Mycorrhizal symbiosis. New York: Academic press; 2010. [Google Scholar]
- 15.Wu N, Li Z, Wu F, Tang M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Scientific reports. 2016;6:37663 10.1038/srep37663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caruso C, Maucieri C, Berruti A, Borin M, Barbera A. Responses of different Panicum miliaceum L. genotypes to saline and water stress in a Marginal Mediterranean environment. Agronomy. 2018:8:8 10.3390/agronomy8010008 [DOI] [Google Scholar]
- 17.Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann Bot. 2009;104:1263–1280. 10.1093/aob/mcp251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrasekaran M, Boughattas S, Hu SJ, Oh SH, Sa TM. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza. 2009;24:611–625. 10.1007/s00572-014-0582-7 [DOI] [PubMed] [Google Scholar]
- 19.Phillips J, Hayman D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc. 1970;55:157–160. 10.1016/S0007-1536(70)80110-3 [DOI] [Google Scholar]
- 20.Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. 10.1111/j.1469-8137.1980.tb04556.x [DOI] [Google Scholar]
- 21.Krüger M, Stockinger H, Krüger C, Schüssler A. DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol. 2009;183:212–223. 10.1111/j.1469-8137.2009.02835.x [DOI] [PubMed] [Google Scholar]
- 22.Dhindsa RA, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;126:93–101. 10.1093/jxb/32.1.93 [DOI] [Google Scholar]
- 23.Mallick N, Mohn EH. Reactive oxygen species: response of algal cells. J Plant Physiol. 2000;157:183–193. 10.1016/S0176-1617(00)80189-3 [DOI] [Google Scholar]
- 24.Mitchell AK. Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree physiol. 1998;18 10.1093/treephys/18.11.749 [DOI] [PubMed] [Google Scholar]
- 25.Bertramson BR. Phosphorus analysis of plant material. Plant Physiol. 1942;17:447 10.1104/pp.17.3.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilde SA, Corey RB, Lyer JG, Voight GK. Soil and plant analysis for Tree Culture. 3st ed New Delhi: Oxford and IBM Puplishing Co; 1985. [Google Scholar]
- 27.Chen S, Li J, Wang S, Hüttermann A, Altman A. Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees Struct Funct. 2001;15:186–194. 10.1007/s004680100091 [DOI] [Google Scholar]
- 28.Wu N, Li Z, Wu F, Tang M. Microenvironment and microbial community in the rhizosphere of dioecious Populus cathayana at Chaka Salt Lake. 2019;19(6):2740–2751. 10.1007/s11368-019-02263-0JSSS-D-18-00886.2 [DOI] [Google Scholar]
- 29.Talaat NB, Shawky BT. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot. 2014;98(1):20–31. 10.1016/j.envexpbot.2013.10.005 [DOI] [Google Scholar]
- 30.Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum Lycopersicum L.) plants. Plant Soil. 2010;331(1–2):313–327. 10.1007/s11104-009-0255-z [DOI] [Google Scholar]
- 31.Sheng M, Tang M, Zhang F, Huang Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza. 2011;21(5):423–430. 10.1007/s00572-010-0353-z [DOI] [PubMed] [Google Scholar]
- 32.Shelke DB, Pandey M, Nikalje GC, Zaware BN, Suprasanna P, Nikam TD. Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotype. Plant Physiol Bioch. 2017;118:519–528. 10.1016/j.plaphy.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 33.Chaves MM, Flexas J, and Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–560. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zhang HQ, Zhang XL, Tang M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front Plant Sci. 2017;8:1739 10.3389/fpls.2017.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araujo GD, Miranda RD, Mesquita RO, Paula SD, Prisco JT, Gomes E. Nitrogen assimilation pathways and ionic homeostasis are crucial for photosynthetic apparatus efficiency in salt-tolerant sunflower genotypes. Plant Growth Regul. 2018;86(3):375–388. 10.1007/s10725-018-0436-y [DOI] [Google Scholar]
- 36.Sheng M, Tang M, Chen H, Yang BW, Zhang FF, Huang YH. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18(6–7):287–296. 10.1007/s00572-008-0180-7 [DOI] [PubMed] [Google Scholar]
- 37.Habibi G. 2017. Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol Plant. 2017;39(10):213 10.1007/s11738-017-2517-3 [DOI] [Google Scholar]
- 38.Albert KR, Boesgaard K, Ro-Poulsen H, Mikkelsen TN, Andersen S, Pilegaard K. Antagonism between elevated CO2, nighttime warming, and summer drought reduces the robustness of PSII performance to freezing events. Environ Exp Bot. 2013;93:1–12. 10.1016/j.envexpbot.2013.03.008 [DOI] [Google Scholar]
- 39.Bagheri V, Shamshiri MH, Alaei H, Salehi H. The role of inoculum identity for growth, photosynthesis, and chlorophyll fluorescence of zinnia plants by arbuscular mycorrhizal fungi under varying water regimes. Photosynthetica 2019;57(2):409–419. 10.32615/ps.2019.048 [DOI] [Google Scholar]
- 40.Nisar F, Gul B, Khan MA, Hameed A. Heteromorphic seeds of coastal halophytes Arthrocnemum macrostachyum and A. indicum display differential patterns of hydrogen peroxide accumulation, lipid peroxidation and antioxidant activities under increasing salinity. Plant Physiol Biochem. 2019;144:58–63. 10.1016/j.plaphy.2019.09.031 [DOI] [PubMed] [Google Scholar]
- 41.Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–216. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- 42.Gabriella F, Vanessa A, Marciel T, Mariana S, Diego G, Leonor C, et al. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018;38(1): 25–36. 10.1093/treephys/tpx105 [DOI] [PubMed] [Google Scholar]
- 43.Frosi G, Barros VA, Oliveira MT, Santos M, Ramos DG, Maia L, et al. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018;38:25–36. 10.1093/treephys/tpx105 [DOI] [PubMed] [Google Scholar]
- 44.Poss JA, Pond E, Menge J. Effect of salinity on mycorrhizal onion and tomato in soil with and without additional phosphate. Plant Soil. 1985;88:307–309. 10.1007/BF02197488 [DOI] [Google Scholar]
- 45.Wang YH, Wang MQ, Li Y, Wu AP, Huang JY. Enhancements of arbuscular mycorrhizal fungi on growth and nitrogen acquisition of Chrysanthemum morifolium under salt stress. PLoS ONE. 2018;13(4):e0196408 10.1371/journal.pone.0196408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashem A, Abd-Allah EF, Alqarawi AA, Ai-Huqail AA, Wirth S, Egamberdieva D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Microbiol. 2016;7:868 10.3389/fmicb.2016.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hidri R, Metoui-Ben Mahmoud O, Debez A, Abdelly C, Barea JM, et al. Modulation of C:N:P stoichiometry is involved in the effectiveness of a PGPR and AM fungus in increasing salt stress tolerance of Sulla carnosa Tunisian provenances. Appl Soil Ecol. 2019;143:161–172. 10.1016/j.apsoil.2019.06.014 [DOI] [Google Scholar]
- 48.Santander C, Aroca R, Ruiz-Lozano JM, Olave J, Cartes P, Borie F, et al. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza. 2017;27(7):639–657. 10.1007/s00572-017-0784-x [DOI] [PubMed] [Google Scholar]
- 49.Teste FP, Jones MD, Dickie IA. Dual-mycorrhizal plants: their ecology and relevance. New Phytol. 2019. 10.1111/nph.16190 [DOI] [PubMed] [Google Scholar]
- 50.Rozentsvet O, Kosobryukhov A, Zakhozhiy I, Tabalenkova G, Nesterov V, Bogdanova E. Photosynthetic parameters and redox homeostasis of Artemisia santonica L. under conditions of Elton region. Plant Physiol Bioch. 2017;118:385–393. 10.1016/j.plaphy.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 51.Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, et al. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Bioch. 2017;118:199–217. 10.1016/j.plaphy.2017.06.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.