Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread rapidly across China. As of 7 March 2020, the infection was reported from 97 countries globally. To date, 103 882 patients have been confirmed to have COVID-19, of whom 3522 have died [1]. Recently, many trials have been designed to determine an effective therapeutic regimen for COVID-19. Of the target regimens, chloroquine therapy is being considered [2]. Several clinical trials in China have shown chloroquine phosphate, an aminoquinoline used in malaria treatment, to be effective against COVID-19 at a dose of 500 mg/day [3]. Chloroquine phosphate also played a promising role in the management of the Zika virus and SARS-CoV outbreaks. Chloroquine acts by increasing the pH of intracellular vacuoles and altering protein degradation pathways through acidic hydrolases in the lysosomes, macromolecule synthesis in the endosomes, and post-translational protein modification in the Golgi apparatus. In macrophages and other antigen-presenting cells, chloroquine interferes with antigen processing, thereby achieving an antirheumatic response [4]. Studies have demonstrated that chloroquine also confers its considerable broad-spectrum antiviral effects via interfering with the fusion process of these viruses by decreasing the pH. In addition, chloroquine alters the glycosylation of the cellular receptors of coronaviruses [5]. Hydroxychloroquine (Fig. 1 ), a less toxic aminoquinoline, has an N-hydroxyethyl side chain in place of the N-diethyl group of chloroquine. This modification makes hydroxychloroquine more soluble than chloroquine. Similar to chloroquine, hydroxychloroquine increases the pH and confers antiviral effects. In addition, hydroxychloroquine has a modulating effect on activated immune cells, downregulates the expression of Toll-like receptors (TLRs) and TLR-mediated signal transduction, and decreases the production of interleukin-6 [6]. Although the antimalarial activity of hydroxychloroquine is equivalent to that of chloroquine, hydroxychloroquine is preferred over chloroquine owing to its lower ocular toxicity [7]. Retinopathy is a dose-limiting adverse effect of hydroxychloroquine, and a safe daily dose appears to correspond to 6.5 mg/kg of ideal body weight and 5.0 mg/kg of actual body weight [8]. Although there are more clinical data on the anti-coronaviral activity of chloroquine than that of hydroxychloroquine, both of these agents are theoretically similar in their antiviral activity [9]. Moreover, chloroquine is not as widely available as hydroxychloroquine in some countries. In addition, chloroquine is associated with greater adverse effects than hydroxychloroquine. For example, in patients with COVID-19, chloroquine can interact with lopinavir/ritonavir, resulting in prolongation of the QT interval. Hence, it is necessary to consider hydroxychloroquine instead of chloroquine when the latter is not available for treating patients with COVID-19. For example, in Iran, there is a serious shortage of chloroquine and hydroxychloroquine can be recommended instead. Other therapeutic agents for COVID-19, such as antiviral agents (oseltamivir, lopinavir/ritonavir, ribavirin, etc.), interferons and intravenous immunoglobulins that do not interfere with hydroxychloroquine, are currently under investigation.
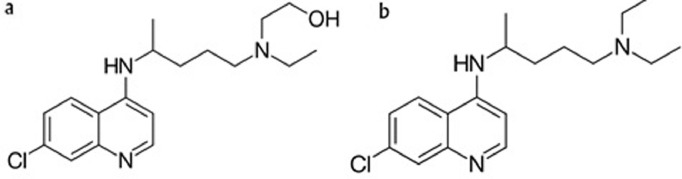
Fig. 1.
Chemical structure of (a) hydroxychloroquine and (b) chloroquine.
Acknowledgments
Declarations
Funding: None.
Competing Interests: None declared.
Ethical Approval: Not required.
Contributor Information
Zahra Sahraei, Email: zahra.sahraei@yahoo.com.
Minoosh Shabani, Email: meinoosh53@yahoo.com.
Shervin Shokouhi, Email: shsh.50@gmail.com.
Ali Saffaei, Email: alisaffaei.ss@gmail.com.
References
- 1.Xu B., Kraemer M.U.G., Open COVID-19 Data Curation Group Open access epidemiological data from the COVID-19 outbreak. Lancet Infect Dis. 2020 Feb 19 doi: 10.1016/S1473-3099(20)30119-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 Feb 19 doi: 10.5582/bst.2020.01047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 3.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plantone D., Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig. 2018;38:653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 5.Lim H.-S., Im J.-S., Cho J.-Y., Bae K.-S., Klein T.A., Yeom J.-S. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorge A.M., Melles R.B., Zhang Y., Lu N., Rai S.K., Young L.H. Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines. Arthritis Res Ther. 2018;20:133. doi: 10.1186/s13075-018-1634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Y.W., Yam W.K., Sun J., Chu J.J.H. An evaluation of chloroquine as a broad-acting antiviral against hand, foot and mouth disease. Antiviral Res. 2018;149:143–149. doi: 10.1016/j.antiviral.2017.11.017. [DOI] [PubMed] [Google Scholar]