Abstract
Long-lived proteins are present in numerous tissues within the human body. With age, they deteriorate, often leading to the formation of irreversible modifications such as peptide bond cleavage and covalent crosslinking. Currently understanding of the mechanism of formation of these crosslinks is limited. As part of an ongoing study, proteomics was used to characterize sites of novel covalent crosslinking in the human lens. In this process, Lys residues were found crosslinked to C-terminal aspartates that had been present in the original protein as Asn residues. Crosslinks were identified in major lens proteins such as αA-crystallin, αB-crystallin and aquaporin 0. Quantification of the level of an AQP0/AQP0 crosslinked peptide showed increased crosslinking with age and in cataract lenses. Using model peptides, a mechanism of crosslink formation was elucidated that involves spontaneous peptide bond cleavage on the C-terminal side of Asn residues resulting in the formation of a C-terminal succinimide. This succinimide does not form crosslinks, but can hydrolyse to a mixture of C-terminal Asn and C-terminal Asp amide peptides. The C-terminal Asp amide is unstable at neutral pH and decomposes to a succinic anhydride. If the side chain of Lys attacks the anhydride, a covalent crosslink will be formed. This multi-step mechanism represents a link between two spontaneous events: peptide bond cleavage at Asn and covalent crosslinking. Since Asn deamidation and cleavage are abundant age-related modifications in long-lived proteins, this finding suggests that such susceptible Asn residues should also be considered as potential sites for spontaneous covalent crosslinking.
Keywords: Age, post-translational modification, protein-protein crosslinking, long-lived proteins, spontaneous decomposition
Introduction
Protein-protein crosslinking is a common feature associated with long-lived proteins (LLPs). It has been described for lens crystallins [1], collagen [2–4], elastin [3], tau [5] and amyloid β peptide within brain plaque [6]. With age, protein-protein crosslinking increases and this is associated with an age-related decrease in tissue function in humans and onset of disease; for example decreased elastiticy/stiffening of the arteries[7], tendons [8]and the progress of cataract [9–11]. In selected cases the structures of protein-protein crosslinks, e.g. di-tyrosine [6] and desmosine [12] have been demonstrated and in these cases the crosslinks are formed intentionally using specific enzymes to enhance the mechanical properties of the proteins and organs in which they reside.
In other tissues the mechanisms of age-associated crosslinking are largely unknown. Untoward protein-protein crosslinking in humans can broadly be categorized by two mechanisms. In one, covalent bonding between proteins is mediated by small metabolites, such as sugars, and results in advanced glycation end products [4,8]. Aldehydes derived from the breakdown of fatty acids are also implicated [13]. In the second mechanism, the breakdown of unstable amino acid residues produces reactive intermediates which ultimately result in protein-protein crosslinks. With age, certain amino acids in LLPs, in particular Asn, Asp, Gln and Ser, break down [14–16] and these spontaneous modifications can result in racemization, deamidation and protein cleavage [17]. Recently, in the human eye lens, a mechanism for crosslinking of aspartic acid and lysine residues via the formation of a succinimide intermediate, was described [18]. As racemisation and isomerisation of Asn is one of the most common modifications present in LLPs [19–22], this discovery provided the first link between this common age-related process and the formation of protein-protein crosslinks.
As part of a continuing project to characterize the mechanisms responsible for covalent crosslinking in LLPs, proteomic analysis of the long-lived proteins in aged human lenses was undertaken. In addition to Lys/Asp crosslinking described above, several crosslinked peptides also involved terminal residues that initially were translated as Asn residues. This manuscript describes a distinctly different mechanistic explanation for this phenomenon.
Materials and Methods:
Human lens sample preparation and analysis:
Frozen human lenses were obtained from NDRI (Philadelphia, PA). All post mortem tissue was obtained from the eye bank de-identified. Human lens work was conducted in compliance with the Declaration of Helsinki. All lenses were isolated from the donor no later than 8 hours post mortem and shipped on dry ice. The lenses were classified as normal or cataract lenses by the eye bank. All lenses received were stored at −80°C until use.
To identify crosslinked peptides, the urea-insoluble fraction from the nucleus region of a 71 year old cataract lens and 68 year old cataract lens were prepared and digested by trypsin (Thermo Fisher Scientific, Rockford, IL) as described previously [23]. Tryptic peptides were fractionated by offline SCX as described previously (24). Briefly, peptides were added to SCX resin (Phenomenex Luna SCX, 5 μm, 100 Å media) and washed with buffer A (5 mM potassium phosphate buffer containing 30% ACN, pH 2.5). Peptides bound on SCX resin were sequentially step-eluted from SCX resins with 40%, 60% and 100% buffer B (5 mM potassium phosphate buffer containing 30% ACN, 350 mM KCl, pH 2.5) balanced with buffer A for 68 y lens and 30%, 40%, 60%, 80% and 100% buffer B for 71 year old lens. 40%-100% buffer B eluates for 71year old lens were analysed by 1D-LC-MS/MS. For 1D-LC-MS/MS analysis, tryptic peptides were separated on a one-dimensional fused silica capillary column (250 mm x 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size). A 70-minute gradient was performed, consisting of the following: 0-60 min, 2-45% ACN (0.1% formic acid); 60-70 min, 45-95% ACN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into a Q Exactive Plus instrument (Thermo Scientific, San Jose, CA) with a nanoelectrospray ionization source. A data-dependent acquisition method consisted of MS1 acquisition from m/z 375-1800 (R=70,000), using an MS AGC target value of 3e6, and a maximum ion time of 80 ms followed by up to 20 MS/MS scans (R=17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 1e5, with a maximum ion time of 100 ms, and an intensity threshold of 4e4. HCD collision energy was set to 28, dynamic exclusion was set to 15 s. The 60% buffer B eluate from 68y lens were analysed by 3 steps MudPIT analysis on Orbitrap Velos mass spectrometer (Thermo Scientific, San Jose, CA) as described previously [23].
To quantify the level of AQP0/AQP0 crosslinking as a function of age, lens region and cataract, twelve human lenses were examined: a young lens group (18, 19, 21 and 22 years old), a middle-aged normal lens group (48, 53, 53 and 56 years old) and a middle-aged cataract lens group (50, 57, 58 and 64 years old). The samples were prepared and analysed on a Q Exactive instrument (Thermo Scientific, San Jose, CA) as described previously (23). For each lens, three different regions (cortex, outer nucleus and inner nucleus) were obtained and water soluble fraction (WSF), urea soluble fraction (USF) and urea insoluble fraction (UIF) was prepared from each region of the lens. AQP0/AQP0 crosslinking was quantified using the data from the UIF of all lenses. WSF, USF and UIF of a 48 year old normal lens and a 50 year old cataract lens were used for identifying truncated peptides as described in data analysis. WSF, USF and UIF of a 50 year old cataract lens were also used for crosslinked peptide identification.
2.2. Data Analysis
For crosslinked peptide identification, the raw data were processed in two steps. Firstly, data from LC-MS/MS analysis of trypsin digested WSF, USF and UIF of a 48 year old normal lens and 50 year old cataract lens were searched using MaxQuant (version 1.6.6) against a custom human lens protein database (generated based on protein identified in [24]). The search parameters include trypsin digestion, semi-specific C-terminus, a maximum of two missed cleavage sites, a static modification of carbamidomethylation of cysteine, variable modification of oxidation of methionine and deamidation of asparagine. Identified proteins with high intensity (top 100 using total spectral counts) were included to generate a database for crosslinking peptide identification. Peptides that have C-terminal Asn residues (truncated and original C-terminus) were generated from the searching results and each of these peptides was then added as a separate entry to the top 100 lens protein database for searching crosslinked peptides. The second step involved analysing for crosslinked peptides using pLink 2.0 [25] to generate candidate crosslinked peptides. The crosslinker setting was set to search for crosslinking of Lys and Asn residues with ammonia loss. Other parameters included trypsin-specific cleavage with a static modification of carbamidomethylation of cysteine, variable modification of oxidation of methionine, acetylation of protein N-terminus and deamidation of Asn, precursor mass deviation of <5 ppm, and fragment ions mass deviation<10 ppm. Manual analysis was then performed to identify crosslinked peptides from the list of candidate peptides generated from pLink. Only crosslinked peptides that involve Lys and C-terminal Asn were considered for manual verification.
For quantification of the abundance of crosslinked AQP0 in different lens regions, the selected ion chromatograms of crosslinked AQP0 peptides (AQP0 227-233 crosslinked to AQP0 239-246) and the linear peptide, AQP0 234-238 were extracted with a +/−10 ppm mass tolerance. The peak areas were calculated within Xcalibur software. The relative level of crosslinking was defined as the ratio of the peak area of the crosslinked peptide to the peak area of uncrosslinked peptide 234-238. Based on the crosslinking mechanism shown in scheme 1, two isomers of crosslinking products can be formed. In addition, isomerization of Asp243 also occurs. Therefore, the crosslinking products are a mixture of multiple isomers as shown in Supplemental Figure 1. All of these peaks have same parent masses and very similar fragmentation patterns. To obtain the peak area of the crosslinked peptide, the peak areas of all isomers were summed. The results are presented as mean ± standard deviation (SD) of 4 independent experiments from four different lenses in each group shown in Figure 2. Statistical analysis was done by one-way analysis of variance (ANOVA) at p<0.05 followed by Tukey’s multiple comparison test.
Scheme 1. Two possible mechanisms of C-terminal cross-link formation from an Asn residue.
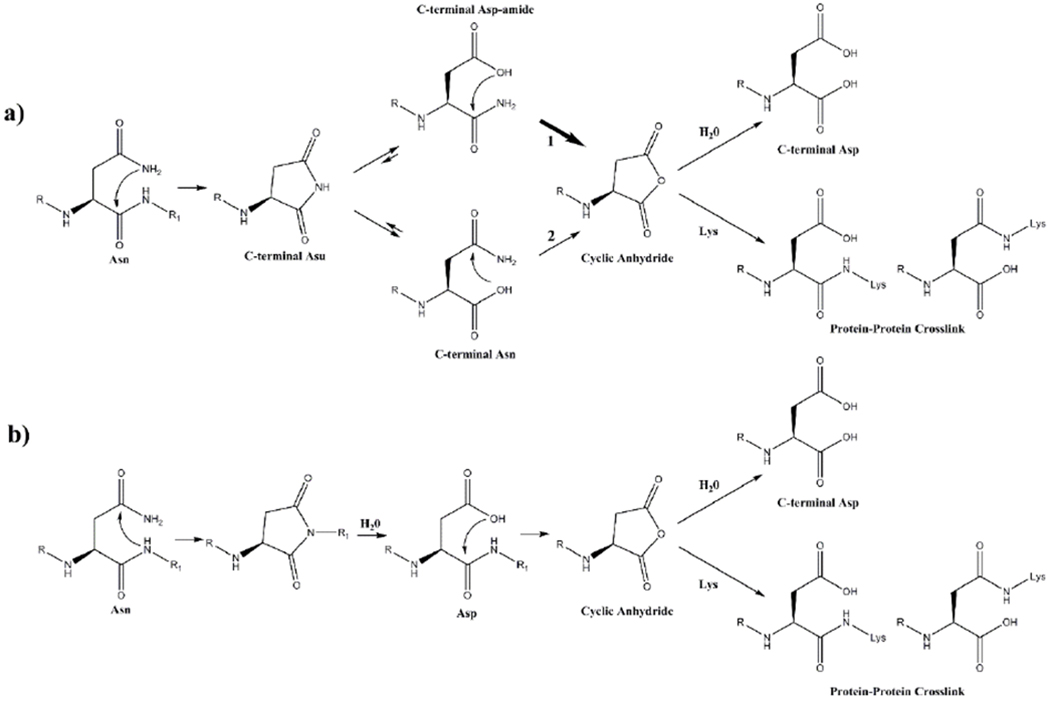
a) Spontaneous cleavage at an Asn residue leads to the formation of a C-terminal succinimide (Asu). This succinimide hydrolyses to yield both a C-terminal Asn and a C-terminal Asp amide. Each of these can deamidate to form a cyclic succinic anhydride. This intermediate can either hydrolyse to Asp/ isoAsp or, in the presence of a nucleophile such as Lys, can form a protein-protein crosslink.
b) Prior to peptide bond cleavage, deamidation of Asn occurs. The newly formed Asp, can then undergo spontaneous cyclisation forming a succinic anhydride intermediate. The cyclic anhydride can react with an amine group, such as that of a Lys residue, forming an Asp-Lys crosslink. The ε-amino group of Lys can potentially attack either carbonyl of the anhydride leading to two isomeric Asp-Lys crosslinks as shown.
Figure 2.
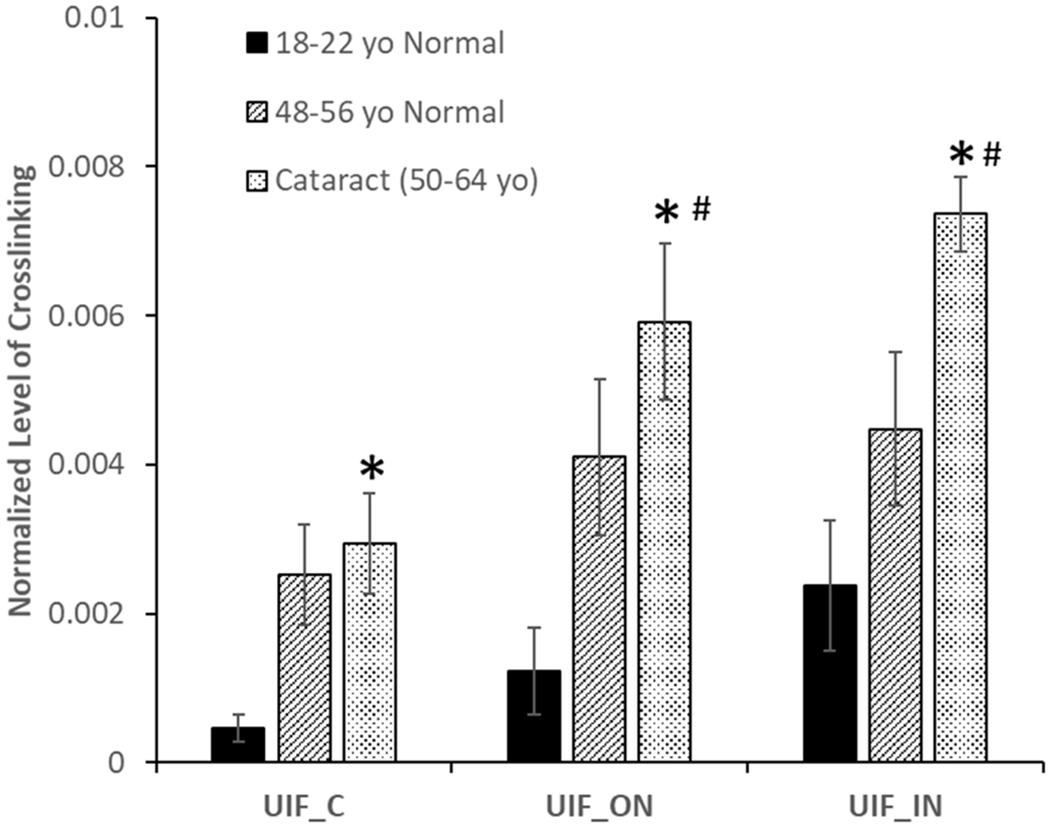
Levels of crosslinking between AQP0 (Asn 246) x AQP0 (Lys 228) in different aged and cataract lenses
An asterisk (*) indicates crosslinking increases in the cataract lenses compared with the young lenses (p<0.05). A pound sign (#) Indicates significant increases in crosslinking in the outer nucleus and inner nucleus regions compared with cortex in the same group of lenses (p< 0.05). n=4 +/− SD (UIF = Urea insoluble fraction, C= cortex, ON=outer nucleus, IN= inner nucleus.
Incubation of Asn peptides
All synthetic peptides were purchased from GME biochemicals (Shanghai, China) at 95% purity. Ac-YSNGF, Ac-YSDGF, Ac-YHNEF, Ac-YHDEF and Ac-YHNPF (1mg/mL) were incubated at pH 5.0 (100mM citric acid), pH 6.7 or pH 7.4 (100mM sodium phosphate) at 60°C. Aliquots were taken at intervals and injected onto a C18 RP HPLC column (Aeris, 2.6μ, XB-C18, 100mm x 2.1mm, Phenomenex). In these and the experiments below, a 50-minute gradient was performed at a flow rate of 0.2mL/min, consisting of the following: 0-5 min, 2% CH3CN; 5-15 min, 20% CH3CN, 15-20min 40% CH3CN, 25-35min 80% CH3CN, 35-50 2% CH3CN. Peptide cleavage products were detected at 216nm and 280nm, collected, lyophilised and their identities confirmed by tandem mass spectrometry using a Thermo LTQ mass spectrometer (Thermo Scientific). Since there was no difference in the MS/MS spectra of C-terminal Asn and Asp-amides, their identity was confirmed by the elution times of synthetic standards. The percentage of cleavage of each peptide was determined by the peak area of cleavage products compared with that of the original peptide at 216nm and 280nm. All experiments were run in triplicate.
Stability of C-terminal Asn and C-terminal Asp-amides
Ac-YHN and Ac-YHD-amide were incubated at 60°C in 100mM sodium phosphate buffer pH 6.7 and pH 7.4. Aliquots were removed for RP-HPLC. Elution times were confirmed using synthetic Ac-YHN, Ac-YHD-amide and Ac-YHD. The percentage of each peptide was determined by the peak area of products compared with that of the original peptide at 280nm. The above experiments were repeated in the presence of a 5 molar excess of PE. All experiments were run in triplicate.
Preparation of C-terminal succinimides
C-terminal succinimide (Asu) derivatives were prepared from Ac-YSN, Ac-YHN and Ac-YVN using a literature procedure [26]. Briefly, 3mg of each peptide was dissolved in 100% MeOH(100uL) in the presence of HCl (2uL). Peptides were incubated at room temperature for 1 hr then lyophilised. Methylation of the C-terminal carboxyl group was confirmed by mass spectrometry. Each methylated peptide was incubated in 100mM phosphate buffer, pH 5.5 for 3 hrs at 90 °C, and the resultant C-terminal Asu was isolated by HPLC and its structure confirmed by MS/MS. C-terminal Asu peptides were incubated at 100 mM sodium phosphate pH 6.7 or pH 7.4 at 37 °C. Aliquots were removed and breakdown of the C-terminal Asu monitored by HPLC. Commercial standards of C-terminal Asn and Asp-amides were used to confirm the identities of breakdown products. All experiments were performed in triplicate.
Preparation of a C-terminal Anhydride and crosslink formation
The C-terminal anhydride of Ac-YHD was prepared as described [27]. Briefly, Ac-YHD was dissolved in acetyl chloride and gently heated with shaking. After 1hr the sample was lyophilised and phenylethylamine (PE) was added in either 50 mM 80% acetonitrile or 100 mM phosphate pH 6.7. Samples were left for 20 min at room temperature and lyophilised. The structure of the C-terminal crosslink with PE was confirmed by MS/MS.
Decomposition of Asn and Asp peptides and C-terminal Asn crosslinking
Ac-YHNEF, Ac-YHDEF, Ac-YSDGF, Ac-YSNGF and Ac-YHNPF (1mg/mL) were incubated in the presence or absence of a 5-molar excess of phenylethylamine (PE) in 100mM phosphate buffer pH 6.7 and pH 7.4 (100mM sodium phosphate) at 60°C. In some cases, 80% CH3CN was used as a solvent. Aliquots were injected onto a C18 RP HPLC column and HPLC peaks were collected and structures confirmed by MS/MS. Experiments were repeated in the presence of corresponding C-terminal Asu, Asn and Asp-amide cleavage products. The crosslink involving PE was quantified by HPLC as a percentage of starting peptide. All experiments were performed in triplicate.
Results:
Identification of crosslinked Asn sites in human lenses
Recently, we reported a novel crosslinking mechanism that involved Lys or an N-terminal amine with terminal Asp residues through the formation of succinic anhydride [23]. Further analysing the data suggested similar crosslinking can also occur on terminal Asp residues that had been Asn residues in their originally translated protein sequence but had undergone deamidation. Therefore, we modified the protein database and searching method as described in the methods section to search for Lys or protein N-terminal amine crosslinked with terminal Asn residues. A list of newly identified crosslinked proteins is highlighted in Table.1. The measured parent masses of these peptides are within 3 ppm of the theoretical masses. When manually confirming the crosslinked peptides, special attention was paid to avoid assigning non-Asn terminal peptide signal to a crosslinked peptide. There is normally a fragment in the tandem mass spectrum that corresponding to cleavage the isopeptide bond formed during crosslinking. Representative tandem mass spectra for two crosslinked peptides can be found in Figure 1. A series of b- or y-ions from each peptide can be seen in the tandem mass spectrum of the crosslinked AQP0/AQP0 peptide. Cleavage of the amide bond formed during crosslinking results in strong fragments at m/z 770.3680 and 832.4890. Similarly, in the αA/αB-crystallin crosslinked peptide, the fragment at m/z 253.0939 corresponds to cleavage of the amide bond formed during crosslinking. Tandem mass spectra for other crosslinked peptides in Table 1 can be found in the supplemental figures 2–6. In addition, several peptides were also identified as potential crosslinked peptides as shown in the Supplemental Table 1. The parent masses matched the assigned crosslinked peptides within 1 ppm mass error and their tandem mass spetra confirm the presence of some residues; however, since two peptides involving in crosslinking are short, further testing is needed to confirm the crosslinking of these proteins.
Table 1.
Sites of crosslinking involving Lys and C-terminal Asn residues in human lens proteins
| Peptide A | Peptide B | [MH]+exp | [MH]+cal | error in ppm |
|---|---|---|---|---|
| AQP0 (Asn 246): GAKPDVSN* | AQP0 (Lys 228): LK*SISER | 1601.849 | 1601.849 | 0.15 |
| AQP0 (Asn 246): GAKPDVSN* | AQP0 (Met1): M*WELR | 1503.7221 | 1503.7260 | 2.59 |
| αA (Asn 101):HN* | αB (Lys 174): EEKPAVTAAPK*K | 1520.810 | 1520.807 | 2.28 |
| αA (Asn 101):HN* | αB (Lys 150): K*QVSGPER | 1152.574 | 1152.576 | 0.192 |
| βB2 (Asn 205): GAFHPSN* | βA4 (Lys 118): LTIFEQENFLGK*K | 2278.1503 | 2278.1502 | 0.4 |
| AQP0 (Asn 246): GAKPDVSN* | AQP0 (Lys 238): LSVLK*GAKPD | 1796.973 | 1796.975 | 1.44 |
Asterisks indicate the residues that are involved in crosslinking. All masses listed are monoisotopic masses.
Figure 1.
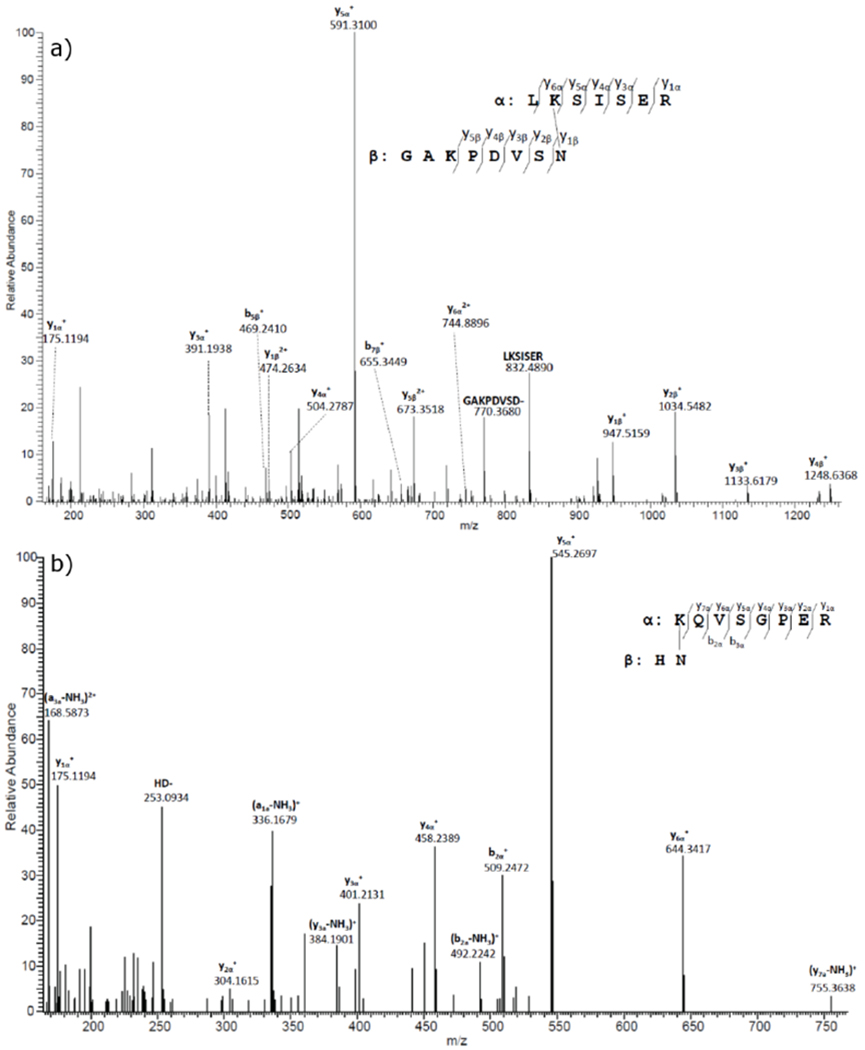
MS/MS spectra of selected crosslinked peptides detected in human lenses.
a) Tandem mass spectrum for the AQP0/AQP0 crosslinked peptide detected in the tryptic digest of UIF. This crosslinked peptide was detected in the majority of the lenses analysed. The tandem mass spectrum shows the crosslink between Asn 246 (GAKPDVSN*) and Lys 228 (LK*SISER). b) Tandem mass spectrum for αA/αB-crystallin crosslinked peptide. The tandem mass spectrum shows the crosslink between αA Asn 101 (HN*) and αB Lys 150 (K*QVSGPER). The loss of NH3 in this spectrum is consistent with that observed under gas-phase conditions [45]
Quantification of crosslinked AQP0/AQP0 in different lens regions
The signal of the crosslinked AQP0/AQP0 peptide shown in Figure 1 can easily be detected in 1D-LC-MS/MS analysis in majorities of the lenses studied, therefore, quantification of the level of crosslinking as a function of age and disease condition is possible. To quantify the level of crosslinking, each lens was dissected into three regions corresponding to distinct ages of the human lens development, inner nucleus (lens fibres present at birth), outer nucleus (lens fibres synthesized during childhood) and the cortex (adult lens fibers). Data were pooled into three groups based on age and cataract and the abundance of crosslinking is shown in Figure 2. Crosslinking increased from the cortex (youngest tissue) to the inner nucleus (oldest tissue) in all three groups of the lenses and the difference reaches statistical significance in cataract lenses (p< 0.05). Comparing the same region from young and middle aged lenses, crosslinking was found to increase with age. Cataract lenses also displayed elevated levels of crosslinking in comparison to age-matched controls in all lens regions. The difference between young lenses and cataract lenses reaches statistical significance. These data highlight the fact that crosslinking in AQP0 occurs early in life and increases with age and cataract.
Breakdown of Asn in peptides
Analysis of the amino acid sequences of the C-terminal crosslinked peptides (Table 1) revealed that the site of crosslinking in each case was a Lys joined covalently to an internal Asn residue through an isopeptide bond that appeared to have been both deamidated and cleaved. Peptide model studies were undertaken to understand the mechanism of formation of these crosslinks. Initially, the pentapeptide, Ac-YHNEF, was employed since this incorporates the Asn cleavage and crosslinking site detected in αA-crystallin (Table 1). The HNE sequence which is present within αA-crystallin, was bookended by Tyr and Phe residues to aid HPLC detection, and the peptide was acetylated to preclude reactions of the N-terminal amine.
Three pHs were employed to mimic physiological conditions; pH 5.0 representative of the interior pH of lysosomes [28], pH 6.7, the pH in the centre of the lens [29] and pH 7.4, the pH of blood and extracellular fluid. The breakdown of Ac-YHNEF was monitored using HPLC. Figure 3 shows the HPLC profile of Ac-YHNEF after 21 days incubation at 60°C, pH 6.7. During the incubation, several HPLC peaks appeared which corresponded to the breakdown of Ac-YHNEF. These included the deamidation products Ac-YHDEF and the internal succinimide (Ac-YH(Asu)EF), in addition to peptide cleavage products, Ac-YHN, Ac-YHD-amide, Ac-YH(Asu), Ac-YHD, EF, and pyroglutamyl Phe (PyroGluF). The structures of these products were confirmed by co-elution of synthetic standards as well as by MS/MS of each HPLC peak.
Figure 3. Decomposition of Ac-YHNEF at pH 6.7.
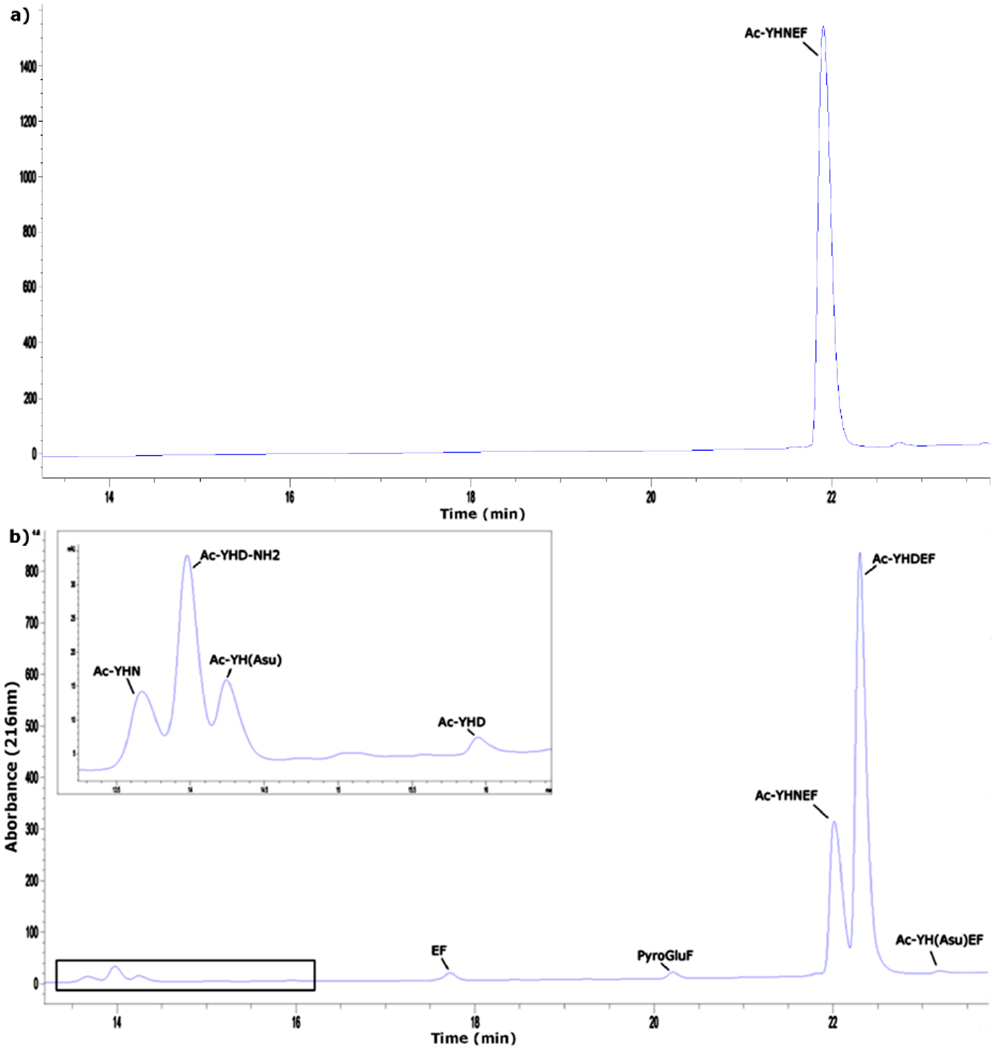
a) HPLC profile of Ac-YHNEF prior to incubation in 100mM phosphate buffer, pH 6.7 b) HPLC profile of Ac-YHNEF after 21 days of incubation at 60°C in 100mM phosphate buffer, pH 6.7. Peaks were identified by co-elution with synthetic peptide standards and MS/MS. Insert; expanded region of boxed HPLC trace. Detection at 216nm.
Ac-YHDEF and Ac-YH(Asu)EF are consistent with the current understanding of the mechanism of Asn deamidation where an internal succinimide forms via attack of the adjacent peptide bond NH on the side chain carbonyl group of Asn and, following hydrolysis of the succinimide, four Asp isomers (L-Asp, L-isoAsp, D-Asp and D-isoAsp) are generated [30]. These Asp isomers were not resolved by the HPLC system.
pH dependence of cleavage at Asn compared with deamidation of Asn
Some peptides that were detected in the HPLC trace clearly reflected cleavage of the peptide bond C-terminal to the Asn residue. The effect of pH on the proportion of Asn deamidated products compared to the cleaved products is depicted in Figure 4. Deamidation of Ac-YHNEF was found to be pH dependent, with the least amount detected at pH 5.0 and the greatest at pH 7.4 (Fig 4a). Cleavage showed a similar pH dependence (Fig 4b). For each pH tested, cleavage accounted for approximately 5-15% of the total amount of deamidation in Ac-YHNEF (Fig 4c). Similar results were found with Ac-YSNGF. The sequence SNG corresponds to the Asn site of crosslinking observed within Aquaporin 0, although deamidation occurred much more readily in this case, and this is most likely due to the fact that a Gly on the C-terminal side of Asn facilitates succinimide formation [31] and therefore deamidation.
Figure 4. A comparison of Asn deamidation and cleavage using Ac-YHNEF and Ac-YSNGF.
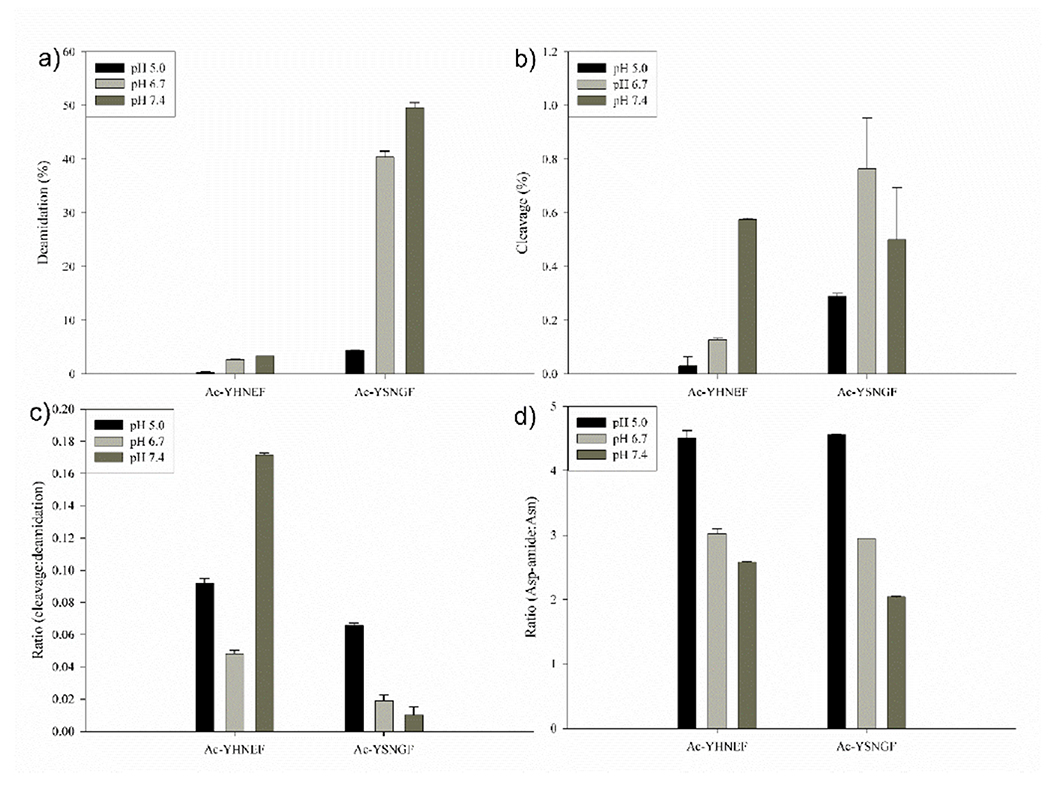
a) Deamidation of peptides at pH 5.0, 6.7 and 7.4. Deamidation of Asn to Asp was calculated using relative HPLC peak areas (see Fig 3); b) Cleavage of the same peptides at pH 5.0, 6.7 and 7.4. c) The ratio of cleavage to deamidation for each peptide after seven days. d) The ratio of C-terminal Asp-amide to Asn formed from original peptides at pH 6.7 and 7.4. All data are for peptides incubated for 7 days at 60°C. Values are n=3 +/− SD.
Intermediates formed during the decomposition of Asn in peptides
As illustrated in Scheme 1, deamidation of Asn, and cleavage at Asn are competing reactions, so it was of interest to examine the effect of factors, such as pH, on the relative rates of these two pathways. The details of the effect of pH on Asn cleavage rates compared to deamidation rates of Asn in the same peptide, will be described later in this manuscript. Quantitatively, the extent of cleavage was always less than the extent of Asn deamidation, typically accounting for 5-15% of total deamidation.
As highlighted in Scheme 1a, when a peptide bond is cleaved C-terminal to an Asn residue, a C-terminal succinimide intermediate is formed. Two products can conceivably be formed by hydrolysis of this terminal succinimide: a C-terminal Asp-amide and a C-terminal Asn (see Scheme 1). Using Ac-YHNEF, analysis of the cleavage products in the mixture showed greater amounts of the Asp amide compared to the corresponding C-terminal Asn (Fig 4d). The dominance of the Asp amide was also observed at each pH with Ac-YSNGF, where very similar ratios were obtained (Fig 4d). C-terminal succinimides of several peptides were studied as described in the next section, to discover if this finding was typical of Asn cleavage.
Properties of C-terminal succinimides
As outlined in Figure 3, the products identified from long-term incubations of an Asn peptide were consistent with the formation of both an internal succinimide (Ac-YH(Asu)EF) and a C-terminal succinimide (Ac-YH(Asu)). Little information is available in the literature on the properties of C-terminal succinimides [26]. In order to assess their stability, three C-terminal succinimides were synthesized using literature protocols [26]. Two peptides corresponding to sites of protein-protein crosslinking in the human lens; Ac-YH(Asu) and Ac-YS(Asu) were prepared, as well as an unrelated peptide Ac-YV(Asu). Initially Asu stability at pH 6.7 and 7.4 was determined (Figure 5a). All three C-terminal Asu peptides were found to be quite stable with half-lives ranging from ~1-3 days at pH 6.7 and ~12-40 hr at pH 7.4 at 37°C. Consistent with previous data [26], the products of hydrolysis at both pHs were C-terminal Asn and C-terminal Asp amide. One example is depicted showing the rate of formation of Ac-YHN and Ac-YHAsp amide from Ac-YH(Asu) (Fig 5b).
Figure 5. Stability of C-terminal succinimides.
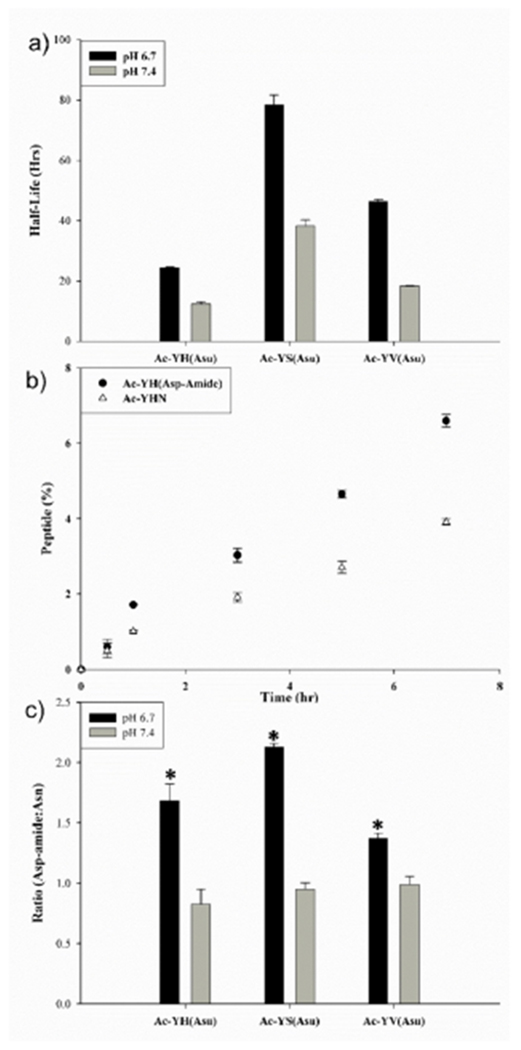
a) Half-lives of Ac-YH(Asu), Ac-YS(Asu) and Ac-YV(Asu) at pH 6.7 and 7.4; b) Time course of formation of Ac-YHN and Ac-YHAsp-amide following incubation of Ac-YH(Asu) at pH 6.7; c) Hydrolysis of Ac-YH(Asu), Ac-YS(Asu) and Ac-YV(Asu) and resultant ratio of Asp-amide to Asn at pH 6.7 and 7.4. All samples were incubated at 37°C and each time point n=3, +/−SD. * indicates p< 0.0005
For all three peptides incubated at pH 7.4, the Asn: Asp-amide ratio was ~1:1(Fig 5c). The relative proportion of Asp amide to C-terminal Asn produced by hydrolysis at pH 6.7 was significantly greater for each peptide when compared to pH 7.4 (p< 0.0005). In comparison to the breakdown products of Ac-YHNEF and Ac-YSNGF (Fig.4d), the ratio of Asn:Asp-amide was lower, though the trend was the same, Asp-amide was the preferred breakdown product. Favoured formation of Asp-amides over the corresponding C-terminal Asn peptides was observed in a number of unrelated peptides (Supplemental Figure 6).
Stability of C-terminal Asn and Asp amides
In the case of both the C-terminal products, nucleophilic attack by the carboxyl group on the amide [32] could result in the nitrogen atom being eliminated as ammonia. A succinic anhydride intermediate would then be formed (Scheme 1a). In aqueous solution, this deamidation would ultimately result in the formation of a C-terminal Asp by hydrolysis of the succinic anhydride. Ac-YHN and Ac-YHD-amide were incubated at pH 6.7 and pH 7.4 and deamidation monitored by HPLC. The data revealed that both Ac-YHN and Ac-YHD-amide deamidate and that this reaction was markedly influenced by pH (Figure 6). Ac-YHD amide deamidated more readily. For example, at pH 6.7 ~ 12% Ac-YHD-amide had deamidated after seven days at 60 °C. By contrast, under the same conditions only ~1.5% of the Ac-YHN had deamidated to Ac-YHD. Using pH 7.4 buffer, deamidation was lowered ~3 fold for Ac-YHD-amide while with Ac-YHN little was observed at pH 7.4 (Fig 6).
Figure 6. Deamidation of Ac-YHD-amide and Ac-YHN.
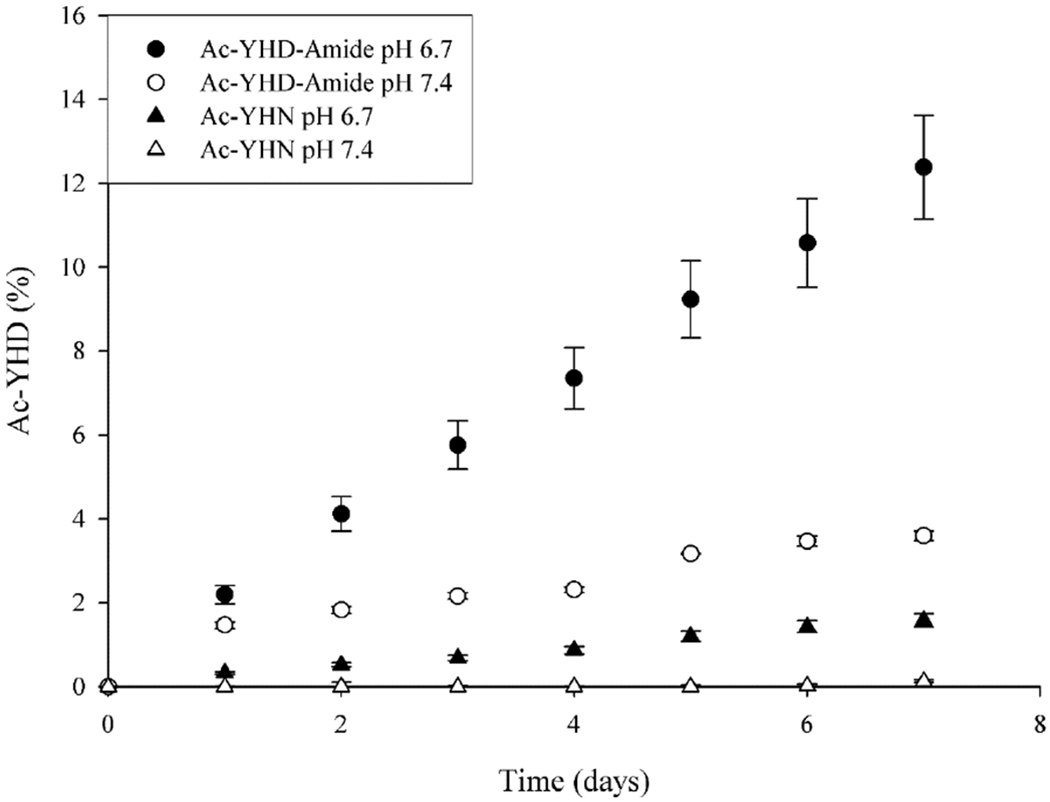
Deamidation to Ac-YHD was detected following incubation of Ac-YHD-amide and Ac-YHN at pH 6.7 and 7.4. Experiments were conducted in triplicate at 60°C
It should also theoretically be possible for C-terminal Asn and C-terminal Asp amides to interconvert via a succinimide intermediate (see Scheme 1). Under the conditions used above, no interconversion was observed over a 12-day period, indicating that interconversion of a C-terminal Asn and C-terminal Asp amide was considerably slower than deamidation of Ac-YHN.
Crosslinking experiments: involvement of an anhydride
As shown in the previous section, breakdown of C-terminal succinimides resulted in the formation of both C-terminal Asn and Asp amides and these products could in turn deamidate presumably via the formation of a C-terminal succinic anhydride intermediate. If such breakdown occurred in the lens, and the succinic anhydride was not immediately hydrolysed to Asp, then this pathway may explain the origin of the Lys crosslinks.
In order to test this proposal, Ac-YHD-amide and Ac-YHN were incubated in the presence, or absence, of a 5-fold molar excess of the lysine mimic, phenylethylamine (PE). When PE is incorporated into the mixture, competition between anhydride hydrolysis, with the resultant formation of Asp, and crosslink formation due to nucleophilic attack by the amine should occur. Figure 7a shows such a trend. When PE was present in the mixture, a crosslink formed linearly with time of incubation. As PE crosslinking occurred, there was a corresponding decrease in the rate of formation of Ac-YHD by comparison to that when PE was absent (Fig 7b). As indicated in the figure, the sum of the two reactions remained relatively constant. This result indicates that only two dominant reactions take place: anhydride hydrolysis and covalent addition of the amine to the anhydride.
Figure 7. Ac-YHD-amide readily forms a crosslink with PE.
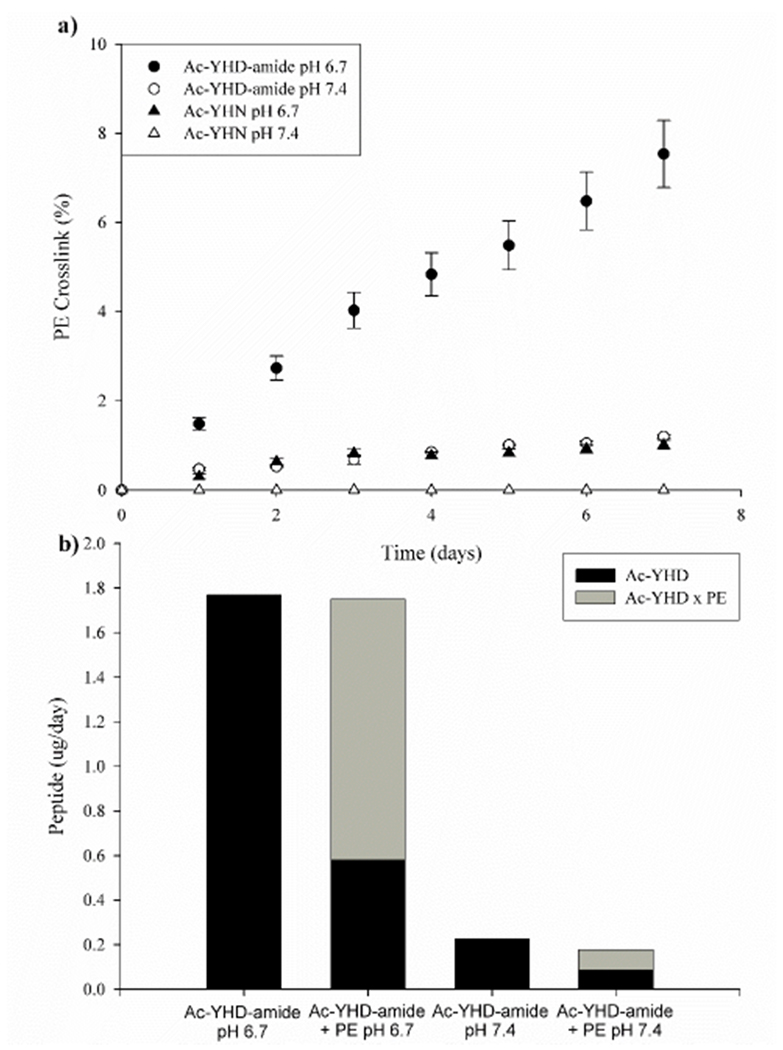
a) Crosslink formation following incubation of Ac-YHD-amide or Ac-YHN with PE at pH 6.7 and 7.4 at 60 °C. b) Bar graphs indicating the rate of Ac-YHD formation at pH 6.7 and 7.4 from Ac-YHD-amide. When the amount of crosslinked adduct is included, the sum of the Ac-YSD and PE adduct approximately equals that of the Ac-YSD in the absence of PE. Experiments were conducted in triplicate at 60°C, pH 6.7 with a five-fold molar excess of PE.
Additional indirect support for the crucial involvement of an anhydride in the crosslinking came when the incubation of Ac-YHD amide with PE was repeated at pH 7.4. At this pH much less of the PE crosslink was detected (Fig 7a). As shown previously (Fig 6), at this pH deamidation does not occur to any appreciable extent, therefore less succinic anhydride formation and less crosslinking was expected. The experiment depicted was repeated using Ac-YSN and Ac-YSD-amide at both pHs with a similar result (Supplemental Figure 7) and the crosslink was also formed with N-Ac-Lys (Supplementary Fig 8). Taken together with the succinimide stability results at pH 6.7 and 7.4 (Fig. 5) these data emphasize the importance of a relatively small change in pH (< 1pH unit) in governing the outcome of these reactions.
The structure of the crosslinked species was confirmed by synthesizing the succinic anhydride directly from Ac-YSD using literature procedures [27] and then reacting it with PE. MS/MS analysis showed that this crosslinked product was identical to the one formed during incubation of Ac-YSD amide with PE (Supplemental Figure 9).
Succinimide reactivity
Since it was possible that the C-terminal succinimide intermediate may react with an amine directly, and then undergo loss of the nitrogen atom, the reactivity of succinimides with PE was investigated. Data in Figure 5a revealed that C-terminal Asu peptides were quite stable at pH 6.7. Ac-YH(Asu) was therefore incubated with PE at pH 6.7 for 48hrs at 60°C. No PE crosslink was detected by HPLC or by mass spectrometry. Only the expected breakdown products of Ac-YH(Asu), i.e. Ac-YHN and Ac-YHD-amide were observed. These experiments were repeated with Ac-YV(Asu) with the same result observed. These experiments indicate that the main avenue of crosslinking involves prior breakdown of the succinimide, rather than direct addition of the amine to the succinimide.
Formation of crosslink from Asp
It is clear from the above experiments that breakdown of a C-terminal succinimide that is produced during peptide bond scission adjacent to Asn, could explain the formation of the crosslinks observed in the lens. Another pathway is possible. It is also feasible that deamidation of Asn to Asp in the intact peptide, followed by cleavage C-terminal to the newly formed Asp residue could also result in the formation of a C-terminal succinic anhydride [30,33,34] (Scheme 1b). Cleavage of Asp containing peptides with the formation of C-terminal succinic anhydrides has been investigated recently, which revealed such a mechanism can lead to C-terminal protein-protein crosslinks [34]. This “prior deamidation’ mechanism was investigated in the following way; first Ac-YHDEF was examined to see if cleavage at the Asp could be detected at pH 6.7 and pH 7.4. The rate of peptide bond cleavage was found to be comparable to that of Ac-YHNEF at pH 6.7 and greater than 10-fold slower at pH 7.4 (Figure 8). Secondly, we investigated if the same crosslinked product could form when Ac-YHDEF were incubated with PE at pH 6.7. It was found that a PE crosslink formed in both cases.
Figure 8. Ac-YHD PE crosslink forms when PE is incubated with Ac-YHNEF.
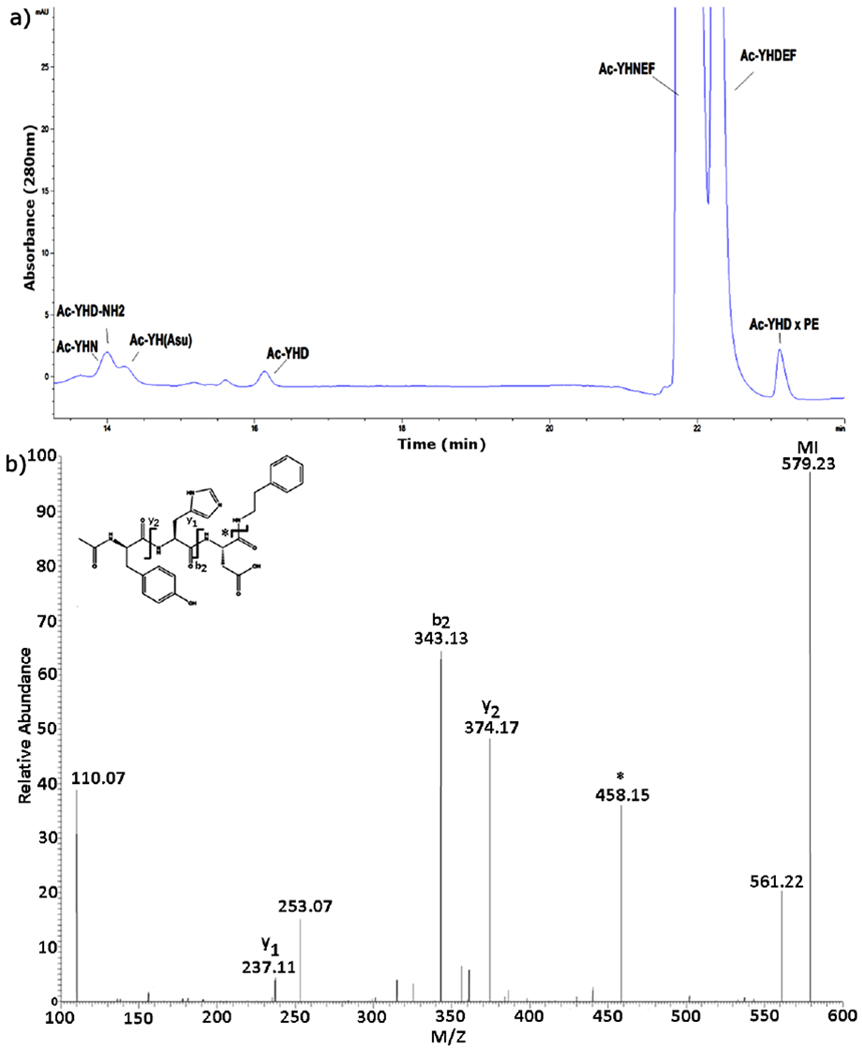
a) HPLC profile of Ac-YHNEF incubated at 60 °C with a five-fold molar excess of PE at pH 6.7 for 3 days. Detection at 280nm b) MS/MS spectrum of the Ac-YHD x PE peak isolated by HPLC. * = loss of PE, MI = molecular ion.
Crosslinking is observed in the absence of Asn deamidation
These findings raised the possibility that all of the crosslinking observed from an Asn peptide may take place via this “prior deamidation” mechanism and that the multi-step pathway involving Asn cleavage, C-terminal succinimide formation and hydrolysis to form the anhydride that was elucidated in peptide experiments, is feasible, but may occur at an insignificant rate compared with prior Asn to Asp conversion.
In order to demonstrate crosslinking without prior Asn deamidation, the Glu residue C-terminal to the Asn was replaced with a Pro residue. Ac-YHNPF is unable to form an internal succinimide, and therefore cannot deamidate to Ac-YHDPF (see mechanism Scheme 1). Consequently, it will only cleave by direct nucleophilic attack of the Asn side chain.
Consistent with this hypothesis no deamidation of the original peptide, Ac-YHNPF, to Ac-YHDPF was detected (Supplemental Fig 10). HPLC analysis of Ac-YHNPF following incubation in pH 6.7 or 7.4 buffers revealed only the presence of Ac-YHD-amide, Ac-YHN and Ac-YH(Asu). Thus, this Asn peptide cleaved directly without any prior deamidation. When PE was added to the Ac-YHNPF incubation an Ac-YHD-PE crosslink was formed. This experiment demonstrated that Asn cleavage can cause crosslinking directly without the intermediary of deamidation of the Asn residue.
Cleavage adjacent to Asn and cleavage adjacent to Asp are markedly affected by pH
Since it is possible to form a C-terminal crosslink starting with either an internal Asn or Asp we investigated the rate of Asn/Asp cleavage in Ac-YHNEF and Ac-YHDEF as a function of pH. pH had a large effect on the rate of peptide bond cleavage. As displayed in Figure 9, the cleavage of Ac-YHNEF occurred more readily at pH 7.4, and as the pH was lowered, the rate of cleavage decreased.
Figure 9. Cleavage at Asp compared with the rate of cleavage at Asn.
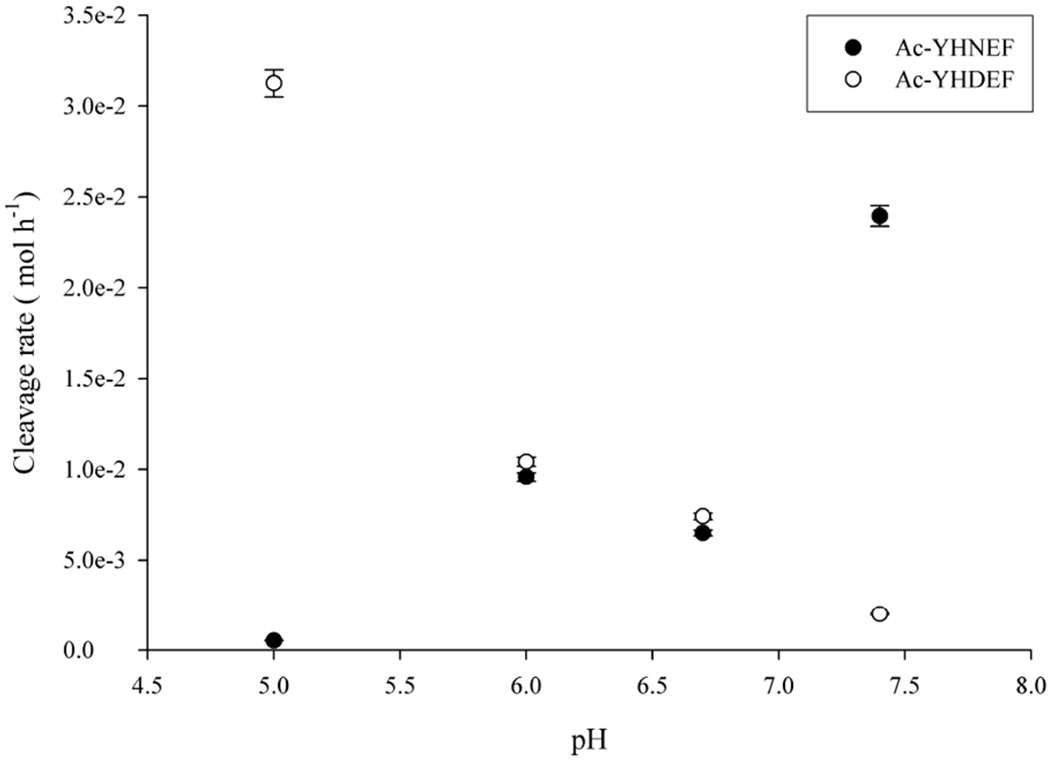
The rate of cleavage of Ac-YHNEF and Ac-YHDEF as a function of pH. Cleavage of Ac-YHNEF was calculated as the sum of the peak areas of Ac-YHD, Ac-YHD-amide, Ac-YHN, and Ac-YH(Asu) by comparison to the amount of original peptide injected on HPLC. Ac-YHDEF cleavage was calculated as above using only Ac-YHD peak area. Each time point n=3. All peak areas were calculated from absorbance at 280nm. Experiments were conducted in triplicate at 60°C.
An opposite trend was observed with Ac-YHDEF with cleavage occurring more readily at lower pHs with limited cleavage detected at pH 7.4. These findings were in agreement with the current understanding of cleavage at Asp residues [35]. At pH 6.7 which corresponds to the pH of the human lens interior, the rates of Ac-YHNEF and Ac-YHDEF cleavage were approximately equivalent, highlighting that both Asn and Asp are potential sites for crosslinking to Lys.
Discussion
This paper describes how spontaneous cleavage at Asn residues can lead to protein-protein crosslinking. Through the use of model peptides a mechanism was elucidated that involves the stepwise breakdown of a C-terminal succinimide; the initial cleavage product formed when the Asn side chain nitrogen atom attacks the carbonyl of the adjacent peptide bond. C-terminal succinimides hydrolysed at neutral pH to yield both Asp amides and C-terminal Asn peptides. These two products can interconvert slowly in a process involving a succinimide intermediate, but importantly for crosslinking, they can also deamidate via an intramolecular reaction that results in the formation of a C-terminal succinic anhydride (Scheme 1). It is the anhydride intermediate that reacts readily with amines and, if this pathway takes place in a protein, can result in covalent crosslinking with a nearby Lys residue.
Several sites of crosslinking that are consistent with this mechanism were characterised in the human lens (Table 1). These crosslinks were found to involve soluble proteins (crystallins), membrane proteins (aquaporin 0), as well as a cytoskeletal protein (filensin). Since this crosslinking is a spontaneous process, the extent of modification would be expected to increase over time. This hypothesis was supported by the increase in APQ0/AQP0 crosslink content from younger cortex to the oldest nuclear fibre cells, in addition to an increase in crosslinking with age for the same anatomical lens region (Fig.2). Increased crosslinking in cataract lenses in comparison to age-matched controls suggests a possible role in cataract onset, although further experiments are required to confirm any link.
It is important to recognise that once a succinic anhydride forms it will be subject to two competing reactions: crosslinking and hydrolysis. Hydrolysis will result in the formation of peptides that contain C-terminal Asp and potentially C-terminal isoAsp residues. Cleavage and formation of C-terminal Asn has previously been reported and correspond to sites of crosslinking found in this study. [36,37]
Seven synthetic peptides that contain Asn were investigated and each one showed evidence of deamidation, as well as peptide bond cleavage on the C-terminal side of the Asn residue following incubation. Typically, at neutral pH, cleavage was found to represent from 1% to 17% of the amount of deamidation (Figure 4) although if a bulky residue such as Val was present on the C-terminal side of the Asn, this could rise to 70% (Supplemental Figure 11). This much higher ratio reflected a marked drop in deamidation, as expected from literature data on deamidation [30], due to the presence of a bulky residue adjacent to the Asn.
Although C-terminal succinic anhydrides hydrolyse readily in aqueous solution, C-terminal succinimides were found to be relatively stable, with half-lives measured in days at pH 6.7 and 7.4 (Figure 6). C-terminal succinimides also showed little, or no, detectable direct reaction with PE. In agreement with this observation, the crosslinks found when PE was incubated with intact Asn peptides corresponded in mass with that of an isopeptide link involving Asp, rather than a direct succinimide-mediated crosslink involving Asn – which would differ in mass by 1 Da. This finding is consistent with the involvement of the anhydride intermediate in crosslinking as outlined above, and the lens peptide data also support this conclusion.
Generally, Asp amides formed by the breakdown of the C-terminal succinimide, were found to be more reactive than the C-terminal Asn peptides (Figures 6 and 7). When Ac-YHD amide was incubated with PE, only two products were observed (Figure 7a): the PE crosslink and the hydrolysis product Ac-YHD. When Ac-YHD amide was incubated with a 5-fold molar excess of PE, quantitative analysis revealed that approximately equal amounts of the PE-crosslink and the hydrolysis product (Ac-YSD) were detected (Figure 7b) and when summed these two products approximately equalled the amount of Ac-YSD formed in the absence of PE. This result indicates that once the anhydride forms from Ac-YSD amide, there are only two significant competing reactions: hydrolysis and nucleophilic attack by the amine.
In agreement with our observations in the human lens, it has been reported that the C-terminal Asn residue of insulin, when stored for extended periods, can form crosslinks via an anhydride [38,39]. These findings imply that LLPs with C-terminal Asn residues may be susceptible to untoward protein-protein crosslinking of the type described in this manuscript.
The role of an anhydride in the crosslinking was further supported by experiments where incubation of Asp amides with PE was performed using methanol or hexafluoroisopropanol as solvents. In both solvents PE adduct formation was greatly reduced and large HPLC peaks corresponding to the esters were observed. The esters formed by reaction of anhydride with the alcohol solvent. Because of this finding, these membrane-mimicking solvents could not be employed; however, 80% acetonitrile was found to be a suitable alternative. Using acetonitrile, deamidation of both Ac-YHN and Ac-YHD amide took place much more rapidly than in aqueous buffers. When PE was added, the rate of crosslinking was also increased (Supplemental Figure 12). It is likely that in the presence of a non-aqueous solvent, such as acetonitrile, the pKa values of the reactive groups, are altered in such a way that intramolecular reactions are facilitated [40,41]. Since this is the case, one prediction would be that Lys crosslinking by this novel mechanism should occur more readily in a membrane environment. In line with this prediction, two sites of crosslinking involving the membrane water channel protein aquaporin 0 were detected in the lens (Table 1).
As outlined in the Results section, it is possible that a C-terminal succinic anhydride could also form via initial deamidation of Asn, followed by an attack on the C-terminal peptide bond by the newly created Asp carboxyl group. Such a process has been examined previously [33,34]. In our hands using an Asn peptide (Ac-YHNEF) and comparing it to the analogous Asp peptide sequence, it was found that both cleavage and the formation of the C-terminal PE crosslink occurred at approximately the same rate at pH 6.7. This suggests that both mechanisms: i) deamidation of Asn to Asp and then cleavage forming a succinic anhydride and ii) direct attack of the Asn side chain forming a C-terminal succinimide and its breakdown to succinic anhydride, could lead to the formation of the observed Lys crosslink (see Scheme 1a and 1b). Evidence that such crosslinking processes may contribute to protein insolubility in the lens and cataract formation, it was found that the relative quantities of the crosslinks were greater in insoluble protein and cataract lenses (Fig 2). Similar results have been found in other crosslinking processes in the lens suggestive that protein-protein crosslinking may play role in the etiology of cataract [42].
Processes that lead to either cleavage/crosslinking or deamidation of Asn are still being explored however a number of environmental and structural factors appear to be important. The flexibility of the protein [43] at the Asn site assists in the formation of the Asu intermediate. Further protein unfolding may occur after cleavage at Asn that may enhance protein-protein crosslinking. The neighbouring amino acids can also affect the breakdown of Asu, with small amino acids such as Gly favouring deamidation [44] while bulker residues towards cleavage. It is probable that these factors and other influence crosslinking, independently, cumulatively or synergistically complicating interpretation of processes that assist in C-terminal Asn crosslink.
In conclusion, this study has revealed a mechanism responsible for novel protein-protein crosslinking. The pathway involves an initial spontaneous cleavage at an Asn residue, with a step-wise breakdown of the C-terminal succinimide ultimately yielding an anhydride. Nucleophilic attack on this anhydride by the amine side chain of Lys results in the formation of a covalent isopeptide bond. Any Asn in a protein that is known to deamidate, appears to also be a potential site of such covalent crosslinking. The discovery of this mechanism represents a link between three of the most well-known processes associated with long-lived proteins: Asn deamidation, peptide bond cleavage and covalent crosslinking.
Supplementary Material
Acknowledgements:
The authors acknowledge the use of the UOW Mass Spectrometry User Resource and Research Facility (MSURRF), University of Wollongong and the Proteomics Core Facility of the Vanderbilt University Mass Spectrometry Research Center.
Funding sources and disclosure of conflicts of interest Funding for this study was provided by National Institutes of Health by grants R01 EY024258 and P30 EY008126. The authors declare no conflict of interest.
References
- 1.Wang Z; Lyons B; Truscott RJW; Schey KL Human protein aging: Modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging cell 2014,13, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsamis A; Krawiec JT; Vorp DA Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. Journal of The Royal Society Interface 2013, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimoto D Aging and cross-linking in human aorta. Biochemical and biophysical research communications 1982, 109, 1264–1269. [DOI] [PubMed] [Google Scholar]
- 4.Haus JM; Carrithers JA; Trappe SW; Trappe TA Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. Journal of Applied Physiology 2007, 103, 2068–2076. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe A; Hong W-K; Dohmae N; Takio K; Morishima-Kawashima M; Ihara Y Molecular aging of tau: Disulfide-independent aggregation and non-enzymatic degradation in vitro and in vivo. Journal of Neurochemistry 2004, 90, 1302–1311. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hilaly YK; Williams TL; Stewart-Parker M; Ford L; Skaria E; Cole M; Bucher WG; Morris KL; Sada AA; Thorpe JR, et al. A central role for dityrosine crosslinking of amyloid-β in alzheimer’s disease. Acta Neuropathologica Communications 2013, 1, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sell DR; Monnier VM Molecular basis of arterial stiffening: Role of glycation - a minireview. Gerontology 2012, 58, 227–237. [DOI] [PubMed] [Google Scholar]
- 8.Verzijl N; DeGroot J; Zaken CB; Braun-Benjamin O; Maroudas A; Bank RA; Mizrahi J; Schalkwijk CG; Thorpe SR; Baynes JW, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis & Rheumatism 2002, 46, 114–123. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z; Lyons B; Truscott RJ; Schey KL Human protein aging: Modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging cell 2014,13, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilley KJ; Pirie A Changes to the proteins of the human lens nucleus in cataract. Experimental eye research 1974, 19, 59–72. [DOI] [PubMed] [Google Scholar]
- 11.Truscott RJ; Augusteyn RC The state of sulphydryl groups in normal and cataractous human lenses. Experimental eye research 1977, 25, 139–148. [DOI] [PubMed] [Google Scholar]
- 12.Akagawa M; Suyama K Mechanism of formation of elastin crosslinks. Connective tissue research 2000, 41, 131–141. [DOI] [PubMed] [Google Scholar]
- 13.Singh R; Barden A; Mori T; Beilin L Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [DOI] [PubMed] [Google Scholar]
- 14.Clarke S Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res 1987, 30, 808–821. [DOI] [PubMed] [Google Scholar]
- 15.Lyons B; Jamie J; Truscott R Spontaneous cleavage of proteins at serine residues. International Journal of Peptide Research and Therapeutics 2011, 17, 131–135. [Google Scholar]
- 16.Hooi MYS; Truscott RJW Racemisation and human cataract. D-ser, d-asp/asn and d-thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age 2011, 33, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truscott RJW; Schey KL; Friedrich MG Old proteins in man: A field in its infancy. Trends Biochem Sci 2016, 41, 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich MG; Wang Z; Schey KL; Truscott RJW Spontaneous crosslinking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate. BiochemicalJournal 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich MG; Hancock SE; Raftery MJ; Truscott RJW Isoaspartic acid is present at specific sites in myelin basic protein from multiple sclerosis patients: Could this represent a trigger for disease onset? Acta Neuropathologica Communications 2016, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooi M; Truscott R Racemisation and human cataract. D-ser, d-asp/asn and d-thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. AGE 2011, 33, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroudas A; Palla G; Gilav E Racemization of aspartic acid in human articular cartilage. Connective tissue research 1992, 28, 161–169. [DOI] [PubMed] [Google Scholar]
- 22.Heo S; Diering GH; Na CH; Nirujogi RS; Bachman JL; Pandey A; Huganir RL Identification of long-lived synaptic proteins by proteomic analysis of synaptosome protein turnover. Proceedings of the National Academy of Sciences 2018, 115, E3827–E3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z; Friedrich MG; Truscott RJW; Schey KL Cleavage c-terminal to asp leads to covalent crosslinking of long-lived human proteins. Biochimica et biophysica acta. Proteins and proteomics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z; Han J; David LL; Schey KL Proteomics and phosphoproteomics analysis of human lens fiber cell membranes. Invest Ophthalmol Vis Sci 2013, 54, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z-L; Meng J-M; Cao Y; Yin J-L; Fang R-Q; Fan S-B; Liu C; Zeng W-F; Ding YH; Tan D, et al. A high-speed search engine plink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nature Communications 2019, 10, 3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao Y; Xu MQ; Paulus H Protein splicing: Characterization of the aminosuccinimide residue at the carboxyl terminus of the excised intervening sequence. Biochemistry 1995, 34, 10844–10850. [DOI] [PubMed] [Google Scholar]
- 27.Fieser LF. Succinic anhydride. Experiments in Organic Chemistry 1941, 104. [Google Scholar]
- 28.Mindell JA Lysosomal acidification mechanisms. Annual Review of Physiology 2012, 74, 69–86. [DOI] [PubMed] [Google Scholar]
- 29.Greiner JV; Kopp SJ; Sanders DR; Glonek T Organophosphates of the crystalline lens: A nuclear magnetic resonance spectroscopic study Investigative ophthalmology & visual science 1981, 21, 700–713. [PubMed] [Google Scholar]
- 30.Geiger T; Clarke S Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. Journal of Biological Chemistry 1987, 262, 785–794. [PubMed] [Google Scholar]
- 31.Robinson NE; Robinson AB Molecular clocks. Proceedings of the National Academy of Sciences 2001, 98, 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catak S; Monard G; Aviyente V; Ruiz-López MF Computational study on nonenzymatic peptide bond cleavage at asparagine and aspartic acid. The Journal of Physical Chemistry A 2008, 112, 8752–8761. [DOI] [PubMed] [Google Scholar]
- 33.Oliyai C; Borchardt RT Chemical pathways of peptide degradation. Iv. Pathways, kinetics, and mechanism of degradation of an aspartyl residue in a model hexapeptide. Pharmaceutical research 1993, 10, 95–102. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z; Friedrich MG; Truscott RJW; Schey KL Cleavage c-terminal to asp leads to covalent crosslinking of long-lived human proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi AB; Sawai M; Kearney WR; Kirsch LE Studies on the mechanism of aspartic acid cleavage and glutamine deamidation in the acidic degradation of glucagon. Journal of Pharmaceutical Sciences 2005, 94, 1912–1927. [DOI] [PubMed] [Google Scholar]
- 36.Voorter CE; de Haard-Hoekman WA; van den Oetelaar PJ; Bloemendal H; de Jong WW Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem 1988, 263, 19020–19023. [PubMed] [Google Scholar]
- 37.Ball LE; Garland DL; Crouch RK; Schey KL Post-translational modifications of aquaporin 0 (aqp0) in the normal human lens: Spatial and temporal occurrence. Biochemistry 2004, 43, 9856–9865. [DOI] [PubMed] [Google Scholar]
- 38.Darrington RT; Anderson BD The role of intramolecular nucleophilic catalysis and the effects of self-association on the deamidation of human insulin at low ph. Pharmaceutical research 1994, 11, 784–793. [DOI] [PubMed] [Google Scholar]
- 39.Hjorth CF; Hubalek F; Andersson J; Poulsen C; Otzen D; Naver H Purification and identification of high molecular weight products formed during storage of neutral formulation of human insulin. Pharmaceutical research 2015, 32, 2072–2085. [DOI] [PubMed] [Google Scholar]
- 40.Gleason NJ; Vostrikov VV; Greathouse DV; Koeppe RE Buried lysine, but not arginine, titrates and alters transmembrane helix tilt. Proceedings of the National Academy of Sciences 2013, 110, 1692–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panahi A; Brooks CL Membrane environment modulates the pk(a) values of transmembrane helices. The Journal of Physical Chemistry. B 2015, 119, 4601–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schey KL; Wang Z; Friedrich MG; Garland DL; Truscott RJW. Spatiotemporal changes in the human lens proteome: Critical insights into long-lived proteins. Progress in retinal and eye research 2019, 100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooi MYS; Raftery MJ; Truscott RJW Racemization of two proteins over our lifespan: Deamidation of asparagine 76 in ys crystallin is greater in cataract than in normal lenses across the age rangeracemization and cataract formation. Invest Ophthalmol Vis Sci 2012, 53, 3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiger T; Clarke S Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem 1987, 262, 785–794. [PubMed] [Google Scholar]
- 45.Kempkes LJM; Martens J; Grzetic J; Berden G; Oomens J Deamidation reactions of asparagine- and glutamine-containing dipeptides investigated by ion spectroscopy. Journal of The American Society for Mass Spectrometry 2016, 27, 1855–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


