Abstract
Background
Ascaris lumbricoides is a common infection, and mainly affects children living in low‐income areas. Water and sanitation improvement, health education, and drug treatment may help break the cycle of transmission, and effective drugs will reduce morbidity.
Objectives
To compare the efficacy and safety of anthelmintic drugs (albendazole, mebendazole, ivermectin) for treating people with Ascaris infection.
Search methods
We searched the Cochrane Infectious Disease Group Specialized Register, CENTRAL, MEDLINE, Embase, LILACS, three other databases, and reference lists of included studies, without language restrictions, up to 4 July 2019.
Selection criteria
Randomized controlled trials (RCT) that compared albendazole, mebendazole, and ivermectin in children and adults with confirmed Ascaris infection.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed risk of bias, and extracted data from the included trials. A third review author checked the quality of data extraction. We used the Cochrane 'Risk of bias' assessment tool to determine the risk of bias in included trials. We used risk ratios (RRs) with 95% confidence intervals (CIs) to compare dichotomous outcomes in treatment and control groups. We used the fixed‐effect model for studies with low heterogeneity and the random‐effects model for studies with moderate to high heterogeneity. We assessed the certainty of the evidence using the GRADE approach. We used the control rate average to provide illustrative cure rates in the comparison groups.
Main results
We included 30 parallel‐group RCTs, which enrolled 6442 participants from 17 countries across Africa, Asia, Central America and the Caribbean, and South America. Participants were from 28 days to 82 years of age, recruited from school, communities, and health facilities. Twenty studies were funded or co‐funded by manufacturers, while 10 studies were independent of manufacturer funding. Twenty‐two trials had a high risk of bias for one or two domains (blinding, incomplete outcome data, selective reporting).
Single dose of albendazole (four trials), mebendazole (three trials) or ivermectin (one trial) was compared to placebo. Parasitological cure at 14 to 60 days was high in all the studies (illustrative cure of 93.0% in the anthelmintic group and 16.1% in the placebo group; RR 6.29, 95% CI 3.91 to 10.12; 8 trials, 1578 participants; moderate‐certainty evidence). Single dose of albendazole is as effective as multiple doses of albendazole (illustrative cure of 93.2% with single dose, 94.3% with multiple doses; RR 0.98, 95% CI 0.92 to 1.05; 3 trials, 307 participants; high‐certainty evidence); or as single dose of mebendazole (illustrative cure of 98.0% with albendazole, 96.9% with mebendazole; RR 1.01, 95% CI 1.00 to 1.02; 6 trials, 2131 participants; high‐certainty evidence). Studies did not detect a difference between a single dose of albendazole and a single dose of ivermectin (cure rates of 87.8% with albendazole, 90.2% with ivermectin; RR 0.99, 95% CI 0.91 to 1.08; 3 trials, 519 participants; moderate‐certainty evidence).
Across all the studies, failure after single dose of albendazole ranged from 0.0% to 30.3%, mebendazole from 0.0% to 22.2%, and ivermectin from 0.0% to 21.6%.
The egg reduction rate (ERR) measured up to 60 days after the treatment was high in all treated groups, regardless of the anthelmintic used (range 96% to 100%). It was not possible to evaluate parasitological cure by classes of infection intensity.
No included trials reported complication or serious adverse events. Other adverse events were apparently similar among the compared anthelmintic groups (moderate‐ to low‐certainty evidence). The most commonly reported other adverse events were nausea, vomiting, abdominal pain, diarrhoea, headache, and fever.
Authors' conclusions
Single‐dose of albendazole, mebendazole, and ivermectin all appeared effective against Ascaris lumbricoides infection, yielding high parasitological cure and large reductions in eggs excreted, with no differences detected between them. The drugs appear to be safe to treat children and adults with confirmed Ascaris infection. There is little to choose between drugs and regimens in terms of cure or adverse events.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Animals; Child; Child, Preschool; Humans; Infant; Middle Aged; Young Adult; Albendazole; Albendazole/administration & dosage; Albendazole/therapeutic use; Anthelmintics; Anthelmintics/administration & dosage; Anthelmintics/therapeutic use; Ascariasis; Ascariasis/drug therapy; Ascaris lumbricoides; Ivermectin; Ivermectin/administration & dosage; Ivermectin/therapeutic use; Mebendazole; Mebendazole/administration & dosage; Mebendazole/therapeutic use; Parasite Egg Count; Placebos; Placebos/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Comparing the effect of medications for treating Ascaris infection
What was the aim of this review?
We aimed to compare the effect of different medications for treating people with Ascaris infection. Albendazole and mebendazole are most commonly used to treat ascariasis. Ivermectin can also be used. We wanted to know if there was anything to choose between these drugs for eradicating the worms and their eggs in stool samples. We included 30 relevant studies.
Key messages
Mebendazole, albendazole, and ivermectin single dose were effective against Ascaris lumbricoides infection, yielding high parasitological cure without any differences detected between them. There were no serious side effects reported.
What was studied in the review?
Ascaris lumbricoides, also known as roundworm, is a soil‐transmitted worm that can infect people. Ascariasis is common worldwide and mainly affects children living in low‐income areas. Interventions against ascariasis include water and sanitation improvement, health education, and medicine treatment for infected individuals. Treatment with medications removes adult worms from the gastrointestinal tract reducing morbidity (illness) and infection transmission. Although many medicines exist to treat people who have worms (anthelmintic drugs), the most effective regimen and the optimal doses are not well known. We assessed studies that compared the use of anthelmintic medications in adults and children, as a single or a combined therapy, and in single or multiple dose regimens.
What were the main results of the review?
We included 30 randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups), enrolling 6442 children and adults aged from 28 days to 82 years, with Ascaris infection. Twenty studies were funded or co‐funded by manufacturers (which may introduce bias), while 10 were independent of manufacturer funding.
Parasitological cure is probably six‐fold more frequent in people receiving anthelmintic medicines when compared to people receiving placebo (treatment with no active ingredient) (moderate‐certainty evidence).
No difference in ascariasis cure was found in comparisons between single dose albendazole with single doses of either mebendazole or ivermectin; and no difference was found between single dose albendazole compared with giving multiple doses.
Severe side effects were not reported. The occurrence of other side effects (feeling sick, being sick, diarrhoea, abdominal discomfort, headache, fever) may be uncommon among the compared anthelmintic medicines (moderate‐ to low‐certainty evidence).
How up‐to‐date is this review?
We searched for studies published up to 4 July 2019.
Summary of findings
Summary of findings for the main comparison. Any anthelmintic drug single dose compared to placebo for treating ascariasis.
| Any anthelmintic drug single dose compared to placebo for treating ascariasis | ||||||
|
Patient or population: children and adults Setting: school and community (United Republic of Tanzania, Haiti, Rwanda, Ethiopia, Guatemala, Republic de Cote d'Ivoire; 1983–2018) Intervention: any anthelmintic drug single dose Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with any anthelmintic drug single dose | |||||
|
Parasitological cure
assessed with: parasitological examination Follow‐up: range 14–60 days |
16 per 100 | 93 per 100 (81 to 98) | RR 6.29 (3.91 to 10.12) | 1578 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | Any anthelmintic as a single dose probably results in a large increase in parasitological cure compared to placebo. |
|
Faecal egg count
assessed with: ERR of epg (GM or AM) Follow‐up: range 14–60 days |
The ERR of GM ranged from 96.1% to 100% in anthelmintic single‐dose group and from 11.7% to 33.9% in placebo group. | — | 1020 (5 RCTs) | ⊕⊕⊕⊕ High | Any anthelmintic as a single dose results in large reduction in faecal egg count compared to placebo. | |
|
Adverse events
assessed with: report Follow‐up: range 14–60 days |
The adverse events reported were few (headache, fever, myalgia, cough, epigastric pain, and diarrhoea) and similar among the groups. | — | 744 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | Any anthelmintic as a single dose probably results in few adverse events compared to placebo. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AM: arithmetic mean egg count; CI: confidence interval; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean egg count; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level: there was a high level of heterogeneity among trials not explained by subgroup analysis (I² = 86%). bDowngraded one level due to risk of performance bias.
Summary of findings 2. Albendazole 400 mg single dose compared to albendazole 400 mg multiple doses for treating ascariasis.
| Albendazole 400 mg single dose compared to albendazole 400 mg multiple doses for treating ascariasis | ||||||
|
Patient or population: children and adults Setting: school and community (People's Republic of China, Kenya, Gabon; March 1990 to December 2008) Intervention: albendazole 400 mg single dose Comparison: albendazole 400 mg multiple doses | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with albendazole 400 mg multiple doses | Risk with albendazole 400 mg single dose | |||||
|
Parasitological cure
assessed with: parasitological examination Follow‐up: range 21–42 days |
94 per 100 | 92 per 100 (87 to 99) | RR 0.98 (0.92 to 1.05) | 307 (3 RCTs) | ⊕⊕⊕⊕ High | Albendazole 400 mg single dose or albendazole multiple doses results in large parasitological cure after the treatment. |
|
Faecal eggs count
assessed with: ERR of epg (GM or AM) Follow‐up: range 21–42 days |
ERR of AM of epg of faeces ranged from 94% to > 99% in albendazole single‐dose group and 87% to > 99.9% in albendazole multiple‐dose group | — | 249 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | Albendazole 400 mg single dose or albendazole multiple doses probably results in a large reduction in the faecal egg count. | |
|
Adverse events
assessed with: report Follow‐up: range 21–42 days |
2 trials reported no adverse events. Few mild adverse events were reported in 1 trial (headache, abdominal cramps, vomiting, diarrhoea, chills, vertigo, fever), and they were similar among groups. | — | 316 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | Albendazole 400 mg single dose or albendazole multiple doses probably results in little to no difference in adverse events. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AM: arithmetic mean egg count; CI: confidence interval; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean egg count; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision: very few participants included.
Summary of findings 3. Albendazole 400 mg single dose compared to mebendazole 500 mg single dose for treating ascariasis.
| Albendazole 400 mg single dose compared to mebendazole 500 mg single dose for treating ascariasis | ||||||
|
Patient or population: children and adults Setting: school and community (Thailand Kingdom, United Republic of Tanzania, People's Republic of China, Republic of Indonesia; August 1991 to November 2012) Intervention: albendazole 400 mg single dose Comparison: mebendazole 500 mg single dose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with mebendazole 500 mg single dose | Risk with albendazole 400 mg single dose | |||||
|
Parasitological cure
assessed with: parasitological examination Follow‐up: range 7–31 days |
97 per 100 | 98 per 100 (97 to 99) | RR 1.01 (1.00 to 1.02) | 2131 (6 RCTs) | ⊕⊕⊕⊕ High | Albendazole 400 mg single dose or mebendazole 500 mg single dose results in large parasitological cure. |
|
Faecal egg count
assessed with: ERR (GM or AM) Follow‐up: range 14–31 days |
ERR was almost 100% in albendazole and mebendazole groups. | — | 1902 (5 RCTs) | ⊕⊕⊕⊕ High | Albendazole 400 mg single dose or mebendazole 500mg single dose results in large reduction in faecal egg count. | |
|
Adverse events
assessed with: report Follow‐up: range 14–31 days |
1 trial reported adverse events in 12.5% of participants in albendazole group and 18.1% in the mebendazole group. The main adverse events reported were headache vomiting, diarrhoea, abdominal discomfort, fatigue. | — | 1902 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | Albendazole 400 mg single dose or mebendazole 500 mg single dose may result in little to no difference in adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AM: arithmetic mean egg count; CI: confidence interval; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean egg count; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of detection and performance bias. bDowngraded one level for imprecision.
Summary of findings 4. Albendazole single dose compared to ivermectin single dose cure rate for treating ascariasis.
| Albendazole single dose compared to ivermectin single dose cure rate for treating ascariasis | ||||||
|
Patient or population: children and adults Setting: school, hospital (People's Republic of China, Haiti, Republic of Philippines; January 1998–2008) Intervention: albendazole 400 mg single dose Comparison: ivermectin 100–400 μg/kg single dose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ivermectin single dose cure rate | Risk with albendazole single dose | |||||
|
Parasitological cure
assessed with: parasitological examination Follow‐up: range 7–35 days |
90 per 100 | 89 per 100 (82 to 97) | RR 0.99 (0.91 to 1.08) | 519 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | Albendazole single dose or ivermectin single dose results in large parasitological cure. |
|
Faecal egg count
assessed with: parasitological examination Follow‐up: range 7–35 days |
The ERR was 93% in albendazole group and 100% in ivermectin group | — | 315 (2 RCTs) | ⊕⊕⊕⊕ High | Albendazole single dose or ivermectin single dose results in large reduction in faecal egg count. | |
|
Adverse outcomes
assessed with: report Follow‐up: range 7–35 days |
No complication and serious adverse events were reported. Other adverse events were mild and self‐limiting such as dizziness, abdominal pain, tiredness, and diarrhoea | — | 204 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Albendazole single dose or ivermectin single dose may result in little to no difference in adverse events. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AM: arithmetic mean egg count; CI: confidence interval; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean egg count; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for inconsistency (I² = 74%); subgroup analysis did not carry out: few trials included. bDowngraded one level for risk of performance and detection bias. cDowngraded one level for imprecision: few events reported.
Background
Ascaris lumbricoides, also known as roundworm, is a soil‐transmitted helminth (STH) that infects humans and animals. It is common worldwide and affects mainly tropical and subtropical areas, such as sub‐Saharan Africa and Southeast Asia (Bethony 2006; WHO 2011). The most affected groups are preschool‐ and school‐age children living in low‐income areas (Xu 1995). A modelling study showed that the prevalence of A lumbricoides declined in some parts of the world after 1990, probably as a result of improvements in living conditions and deworming programmes (Pullan 2014). However, ascariasis remains one of the most prevalent diseases affecting around 738 million to 872 million people worldwide (GBD 2017).
A lumbricoides infection rarely causes direct mortality, but it contributes to chronic lifetime morbidity. The morbidity attributable to Ascaris infection is difficult to measure considering the non‐specificity of clinical manifestation (Campbell 2016; Pullan 2014). Complications related to Ascaris infection may cause up to 60,000 deaths annually (WHO 2011).
Ascariasis is transmitted through the faecal–oral route. Infection occurs when embryonated eggs that contaminate food, utensils, or hands are ingested. The eggs hatch in the small intestine, releasing the larvae that pass through the intestinal wall and migrate through the liver and heart, up to the lungs. In the lung passage, the larvae are expectorated and swallowed, passing through the gastrointestinal tract until they arrive at the small intestine, where they mature into adult worms and produce new eggs which are expelled with faeces contaminating the environment (CDC 2009; WHO 2001; WHO 2011). Reinfection occurs only when contaminated eggs are ingested, since these parasites do not multiply in the human host (WHO 2011). The distribution of A lumbricoides in the community can be either aggregated or over dispersed, with most people who are infected harbouring few worms, and a small proportion of people who are infected harbouring a very high number of worms (Holland 2009).
The relationship between A lumbricoides infection and socioeconomic variables is intense, as STH infections are linked to a lack of sanitation and poverty (Stepek 2006; WHO 2011). Other factors such as unhygienic housing conditions, precarious health care, and poor educational or financial resources result in difficulties in ascariasis management, especially among economically disadvantaged groups (Bethony 2006; WHO 2001; WHO 2002; WHO 2005; WHO 2011).
Description of the condition
In general, people infected with A lumbricoides are asymptomatic. However, the infection can manifest as abdominal discomfort, anorexia, diarrhoea, and vomiting (Bethony 2006; Jardim‐Botelho 2008), and is associated with both chronic and acute morbidity, particularly in growing children. Specialists consider nutritional impairment as a common condition, mainly manifested by anaemia. A lumbricoides infection can also result in an allergic inflammatory response to parasites and parasite antigens in people who are infected. A classic example is the asthma‐like illness, Loeffler's syndrome, caused by the passage of A lumbricoides larvae through the lungs. Also, exposure to A lumbricoides can cause or increase asthma symptoms and bronchial hyperreactivity (Cooper 2009; Leonardi‐Bee 2006). A lumbricoides is a persistent parasite and may have impact on a person's immune responses to other pathogens. The bystander chronic infection is associated with increased susceptibility to other pathogens as well as reduced vaccine efficacy (Stelekati 2012). Despite the large number of studies, the potential interaction of intestinal helminths and other pathogens remains controversial. Studies focusing on the coinfection of A lumbricoides and Plasmodium yield conflicting conclusions. In some studies, the interaction results in worsening of a specific clinical condition whereas other studies demonstrate it may protect severe manifestation (Degarege 2016; Fenton 2013).
In general, most of the affected individuals have mild Ascaris infections. However, children may have high parasitic burden resulting in increased morbidity and complications (de Silva 2015). Complications of A lumbricoides infection are related to intestinal or biliary obstruction, or both, that lead to pancreatitis, cholecystitis, cholangitis, appendicitis, intestinal volvulus, perforation of an intestinal segment, and peritonitis (Hefny 2009; Khuroo 1990; Pawlowski 1985). Notably, the same clinical features can occur in people infected with Ascaris suum, which is a similar species with characteristics that make it very difficult to distinguish from A lumbricoides infection (Crompton 1989). It is likely that both species co‐occur especially in places where pigs and humans coexist (Kofie 1983; Maruyama 1997).
Helminth infection may cause damage to the intestinal mucosa, resulting in malabsorption of nutrients. Also, the helminth competes for nutritional resources with its human host (Hall 2008; Stepek 2006; WHO 2011), and can cause lactose intolerance (Hall 2008; Stephenson 2000). Poor school attendance and low cognitive performance are associated with ascariasis infection in school‐aged children. Comparisons between infected and uninfected children have shown a lower academic performance of infected children at school, mainly when the children harboured moderate to heavy infections (Bethony 2006; De Silva 2003; Stepek 2006; Stephenson 2000; WHO 2000; WHO 2011). Treatment of A lumbricoides infection, either alone or in combination with treatment for other helminth infections, is associated with improvements in appetite, weight gain, and physical fitness in school children (Hall 2008). A decrease in infection incidence and an improvement in nutritional status are likely to lead to improvements in children's school performance (Stepek 2006).
Diagnosis
Peripheral eosinophilia occurs during migration of A lumbricoides larvae through the infected person's lungs, but sometimes appears at other stages of A lumbricoides infection (Ehrhardt 2008). In individuals with heavy infections, a mass of worms may be detectable following X‐ray of the abdomen. The worms contrast against the gas in the bowel, typically producing a 'whirlpool' effect (Reeder 1998). Ultrasound and endoscopy are useful for diagnosis of hepatobiliary and pancreatic duct involvement (Reeder 1998). Computed tomographic (CT) scanning or magnetic resonance imaging (MRI) may identify worms in the liver or bile ducts, but are not usually necessary (Khuroo 1985; Khuroo 1990).
Parasitological diagnosis of ascariasis is made by examining stool specimens for the microscopic identification of eggs. Characteristic eggs may be seen on direct examination of faeces or by using concentration techniques (CDC 2009). Faecal smears and the Kato technique, also referred to as Kato thick smear examination, consist of the microscopic examination of a known amount of faecal material that allows an egg count to be performed (Katz 1972; Santos 2005; WHO 2001; WHO 2011). This method is widely used to confirm ascariasis infection and is recommended by the World Health Organization (WHO) as the standard method for evaluating prevalence and intensity of soil‐transmitted helminthiasis in endemic communities. It is an easy technique to use in field situations or when a great number of specimens need to be examined. However, it requires well‐trained laboratory technicians and quality control measures to ascertain accurate diagnosis of ascariasis and other helminth infections (Bergquist 2009; Montresor 1998; Pawlowski 1985). The sensitivity of faecal smears decrease with low‐intensity infection and with liquid stool samples. The stool filtration method, which has been previously described for finding Schistosoma mansoni eggs in stool samples, is an option to detect A lumbricoides eggs (Bell 1975). Intensity of infection is measured in terms of eggs per gram (epg) of faeces and is classified as a light‐intensity infection (between one and 4999 epg), moderate‐intensity infection (between 5000 and 49,999 epg), or heavy‐intensity infection (more than 50,000 epg) based on the report of WHO Expert Committee (WHO 2002). Adult worms are occasionally present in the stools. They may pass through the mouth, nose, or rectum and are recognizable by their macroscopic characteristics (WHO 2011). An increasing number of studies have presented the results of development and standardization of molecular tests for intestinal pathogens (Ayana 2019; Cools 2019; Papaiakovou 2019). However, until 2019, molecular diagnosis for A lumbricoides was mainly restricted to research settings with no commercial tests available (Khurana 2017; O'Connell 2016).
Description of the intervention
Interventions against worm infection include deworming using anthelmintic drugs, water and sanitation improvement, and health education. The WHO recommends three public health drug treatment policies (WHO 2011; WHO 2017a).
Selective: individual deworming based on a diagnosis of infection.
Targeted: group deworming where a specific risk group is treated without prior diagnosis.
Universal: population deworming in which the whole community is treated irrespective of infection status.
The WHO considers the target groups for drug treatment to be preschool‐age children (aged between one and five years), school‐age children (aged between six and 15 years), women of childbearing age including pregnant women in the second and third trimesters and breastfeeding women, and adults in certain high‐risk occupations (such as tea‐pickers and miners).
The recommended frequency of treatment is once per year for low‐risk communities with between 20% and 50% infection prevalence, or twice per year for high‐risk communities with more than 50% infection prevalence (WHO 2011). Infections of heavy intensity are absent when the prevalence of any STH infection is less than 20% (Montresor 2015). However, the advantages to recommend universal (also called mass or whole community) deworming or targeted deworming for STHs is still controversial. One systematic review and meta‐analysis compared the effect of universal and targeted anthelmintic delivery strategies on STH prevalence in school‐aged children (Clarke 2017). The results of this meta‐analysis suggest that universal deworming programmes led to a greater reduction in the prevalence of STHs rather than targeted strategy (Clarke 2017). According to another systematic review and meta‐analysis, treating children known to have worm infection may achieve nutritional benefits for the individual. However, universal treatment seems to have little or no effect on haemoglobin levels, nutritional status, school performance, or survival rates among children in endemic area (Taylor‐Robinson 2019).
Anthelmintic drugs for treating ascariasis
The current WHO Model List of Essential Medicine for treating intestinal helminths includes seven drugs: albendazole, mebendazole, levamisole, ivermectin, niclosamide, praziquantel, and pyrantel (WHO 2017b). The benzimidazoles drugs (i.e. albendazole and mebendazole), are used to treat a variety of parasitic infestations by interfering with the parasitic worm microtubular system (Utzinger 2004). They are considered the mainstay drugs for roundworm and hookworm treatment. They are low cost, safe, easily administered, and children do not need to be weighed. Dosage is the same for children and adults. Albendazole 400 mg once a day and mebendazole 100 mg orally twice daily for three days or 500 mg orally once are given.
The accumulated scientific knowledge shows high efficacy, resulting in large‐scale use of these drugs for treatment and preventive chemotherapy (Bennett 2000; Keiser 2008). Albendazole and mebendazole are donated to national ministries of health through WHO in endemic countries for the treatment of school‐age children (WHO 2012; WHO 2017a). Single‐dose albendazole achieves high cure rates againstA lumbricoides infection. However, there are differences in the cure rates obtained among trials (Venkatesan 1998; Vercruysse 2011a).
Mebendazole is an equivalent alternative to albendazole and may cause the same adverse effects, such as transient gastrointestinal discomfort, headache, and leukopenia. Levamisole and pyrantel pamoate act as nicotinic acetylcholine receptor agonists (Utzinger 2004). Levamisole has been studied less intensively, and the availability of this drug is limited, but it is currently considered a safe and effective drug. In mass treatment, it showed significant differences pre‐ and post‐treatment egg count values (Asaolu 1991). Pyrantel pamoate is cited in the WHO Model List of Essential Medicine for treating intestinal helminths (WHO 2017b). It is considered an effective single‐dose drug for treating ascariasis in one systematic review and meta‐analysis (Keiser 2008). Ivermectin is most commonly used to treat lymphatic filariasis, onchocerciasis, loiasis, and strongyloidiasis. It is also moderately effective against Trichuris trichiura and is approved for treating human ascariasis. It causes paralysis of adult worms and seems to be effective. Piperazine citrate acts by paralyzing the worms, which aids expulsion from the infected person's body (del Castillo 1964). However, it is now being withdrawn from the market as other alternative drugs are less toxic and more efficacious. Nitazoxanide is a new antiprotozoal drug reported as an effective choice against a broad range of parasites, including A lumbricoides (Galvan‐Ramirez 2007). This drug has been listed as a potential candidate for human‐soil transmitted helminthiasis and further research has been suggested (Diaz 2003). Anthelmintic drugs not registered for treating ascaris but occasionally compared with these drugs are praziquantel and diethylcarbamazine (Long 2007; WHO 2000).
How the intervention might work
Ascariasis causes a high disease burden worldwide. Health education, access to good‐quality water, and improvements in basic sanitation are crucial to reduce the number of people infected globally. Drug treatment for infected individuals, in combination with other public health measures, is necessary to break the cycle of transmission (Bethony 2006; WHO 2005). Infected individuals should be treated with anthelmintic drugs to remove adult worms from the gastrointestinal tract aiming to reduce morbidity and infection transmission (Bethony 2006). In preventive chemotherapy programmes, the purpose of anthelmintics administration is to control morbidity by maintaining the intensity of the infection low (WHO 2001).
Some randomized trials suggest that poor cognitive performance, malnutrition, and anaemia may be potentially reversible following treatment with anthelmintic drugs (Hall 2008; Stepek 2006). Even when a person has concomitant infections, such as hookworm, T trichiura, or Schistosoma haematobium infection, treatment may improve nutritional status (Stephenson 2000). One systematic review suggested that selective deworming probably increases weight gain (low‐quality evidence) and may increase haemoglobin in children confirmed to have worms based on screening. According to this review there is limited evidence of other benefits on selective deworming (Taylor‐Robinson 2019).
Figure 1 shows a logic diagram of relationship between anthelmintic use and expected outcomes.
1.
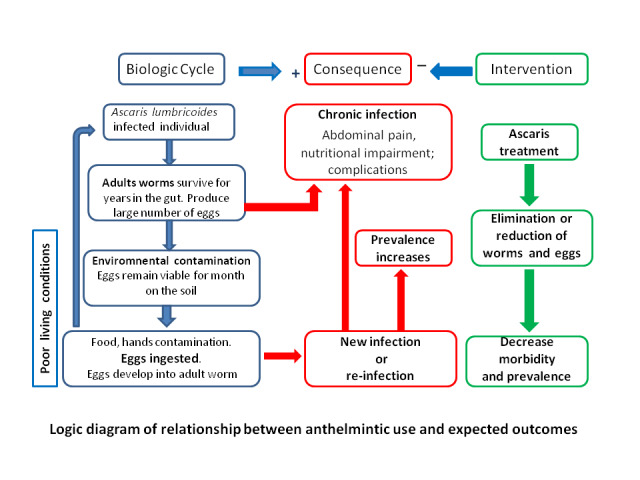
Logic diagram of relationship between anthelmintic use and expected outcomes.
Why it is important to do this review
Ascariasis remains a neglected disease despite its global distribution and the high number of infected individuals. It is still one of the most prevalent STH in the world. A lumbricoides, like other helminth infections, can affect the immune system and alter susceptibility to other parasitic diseases, such as malaria. The potential interaction between STH and malaria is complex. Previous studies suggest that large‐scale deworming programmes can have a protective effect on malaria morbidity in children (Stelekati 2012). One systematic review and meta‐analysis suggested that STH infection is associated with an increased prevalence and density of asymptomatic/uncomplicated Plasmodium falciparum infection but with a decreased occurrence of anaemia (Degarege 2016).
The main goals of deworming programmes are to reduce the number of people who have heavy infections; reduce environmental contamination and risk of infection for other people; reduce micronutrient loss (e.g. iron loss through intestinal bleeding in hookworm infection); and improve nutritional status, cognitive functions, and learning abilities (WHO 2011).
Some specialists believe that wide‐scale administration of anthelmintic drugs will exert increasing drug pressure on parasite populations and favour parasite genotypes resistant to anthelmintic drugs (Vercruysse 2011a). Occurrence of resistance to anthelmintic drugs in nematode populations has been described in veterinary medicine. It highlights the potential for selecting drug‐resistant worms when chemotherapy programmes are widely adopted (Wolstenholme 2004). For example, reduction in the efficacy of mebendazole compared with historical controls has been documented in studies in Vietnam (Flohr 2007).
The WHO has highlighted the need to closely monitor anthelmintic drug efficacy (Vercruysse 2011a). Currently, there have been few research‐based studies about anthelmintic drugs, a very limited number of drugs that do not meet all needs in terms of efficacy, and there are no new anthelmintic drugs in late‐stage development (Geary 2010).
One network meta‐analysis evaluated the efficacy of mebendazole, albendazole, levamisole, and pyrantel pamoate against A lumbricoides, hookworms and T trichiura. It included 55 randomized controlled trials (RCTs) to assess the cure rate and 46 RCTs to assess the egg reduction rates (ERR), with a single‐dose of anthelmintic drugs (Moser 2017b). In this network meta‐analysis, all drugs presented high efficacy against Ascaris.
Although using different methodological approaches, these two systematic reviews published with an interval of about 10 years (Keiser 2008; Moser 2017b) focus on the same anthelmintic drugs. Another meta‐analysis using individual patient data analysis evaluated the efficacy and safety of co‐administered ivermectin plus albendazole for treating STH. According to this systematic review, the coadministration resulted in no benefit on cure and ERRs over albendazole alone for A lumbricoides (Palmeirim 2018b).
Some anthelmintic drugs, for example nitazoxanide and ivermectin, potentially effective againstA lumbricoides, have not been evaluated in previous systematic reviews. Although many anthelmintic drugs exist, the most effective regimen and the optimal doses to treat ascariasis are not well known. In this sense, further systematic reviews are necessary to evaluate efficacy and safety of these drugs.
Objectives
To compare the efficacy and safety of anthelmintics (albendazole, mebendazole, ivermectin) for treating people with Ascaris infection.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs.
Types of participants
Participants were adults and children with infection by A lumbricoides confirmed by direct examination of faeces or by using concentration techniques.
We excluded anthelmintic drugs used for treating ascaris exclusively in pregnant women and in people with HIV infection.
Types of interventions
Intervention
We included the most currently used drugs for treating A lumbricoides: albendazole and mebendazole. We also included ivermectin and nitazoxanide. We decided not to include other anthelmintic drugs as initially proposed in the protocol (Conterno 2013) (levamisole, pyrantel‐oxantel pamoate, piperazine) because they are not currently among the main drugs recommended to treat ascariasis. See Differences between protocol and review.
We included studies examining the use of drugs either as a monotherapy or as a combined therapy, in single dose or multiple dose regimens.
When additional interventions were used, they had been given to the control and intervention groups. The additional interventions included, but were not limited to, education, micronutrient supplementation, malaria chemoprevention, or use of other drugs.
We did not include studies evaluating repeat treatments with anthelmintic drugs, and studies comparing different deworming programmes where it was not possible to know the number of participants with A lumbricoides pre‐ and post‐treatment, or when the effect was measured after multiple treatment rounds.
Control
No intervention, placebo, different doses of any of the drugs, or a different combination of drugs.
Types of outcome measures
Primary outcomes
Parasitological cure.
We defined parasitological cure as the eradication of parasites from stool samples. We calculated parasitological cure as the percentage of people with positive A lumbricoides eggs before the treatment who had negative eggs from stool samples after the treatment.
Secondary outcomes
Faecal egg count (FEC) pre‐ and post‐treatment, or egg reduction rate (ERR). See Differences between protocol and review.
FEC was measured by geometric mean (GM) or arithmetic mean (AM) of epg of faeces.
ERR compares the mean epg count pre‐ and post‐treatment expressed as a percentage (1 – mean post‐deworming epg/mean pre‐deworming epg) (Vercruysse 2011b; WHO 2011).
We excluded effects on nutritional indicators, haemoglobin, and school performance. There is a specific systematic review about this topic already published (Taylor‐Robinson 2019).
Adverse events
Any type of complication (intestinal or biliary obstruction, pancreatitis, cholecystitis, cholangitis, appendicitis, intestinal volvulus, perforation of an intestinal segment and peritonitis, etc.).
Serious adverse events (hospitalizations, life‐threatening events, or death).
Other adverse events.
Search methods for identification of studies
Electronic searches
Vittoria Lutje, the Cochrane Infectious Diseases Group (CIDG) Information Specialist, performed the literature searches in the CIDG Specialized Register, CENTRAL, MEDLINE, Embase, LILACS, three other databases, and reference lists of included studies, without language restrictions or publication status (published, unpublished, in press, and in progress), up to 4 July 2019, using the search terms detailed in Appendix 1. We also searched the metaRegister of Controlled Trials and the WHO Clinical Trials Search Portal using 'ascariasis*"' or 'roundworm''' search terms, without language restrictions, up to 4 July 2019.
Searching other resources
We checked the reference lists of all trials and relevant articles identified by the above methods.
Data collection and analysis
Selection of studies
Two review authors (LOC and RAMBA or MDT or IC) independently screened all citations and abstracts identified by the search against the inclusion criteria. Two review authors (LOC and MDT or IC or RAMBA) independently obtained and assessed potentially eligible articles for inclusion in the review using a pre‐designed eligibility form based on the inclusion criteria. We resolved any disagreements through discussion. We documented the reasons for the exclusion of studies that did not meet the inclusion criteria.
For multiple publications from the same trial, we considered only one data set.
Data extraction and management
Two review authors (LOC and RAMBA or MDT or IC) extracted data independently from included studies using a data extraction form. We resolved any differences through discussion. A third review author checked the quality of data extraction (RAMBA). Overall, we extracted the number of participants (A lumbricoides confirmed) randomized and analyzed in each treatment group of each trial, characteristics of participants, characteristics of interventions, characteristics of outcome measures, date of trial, location of trial, sponsor of trial, design, interventions (treatment, days, doses), outcomes (prevalence pre‐ and post‐treatment, cure rate, epg of faeces before and after the treatment, ERR, adverse events). We calculated the follow‐up loss in each group.
For dichotomous outcomes, we extracted the number of participants with the event.
For continuous outcomes, we extracted means and standard deviation (SD) when reported. Otherwise, we tried to extract medians and ranges and entered them into tables. Where change from baseline results were presented alongside results purely based on the end value, we only extracted the change from baseline results.
ERRs were extracted when possible and reported as point estimates but, due to differences in the reported mean (GM versus AM) and lack of reported SDs, it was not possible to conduct a meta‐analysis with these measures. The quantitative analysis of adverse events was not carried out because the small number of studies in each comparison that reported them. We presented AM and GM pre‐ and post‐treatment, ERR, and adverse events in additional tables.
We planned for cluster‐randomized trials that adjusted for clustering in the analysis, to extract a measure of effect and its standard error and to extract the average cluster size, intracluster correlation coefficient (ICC), number of clusters, and cluster type (Higgins 2011a). For cluster RCTs that did not adjust for clustering, we planned to attempt to adjust the results for clustering by estimating the design effect calculated as 1 + (m – 1) × ICC, where m was the mean cluster size. To make the adjustment, we planned to estimate a treatment effect that does not adjust for clustering and then multiply the standard errors of the estimate by the square root of the design effect.
When the true ICC was unknown, we intended to estimate it from other included cluster‐RCTs (Higgins 2011b).
One review author (LOC) entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014), which was checked by a second review author (RAMBA).
Assessment of risk of bias in included studies
Two review authors (LOC and MDT or IC or RAMBA) independently assessed the risk of bias in the included trials. We assessed the following domains: sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other biases.
For each of these domains, we placed a judgement of risk of bias as low, high, or unclear/unknown (Appendix 2). We resolved any disagreements through discussion.
We planned for RCTs randomized by cluster to assess several additional components including: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and compatibility with RCTs randomized by individual.
Measures of treatment effect
We used the risk ratio (RR) to compare the treatment and control groups for dichotomous outcomes. We presented all treatment effects with 95% confidence intervals (CIs). We used a fixed‐effect model if there was no moderate or substantial heterogeneity. If there was clinical heterogeneity or if we detected substantial statistical heterogeneity, we used a random‐effects model. We planned to summarize continuous data (means and SDs) using mean differences (MDs).
Unit of analysis issues
We did not include cluster‐RCTs. See Data extraction and management for our intended methods should we have found such studies.
Certainty of the evidence
We used the principles of the GRADE system to assess the certainty of the evidence associated with all main outcomes (Schünemann 2011). The GRADE approach appraises the certainty of a body of evidence considering within study risk of bias, the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias. We constructed 'Summary of findings' tables using the GRADEpro software (GRADEpro).
Dealing with missing data
We assessed missing outcomes data and reported the proportion of participants lost to follow‐up for each study. We used the number of available participants at the time point at which the outcome was measured as the denominator.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plot for overlapping CIs and outlying data and we used the Chi² test with a P value of 0.1 to indicate statistic significantly heterogeneity, and the I² statistic. We used an I² statistic of 50% to denote moderate heterogeneity and 75% or greater to denote substantial heterogeneity. We intended to investigate possible causes of heterogeneity in subgroup analyses.
Assessment of reporting biases
We planned to construct funnel plots to assess publication bias, but did not as there was a limited number of trials in each analysis.
Data synthesis
We used RevMan 5 to perform analyses (Review Manager 2014). We combined the primary outcome, parasitological cure, from the individual trials in a meta‐analysis to provide a pooled effect estimate because the studies were sufficiently similar in terms of anthelmintic drug and doses used. We carried out analyses according to the comparison (as in the Types of interventions section), by the time of follow‐up (up to 60 days and more than 60 days after the treatment in the comparisons: any anthelmintic dose single versus placebo (Analysis 1.1), albendazole single dose versus albendazole multiple doses (Analysis 2.1), and by region (Analysis 3.1)
1.1. Analysis.
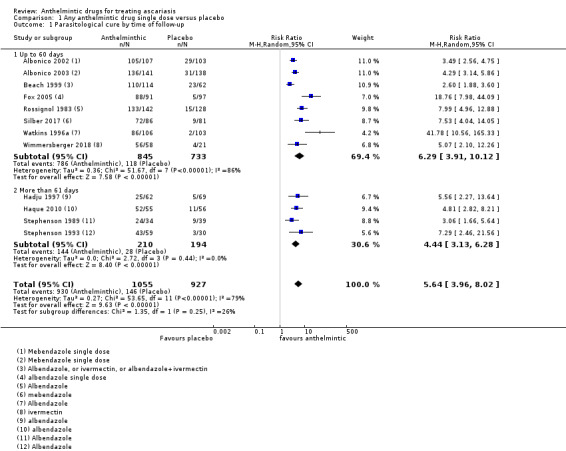
Comparison 1 Any anthelmintic drug single dose versus placebo, Outcome 1 Parasitological cure by time of follow‐up.
2.1. Analysis.
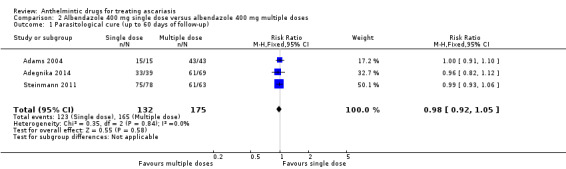
Comparison 2 Albendazole 400 mg single dose versus albendazole 400 mg multiple doses, Outcome 1 Parasitological cure (up to 60 days of follow‐up).
3.1. Analysis.
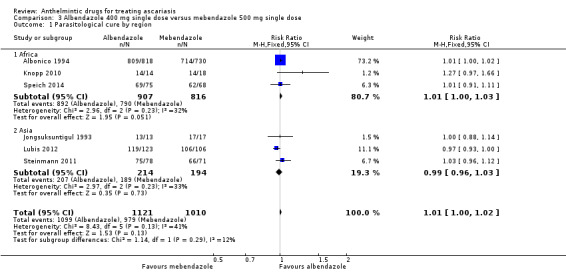
Comparison 3 Albendazole 400 mg single dose versus mebendazole 500 mg single dose, Outcome 1 Parasitological cure by region.
We performed fixed‐effect meta‐analysis when there was no moderate or substantial heterogeneity, and random‐effects meta‐analysis if the assessment results revealed heterogeneity and the heterogeneity could not be explained by performing subgroup analysis (Higgins 2011b).
We included only a single pair‐wise comparison in each meta‐analysis of studies with multiple intervention groups. When we considered all intervention groups to be eligible for the same meta‐analysis, we combined the groups creating a single pair‐wise comparison. We combined all relevant experimental intervention groups into a single group and all relevant control groups into a single control group.
We presented AM and GM pre‐ and post‐treatment, ERR, and adverse events in additional tables, because they could not be pooled (medians, means without measure of variance, ranges) (Table 5; Table 6).
1. Egg reduction rates of epg of faeces.
| Study ID | Time point | Anthelmintic | Number | Geometric mean (epg faeces) | Arithmetic mean (epg faeces) | ||||
| Baseline | Follow‐up | ERR % (95% CI) | Baseline (range) | Follow‐up | ERR % (95% CI) | ||||
| Adams 1994 | 63 days | Albendazole 400 mg 3 consecutive days | 9 | 17 | 1 | 94 | 10,701 | 1 | 100 |
| Placebo | 7 | 13 | 6 | 54 | 7575 | 8440 | –11 | ||
| Adegnika 2014 | 42 days | Albendazole 400 mg single dose | 39 | — | — | — | 4794 (2494–8826) | 188 (24–1516) | 94 (88 to 100) |
| Albendazole 400 mg single dose 2 consecutive days | 32 | — | — | — | 5409 (2554–10,118) | 1136 (71–18,160) | 87 (74 to 100) | ||
| Albendazole 400 mg single dose 3 consecutive days | 37 | — | — | — | 4734 (2519–8626) | 180 (8–3504) | 99 (97 to 100) | ||
| Albonico 1994 | 18 to 31 days (mean 22.5 days) | Mebendazole 500 mg single dose | 730 | 164 | 0.08 | 99.3 (99.2 to 99.5) | — | — | — |
| Albendazole 400 mg single dose | 818 | 239 | 0.05 | 99.6 (99.4 to 99.7) | — | — | — | ||
| Albonico 2002 | 21 days (range 20 to 23 days) | Mebendazole 500 mg single dose | 107 | 5 | 0.1 | 96.1 (94.3 to 97.9) | — | — | — |
| Placebo | 103 | 5 | 4 | 18.1 (‐2.7 to 34.8) | — | — | — | ||
| Albonico 2003 | 21 days | Mebendazole 500 mg single dose | 141 | 114 | 0.2 | 99.0 (98.2 to 99.4) | — | — | — |
| Placebo | 138 | 96 | 63 | 33.9 (0.4 to 56.1) | — | — | — | ||
| Beach 1999 | 35 days | Albendazole 400 mg single dose | 62 | 284 | NR | 100 | (40–20,960) | — | — |
| Ivermectin 200–400 μg/kg (mean 282.7 μg/kg) | 52 | 427 | NR | 100 | (40–8960) | — | — | ||
| Albendazole 400 mg single dose + ivermectin 200–400 μg/kg | 73 | 334 | NR | 100 | (40–26,640) | — | — | ||
| Placebo (vitamin C 250 mg) | 62 | 352 | NR | 32.9 | (40–19,560) | — | — | ||
| Belizario 2003 | 7 to 14 days | Albendazole 400 mg single dose + placebo | 99 | — | — | — | 21,656 | 1520 | 93.0 |
| Ivermectin 200 μg/kg bodyweight + placebo | 102 | — | — | — | 36,486 | 2072 | 94.3 | ||
| Albendazole 400 mg + ivermectin 200 μg/kg bodyweight | 105 | — | — | — | 41,011 | 199 | 99.5 | ||
| Fox 2005 | 35 days | Albendazole 400 mg single dose | 91 | 535 | NR | 98.8 | (40–34,800) | — | — |
| Placebo (vitamin C 250 mg) | 97 | 393 | NR | 11.7 | (40–24,000) | — | — | ||
| Hadju 1997 | 90 days | Albendazole 400 mg single dose | 62 | 5058 | 52 | 100 | — | — | — |
| Albendazole 400 mg twice | 67 | 6026 | 24 | 99 | — | — | — | ||
| Placebo | 69 | 4518 | 1803 | 60 | — | — | — | ||
| Haque 2010 | 120 days | Albendazole 400 mg single dose + placebo | 55 | — | — | — | 4923 ± 551 | 19 ± 12 | — |
| Placebo + placebo | 56 | — | — | — | 4689 ± 426 | 4525 ± 738 | — | ||
| Jongsuksuntigul 1993 | 14 days | Mebendazole 300 mg single dose | 26 | — | — | — | NR | NR | NR |
| Mebendazole 500 mg single dose | 17 | — | — | — | 20,986 | 0 | 100 | ||
| Albendazole 400 mg single dose | 13 | — | — | — | 3710 | 0 | 100 | ||
| Knopp 2010 | 21 days | Albendazole 400 mg single dose + placebo | 14 | 3401 | 0 | — | — | — | — |
| Albendazole 400 mg single dose + ivermectin 200 μg/kg | 14 | 1839 | 1 | — | — | — | — | ||
| Mebendazole 500 mg + placebo | 18 | 2601 | 5 | — | — | — | — | ||
| Mebendazole 500 mg + ivermectin 200 μg/kg | 18 | 381 | 0 | — | — | — | — | ||
| Legesse 2002 | 21 days | Mebendazole 100 mg twice for 3 days | 153 | 669 | 0.3 | 99.8 | — | — | — |
| Albendazole 400 mg | 234 | 1843 | 0.1 | 99.9 | — | — | — | ||
| Legesse 2004 | 21 days | Mebendazole 100 mg twice a day for 3 days | 325 | NR | NR | NR | — | — | — |
| Albendazole 400 mg single dose | 107 | 6982 | 305.1 | 99.9 | — | — | — | ||
| Nokes 1992 | 10 to 30 days | Albendazole 400 mg daily for 3 consecutive days | 38 | — | — | — | 36,012 ± 65,120 | 2 ± 17 | 99.9 |
| Placebo | 22 | — | — | — | 24,298 ± 43,890 | 25,725 ± 38,544 | NR | ||
| Ortiz 2002 | 21 to 30 days | Albendazole a single 10 mL dose of a 200 mg/5 mL suspension | 35 | 1291 | 1 | 99.9 | — | — | — |
| Nitazoxanide 100 mg/5 mL (2–3 years of age), 200 mg/10 mL (4–11 years of age) in the morning and evening for 3 consecutive days with food | 28 | 1978 | 1 | 99.9 | — | — | — | ||
| Palmeirim 2018a | 21 days | Mebendazole 500 mg single dose | 47 | 2691 | 0 | 100% | 14,597.6 | 0 | 100% |
| Mebendazole 100 mg twice a day for 3 consecutive days | 51 | 4095.9 | 0.2 | 100% | 14,859.9 | 130.9 | 99.9% | ||
| Silber 2017 | 17 to 21 days | Mebendazole 500 mg single dose (chewable) | 86 | NR | NR | 97.9 | — | — | — |
| Identical placebo (chewable) | 81 | NR | NR | 19.2 | — | — | — | ||
| Speich 2014 | 18 to 23 days | Albendazole 400 mg single dose | 75 | 2426 | 1 | 100 (99.9 to 100) | — | — | — |
| Mebendazole 500 mg single dose | 68 | 1876 | 1 | 99,9 (99.8 to 100) | — | — | — | ||
| Steinmann 2011 | 21 days | Albendazole 400 mg single dose | 78 | 8442 | 0.1 | > 99.9 (> 99.9 to 100) | — | — | — |
| Mebendazole 500 mg single dose | 71 | 7855 | 0.5 | > 99.9 (> 99.9 to > 99.9) | — | — | — | ||
| Albendazole 400 mg single dose over 3 consecutive days | 63 | 6485 | 0.2 | > 99.9 (99.9 to 100) | — | — | — | ||
| Mebendazole 500 mg single dose over 3 consecutive days | 72 | 8435 | 0.2 | > 99.9 (> 99.9 to > 99.9) | — | — | — | ||
| Stephenson 1989 | 180 | Albendazole 200 mg 2 tablets single dose | 34 | 86 | 2 | NR | 32,996 | 2959 | 91 |
| Placebo | 39 | 284 | 72 | NR | 32,044 | 24,400 | 24 | ||
| Stephenson 1993 | 108 days | Albendazole 200 mg 3 tablets (600 mg) single dose | 33 | 33 | 0.4 | 99 | 16,074 | 39 | 99.8 |
| Placebo | 30 | 20 | 17 | 15 | 8470 | 12,379 | –46 | ||
| Watkins 1996a | 14 days | Albendazole | 106 | 21,677 | 964 | NR | 38,485 | 10,000 | NR |
| Placebo | 101 | 21,528 | 23,014 | NR | 37,442 | 45,984 | NR | ||
| Wen 2008 | 30 days | Albendazole 6.7 mg/kg (2 tablets) single dose | 102 | — | — | — | 7438 (1245 to 16,936) |
110 | 98.5 |
| Ivermectin 0.1 mg/kg (1 tablet) single dose | 102 | — | — | — | 7286 (1195 to 15,235) |
0 | 110 | ||
| Wimmersberger 2018 | 21 days | Ivermectin 100 μg/kg | 14 | 2809.9 | 0 | 100% | — | — | — |
| Ivermectin 200 μg/kg | 23 | 1565.8 | 0 | 100% | — | — | — | ||
| Ivermectin 400 μg/kg | 13 | 2037.3 | 0 | 100% | — | — | — | ||
| Ivermectin 600 μg/kg | 8 | 2826.8 | 0 | 100% | — | — | — | ||
| Placebo (children aged 2–5 years) | 10 | 3694.0 | 575 | 84.4% | — | — | — | ||
| Placebo (children aged 6–12 years) | 11 | 2037.3 | 64.2 | 68.3% | — | — | — | ||
| Yap 2013 | 30 days | Albendazole 400 mg single dose for 3 days | 94 | 15,850 (10,834 to 23,189) |
1.3 (1.0 to 1.7) | — | — | — | — |
| Placebo single dose for 3 days | 87 | 19,101 (13,198 to 27,644) |
21,001 (12,835 to 34,362) |
— | — | — | — | ||
CI: confidence interval; epg: eggs per gram; ERR: eggs reduction rate; NR: not reported.
2. Adverse events.
| Study ID | Timepoint | Anthelmintic | N | Adverse events monitoring | Summary of adverse events finds |
| Adams 2004 | 30 days | Albendazole 400 mg one single dose | 31 | Not reported | "No adverse drug‐related effects were reported or detected in any treatment group" |
| Albendazole 400 mg two consecutive days | 43 | ||||
| Albendazole 400 mg three consecutive days | 39 | ||||
| Adegnika 2014 | 42 days | Albendazole 400 mg one single dose | 39 | "Study participants were followed‐up passively every day and actively every 2 weeks for any adverse events, including nausea, vomiting, abdominal pain, headaches, fever, fatigue, rash, dizziness, or temporary hair loss" | "There were no clinically important adverse events attributable to the study drug during the course of the study". |
| Albendazole 400 mg one single dose two consecutive days | 32 | ||||
| Albendazole 400 mg one single dose three consecutive days | 37 | ||||
| Albonico 1994 | 18 to 31 days | Mebendazole 500 mg one single dose | 730 | In the initial part of the trial (the first 1360 children), children found to be relatively infected with one of helminths were questioned in private by health worker, using an open ended questionnaire 7 days after treatment, about any problems or symptoms experienced after consumption of the drugs | “The frequencies of the different symptoms reported by the children (for the 7 days following treatment) were not significantly different between the 2 treatment groups. The percentages of children reporting symptoms, other than passing worms, following albendazole and mebendazole treatment, respectively, were: headache, 9.7% and 12.7%; abdominal discomfort, 9.0% and 9.3%; diarrhoea 4.9% and 3.4%; nausea, 0.7% and 8%; itching, 1.4% and 0.8%; rash, 1.4% and 0.0%; fever, 0% and 1.7%; and vomiting, 0% and 0.8%” |
| Albendazole 400 mg one single dose | 818 | ||||
| Albonico 2002 | 21 days (range 20 to 23 days) | Mebendazole 500 mg single dose | 107 | "Parents and children were instructed to report to the teacher and refer to the nearest health centre any severe adverse effects occurring in the week after treatment" | No adverse events were reported after any of the treatments |
| Placebo | 103 | ||||
| Albonico 2003 | 21 days | Mebendazole 500 mg single dose | 141 | “Parents and children were instructed to report to the teacher and refer to the nearest health centre with any severe adverse effects that occurred in the week after treatment” | “Although adverse effects were not investigated actively, no adverse events were reported after any single or combined treatment in the week following the administration of anthelminthics” |
| Placebo | 138 | ||||
| Fox 2005 | 35 days | Albendazole 400 mg single dose | 91 | “Every day for seven days after treatment, a clinician who was blinded as to treatment group questioned and examined the children at school for adverse reactions” | “The percentage of children reporting symptoms, following albendazole and placebo respectively were: headache, 24% and 28%; self reported or documented fever, 20% and 23%; Myalgia, 2% and 16%; cough, 2% and 16%.” |
| Placebo (vitamin C 250 mg) | 97 | ||||
| Jongsuksuntigul 1993 | 14 days | Mebendazole 300 mg single dose | 26 | “Each participants was given a questionnaire to record the severely and duration of any treatment induced side effects” | No side effects were reported among the four participant groups |
| Mebendazole 500 mg single dose | 17 | ||||
| Albendazole 400 mg single dose | 13 | ||||
| Knopp 2010 | 21 days | Albendazole 400 mg single dose + placebo | 14 | At 48 hours after treatment, AEs due to the treatment were assessed by a pre‐tested questionnaire. Children were interviewed by trained personnel of the Helminth Control Laboratory Unguja (HCLU) | The main symptoms reported were: abdominal cramps (range from 11% to 14.6%), fatigue (range from 6,4% to 2,8%), headache (range from 3.5% to 5.9%), diarrhoea (range from 2.8 to 4.2%), vertigo (range from 1.7% to 4.4%) without difference among the groups. |
| Albendazole 400 mg single dose+ ivermectin 200 mcg/kg | 14 | ||||
| Mebendazole 500 mg + placebo | 18 | ||||
| Mebendazole 500 mg + ivermectin 200 mcg/kg | 18 | ||||
| Legesse 2002 | 21 days | Mebendazole 100 mg twice a day for three days | 153 | “All treated individuals were interview for any symptoms or complaints experienced after receiving the treatment. For children under five years, information was obtained from their parents or guardians” | The percentage of children reporting symptoms, following albendazole and mebendazole treatment, were respectively: headache, 3.4% and 2%; abdominal comfort,7.1% and 3%; vomiting 2.6% and 0%; diarrhoea 8.9% and 1%; fever, 0.4% and 0.5%; worm expulsion through mouth, 1.5% and 0.5%; and worm expulsion through faeces, 52.6% and 55.0%. |
| Albendazole 400 mg single dose | 234 | ||||
| Ortiz 2002 | 21 to 30 days | Nitazoxanide 100 mg/5 mL (2 to 3 years of age), 200 mg/10 mL (4 to 11 years of age) in the morning and evening for 3 consecutive days with food | 28 | The guardians of the children were given instructions for recording the occurrence of adverse events | The percentage of children reporting symptoms, following nitazoxanide and albendazole treatment, respectively were: abdominal pain 8.6% and 1.9%; diarrhoea 1.9% and 0.0%; nausea 1.0% and 1.9%; vomiting 0.0% and 1.9%; headache 1.0% and 0.0% |
| Albendazole a single 10 mL dose of a 200 mg/5 mL suspension | 35 | ||||
| Palmeirim 2018a | 21 to 30 days | Mebendazole 100 mg twice a day for 3 consecutive days plus placebo | 47 | Tolerability (number of adverse events) assessed 3, 24, and 48 hours post‐treatment | “Children in the multiple dose treatment arm reported slightly more adverse events than those in the single dose arm. In total, throughout all adverse event assessment time points, 34 children (37%) reports), headache (46 reports) and diarrhoea (17 reports) during all treatment points. All events were mild.” |
| Mebendazole 500 mg single dose plus placebo | 51 | ||||
| Rossignol 1983 | 21 days | Albendazole 200 mg twice daily or 400 mg once daily for adults and 100 mg twice daily for children under 12 years old | 142 | “The same physical examination and laboratory investigations were carried out 24 to 72 hours after the last treatment, and each patient was carefully questioned about side effects” | The number of children reporting symptoms, following albendazole and placebo treatment, respectively were: dizziness 3 and 5; epigastric pain 30 and 22; diarrhoea 8 and 4; vomiting 2 and 1; headache 8 and 10; pruritus 2 and 1; fever 1 and 1; dry mouth 1 and 0 |
| Placebo | 128 | ||||
| Silber 2017 | 17 to 21 days | Mebendazole 500 mg single dose (chewable) | 86 | “The safety analysis set consisted of all randomized subjects who received 1 dose of study agent (mebendazole or placebo) at baseline. An adverse event is any untoward medical occurrence in a subject who received study drug without regard to possibility of causal relationship. An serious adverse event (SAE) is an AE resulting in any of the following outcomes or deemed significant for any other reason: death; initial or prolonged inpatient hospitalization; life‐threatening experience (immediate risk of dying); persistent or significant disability/incapacity; congenital anomaly. End point timeframe: Up to Visit 3 (Day 19 +/‐2)” | The percentage of subjects presenting adverse effects, following mebendazole and placebo, were respectively: cough 0.69% and 1.43%; night blindness 0.00% and 0.71%; abdominal distension 1.39% and 0.71%; abdominal pain 0.69% and 0.71%; rash pruritic 0.69% and 0.00%; vitamin A deficiency 0.69% and 0.00%; conjunctivitis 0.00% and 0.71%; conjunctivitis bacterial 0.00% and 0.71%; gastroenteritis 0.69% and 0.00%; nasopharyngitis 0.69% and 1.43%; tinea infection 0.69% and 0.00%; tonsillitis 0.00% and 0.71% |
| Identical placebo (chewable) | 81 | ||||
| Speich 2014 | 18 to 23 days | Albendazole 400 mg single dose | 69 | “Adverse events were assessed and graded by means of active questioning at four time points after treatment — at 3 hours and 24 hours after the first and second treatments” | “No serious side events were note. Number of participants with adverse events: albendazole group: 3 hours after the treatment 15/120 (12.5%); 24 hours after the treatment 12/120 (10.0%); mebendazole group: 3 hours after the treatment 8/116 (6.9%); 24 hours after the treatment 21/116 (18.1%)” |
| Mebendazole 500 mg single dose | 75 | ||||
| Steinmann 2011 | 21 days | Albendazole 400 mg single dose | 78 | “On the second morning – 36 hours after the first dosing – all participating households were visited and participants actively solicited to report any potential adverse events” | "Thirteen study participants (4.1%) reported between one and five adverse events following drug administration. Four of these individuals were treated with a single dose (3 with mebendazole, 1 with albendazole) while the remaining nine were treated with triple mebendazole (N=5) or triple albendazole (N=4). Adverse events included headache (N=3; all mebendazole), abdominal cramps (N=3; 2 mebendazole, 1 albendazole) and the closely related ‘‘full stomach’’ (N=2; mebendazole), and waist pain (N =1; albendazole). Two individuals each reported vomiting, including production of A. lumbricoides worms (1 albendazole, 1 mebendazole), diarrhoea (2 mebendazole), fatigue (1 albendazole, 1 mebendazole), and chills (2 mebendazole). Vertigo (albendazole), throat pain (albendazole), fever (mebendazole), and a swollen face (mebendazole) were each reported once." |
| Mebendazole 500 mg single dose | 71 | ||||
| Albendazole 400 mg single dose over three consecutive days | 63 | ||||
| Mebendazole 500 mg single dose over three consecutive days | 72 | ||||
| Wen 2008 | 30 days | Ivermectin 0.1 mg/kg (one tablet) single dose | 102 | “During hospitalizations, medical history and health checks including ultrasound and X‐ray, and basic laboratory tests were carried out before treatment and enquiry and physical examination were done 24 H post‐treatment. If any side effects occurred, the participants and the laboratory indices were carefully observed for days until the symptoms disappeared” | “Overall, 8 out of 408 (1.96%) cases receiving ivermectin treatment showed side‐effects that included dizziness (N = 4), abdominal pain (N = 2) and tiredness (N = 2) 2–12 hour after drug administration. These side effects were mild and transient, and no special treatment was provided. For albendazole, a total of 9 out of 408 (2.21%) had side effects including dizziness (N = 3), vomiting (N = 3, one with Ascaris worms), and diarrhoea (N= 3). No significant difference (2 = 0.061, P = 0.806) between the two treatments in terms of side effects was shown. There were no significant differences before and post‐treatment in the laboratory tests including hematology, urinalysis, liver and renal functions and electrocardiograms for all participants. Those with side effects in the trial did not show abnormal laboratory test at 24 H followed‐up” |
| Albendazole 6.7 mg/kg (two tablets) single dose | 102 | ||||
| Wimmersberger 2018 | 21 days | Ivermectin 100 μg/kg | 14 | “In the present study, it was well tolerated in both age groups at all doses studied. Data from blood samples taken at baseline and 72 hours after treatment did not reveal any significant hematotoxic, nephrotoxic or hepatotoxic effect”. | |
| Ivermectin 200 μg/kg | 23 | ||||
| Ivermectin 400 μg/kg | 13 | ||||
| Ivermectin 600 μg/kg | 8 | ||||
| Placebo | 21 |
We planned to include cluster‐RCTs pooling the results from trials that randomized individuals and results from cluster RCTs that adjusted for clustering in meta‐analysis, using the generic inverse variance method. We intended to present results from trials that did not adjust for clustering in the text or additional tables and labelled as "other results."
We carried out the following comparisons.
Comparison 1: any anthelmintic drug single dose versus placebo.
Comparison 2: albendazole 400 mg single dose versus albendazole 400 mg multiple doses.
Comparison 3: albendazole 400 mg single dose versus mebendazole 500 mg single dose.
Comparison 4: albendazole 400 mg single dose versus ivermectin 100 μg/kg to 400 μg/kg single dose.
Other comparisons.
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity conducting the following subgroup analyses: age (preschool children, school children, and adults), period of follow‐up, intensity of infection (according to WHO classification), geographical region (Asia, Africa, Mediterranean basin, and South America), and decade of studies publication. We performed subgroup analysis by period of follow‐up (Analysis 2.1) and region (Analysis 3.1).
Sensitivity analysis
We intended to perform the following sensitivity analyses, but the number of studies identified were insufficient.
Assess the effect of including only cluster designs.
Assess the effect of including studies at 'low risk of bias' overall versus those identified at 'high risk of bias' overall (Higgins 2011a).
Exclude studies with high levels of missing data (percentage of participants lost greater than 30%, or where differences between the groups exceed 10%, or both).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies tables.
Results of the search
The electronic search generated 265 citations and 10 additional records were identified through other sources. We screened the title and abstracts and selected 162 as potentially relevant and assessed the full text. Thirty trials met the inclusion criteria and were included in the qualitative and quantitative analyses (meta‐analysis). We illustrated the selection process in a flow diagram (Figure 2).
2.
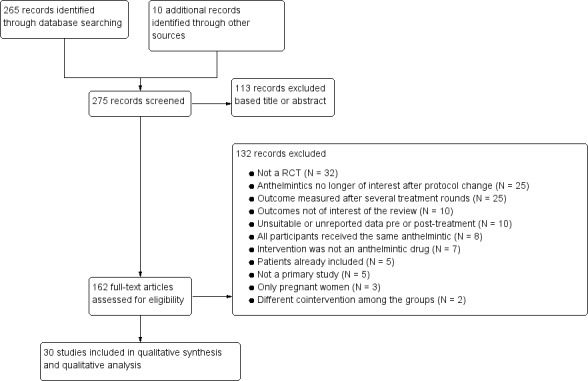
Study flow diagram.
Included studies
We included 30 parallel‐group randomized trials (see Characteristics of included studies table).
Two trials were conducted between 1981 and 1990 (Rossignol 1983; Stephenson 1989), eight between 1991 and 2000 (Adams 1994; Albonico 1994; Beach 1999; Hadju 1997; Jongsuksuntigul 1993; Nokes 1992; Stephenson 1993; Watkins 1996a), 12 between 2001 and 2010 (Adams 2004; Albonico 2002; Albonico 2003; Belizario 2003; Fox 2005; Haque 2010; Knopp 2010; Legesse 2002; Legesse 2004; Ortiz 2002; Wen 2008; Zani 2004), and eight after 2011 (Adegnika 2014; Lubis 2012; Palmeirim 2018a; Silber 2017; Speich 2014; Steinmann 2011; Yap 2013; Wimmersberger 2018).
Location
Fifteen studies were undertaken in the African continent, eight in Asia, four in Central America and the Caribbean, two in South America, and one study was multicontinental. The countries included were: China (three trials); Ethiopia (two trials); Haiti (two trials); Indonesia (two trials); Kenya (three trials); Tanzania (six trials); and Bangladesh, Brazil, Côte d'Ivoire, Gabon, Guatemala, Jamaica, Peru, Philippines, South Africa, and Thailand (one trial each). One trial included two countries (Rwanda and Ethiopia) (Silber 2017), and one trial was multicentre including 11 countries (Rossignol 1983).
Eleven trials recruited the participants from schools, five trials from communities, and one trial from a health facility. Three trials did not report how the participants were recruited.
Participants
The total number of participants enrolled in the selected studies was 16,475, of whom 7647 had a positive parasitological examination for A lumbricoides, and 6442 were included in the review. We included only participants with pretreatment positive parasitological examinations for A lumbricoides, treated with one of the anthelmintic drugs included in the study, and with cure control data available after the first treatment.
All participants were screened before the treatment was given. In two trials, 100% of participants had A lumbricoides (Haque 2010; Lubis 2012). The percentage of participants with A lumbricoides ranged from 12% (Knopp 2010) to 85.8% (Yap 2013) in the other trials.
The age of participants varied from 28 days to 82 years. Twenty‐four trials included participants under 18 years old (Adams 1994; Adams 2004; Adegnika 2014; Albonico 1994; Albonico 2002; Albonico 2003; Beach 1999; Belizario 2003; Fox 2005; Hadju 1997; Haque 2010; Knopp 2010; Lubis 2012; Nokes 1992; Ortiz 2002; Palmeirim 2018a; Silber 2017; Speich 2014; Steinmann 2011; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wimmersberger 2018; Yap 2013), and six studies included participants under and over 18 years (Jongsuksuntigul 1993; Legesse 2002; Legesse 2004; Rossignol 1983; Wen 2008; Zani 2004).
Six trials classified the intensity of infection. Three trials considered light infection as from 1 to 4999 epg of faeces, moderate as 5000 to 9999 epg, and heavy as more than 10,000 epg (Albonico 1994; Albonico 2002; Albonico 2003). The trial authors presented the values in graphs.
Two trials considered light infection as from 1 to 4999 epg, moderate as 5000 to 49,999 epg, and heavy as more than 50,000 epg (Speich 2014; Watkins 1996a). In Speich 2014, 51.8% of participants had light infection, 46.6% moderate, and 1.6% heavy infection. Watkins 1996a reported that more than 50% of participants had greater than 10,000 and less than 50,000 epg, and 25% had 50,000 epg or greater.
In 25 trials the participants had multiple other helminth infections (T trichiura,Enterobius vermicularis, hookworm). In two trials, they were also treated for lymphatic filariasis caused by Wuchereria bancrofti (Beach 1999; Fox 2005), and in three trials for Schistosoma spp (Legesse 2002; Legesse 2004; Wimmersberger 2018).
Intervention
Twenty‐four studies included albendazole in one of the treatment arms, 12 trials included mebendazole, four trials included ivermectin, and one trial included nitazoxanide.
Albendazole
Eleven trials compared albendazole to placebo (Adams 1994; Beach 1999; Fox 2005; Hadju 1997; Haque 2010; Nokes 1992; Rossignol 1983; Stephenson 1989; Stephenson 1993; Watkins 1996a; Yap 2013), nine trials to mebendazole (Albonico 1994; Jongsuksuntigul 1993; Knopp 2010; Legesse 2002; Legesse 2004; Lubis 2012; Speich 2014; Steinmann 2011; Zani 2004), three trials to ivermectin (Beach 1999; Belizario 2003; Wen 2008), and one trial to nitazoxanide (Ortiz 2002).
Albendazole dose was 400 mg single dose in 17 trials. Four trials compared different doses of albendazole (400 mg once a day to 400 mg each two consecutive days or 400 mg each three consecutive days) (Adams 2004; Adegnika 2014; Hadju 1997; Steinmann 2011). The dose of albendazole was 600 mg single dose in one study (Stephenson 1993).
Mebendazole
Three trials compared mebendazole to placebo (Albonico 2002; Albonico 2003; Silber 2017). Nine trials used mebendazole 500 mg single dose (Albonico 1994; Albonico 2002; Albonico 2003; Knopp 2010; Legesse 2002; Lubis 2012; Palmeirim 2018a; Speich 2014). One trial used mebendazole 300 mg single dose in one of the comparison arms (Jongsuksuntigul 1993). Four trials used mebendazole 200 mg each three consecutive days (Legesse 2002; Legesse 2004; Steinmann 2011; Zani 2004). One trial compared mebendazole 500 mg single dose to mebendazole 200 mg each three consecutive days (Palmeirim 2018a).
Ivermectin
Two studies compared albendazole 400 mg single dose to ivermectin or to ivermectin plus albendazole (Beach 1999; Belizario 2003); the doses of ivermectin were 200 μg/kg to 400 μg/kg. One trial compared albendazole 6.7 mg/kg to ivermectin 100 μg/kg (Wen 2008). One trial compared different doses of ivermectin with placebo (Wimmersberger 2018).
Control
Fifteen studies used placebo (Adams 1994; Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Hadju 1997; Haque 2010; Nokes 1992; Rossignol 1983; Silber 2017; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wimmersberger 2018; Yap 2013). Two trials used vitamin C as placebo (Beach 1999; Fox 2005); see Characteristics of included studies table.
Study designs
Twenty‐nine studies were parallel‐group randomized trials and the individual was the randomization unit. One trial had a factorial‐randomized clinical trial design (Haque 2010).
Outcomes
Twenty‐five trials diagnosed A lumbricoides by Kato‐Katz or modified Kato‐Katz, two studies by a modification to the method of Stoll (Beach 1999; Fox 2005), and three trials did not report the methods used for diagnosis (Adams 2004; Haque 2010; Silber 2017).
All included trials reported the prevalence pre‐ and post‐treatment and it was possible to calculate the parasitological cure.
Twenty‐five studies did the parasitological examination for cure control between seven and 60 days post‐treatment (Adams 2004; Adegnika 2014; Albonico 1994; Albonico 2002; Albonico 2003; Beach 1999; Belizario 2003; Fox 2005; Jongsuksuntigul 1993; Knopp 2010; Legesse 2002; Legesse 2004; Lubis 2012; Nokes 1992; Ortiz 2002; Palmeirim 2018a; Rossignol 1983; Silber 2017; Speich 2014; Steinmann 2011; Watkins 1996a; Wen 2008; Wimmersberger 2018; Yap 2013; Zani 2004), and five trials did the cure control between 61 and 180 days after the treatment (Adams 1994; Hadju 1997; Haque 2010; Stephenson 1989; Stephenson 1993).
Twenty‐five trials reported the AM or GM of epg pre‐ and post‐treatment (Adams 1994; Adegnika 2014; Albonico 1994; Albonico 2002; Albonico 2003; Beach 1999; Belizario 2003; Fox 2005; Hadju 1997; Haque 2010; Jongsuksuntigul 1993; Knopp 2010; Legesse 2002; Legesse 2004; Nokes 1992; Ortiz 2002; Palmeirim 2018a; Speich 2014; Steinmann 2011; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wen 2008; Wimmersberger 2018; Yap 2013). Twenty‐three trials reported the ERRs (Table 5). Only five trials reported the impact of treatment stratified by infection intensity (mild, moderate, or heavy) (Albonico 1994; Albonico 2002; Albonico 2003; Rossignol 1983; Speich 2014).
Seventeen trials reported adverse events (Adams 2004; Adegnika 2014; Albonico 1994; Albonico 2002; Albonico 2003; Fox 2005; Jongsuksuntigul 1993; Knopp 2010; Legesse 2002; Ortiz 2002; Palmeirim 2018a; Rossignol 1983; Silber 2017; Speich 2014; Steinmann 2011; Wen 2008; Wimmersberger 2018) (Table 6).
Several trials reported the prevalence pre‐ and post‐treatment of other helminths: T trichiura (25 trials), hookworm (17 trials), Enterobius vermicularis (two trials),Schistosoma mansoni (three trials), Wuchereria bancrofti (two trials), but we did not include these outcomes in this review.
The main objective of 10 trials was to evaluate the impact of anthelmintic treatment on anthropometric measurements, school performance, appetite, and haemoglobin level (Adams 1994; Adams 2004; Adegnika 2014; Beach 1999; Fox 2005; Hadju 1997; Nokes 1992; Stephenson 1989; Stephenson 1993; Watkins 1996a). From these trials, we included in this review only the data related to pre‐ and post‐treatment prevalence of A lumbricoides.
Other outcomes evaluated were beta‐carotene level (Haque 2010), egg maturation (Lubis 2012), and reinfection (Yap 2013), but we did not include in this review.
Excluded studies
We excluded 132 studies. The main reasons for exclusion were: 32 were not randomized trials, 25 compared anthelmintics that were not of interest in this review, and 25 trials carried out cure control after several treatment rounds. See the other reasons for exclusion in Characteristics of excluded studies table.
Ongoing studies
Two trials are ongoing (see Characteristics of ongoing studies table).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 3 and summarized in Figure 4. The Characteristics of included studies table shows details of the risk of bias. When the studies did not describe adequately the method to allow the judgement of the of risk of bias, it was classified as unclear.
3.
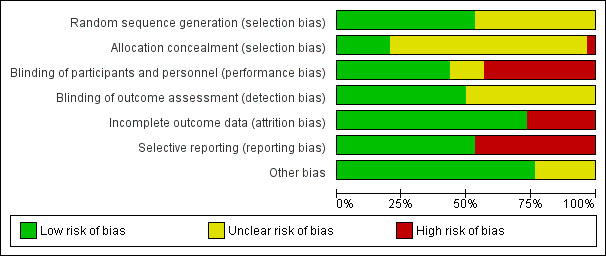
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
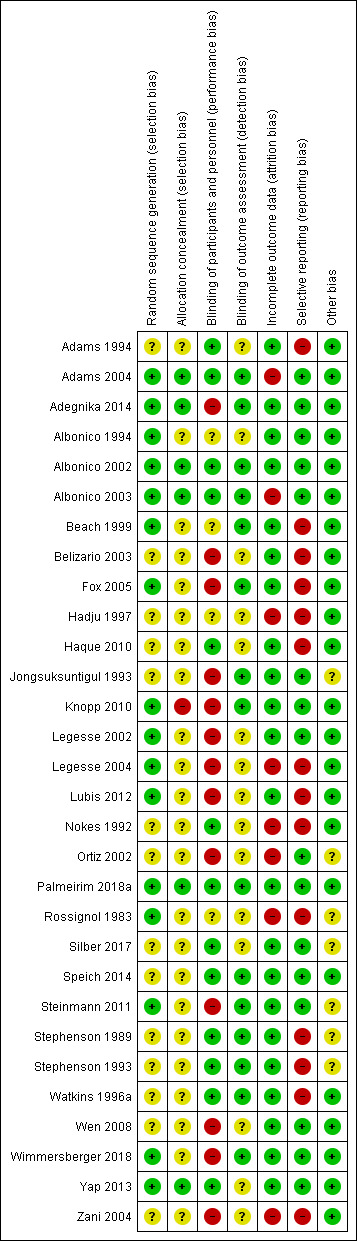
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Sixteen trials described adequate sequence generation methods, and the risk of bias was considered low (Adams 2004; Adegnika 2014; Albonico 1994; Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Knopp 2010; Legesse 2002; Legesse 2004; Lubis 2012; Palmeirim 2018a; Rossignol 1983; Steinmann 2011; Wimmersberger 2018; Yap 2013). The other trials did not reported details to judge the risk of selection bias and were considered unclear.
Six trials described adequate allocation concealment, and were considered of low risk of selection bias (Adams 2004; Adegnika 2014;Albonico 2002; Albonico 2003; Palmeirim 2018a; Yap 2013). One trial was considered of high risk of selection bias (Knopp 2010).
Blinding
Thirteen trials blinded participants and personnel (Adams 1994; Adams 2004; Albonico 2002; Albonico 2003; Haque 2010; Nokes 1992; Palmeirim 2018a; Silber 2017; Speich 2014; Stephenson 1989; Stephenson 1993; Watkins 1996a; Yap 2013). The risk of performance bias was unclear in four trials (Albonico 1994; Beach 1999; Hadju 1997; Rossignol 1983), and high in 13 trials (Adegnika 2014; Belizario 2003; Fox 2005; Jongsuksuntigul 1993; Knopp 2010; Legesse 2002; Legesse 2004; Lubis 2012; Ortiz 2002; Steinmann 2011; Wen 2008; Wimmersberger 2018; Zani 2004) (Figure 3).
Fifteen trials blinded the outcome assessor (Adams 2004; Adegnika 2014; Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Jongsuksuntigul 1993; Knopp 2010; Palmeirim 2018a; Speich 2014; Steinmann 2011; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wimmersberger 2018). There was insufficient information for judgement in the other trials, and the risk of detection bias was unclear.
Incomplete outcome data
Eight trials were at high risk of attrition bias because more than 20% of participants lost follow‐up, or there was great imbalance of lost follow‐up among the treatment groups (Adams 2004; Albonico 2003; Hadju 1997; Legesse 2004; Nokes 1992; Ortiz 2002; Rossignol 1983; Zani 2004). The remaining trials were at low risk of attrition bias.
Selective reporting
Fourteen trials did not report the adverse events after anthelmintic treatment (Adams 1994; Beach 1999; Belizario 2003; Fox 2005; Hadju 1997; Haque 2010; Legesse 2004; Lubis 2012; Nokes 1992; Rossignol 1983; Stephenson 1989; Stephenson 1993; Watkins 1996a; Zani 2004). We considered these trials at high risk of reporting bias because we judged that adverse events after anthelmintic treatment should be reported. In one study, authors described the results of placebo group just for "adult" patients and it was considered at high risk too (Rossignol 1983). The remaining trials were at low risk of reporting bias.
Other potential sources of bias
Seven trials were at unclear risk of other bias (Jongsuksuntigul 1993; Ortiz 2002; Rossignol 1983; Silber 2017; Steinmann 2011; Stephenson 1989; Stephenson 1993). The other trials were at low risk of other bias. See Characteristics of included studies table.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We could not conduct quantitative analysis comparing the FEC pre‐ and post‐treatment or ERR because of insufficient number of studies reporting egg counts in the same format (AM or GM, including SD). In addition, quantitative analysis of adverse events were not possible because of the small number of studies in each comparison that reported them.
Comparison 1: any anthelmintic drug single dose versus placebo
Twelve studies compared any anthelmintic single dose with placebo (Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Hadju 1997; Haque 2010; Rossignol 1983; Silber 2017; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wimmersberger 2018).
Six trials were conducted in the African continent (Albonico 2002; Albonico 2003; Stephenson 1989; Silber 2017; Stephenson 1993; Wimmersberger 2018); three trials in Central America and the Caribbean (Beach 1999; Fox 2005; Watkins 1996a); and two in Asia (Hadju 1997; Haque 2010). Rossignol 1983 included participants from different continents.
Eleven trials included participants between 28 days and 18 years old (Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Hadju 1997; Haque 2010; Silber 2017; Stephenson 1989; Stephenson 1993; Watkins 1996a; Wimmersberger 2018).
Six trials used albendazole 400 mg single dose in experimental arms (Fox 2005; Hadju 1997; Haque 2010; Rossignol 1983; Stephenson 1989; Watkins 1996a); one trial used albendazole 600 mg single dose (Stephenson 1993); three trials used mebendazole 500 mg single dose (Albonico 2002; Albonico 2003; Silber 2017); and one trial used ivermectin 200 μg to 400 μg/kg or albendazole 400 mg single dose in the experimental arms (Beach 1999).
1.1. Parasitological cure
Eight trials performed the parasitological examination between 14 and 60 days after the treatment (Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Rossignol 1983; Silber 2017; Watkins 1996a; Wimmersberger 2018). The cure rate measured was 93.0% in the any anthelmintic single‐dose group compared to 16.1% in the placebo group (RR 6.29, 95% CI 3.91 to 10.12; I² = 86%; Analysis 1.1).
The treatment failure rate in any anthelmintic single‐dose group ranged from 1.9% (Albonico 2002) to 18.8% (Watkins 1996a), and in the placebo group ranged from 62.9% (Beach 1999) to 98% (Watkins 1996a).
Four trials performed the parasitological examination between 61 and 180 days after the treatment (Hadju 1997; Haque 2010; Stephenson 1989; Stephenson 1993). The parasitological cure was 68.6% in the any anthelmintic group and 14.4% in the placebo group (RR 4.44, 95% CI 3.13 to 6.28; I² = 0%; Analysis 1.1). Treatment failure was 31.4% in the any anthelmintic group and 85.6% in the placebo group.
1.2. Faecal egg count
See Table 5.
Nine trials reported the ERR of GM of epg of faeces but they provided data in a form that we could not use in a meta‐analysis (Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Hadju 1997; Haque 2010; Silber 2017; Stephenson 1989; Stephenson 1993).
Five trials (1020 participants) reported the ERR of GM of faeces between 14 and 60 days after the treatment and ranged from 96.1% to 100% in the any anthelmintic single‐dose group and from 11.7% to 33.9% in the placebo group (Albonico 2002; Albonico 2003; Beach 1999; Fox 2005; Silber 2017).
Four trials (404 participants) reported the ERR of GM epg of faeces between 90 and 180 days after the treatment and ranged from 91.0% to 100% in the any anthelmintic group and from 15.0% to 60.0% in the placebo group (Hadju 1997; Haque 2010; Stephenson 1989; Stephenson 1993).
1.3. Adverse outcomes
See Table 6.
Four trials (744 participants) investigated adverse events (Albonico 2002; Albonico 2003; Fox 2005; Silber 2017). The adverse events reported were few (headache, fever, myalgia, cough, epigastric pain, and diarrhoea) and similar among the groups.
Comparison 2: albendazole 400 mg single dose versus albendazole 400 mg multiple doses
Four studies were included, one trial was carried out in South Africa (Adams 2004), one in Gabon (Adegnika 2014), one in Indonesia (Hadju 1997), and one in China (Steinmann 2011). The trials included children and adults, and 194 participants received albendazole 400 mg single dose and 251 received albendazole 400 mg multiple doses.
2.1. Parasitological cure
Three trials determined the parasitological cure between 21 and 42 days (Adams 2004; Adegnika 2014; Steinmann 2011). The cure rate was 93.2% among participants who received albendazole 400 mg single dose compared to 94.3% among participants who received albendazole 400 mg multiple doses (RR 0.98, 95% CI 0.92 to 1.05; 307 participants; Analysis 2.1).
The failure of treatment range from 0.0% (Adams 2004) to 15.4% (Adegnika 2014) in albendazole single‐dose group, and from 0.0% (Adams 2004) to 11.6% (Adegnika 2014) in the albendazole multiple‐dose group.
One trial determined parasitological cure at 90 days after the treatment (Hadju 1997). The cure rate was 40.3% among participants who received albendazole 400 mg single dose compared to 50.7% among participants who received albendazole 400 mg multiple dose (RR 0.79 95% CI 0.54 to 1.17; 129 participants).
2.2. Faecal egg count
See Table 5.
Two trials reported the ERR of AM of epg of faeces determined between 21 and 42 days after the treatment (Adegnika 2014; Steinmann 2011). It ranged from 94% to greater than 99% in the albendazole single‐dose group and from 87% to greater than 99.9% in the albendazole multiple doses group.
One trial reported the ERR of GM of epg of faeces at 90 days after the treatment was 100% in the albendazole single‐dose group and 99% in the albendazole multiple‐dose group (Hadju 1997).
2.3. Adverse outcomes
See Table 6.
Three trials (316 participants) investigated the occurrence of adverse events (Adams 2004; Adegnika 2014; Steinmann 2011). In one trial, few participants of two groups reported headache, abdominal cramps, vomiting, diarrhoea, chills, vertigo, throat pain, and fever (Steinmann 2011). Two studies did not observed adverse events among the participants (Adams 2004; Adegnika 2014).
Comparison 3: albendazole 400 mg single dose versus mebendazole 500 mg single dose
We included six trials: three carried out in Tanzania (Albonico 2002; Knopp 2010; Speich 2014), one in Thailand (Jongsuksuntigul 1993), one Indonesia Lubis 2012), and one in China (Steinmann 2011). Albendazole 400 mg single dose was used in 1121 participants and mebendazole 500 mg single dose in 1010 participants. Five studies included children up to 14 years old, and one trial included children and adults up to 82 years old (Jongsuksuntigul 1993).
3.1. Parasitological cure
All trials determined the parasitological cure up to 31 days after the treatment, and it was achieved in 98.0% of participants who received albendazole compared to 96.9% of participants who received mebendazole (RR 1.01, 95% CI 1.00 to 1.02; 6 trials, 2131 participants; Analysis 3.1). There was low heterogeneity among the trials (I² = 33%, P = 0.23; fixed‐effect model).
The result was consistent by region (Africa: RR 1.01, 95% CI 1.00 to 1.03; 3 trials, 1723 participants; Asia: RR 0.99, 95% CI 0.96 to 1.03; 3 trials, 408 participants; test for subgroup difference: Chi² = 1.14, P = 0.29; Analysis 3.1).
The failure rates after the treatment in the albendazole single‐dose group ranged from 0.0% (Albonico 1994) to 8.0% (Speich 2014), and in the mebendazole single‐dose group from 0.0% (Lubis 2012) to 22.2% (Knopp 2010).
3.2. Faecal egg count
See Table 5.
Five trials reported the intensity of infection (GM or AM egg counts) and the ERR, but it was not possible to pool the data due to the different unit used, and the SD was not reported (Albonico 1994; Jongsuksuntigul 1993; Knopp 2010; Speich 2014; Steinmann 2011). It was not possible to estimate the effect of intervention by the infection intensity due to the different classification used by the studies.
The ERR of GM and AM of epg of faeces was high in all trials, and it was almost 100% for both drugs albendazole and mebendazole (5 trials, 1902 participants).
3.3. Adverse outcomes
See Table 6.
Five trials investigated the occurrence of adverse events (Albonico 1994; Jongsuksuntigul 1993; Knopp 2010; Speich 2014; Steinmann 2011).
In the Speich 2014 study, the frequency of adverse events in the albendazole group was 12.5% and in the mebendazole group was 18.1%.
The main adverse events reported in three trials were headache, vomiting, diarrhoea, abdominal discomfort, and fatigue (Albonico 1994; Knopp 2010; Steinmann 2011). There were no adverse events detected among the participants in Jongsuksuntigul 1993.
Comparison 4: albendazole 400 mg single dose versus ivermectin 100 μg/kg to 400 μg/kg single dose
Three studies compared albendazole single dose versus ivermectin single dose (Beach 1999; Belizario 2003; Wen 2008). One trial was carried out in Haiti (Beach 1999), one in the Philippines (Belizario 2003), and one in China (Wen 2008). A total of 263 participants received albendazole 400 mg single dose and 256 received ivermectin 100 μg/kg to 400 μg/kg single dose.
Two trials included only participants up to 12 years old (Beach 1999; Belizario 2003), and one trial between 6 and 70 years old (Wen 2008).
4.1. Parasitological cure
The parasitological cure rate was 87.8% among the participants who received albendazole single dose compared to 90.2% of participants who received ivermectin single dose (RR 0.99, 95% CI 0.91 to 1.08; 3 trials, 519 participants; Analysis 4.1). There was moderate heterogeneity among the trials (I² = 74%, P = 0.02; random‐effects model).
4.1. Analysis.
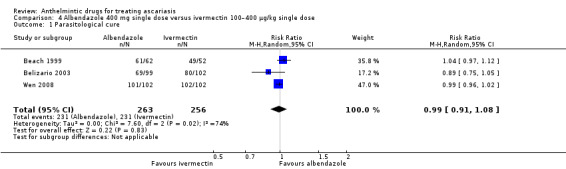
Comparison 4 Albendazole 400 mg single dose versus ivermectin 100–400 μg/kg single dose, Outcome 1 Parasitological cure.
The failure rate in the albendazole single‐dose group ranged from 1% (Wen 2008) to 32% (Belizario 2003), and from 0% (Wen 2008) to 21.6% (Belizario 2003) in the ivermectin group.
4.2. Faecal egg count
See Table 5.
One trial reported the ERR of AM epg of faeces, which was 93.0% in albendazole single‐dose group and 94.3% in ivermectin single‐dose group (Belizario 2003). In other trial, the ERR of GM epg of faeces was 100% in both groups (Beach 1999).
4.3. Adverse events
See Table 6.
Only Wen 2008 reported adverse events (dizziness, abdominal pain, and tiredness), with no difference between the groups.
Other comparisons
One trial from Peru compared albendazole 400 mg single dose versus nitazoxanide single dose (100 mg/5 mL for children aged 2 to 3 years, and 200 mg/10 mL for children aged 4 to 11 years) (Ortiz 2002). The parasitological cure rates were 91.4% in albendazole group and 89.3% in the nitazoxanide group (RR 0.98, 95% CI 0.83 to 1.15). The ERR of AM of epg of faeces was 99.9% in both groups (Table 5). A small percentage of children presented with adverse events, mainly abdominal pain, diarrhoea, vomiting, and headache (Table 6).
One trial carried out in Tanzania compared mebendazole 500mg single dose to mebendazole 100 mg twice a day for three consecutive days (Palmeirim 2018a). The parasitological cure measured between 18 and 22 days was 100% in the mebendazole single‐dose group and 98% in the mebendazole multiple‐dose group (RR 1.02, 95% CI 0.96 to 1.08; 98 participants). The ERR was 100% the mebendazole single‐dose group and 99.1% in the mebendazole multiple‐dose group (Table 5). The adverse events were mild and similar between groups (Table 6).
Four trials compared albendazole 400 mg single dose to mebendazole 200 mg for three consecutive days (Legesse 2002; Legesse 2004; Steinmann 2011; Zani 2004; Appendix 3). The parasitological cure rates were 97.0% in albendazole single‐dose group and 95.3% in mebendazole multiple‐dose group (RR 1.01, 95% CI 0.98 to 1.04; 1052 participants; Analysis 5.1). The ERR of epg ranged from 99.0% to more than 99.9% in both group (Table 5). The most common reported adverse events were vomiting, headache, diarrhoea, and worm expulsion through mouth and faeces (Table 6).
5.1. Analysis.
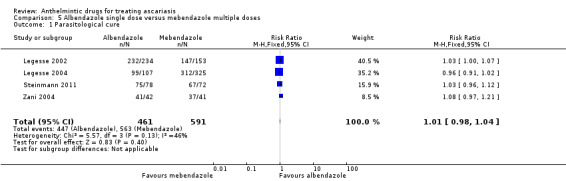
Comparison 5 Albendazole single dose versus mebendazole multiple doses, Outcome 1 Parasitological cure.
Discussion
Summary of main results
See Table 1; Table 2; Table 3; and Table 4.
We undertook this Cochrane Review to assess the efficacy of albendazole, mebendazole, and ivermectin in the parasitological cure of A lumbricoides infection. Thirty RCTs, including 6442 participants from Africa, Asia, Central America and the Caribbean, and South America were evaluated for the treatment of Ascaris infection confirmed by parasitological examination. Trials recruited people from schools, communities, and health facilities. The age of participants varied from 28 days to 82 years. All drugs achieved a high cure rate with few adverse events reported. The certainty of evidence was graded as high to moderate. The incidence of mild adverse events was small and similar among anthelmintic drugs. Twenty‐two trials had high risk of bias for one or two domains (blinding, incomplete outcome data, selective reporting).
The parasitological cure of ascariasis was six times higher in the group receiving any anthelmintic compared to those receiving placebo (93.0% versus 16.1%; moderate‐certainty evidence).
Three main comparisons (albendazole single dose versus albendazole multiple dose, albendazole single dose versus mebendazole single dose, and albendazole single dose versus ivermectin single dose) reported cure control up to 60 days after treatment and parasitological cure ranged from 87.8% to 98.0% with no difference among the compared drugs and doses. The cure rate ranged from 40.3% to 72.8% among trials that measured parasitological cure after 60 days of the treatment.
For parasitological cure, single‐dose albendazole appears to be as effective as multiple doses of albendazole or single‐dose mebendazole (high‐certainty evidence). Single‐dose albendazole is probably as effective as single‐dose ivermectin (moderate‐certainty evidence).
The failure rates after single dose of albendazole ranged from 0.0% to 30.3%, mebendazole from 0.0% to 22.2% and ivermectin from 0.0% to 21.6%.
The ERR measured up to 60 days after treatment was high in all treated groups, regardless of the anthelmintic used (96.0% to 100%), but it was not possible to conduct a meta‐analysis or to evaluate the impact of anthelmintic drugs according to the intensity of the infection (high‐ to moderate‐certainty evidence).
There were no reports of complications or serious adverse events. The incidence of mild adverse events was small and similar among anthelmintic drugs, but it was not possible to perform a meta‐analysis (moderate‐ to low‐certainty evidence). The most commonly reported adverse events were nausea, vomiting, abdominal pain, diarrhoea, headache, and fever.
Overall completeness and applicability of evidence
The elimination of worms and the reduction of egg burden are essential to decrease morbidity and transmission of Ascaris infection on individual and community level in different epidemiological scenarios. In the present review, the clinical trials analysed included children and adults from Africa, Asia, Central America and the Caribbean, and South America. Twelve studies were carried out in Africa (Ethiopia, Gabon, Kenya, Rwanda, South Africa, Tanzania), eight in Asia (Bangladesh, China, Indonesia, Philippines), four in Central America and the Caribbean (Haiti, Guatemala, Jamaica), two in South America (Peru, Brazil), and one was multicontinental. Given this spread, the results of this review can probably be applied to children and adults living in countries with moderate‐to‐high ascariasis endemicity.
In the included trials, the participants had the diagnosis of ascariasis confirmed by parasitological examination before treatment, mainly using the Kato‐Katz or modified Stoll technique.
We did not include ERR in the quantitative analysis due to differences in the reported mean (geometric or arithmetic) and the lack of measures of variation in some trials. Therefore, solid conclusions about these measures could not be drawn.
The trials were analysed together according the time of follow‐up. The cure rate was lower among the trials that measured parasitological cure between 60 and 180 days after treatment, probably because the prolonged follow‐up time could falsely reduce the effectiveness of the treatment, since the treated participants could be reinfected and eliminate eggs within this period.
The results of this review suggest that there were no difference in parasitological cure rates among the compared anthelmintic drugs and doses for Ascaris treatment. Mebendazole, albendazole, and ivermectin, were effective againstA lumbricoides infection, yielding high parasitological cure rates without differences among them. It was not possible to generate a summary on effects estimate of FEC pre‐ and post‐treatment, neither for the effect of anthelmintic drugs by the intensity of the infection, nor for the adverse outcomes.
Despite the concern regarding Ascaris resistance to anthelmintics currently in use, the data from this review suggest that mebendazole, albendazole, and ivermectin remain highly effective for the treatment of people with documented infection for this parasite yielding small failure rates for these drugs.
Three of the anthelmintic drugs evaluated are the most currently used at usual prescribed doses (albendazole 400 mg single dose, mebendazole 500 mg, and ivermectin 200 μg/kg), and they are on WHO Model List of Essential Medicine for treating intestinal helminths (WHO 2017a).
Certainty of the evidence
We assessed the certainty of the evidence across trials using the GRADE approach, and reported the outcomes in 'Summary of findings' tables.
The certainty of the evidence for parasitological cure comparing the different anthelmintic drugs was high to moderate. The certainty of evidence was downgraded mainly due to the concern about inconsistency. However, the results were consistent by geographical region in the main comparisons between albendazole single dose and mebendazole single dose. We could not evaluate the risk of publication bias because of the few studies included in each comparison.
The estimate of ERR was graded as high‐ to moderate‐certainty evidence and downgraded mainly due to imprecision. Meta‐analysis was not possible for these outcomes.
The certainty of the evidence for other adverse events was moderate to low and downgraded mainly due to concern of risk of performance, detection, and reporting bias. Meta‐analysis was not possible for this outcome due to insufficient number of studies reporting them.
Potential biases in the review process
We attempted to limit bias in the review process. Vittoria Lutje, the CIDG Information Specialist, performed the literature searches, and we checked the reference list of relevant studies. It is unlikely that these searches missed any major trials. We were able to obtain all published and unpublished selected studies.
At least two review authors selected the studies and extracted data. A third review author discussed the disagreement and double‐checked the data extraction. We excluded one RCT that met the inclusion criteria from the quantitative analysis because it used a different anthelmintic dose from all other trials. We did not conduct an intention‐to‐treat analysis, indeed the data represented the number of events and participants with available data.
We excluded studies that assessed parasitological cure after several rounds of treatment since the objective of this review was to evaluate the efficacy of anthelmintic drugs after a single treatment at the individual level. Our aim was not to evaluate the deworming programmes, whose periodicity considers the probability of reinfection or of new infections between treatment rounds. We believe that the exclusion of these studies did not impact negatively the results of the review.
Most studies that did not report adverse events were conducted more than 10 years ago, so we decided not to contact the authors for additional information.
We could not evaluate the risk of publication bias because there were too few trials included in each main analysis, therefore, we cannot rule out publication bias.
Agreements and disagreements with other studies or reviews
We identified three reviews that evaluated the parasitological cure of anthelmintic drugs compared to placebo for treating Ascaris (Keiser 2008; Moser 2017b; Mrus 2017). However, these reviews had wider inclusion criteria, included not only RCTs, and they did not directly compare different anthelmintic drugs or different doses of anthelmintics as we did in this review.
We also identified two large uncontrolled trials that evaluated the parasitological cure after treatment with anthelmintic drugs (Levecke 2014; Vercruysse 2011a). Vercruysse 2011a was conducted in seven countries and included 1834 children treated with albendazole single dose. Levecke 2014 was conducted in six countries and included 1209 children treated with a single dose of mebendazole. These trials did not use placebo or different anthelmintic drug or different doses of anthelmintic drug to determine the efficacy of albendazole and mebendazole. The present review included only studies with control groups.
Authors' conclusions
Implications for practice.
The head‐to‐head comparisons suggested that there is little to choose between the drugs evaluated in terms of cure: mebendazole, albendazole, and ivermectin all yielded high parasitological cure with no differences between them. We do not know in Ascaris‐confirmed infections the effect of anthelmintic drugs on egg reduction rate (ERR) according to the class of infection intensity. High‐ to moderate‐certainty evidence suggests that albendazole, mebendazole, and ivermectin are safe drugs to treat children and adults with confirmed Ascaris infection, showing low failure rates.
Implications for research.
These drugs are effective and it is unclear whether outstanding questions remain.
Acknowledgements
The Academic Editor is Dr Emmanuel Effa.
The CIDG editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank Anne‐Marie Stephani, former CIDG Managing Editor, for support during this review and Vittoria Lutje, CIDG Information Specialist, who conducted the literature searches.
We acknowledge Marcos Vinicius Fernandes Garcia and Natália Sayuri Mukai for participating in the protocol of this Cochrane Review; and Paul Garner, Celia Holland, Tonya Esterhuizen, and Sarah Donegan for their comments in the review protocol (Conterno 2013).
The protocol was funded by the São Paulo Research Foundation (FAPESP) (Conterno 2013).
Appendices
Appendix 1. Search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINE | Embase | LILACS |
| 1 | Ascari* | Ascariasis [MeSH] | Ascariasis [MeSH] | Ascariasis [Emtree] | Ascari$ |
| 2 | albendazole | Ascaris [Mesh] | Ascaris [Mesh] | Ascaris ti, ab | albendazole |
| 3 | mebendazole | Ascari* ti, ab | Ascari* ti, ab | 1 OR 2 | mebendazole |
| 4 | levamisole | 1 OR 2 OR 3 | 1 OR 2 OR 3 | Albendazole [Emtree] | levamisole |
| 5 | ivermectin | Albendazole [MeSH] | Albendazole [MeSH] | Mebendazole [Emtree] | ivermectin |
| 6 | pyrantel | Mebendazole [MeSH] | Mebendazole [MeSH] | Levamisole [Emtree] | pyrantel |
| 7 | piperazine | Levamisole [MeSH] | Levamisole [MeSH] | Ivermectin [Emtree] | piperazine |
| 8 | nitazoxanide | Ivermectin [MeSH] | Ivermectin [MeSH] | Pyrantel palmoate ti, ab | nitazoxanide |
| 9 | tetrachlorethylene | Pyrantel palmoate [MeSH] | Pyrantel palmoate [MeSH] | Piperazine citrate [Emtree] | Tetrachlorethylene |
| 10 | thiabendazole | Piperazine citrate ti, ab | Piperazine citrate ti, ab | Nitazoxanide [Emtree] | Thiabendazole |
| 11 | tiabendazole | Nitazoxanide ti, ab | Nitazoxanide ti, ab | Tetrachlorethylene [Emtree] | 2‐10/OR |
| 12 | 2‐11/OR | Tetrachlorethylene [MeSH] | Tetrachlorethylene [MeSH] | Thiabendazole ti, ab | 1 AND 11 |
| 13 | 1 AND 12 | Thiabendazole [MeSH] | Thiabendazole [MeSH] | Tiabendazole [Emtree] | |
| 14 | Tiabendazole ti, ab | Tiabendazole ti, ab | 4‐13/OR | ||
| 15 | 5‐14/OR | 5‐14/OR | 3 AND 14 | ||
| 16 | 4 AND 15 | 4 AND 15 | Limit 15 to Humans | ||
| 17 | Limit 16 to Human | ||||
| aCochrane Infectious Diseases Group (CIDG) Specialized Register | |||||
Appendix 2. 'Risk of bias' assessments
| Potential bias | Authors' judgement |
|
Random sequence generation (selection bias) |
High – not randomized or quasi‐randomized Unclear – randomized stated, but method not reported Low – described method of randomization |
| Allocation concealment (selection bias) | High – not concealed, open‐label trial for individually randomized or method of concealment not adequate Unclear – details of method not reported or insufficient details Low – central allocation, sequentially numbered opaque sealed envelopes |
|
Blinding (performance bias and detection bias) |
High – personnel, participants, or outcome assessors not blinded Unclear – no details or insufficient details reported Low – personnel, participants, and outcome assessors blinded |
| Incomplete outcome data (attrition bias) | High – losses to follow‐up not evenly distributed across intervention and control group, high attrition rate (≥ 20% for the main outcome) Unclear – no details reported, insufficient details reported Low – no losses to follow‐up, losses < 20% and evenly distributed across groups, intention‐to‐treat analysis used Note: for cluster randomized controlled trials, the loss relates to the clusters |
| Selective reporting (reporting bias) | High – did not fully report measured or relevant outcomes Unclear – insufficient information reported to judge Low – all expected outcomes were reported |
| Other bias | Low – no obvious other source of bias of concern to review authors High – major source of bias such as unexplained differences in baseline characteristics |
Appendix 3. Summary of findings table 5: albendazole single dose compared to mebendazole multiple doses for treating ascariasis
| Albendazole single dose compared to mebendazole multiple doses for treating ascariasis | ||||||
|
Patient or population: treating ascariasis Setting: school and community Intervention: albendazole single dose Comparison: mebendazole multiple doses | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with mebendazole multiple doses | Risk with albendazole single dose | |||||
|
Parasitological cure assessed with: parasitological examination Follow‐up: range 21–30 days |
95 per 100 | 96 per 100 (93 to 99) | RR 1.01 (0.98 to 1.04) | 1052 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Albendazole single dose is probably as effective as mebendazole multiple doses for treating ascariasis. |
|
Faecal egg count assessed with: ERR (GM or AR) Follow‐up: range 21–30 days |
ERR of epg of faeces was almost 100% in albendazole single dose and mebendazole multiple doses | 969 (3 RCTs) | ⊕⊕⊕⊕ High | Albendazole single dose and mebendazole multiple doses result in large reductions in faecal eggs count. | ||
| Adverse outcomes Follow‐up: range 21–30 days | The percentage of children reporting symptoms, following albendazole and mebendazole treatment was small and included headache abdominal comfort, vomiting diarrhoea, fever, and worm expulsion through mouth. | 537 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | There may be little to no difference in adverse outcomes for albendazole single dose compared to mebendazole multiple doses. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AM: arithmetic mean egg count; CI: confidence interval; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean egg count; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
Footnotes aDowngraded one level for inconsistency (I² = 46%). bDowngraded one level for risk of performance bias. cDowngraded one level for imprecision: very few events reported. | ||||||
Data and analyses
Comparison 1. Any anthelmintic drug single dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure by time of follow‐up | 12 | 1982 | Risk Ratio (M‐H, Random, 95% CI) | 5.64 [3.96, 8.02] |
| 1.1 Up to 60 days | 8 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 6.29 [3.91, 10.12] |
| 1.2 More than 61 days | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 4.44 [3.13, 6.28] |
Comparison 2. Albendazole 400 mg single dose versus albendazole 400 mg multiple doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure (up to 60 days of follow‐up) | 3 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.05] |
Comparison 3. Albendazole 400 mg single dose versus mebendazole 500 mg single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure by region | 6 | 2131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [1.00, 1.02] |
| 1.1 Africa | 3 | 1723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [1.00, 1.03] |
| 1.2 Asia | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
Comparison 4. Albendazole 400 mg single dose versus ivermectin 100–400 μg/kg single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.08] |
Comparison 5. Albendazole single dose versus mebendazole multiple doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure | 4 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 1994.
| Methods |
Design: parallel group randomized trial Duration of study: March to May 1990 Duration of follow‐up: 63 days |
|
| Participants |
Country: Kenya Setting: school Number included in study: 56 Age: 5–10 years Sex: 31 girls, 25 boys Inclusion criteria: children in nursery and standard 1 classes of Mvindeni Primary School in Kwale District Coast Province Kenya, who had more than 500 epg of T trichiura or > 1000 epg of A lumbricoides or hookworm, prepubertal and > 5 years old Lost at follow‐up: 1 (1.8%) Number positive for A lumbricoides: 16 Number included in review: 16 Exclusion criteria: children with severe anaemia |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, pre‐ and post‐treatment AM and GM epg, ERR. Outcomes not included in review: efficacy of anthelmintic treatment for T trichiura and hookworm and anthropometric measurements, activity and appetite, haemoglobin concentration |
|
| Notes |
Diagnostic technique: Modified Kato‐Katz Funding support: Thrasher Research Fund, SmithKline Beecham, Ltd. and NIH Nutrition Training Grant 2‐T32‐DK07158 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were grouped according to sex and paired according to hookworm intensity; one of each pair was allocated at random to the albendazole‐treated group or the placebo group." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "three 400 mg doses of either albendazole (SmithKline Beecham, Brentford, Middlesex, U.K.) or an identical‐appearing placebo were administered to each child on three consecutive school days (MIMS Africa 1989)." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 (1.8%) participant lost to follow‐up and data not considered in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No obvious source of other bias. |
Adams 2004.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Duration of follow‐up: 30 days |
|
| Participants |
Country: Republic of South Africa Setting: school Number included in study: 150 Age: 6–16 years (mean 10 years) Sex: 75 girls, 75 boys Inclusion criteria: pupils at a primary school serving a wine‐producing area were eligible to receive albendazole if they were infested by T trichiura. Children not infected by any species of helminth were suitable for the placebo group. Exclusion criteria: chronic prescription medication, clinically evident illness, or both Lost at follow‐up: 37 (24.6%) Number positive for A lumbricoides: 58 Number included in review: 58 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment cure rates and adverse events Outcomes not included in review: efficacy of anthelmintic treatment for T trichiura not reported |
|
| Notes |
Diagnostic technique: "Standard methods" Funding support: The Peninsula School Feeding Association; Anglo American Chairman's Fund, AngloGold Fund, De Beers Fund, and AusAID supported operational and developmental research to implement crèche‐ and school‐based deworming, health education and sanitation in impoverished communities in the south‐western Cape. GlaxoSmithKline donated the albendazole and placebo tablets. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician operating independently of the researchers in the field used random permutations to allocate the 150 Trichuris infected children into 3 groups, which were again randomized to the different doses of albendazole." |
| Allocation concealment (selection bias) | Low risk | Quote: "Set of three blister packs for each code recipient were prepared in laboratory. Packs were marked for use on day 1, 2 and 3 respectively." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo tablets matched the albendazole tablets in appearance, as did the blister packs. All treatments comprised 1 tablet a day for 3 days. At the school, neither the person administering the treatment, nor the child receiving the tablet, was aware of the dose." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Faecal samples were coded and microscopist who processed and examined the specimens for helminth eggs were unaware which treatment group any sample corresponded to. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 (24.7%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Low risk | No other obvious source of bias. |
Adegnika 2014.
| Methods |
Design: parallel group randomized trial Duration of study: August 2010 to June 2011 Duration of follow‐up: 42 days |
|
| Participants |
Country: Gabon Setting: school Number included in study: 175 children Age: 4–14 years (mean 8.7 years) Sex: not reported Inclusion criteria: aged 4–14 years and ≥ 5 eggs or larvae of A lumbricoides,T trichiura, or hookworm Exclusion criteria: known HIV infection, allergy to albendazole, severe anaemia, and any other underlying severe physical condition Lost to follow‐up: 0 (0%) Number positive for A lumbricoides: 108 Number included in review: 108 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rate, pre‐ and post‐treatment AM epg, ERR rate, and adverse events Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris, hookworm, and mean haemoglobin |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: EDCTP Senior Fellowship TA 11 40200025, the Deutsche Forschungsgemeinschaft‐funded project Deutsch‐Afrikanische Kooperationsprojekte in der Infektiologie (DFG‐Projekt KR 1150/6‐1), and EU‐funded project Immunological Interplay between Poverty Related Diseases and Helminth Infections: An African‐European Research Initiative (IDEA) (HEALTH‐F3‐2009‐241642). "Targeted Development of a New Generation Vaccine for Schistosomiasis" ("TheSchistoVac") (Health‐2009‐242107) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomizations code was generated with the use of R statistical software by an investigator not involved in patient study procedures." |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was kept concealed on a password‐protected personal computer inaccessible to study staff. The treatment group assignments were communicated to the study staff after study numbers were given to the eligible subjects and shortly before the beginning of treatment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 2 laboratory technicians independently read slides and were blinded to assigned drug regimen. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Low risk | No other obvious source of bias. |
Albonico 1994.
| Methods |
Design: parallel group randomized trial Duration of study: October 1992 to February 1993 Duration of follow‐up: 18–31 days(mean 22.5 days) |
|
| Participants |
Country: United Republic of Tanzania Setting: school Number included in study: 2650 Age: 6–12 years (mean 10 years) Sex: not reported Inclusion criteria: school children aged 6–12 years who had never been treated for intestinal helminths Exclusion criteria: not reported Lost to follow‐up: 356 (13.4%) Number positive for A lumbricoides: 1548 Number included in review: 1548 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR, adverse events Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris, and hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: World Health Organization Programme of Intestinal Parasitic Infection Division of Communicable Diseases and by Direzione Generale per la Cooperazione allo Sviluppo, Italian Ministry of Foreign Affair |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the start of the trial, sequentially numbered envelopes were prepared, each envelope containing a single dose of one of 2 antihelminthic drugs. Half of envelopes, selected using computer generated random numbers, contained albendazole (400 mg (SmithKline Beecham) and other half mebendazole 500 mg (Jansen Pharmaceutica)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "About 110 faecal specimens were collected each day, allocated a trial number sequentially, and whichever treatment was in the envelope with that number was administered to the child on the spot." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details not reported. Quote: "single blind randomized clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 354 (13.4%) participants lost at follow‐up, and not included in analysis; 11% (148) in albendazole group and 16% (206) in mebendazole group. Loss was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Low risk | No other obvious source of bias. |
Albonico 2002.
| Methods |
Design: parallel group randomized trial Duration of study: September to October 2000 Duration of follow‐up: mean 21 days (range 20–23 days) |
|
| Participants |
Country: United Republic of Tanzania Setting: school Number included in study: 1435 Age: 8–13 years (mean 9.4 years) Sex: 795 girls, 640 boys Inclusion criteria: 1st and 2nd grade school children from 7 primary public school randomly selected among 72 schools Exclusion criteria: significant comorbidities (e.g. severe diarrhoea, severe anaemia, high fever); and had received anthelmintic treatment in previous month Lost at follow‐up: 106 (7.4%) Number of participants positive for A lumbricoides: 310 Number of participants included in review: 210 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates, pre‐ and post‐treatment GM epg, ERR, adverse events Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris; efficacy of pyrantel‐oxantel for Ascaris |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Parasitic and Vector Control, Division of Communicable Diseases, World Health Organization Pharmamed (Malta) donated placebo and mebendazole, and Pfizer (Indonesia) donated pyrantel‐oxantel. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was blocked on weight and a computer‐generated program was used to create 3 randomized treatment list." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatments were placed in sealed, opaque envelopes and coded with a number." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo pills resembled mebendazole in colour, size, taste, and shape." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All laboratory investigations were blinded, i.e. the technicians examining the slides were unaware of the treatment regimen of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 106 (7.4%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No other obvious source of bias. |
Albonico 2003.
| Methods |
Design: parallel group randomized trial Duration of study: August 1999 Duration of follow‐up: 21 days |
|
| Participants |
Country: United Republic of Tanzania Setting: school Number included in study: 1137 Age: 7–18 years (mean 11.5 years) Sex: girls 625, boys 512 Inclusion criteria: children in 1st grade (Standard 1) and 5th grade (Standard 5) of 10 public school on Pemba Island. Exclusion criteria: no parental or guardian permission to participate, no stool sample, significant comorbidities (e.g. severe diarrhoea, severe anaemia, or high fever), or had recently transferred to the school from an area outside Zanzibar. Lost at follow‐up: 233 (20.5%) Number positive for A lumbricoides: 538 Number included in review: 279 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates, pre‐ and post‐treatment AM and GM epg, ERR, adverse events Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris and hookworms; mebendazole + levamisole efficacy for Ascaris; levamisole efficacy for Ascaris |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Parasitic Disease and Vector Control, Prevention and Eradication, World Health Organization, Geneva. Pharmamed (Malta) and Janssen (Belgium) donated placebo and mebendazole, Zeneca (UK) donated levamisole. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was blocked on weight, and a computer‐generated programme was used to create two randomized treatment list: one for children who weighed 15–20 kg, who were to receive one tablet of 40 mg levamisole, and another for children who weighed 21–60 kg, who were to receive two tablets (80 mg)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatments given were placed in sealed, opaque envelopes and were coded with a number. Children were identified by these numbers only throughout the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo pills resembled mebendazole colour, size, taste and shape." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All laboratory investigators were blinded." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 233 (20.5%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | Quote: "Although adverse effects were not investigated actively, no adverse events were reported after any single or combined treatment in the week following the administration of anthelminthics." |
| Other bias | Low risk | No other obvious source of bias. |
Beach 1999.
| Methods |
Design: parallel group randomized trial Duration of study: started January 1996 Duration of follow‐up: 35 days |
|
| Participants |
Country: Haiti Setting: school Number included in study: 965 Age: 5–11 years Sex: 407 girls, 446 boys Inclusion criteria: aged 5–11 years; anthropometric measurements before and 4 months after treatment; stool specimens before and 5 weeks after treatment; random assignment to a treatment group; and height, weight, and age within limits of the anthropometric database Exclusion criteria: haematocrit levels < 22% Lost at follow‐up: 112 (11.6%) Number positive for A lumbricoides: 249 Number included in review: 249 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, prevalence reduction, pre‐ and post‐treatment AM and GM epg, ERR Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris, hookworm,and W bancrofti microfilariae; nutritional and anthropometric measures; data after 4 months of treatment |
|
| Notes |
Diagnostic technique: modified Stoll method Funding support: United States Agency for International Development; Merck Inc. donated the ivermectin and SmithKline Beecham donated the albendazole. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All eligible students were assigned, using a random number table, to four treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details not reported for parasitological cure outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Laboratory personnel, measurement teams, and personnel evaluating students for adverse reactions were blinded to the treatment status of the children." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 112 (11.6%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No other obvious source of bias. |
Belizario 2003.
| Methods |
Design: parallel group randomized trial Duration of study: second half of 1998 Duration of follow‐up: 7–14 days |
|
| Participants |
Country: Republic of the Philippines Setting: school Number included in study: 784 Age: 6–12 years Sex: not reported Inclusion criteria: boys or girls; aged 6–12 years; informed consent; A lumbricoides or T trichiura (or both) eggs in stool samples; and compliance with protocol, requiring stool samples at the specified times after treatment Exclusion criteria: previous hypersensitivity reaction to benzimidazole, ivermectin, diethylcarbamazine, or any related compound; other helminths; without the target helminths listed; diarrhoea disease; receipt of any anthelmintic in the 2 weeks before enrolment; receipt of an anthelmintic during the study period; and concomitant infection or underlying disease compromising evaluation of the response to the medications being studied Lost at follow‐up: 29 (3.7%) Number positive for A lumbricoides: 528 Number included in review: 306 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rate, pre‐ and post‐treatment AM epg, ERR Outcomes not included in review: efficacy of anthelmintic treatment for Trichuris; efficacy of diethylcarbamazine and albendazole + diethylcarbamazine for A lumbricoides. |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Placebo resembling albendazole was used together with the one‐drug treatments in order to make it appear that all pupils were receiving a combination of two drugs." Comment: ivermectin not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 (3.7%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No obvious source of bias. |
Fox 2005.
| Methods |
Design: parallel group randomized trial Duration of study: October 1998 to May 1999 Duration of follow‐up: 35 days |
|
| Participants |
Country: Haiti Setting: school Number included in study: 1292 Age: 5–11 years(mean 7.7 years) Sex: 656 girls, 636 boys Inclusion criteria: aged 5–11 years, anthropometric measurements collected before and 6 months after treatment, stool specimens collected before and 5 weeks after treatment, microfilaria smears prepared before and 6 months after treatment, and random assignment to a treatment group Exclusion criteria: not reported Lost at follow‐up: 43 (3.3%) Number positive for A lumbricoides: 383 Number included in review: 188 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment AM epg, ERR, adverse events Outcomes not included in review: anthelminthic efficacy for Trichuris, hookworm, and W bancrofti; diethylcarbamazine and diethylcarbamazine + albendazole efficacy for A lumbricoides; nutritional and anthropometric measurements |
|
| Notes |
Diagnostic technique: Stoll modified method Funding support: emerging Infections Program of the Centers for Disease Control and Prevention and by an Institutional Strengthening Grant from the World Health Organization to the Hôpital Sainte Croix. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all eligible students were assigned using a random number table to one of four treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | No detail reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "given by CDC staff either a placebo (250mg of vitamin C), 400 mg of ALB [albendazole] (Zentel; SmithKline Beecham, Philadelphia, PA or generic drug; BeltaPharm Srl., Milan, Italy), a single 6 mg/kg dose of DEC [diethylcarbamazine] (Hetrazan; Lederle, Grosport, Hampshire, United Kingdom), or a combination of DEC and ALB." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Laboratory personnel, measurement teams, and personnel evaluating students for adverse reactions were blinded to the treatment status of the children." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 (3.3%) participants lost at follow‐up, and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events just reported for children with heavy microfilaria infection. |
| Other bias | Low risk | No obvious other source of bias. |
Hadju 1997.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Duration of follow‐up: 90 days |
|
| Participants |
Country: Republic of Indonesia Setting: school Number included in study: 507 Age: ≤ 10 years Sex: not reported Inclusion criteria: primary school children attending to grades 1, 2, and 3 Exclusion criteria: children aged > 11 years old or with signs of puberty, with signs of severe protein energy malnutrition, with deformity or congenital abnormality Lost at follow‐up: 177 (34.9%) Number positive for A lumbricoides: 308 Number included in review: 198 |
|
| Interventions |
Treatment strategy: screening and treat all participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR Outcomes not included in review: anthropometric measures; efficacy of pyrantel pamoate for A lumbricoides |
|
| Notes |
Diagnostic technique: modified Kato‐Katz Funding support: Directorate of Higher Education, Government of Indonesia; SmithKline Beecham Ltd. in the UK produced the placebo and albendazole. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All eligible children were randomized to five treatments groups. Randomization was based on sex and eggs counts of Ascaris." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The placebo table was similar to the albendazole tablet but pyrantel was different, Children and field workers were not informed of the actual name of both drugs. Each treatment was put in three different boxes label A, B, C. No one except the principal investigator was made aware of the labels." Comment: no details if albendazole twice group received 2 days of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 177 children lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No other obvious source of bias. |
Haque 2010.
| Methods |
Design: parallel group randomized trial (factorial) Duration of study: not reported Follow‐up: 120 days |
|
| Participants |
Country: Bangladesh Setting: community Number included in study: 248 Age: 24–60 months Sex: 121 boys, 100 girls Inclusion criteria:Ascaris infection was prerequisite for enrolment to study; children apparently healthy without a history of chronic illness; without hookworm infection, and willing to take daily β‐carotene capsule and 2 doses of albendazole during study Exclusion criteria: severe malnutrition, clinical vitamin A deficiency (as indicated by corneal involvement), chronic diseases, or persistent diarrhoea Lost at follow‐up: 27 (10.9%) Number positive for A lumbricoides: 248 Number included in review: 111 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment AM epg, ERR Outcomes not included in review: efficacy of albendazole + β‐carotene and β‐carotene + placebo for A lumbricoides; anthelmintic efficacy for Trichuris; β‐carotene levels |
|
| Notes |
Diagnostic technique: not reported Funding support: Thrasher Research Fund, USA. Eskayef Bangladesh Ltd. provided albendazole |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization was used for recruiting children in the treatment group and placebo (control) groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo forms for both albendazole tablets and β‐carotene capsules were of identical size, shape, and colour." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27 (10.9%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | The pharmaceutical industry donated the drug. |
Jongsuksuntigul 1993.
| Methods |
Design: parallel group randomized trial Duration of study: 5–30 August 1991 Follow‐up: 14 days |
|
| Participants |
Country: Thailand Kingdom Setting: community Number included in study: 196 Age: 3–80 years Sex: 98 male, 98 female Inclusion criteria: single or multiple infections of hookworm, Ascaris, and Trichuris Exclusion criteria: pregnant women and breastfeeding mothers Lost at follow‐up: 0 (0%) Number positive for A lumbricoides: 56 Number included in review: 56 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR adverse events Outcomes not included in review: anthelmintic efficacy for hookworm, Trichuris, andEnterobius |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: SmithKline Beecham Pharmaceuticals Ltd and Janssen Pharmaceutica Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported. Quote: "patients were randomly assigned into four treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Drugs had different appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The laboratory technicians were blind to the respective treatment of each patient group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Pharmaceutical support unclear. |
Knopp 2010.
| Methods |
Design: parallel group randomized trial Duration of study: March to May 2009 Follow‐up: 21 days |
|
| Participants |
Country: Tanzania Setting: school Number included in study: 610 Age: mean 11 years Sex: 55% girls Inclusion criteria: children attending grades 1–7 in the 2 schools with written informed consent provided by parents or guardians, aged ≥ 5 years, sufficiently large stool sample to perform duplicate Kato‐Katz thick smears at baseline survey, infection with T trichiura, and submission of second stool sample subjected to duplicate Kato‐Katz thick smears before treatment Exclusion criteria: pregnant (for girls), as verbally assessed by medical personnel; presence of systemic illnesses (e.g. fever or severe illness); and anthelmintic treatment within the previous 4 weeks Lost at follow‐up: 62 (10.0%) Number positive for A lumbricoides: 73 Number included in review: 64 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris and hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Commission for Research Partnerships with Developing Countries (through the Swiss Agency for Development and Cooperation–sponsored program "Jeunes Chercheurs" to S.K.), the Swiss National Science Foundation (project PPOOB‐102883 and PPOOB‐119129), the EU (FP6 STREP CONTRAST project, contract 032203), and Burckhardt Foundation Basel. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The trial statistician was provided with the list of identification numbers of 618 T.trichiura‐positive children and generated a computer‐based random allocation sequence (numbers 1–4)." |
| Allocation concealment (selection bias) | High risk | Quote: "The numbers were decoded for each school by 1 of 2 researchers (S.K. for Kilombero and B.S. for Kinyasini) to assign children either to albendazole (400 mg; Laboratoria Wolfs) plus placebo (Hermes Edulcorants), albendazole plus ivermectin (200 mg/kg; Merck), mebendazole (500 mg; Janssen‐Cilag) plus placebo, or mebendazole plus ivermectin." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Trial medications were prepared in identical envelopes labelled with unique identification numbers and sealed. Because ivermectin is administered according to patient weight, ivermectin and placebo tablets were counted and packed according to children's weight. The gravure on the albendazole or mebendazole tablets was not identical, and placebos were slightly smaller than ivermectin tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All laboratory personnel, including the outcome assessors, were masked to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62 (10.0%) participants lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No other obvious source of bias. |
Legesse 2002.
| Methods |
Design: parallel group randomized trial Duration of study: November 2000 Follow‐up: 21 days |
|
| Participants |
Country: Ethiopia Setting: community Number included in study: 520 Age: 2–80 years Sex: 221 male, 299 female Inclusion criteria: not reported Exclusion criteria: not reported Lost at follow‐up: 52 (11.0%) Number positive for A lumbricoides: 387 Number included in review: 387 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris andS mansoni |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A sequentially numbered list of individuals positive for Ascaris lumbricoides and/or Trichuris trichiura infections was prepared. The list was randomly divided into treatment group using random numbers obtained from a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 (11.0%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcome were reported. |
| Other bias | Low risk | No other obvious source of bias. |
Legesse 2004.
| Methods |
Design: parallel group randomized trial Duration of study: March 2003 Follow‐up: 21 days |
|
| Participants |
Country: Ethiopia Setting: school Number included in study: 661 Age: 6–19 years (mean 10.6 years) Sex: female 254, male 280 Inclusion criteria: did not receive any anthelmintic drugs in the past 3 months; positive for ≥ 1 helminth infections Exclusion criteria: not reported Lost at follow‐up: 127 (19.2%) Number positive for A lumbricoides: 432 Number included in review: 432 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR Outcomes not included in review: anthelmintic efficacy for Trichuris andS mansoni |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: mebendazole donated by Dr AR Hashim, Manager of East African Pharmaceuticals in Addis Ababa. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A sequentially numbered list of students positive for at least one of the two helminth infections (Ascariasis or Trichuriasis) was prepared and randomly divided into four treatment groups using random numbers obtained from a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 127 (19.2%) participants lost at follow‐up and not included in analysis. Balanced lost among groups. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No other obvious source of bias. |
Lubis 2012.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Follow‐up: 7 days |
|
| Participants |
Country: Republic of Indonesia Setting: school Number included in study: 229 Age: not reported (primary school children) Sex: not reported Inclusion criteria: primary school children with single infection of A lumbricoides or mixed infections of A lumbricoides with other soil‐transmitted helminths Exclusion criteria: children infected by single infections of T trichiura, hookworm, orEnterobius vermicularis Lost at follow‐up: 0 (0%) Number analysed for primary outcome of review: 229 Number positive for A lumbricoides: 229 Number included in review: 229 |
|
| Interventions |
Treatment strategy: screening and treat the positive participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates, ERR Outcomes not included in review: observation and counting of the egg maturation stages taken from the egg culture. |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infected children were randomly assigned using random‐number table into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment drugs had different appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized children included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No other obvious source of bias. |
Nokes 1992.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Follow‐up: 10–30 days |
|
| Participants |
Country: Jamaica Setting: school Number included in study: 140 Age: 9–12 years Sex: 35 boys, 68 girls Inclusion criteria: children from 3 schools in Mandeville with Trichuris egg counts > 1900, but low hookworm counts on 2 occasions before the trial separated by 3 months Exclusion criteria: twins, children with severe illness, physical disabilities, and neurological disorders Lost at follow‐up: 37 (26.4%) Number positive for A lumbricoides: 60 Number included in review: 60 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates, pre‐ and post‐treatment AM epg, ERR Outcomes not included in review: anthelmintic efficacy for Trichuris andNecator americanus; data of uninfected control group; measures of cognitive function |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Rotary International Scholarship, Wellcome Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those selected were randomly assigned to treatment or placebo groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind controlled trial;" "identical placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 (26.4%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No obvious other source of bias. |
Ortiz 2002.
| Methods |
Design: parallel group randomized trial Duration of study: 2000 Follow‐up: 21–30 days |
|
| Participants |
Country: Peru Setting: not reported Age: 4–11 years (mean 7.9 years) Sex: not reported Number included in study: 210 Inclusion criteria: aged 2–11 years with eggs of A lumbricoides,T trichiura,or Hymenolepis nana in a faecal sample Exclusion criteria: not reported Lost at follow‐up: 22 (10.5%) Number positive for A lumbricoides: 70 Number included in review: 63 |
|
| Interventions |
Treatment strategy: screening and treat the positive
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris and Hymenolepis; efficacy of praziquantel for A lumbricoides |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Romark Laboratories (Tampa, Florida, USA) provided the nitazoxanide suspension and gave financial support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the children were randomized to treatment with either nitazoxanide suspension or the comparator drug." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 (10.5%) participants lost at follow‐up, dropouts not balanced among groups and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcome were reported. |
| Other bias | Unclear risk | Financial support by Romark laboratories. |
Palmeirim 2018a.
| Methods |
Design: parallel group randomized trial Duration of study: July to September 2017 Follow‐up: 21–30 days |
|
| Participants |
Country: Tanzania Setting: school Age: 6–12 years (mean 10.1 years) Sex: 46% girls Number included in study: 186 Inclusion criteria: 2 stool samples positive for hookworm eggs in the stool (≥ 100 epg or ≥ 2 Kato‐Katz thick smears slides with > 1 hookworm egg) Exclusion criteria: had menarche (for females); presence of severe anaemia (haemoglobin 8.0 g/dL considered severe anaemia); had any known or reported history of chronic illness such as cancer, diabetes, chronic heart, liver, or renal disease; received any recent anthelminthic treatment (within past 4 weeks); had any known allergy to mebendazole or albendazole Lost at follow‐up: 1 (0.5%) Number positive for A lumbricoides: 98 Number included in review: 98 |
|
| Interventions |
Treatment strategy: screening and treat the positive
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment GM epg, ERR, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris and hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: PATH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The trial statistician (JH), who was not involved in any field work, provided a computer‐generated stratified (by baseline infection intensities), block randomisation code (blocks of size ten). Participant were allocated 1:1 to one of the two treatment arms: single dose (500 mg) or multiple dose (100 mg twice a day during three consecutive days) of mebendazole." |
| Allocation concealment (selection bias) | Low risk | Quote: "the envelopes containing the drugs were in bags of ten and, within each group of ten, envelopes were stacked on each other, preventing the administrator from seeing the next envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Matching placebos were manufactured at the University of Basel (100 mg mebendazole matching placebo) or purchased at Fagron, Germany (500 mg mebendazole matching placebo). Trial medications were prepared in advance in identical plastic envelopes labelled with the children's unique treatment identification numbers and sealed. The treatment lasted 3 days and children received tablets at six different time points (mornings and evenings of each of the 3 days). At the first time point, all participants received two tablets: either 500 mg mebendazole plus 100 mg mebendazole matching placebo, or 500 mg mebendazole matching placebo and 100 mg mebendazole; at the remaining Five time points, children only received one tablet: either 100 mg mebendazole or matching placebo, depending to which treatment arm they were allocated." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, caregivers, outcome assessors, and trial statistician were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 (1.0%) participant lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No obvious other source of bias. |
Rossignol 1983.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Follow‐up: 21 days |
|
| Participants |
Country: 11 countries (France, Morocco, Mali, Senegal, Nigeria, Central African Republic, Kenya, Brazil, Peru, Mexico, Philippines) Setting: not reported Number included in study: 1100 Age: 3–79 years (only adult data included) Sex: 525 male, 345 female Inclusion criteria: people harbouring nematodes and cestodes Exclusion criteria: people receiving or who had received anthelmintics during the 21 days before commencing the study, those with an acute illness (with or without fever), pregnant females, nursing mothers, children under 3 years of age, diagnosed epilepsy cases and people with generalized active skin conditions. In general, people who experienced high sensitivity to any drug or were receiving long‐term therapy or having chronic illnesses or proteinuria Follow‐up: 230 (20.9%) Number positive for A lumbricoides: 270 Number included in review: 270 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates and adverse events Outcomes not included in review: anthelmintic efficacy for T trichiura,Strongyloides stercoralis, and hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Smith, Kline & French Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Albendazole or placebo tablets were made available to patients according to randomised numbers under a code established by the manufacturer." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 230 (20.9%) participants lost at follow‐up, and not included in review. |
| Selective reporting (reporting bias) | High risk | Authors described the results of placebo group just for "adult" participants. |
| Other bias | Unclear risk | Smith, Kline & French Laboratories and their area medical directors helped during the multicentre study and provided albendazole 100 mg and placebo tablets. |
Silber 2017.
| Methods |
Design: parallel group randomized trial Duration of study: December 2014 and September 2015 Follow‐up: 17–21 days |
|
| Participants |
Country: Ethiopia and Rwanda Setting: community Number included in study: 295 Age: 28 days to 17 years Sex: 143 boys, 152 women Inclusion criteria: not reported Exclusion criteria: not reported Lost at follow‐up: 17 (5.8%) Number positive for A lumbricoides: 167 Number included in review: 167 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates, adverse events Outcomes not included in review: anthelmintic efficacy for T trichiura, plasma concentration of mebendazole |
|
| Notes |
Diagnostic technique: not reported Funding support: Janssen Research & Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double blind randomized trial." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical chewable tables of mebendazole and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 (5.8%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Project carried out by Janssen Research & Development. |
Speich 2014.
| Methods |
Design: parallel group randomized trial Duration of study: September to November 2012 Follow‐up: 18–23 days |
|
| Participants |
Country: Tanzania Setting: school Number included in study: 480 Inclusion criteria: children who were positive for either T trichiura or hookworm Age: 6–14 years Sex: 247 boys, 233 women Exclusion criteria: children who had any systemic illness (e.g. clinical malaria or hepatosplenic schistosomiasis), as assessed by a medical doctor at the initial clinical assessment Lost at follow‐up: 22 (4.6%) Number positive for A lumbricoides: 309 Number included in review: 143 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rate, pre‐ and post‐treatment GM epg, adverse events Outcomes not included in review: oxantel pamoate, oxantel pamoate + albendazole efficacy for Ascaris, anthelminthic efficacy for T trichiura and hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Medicor Foundation and the Swiss National Science Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomly assigned, with the use of block sizes of four, to receive one of four treatments." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Children, study‐site investigators were unaware of the study‐group assignments. In the first day children were given either oxantel pamoate or identical placebo tablets. On the second day, children were administered two tablets albendazole or placebo table plus mebendazole." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Laboratory technicians were unaware of the treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 (4.6%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No obvious source of other bias. |
Steinmann 2011.
| Methods |
Design: parallel group randomized trial Duration of study: October to December 2008 Follow‐up: 21 days |
|
| Participants |
Country: People's Republic of China Setting: community Number included in study: 378 Age: > 5 years Sex: 163 male, 151 female Inclusion criteria: all residents of Nongyang aged ≥ 5 years Exclusion criteria: presence of diagnosed or perceived chronic disease or other conditions likely to interfere with anthelmintic treatment (e.g. hypersensitivity to anthelmintics), pregnancy (verbally assessed at enrolment and again before treatment), recent history of anthelmintic treatment, and participation in other trials (within 1 month) Lost at follow‐up: 64 (16.9%) Number positive for A lumbricoides: 284 Number included in review: 284 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment AM and GM epg, ERR, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris, hookworm,and Taenia spp, median epg of faeces |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Novartis Foundation, Stanley Thomas Johnson Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All participants were randomly assigned either to the albendazole or the mebendazole arm of the study. In an independent randomization step, single or triple dose treatment using two computer‐generated random sequences of 0 and 1 which were aligned with the list of participants in ascending order of their identification numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For each day of treatment, an envelope of the type locally used to hand out drugs was labelled with the name, identification number, and number of treatment, loaded with the appropriate drugs, and sealed, single or 3 consecutive days." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No placebo drugs were given to individuals assigned to single dose treatment (open label)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessors‐blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64 (16.9%) participants lost at follow‐up and not included in analysis. Balanced lost among groups. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | PS is supported by the Novartis Foundation through a personal stipend. |
Stephenson 1989.
| Methods |
Design: parallel group randomized trial Duration of study: 1989 Follow‐up: 180 days |
|
| Participants |
Country: Kenya Setting: school Number included in study: 17 Age: 6–16 years Sex: 96 boys, 75 girls Inclusion criteria: all available children in the lower grades (Standards I and II) in Mvindeni Primary School in Kwale District, Coast Province, Kenya Exclusion criteria: severe anaemia at stool examination 1 (haemoglobin < 8.0 g/dL) Lost at follow‐up: 21 (12.3%) Number positive for A lumbricoides: 73 Number included in review: 73 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, AM and GM epg Outcomes not included in review: anthropometric measures, anthelmintic efficacy for T trichiura and hookworm |
|
| Notes |
Diagnostic technique: modified Kato‐Katz Funding support: Smith Kline & French Laboratories, Ltd., and the Edna McConnellClark Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the children were allocated at random within sex to albendazole (A) or placebo (PL) groups and treated." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Control group received identical placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examinations were carried out with the same team of worker and were done in a blind fashion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21 (12.3%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Unclear risk | Financial support: Smith Kline & French Laboratories, Ltd. |
Stephenson 1993.
| Methods |
Design: parallel group randomized trial Duration of study: September 1989 to July 1990 Follow‐up: 108 days |
|
| Participants |
Country: Kenya Setting: school Number included in study: 328 Age: mean 10.5 years Sex: not reported Inclusion criteria: all available children in the lower grades (Standards I–V) in Mvindeni Primary School in Kwale District, Coast Province, Kenya Exclusion criteria: children with heavy hookworm egg counts (> 20,000 epg of faeces) at examination 1 or 2 Lost at follow‐up: 44 (13.4%) Number positive for A lumbricoides: 89 Number included in review: 89 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, AM and GM epg Group 1 and 2 analysed together in review after the first round of treatment Outcomes not included in review: outcomes after the second round of treatment, anthropometric measures, anthelmintic efficacy for T trichiura and hookworm |
|
| Notes |
Diagnostic technique: modified Kato‐Katz Funding support: supported in part by Thrasher Research Fund and SmithKline Beecham, Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At exam 1, children were allocated at random within sex by descending hookworm egg count to placebo one dose or two dose groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examinations were carried out with the same team of workers and were done in a blind fashion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 (13.4%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Unclear risk | Quote: "Supported in part by Thrasher Research Fund and SmithKline Beecham, Ltd." |
Watkins 1996a.
| Methods |
Design: parallel group randomized trial Duration of study: February to October 1993 Follow‐up: 14 days |
|
| Participants |
Country: Republic of Guatemala Setting: school Number included in study: 246 Age: ≤ 12 years(mean 9.8 years) Sex: not reported Inclusion criteria: girls and boys aged ≤ 12 years who had not taken any deworming medicine in the past year Exclusion criteria: pregnancy Lost at follow‐up: 22 (8.8%) Number positive for A lumbricoides: 209 Number included in review: 209 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris cure rates and pre‐ and post‐treatment AM and GM epg Outcomes not included in review: anthropometric measures, anthelmintic efficacy for Trichuris |
|
| Notes |
Diagnostic technique: modified Kato‐Katz Funding support: Pew Charitable Trusts, the US Agency for International Development University Development and Linkage Program, the Children's Miracle Network Telethon, and the ARCS Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were stratified by sex and age and then randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The children and field workers were unaware of treatment group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Although the 2‐week posttreatment egg examination made it clear to the study director, which treatment was which, this information was not communicated to the field workers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 (8.8%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No obvious source of other bias. |
Wen 2008.
| Methods |
Design: parallel group randomized trial Duration of study: not reported Follow‐up: 30 days |
|
| Participants |
Country: People's Republic of China Setting: community (multicentre) Age: 6–70 years Sex: 79 male, 125 female Number included in study: 816 Inclusion criteria: faecal egg‐positive farmers and children > 6 years of age from rural areas Exclusion criteria: other diseases such as hepatic, renal, and cardiovascular diseases; and pregnant or lactating women Follow‐up: 0 (0%) Number positive for A lumbricoides: 204 Number included in review: 204 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, pre‐ and post‐treatment AM epg, ERR, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris, hookworm, and Enterobius vermicularis |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "individuals confirmed with intestinal nematode infections were chosen and stratified by age, sex, and intensity of the infection, and then were randomly assigned into treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment drugs had different appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No obvious source of other bias. |
Wimmersberger 2018.
| Methods |
Design: parallel group randomized trial Follow‐up: 21 days |
|
| Participants |
Country: République de Côte d'Ivoire Setting: rural school from 7 village Age: 2–12 years Sex: 46% girls Number included in study: 302 Inclusion criteria: children with T trichiura infection intensities of > 60 epg of stool in preschool aged and 100 epg in school age Exclusion criteria: children with any systemic illness (e.g. symptomatic malaria, severe anaemia defined as haemoglobin < 70 g/L in preschool‐aged and < 80 g/L in school‐aged children, underwent any anthelminthic treatment within the past 4 weeks, or were allergic to ivermectin Follow‐up: 10 (3.3%) Number positive for A lumbricoides: 79 Number included in review: 79 |
|
| Interventions |
Treatment strategy: screening and treat all included participants Children aged 2–5 years
Children aged 6–12 years
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, GM epg, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris, hookworm, andS mansoni |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Bill and Melinda Gates Foundation (Grant number: OPP1153928) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using a computer‐generated code with varying random blocks sizes of four or eight for school aged children and of three or six for pre school aged children stratified by their baseline infection intensities (light or moderate plus heavy infection according to WHO guidelines." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Study‐site investigators were aware of the study‐group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "laboratory technicians were blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (3.3%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No obvious source of other bias. |
Yap 2013.
| Methods |
Design: parallel group randomized trial Follow‐up: 30 days |
|
| Participants |
Country: People's Republic of China Setting: school Age: 9–12 years Sex: not reported Number included in study: 211 Inclusion criteria: submission of 2 stool samples at baseline; detection of ≥ 1 soil‐transmitted helminth egg in the samples; no major systemic illnesses as determined by a medical doctor from the Bulangshan township hospital; no known allergy to albendazole; no deworming treatment over the previous 6 months; no participation in other clinical trials; and residency in the study area for ≥ 1 year before enrolment, as assessed by a parental questionnaire Exclusion criteria: not reported Follow‐up: 17 (8.1%) Number positive for A lumbricoides: 181 Number included in review: 181 |
|
| Interventions |
Treatment strategy: screening and treat all included participants
|
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates, AM epg, adverse events Outcomes not included in review: anthelmintic efficacy for Trichuris, hookworm, and reinfection rates 4 and 6 months after treatment |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: Swiss Tropical and Public Health Institute in Basel, and the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention in Shanghai |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was determined by a statistician using block randomization with randomly varying block sizes of 2, 4, and 6 to ensure that both treatment arms had similar sample sizes." |
| Allocation concealment (selection bias) | Low risk | Quote: "The assigned random number for each child corresponded to the treatment number on the sealed envelope and thus, determined the type of treatment to be allocated to the child." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Triple‐dose non flavoured albendazole (400 mg) and placebo treatments (tablets matched in colour, size, taste, and shape) were prepared by staff not involved in the field work, in sealed envelopes marked with unique identifiers." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 (8.1%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No obvious source of other bias. |
Zani 2004.
| Methods |
Design: parallel group randomized trial Duration of study: March 2001 to March 2002 Follow‐up: 30 days |
|
| Participants |
Country: Brazil Setting: community Number included in study: 151 Age: 2–82 years Sex: not reported Inclusion criteria: people positive for A lumbricoides,T trichiura, or hookworms Exclusion criteria: pregnant or lactating women, children aged < 2 years, and people with intense infection involving expulsion of worms through the mouth and faeces, did not receive any medication Number positive for A lumbricoides: 83 Number included in review: 83 |
|
| Interventions |
Treatment strategy: screened, randomized and treated all included participants
Participants positive for S mansoni were treated with praziquantel (60 mg/kg, Farmanguinhos/Fiocruz, 1 week after the treatment for helminthiasis) |
|
| Outcomes |
Outcomes included:Ascaris prevalence pre‐ and post‐treatment, cure rates Outcomes not included in review: anthelmintic efficacy for Trichuris, hookworm |
|
| Notes |
Diagnostic technique: Kato‐Katz Funding support: World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each group was randomly assigned to treatment with one or the other anthelmintic drug." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 (21.2%) participants lost at follow‐up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No obvious source of other bias. |
A lumbricoides:Ascaris lumbricoides; AM: arithmetic mean; epg: eggs per gram; ERR: egg reduction rate; GM: geometric mean; n: number of participants; S mansoni: Schistosoma mansoni; T trichiura:Trichuris trichiura;W bancrofti: Wuchereria bancrofti.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adriko 2018 | Not an RCT. |
| Al‐Mekhlafi 2014 | All participants received albendazole. |
| Alavi Majd 2014 | Systematic review. |
| Albonico 1995 | Study derived from Albonico 1994, participants already included. |
| Albonico 1999 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Albonico 2013 | All participants received albendazole. |
| Amato 1983 | Not an RCT. |
| Anto 2019 | Comparison group received albendazole or mebendazole with levamisole that was not of interest for this review. |
| Awasthi 2000 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Awasthi 2001 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Bartoloni 1993 | It was not possible to know how many participants were treated |
| Bassily 1984 | Not an RCT. |
| Behnke 1994 | Not an RCT. |
| Belew 2015 | All participants received albendazole. |
| Bell 1971 | Study compared pyrantel pamoate, piperazine phosphate, and placebo that were not of interest for this review. |
| Boivin 1993 | Ascaris lumbricoides prevalence not reported after treatment. |
| Brutus 2006 | Intervention group received levamisole that was not of interest for this review. |
| Brutus 2007 | Intervention group received levamisole that was not of interest for this review. |
| Campbell 2014 | Systematic review. |
| Carmona‐Fonseca 2015 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Cervoni 1971 | Not an RCT. |
| Clarke 2016 | Systematic review. |
| Cleary 2007 | Not an RCT. |
| Coulaud 1984 | Not an RCT. |
| Coulibaly 2018 | Not an RCT. |
| De Guimaraes 2001 | Comparison group received placebo that was not of interest for this review. |
| Donnen 1998 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Dossa 2001 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Ebenezer 2013 | Control group did not receive the same cointervention. |
| Edelduok 2013 | Not an RCT. |
| El‐Masry 1983 | Not an RCT. |
| Farahmandian 1972 | Not an RCT. |
| Farahmandian 1977 | Study compared pyrantel pamoate, levamisole, thiabendazole, and bephenium hydroxynaphthoate that were not of interest for this review. |
| Fernandes 1981 | All participants received the same dose of different formulations of mebendazole. |
| Freeman 2013 | Intervention was not the anthelminthic treatment. |
| Friis 2003 | No outcome of interest for this review. |
| Gilgen 2001 | No outcome of interest for this review. |
| Gizaw 2019 | Intervention was not the anthelminthic treatment. |
| Goodwin 1954 | Not an RCT. |
| Goodwin 1958 | Not an RCT. |
| Greemberg 1981 | Comparison group treated with piperazine citrate that was not of interest for this review. |
| Gupta 1982 | Ascaris lumbricoides prevalence reported after 2 treatment rounds. |
| Gutierrez 1986 | Comparison group received pyrantel oxantel that was not of interest for this review. |
| Gyorkos 2013b | Intervention was not anthelmintic treatment. |
| Gyorkos 2013a | All participants received albendazole. |
| Hadidjaja 1998 | Cluster‐RCT with no comparable groups at baseline. |
| Hadju 1996 | Comparison group received pyrantel pamoate that was not of interest for this review. |
| Hall 1994 | Ascaris lumbricoides prevalence not reported for each intervention group. |
| Hatchuel 1973 | Not an RCT. |
| Hoang 1993 | Comparison group received pyrantel analogue that was not of interest for this review. |
| Holland 1996 | Intervention group received levamisole that was not of interest for this review. |
| Hurlimann 2018 | Intervention was not the anthelminthic treatment. |
| Islam 1976 | Comparison group received pyrantel pamoate that was not of interest for this review. |
| Jalal 1998 | Comparison groups received different cointerventions. |
| Jancloes 1979 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Jinabhai 2001 | Treatment efficacy reported of a subsample of participants. |
| Joseph 2015 | Intervention groups received anthelmintic treatment at different follow‐up times. |
| Kale 1981 | Intervention groups received pyrantel pamoate and piperazine citrate + bephenium hydroxynaphthoate that were not of interest for this review. |
| Kale 1982 | Intervention groups received different doses of pyrantel pamoate that was not of interest for this review. |
| Karyadi 1996 | Not an RCT. |
| Kepha 2017 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Kirwan 2009 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Kirwan 2010 | No outcomes of interest for this review. |
| Kugo 2018 | Intervention group received carica papaya fruit seeds that were not of interest for this review. |
| Lai 1995 | Intervention groups received pyrantel pamoate that was not of interest for this review. |
| Le Huong 2007 | Ascaris lumbricoides prevalence reported after 2 treatment rounds. |
| Lechat 1974 | Intervention groups received levamisole that was not of interest for this review. |
| Lionel 1969 | Study compared levamisole to piperazine that were not of interest for this review. |
| Lynch 1997 | Not outcomes of interest for this review. |
| Maipanich 1997 | Not an RCT. |
| Mani 2002 | Not an RCT. |
| Marti 1996 | Ascaris lumbricoides pretreatment prevalence were not reported by each intervention group. |
| Martin 2018 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Mascie‐Taylor 1999 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Means 2018 | Not a primary study. |
| Meyrowitsch 2001 | Not an RCT. |
| Miller 1978 | Not an RCT. |
| Moens 1978 | Review. |
| Moser 2017a | No outcome of interest for this review. |
| Muchiri 2001 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Murray 1978 | Not an RCT. |
| Naquira 1989 | Not an RCT. |
| Ndibazza 2010 | Deworming during pregnancy. |
| Ndibazza 2012 | Deworming during pregnancy. |
| Ndyomugyenyi 2008 | Deworming during pregnancy. |
| Newlove 2011 | No outcomes of interest for this review. |
| Nokes 1999 | It was not possible to know the number of participants in each intervention groups. |
| Nontasut 1997 | Not an RCT. |
| Northrop‐Clewes 2001 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Okoyo 2016 | Not an RCT. |
| Pene 1982 | Ascaris lumbricoides prevalence after treatment not reported in placebo group. |
| Persson 2001 | No outcome of interest for this review. |
| Pickering 2019 | Intervention was not anthelmintic. |
| Pond 1970 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Pullan 2019 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Rahman 1996 | Ascaris lumbricoides prevalence not reported for each intervention group. |
| Rahman 1998 | Not an RCT. |
| Raj 1998 | Not an RCT. |
| Reddy 1986 | Intervention group received L‐tetramisole that was not of interest for this review. |
| Restrepo 1987 | Ascaris lumbricoides pretreatment prevalence not reported. |
| Rousham 1994 | Not an RCT. |
| Sarmah 1988 | Comparison group received pyrantel that was not of interest for this review. |
| Seftel 1968 | Intervention groups received piperazine or tetramisole that were not of interest for this review. |
| Seo 1980 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Sileshi 2017 | All participants received the same anthelmintic. |
| Sinniah 1981 | Intervention groups received pyrantel pamoate, oxantel pyrantel, and levamisole that were not of interest for this review. |
| Sood 1975 | Intervention groups received pyrantel and tetramisole that were not of interest for this review. |
| Speich 2016 | Follow‐up study of Speich 2014; participants already included in this review. |
| Staal 2018 | Not an RCT. |
| Steinmann 2008 | Intervention groups received pyrantel and tribendimidine that were not of interest for this review. |
| Stephenson 1990 | RCT derived from Stephenson 1989. Participants already included in the review. |
| Stoltzfus 1997 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Stoltzfus 2001 | Ascaris lumbricoides prevalence reported after multiple treatment rounds. |
| Supali 2017 | Not an RCT. |
| Tankhiwale 1989 | Comparison group received pyrantel that was not of interest for this review. |
| Tanumihardjo 2004 | All participants received albendazole. |
| Taylor 2001 | Ascaris lumbricoides prevalence reported after 2 anthelmintic rounds. |
| Thein‐Hlaing 1991a | Ascaris lumbricoides prevalence reported after 3 anthelmintic rounds. |
| Thein‐Hlaing 1991b | No outcome of interest for this review. |
| Urjel 1985 | Not an RCT. |
| Vaz Nery 2019 | All participants received albendazole. |
| Walson 2008 | No outcome of interest for this review. |
| Walson 2010 | No outcome of interest for this review. |
| Wang 1987 | Not an RCT. |
| Watkins 1996b | Participants already included in Watkins 1996a. |
| Wesche 1994 | Intervention groups received pyrantel pamoate that was not of interest for this review. |
| Whitworth 1991 | Ascaris lumbricoides prevalence reported after multiple anthelmintic rounds. |
| Willett 1979 | Intervention groups received levamisole that was not of interest for this review. |
| Williams 1997 | Not an RCT. |
| Wright 2009 | Ascaris lumbricoides prevalence reported after 3 anthelmintic rounds. |
| Yangco 1981 | Not an RCT. |
| Yap 2014 | Participants already included in this review. |
RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT02636803.
| Trial name or title | An open comparative study of the efficacy of different doses of oxfendazole compared to single dose albendazole in the treatment of Trichuris trichiura infection in adults |
| Methods | Randomized clinical trial |
| Participants |
Estimated enrolment: 200 Age: 16–65 years Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | November 2017 |
| Contact information | Robert H Gilman, rgilman@johnshopkins.edu |
| Notes | Katz test 21 days following treatment |
TCTR20190111001.
| Trial name or title | Assessment of efficacy of anthelmintic drugs against soil‐transmitted helminths in Thailand |
| Methods | Randomized trial (TCTR20190111001) |
| Participants |
Number of participants: 252 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Cure rate 3 weeks after treatment Egg reduction rate Modified Kato‐Katz thick smear, Harada‐mori |
| Starting date | Not reported |
| Contact information | Vivornpun Sanprasert Department of Parasitology, Faculty of Medicine, Chulalongkorn University 1873 Rama IV Road, Pathumwa, Bangkok 10330, Thailand |
| Notes |
Funding: Department of Parasitology, Faculty of Medicine, Chulalongkorn University 1873 Rama IV Road, Pathumwa, Bangkok, 10330, Thailand +6622564387 17; vivornpun@chula.md |
Differences between protocol and review
We defined better faecal egg count (FEC) and included the egg reduction rate (ERR) as comparison between pre‐ and post‐treatment eggs count.
We decided to include only the main drugs currently used (albendazole, mebendazole, ivermectin, and nitazoxanide) and excluded the studies or participants who received levamisole, pyrantel oxantel, or piperazine.
We excluded trials that evaluated anthelmintic treatment only for pregnant women because that it was subject of another review (Salam 2015).
We excluded studies when the parasitological cure after the first treatment was not reported, or those that compared different deworming programmes, as it was subject of another review (Taylor‐Robinson 2019)
We excluded the studies or outcomes when they were reported only in graphic form.
The author team has changed since protocol publication: Marcos Vinicius Fernandes and Garcia and Natália Sayuri Mukai participated in the protocol. Lucieni Oliveira Conterno, Marilia Dalva Turchi, Ione Corrêa, and Ricardo AMB Almeida carried out the review.
Contributions of authors
LOC conceived, designed, and co‐ordinated the review, extracted data, assessed the risk of bias, and analyzed and interpreted data. She wrote and edited the review and is the guarantor of the review.
MDT updated the background, extracted data, assessed the risk of bias, analyzed and interpreted data. She wrote and edited the review and approved the final review prior to submission.
IC extracted data, assessed the risk of bias, and approved the final review prior to submission.
RAMBA extracted data, assessed the risk of bias, checked the quality of the data extraction, and analyzed and interpreted data. He wrote and edited the review and approved the final review prior to submission.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
Marília Medical School – FAMEMA, Brazil.
Institute of Tropical Pathology and Public Health – Federal University of Goias, Brazil.
Universidade Estadual Paulista (UNESP), Faculdade de Medicina de Botucatu, Botucatu, Brazil.
External sources
-
Department for International Development (DFID), UK.
Project number 300342‐104
-
Fundação de Amparo à Pesquisa do Estado de São Paulo ‐ FAPESP, Brazil.
Lucieni Oliveira Conterno received grant support from FAPESP for protocol development
-
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Turchi MD has a research scholarship from the National Council of Technological and Scientific Development (CNPq) and is a member of the Institute for Health Technology Assessment – Brazil (IATS)
Declarations of interest
LOC has no known conflicts of interest.
MDT has no known conflicts of interest.
IC has no known conflicts of interest.
RAMBA has no known conflicts of interest.
New
References
References to studies included in this review
Adams 1994 {published data only}
- Adams EJ, Stephenson LS, Latham MC, Kinoti S. Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. Journal of Nutrition 1994;124(8):1199‐206. [DOI] [PubMed] [Google Scholar]
Adams 2004 {published data only}
- Adams VJ, Lombard CJ, Dhansay MA, Markus MB, Fincham JE. Efficacy of albendazole against the whipworm Trichuris trichiura – a randomized, controlled trial. South African Medical Journal 2004;94(12):972‐6. [PubMed] [Google Scholar]
Adegnika 2014 {published data only}
- Adegnika AA, Zinsou JF, Issifou S, Ateba‐Ngoa U, Kassa KF, Feuga LN, et al. Randomized, controlled, assessor‐blind clinical trial to assess the efficacy of single‐ versus repeated‐dose albendazole to treat Ascaris lumbricoides, Trichuris trichiura, and Hookworm Infection. Antimicrobial Agents and Chemotherapy 2014;58(5):2535‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albonico 1994 {published data only}
- Albonico M, Smith PG, Hall A, Chwaya HM, Alawi KS, Savioli L. A randomized controlled trial comparing mebendazole and albendazole against Ascaris, Trichuris and hookworm infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(5):585‐9. [DOI] [PubMed] [Google Scholar]
Albonico 2002 {published data only}
- Albonico M, Bickle Q, Haji HJ, Ramsan M, Khatib KJ, Montresor A, et al. Evaluation of the efficacy of pyrantel‐oxantel for the treatment of soil‐transmitted nematode infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(6):685‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albonico 2003 {published data only}
- Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bulletin of the World Health Organization 2003;81(5):343‐52. [PMC free article] [PubMed] [Google Scholar]
Beach 1999 {published data only}
- Beach MJ, Streit TG, Addiss DG, Prospere R, Roberts JM, Lammie PJ. Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian schoolchildren. American Journal of Tropical Medicine and Hygiene 1999;60(3):479‐86. [DOI] [PubMed] [Google Scholar]
Belizario 2003 {published data only}
- Belizario VY, Amarillo ME, Leon WU, Reyes AE, Bugayong MG, Macatangay BJ. A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bulletin of the World Health Organization 2003;81(1):35‐43. [PMC free article] [PubMed] [Google Scholar]
Fox 2005 {published data only}
- Fox LM, Furness BW, Haser JK, Desire D, Brissau JM, Milord MD, et al. Tolerance and efficacy of combined diethylcarbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. Bulletin of the World Health Organization 2005;73(1):115‐21. [PubMed] [Google Scholar]
Hadju 1997 {published data only}
- Hadju V, Satriono, Abadi K, Stephenson LS. Relationships between soil‐transmitted helminthiases and growth in urban slum schoolchildren in Ujung Pandang Indonesia. International Journal of Food Sciences and Nutrition 1997;48:85‐93. [DOI] [PubMed] [Google Scholar]
Haque 2010 {published data only}
- Haque R, Ahmed T, Wahed MA, Mondal D, Rahman AS, Albert MJ. Low‐dose beta‐carotene supplementation and deworming improve serum vitamin A and beta‐carotene concentrations in preschool children of Bangladesh. Journal of Health, Population, and Nutrition 2010;28(3):230‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jongsuksuntigul 1993 {published data only}
- Jongsuksuntigul P, Jeradit C, Pornpattanakul S, Charanasri U. A comparative study on the efficacy of albendazole and mebendazole in the treatment of ascariasis, hookworm infection and trichuriasis. Southeast Asian Journal of Tropical Medicine and Public Health 1993;24(4):724‐9. [PubMed] [Google Scholar]
Knopp 2010 {published data only}
- Knopp S, Mohammed KA, Speich B, Hattendorf J, Khamis S, Khamis AN, et al. Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial. Clinical Infectious Diseases 2010;51(12):1420‐8. [DOI] [PubMed] [Google Scholar]
Legesse 2002 {published data only}
- Legesse M, Erko B, Medhin G. Efficacy of albendazole and mebendazole in the treatment of Ascaris and Trichuris infections. Ethiopian Medical Journal 2002;40(4):335‐43. [PubMed] [Google Scholar]
Legesse 2004 {published data only}
- Legesse M, Erko B, Medhin G. Comparative efficacy of albendazole and three brands of mebendazole in the treatment of ascariasis and trichuriasis. East African Medical Journal 2004;81(3):134‐8. [DOI] [PubMed] [Google Scholar]
Lubis 2012 {published data only}
- Lubis IN, Pasaribu S, Lubis CP. Current status of the efficacy and effectiveness of albendazole and mebendazole for the treatment of Ascaris lumbricoides in North‐Western Indonesia. Asian Pacific Journal of Tropical Medicine 2012;2012(8):605‐9. [DOI] [PubMed] [Google Scholar]
Nokes 1992 {published data only}
- Nokes C, Grantham‐McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology 1992;104:539‐47. [DOI] [PubMed] [Google Scholar]
Ortiz 2002 {published data only}
- Ortiz JJ, Chegne NL, Gargala G, Favennec L. Comparative clinical studies of nitazoxanide, albendazole and praziquantel in the treatment of ascariasis, trichuriasis and hymenolepiasis in children from Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(2):193‐6. [DOI] [PubMed] [Google Scholar]
Palmeirim 2018a {published data only}
- Palmeirim MS, Ame SM, Ali SM, Hattendorf J, Keiser J. Efficacy and safety of a single dose versus a multiple dose regimen of mebendazole against hookworm infections in children: a randomised, double‐blind trial. EClinical Medicine 2018;1:7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 1983 {published data only}
- Rossignol JF, Maisonneuve H. Albendazole: placebo controlled study in 870 patients with intestinal helminthiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983;77(5):707‐11. [DOI] [PubMed] [Google Scholar]
Silber 2017 {published data only}
- Silber SA, Diro E, Workneh N, Mekonnen Z, Levecke B, Steinmann P, et al. Efficacy and safety of a single‐dose mebendazole 500 mg chewable, rapidly‐disintegrating tablet for Ascaris lumbricoides and Trichuris trichiura infection treatment in pediatric patients: a double‐blind, randomized, placebo‐controlled, phase 3. American Journal of Tropical Medicine and Hygiene 2017;97(6):1851‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speich 2014 {published data only}
- Speich B, Ame SM, Ali SM, Alles R, Huwyler J, Hattendorf J, et al. Oxantel pamoate–albendazole for Trichuris trichiura infection. New England Journal of Medicine 2014;370(7):610‐20. [DOI] [PubMed] [Google Scholar]
Steinmann 2011 {published data only}
- Steinmann P, Utzinger J, Du ZW, Jiang JY, Chen JX, Hattendorf J, et al. Efficacy of single‐dose and triple‐dose albendazole and mebendazole against soil‐transmitted helminths and Taenia spp.: a randomised controlled trial. PloS One 2011;6(9):e25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 1989 {published data only}
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Brigham H. Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. American Journal of Tropical Medicine and Hygiene 1989;41(1):78‐87. [PubMed] [Google Scholar]
Stephenson 1993 {published data only}
- Stephenson LS, Latham MD, Adams EJ, Perte A. Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once‐ or twice‐yearly treatment with albendazole. American Institute of Nutrition 1993;123(4):656‐65. [DOI] [PubMed] [Google Scholar]
Watkins 1996a {published data only}
- Watkins WE, Pollit E. Effect of removing Ascaris on the growth of Guatemalan schoolchildren. Pediatrics 1996;97(6):871‐6. [PubMed] [Google Scholar]
Wen 2008 {published data only}
- Wen LY, Yan XL, Sun FH, Fang YY, Yang MJ, Lou LJ. A randomized, double‐blind, multicenter clinical trial on the efficacy of ivermectin against intestinal nematode infections in China. Acta Tropica 2008;103(8):190‐4. [DOI] [PubMed] [Google Scholar]
Wimmersberger 2018 {published data only}
- Wimmersberger D, Coulibaly JT, Schulz J, Puchkow M, JörgHuwyler J, N'Gbesso Y, et al. Efficacy and safety of ivermectin against Trichuris trichiura in preschool‐ and school‐aged children: a randomized controlled dose‐finding trial. Clinical Infectious Diseases 2018;67(8):1247‐55. [DOI] [PubMed] [Google Scholar]
Yap 2013 {published data only}
- Yap P, Du ZW, Wu FW, Jiang JY, Chen R, Zhou XN, et al. Rapid re‐infection with soil‐transmitted helminths after triple‐dose albendazole treatment of school‐aged children in Yunnan, People's Republic of China. American Journal of Tropical Medicine and Hygiene 2013;89(1):23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zani 2004 {published data only}
- Zani LC, Favre TC, Piere OS, Barbosa CS. Impact of antihelminthic treatment on infection by Ascaris lumbricoides, Trichuris trichiura e Hookworm in Covas, a rural community of Pernambuco Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2004;46(2):63‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adriko 2018 {published data only}
- Adriko M, Tinkitina B, Arinaitwe M, Kabatereine NB, Nanyunja M, Tukahebwa E. Impact of a national deworming campaign on the prevalence of soil‐transmitted helminthiasis in Uganda (2004‐2016): implications for national control programs. PLoS Neglected Tropical Diseases 2018;12(7):e0006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alavi Majd 2014 {published data only}
- Alavi Majd H, Najafi Ghobadi K, Akbarzadeh Baghban A, Ahmadi N, Sajjadi E. Detecting and accommodating outliers in meta‐analysis for evaluating effect of albendazole on ascaris lumbricoides Infection. Iranian Red Crescent Medical Journal 2014;16(5):e17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albonico 1995 {published data only}
- Albonico M, Smith PG, Ercole E, Hall A, Chwaya HM, Alawi KS, et al. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(5):538‐41. [DOI] [PubMed] [Google Scholar]
Albonico 1999 {published data only}
- Albonico M, Stoltzfus RJ, Savioli L, Chwaya HM, d'Harcourt E, Tielsch JM. A controlled evaluation of two school‐based anthelminthic chemotherapy regimens on intensity of intestinal helminth infections. International Journal of Epidemiology 1999;28(3):591‐6. [DOI] [PubMed] [Google Scholar]
Albonico 2013 {published data only}
- Albonico M, Rinaldi L, Sciascia S, Morgoglione ME, Piemonte M, Maurelli MP, et al. Comparison of three copromicroscopic methods to assess albendazole efficacy against soil‐transmitted helminth infections in school‐aged children on Pemba Island. Transaction of Real Society of Tropical Medicine Hygiene 2013;107(8):493‐501. [DOI] [PubMed] [Google Scholar]
Al‐Mekhlafi 2014 {published data only}
- Al‐Mekhlafi HM, Anuar TS, Al‐Zabedi EM, Al‐Maktari M, T Mahdy M, Ahmed A, et al. Does vitamin A supplementation protect schoolchildren from acquiring soil‐transmitted helminthiasis? A randomized controlled trial. Parasites & Vectors 2014;7:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amato 1983 {published data only}
- Amato NV, Moreira AA, Campos R, Lazzaro ES, Chiaramelli MC, Pinto PL, et al. Tratamento da ancilostomiase, ascaridiase e tricocefaliase por meio do albendazol ou do mebendazol. Revista do Instituto de Medicina Tropical de São Paulo 1983;25(6):294‐9. [PubMed] [Google Scholar]
Anto 2019 {published data only}
- Anto EJ, Nugraha SE. Efficacy of albendazole and mebendazole with or without levamisole for ascariasis and trichuriasis. Open Access Macedonian Journal of Medical Sciences 2019;7(8):1299‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Awasthi 2000 {published data only}
- Awasthi S, Pande VK, Fletcher RH. Effectiveness and cost‐effectiveness of albendazole in improving nutritional status of pre‐school children in urban slums. Indian Pediatrics 2000;37(1):19‐29. [PubMed] [Google Scholar]
Awasthi 2001 {published data only}
- Awasthi S, Pande VK. Six‐monthly de‐worming in infants to study effects on growth. Indian Journal of Pediatrics 2001;68(9):823‐7. [DOI] [PubMed] [Google Scholar]
Bartoloni 1993 {published data only}
- Bartoloni A, Guglielmetti P, Cancrini G, Gamboa H, Roselli M, Nicoletti A, et al. Comparative efficacy of a single 400 mg dose of albendazole or mebendazole in the treatment of nematode infections in children. Tropical and Geographical Medicine 1993;45(3):114‐6. [PubMed] [Google Scholar]
Bassily 1984 {published data only}
- Bassily S, El‐Masry NA, Trabolsi B, Farid Z. Treatment of ancylostomiasis and ascariasis with albendazole. Annals of Tropical Medicine and Parasitology 1984;78(1):81‐2. [DOI] [PubMed] [Google Scholar]
Behnke 1994 {published data only}
- Behnke JM, Pritchard DI, Wakelin D, Park JR, McNicholas AM, Gilbert FS. Effect of ivermectin on infection with gastro‐intestinal nematodes in Sierra Leone. Journal of Helminthology 1994;68(3):187‐95. [DOI] [PubMed] [Google Scholar]
Belew 2015 {published data only}
- Belew S, Getachew M, Suleman S, Mohammed T, Deti H, D'Hondt M, et al. Efficacy and quality of two albendazole brands commonly used against soil‐transmitted helminth infections in school children in Jimma Town, Ethiopia. PLoS Neglected Tropical Diseases 2015;9(9):e0004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1971 {published data only}
- Bell WJ, Nassif S. Comparison of pyrantel pamoate and piperazine phosphate in the treatment of ascariasis. American Journal of Tropical Medicine and Hygiene 1971;20(4):584‐8. [DOI] [PubMed] [Google Scholar]
Boivin 1993 {published data only}
- Boivim MJ, Giordani B. Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. Journal of Pediatric Psychology 1993;18(2):249‐64. [DOI] [PubMed] [Google Scholar]
Brutus 2006 {published data only}
- Brutus L, Watier L, Briand V, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Parasitic co‐infections: does Ascaris lumbricoides protect against Plasmodium falciparum infection?. American Journal of Tropical Medicine and Hygiene 2006;75(2):194‐8. [PubMed] [Google Scholar]
Brutus 2007 {published data only}
- Brutus L, Watier L, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Confirmation of the protective effect of Ascaris lumbricoides on Plasmodium falciparum infection: results of a randomized trial in Madagascar. American Journal of Tropical Medicine and Hygiene 2007;77(6):1091‐5. [PubMed] [Google Scholar]
Campbell 2014 {published data only}
- Campbell SJ, Savage GB, Gray DJ, Atkinson JA, Soares Magalhães RJ, Nery SV, et al. Water, sanitation, and hygiene (WASH): a critical component for sustainable soil‐transmitted helminth and schistosomiasis control. PLoS Neglected Tropical Diseases 2014;8(4):e2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carmona‐Fonseca 2015 {published data only}
- Carmona‐Fonseca J, Correa‐Botero A. Effect of albendazole and vitamin A treatment on intestinal helminths and anaemia in children from Urabá (Antioquia, Colombia) [Effecto del albendazol y la vitamina A periódicos sobre helmintos intestinales y anemia em niños del Urabá Antioqueño (Colombia)]. Biosalud 2015;14(1):9‐25. [Google Scholar]
Cervoni 1971 {published data only}
- Cervoni WA, Oliver‐Gonzçalez J. Clinical evaluation of pyrantel pamoate in helminthiasis. American Journal of Tropical Medicine and Hygiene 1971;20(4):589‐91. [DOI] [PubMed] [Google Scholar]
Clarke 2016 {published data only}
- Clarke NE, Clements AC, Doi SA, Wang D, Campbell SJ, Gray D, et al. Differential effect of mass deworming and targeted deworming for soil‐transmitted helminth control in children: a systematic review and meta‐analysis. Lancet 2016;389(10066):287‐97. [DOI] [PubMed] [Google Scholar]
Cleary 2007 {published data only}
- Cleary JD, Graham D, Lushbaugh WB, Nolan RL, Chapman SW. Single low‐dose mebendazole administered quarterly for ascaris treatment. American Journal of Medical Science 2007;333(6):340‐5. [DOI] [PubMed] [Google Scholar]
Coulaud 1984 {published data only}
- Coulaud JP, Rossignol JF. Albendazole: a new single dose anthelmintic‐study in 1455 patients. Acta Tropica 1984;41:87‐90. [PubMed] [Google Scholar]
Coulibaly 2018 {published data only}
- Coulibaly G, Ouattara M, Dongo K, Hürlimann E, Bassa FK, Koné N, et al. Epidemiology of intestinal parasite infections in three departments of south‐central Côte d'Ivoire before the implementation of a cluster‐randomised trial. Parasite Epidemiology Control 2018;3(2):63‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Guimaraes 2001 {published data only}
- Guimaraes DL, Llanos RS, Acevedo JH. Ascariasis: comparison of the therapeutic efficacy between paico and albendazole in children from Huaraz [Ascaridiasis: comparación de la eficacia terapeutica entre paico y albendazol en niños de Huaraz]. Revista de Gastroenterología del Perú 2001;21(3):212‐19. [PubMed] [Google Scholar]
Donnen 1998 {published data only}
- Donnen P, Brasseur D, Dramaix M, Vertongen F, Zihindula M, Muhamiriza M, et al. Vitamin A supplementation but not deworming improves growth of malnourished preschool children in eastern Zaire. Journal of Nutrition 1998;128(8):1320‐7. [DOI] [PubMed] [Google Scholar]
Dossa 2001 {published data only}
- Dossa RA, Ategbo EA, Koning FL, Raaij JM, Hautvast JG. Impact of iron supplementation and deworming on growth performance in preschool Beninese children. European Journal of Clinical Nutrition 2001;55:223‐8. [DOI] [PubMed] [Google Scholar]
Ebenezer 2013 {published data only}
- Ebenezer R, Gunawardena K, Kumarendran B, Pathmeswaran A, Jukes MC, Drake LJ, et al. Cluster‐randomised trial of the impact of school‐based deworming and iron supplementation on the cognitive abilities of schoolchildren in Sri Lanka's plantation sector. Tropical Medicine & International Health 2013;18(8):942‐51. [DOI] [PubMed] [Google Scholar]
Edelduok 2013 {published data only}
- Edelduok EG, Eke FN, Evelyn NE, Atama CI, Eyo JE. Efficacy of a single dose albendazole chemotherapy on human intestinal helminthiasis among school children in selected rural tropical communities. Annals of Tropical Medicine and Public Heath 2013;6(4):413‐7. [Google Scholar]
El‐Masry 1983 {published data only}
- El‐Masry NA, Trabolsi B, Bassily S, Farid Z. Albendazole in the treatment of Ancylostoma duodenale and Ascaris lumbricoides infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983;77(2):160‐1. [DOI] [PubMed] [Google Scholar]
Farahmandian 1972 {published data only}
- Farahmandian I, Sahba GH, Arfaa F, Jalali H. A comparative evaluation of the therapeutic effect of pyrantel pamoate and bephenium hydroxynaphthoate on Ancylostoma duodenale and other intestinal helminths. Journal of Tropical Medicine and Hygiene 1972;75(10):205‐7. [PubMed] [Google Scholar]
Farahmandian 1977 {published data only}
- Farahmandian I, Arfaa F, Jalali H, Reza M. Comparative studies on the evaluation of the effect of new anthelminthics on various intestinal helminthiasis in Iran. Effects of anthelminthics on intestinal helminthiasis. Chemotherapy 1977;23(2):98‐105. [DOI] [PubMed] [Google Scholar]
Fernandes 1981 {published data only}
- Fernandes P, Baranski MC. Comparative therapeutic efficacy of mebendazole of different origin [Comparação da eficácia terapêutica de diferentes preparados de mebendazol]. Folha Médica 1981;82(4):431‐3. [Google Scholar]
Freeman 2013 {published data only}
- Freeman MC, Clasen T, Brooker SJ, Akoko DO, Rheingans R. The impact of a school‐based hygiene, water quality and sanitation intervention on soil‐transmitted helminth reinfection: a cluster‐randomized trial. American Journal of Tropical Medicine and Hygiene 2013;89(5):875‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friis 2003 {published data only}
- Friis H, Mwaniki D, Omondi B, Muniu E, Thiong'o F, Ouma J, et al. Effects on haemoglobin of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 2003;57(4):573‐9. [DOI] [PubMed] [Google Scholar]
Gilgen 2001 {published data only}
- Gilgen DD, Mascie‐Taylor CG, Rosetta LL. Intestinal helminth infections, anaemia and labour productivity of female tea pluckers in Bangladesh. Tropical Medicine and International Health 2001;6(6):449‐57. [DOI] [PubMed] [Google Scholar]
Gizaw 2019 {published data only}
- Gizaw Z, Addisu A, Dagne H. Effects of water, sanitation and hygiene (WASH) education on childhood intestinal parasitic infections in rural Dembiya, northwest Ethiopia: an uncontrolled before‐and‐after intervention study. Environmental Health and Preventive Medicine 2019;24(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodwin 1954 {published data only}
- Goodwin LG, Staden OD. Treatment of roundworm with piperazine citrate: "antepar". British Medical Journal 1954;4(2):1332‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodwin 1958 {published data only}
- Goodwin LG, Standen OD. Treatment of ascariasis with various salts of piperazine. British Medical Journal 1958;18(1):131‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greemberg 1981 {published data only}
- Greenberg BL, Gilman RH, Shapiro H, Gilman JB, Mondal G, Maksud M, et al. Single dose piperazine therapy for Ascaris lumbricoides: an unsuccessful method of promoting growth. American Journal of Clinical Nutrition 1981;34(11):2508‐15. [DOI] [PubMed] [Google Scholar]
Gupta 1982 {published data only}
- Gupta MC, Urrutia JJ. Effect of periodic anti ascaris and anti giardia treatment on nutritional status of preschool children. American Journal of Clinical Nutrition 1982;36(1):79‐86. [DOI] [PubMed] [Google Scholar]
Gutierrez 1986 {published data only}
- Gutierrez FP, Solís JM, Delgado GL. Single dose antihelmintic: comparative study with single dose, between oxantel/pyrantel and albendazole in ascariasis, trochocephalus and hookworm [Dosis unica antihelmintica: estudio comparativo con dosis unica, entre oxantel/pirantel y albendazol en ascariasis, tricocefalosis y uncinarias]. Revista Medica de Costa Rica 1986;53(494):5‐12. [Google Scholar]
Gyorkos 2013a {published data only}
- Gyorkos TW, Maheu‐Giroux M, Blouin B, Saavedra L, Casapía M. Efficacy of a single dose of albendazole for soil‐transmitted helminth infections in school children of a village in Iquitos, Perú [Eficacia del Albendazol en dosis única sobre las infecciones por helmintos transmitidos por el suelo en escolares de una comunidad de Iquitos, Perú]. Revista Peruana de Medicina Experimental y Salud Publica 2013;30(4):601‐7. [PubMed] [Google Scholar]
Gyorkos 2013b {published data only}
- Gyorkos TW, Maheu‐Giroux M, Blouin B, Casapia M. Impact of health education on soil‐transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster‐randomized controlled trial. PLoS Neglected Tropical Diseases 2013;12(7):e2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadidjaja 1998 {published data only}
- Hadidjaja P, Bonang E, Suyardi MA, Abidin SA, Ismid IS, Margono SS. The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides. American Journal of Tropical Medicine and Hygiene 1998;59(5):791‐5. [DOI] [PubMed] [Google Scholar]
Hadju 1996 {published data only}
- Hadju V, Stephenson LS, Abadi K, Mohammed HO, Bowman DD, Parker RS. Improvements in appetite and growth in helminth‐infected schoolboys three and seven weeks after a single dose of pyrantel pamoate. Parasitology 1996;113(Pt 5):497‐504. [DOI] [PubMed] [Google Scholar]
Hall 1994 {published data only}
- Hall A, Nahar Q. Albendazole and infections with Ascaris lumbricoides and Trichuris trichiura in children in Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(1):110‐2. [DOI] [PubMed] [Google Scholar]
Hatchuel 1973 {published data only}
- Hatchuel W, Isaãcson M, Villiers DJ. Pyrantel pamoate in roundworm infestations: a comparative trial with piperazine citrate given in a single dose. South African Medical Journal 1973;47(3):91‐3. [PubMed] [Google Scholar]
Hoang 1993 {published data only}
- Hoang TK, Bronshtein AM, Zang NT, Nguyen VT, Sabgaida TP. The first experience with embovin therapy of intestinal nematodes in a focus of the disease in Vietnam. Meditsinskaya Parazitologiya i Parazitarnye Bolezni 1993;0(2):27‐8. [PubMed] [Google Scholar]
Holland 1996 {published data only}
- Holland CV, Asaolu SO, Crompton DW, Whitehead RR, Coombs I. Targeted anthelminthic treatment of school children: effect of frequency of application on the intensity of Ascaris lumbricoides infection in children from rural Nigerian villages. Parasitology 1996;113:87‐95. [DOI] [PubMed] [Google Scholar]
Hurlimann 2018 {published data only}
- Hurlimann E, Silué KD, Zouzou F, Ouattara M, Schmidlin T, Yapi RB, et al. Effect of an integrated intervention package of preventive chemotherapy, community‐led total sanitation and health education on the prevalence of helminth and intestinal protozoa infections in Cote d'Ivoire. Parasites & Vectors 2018;11(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 1976 {published data only}
- Islam N, Chowdhury NA. Mebendazole and pyrantel pamoate as broad spectrum anthelmintics. Southeast Asian Journal of Tropical Medicine and Public Health 1976;1:81‐4. [PubMed] [Google Scholar]
Jalal 1998 {published data only}
- Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of beta‐carotene, fat intake, and anthelmintic drug treatment. American Journal of Clinical and Nutrition 1998;68(3):623‐9. [DOI] [PubMed] [Google Scholar]
Jancloes 1979 {published data only}
- Jancloes MF, Cornet P, Thienpont D. Mass control of ascariasis with single oral doses of levamisole. A controlled comparison in 3,056 subjects between three incomplete population coverage. Tropical and Geographic Medicine 1979;31(1):111‐21. [PubMed] [Google Scholar]
Jinabhai 2001 {published data only}
- Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. Epidemiology of helminth infections: implications for parasite control programmes, a South African perspective. Public Health and Nutrition 2001;4(6):1211‐9. [DOI] [PubMed] [Google Scholar]
Joseph 2015 {published data only}
- Joseph SA, Casapía M, Montresor A, Rahme E, Ward BJ, Marquis GS, et al. The Effect of deworming on growth in one‐year‐old children living in a soil‐transmitted helminth‐endemic area of Peru: a randomised controlled trial. PLoS Neglected Tropical Diseases 2015;9(10):e0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kale 1981 {published data only}
- Kale OO. Controlled comparative study of the efficacy of pyrantel pamoate and a combined regimen of piperazine citrate and bephenium hydroxynaphthoate in the treatment of intestinal nemathelminthiases. African Journal of Medicine and Medical Sciences 1981;10(1‐2):63‐7. [PubMed] [Google Scholar]
Kale 1982 {published data only}
- Kale OO, Bammeke AO, Nwankwo EO. Field trials of pyrantel pamoate (Combantrin) in Ascaris, hookworm and Trichuris infections. African Journal of Medicine and Medical Sciences 1982;11(1):23‐31. [PubMed] [Google Scholar]
Karyadi 1996 {published data only}
- Karyadi E, Gross R, Sastroamidjojo S, Dillon D, Richards AL, Sutanto I. Anthelminthic treatment raises plasma iron levels but does not decrease the acute‐phase response in Jakarta school children. Southeast Asian Journal of Tropical Medicine and Public Health 1996;4(6):742‐53. [PubMed] [Google Scholar]
Kepha 2017 {published data only}
- Kepha S, Mwandawiro CS, Anderson RM, Pullan RL, Nuwaha F, Cano J, et al. Impact of single annual treatment and four‐monthly treatment for hookworm and Ascaris lumbricoides, and factors associated with residual infection among Kenyan school children. Infectious Diseases of Poverty 2017;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 2009 {published data only}
- Kirwan P, Asaolu SO, Molloy SF, Abiona TC, Jackson AL, Holland CV. Patterns of soil‐transmitted helminth infection and impact of four‐monthly albendazole treatments in preschool children from semi‐urban communities in Nigeria: a double‐blind placebo‐controlled randomised trial. BMC Infectious Diseases 2009;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 2010 {published data only}
- Kirwan P, Jackson AL, Asaolu SO, Molloy SF, Abiona TC, Bruce MC, et al. Impact of repeated four‐monthly anthelmintic treatment on Plasmodium infection in preschool children: a double‐blind placebo‐controlled randomised trial. BMC Infectious Diseases 2010;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kugo 2018 {published data only}
- Kugo M, Keter L, Maiyo A, Kinyua J, Ndemwa P, Maina G, et al. Fortification of carica papaya fruit seeds to school meal snacks may aid Africa mass deworming programs: a preliminary survey. BMC Complementary and Alternative Medicine 2018;18(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 1995 {published data only}
- Lai KP, Kaur H, Mathias RG, Ow‐Yang CK. Ascaris and Trichuris do not contribute to growth retardation in primary school children. Southeast Asian Journal of Tropical Medicine and Public Health 1995;26(2):322‐8. [PubMed] [Google Scholar]
Lechat 1974 {published data only}
- Lechat MF, Jancloes MF, Galambos F, Cornet P, Thienpont D. Control by levamisole of ascariasis and ancylostomiasis in rural areas. Tropical and Geographical Medicine 1974;26(4):441‐5. [PubMed] [Google Scholar]
Le Huong 2007 {published data only}
- Huong T, Brouwer ID, Nguyen KC, Burema J, Kok FJ. The effect of iron fortification and de‐worming on anaemia and iron status of Vietnamese schoolchildren. British Journal of Nutrition 2007;97:955‐62. [DOI] [PubMed] [Google Scholar]
Lionel 1969 {published data only}
- Lionel ND, Mirando EH, Nanayakkara JC, Soysa PE. Levamisole in the treatment of ascariasis in children. British Medical Journal 1969;4(5679):340‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 1997 {published data only}
- Lynch NR, Palenque M, Hagel I, DiPrisco MC. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. American Journal of Respiratory Critical Care Medicine 1997;156(1):50‐4. [DOI] [PubMed] [Google Scholar]
Maipanich 1997 {published data only}
- Maipanich W, Pubampen S, Sa‐nguankiat S, Nontasut P, Waikagul J. Effect of albendazole and mebendazole on soil‐transmitted helminth eggs. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(2):321‐5. [PubMed] [Google Scholar]
Mani 2002 {published data only}
- Mani TR, Rajendran R, Munirathinam A, Sunish IP, Md Abdulla, Augustin DJ, et al. Efficacy of co‐administration of albendazole and diethylcarbamazine against geohelminthiases: a study from South India. Tropical Medicine and International Health 2002;7(6):541‐8. [DOI] [PubMed] [Google Scholar]
Marti 1996 {published data only}
- Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, et al. A comparative trial of a single‐dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil‐transmitted helminth infections in children. American Journal of Tropical Medicine and Hygiene 1996;55(5):477‐81. [DOI] [PubMed] [Google Scholar]
Martin 2018 {published data only}
- Martin I, Djuardi Y, Sartono E, Rosa BA, Supali T, Mitreva M, et al. Dynamic changes in human‐gut microbiome in relation to a placebo‐controlled anthelminthic trial in Indonesia. PLoS Neglected Tropical Diseases 2018;12(8):e0006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascie‐Taylor 1999 {published data only}
- Mascie‐Taylor CG, Alam M, Montanari RM, Karim R, Ahmed T, Karim E, et al. A study of the cost effectiveness of selective health interventions for the control of intestinal parasites in rural Bangladesh. Journal of Parasitology 1999;85(1):6‐11. [PubMed] [Google Scholar]
Means 2018 {published data only}
- Means AR, Lieshout L, Brienen E, Yuhas K, Hughes JP, Ndungu P, et al. Combined effectiveness of anthelmintic chemotherapy and WASH among HIV‐infected adults. PLoS Neglected Tropical Diseases 2018;12(1):e0005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyrowitsch 2001 {published data only}
- Meyrowitsch DW, Simonsen PE. Efficacy of DEC against Ascaris and hookworm infections in schoolchildren. Tropical Medicine and International Health 2001;6(9):739‐42. [DOI] [PubMed] [Google Scholar]
Miller 1978 {published data only}
- Miller MJ, Farahmandian I, Arfaa F, Katz N, Winsor E, Bennett E. An evaluation of levamisole for treatment of ascariasis. Southern Medical Journal 1978;71(2):137‐40. [DOI] [PubMed] [Google Scholar]
Moens 1978 {published data only}
- Moens M, Dom J, Burke WE, Schlossberg S, Schuermans V. Levamisole in ascariasis. A multicenter controlled evaluation. American Journal of Tropical Medicine and Hygiene 1978;27(5):897‐904. [DOI] [PubMed] [Google Scholar]
Moser 2017a {published data only}
- Moser W, Coulibaly JT, Ali SM, Ame SM, Amour AK, Yapi RB, et al. Efficacy and safety of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel pamoate, and albendazole plus oxantel pamoate against hookworm and concomitant soil‐transmitted helminth infections in Tanzania and Côte d'Ivoire: a randomized, controlled, single‐blinded, non‐inferiority trial. Lancet Infectious Diseases 2017;17(11):1162‐71. [DOI] [PubMed] [Google Scholar]
Muchiri 2001 {published data only}
- Muchiri EM, Thiong'o FW, Magnussen P, Ouma JH. A comparative study of different albendazole and mebendazole regimens for the treatment of intestinal infections in school children of Usigu Division, western Kenya. Journal of Parasitology 2001;87(2):413‐9. [DOI] [PubMed] [Google Scholar]
Murray 1978 {published data only}
- Murray J, Murray A, Murray M, Murray C. The biological suppression of malaria: an ecological and nutritional interrelationship of a host and two parasites. American Journal of Clinical Nutrition 1978;31(8):1363‐6. [DOI] [PubMed] [Google Scholar]
Naquira 1989 {published data only}
- Naquira C, Jimenez G, Guerra JG, Bernal R, Nalin DR, Neu D, et al. Ivermectin for human strongyloidiasis and other intestinal helminths. American Journal of Tropical Medicine and Hygiene 1989;40(3):304‐9. [DOI] [PubMed] [Google Scholar]
Ndibazza 2010 {published data only}
- Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, Oweka J, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled Trial. Clinical of Infectious Diseases 2010;50(4):531‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndibazza 2012 {published data only}
- Ndibazza J, Mpairwe H, Webb EL, Mawa PA, Nampijja M, Muhangi L, et al. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PloS One 2012;7(12):e50325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndyomugyenyi 2008 {published data only}
- Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil‐transmitted helminths in pregnancy and adverse events: a randomised open label controlled intervention trial in Masindi district, western Uganda. Americam Journal of Tropical Medicine and Hygiene 2008;79(6):856‐63. [PubMed] [Google Scholar]
Newlove 2011 {published data only}
- Newlove T, Guimaraes LH, Morgan DJ, Alcantara L, Glesby MJ, Carvalho EM, et al. Antihelminthic therapy and antimony in cutaneous leishmaniasis: a randomised, double‐blind, placebo‐controlled trial in patients co‐infected with helminths and leishmania braziliensis. American Journal of Tropical Medicine and Hygiene 2011;84(4):551‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nokes 1999 {published data only}
- Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, et al. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. American Journal of Tropical Medicine and Hygiene 1999;60(4):556‐65. [DOI] [PubMed] [Google Scholar]
Nontasut 1997 {published data only}
- Nontasut P, Waikagul J, Muennoo C, Sanguankait, N, Maipanich W. Minimum effective doses of mebendazole in treatment of soil‐transmitted helminths. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(2):326‐8. [PubMed] [Google Scholar]
Northrop‐Clewes 2001 {published data only}
- Northrop‐Clewes CA, Rousham EK, Mascie‐Taylor CN, Lunn PG. Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. Americam Journal of Nutrition 2001;73(1):53‐60. [DOI] [PubMed] [Google Scholar]
Okoyo 2016 {published data only}
- Okoyo C, Nikolay B, Kihara J, Simiyu E, Garn JV, Freeman MC, et al. Monitoring the impact of a national school based deworming programme on soil‐transmitted helminths in Kenya: the first three years, 2012 ‐ 2014. Parasites & Vectors 2016;9(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pene 1982 {published data only}
- Pene P, Mojon M, Garin JP, Coulaud JP, Rossignol JF. Albendazole : a new broad spectrum anthelmintic. Double‐blind multicenter clinical trial. American Journal of Tropical Medicine and Hygiene 1982;31(2):236‐66. [DOI] [PubMed] [Google Scholar]
Persson 2001 {published data only}
- Persson V, Ahmed F, Gebre‐Medhin M, Greiner T. Increase in serum beta‐carotene following dark green leafy vegetable supplementation in mebendazole‐treated school children in Bangladesh. European Journal of Clinical Nutrition 2001;55(1):1‐9. [DOI] [PubMed] [Google Scholar]
Pickering 2019 {published data only}
- Pickering AJ, Njenga SM, Steinbaum L, Stwarthout J, Lin A, Arnold BF, et al. Effects of single and integrated water, sanitation, handwashing, and nutrition interventions on child soil‐transmitted helminth and Giardia infections: a cluster‐randomized controlled trial in rural Kenya. PLoS Medicine 2019;16(6):e1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pond 1970 {published data only}
- Pond HS, Bokat RB, Johnson JP, Knight JL, Healy GR, Gleason NN, et al. Mass treatment for ascariasis: value of prophylactic use of piperazine in groups heavily infected with Ascaris lumbricoides. Southern Medical Association 1970;63(5):599‐602. [PubMed] [Google Scholar]
Pullan 2019 {published data only}
- Pullan RL, Halliday KE, Oswald WE, McHaro C, Beaumont E, Kepha S, et al. Effects, equity, and cost of school‐based and community‐wide treatment strategies for soil‐transmitted helminths in Kenya: a cluster‐randomised controlled trial. Lancet 2019;393(10185):2039‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 1996 {published data only}
- Rahman WA. Comparative trials using albendazole and mebendazole in treatment of soil‐transmitted helminths in schoolchildren on Penang, Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 1996;27(4):765‐7. [PubMed] [Google Scholar]
Rahman 1998 {published data only}
- Rahman WA. Helminthic infections of urban and rural school children in Penang Island, Malasia: implications for control. Southeast Asian Journal of Tropical Medicine and Public Health 1998;29(3):596‐8. [PubMed] [Google Scholar]
Raj 1998 {published data only}
- Raj SM, Naing NN. Ascariasis, trichuriasis, and growth of schoolchildren in Northeastern Peninsular Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 1998;29(4):729‐34. [PubMed] [Google Scholar]
Reddy 1986 {published data only}
- Reddy V, Vijayaraghsvsn K, Mathur KK. Effect of deworming and vitamin A administration on serum vitamin A levels in preschool children. Journal of Tropical Pediatrics 1986;32(4):196‐9. [DOI] [PubMed] [Google Scholar]
Restrepo 1987 {published data only}
- Restrepo M, Isaza D. Estudio comparativo de flubendazol, oxantel‐pirantel, albendazol y mebendazol en el tratamiento de helmintos transmitidos por el suelo. Acta Médica Colombiana 1987;12(5):344‐51. [Google Scholar]
Rousham 1994 {published data only}
- Rousham EK, Mascie‐Taylor CG. An 18‐month study of the effect of periodic anthelminthic treatment on the growth and nutritional status of pre‐school children in Bangladesh. Annals of Human Biology 1994;21(4):315‐24. [DOI] [PubMed] [Google Scholar]
Sarmah 1988 {published data only}
- Sarmah HC. A randomised controlled trial of pyrantel and mebendazole in children with enterobiasis and concomitant ascariasis. Indian Pediatrics 1988;25(6):544‐7. [PubMed] [Google Scholar]
Seftel 1968 {published data only}
- Seftel HC, Heinz HJ. Comparison of piperazine and tetramisole in the treatment of ascariasis. British Medical Journal 1968;4(5623):93‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seo 1980 {published data only}
- Seo BS, Cho SY, Chai JY, Hong ST. Comparative efficacy of various interval mass treatment of Ascaris lumbricoides infection in Korea. Kisaengch'unghak Chapchi [Korean Journal of Parasitology] 1980;18(2):145‐51. [DOI] [PubMed] [Google Scholar]
Sileshi 2017 {published data only}
- Sileshi B, Suleman S, Wynendaele E, Kosgei A, Duchateau L, Siegeleer B, et al. Poor dissolution of anthelminthic medicines: a risk for reduced efficacy. Tropical Medicine and International Health 2017;22(S1):353. [Google Scholar]
Sinniah 1981 {published data only}
- Sinniah B, Sinniah D. The anthelmintic effects of pyrantel pamoate, oxantel‐pyrantel pamoate, levamisole and mebendazole in the treatment of intestinal nematodes. Annals of Tropical Medicine and Parasitology 1981;75(3):315‐21. [DOI] [PubMed] [Google Scholar]
Sood 1975 {published data only}
- Sood S, Kini AS, Master J. Comparison of pyrantel and tetramisole in ascariasis. Indian Pediatrics 1975;12(8):689‐92. [PubMed] [Google Scholar]
Speich 2016 {published data only}
- Speich B, Moser W, Ali SM, Ame SM, Albonico M, Hattendorf J, et al. Efficacy and reinfection with soil‐transmitted helminths 18‐weeks post‐treatment with albendazole‐ivermectin, albendazole‐mebendazole, albendazole‐oxantel pamoate and mebendazole. Parasites & Vectors 2016;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staal 2018 {published data only}
- Staal SL, Hogendoorn SK, Voets SA, Tepper RC, Veenstra M, Vos II, et al. Prevalence of atopy following mass drug administration with albendazole: a study in school children on Flores Island, Indonesia. International Archives of Allergy and Immunology 2018;177(3):192‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinmann 2008 {published data only}
- Steinmann P, Zhou XN, Du ZW, Jiang JY, Xiao SH, Wu ZX, et al. Tribendimidine and albendazole for treating soil‐transmitted helminths, Strongyloides stercoralis and Taenia spp.: open‐label randomized trial. PLoS Neglected Tropical Diseases 2008;2(10):e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 1990 {published data only}
- Stephenson LS, Latham MC, Kinoti SN, Kurz KM, Brigham H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(2):277‐82. [DOI] [PubMed] [Google Scholar]
Stoltzfus 1997 {published data only}
- Stoltzfus RJ, Albonico M, Tielsch JM, Chwaya HM, Savioli L. School‐based deworming program yields small improvement in growth of Zanzibari school children after one year. Journal of Nutrition 1997;127(11):2187‐93. [DOI] [PubMed] [Google Scholar]
Stoltzfus 2001 {published data only}
- Stoltzfus RJ, Kvalsvig JD, Chwaya HW, Montresor A, Albonico M. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind placebo controlled study. British Medical Journal (Clinical Research Ed.) 2001;323(7326):1389‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supali 2017 {published data only}
- Supali T, Djuardi Y, Christian M, Iskandar E, Maylasari R, Wondmeneh S, et al. A single dose of ivermectin, dec plus albendazole is superior to dec plus albendazole for treatment of Trichuris trichiura in Indonesia. American Journal of Tropical Medicine and Hygiene 2017;97:614. [Google Scholar]
Tankhiwale 1989 {published data only}
- Tankhiwale SR, Kukade AL, Sarmah HC, Salunkhe DS, Kulkarni AS. Single dose therapy of ascariasis – a randomized comparison of mebendazole and pyrantel. Journal of Communicable Diseases 1989;21(1):71‐4. [PubMed] [Google Scholar]
Tanumihardjo 2004 {published data only}
- Tanumihardjo SA, Permaesih D, Muhilal. Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. European Journal of Clinical Nutrition 2004;58(9):1223‐30. [DOI] [PubMed] [Google Scholar]
Taylor 2001 {published data only}
- Taylor M, Jinabhai CC, Couper I, Kleinschmidt I, Jogessar VB. The effect of different anthelmintic treatment regimens combined with iron supplementation on the nutritional status of schoolchildren in KwaZulu‐Natal, South Africa: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(2):211‐6. [DOI] [PubMed] [Google Scholar]
Thein‐Hlaing 1991a {published data only}
- Thein‐Hlaing, Than‐Saw, Myat‐Lay‐Kyin. The impact of three‐monthly age‐targeted chemotherapy on Ascaris lumbricoides infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(4):519‐22. [DOI] [PubMed] [Google Scholar]
Thein‐Hlaing 1991b {published data only}
- Thein‐Hlaing, Thane‐Toe, Than‐Saw, Myat‐Lay‐Kyin, Myint‐Lwin. A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(4):523‐8. [DOI] [PubMed] [Google Scholar]
Urjel 1985 {published data only}
- Urjel D, Recacoechea SM, Darras C, Zuna V, Cardozo C, Carrasco J. Efficacy after 6 months of different therapeutic schedules of mebendazole in infections by A. lumbricoides, Ancylostomidae sp and T. trichiura in school children [Eficacia a los seis meses de diferentes esquemas terapeuticos de mebendazol en las infecciones por A. lumbricoides, ancylostomidae SP y T. trichiura en niños de edad escolar]. Boletín Científico del CENETROP 1985;11(1):13‐20. [Google Scholar]
Vaz Nery 2019 {published data only}
- Vaz Nery S, Traub RJ, McCarthy JS, Clarke NE, Amaral S, Llewellyn S, et al. WASH for WORMS: a cluster‐randomized controlled trial of the impact of a community integrated water, sanitation, and hygiene and deworming intervention on soil‐transmitted helminth infections. American Journal of Tropical Medicine and Hygiene 2019;100(3):750‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2008 {published data only}
- Walson JL, Otieno PA, Mbuchi M, Richardson BA, Lohnman‐Payne B Macharia SW, et al. Albendazole treatment of HIV‐1 helminth co‐infection: a randomised, double blind, placebo‐controlled trial. AIDS 2008;22(13):1601‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2010 {published data only}
- Walson JL, Stewart BT, Sangare L, Mbogo LW, Otieno PA, Piper BK, et al. Prevalence and correlates of helminth co‐infection in Kenyan HIV‐1 infected adults. PLoS Neglected Tropical Diseases 2010;4(3):e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1987 {published data only}
- Wang BR, Wang HC, Li LW, Zhang XL, Yue JQ, Wang GX, et al. Comparative efficacy of thienpydin, pyrantel pamoate, mebendazole and albendazole in treating ascariasis and enterobiasis. Chinese Medical Journal 1987;100(11):928‐30. [PubMed] [Google Scholar]
Watkins 1996b {published data only}
- Watkins WE, Cruz JR, Pollitt E. The effects of deworming on indicators of school performance in Guatemala. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(2):156‐60. [DOI] [PubMed] [Google Scholar]
Wesche 1994 {published data only}
- Wesche D, Lutz S, Barnish G. Comparative study of chewable pyrantel pamoate: should standards for chewable tablets be revised?. Papua and New Guinea Medical Journal 1994;37(1):12‐4. [PubMed] [Google Scholar]
Whitworth 1991 {published data only}
- Whitworth JA, Morgan D, Maude GH, McNicholas AM, Taylor DW. A field study of the effect of ivermectin on intestinal helminths in man. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(2):232‐4. [DOI] [PubMed] [Google Scholar]
Willett 1979 {published data only}
- Willett WC, Kilama WL, Kihamia CM. Ascaris and growth rates: a randomized trial of treatment. American Journal of Public Health 1979;69(10):987‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1997 {published data only}
- Williams RA, Koroma MM, Hodges M. Comparison of albendazole and levamisole chemotherapy on prevalence and intensity of common soil‐transmitted helminth infection in school children, Sierra Leone. West African Journal of Medicine 1997;16(3):179‐83. [PubMed] [Google Scholar]
Wright 2009 {published data only}
- Wright VJ, Ame SM, Haji HS, Weir RE, Goodman D, Pritchard DI, et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Neglected Tropical Diseases 2009;3(5):e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yangco 1981 {published data only}
- Yangco BG, Klein TW, Deresinski SC, Vickery AC, Craig CP. Flubendazole and mebendazole in the treatment of trichuriasis and other helminthiases. Clinical Therapeutics 1981;4(4):285‐90. [PubMed] [Google Scholar]
Yap 2014 {published data only}
- Yap P, Wu FW, Du ZW, Hattendorf J, Chen R, Jiang JY, et al. Effect of deworming on physical fitness of school‐aged children in Yunnan, China: a double‐blind, randomized, placebo‐controlled trial. PLoS Neglected Tropical Diseases 2014;8(7):e2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02636803 {unpublished data only}
- NCT02636803. Study of the efficacy of oxfendazole compared to albendazole in the treatment of Trichuris trichiura infection in adults. clinicaltrials.gov/ct2/show/NCT02636803 (first received 22 December 2015).
TCTR20190111001 {unpublished data only}
- Sanprasert V. Assessment of efficacy of anthelmintic drugs against soil‐transmitted helminths in Thailand. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=4332/%20TCTR20190111001 (first received 10 January 2019).
Additional references
Asaolu 1991
- Asaolu SO, Holland CV, Crompton DW. Community control of Ascaris lumbricoides in rural Oyo State, Nigeria: mass, targeted and selective treatment with levamisole. Parasitology 1991;103(Pt 2):291‐8. [DOI] [PubMed] [Google Scholar]
Ayana 2019
- Ayana M, Cools P, Mekonnen Z, Biruksew A, Dana D, Rashwan N, et al. Comparison of four DNA extraction and three preservation protocols for the molecular detection and quantification of soil‐transmitted helminths in stool. PLoS Neglected Tropical Diseases 2019;13(10):e0007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1975
- Bell DR. Diagnosis of parasitic diseases by filtration. Annales de la Société Belge de la Médicine Tropicale 1975;55(5):489‐96. [PubMed] [Google Scholar]
Bennett 2000
- Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitology Today 2000;16(2):71‐4. [DOI] [PubMed] [Google Scholar]
Bergquist 2009
- Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when?. Trends in Parasitology 2009;25(4):151‐6. [DOI] [PubMed] [Google Scholar]
Bethony 2006
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil‐transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 2006;367(9521):1521‐32. [DOI] [PubMed] [Google Scholar]
Campbell 2016
- Campbell SJ, Nery SV, Doi SA, Gray DJ, Soares Magalhães RJ, McCarthy JS, et al. Complexities and perplexities: a critical appraisal of the evidence for soil‐transmitted helminth infection‐related morbidity. PLoS Neglected Tropical Diseases 2016;10(5):e0004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2009
- Centers for Disease Control and Prevention. Parasites and health: ascariasis. dpd.cdc.gov/DPDx/HTML/Ascariasis.htm. Atlanta, GA, U.S., (accessed 5 December 2011).
Clarke 2017
- Clarke NE, Clements AC, Doi SA, Wang D, Campbell SJ, Gray D, et al. Differential effect of mass deworming and targeted deworming for soil‐transmitted helminth control in children: a systematic review and meta‐analysis. Lancet 2017;389(10066):287‐97. [DOI] [PubMed] [Google Scholar]
Cools 2019
- Cools P, Vlaminck J, Albonico M, Ame S, Ayana M, José Antonio BP, et al. Diagnostic performance of a single and duplicate Kato‐Katz, Mini‐FLOTAC, FECPAKG2 and qPCR for the detection and quantification of soil‐transmitted helminths in three endemic countries. PLOS Neglected Tropical Diseases 2019;13(8):e0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2009
- Cooper PJ. Interactions between helminth parasites and allergy. Current Opinion in Allergy and Clinical Immunology 2009;9(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crompton 1989
- Crompton DW, Nesheim MC, Pawlowski ZS, editor(s). Biology of Ascaris lumbricoides. Ascariasis and Its Prevention and Control. London: Taylor and Francis, 1989:9‐44. [Google Scholar]
de Silva 2015
- Silva N, Ahmed BN, Casapia M, Silva HJ, Gyapong J, Malecela M, et al. Cochrane reviews on deworming and the right to a healthy, worm‐free life. PLoS Neglected Tropical Diseases 2015;9(10):e0004203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Degarege 2016
- Degarege A, Degarege D, Veledar E, Erko B, Nacher M, Beck‐Sague CM, et al. Plasmodium falciparum Infection status among children with schistosoma in Sub‐Saharan Africa: a systematic review and meta‐analysis. PLoS Neglected Tropical Diseases 2016;10(12):e0005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
del Castillo 1964
- Castillo D, Mello WC, Morales T. Mechanism of the paralysing action of piperazine on ascaris muscle. British Journal of Pharmacology and Chemotherapy 1964;22(3):463‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2003
- Diaz E, Mondragon J, Ramirez E, Bernal R. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. American Journal of Tropical Medicine and Hygiene 2003;68(4):384‐5. [PubMed] [Google Scholar]
Ehrhardt 2008
- Ehrhardt S, Burchard GD. Eosinophilia in returning travellers and migrants. Deutsches Ärzteblatt International 2008;105(46):801‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenton 2013
- Fenton A. Dances with worms: the ecological and evolutionary impacts of deworming on co infecting pathogens. Parasitology 2013;140:1119‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flohr 2007
- Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J, et al. Low efficacy of mebendazole against hookworm in Vietnam: two randomised controlled trials. American Journal of Tropical Medicine and Hygiene 2007;76(4):732‐6. [PubMed] [Google Scholar]
Galvan‐Ramirez 2007
- Galvan‐Ramirez ML, Rivera N, Loeza ME, Avila X, Acero J, Troyo R, et al. Nitazoxanide in the treatment of Ascaris lumbricoides in a rural zone of Colima, Mexico. Journal of Helminthology 2007;81(3):255‐9. [DOI] [PubMed] [Google Scholar]
GBD 2017
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geary 2010
- Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. International Journal for Parasitology 2010;40(1):1‐13. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Hall 2008
- Hall A, Hewitt G, Tuffrey V, Silva N. A review and meta‐analysis of the impact of intestinal worms on child growth and nutrition. Maternal & Child Nutrition 2008;4(Suppl 1):118‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hefny 2009
- Hefny AF, Saadeldin YA, Abu‐Zidan FM. Management algorithm for intestinal obstruction due to ascariasis: a case report and review of the literature. Turkish Journal of Trauma & Emergency Surgery 2009;15(3):301‐5. [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 2009
- Holland CV. Predisposition to ascariasis: patterns, mechanisms and implications. Parasitology 2009;136(12):1537‐47. [DOI] [PubMed] [Google Scholar]
Jardim‐Botelho 2008
- Jardim‐Botelho A, Brooker S, Geiger SM, Fleming F, Souza Lopes AC, Diemert DJ, et al. Age patterns in under nutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Tropical Medicine and International Health 2008;13(4):458‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1972
- Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick‐smear technique in Schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1972;14(6):397‐400. [PubMed] [Google Scholar]
Keiser 2008
- Keiser J, Utzinger J. Efficacy of current drugs against soil‐transmitted helminth infections: systematic review and meta‐analysis. Journal of the American Medical Association 2008;299(16):1937‐48. [DOI] [PubMed] [Google Scholar]
Khurana 2017
- Khurana S, Sethi S. Laboratory diagnosis of soil transmitted helminthiasis. Tropical Parasitology 2017;7(2):86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khuroo 1985
- Khuroo MS, Zargar SA. Biliary ascariasis. A common cause of biliary and pancreatic disease in an endemic area. Gastroenterology 1985;88(2):418‐23. [PubMed] [Google Scholar]
Khuroo 1990
- Khuroo MS, Zargar SA, Mahajan R. Hepatobiliary and pancreatic ascariasis in India. Lancet 1990;335(8704):1503‐6. [DOI] [PubMed] [Google Scholar]
Kofie 1983
- Kofie BA, Dipeolu OO. A study of human and porcine ascariasis in a rural area of South‐West Nigeria. International Journal of Zoonoses 1983;10(1):66‐70. [PubMed] [Google Scholar]
Leonardi‐Bee 2006
- Leonardi‐Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta‐analysis. American Journal of Respiratory and Critical Care Medicine 2006;174(5):514‐23. [DOI] [PubMed] [Google Scholar]
Levecke 2014
- Levecke B, Montresor A, Albonico M, Ame SM, Behnke JM, Bethony JM, et al. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil‐transmitted helminths are endemic. PLoS Neglected Tropical Diseases 2014;8(10):e3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2007
- Long KZ, Rosado JL, Montoya Y, de Lourdes Solano M, Hertzmark E, DuPont HL, et al. Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics 2007;120(4):e846‐55. [DOI] [PubMed] [Google Scholar]
Maruyama 1997
- Maruyama H, Nawa Y, Noda S, Mimori T. An outbreak of ascariasis with marked eosinophilia in the southern part of Kyushu District, Japan, caused by infection with swine ascaris. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(Suppl 1):194‐6. [PubMed] [Google Scholar]
Montresor 2015
- Montresor A, À Porta N, Albonico M, Gabrielli AF, Jankovic D, Fitzpatrick C, et al. Soil‐transmitted helminthiasis: the relationship between prevalence and classes of intensity of infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;109(4):2672‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montresor 1998
- Montresor A, Crompton DW, Hall A, Bundy DA, Savioli L. Guidelines for the evaluation of soil‐transmitted helminthiasis and schistosomiasis at community level. whqlibdoc.who.int/hq/1998/WHO_CTD_SIP_98.1.pdf (accessed prior to 31 January 2020).
Moser 2017b
- Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta‐analysis. BMJ 2017;358:j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mrus 2017
- Mrus J, Baeten B, Engelen M, Silber SA. Efficacy of single‐dose 500 mg mebendazole in soil‐transmitted helminth infections: a review. Journal of Helminthology 2017;18:1‐10. [DOI] [PubMed] [Google Scholar]
O'Connell 2016
- O'Connell EM, Nutman TB. Molecular diagnostics for soil‐transmitted helminths. American Journal of Tropical Medicine and Hygiene 2016;95(3):508‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmeirim 2018b
- Palmeirim MS, Hürlimann E, Knopp S, Speich B, Belizario V Jr, Joseph SA, et al. Efficacy and safety of co‐administered ivermectin plus albendazole for treating soil‐transmitted helminths: a systematic review meta‐analysis and individual patient data analysis. PLoS Neglected Tropical Diseases 2018;12(4):e0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papaiakovou 2019
- Papaiakovou M, Wright J, Pilotte N, Chooneea D, Schär F, Truscott JE, et al. Pooling as a strategy for the timely diagnosis of soil‐transmitted helminths in stool: value and reproducibility. Parasites & Vectors 2019;12(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pawlowski 1985
- Pawlowski ZS. Public health practice: ascariasis control. World Health Forum 1985;6(3):254‐6. [Google Scholar]
Pullan 2014
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors 2014;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeder 1998
- Reeder MM. The radiological and ultrasound evaluation of ascariasis of the gastrointestinal, biliary, and respiratory tracts. Seminars in Roentgenology 1998;33(1):57‐78. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salam 2015
- Salam RA, Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil‐transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews (Online) 2015;6:CD005547. [DOI] [PubMed] [Google Scholar]
Santos 2005
- Santos FL, Cerqueira EJ, Soares NM. Comparison of the thick smear and Kato‐Katz techniques for diagnosis of intestinal helminth infections. Revista da Sociedade Brasileira de Medecina Tropical 2005;38(2):196‐8. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Stelekati 2012
- Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host & Microbe 2012;12(4):458‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stepek 2006
- Stepek G, Buttle DJ, Duce IR, Behnke JM. Human gastrointestinal nematode infections: are new control methods required?. International Journal of Experimental Pathology 2006;87(5):325‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 2000
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology 2000;121(Suppl):S23‐38. [DOI] [PubMed] [Google Scholar]
Taylor‐Robinson 2019
- Taylor‐Robinson DC, Maayan N, Donegan S, Chaplin M, Garner P. Public health deworming programmes for soil‐transmitted helminths in children living in endemic areas. Cochrane Database of Systematic Reviews 2019, Issue 9. [DOI: 10.1002/14651858.CD000371.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Utzinger 2004
- Utzinger J, Keiser J. Schistosomiasis and soil‐transmitted helminthiasis: common drugs for treatment and control. Expert Opinion on Pharmacotherapy 2004;5(2):263‐85. [DOI] [PubMed] [Google Scholar]
Venkatesan 1998
- Venkatesan P. Albendazole. Journal of Antimicrobial Chemotherapy 1998;41(2):145‐7. [DOI] [PubMed] [Google Scholar]
Vercruysse 2011a
- Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, et al. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil‐transmitted helminths are endemic. PLoS Neglected Tropical Diseases 2011;5(3):e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vercruysse 2011b
- Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, et al. Is anthelmintic resistance a concern for the control of human soil‐transmitted helminths?. International Journal for Parasitology 2011;1(1):14‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. The use of essential drugs. Ninth report of the WHO Expert Committee. WHO Technical Report Series, No. 895 2000:1‐61. [PubMed]
WHO 2001
- World Health Organization. Water related diseases: ascariasis. www.who.int/water_sanitation_health/diseases/ascariasis/en/ (accessed 20 September 2010).
WHO 2002
- World Health Organization. Prevention and control of schistosomiasis and soil‐transmitted helminthiasis: report of a WHO expert committee. WHO Technical Report Series No. 912 2002:1‐63. [PubMed]
WHO 2005
- World Health Organization. Deworming for health and development. Report of the third global meeting of the partners for parasite control. Geneva, 29‐30 November 2004. apps.who.int/iris/bitstream/handle/10665/69005/WHO_CDS_CPE_PVC_2005.14.pdf (accessed prior to 31 January 2020).
WHO 2011
- World Health Organization. Helminth control in school‐age children: a guide for managers of control programmes. WHO Technical Report Series 2011:1‐90.
WHO 2012
- World Health Organization. Soil‐transmitted helminthiases: eliminating soil‐transmitted helminthiases as a public health problem in children. Progress report 2001‐2010 and strategic plan 2011‐2020. whqlibdoc.who.int/publications/2012/9789241503129_eng.pdf (accessed prior to 31 January 2020.
WHO 2017a
- World Health Organization. Soil‐transmitted helminth infections. www.who.int/mediacentre/factsheets/fs366/en/ (accessed 19 August 2017).
WHO 2017b
- World Health Organization. The selection and use of essential medicines. WHO Technical Report Series 2017;1006:1‐604. [PubMed] [Google Scholar]
Wolstenholme 2004
- Wolstenholme AJ, Fairweather I, Prichard R, Samson‐Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends in Parasitology 2004;20(10):469‐76. [DOI] [PubMed] [Google Scholar]
Xu 1995
- Xu LQ, Yu SH, Jiang ZX, Yang JL, Lai LQ, Zhang XJ, et al. Soil‐transmitted helminthiases: nationwide survey in China. Bulletin of the World Health Organization 1995;73(4):507‐13. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Conterno 2013
- Conterno LO, Garcia MVF, Mukai NS. Anthelmintic drugs for treating ascariasis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010599] [DOI] [PMC free article] [PubMed] [Google Scholar]


