Abstract
Ever since the first case was reported at the end of 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated coronavirus disease 2019 (COVID-19) has become a serious threat to public health globally in short time. At this point in time, there is no proven effective therapy. The interactions with concomitant disease are largely unknown, and that may be particularly pertinent to inherited arrhythmia syndrome. An arrhythmogenic effect of COVID-19 can be expected, potentially contributing to disease outcome. This may be of importance for patients with an increased risk of cardiac arrhythmias, either secondary to acquired conditions or comorbidities or consequent to inherited syndromes. Management of patients with inherited arrhythmia syndromes such as long QT syndrome, Brugada syndrome, short QT syndrome, and catecholaminergic polymorphic ventricular tachycardia in the setting of the COVID-19 pandemic may prove particularly challenging. Depending on the inherited defect involved, these patients may be susceptible to proarrhythmic effects of COVID-19–related issues such as fever, stress, electrolyte disturbances, and use of antiviral drugs. Here, we describe the potential COVID-19–associated risks and therapeutic considerations for patients with distinct inherited arrhythmia syndromes and provide recommendations, pending local possibilities, for their monitoring and management during this pandemic.
Keywords: Brugada syndrome, Catecholaminergic polymorphic ventricular tachycardia, COVID-19, Long QT syndrome, SARS-CoV-2, Short QT syndrome
A. Introduction
Ever since the first case was reported at the end of 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) virus and associated coronavirus disease 2019 (COVID-19) has spread throughout the world and has become a pandemic. In particular, the high transmission rate of the virus has made it a threat to public health globally.1 , 2 Currently, there is no proven effective therapy against the virus, and the effect on other diseases is also uncertain.
SARS-CoV-2 is an RNA virus, a member of the coronavirus family of viruses, similar to SARS-CoV.3 Like SARS-CoV, SARS-CoV-2 infects humans by binding to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of the cell through its spike domain.3 Infected patients present with a variety of manifestations. The most common clinical symptom is fever (88.7%). Other symptoms include cough (67.8%), shortness of breath (18.7%), myalgia or arthralgia (14.9%), headache (13.6%), diarrhea (3.8%), sore throat (13.9%), and sputum production (33.7%) and fatigue (38.1%).4 Studies have shown that while the vast majority of patients have minor symptoms, it is also possible for infected cases to become critically ill, especially older individuals (older than 60 years) or patients with comorbidities.1 , 2 Severely affected patients may have acute respiratory distress (15.6%), which requires invasive mechanical ventilation (14.5%) and extracorporeal membrane oxygenation (2.9%).4
B. Possible cardiac effects of the SARS-COV-2
A registry of 1099 cases with COVID-19 reported a higher prevalence of hypertension (23.7% vs 13.4%) and coronary artery disease (5.8% vs 1.8%) in severely affected vs nonseverely affected patients.4 Another study of 138 hospitalized patients with COVID-19 compared patients admitted to the intensive care unit (ICU) and those not admitted to the ICU. Higher rates of hypertension (58.3% vs 21.6%; P < .001) and cardiovascular disease (25.0% vs 10.8%; P = .04) were observed in patients admitted to the ICU.1 This indicates that patients with preexisting cardiovascular disease may have a worse prognosis than do others, although age could be one of the confounders. Furthermore, it is essential to understand that although most clinical presentations relate to the respiratory system, the disease may also affect the cardiovascular system.5 Besides the respiratory system, ACE2 is expressed in the human cardiovascular system including the heart6 and a number of mechanisms have been put forward whereby SARS-CoV-2 may cause myocardial injury. These include mechanisms involving derangement of ACE2 signal pathways (animal studies have shown that cellular ACE2 levels decrease upon SARS-CoV infection),6 cytokine storm, and myocarditis.7 , 8 The occurrence of myocardial involvement and severity thereof varies among affected individuals. While myocardial damage evidenced by high cardiac markers such as high-sensitivity cardiac troponin I has been recognized9 and fulminant myocarditis has been reported,8 whether cardiovascular complications include malignant arrhythmias is not yet known. In the aforementioned study of 138 hospitalized patients with COVID-19, arrhythmia (not further specified) was reported in 17% of total patients and in 16 of 36 patients admitted to the ICU.1 Therefore, an arrhythmogenic effect of COVID-19 could be expected, potentially contributing to disease outcome. This may be of importance for patients with an increased risk of cardiac arrhythmias, either secondary to acquired conditions or comorbidities or consequent to inherited syndromes. Management of patients with inherited arrhythmia syndromes such as long QT syndrome (LQTS), Brugada syndrome (BrS), short QT syndrome (SQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT) in the setting of the COVID-19 pandemic may prove particularly challenging. Depending on the inherited defect involved, these patients may be susceptible to proarrhythmic effects of COVID-19–related issues such as fever, stress, electrolyte disturbances, and use of antiviral drugs. Hence, additional precautions and preventive measures are recommended, including electrocardiographic (ECG) monitoring, aggressive antipyretic treatment, and more stringent social distancing, to prevent infection.10 Here, we describe the potential COVID-19–associated risks and therapeutic considerations for patients with distinct inherited arrhythmia syndromes and provide recommendations for their monitoring and management during this pandemic.
C. LQTS
LQTS is characterized by abnormally prolonged ventricular repolarization and an increased risk of the malignant arrhythmia torsades de pointes and ventricular fibrillation that may lead to sudden death. LQTS is an inheritable condition caused by pathogenic variants in genes encoding ion channels (primarily KCNQ1, KCNH2, and SCN5A). An often-faced clinical situation, however, is acquired QT prolongation, which occurs, for instance, during myocardial ischemia, hypothermia, hypokalemia, sepsis, or as a result of treatment with a wide range of drugs. Severe corrected QT (QTc) prolongation due to these conditions might similarly result in malignant arrhythmias. Rather commonly, patients who have severe forms of acquired QT prolongation also have a genetic predisposition for QTc prolongation,11 , 12 but without such extreme provocation, these patients generally have normal QT intervals. In fact, many patients with LQTS may also have QT intervals within normal limits in resting conditions,13 although this still puts them at a higher risk of malignant arrhythmias,14 especially during provocations such as the use of QTc-prolonging drugs.15 Whereas severe forms of inherited LQTS often surface during (early) childhood (from infants to adolescents),14 , 16 acquired QT prolongation generally occurs in older patients because these critical provocative events more often occur in older patients.
C.1.LQTS and COVID-19
There are several issues that require attention when discussing COVID-19 in relation to inheritable or acquired QT prolongation.
The most important determinant of the risk of malignant arrhythmias in patients with LQTS or in acquired QT prolongation is the use of ≥1 QTc-prolonging drugs in the setting of severe manifestations of COVID-19. Many drugs (with either cardiac or noncardiac indications) have the ability to block cardiac potassium currents, impairing ventricular repolarization with subsequent prolongation of the QT interval and an increased risk of malignant arrhythmias.15 In addition, many drugs may alter drug metabolism, for example, because of the inhibition of cytochrome P450 3A4 (CYP3A4), which may further increase the plasma levels of QT-prolonging drugs and the risk of malignant arrhythmias. Of special interest in COVID-19 is that there are indications that chloroquine and hydroxychloroquine might be of value.17
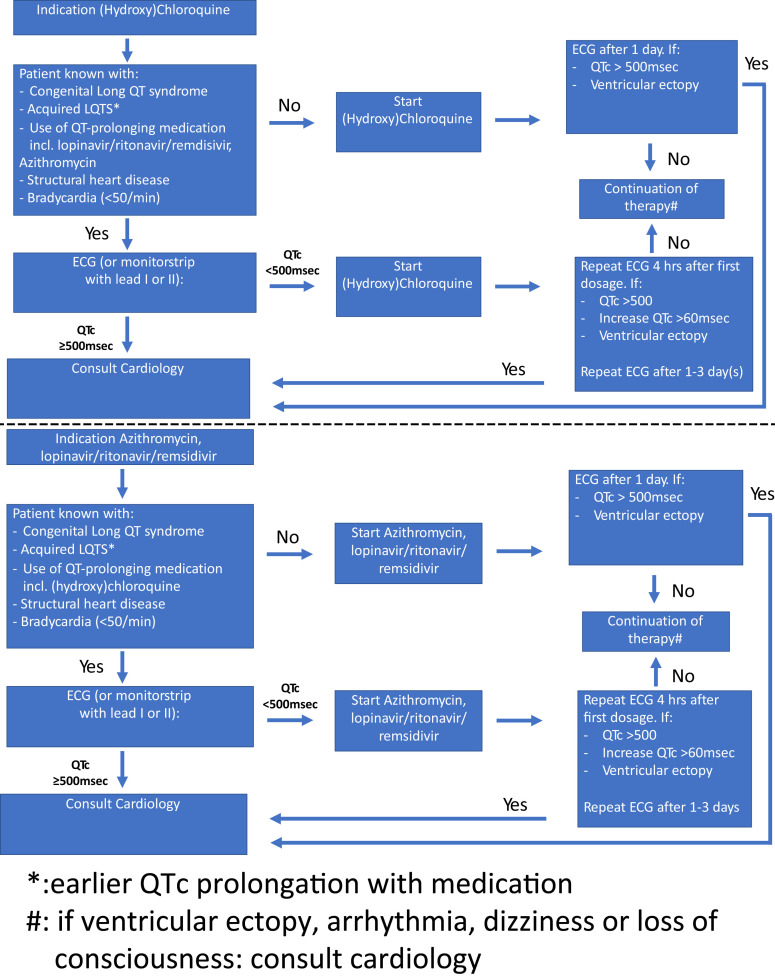
Chloroquine is one of the most widely used antimalarial drugs worldwide, but it has also been investigated as a potential broad-spectrum antiviral drug.18 Among its mechanisms, chloroquine appears to interfere with the terminal glycosylation of ACE2 and may thus negatively influence virus-receptor binding and abrogate infection.19, 20, 21 However, chloroquine is closely related to quinidine, and while the latter is used as an antiarrhythmic drug in BrS and idiopathic forms of ventricular fibrillation, it is also well known for its QT-prolonging effects and has been associated with QT interval-related malignant arrhythmias. Luckily, the QT-prolonging effect of chloroquine is modest, and in general, it does not result in clinically significant QT prolongation in patients without LQTS.22 Hydroxychloroquine sulfate, a less toxic derivative of chloroquine, is widely used in the chronic treatment of autoimmune diseases without significant effects on ECG parameters23 and was recently shown to also efficiently inhibit SARS-CoV-2 infection in vitro.24 However, both chloroquine and hydroxychloroquine are metabolized by CYP3A4, and COVID-19 treatment with (hydroxy)chloroquine can be combined with additional antiviral treatments such as ritonavir plus lopinavir (both potent CYP3A4-inhibiting drugs; their combination is associated with QT prolongation), azithromycin (besides being a macrolide antibiotic, it is investigated for its antiviral properties, with also (weak) CYP3A4 inhibition and associated with QT prolongation),25 , 26 or remdesivir (an investigational drug for which metabolism and possible QT-prolonging effects are not yet resolved). Combining (hydroxy)chloroquine with these drugs might thus result in higher plasma levels and significant QT prolongation. Hence, we advise monitoring QT intervals and cardiac rhythm if initiating these drugs given the increased risk of malignant arrhythmias (Figure 1 ). In addition, physicians should be aware of the α-blocking effects of (hydroxy)chloroquine, which might result in hypotension.
Figure 1.
Flowchart of the proposed guidance for corrected QT (QTc) interval monitoring in patients receiving (hydroxy-)chloroquine and/or antiviral drugs and/or azathromycin. It should be noted that not every patient with long QT syndrome (LQTS) has the same risk. The length of the QTc interval is of importance (as is implicit in the flowchart), but also sex, age, and genotype are important. Patients with LQTS type 2 may be at higher risk than patients with LQTS type 1, for example. The consulted cardiologist should have sufficient experience with QT-related arrhythmic problems. ECG = electrocardiography.
Another issue is fever. The effect of fever is much less evident in patients with LQTS in contrast to patients with, for example, BrS (see below). A possible exception is LQTS type 2 patients with specific mutations who are presenting with fever-triggered arrhythmias, which are based on temperature-sensitive mutant channels (ie, less current with higher temperature).27 As most patients hospitalized for COVID-19 have fever,4 patients with known LQTS will thus generally not be at increased risk. The separate contribution of fever in acquired QT prolongation is not well known, but sepsis is a denominator of risk of acquired QT prolongation28 and septic shock is one of the clinical scenarios in COVID-19.4
Finally, interpretation of the QT interval is not easy,29 but guidance is available.13 While patients with COVID-19 admitted to the ICU will often have continuous ECG monitoring available, ECG monitoring of inpatients who are being treated in an airborne infection isolation room can be challenging. Nevertheless, if possible, we advise (Figure 1) to monitor QT intervals at baseline and at 4 hours after the administration of (hydroxy)chloroquine and/or antiviral therapy in patients with congenital or acquired LQTS, patients already taking other QT-prolonging drugs, and patients with structural heart disease or bradycardia. A second ECG is recommended after 1–3 days. In all other patients, QTc interval monitoring should be performed 24 hours after the initiation of therapy. During the course of (hydroxy)chloroquine and/or antiviral therapy, QTc interval monitoring is furthermore indicated in case of worsening kidney/liver function and electrolyte disorders (in particular, K+, Ca2+, and Mg2+), especially in patients with LQTS or patients with abnormal QT intervals at baseline. Of particular concern is COVID-19–associated diarrhea, which may lead to hypokalemia with adverse effects on the QTc interval. In addition, β-blocker treatment should be considered if the patient is not yet treated. Cardiologists throughout Europe, Canada, and the United States have initiated a QT interval registry for patients with COVID-19 treated with chloroquine, hydroxychloroquine, and/or antiviral drugs and contribution is open to all.
In summary, we advise (Figure 1)
-
1.
QTc interval monitoring when using (hydroxy)chloroquine in patients with COVID-19
-
2.
QTc interval monitoring when using or combining antiviral drugs in patients with COVID-19
-
3.
QTc interval monitoring in patients with known LQTS, acquired QT prolongation, or conditions associated with acquired QT prolongation (eg, use of other QT-prolonging drugs, structural heart disease, bradycardia [<50 beats/min], and liver and renal disease)
-
4.
When the QTc interval is >500 ms, we advise consultation with a cardiologist (“QT specialist”) for guidance (which might, eg, result in intensified monitoring, raising potassium levels, and/or discontinuation of ≥1 QT-prolonging drugs)
-
5.
Patients with acquired LQTS or patients using a combination of QT-prolonging drugs should have a high serum potassium level. Avoiding hypokalemia is not enough, and the adagium should be “a serum potassium of 5 is better than 4.”30
D. BrS
BrS is a familial arrhythmia syndrome characterized by the type 1 Brugada ECG pattern in the right precordial leads of the ECG (coved-type ST-segment elevation and T-wave inversion in lead V1 and/or V2) and an increased risk of ventricular fibrillation and sudden cardiac death. Up to 30% of patients with BrS carry a loss-of-function pathogenic variant (mutation) in SCN5A, the gene that encodes the cardiac sodium channel, as the pathophysiological substrate of their disease.31 The most frequently used drugs for patients with SARS-CoV-2 and COVID-19 are not on the list of drugs to be avoided by patients with BrS (the exception being propofol).32 However, attention to the management of patients with BrS is relevant in the setting of the SARS-CoV-2 outbreak since ECG manifestations of the disorder may be uncovered during fever and since fever has been unequivocally associated with life-threatening arrhythmic events (LTEs) in patients with the disorder.33
The importance of fever in patients with BrS is now well established.33, 34, 35 In 24 patients with BrS, 3 of whom had a fever-triggered cardiac arrest, the increase in body temperature reduced the PR interval in control individuals, but increased PR interval, QRS width, and the maximum J point in patients with BrS.34 Another study showed that fever-associated BrS seems to be associated with a higher future risk of LTEs compared to drug-induced type 1 Brugada ECG pattern.35 Finally, fever seems to be particularly relevant in children.33 Indeed, in a registry with symptomatic patients with BrS (the Survey on Arrhythmic Events in Brugada Syndrome (SABRUS) registry), ∼6% of LTEs were associated with fever and the highest rate of fever-triggered LTEs was observed in the very young (5 years or younger; 65%). In the age range 16–70 years, only 4% of the LTEs was related to fever. In the elderly (older than 70 years), this percentage increased to 25%.33
In the setting of fever, the presence of a pathogenic variant in SCN5A may be particularly relevant. In a single-center series of 111 patients with BrS, 22 presented with a cardiac arrest, and 4 of these cases were fever related. Three of these 4 patients harbored a pathogenic variant in SCN5A.34 In the SABRUS registry, the percentage of SCN5A pathogenic variants was 77% in children and 27% in adults with an LTE.33 The authors also performed an analysis of all published cases (up to 2018) with fever-triggered LTEs (40 patients in 22 reports) which revealed that the presence of a putatively pathogenic variant in SCN5A was found in 13 of 19 patients tested (68%).33 Moreover, in a multicenter pediatric population of 106 patients, 10 patients had an LTE during follow-up, which was triggered by fever in 27%; all the 10 patients were positive for a pathogenic SCN5A variant.36 Finally, preliminary data in a pediatric cohort indicated that mainly children with an SCN5A mutation developed a type 1 Brugada ECG pattern during fever (43.8% of children who developed a type 1 Brugada ECG pattern during fever had an SCN5A mutation vs 4.2% of children without a type 1 Brugada ECG pattern during fever) and had events during follow-up (7 of 21 vs 0 of 47).37 These studies collectively indicate that sodium channel function is sensitive to temperature. This sensitivity may be due to altered temperature-sensitive kinetics, in particular accelerated inactivation,38 and/or decreased sodium channel expression at higher temperatures.39 Also in other sodium channel–mediated diseases, increased temperature sensitizes patients to disease-related symptoms.40 , 41
On the basis of the above, we feel that the following recommendations are pertinent:
-
1.
All patients with BrS should self-treat with paracetamol/acetaminophen immediately if they develop signs of fever and self-isolate.
-
2.Patients without an implantable cardioverter-defibrillator (ICD) who are at higher risk because of fever include
-
a.sodium channel disease with or without a type 1 Brugada ECG pattern,
-
b.children, young adults (younger than 26 years old), and the elderly (older than 70 years) with BrS, and
-
c.all patients with a spontaneous type 1 Brugada ECG pattern and/or cardiac syncope.
-
a.
-
3.
If these higher-risk patients develop a high fever (>38.5°C) despite paracetamol treatment, they will need to attend the emergency department.∗ The emergency department must be forewarned to allow assessment by staff with suitable protective equipment. Assessment should include an ECG† and monitoring for arrhythmia. If an ECG shows the type 1 Brugada ECG pattern, then the patient will need to be observed until fever and/or the ECG pattern resolves. If all ECGs show no sign of the type 1 Brugada ECG pattern, then they can go home to self-isolate.
-
4.
Patients who are not part of the higher-risk group and have a drug-induced type 1 Brugada ECG pattern, no symptoms of syncope, and no sign of a spontaneous type 1 Brugada ECG pattern at any other time are at lowest risk and can afford to self-isolate at home. The risk of visiting the emergency department and contracting COVID-19 is likely to outweigh the risk of an LTE. Attendance at hospital should then be dictated by other clinical features, such as palpitations or (pre-)syncope. The same advice holds for patients with an ICD.
Management in the hospital should include monitoring of ECG abnormalities and arrhythmia as well as efforts to reduce the body temperature (with antipyretic drugs, preferably paracetamol/acetaminophen, or eventually ibuprofen). More generally, patients with BrS, in particular those with a pathogenic or likely pathogenic variant in SCN5A, are advised to self-isolate in their private environment.
E. SQTS
SQTS is a familial arrhythmia syndrome characterized by short QT intervals on the ECG and a significant rate of ventricular arrhythmias.42 It is a heterogeneous disease caused by pathogenic variants in at least 3 different potassium channel genes KCNH2, KCNQ1, and KCNJ2 and the cardiac chloride–bicarbonate exchanger gene SLC4A3. 43 It is an extremely rare disease; in a recent systematic literature review, only 110 cases were described.44 No specific triggers for LTE, including fever, have been described. Hence, on the basis of current knowledge, patients with SQTS do not seem to be at particular risk when they are affected by COVID-19.
Potential drugs for patients with COVID-19, such as chloroquine, might actually be beneficial for patients with SQTS because of lengthening of their QT interval, as has been suggested by modeling data for SQTS type 1 (KCNH2-related45) and type 3 (KCNJ2 related45 , 46). There are no clinical data as far as we are aware.
Therefore, we do not believe that there is a particular concern when patients with SQTS are infected with SARS-CoV-2.
F. CPVT
CPVT is a familial arrhythmia syndrome characterized by adrenergic-related ventricular arrhythmias (ie, during exercise or stress).42 It is a heterogeneous disease with pathogenic variants in RYR2 encoding the human ryanodine receptor 2 as the most important contributor.47 The first-line treatment comprises intensive β-blocker therapy. In insufficiently responsive cases, flecainide should be added or left sympathetic denervation should be conducted.42 , 47 ICD therapy should be avoided.48
As mentioned above, exercise and emotional circumstances constitute specific triggers for LTE. An increased heart rate alone (pacing-induced), as an important symptom of fever, does not appear to be sufficient for the induction of ventricular arrhythmias.49 Fever as a specific trigger has not been described. Whether the stressful circumstances that patients with COVID-19 find themselves in will lead to an increased burden of arrhythmias can only be speculated upon.
The antiviral therapy proposed for COVID-19 is not expected to lead to increased risk. The only potential deleterious pharmacological interaction in these patients is drugs with α- or β-adrenoceptor mimetic activity, which may be used in cases in need of hemodynamic support. Intravenous epinephrine has been used to unmask ventricular arrhythmias, and initial data suggested that epinephrine was more effective than exercise testing in unmasking ventricular arrhythmias.50 However, later studies revealed a low sensitivity and high specificity (with the exercise test as the gold standard51). Nevertheless, based on their pathophysiological mechanism of action, epinephrine, isoproterenol, and dobutamine, all α and/or B1 receptor agonists, should probably be avoided. Milrinone, the most widely used phosphodiesterase 3 inhibitor, acts by decreasing the degradation of cyclic adenosine monophosphate. This may potentially stimulate the RyR2 receptor and thus must be used with caution. However, with the continuation of β-blockers (as we recommend; see below), this may not be that relevant because β-blockers suppress milrinone-induced increased Ca2+ leak.52 Patients with CPVT, in particular those who were symptomatic before diagnosis, should stay on their β-blocker treatment with or without flecainide as long as it is tolerated hemodynamically. Flecainide does have interactions with ritonavir/lopinavir and chloroquine, yet we believe that it is an important enough therapy not to discontinue in these particularly stressful circumstances.
On the basis of the above, we also suggest avoidance of epinephrine in the setting of a ventricular tachycardia/ventricular fibrillation arrest, if possible. This is probably the only resuscitation setting where epinephrine is contraindicated.53
Conclusion
Patients with inherited arrhythmia syndromes may be at an increased proarrhythmic risk in the setting of COVID-19 infection, necessitating additional precautions and specialized management. Preventive measures should include stringent social distancing to prevent infection, aggressive antipyretic treatment to reduce fever in patients with BrS, and ECG monitoring in patients with LQTS treated with antiviral drugs. In the meantime, some larger studies on the therapeutic effect of (H)CQ have not been able to confirm the beneficial effects of these drugs, although one may still consider using the drug for prevention. In the meantime, some larger studies on the therapeutic effect of (H)CQ have not been able to confirm the beneficial effects of these drugs, although one may still consider using the drug for prevention.54
Acknowledgments
We acknowledge the support from “the Netherlands CardioVascular Research Initiative”: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences (PREDICT-2). This work was also supported by grants from Yin Shu-Tien Foundation Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program(No.107-V-B-014 and No. 108-V-A-013 to CI Wu).
Footnotes
Attendance at the emergency department may require regulation according to the capacity of service and risk of SARS-CoV-2 infection.
Ideally 3 different ECGs with leads V1 and V2 in the fourth, third, and second intercostal spaces.
Contributor Information
Cheng-I Wu, Email: c.wu@amsterdamumc.nl.
Arthur A.M. Wilde, Email: a.a.wilde@amsterdamumc.nl.
References
- 1.Wang D., Hu B., Hu C. clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin [published online ahead of print March 16, 2020]. Eur Heart J. ehaa190. https://doi.org/10.1093/eurheartj/ehaa190
- 9.Wu C., Hu X., Song J. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) [published online ahead of print February 29, 2020]. medRxiv. https://doi.org/10.1101/2020.02.26.20028589
- 10.Bedford J., Enria D., Giesecke J. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang P., Kanki H., Drolet B. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 12.Paulussen A.D., Gilissen R.A., Armstrong M. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med (Berl) 2004;82:182–188. doi: 10.1007/s00109-003-0522-z. [DOI] [PubMed] [Google Scholar]
- 13.Vink A.S., Neumann B., Lieve K.V.V. Determination and interpretation of the QT interval. Circulation. 2018;138:2345–2358. doi: 10.1161/CIRCULATIONAHA.118.033943. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg I., Horr S., Moss A.J. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011;57:51–59. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postema P.G., Neville J., de Jong J.S.S.G., Romero K., Wilde A.A.M., Woosley R.L. Safe drug use in long QT syndrome and Brugada syndrome: comparison of website statistics. Europace. 2013;15:1042–1049. doi: 10.1093/europace/eut018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenberg I., Moss A.J., Bradley J. Long-QT syndrome after age 40. Circulation. 2008;117:2192–2201. doi: 10.1161/CIRCULATIONAHA.107.729368. [DOI] [PubMed] [Google Scholar]
- 17.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D., Dai S.-M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. Article 105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 23.Costedoat-Chalumeau N., Hulot J.S., Amoura Z. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007;46:808–810. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018;2018:1574806. doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z., Prinsen J.K., Bersell K.R. Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin A.S., Herfst L.J., Delisle B.P. Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118:2552–2561. doi: 10.1172/JCI35337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tisdale J.E., Jaynes H.A., Kingery J.R. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viskin S., Rosovski U., Sands A.J. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–574. doi: 10.1016/j.hrthm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Yang T., Roden D.M. Extracellular potassium modulation of drug block of IKr: implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- 31.Antzelevitch C., Yan G.X., Ackerman M.J. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 2016;13:e295–e324. doi: 10.1016/j.hrthm.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs.org). Heart Rhythm 2009;6:1335-1341. [DOI] [PMC free article] [PubMed]
- 33.Michowitz Y., Milman A., Sarquella-Brugada G. Fever-related arrhythmic events in the multicenter Survey on Arrhythmic Events in Brugada Syndrome. Heart Rhythm. 2018;15:1394–1401. doi: 10.1016/j.hrthm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Amin A.S., Meregalli P.G., Bardai A., Wilde A.A.M., Tan H.L. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216–218. doi: 10.7326/0003-4819-149-3-200808050-00020. [DOI] [PubMed] [Google Scholar]
- 35.Mizusawa Y., Morita H., Adler A. Prognostic significance of fever-induced Brugada syndrome. Heart Rhythm. 2016;13:1515–1520. doi: 10.1016/j.hrthm.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Andorin A., Behr E.R., Denjoy I. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm. 2016;13:1274–1282. doi: 10.1016/j.hrthm.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Peltenburg P, Vink AS, Blom NA, Rammeloo LAJ, Clur SAB. Fever in children at-risk for the Brugada syndrome. Poster HRS 2019 (S-PO04-217); 10.1016/j.hrthm.2019.04.017. [DOI]
- 38.Dumaine R., Towbin J.A., Brugada P. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 39.Wan X., Wang Q., Kirsch G.E. Functional suppression of sodium channels by β1-subunits as a molecular mechanism of idiopathic ventricular fibrillation. J Mol Cell Cardiol. 2000;32:1873–1884. doi: 10.1006/jmcc.2000.1223. [DOI] [PubMed] [Google Scholar]
- 40.Escayg A., MacDonald B.T., Meisler M.H. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+ 2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 41.Novella S.P., Hisama F.M., Dib-Hajj S.D., Waxman S.G. A case of inherited erythromelalgia. Nat Clin Pract Neurol. 2007;3:229–234. doi: 10.1038/ncpneuro0425. [DOI] [PubMed] [Google Scholar]
- 42.Priori S.G., Wilde A.A.M., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Thorsen K., Dam V.S., Kjaer-Sorensen K. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat Commun. 2017;8:1696. doi: 10.1038/s41467-017-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raschwitz L.S., El-Battrawy I., Schlentrich K. Differences in short QT syndrome subtypes: a systematic literature review and pooled analysis. Front Genet. 2020;10:1312. doi: 10.3389/fgene.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo C., Wang K., Liu T., Zhang H. Computational analysis of the action of chloroquine on short QT syndrome variant 1 and variant 3 in human ventricles. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:5462–5465. doi: 10.1109/EMBC.2018.8513572. [DOI] [PubMed] [Google Scholar]
- 46.El Harchi A., McPate M.J., Zhang Yh, Zhang H., Hancox J.C. Action potential clamp and chloroquine sensitivity of mutant Kir2.1 channels responsible for variant 3 short QT syndrome. J Mol Cell Cardiol. 2009;47:743–747. doi: 10.1016/j.yjmcc.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Werf C., Wilde A.A.M. Catecholaminergic polymorphic ventricular tachycardia: from bench to bedside. Heart. 2013;99:497. doi: 10.1136/heartjnl-2012-302033. [DOI] [PubMed] [Google Scholar]
- 48.van der Werf C., Lieve K.V., Bos J.M. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J. 2019;40:2953–2961. doi: 10.1093/eurheartj/ehz309. [DOI] [PubMed] [Google Scholar]
- 49.Danielsen T.K., Manotheepan R., Sadredini M. Arrhythmia initiation in catecholaminergic polymorphic ventricular tachycardia type 1 depends on both heart rate and sympathetic stimulation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krahn Andrew D., Gollob M., Yee R. Diagnosis of unexplained cardiac arrest. Circulation. 2005;112:2228–2234. doi: 10.1161/CIRCULATIONAHA.105.552166. [DOI] [PubMed] [Google Scholar]
- 51.Marjamaa A., Hiippala A., Arrhenius B. Intravenous epinephrine infusion test in diagnosis of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2012;23:194–199. doi: 10.1111/j.1540-8167.2011.02188.x. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi S., Susa T., Ishiguchi H. A low-dose β1-blocker in combination with milrinone improves intracellular Ca2+ handling in failing cardiomyocytes by inhibition of milrinone-induced diastolic Ca2+ leakage from the sarcoplasmic reticulum. PLoS One. 2015;10 doi: 10.1371/journal.pone.0114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellamy D., Nuthall G., Dalziel S., Skinner J.R. Catecholaminergic polymorphic ventricular tachycardia: the cardiac arrest where epinephrine is contraindicated. Pediatr Crit Care Med. 2019;20:262–268. doi: 10.1097/PCC.0000000000001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Offerhaus J.A., Wilde A.A.M., Remme C.A. Prophylactic (hydroxy)chloroquine in COVID-19: potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020;17:1480–1486. doi: 10.1016/j.hrthm.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]