Abstract
Objective
To determine the contributions of apraxia of speech (AOS) and anomia to conversational dysfluency.
Methods
In this observational study of 52 patients with chronic aphasia, 47 with concomitant AOS, fluency was quantified using correct information units per minute (CIUs/min) from propositional speech tasks. Videos of patients performing conversational, how-to and picture-description tasks, word and sentence repetition, and diadochokinetic tasks were used to diagnose AOS using the Apraxia of Speech Rating Scale (ASRS). Anomia was quantified by patients' scores on the 30 even-numbered items from the Boston Naming Test (BNT).
Results
Together, ASRS and BNT scores accounted for 51.4% of the total variance in CIUs/min; the ASRS score accounted for the majority of that variance. The BNT score was associated with lesions in the left superior temporal gyrus, left inferior frontal gyrus, and large parts of the insula. The global ASRS score was associated with lesions in the left dorsal arcuate fasciculus (AF), pre- and post-central gyri, and both banks of the central sulcus of the insula. The ASRS score for the primary distinguishing features of AOS (no overlap with features of aphasia) was associated with less AF and more insular involvement. Only ∼27% of this apraxia-specific lesion overlapped with lesions associated with the BNT score. Lesions associated with AOS had minimal overlap with the frontal aslant tract (FAT) (<1%) or the extreme capsule fiber tract (1.4%). Finally, ASRS scores correlated significantly with damage to the insula but not to the AF, extreme capsule, or FAT.
Conclusions
Results are consistent with previous findings identifying lesions of the insula and AF in patients with AOS, damage to both of which may create dysfluency in patients with aphasia.
Several factors affect fluency in aphasia.1–4 These include apraxia of speech (AOS) and anomia or word finding problems. AOS is an acquired motor speech disorder in which the intent, linguistic representations, and underlying motor ability for speech are intact, but the ability to correctly program speech movements is impaired.5 The articulatory groping (attempts to achieve initial articulatory configurations) and inconsistent errors (phoneme substitutions, additions, prolongations, repetitions, and distortions) found in AOS5 have been linked to impairments in both feedforward control6 (i.e., the motor commands to produce the intended acoustic output) and feedback control.7
However, the specific brain regions associated with AOS and anomia are still a matter of debate. Several studies have documented lesions to the insula in patients with AOS8,9 and no insula lesions in patients without AOS, but others have shown no association between AOS and insula damage.10–12 Still, others have documented patients with AOS with spared insula, patients with AOS with damaged insula, and patients with damage to the insula but no AOS.13 Damage to certain white matter fiber tracts associated with language has also been linked to dysfluency. Lesion load of the arcuate fasciculus (AF)14 and damage to the overlapping portion of the anterior AF and the ventral/posterior frontal aslant tract (FAT) have both been shown to predict fluency.11
We sought to investigate how much AOS and anomia each contribute to fluency and identify the location/overlap of lesions associated with each. Understanding their unique contributions to dysfluency is crucial if we are to provide the most effective therapy.
Methods
Participants
Fifty-two stroke survivors (6F) with chronic aphasia were recruited from the stroke service at Boston's Beth Israel Deaconess Medical Center, referred by outside physicians, or through ClinicalTrials.gov. Eligibility criteria included ≥6 months postonset of left hemisphere stroke, age 18–80 years, right-handedness, and persistent expressive aphasia despite receiving standard speech therapy. Exclusion criteria included bihemispheric or brainstem infarcts, history of previous stroke, other neurologic disorders, or significant cognitive impairments (i.e., ≤50% correct on Raven Colored Progressive Matrices15).
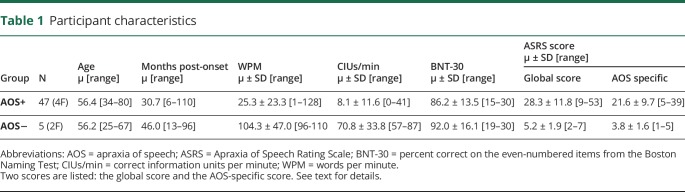
Levels of impairment ranged from mild (minimal loss of fluency) to severe (inability to produce any meaningful speech) (table 1). Words/minute (wpm), calculated from picture-description and conversation tasks, ranged from 1 to 128 wpm. Generally, individuals with AOS were less fluent than individuals without AOS.
Table 1.
Participant characteristics
Standard protocol approvals, registrations, and patient consents
All participants were screened for 1 of 2 treatment protocols approved by the IRB of Beth Israel-Deaconess Medical Center, and all patients gave written informed consent before enrollment.
Behavioral assessments
We used the Apraxia of Speech Rating Scale (ASRS16) to code video of our patients performing conversational interviews; how-to descriptions; picture description (e.g., “Cookie Theft”) and word/sentence repetition from Boston Diagnostic Aphasia Examination17; and diadochokinesis tasks from the apraxia battery for adults, second edition18 (ABA-2). The ASRS contains 16 items identifying characteristic features of AOS. Each item is rated from 0 (not present) to 4 (nearly always evident and marked in severity). The global ASRS score is the sum of all 16 items, range: 0–64. Global scores >8 indicate a diagnosis of AOS.
Signs of AOS on the ASRS fall into 4 categories: (1) primary distinguishing features of AOS (no overlap with dysarthria or aphasia), (2) distinguishing features unless dysarthria present, (3) distinguishing features unless aphasia present, and (4) distinguishing features unless dysarthria and/or aphasia present. In our analyses, we used both the global score and an AOS-specific score, consisting of categories (1) and (2). Reliability figures appear below.
Performance on the Boston Naming Test19 (BNT) was used to quantify anomia. Although patients were administered the full 60-item BNT, participants' scores on the 30 even-numbered items (BNT-30), a Common Data Element recommended by the National Institute of Neurological Diseases and Stroke,20 were used for this analysis.21
We used correct information units per minute (CIUs/min),22 which combines word-production efficiency and informativeness, to quantify fluency. Hierarchical multivariable linear regression (SPSS v.25) was used to determine the contribution of the global ASRS score and BNT-30 score to variance in CIUs/min. All hypotheses were 2 sided.
Imaging analyses
Structural MRI was performed using a 3-T GE scanner. Images were acquired using a T1-weighted, 3-dimensional, magnetization-prepared, rapid-acquisition, gradient-echo volume acquisition with a voxel resolution of 0.93 × 0.93 ×1.5 mm. To perform voxel-based lesion-symptom mapping (VLSM) analyses, lesion masks were first drawn manually on T1-weighted images by an experienced investigator blinded to the question of interest. Masks were normalized using SPM8.23 Figure 1 shows the overlap of patients' lesions.
Figure 1. Patients' initial left-hemisphere lesion overlays.
Lesion density map superimposed on a canonical T1-weighted image. Red indicates areas of greatest overlap; blue, least overlap.
Normalized lesions were submitted to statistical mapping analyses using VLSM algorithms in nonparametric mapping/MRIcron software.24 Voxel-wise Brunner-Munzel nonparametric tests (z-scores)24 were performed to compare continuous ASRS and BNT-30 scores in each left hemisphere voxel. False discovery rate correction was set at p ≤ 0.01 and critical threshold at 19% (i.e., lesioned voxels had to be present in at least 10/52 cases, a conservative criterion recommended for our sample size25). The resulting AOS and anomia maps were then compared to the anatomic atlas (WFU PickAtlas26) and to canonical tracts of the AF, FAT, and EmC. These canonical, probabilistic, left hemisphere tracts (thresholded at 25%) were reconstructed from high-resolution diffusion tensor images of 12 right-handed elderly control subjects.14 This allowed us to determine whether the lesions associated with AOS and anomia were colocalized with known white matter tracts such as the AF, which has been shown to form a link between articulatory movements and perceived feedback of acoustic output,27,28 or the FAT, which has been associated with speech initiation and stuttering-like dysfluencies.29,30 The EmC, a fiber tract considered part of the dorsal language system27 and thought to be closely linked to the AF, connects posterior perisylvian language regions to speech-motor regions in the inferior frontal gyrus (IFG) in addition to connections to the claustrum and the insula.31
Data availability
Deidentified data will be shared upon request from any qualified investigator.
Results
Behavioral assessments
Ten percent of patients' videos were independently coded by 2 investigators (K.C. and S.P.). A 2-way random-effects intraclass correlation coefficient (ICC) for absolute agreement on single measures yielded a between-judge ICC of 0.897 on the overall ASRS score, indicating excellent reliability on identification of features of AOS. ICC values on individual ASRS items ranged from 0.529 (distorted sound substitutions) to 0.986 (syllable segmentation in multisyllable words). The remaining videos were subsequently scored by 1 investigator (K.C.). Of note, 90.4% of our participants (47/52) met the criteria for AOS (global ASRS score >8). No participants were rated as having dysarthria.
Next, a hierarchical multivariable regression was performed to determine how much global ASRS score (first step) and BNT-30 score (second step) contributed to variance in CIUs/min. The global ASRS score and BNT-30 score were not significantly correlated (r = −0.139, p = 0.17). The overall model was significant: F(2,46) = 24.295, p < 0.001. Together, the ASRS score and BNT-30 score accounted for approximately half of the variance in CIUs/min (R2 = 0.514). However, the BNT-30 score only increased R2 by 0.044 (p = 0.05), indicating that the ASRS score accounted for the majority of the variance in fluency. The variance inflation factor was 1.020, indicating no collinearity between the 2 independent variables. Regression coefficients appear in table 2.
Table 2.
Regression parameters for fluency

Imaging results
Initial analyses were performed using the global ASRS score (figure 2). Lesioned voxels associated with the global ASRS score mapped to the longitudinal portion of the left dorsal AF; the middle to inferior portions of the pre- and post-central gyri; and along the short posterior insular gyrus and the long anterior insular gyrus, parallel to the central sulcus of the insula (table 3).
Figure 2. Left-hemisphere lesion location corresponding to global ASRS score.
Red indicates lesioned voxel locations associated with the global score from the apraxia of speech rating scale (ASRS) superimposed onto a canonical T1-weighted image.
Table 3.
VLSM p < 0.01 clusters
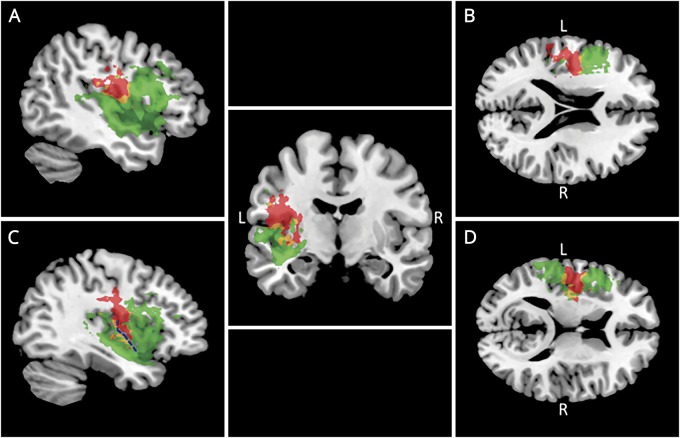
A second analysis identified voxels associated with the AOS-specific score, calculated from parts 1 and 2 of the ASRS. The AOS-specific score (red, figure 3) mapped to a larger number of lesioned voxels than the global ASRS score, especially in the insula. Regions associated with the AOS-specific score included less longitudinal left dorsal AF and pre- and post-central gyri, but more putamen and regions on both sides of the central sulcus of the insula. There was only minimal overlap with the FAT (∼1%) and EmC (1.4%) lesioned voxels mapping to the global or AOS-specific ASRS score.
Figure 3. Left-hemisphere lesion location corresponding to AOS-specific ASRS and BNT-30 scores.
Red indicates lesion corresponding to the AOS-specific score from the apraxia of speech rating scale. Green: lesion corresponding to BNT-30 score. Yellow: overlap. Panels A through D depict different sagittal and axial slices, which show the lesion overlap on a canonical T1-weighted brain. The blue dotted line in the lower left section (C) indicates the central sulcus of the insula. AOS = apraxia of speech.
Of note, 72.3% of the clusters associated with the AOS-specific score (red, figure 3) were distinct from the region associated with the BNT-30 score (green, figure 3). The remaining 27.7% of the clusters associated with the AOS-specific score and BNT-30 score overlapped (yellow, figure 3).
By contrast, lesions linked with the BNT-30 score were located along the entire supratemporal gyrus, in the IFG, in the lentiform nucleus/extranuclear regions, and in regions of the insula distinct from those adjacent to the central sulcus of the insula (figure 3; bottom left). Table 3 details the number and percentage of lesioned voxels corresponding to each score type and associates clusters of voxels with their anatomical regions.
In addition to the VLSM analysis, we also assessed the degree of correlation between ASRS scores (global and AOS specific) and the degree of damage to specific left hemisphere brain structures (insula, AF, EmC, and FAT). Pearson r was significant only for both scores and the percent of the insula that was lesioned (global score: r = 0.344, p = 0.021; AOS-specific score: r = 0.351, p = 0.011). Neither ASRS scores were significantly correlated with weighted lesion load of the AF, EmC, or FAT. Table 4 details the correlation results.
Table 4.
Pearson r between ASRS scores and damage to the insula, AF, EmC, and FAT
Discussion
We examined the contributions of signs of AOS and anomia to dysfluency in patients with post-stroke aphasia, focusing on 2 components of dysfluency that are differentially diagnosable using separate test instruments and thought to arise from damage to different brain regions. We compared lesion locations for ASRS and BNT-30 scores, identifying the anatomic regions and white matter tracts that, when damaged, produce signs of each disorder. Several findings emerged.
As expected, fluency was affected by both AOS and anomia, which together accounted for approximately half of the variance in CIUs/min. Presumably, the remainder can be explained by previously identified factors1: syntactic production, language comprehension, and working memory. However, we found that AOS, not anomia, was the major contributor to dysfluency.
Second, lesions associated with AOS and anomia were largely nonoverlapping. This is consistent with previous findings11 of little overlap in lesions corresponding to AOS and an overall measure of aphasia (Western aphasia battery [WAB-R] score32). The present results are consistent with those showing that damage to both the insula and the anterior AF (Broca area) gives rise to AOS.13 Similarly, our results represent middle ground between studies showing an association between AOS and damage to the insula8,9 and those linking AOS to damage in the pre- and post-central gyri,10,11 adding nuance to what might be considered contradictory findings. The significant correlation of ASRS scores with damage to the insula but not to the AF, EmC, or FAT supports the idea that insular damage contributes to AOS.
We interpret our finding that lesions associated with AOS included the insula in light of research on the putative function of the insula in speech production. One group33 found activation along the precentral gyrus of the insula during production of syllable sequences, concluding that the anterior insula integrates lower-level aspects of speech-motor planning with abstract representations of speech sounds for sequence planning, supplying contextual information to the speech-sound map. Others identified the anterior-dorsal portion of the insula as being involved in language tasks, musical processing, and perception of vocalization, speculating that it functions as an error detection or sensorimotor integration region.34 Still, others found that the superior precentral gyrus of the insula was, in fact, most strongly activated by nonspeech oral movements.35
Thus, it may be that while the AF is involved in bidirectional interaction of perceptual and auditory-motor mapping, the insula may support that function by integrating sensory and motor function at a more fundamental level. This implies that patients with AOS with greater ability to self-monitor may have less AF damage, whereas injury to the insula may more strongly affect the ability to produce correct motor patterns for output that integrates sensory feedback. Because the insular region is a phylogenetically preserved older brain region containing partly a 3-layered archicortex, it may be more involved in the fundamental operations of sensorimotor integration of vocal sounds than are other higher-order, more recently evolved brain regions such as the parietal and frontal opercula. The latter might support more sophisticated, complex models of feedforward/feedback speech-motor operations.
Findings that scores on tests of both word-finding and fluency loaded onto the same factor in a principal components analysis,36 and that deficits in these areas largely associated with lesions in the IFG and the anterior insula, are also relevant to our results. They confirm that both anomia and apraxia contribute to dysfluency in aphasia and are consistent with the idea that damage to the insula and IFG is associated with these difficulties. Note, however, that speech repetition ability (ABA score) was associated with a phonology/working memory factor, not word-finding/fluency,36 and with lesions in the ventral motor and somatosensory cortex, supramarginal gyrus, and posterior planum temporale. Arguably, these regions include the AF—lesions of which were found to be associated with AOS. This is consistent with its putative role in generating a forward model of speech production.
Our finding that lesions associated with AOS did not overlap with the FAT contradicts research identifying damage to the intersection of the AF and FAT as reducing speech fluency (measured by the WAB-R score37). However, the WAB-R score takes into account factors beyond the ability to initiate and sequence speech movements (a putative function of the FAT): patients' ability to produce propositional phrases (a semantic task), sentences with normal syntax, and paraphasia-free speech. By contrast, signs of AOS identified by the ASRS involve phonetic errors (e.g., phoneme distortions) and prosodic errors (e.g., slow speech rate and syllable segmentation). Thus, the specific construct used for fluency appears important for lesion-mapping studies, and discrepant findings may be due to dysfluency's multifactorial nature. Furthermore, the fact that lesions associated with the BNT-30 score did include the FAT suggests that the region of overlap between the AF and FAT may be more involved in language production than previously thought.
A final issue concerns differential diagnosis and treatment planning. Although AOS and anomia represent very different types of spoken language impairments, AOS can affect patients' performance on the BNT and anomia can affect performance on the ASRS. Inability to sequence correct movements in naming tasks can result in distorted production of words, nonwords, or complete inability to produce the word. In addition, features such as phoneme distortions, articulatory groping, and speech initiation difficulty are common to both disorders.
It is thus clinically important to determine the degree to which AOS contributes to individual aphasic patients' dysfluency to provide individualized treatment that capitalizes on their strengths and targets their specific challenges. Treatment for AOS incorporates repetition of syllables38 and words39 using principles of motor learning40 and includes feedback on production accuracy. Treatment centers on rebuilding the forward model of speech production by providing multiple opportunities to practice the speech movement sequences, using both massed practice (many repetitions of the same target in a row) and distributed practice (practice of several different stimuli within a therapy session).41
The extent of damage to the insula may affect the degree of improvement a patient with aphasia with AOS can expect from therapy. If AF lesions are associated with damage to the feedforward model of speech production, and insular lesions are associated with diminished ability to use auditory or sensory feedback to improve speech accuracy, patients with damage to the AF or insula alone may show greater improvement than those with AF and insula lesions. This hypothesis should be investigated in future research.
A limitation of this study is the limited number of participants (n = 52). However, a recent study examined the relationship of sample size variation to reproducibility of the link between insular damage and decreased speech fluency.42 Of note, 71.2% of resamples for n = 60 in that study resulted in a significant finding, with a mean effect size (R2) of 0.15 (range 0.07–0.52). Thus, our significant finding is likely accurate, although our R2 of 0.47 for global ASRS score is on the high side of this range.
Despite the challenges of distinguishing AOS from anomia, the fact that lesions associated with ASRS and BNT-30 scores were largely nonoverlapping supports the construct validity of and lends confidence to the use of these assessments as measures of AOS and anomia, respectively.
Appendix. Authors

Study funding
This research was supported by grants to GS from the NIH (R01 DC008796, R01 DC008796-02S1, and R01 DC009823), the Grammy Foundation, the Richard and Rosalyn Slifka Family Fund, the Tom and Suzanne McManmon Family Fund, and the Mattina R. Proctor Foundation. K.C. was supported by NIDCD T32 DC013017 (PI: C. A. Moore). S.P. was supported by Postdoctoral Awards from the Canadian Institutes of Health Research.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Nozari N, Faroqi-Shah Y. Investigating the origin of nonfluency in aphasia: a path modeling approach to neuropsychology. Cortex 2017;95:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haley K, Wertz R, Ohde R. Single word intelligibility in aphasia and apraxia of speech. Aphasiology 1998;12:715–730. [Google Scholar]
- 3.Haley K, Ohde R, Wertz R. Single word intelligibility in aphasia and apraxia of speech: a phonetic error analysis. Aphasiology 2000;14:179–201. [Google Scholar]
- 4.Haley K, Jacks A, Cunningham K. Error variability and the differentiation between apraxia of speech and aphasia with phonemic paraphasia. J Speech Lang Hear Res 2013;56:891–905. [DOI] [PubMed] [Google Scholar]
- 5.McNeil M, Robin D, Schmidt R. Apraxia of speech: definition and differential diagnosis. In Mc Neil M, editor. Clinical Management of Sensorimotor Speech Disorders, New York: Thieme Medical Publishers, Inc; 2009:267–286. [Google Scholar]
- 6.Feenaughty L, Basilakos A, Bonilha L, et al. Non-fluent speech following stroke is caused by impaired efference copy. Cogn Neurosci 2017. 2017;34:333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartle-Meyer C, Murdoch B. A kinematic investigation of anticipatory lingual movement in acquired apraxia of speech. Aphasiology 2010;24:623–642. [Google Scholar]
- 8.Dronkers NF. A new brain region for coordinating speech articulation. Nature 1996;384: 159–161. [DOI] [PubMed] [Google Scholar]
- 9.Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang 2006;97:343–350. [DOI] [PubMed] [Google Scholar]
- 10.Hillis AE, Work M, Barker PB, Jacobs M, Breese E, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain 2004;127:1479–1487. [DOI] [PubMed] [Google Scholar]
- 11.Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke 2015;46:1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itabashi R, Nishio Y, Kataoka Y, et al. Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke 2016;47:31–36. [DOI] [PubMed] [Google Scholar]
- 13.Moser D, Basilakos A, Fillmore P, Fridriksson J. Brain damage associated with apraxia of speech: evidence from case studies. Neurocase 2016;22:346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Marchina S, Norton A, Wan C, Schlaug G. Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci 2013;7: article 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raven JC. Colored Progressive Matrices. Oxford: Oxford Psychologist's Press; 1995. [Google Scholar]
- 16.Strand E, Duffy J, Clark H, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord 2014;51:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodglass H, Kaplan E, Barresi B. BDAE: The Boston Diagnostic Aphasia Examination. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 18.Dabul B. Apraxia Battery for Adults. Austin: PRO-ED; 1979. [Google Scholar]
- 19.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 20.Grinnon ST, Miller K, Marler JR, et al. National Institute of Neurological Disorders and Stroke Common Data Element Project—approach and methods. Clin Trials 2012;9:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson A, Wong S, Gracer T, Ozonoff A, Green R, Stern R. Geriatric performance on an abbreviated version of the Boston naming test. Appl Neuropsychol 2007;14:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas L, Brookshire R. A system for quantifying the informativeness and efficiency of connected speech of adults with aphasia. J Speech Lang Hear Res 1993;36:338–350. [DOI] [PubMed] [Google Scholar]
- 23.Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. New York: Elsevier; 2011. [Google Scholar]
- 24.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 25.Medina J, Kimberg DY, Chatterjee A, Coslett HB. Inappropriate usage of the Brunner–Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia 2010;48:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 27.Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 2008;105:18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado IL, Moritz-Gasser S, Duffau H. Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct 2011;216:263–274. [DOI] [PubMed] [Google Scholar]
- 29.Vassal F, Boutet C, Lemaire JJ, Nuti C. New insights into the functional significance of the frontal aslant tract: an anatomofunctional study using intraoperative electrical stimulations combined with diffusion tensor imaging-based fiber tracking. Br J Neurosurg 2014;28:685–687. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita M, de Champfleur NM, Deverdun J, et al. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct Funct 2015;220:3399–3412. [DOI] [PubMed] [Google Scholar]
- 31.Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 2009;213:343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kertesz A. The Western Aphasia Battery-Revised. New York: Grune and Stratton; 2007. [Google Scholar]
- 33.Bohland J, Guenther F. An fMRI investigation of syllable sequence production. Neuroimage 2006;32:821–841. [DOI] [PubMed] [Google Scholar]
- 34.Mutschler I, Wieckhorts B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neurosci Lett 2009;457:66–70. [DOI] [PubMed] [Google Scholar]
- 35.Fedorenko E, Fillmore P, Smith K, Bonilha L, Fridriksson J. The superior precentral gyrus of the insula does not appear to be functionally specialized for articulation. J Neurophysiol 2015;113:2376–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacey EH, Skipper-Kallal LM, Xing S, Fama ME, Turkeltaub PE. Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabil Neural Repair 2017;31:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basilakos A, Fillmore P, Rorden C, Guo D, Bonilha L, Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front Hum Neurosci 2014;8:Article 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Merwe A. A speech motor learning approach to treating apraxia of speech: rationale and effects of intervention with an adult with acquired apraxia of speech. Aphasiology 2011;25:1174–1206. [Google Scholar]
- 39.Buchwald A, Miozzo M. Phonological and motor errors in individuals with acquired speech sound production impairment. J Speech Lang Hear Res 2012;55:S1573–S1586. [DOI] [PubMed] [Google Scholar]
- 40.Maas E, Robin D, Austermann Hula S, et al. Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol 2008;17:277–298. [DOI] [PubMed] [Google Scholar]
- 41.Wambaugh JL, Duffy JR, McNeil MR, Robin DA, Rogers MA. Treatment guidelines for acquired apraxia of speech: treatment descriptions and recommendations. J Med Speech Lang Pathol 2006;14:33–67. [Google Scholar]
- 42.Lorca-Puls D, Gajardo-Vidal A, White J, et al. The impact of sample size on the reproducibility of voxel-based lesion-deficit mappings. Neuropsychologia 2018;115:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data will be shared upon request from any qualified investigator.