Abstract
COVID-19 emerges as a pandemic disease with high mortality. Development of effective prevention and treatment is an urgent need. We reviewed TH17 responses in patients with SARS-CoV-2 and proposed an FDA approved JAK2 inhibitor Fedratinib for reducing mortality of patients with TH17 type immune profiles.
Keywords: COVID-19, SARS-CoV-2, Cytokine storm, TH17, JAK2 inhibitor
COVID-19 (previously termed as 2019-nCoV), a novel coronavirus disease with high mortality, emerges as a pandemic disease. As of Mar. 8, 2020, COVID-19 has spread to 102 countries and caused 3584 deaths out of 105,586 confirmed cases [WHO, Coronavirus disease 2019 (COVID-19) Situation Report – 48]. There is no existing treatment specific for COVID-19. Current treatments are largely symptomatic. Development of effective prevention and treatment is an urgent need, especially for the life-threatening severe cases.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many COVID-19 patients develop acute respiratory distress syndrome (ARDS), which leads to pulmonary edema and lung failure, and have liver, heart, and kidney damages.1 , 2 These symptoms are associated with a cytokine storm, manifesting elevated serum levels of IL-1β, IL-2, IL-7, IL-8, IL-9, IL-10, IL-17, G-CSF, GM-CSF, IFNγ, TNFα, IP10, MCP1, MIP1A and MIP1B.1 Compared with non-ICU patients, ICU patients have even higher levels of IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP1A, and TNFα.1 Amongst these, several cytokines are involved in TH17 type responses. IL-1β and TNFα (TH17 and TH1 cells highly express TNFα), both promote TH17 responses and vascular permeability and leakage. TH17 cells themselves produce IL-17, GM-CSF (GM-CSF is mainly associated with TH1 cells in human), IL-21 and IL-22 (currently, there are no data on IL-21 and IL-22). IL-17 has broad pro-inflammatory effects on induction of cytokines G-CSF (responsible for granulopoiesis and recruitment of neutrophils), IL-1β, IL-6, TNFα (the latter 3 cause systemic inflammatory symptoms, including fever); chemokines KC, MIP2A, IL-8, IP10, MIP3A (attracting and recruiting more immune infiltrates); and matrix metalloproteinases (participating in tissue damage and remodeling). IL-17 (and GM-CSF) are associated with autoimmune and inflammatory diseases. IL-21 is required for TH17 cell maintenance and germinal center responses in a STAT3 dependent manner. IL-22, in collaborate with IL-17 and TNFα, is known to induce antimicrobial peptides in the mucosal organs. In addition to antimicrobial peptides, IL-22 upregulates mucins, fibrinogen, anti-apoptotic proteins, serum amyloid A, and LPS binding protein3; therefore, IL-22 may contribute to the formation of life-threatening edema enriched with mucins and fibrin, seen in SARS-CoV-22 and SARS-CoV patients.4 Xu et al. showed that peripheral blood of a patient with severe COVID-19 had a strikingly high number of CCR6+ TH17 cells,2 further supporting a TH17 type cytokine storm in this disease. Elevated TH17 (as well as TH1) responses or enhanced IL-17-related pathways are also observed in MERS-CoV and SARS-CoV patients.5 , 6 In MERS-CoV patients, higher IL-17 with lower IFNγ and IFNα have worse outcome than the reversed phenotype.5 Pandemic H1N1 influenza virus also induces strong TH17 (and TH1) responses.7 In a mouse model, H1N1 causes acute lung injury in an IL-17-dependent manner.8 Taken together, the TH17 type response contributes to the cytokine storm in pulmonary viral infection including SARS-CoV-2, which results in tissue damage and likely promotes pulmonary edema; targeting the TH17 pathway may benefit the patients with TH17 dominant immune profiles.
Since it will take several years to develop specific drugs to treat COVID-19, repurposing currently marketed drugs would provide valuable opportunities. There are several antibody-based TH17 blockades (anti-IL-17, anti-IL-17R and anti-IL-12/23p40) available; however, the antibody-based treatment is expensive and has only a narrow spectrum of effects. Several RORγt (and RORα) inhibitors currently on clinic trials would be promising TH17 blockers in a near future. Here, we propose an alternative method to inhibit TH17 responses.
STAT3, a transcription factor, mediates IL-6 and IL-23 signals for TH17 cell initial differentiation and effector function. Both IL-6 and IL-23 activate STAT3 through JAK2 (IL-6 also uses JAK1),9 whereas IL-21 activates STAT3 (and STAT1 and STAT5) through JAK1 and JAK3. We postulate that JAK2 inhibitors can be used to restrict the proinflammatory function of existing TH17 cells. In addition to JAK2 inhibitors, several FDA approved STAT3 inhibitors are also promising but may affect IL-21 signals in B cells. Type I interferons are important in anti-viral immunity, but type I interferons employ JAK1 and TYK2 to activate STAT1 and STAT2. Therefore, specific JAK2 inhibitors would not disrupt the signals of type I interferons.
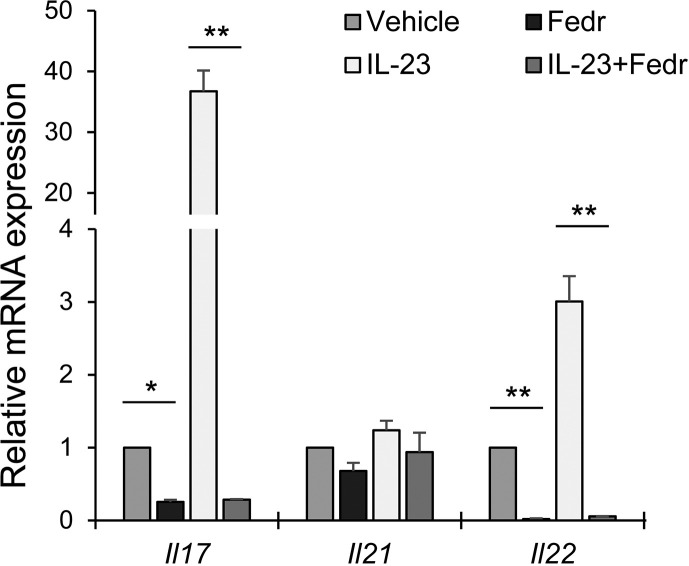
We tested Fedratinib (SAR302503, TG101348), a JAK2 inhibitor approved by FDA for myeloproliferative neoplasms, on TH17 cell cytokine production. Fedratinib is specific for JAK2 but does not affect JAK1, JAK3 and TYK2. We found that Fedratinib treatment decreased the expression of IL-17 by murine TH17 cells, and this suppressive effect was even more profound when IL-23 was added (Fig. 1 ). In addition, Fedratinib also inhibited the expression of IL-22 by TH17 cells (Fig. 1). Besides, Fedratinib only has marginal effects on IL-21 expression (Fig. 1), suggesting that Fedratinib does not compromise IL-21 mediated B cell function. In addition, GM-CSF also uses JAK2 to transduce signals; therefore, JAK2 inhibitor would also suppress GM-CSF function. In a murine model of multiple sclerosis, a TH17 and TH1-driven autoimmune brain disease, subcutaneous administration of JAK2 inhibitor tyrphostin B42, during the disease induction, greatly decreased the disease severity.10 In summary, JAK2 inhibitor Fedratinib can suppress the production of several TH17 signature cytokines (and likely also the effects of IL-6 on other types of cells), therefore promising to prevent the deteriorating outcomes of TH17 associated cytokine storm in COVID-19 and other severe viral infections. The JAK2 inhibitor can also be used in combination of anti-viral drugs and supportive treatments. Because JAK2 inhibition is reversible, transient treatment with this inhibitor before the disease transition from serious to critical or during the critical phase would not affect TH17 responses essential for innate immune responses and immunity against extracellular pathogens.
Figure 1.
Quantitative RT-PCR of cytokine mRNAs in murine TH17 cells. In vitro differentiated TH17 cells were activated with plate-bound anti-CD3 and anti-CD28 and treated with or without IL-23 in the presence of 2 μM Fedratinib (Fedr) or a vehicle (saline) for 4 h. The results were normalized to an internal control Actb and the vehicle treatment was set as 1. Data (mean and s.d.) represent 2 experiments (N = 3 in each group). Two side student T test, ∗, p ≤ 0.05; ∗∗, p ≤ 0.005.
Declaration of Competing Interest
All authors have no conflicts of interest to declare.
Acknowledgments
XOY is supported by NIH AI142200.
Abbreviations
- COVID-19
coronavirus disease-2019
- TH
T helper cell
- SARS
severe acute respiratory syndrome
- CoV
coronavirus
- MERS
Middle East Respiratory Syndrome
- FDA
U.S. Food and Drug Administration
- JAK
Janus kinase
- ARDS
acute respiratory distress syndrome
- IL
interleukin
- IL-17R
interleukin 17 receptor
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
Granulocyte-Macrophage Colony Stimulating Factor
- IFN
interferon
- TNF
tumor necrosis factor
- IP10
Interferon gamma-induced protein 10
- MCP1
Monocyte Chemoattractant Protein-1
- MIP1
macrophage inflammatory protein 1
- ICU
intensive care unit
- CCR
CC chemokine receptor
- ROR
RAR-related orphan receptor
- STAT
signal transducer and activator of transcription protein
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zenewicz L.A. IL-22: there is a gap in our knowledge. Immunohorizons. 2018;2:198–207. doi: 10.4049/immunohorizons.1800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse G.M., To K.F., Chan P.K., Lo A.W., Ng K.C., Wu A. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josset L., Menachery V.D., Gralinski L.E., Agnihothram S., Sova P., Carter V.S. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio. 2013;4 doi: 10.1128/mBio.00165-13. e00165-00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramirez P. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Yang P., Sun Y., Li T., Wang C., Wang Z. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabbi M., Carbotti G., Ferrini S. Dual roles of IL-27 in cancer biology and immunotherapy. Mediat Inflamm. 2017;2017:3958069. doi: 10.1155/2017/3958069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bright J.J., Du C., Sriram S. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999;162:6255–6262. [PubMed] [Google Scholar]