Highlights
-
•
Summarize the study of the role of chemokines and their receptors in multiple sclerosis (MS) patients and MS animal models.
-
•
Discuss their potential significance in inflammatory injury and repair of MS.
-
•
Summarize the progress in the research of MS antagonists in recent years with chemokine receptors as targets.
Keywords: Chemokines, Chemokine receptors, Multiple sclerosis
Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease that is characterized by leukocyte infiltration and subsequent axonal damage, demyelinating inflammation, and formation of sclerosing plaques in brain tissue. The results of various studies in patients indicate that autoimmunity and inflammation make an important impact on the pathogenesis of MS. Chemokines are key mediators of inflammation development and cell migration, mediating various immune cell responses, including chemotaxis and immune activation, and are important in immunity and inflammation, therefore we focus on chemokines and their receptors in multiple sclerosis. In this article, we summarize the study of the role of prominent chemokines and their receptors in MS patients and MS animal modelsand discuss their potential significance in inflammatory injury and repair of MS. We have also summarized the progress in the treatment of multiple sclerosis antagonists in recent years with chemokine receptors as targets.
1. Introduction
Multiple sclerosis is an immune-mediated, chronic, demyelinating disorder, which is commonly considered to result from the interaction of environmental and genetic factors that still unclear [26], [100]. It’s the most common neurological disease among young adults and meanwhile a major cause of physical disability among young adults [26], [85]. Current MS phenotypic classifications contain RRMS, CIS, PPMS and SPMS, which means relapsing-remitting multiple sclerosis, clinically isolated syndrome, primary-progressive multiple sclerosis, and secondary-progressive multiple sclerosis respectively [71]. Among them, RRMS accounts for the majority of patients (about 80%) [87]. The hallmark of MS is leukocyte infiltration of brain tissue and subsequent axonal injury, demyelination inflammation and the formation of sclerosing plaques [54].
Abnormal inflammatory response initiated by CD4 + T cells promotes tissue damage of the CNS in EAE and MS. Among the CD4 + T cells, Th1 cells and Th17 cells are dominant, the former producing IFN-γ (interferon-γ), the latter secreting IL-17A, IL-17F, IL-21, IL-22, and GM- CSF (granulocyte–macrophage colony-stimulating factor) [60], [123]. After the secreted substances enter the central nervous system, they activate astrocytes and microglia, produce a large number of chemokines and cytokines, and recruit peripheral immune cells to sites of inflammation [123]. The use of CXCR3 and CCR6 as markers of identification of Th17 cells not only reflects their pro-inflammatory status but also reflects their ability to migrate to localized sites of inflammation [118]. The initiation of T cell entry into the CNS is governed by integrin-dependent vascular adhesion and chemokine-driven trans-blood–brain barrier [1]. The pathophysiology of MS is not fully understood. It appears that the autoimmune activity of Th17 cell-mediated aggressive attacks is one of the key mechanisms. Th1 cells secrete cytokines (IL-4, IL-10) leading to activation of macrophages and cytotoxicity while cytokines (IFN-γ) produced by Th2 cells lead to activation of B cells and induction of antibody production. Th1 cytokines inhibit Th2 development, and Th2-related cytokines inhibit Th1 responses. The results of some studies indicate that a specific Th1-mediated immune response contributes to the pathogenesis of MS disease and that inhibition of Th1 response is protective against the development of MS, and it is reported that the transition from Th1 to Th2 cytokine has a beneficial effect on the clinical course of MS [46].
Although most research on the pathogenesis of MS has focused on the role of CD4 T cells, in fact, compared to most animal models, the main T cells found in the central nervous system of MS patients are CD8 T cells, suggesting that CD8 T Cells may play an important role in human disease [52]. There is growing evidence indicates that successful treatments are often related to changes in many other lymphocyte subpopulations, including B cells, NK cells, Lti cells, other ILC cells, γδT cells, NKT cells, MAIT cells and innate B cells. These findings not only indicate that multiple lymphocyte subpopulations are involved in the pathogenesis of the disease, but also identify the cells as potential targets for immunotherapy [117].
Classically studied animal models of MS are (1) allergic encephalomyelitis/experimental autoimmune (EAE); (2) virus-induced models and (3) toxin-induced demyelination models [90].Experimental autoimmune encephalomyelitis (abbreviated as EAE), an experimental animal model of human MS, is obtained by using myelin oligodendrocyte protein(MOG), proteolipid protein(PLP) or myelin basic protein(MBP) immuning and inducing in certain susceptible experimental animals such as mice and other rodents [44]. Besides, EAE can be triggered by the adoptive transfer of myelin antigen-specific T cells, CD8 + T lymphocytes and Th1 and Th17 type CD4 + cells [66], [87]. The pathogenesis of EAE consists of two phases. First, the EAE priming phase: DC migrates to peripheral lymph nodes for self-antigen presentation, induces encephalitogenic Th cells. The second point is encephalitis Th cells are accumulated in the CNS to re-occur cognate antigen on local and CNS-invasive APC(antigen-presenting cells, homologous antigen) [102]. The pathological characteristics of experimental autoimmune encephalomyelitis are not uniform because they vary widely depending on the type of animal used and the type of epitope. The EAE model is relevant for RRMS mostly. MOG induces chronic progressive disease which is closer to SPMS in C57BL/6 mice whereas chronic relapsing-remitting disease in NOD/Lt and SJL mice and Lewis rats [87], non-classical chronic relapsing EAE in PL/J mice. MOG35-55-immunized NOD mice will first develop an acute episode of EAE and then undergo a secondary progressive EAE course. This model can be used to simulate the secondary progressive disease of some MS patients [39]. In DA (Dark Agouti) rats, recombinant rat MOG can be used to induce EAE, which is characterized by demyelinating and spinal cord injury with subcutaneous and perivascular inflammatory infiltration [25]. In SJL/J mice, PLP139-151 induced disease mimicking relapsing-remitting EAE [21], [25]. MBP can induce acute self-limiting or chronic recurrent disease / progressive disease in guinea pigs. T cell clones reactive with MBP peptides induce EAE in SJL/J mice, which is characterized by acute paralysis attacks, and mice can be partially or fully recovered [39]. Furthermore, spinal cord homogenate can be used to induce EAE in Biozzi ABH mice, recapitulating the clinical characteristics of MS, including secondary progressive disability and relapsing-remitting episodes, which can address the role of an adaptive immune response in neurodegeneration [55].
Mouse Hepatitis (corona) Virus (MHV) and Theiler’s virus (TMEV) are the two most widely used viral in inducing chronic encephalomyelitis as MS animal models. Systemic exposure of animals to cuprizone(CPZ) is most commonly used for toxic demyelination models, other demyelination models caused by focal toxins, including focal injections of lysolecithin or ethidium bromide in specific white matter tracts and focal injections of some other toxins not widely in use, such as ionomycin, diphtheria toxin or 6-aminonicotinamide in the spinal cord [13], [63]. Among them, the CPZ model simulates the acute and chronic courses of MS and may be a useful tool for the development of new therapies that protect oligodendrocytes and stimulate myelin regeneration. The TMEV infection model is an animal model that specializes in studying the mechanisms of virus-mediated acute and primary progressive MS [87].Chemokines regulate lymphoid tissue development, lymphocyte transport, and homing, and leukocyte maturation as we all know [10]. In autoimmune diseases such as MS, characterized by uncontrolled inflammatory states and increased immune cell infiltration into tissue parenchyma, upregulated expression of chemokines is associated with elevated infiltration of macrophages, monocytes, and lymphocytes in lesions and plaques [62]. The levels of specific chemokine ligands such as CCL2, CCL5, CXCL12, and CX3CL1 continue to rise, resulting in sustained infiltration of immune cells [22], [97]. Many chemokines that are produced locally in the tissue, attract different kinds of leukocytes to the sites of infection and inflammation via selective receptors [10]. Chemokines could induce and activate leukocyte adhesion molecules, establishing a chemotactic concentration gradient that causes transendothelial monolayer recruitment [64], [110]. The induction of proteolytic enzymes promotes the opening of the BBB and mediates the retention of leukocytes in the central nervous system. Chemokines can also increase adhesion and migration of target cells through the BBB via interacting with leukocyte integrins [12], [106]. For example, autoreactive T cells targeting myelin components are triggers for MS disease and that’s a vital step for the transport of inflammatory T cells to the CNS in MS. A lot of reports have analyzed the role of chemokines and chemokine receptors in the intrathecal accumulation of T cells in MS [111].
2. Chemokines and chemokine receptors
Chemokines are small proteins that own conserved sequence and structural features, which usually have 70–80 amino acid residues, typically 8–10 kDa in MW(molecular weight) [113]. Human and other mammalian genomes encode approximately 50 chemokines respectively. Most Chemokines contain four cysteine residues, naturally, they are divided into four classes according to structure character that the spacing between the first two of cysteine residue: CC(β), CXC(α), CX3C(γ) and C(δ) [113], [121]. In the first three subfamilies, the two cysteine residues are spaced out by 0, 1, and 3 residues separately, while in the XC subfamily the second cysteine residues do not exist [113]. The systematic names of chemokines consist of a prefix like CCL, CXCL, CX3CL or CL followed by an identifying number. Chemokine receptors are named according to which group of chemokines they bind [68], therefore their systematic names consist of a prefix like CCR, CXCR, CX3CR or CR followed by an identification number. In the system nomenclature, the “L” signifies a ligand while the “R” denotes a receptor. For example, CCL2 and CCR2, are named based on the above rules [113].
Besides the above criteria, chemokines may be categorized according to their biological roles [113]. Inflammatory chemokines are those activated and upregulated under inflammatory conditions and have roles in response to tissue damage, infection, and other physiological abnormalities in both adaptive and innate immunity, including CXCL1-3 and CXCL5-8 [86]. Their expression is temporary, until the resolution of the situation [3], [127]. Homeostatic chemokines expressed in lymphoid or other organs constitutively, including CXCL12和CXCL13 [86]. They mediate the homeostatic migration and homing of different cells containing lymphocytes, which is needed for the proper function of the immune system. In addition, developmental processes that related to the patterning of cells and complex movements often involve homeostatic chemokines [3], [127]. There is also a third class of bifunctional chemokines that are elevated in case of inflammatory conditions and are participated in normal immune defense. This group includes CXCL9, 10, 11,16, CCL1, CCL20, CCL25, etc [86], [99].
Chemokines are critical mediators of inflammation, development and cell migration during routine immune surveillance [3], which can be secreted by activated T lymphocytes and regulate the accumulated and migration of monocytes/macrophages and lymphocytes to damaged CNS districts and promote the differentiation, therefore maintain the immune response process [126]. Chemokine receptors are γ subfamily rhodopsin GPCRs (G protein-coupled receptors). This family includes members such as angiotensin, somatostatin, fMLP, bradykinin and adrenomedullin receptors (ADMRs) [27]. A great majority of chemokines bind and activate multiple receptors and in turn, a great majority of chemokine receptors respond to more than one chemokines [113]. Chemokines bind to GPCRs(chemokine receptors more precisely) cause conformational changes that touch off intracellular signaling pathways involved in cell activation and movement [27], thus mediates various leukocyte responses, including chemotaxis and immune activation [123].
Different chemokine/chemokine receptors represent different immune responses types:
CCR1, CCR5, and CXCR3 are a feature of Th-1 immune response, and CCR3, CCR4, and CCR10 are the feature of Th-2 immune response [1]. In addition, CCR4 and CCR6 are highly expressed in Th17 cells while CCR6 and CXCR3 are highly expressed in Th17.1 cells [1], [67]. In humans and mice, chemokine receptors have different expression levels in memory T cells and effector cell subsets, providing specificity for cell trafficking in both homeostasis and inflammation. CCR6 directs Th17 cells into the CNS and CXCR7 inhibits T cell migration. CCR7 migrates naive T cells and central memory T cells to peripheral lymph nodes [66]. Small molecule-mediated upregulation of CCR7 can ameliorate EAE through this role at the initial stage of the disease, providing a promising strategy for the more effective disease therapies development of MS [66]. CNS white matter trauma in MS and acute EAE shows high levels of CCL2, 3, 4, 5, and CCL7, which are involved in the aggregation and activation of leukocytes expressing CCR2, CCR4, 5 and CCR6 receptors [123].
3. CC family and their receptors in MS
The CC class chemokine is the largest of the four chemokines [95], consisting of at least CCL1-28, 28 chemokine members which signal through CCR1-10. CC chemokines have chemotactic effects on monocytes and lymphocyte subsets, but also stimulate eosinophils, basophils and natural killer cells [86]. Chemokine receptors that bind to CC class chemokines are mainly expressed by monocyte-macrophages and T cells, the cell types are related to chronic inflammation such as multiple sclerosis that with persistent leukocyte infiltration into the site of inflammation [122].
3.1. CCL2/Ccr2
CCL2, also known as MCP-1, is a potent chemokine for the recruitment process of macrophages, monocytes, activated T cells and NK cells to the CNS [47]. MCP is an important member of the CC subfamily. To date, five MCPs are identified and classified: from MCP-1to MCP-5, which means CCL2, CCL8, CCL7, CCL13, and CCL12. Among MCPs, MCP-1was the first one to be discovered and is the most characteristic CC subclass chemokine in humans [15]. CCL2 can be produced by cells such as endothelial cells, astrocytes, macrophages and microglia [47]. One study showed that the efflux of CCL2 may be mediated by the astrocyte ABC transporters (MRP-1 and P-glycoprotein), thereby promoting the migration of immune cells across the brain endothelial cells, involved in the process of neuroinflammation [57].
CCR2 is crucial for the induction of acute EAE. It has been determined that all five MCPs play their roles through interaction with the CCR2 receptor [15]. Due to the highest affinity of CCL2 for CCR2, most studies have focused on it [128]. There are two alternative splicing types of CCR2 in humans, CCR2A and CCR2B, which are 360 and 374 amino acids, respectively [15]. CCR2 is expressed on T cell subsets, immature dendritic cells, and monocytes, mediating their migration to endogenous CC chemokines such as CCL2 [124]. The endogenous ligands for CCR2 in humans are CCL2, CCL7, CCL8, CCL11, CCL13, CCL16, CCL24, and CCL26. It is worth noting that the first six are agonists while the last two are antagonists [113]. The endogenous ligands in mice for CCR2 are CCL2, CCL7, CCL8, CCL11 and in rats are CCL2, CCL7, CCL11. (http://guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId = 59 (accessed 19 September 2019))
CCL2 levels in CNS are reduced during increased disease activity in MS [82]. According to the discovery of Franciotta D et al., CCL2 can be constitutively produced in the brain and CCL2 CSF levels in acute MS are lower than those in stable MS [34]. As demonstrated by Gd-enhanced lesions on MRI, RRMS patients with acute exacerbations had significantly lower CCL2 levels in CSF and serum than those without enhanced lesions [107]. However, in another study, the results were determined to have higher levels of CCL2 in CSF in patients with RRMS compared to controls. This difference between studies may be due to differences between populations, differentiation in CCL2 production at unequal stages of the disease, rarely using healthy controls or the different components of the control group in the study [83]. MS relapses have been shown to be induced by the depression of Th2 cytokines and predominance of Th1, while CCL2 can affect T cell differentiation and adjust the Th1/Th2 cytokine profiles. Thus, the decreased CCL2 levels in CSF might reflect an increase in Th1 activity during an acute episode [107]. CCL2 mRNA expression levels are dramatically increased in both the spinal cord and brain and CCL2 protein expression levels are significantly upregulated in the brain during the relapsing phase of MS. The CCL2 expression has a biologically relevant function in the relapsing EAE disease process due to the ability to improve relapsing clinical disease with anti- CCL2 treatment. The inhibition of relapsing EAE is relevant to a decreased influx of macrophages [51].
Data demonstrate that the level of CNS CCL2 protein production is low in preclinical B6129PF2/J or C57BL/6J mice, but as the disease rises (MOG induced) sharply through the initial clinical symptoms to the development of acute disease peaks [32]. Hulkower et al. not only demonstrated that CCL2 (MCP-1) mRNA was expressed with close temporal relation to symptom onset in the CNS of rats, but also noted that CCL2 mRNA could no longer be detected when the animals entered remission. It’s a reasonable hypothesis that the encephalitogenic T cells secrete certain proinflammatory cytokines contains IFN-g and TNF-a, which is able to induce astrocytes to express CCL2(MCP-1) and RANTES etc. leads to the recruitment of additional mononuclear cells [51]. Upregulation of CCL2 in activated oligodendrocyte progenitors near the demyelinating region,it’s possible that CCL2 differentiates into mature myelin to form oligodendrocytes by enhancing the activity of oligodendrocyte progenitor cells, thereby allowing remyelination [31]. CCL2 and CCL3 play a role in a region-specific manner in cuprizone-induced demyelination(in C57BL/6J mice) and astrocyte activation, with special effects on gray matter [47]. In addition, current MS therapies (6-methylprednisolone or IFN-β1a treatment) do not modify the circulating levels of CCL2 [34]. It is known that the proportion of CCR2+ Ly6Chigh cells in the CNS is positively correlated with the EAE clinical score. The recruitment of the proinflammatory CCR2+Ly6Chigh monocyte subpopulation involves chemokine CCL2 [49]. In addition, CCR2-/- mice did not illustrate CNS histopathology or clinical EAE(MOG induced in B6129PF2/J or C57BL/6J mice) and decreased obviously in T cell infiltrating when compared with control mice [32]. Recent evidence suggests that the highly phagocytic and inflammatory macrophages produced by blood-derived CCR2rfp/+ monocytes elicit demyelination in EAE. According to Nielsen et.al., assessment of the demyelinating effect of CCR2+ monocytes on cortical shows: Th/+ CCR2−/− mice developed EAE later than Th/+ CCR2+/+ mice, but their severity is comparable. More importantly, both perivascular cortical and subpial demyelination(MOG induced in C57BL/6J mice) of Th/+ CCR2−/− mice were significantly reduced compared with Th/+ CCR2+/+ mice [61]. The CCR2 + 190 G/A(rs1799864) polymorphism might elevate susceptibility to multiple sclerosis, but the effect appears to be limited to the individuals with no primary risk allele HLA-DRB1 * 15:01 [48].
3.2. CCL3/ Ccr1
MIP-1 includes MIP-1alpha (CCL3), MIP-1β(CCL4), MIP-1delta (CCL9/10) and MIP-1 gamma (CCL15), which are produced by various cells, especially macrophages, lymphocytes, and dendritic cells. MIP-1 proteins act through CCR1, CCR3-5 on B cells, T cells, DCs, and eosinophils, are best known for chemotaxis and pro-inflammatory effects, but also promote homeostasis [73], [101].In detail, the functions of CCL3 and CCL4 are chemotaxis, phagocytic, synthesis of inflammatory mediators and degranulation in target cells during the acute phase of inflammation, and the activation and recycling of leukocytes [109]. CCL3 is an effective chemokine of lymphocytes and monocytes, one of the most unique features is its ability to coordinate the compartmentalization and mobilization of MPC (myeloid precursor cells). Preclinical studies further demonstrate that CCL3 is released after the induction of the Th1 response [101].
CCR1 is expressed on a great amount leukocytes including dendritic cells, monocytes, NK cells, T cells, basophils, and eosinophils. CCR1 is considered a target for MS due to the observation of perivascular staining of CCL3 and CCR1 in immunohistochemical studies of biopsy of the deceased [2]. The chemokines CCL3, CCL5 and CCL7 and their receptor CCR1 are strongly expressed in the brain inflammatory foci of patients [30].
In a study by Mohammad Soleimani et al., they found an obvious increase in CSF levels of CCL3 in RMSS patients compared with the control group [109]. Eltayeb, S et al found that the use of a DNA vaccination protocol to generate an immunologically neutralizing antibody against CCL3 effectively inhibits disease induction in DA rat EAE(MOG induced). The deletion of the CCR1 gene results in partial disease protection in the MS mouse model. The above data provide a strong argument that CCR1-expressing macrophages activated through CCL3-mediated is critical for neuroinflammatory processes in EAE [30].
3.3. CCL5/Ccr5
CCL5 also has the name of RANTES, which is a marker for M1 macrophages. Infiltrating T cells are able to express and secrete chemokine CCL5 [38], but CCL5 is mainly expressed in perivascular cells, vascular endothelial cells and astrocytes [31]. CCL5 is possible to adjust glutamate release and plasticity in the MS brain with associated effects for the disease clinical manifestations [79] and was detected in active demyelinated MS lesions. CCL5 and its receptor CCR5 have strong monocyte chemotaxis [92]. CCR5 is mainly expressed on immature dendritic cells, T cells, and a small number of monocytes [2] and is expressed on most monocytes, macrophages and CD8 + T cells in inflammatory MS lesions [31]. There are two ways for CCR5 to be involved in the pathogenesis of MS. One is the increase in the number of CCR5-positive INF-γ-secreting T cells in the circulation of MS patients, and the other is that the monocytes having phagocytosis function greatly enriched CCR5 expression in CSF and peripheral blood [72].
In the research of E. Sindern et al., CCL5 was not detected in the CSF of RR-MS, but CCL5 release in serum was increased [107]. However, in the 2015 study by Mori F et al., including RRMS patients, CCL5 level was higher in CSF in patients than in the control group, and CCL5 increased during MS disease activity [79]. Other than that, one research by Sørensen et al. confirmed the presence of elevated CCL5 in cerebrospinal fluid in patients with acute MS and the fact that lymphocytes expressing CCR5 in the active MS lesions of autopsied brain tissue. The above pieces of evidence suggesting the role of CCL5 in the development of MS [107] .
C57BL/6J mice infected with mouse hepatitis virus (MHV) in the brain can reproducibly result in acute encephalomyelitis and develop into chronic demyelinating disease. In the chronic disease stage, neuropathology is primarily immune-mediated, similar to human demyelinating disease MS (multiple sclerosis). Using this animal model, CCL5 is significantly expressed in the CNS during acute disease, and anti-CCL5 mAb-mediated neutralization results in delayed viral clearance with concomitant reduction of CCL5-induced migration of virus-specific T cells and activated macrophages, T cell and macrophage infiltration decreased and demyelination decreased. Experiments show that CCL5 contributes to macrophage entry and demyelination [38]. However, in another animal model, EAE model of MS, the immunological neutralization of CCL5 has no modulating effect. The use of DNA vaccination protocols to generate immunoneutralization antibodies against CCL5 also failed to inhibit disease induction in rat EAE(MOG induced in DA rats). CCR5 genomics deletion also did not protect EAE mice [30], [96].
The impact of CCL5 genetic polymorphism (high producer allele) is relevant to the worse progression of MS disability, and low producer alleles are associated with a decreased risk of severe axonal loss [126]. The expression of CCL5 may be disrupted by gene mutations and downregulation of its receptor CCR5. For example, a genetic polymorphism, ie, a single nucleotide substitution (A to G) at 403 position of the CCL5 gene promoter results in lower expression of CCL5, or in the 303 position of its receptor CCR5 promoter sequence, X32 (32 base pair deletion) of CCR5 gene coding region is associated with a lower MS severity index [79]. Although a significant increase in CCR5 expression has been found in MS patients, it appears that besides the CCR5Δ32 mutation, other haplotypes or polymorphisms, as well as other possible epigenetic and genetic factors, are vital for CCR5 expression in multiple sclerosis patients [37]. In addition, based on a summary of one review, there appears to be no consensus on the association of CCR5Δ32 mutations with MS, and the differences in the reports may reflect the genetic background of the study population and their exposure to environmental factors [37].
3.4. Ccl18
CCL18 also called alternative macrophage activation associated CC chemokine, AMAC-1; or Pulmonary and Activation-Regulated Chemokine, PARC [104]. CCL18 is one specific marker of M2 macrophages and is the highest chemokines expression level in several human chronic inflammatory diseases and are synthesized by dendritic cells and monocytes during infection or inflammation [116]. It has the function of attracting T cells, inducing a CD4 + CD25 + FoxP3 + regulatory T cell phenotype, which may be an important factor in inhibiting the local pro-inflammatory immune response in the CNS [74].
CCL18 levels correlate with neurodegenerative and inflammatory brain MRI results: CCL18 levels were higher in progressive multiple sclerosis compared to healthy individuals and RR-MS, and various correlations with MRI measurements were discovered. Higher CCL18 levels were related to decreased DGM(deep grey matter), NCV(normalized cortical volume) and thalamic volume, and increased T2-LV(lesion volume) and LVV(lateral ventricular volume). These shreds of evidence support the function of CCL18 in the progression of MS, but no correlation between CCL18 levels and EDSS(expanded disability status scale) as well as disease duration was found [126]. However, another study suggests that CCL18 can inhibit CCR1, CCR2, CCR4 and CCR5-mediated chemotaxis [58].
CCL18 was identified as one of the top 3 up-regulated genes on the verge of chronic active MS lesions, where there is a large amount of foam and microglia/macrophages that accumulate myelin. This is consistent with previous research by Boven LA et al [41], [112]. CCL18 recruits a subset of human regulatory T cells and inhibits the proliferation of effector T cells through the IL-10 production in vitro. CCL18 expressed by macrophages also ingests myelin, producing an immunosuppressive phenotype [41]. Incubation of microglia with dexamethasone resulted in a significant upregulation of CCL18 [74]. CCL18 is considered to be an anti-inflammatory factor [18].
3.5. CCL20/Ccr6
CCL20 is produced by many kinds of cells, including endothelial cells and macrophages that respond to stimuli such as IL-6, IFN-γ, TNF-α, IL-1β and so on [65]. CCL20 attracts lymphocytes but does not attract monocytes [1]. CCL20 is expressed in a site called the choroid plexus where T cells enter the CNS. CCR6, the receptor of CCL20, is selectively expressed by CD4 + T cells that produce the cytokine IL-17. Thus, the CCL20-CCR6 interaction is involved in the BBB disruption and follow-up migration of pathogenic T cells into the CNS [29]. Moreover, IL-17 secreted by circulating Th17 cells expands the pro-inflammatory response via NF-κB signaling to enhance CCL20 transcription [29].
In one study by El Sharkawi FZ et al., serum CCL20 levels in patients with SPMS and RRMS are obviously higher than healthy subjects. This trend is identical with the results of the 2017 research of CCL20 serum levels in RRMS patients and controls by Li R et al, as well as the results that higher CCL20 serum levels in patients with SPMS, RRMS and PPMS in a 2014 study by Jafarzadeh A et al. [46], [65]. The study also shows that serum levels of CCL20 were associated with a progression index and showed a higher than remission period during relapse, while treatment type did not influence chemokine levels. This is also consistent with previous research results. However, serum CCL20 levels were higher during remission than in relapse significantly according to Kalinowska-Lyszczarz et al. [29]. One study showed a negative correlation between SIRT1 activity levels and serum CCL20 levels in patients. It is speculated that SIRT1 may down-regulate the transcriptional activity of NF-κB by deacetylating p65 in the promoter region of the CCL20 gene, thereby decreasing the expression of CCL20. This speculation requires further research to prove [65]. Serum CCL20 levels are not affected by disease patterns, gender and treatment options [46]. The researchers found that anti-CCL20 levels were inversely associated with clinical severity of EAE, and MS patients had lower antibodies levels which against CCL20 than healthy controls. Moreover, high levels of anti-CCL20 antibodies in human serum have biological activities that inhibit the TH17 CCR6 positive T cells migration. Based on experimental results, Abraham M et al. hypothesized that natural vaccination against CCL20, which can protect humans from autoimmunity MS development. It is further supposed that exposure to pathogens early in life might result in the production of CCL20 by autoantibodies and trigger a protective effect against MS development [1]. In the research of Jafarzadeh A et al., the CCL20 and CCR6 expression levels in the spinal cord of EAE mice (MOG induced in C57BL/6J mice) administered with PBS were evidently elevated compared to the control group [43], [44].
The results of the study of the concomitant effects of single nucleotide polymorphism (SNP) (-786 T > C(rs6749704))on MS susceptibility showed that the CT genotype frequency of rs6749704 in CCL20 gene and the C allele frequency of multiple sclerosis patients were obviously higher than the control group. The accompanying polymorphism of the gene in MS patients in Egypt shows an increased risk of MS rather than an individual locus [29]. Another study from Iran showed that the rs6749704 polymorphism is associated with the SPMS model [45].
3.6. CCL22/Ccr4
CCL22 is a chemokine for the accumulation and movement of CCR4-expressing cells (contains Th2/Treg cells) into the site of inflammation [44] and can be produced by microglia and astrocytes [81]. Macrophage-derived chemokines (CCL22) are produced by monocyte-derived dendritic cells and macrophages upon stimulation with anti-CD40 antibodies or microbial products and are downregulated by Th1 type cytokines, but upregulated by Th2 type cytokines [46].
CCR4 has been identified as one of the risk genes for MS and found on Treg, Th2 and Th17 cells. In particular, CCR4 is a marker for Th2 cells [2]. Since Th2 cells produce anti-inflammatory cytokines, CCL22 participated in the accumulation of a subset of cells with anti-inflammatory functions in the brain. Regulatory T cells suggest that CCL22 may involve in counteracting pro-inflammatory Th1 responses and ultimately lead to a reduced degree of myelin damage [36].
Decreased serum CCL22 levels have been reported in multiple sclerosis patients [46]. In a 2008 research, CCL22 levels of CSF in women were higher than in men. CCL22 may only have an effect on the MS development in women, affecting the brain recruitment of Th2 cells, which can produce anti-inflammatory cytokines [35]. In a 2014 study, women MS patients showed lower serum CCL22 levels, representing that a decrease in chemokine levels might have a great impact on the MS pathogenesis of women [46]. Compared with OND patients (non-inflammatory neurological diseases), the CSF level of CCL22 was evidently increased in MS patients at baseline [75]. The trend of the decrease in the A allele of CCL22 C/A SNP was put forward in MS patients compared with the control group [36]. CCL22 may primarily attract Treg cells, shutting down the adaptive immune response effectively [20]. The expression levels of CCR4 and CCL22 in the spinal cord of EAE mice(MOG induced in C57BL/6J mice) were significantly increased [43], [44]. CCL22 expression is related to increased severity of EAE, and anti-CCL22 treatment may improve EAE development, possibly by regulating inflammatory macrophage accumulation [28].
4. CXC family and their receptors in MS
In the CXCL subfamily, chemokines can be divided into ELR + ve (ELR positive) group and ELR-ve (ELR negative) group. The former has a characteristic three-sequence ELR (Glu-Leu-Arg), but the latter does not have. ELR + ve chemokines (eg., CXCL1-3, CXCL7-8) combine with CXCR2 and typically attract neutrophil and activate neutrophil degranulation, which causing a release of myeloperoxidase and some other enzymes [86], [120]. ELR negative CXC chemokines (including CXCL4, 10 and CXCL12-13) combine with CXCR3-5, control the chemotaxis of lymphocytes together with XCL chemokines [120].
4.1. CXCL1/ CXCL5/ Cxcr2
Rumble JM et al. demonstrated for the first time that the peak level of plasma CXCL5, the ELR + CXC chemokine, is consistent with the development of new inflammatory lesions in relapsing MS patients. It was also demonstrated that the expression of CXCL1 and CXCL5 is associated with radiological and clinical measurements of CNS lesions in MS [98].
The expression of inflammation-inducible receptor CXCR2 in the endothelium was increased in MS plaques, and Haarmann A et al. demonstrated that CXCL5 and CXCL8 interfere with endothelial cell membrane barrier morphology and function via CXCR2. Inhibition of CXCR2 in brain endothelial cells prevents the decomposition of BBB in EAE but does not directly affect vascular permeability [40]. The oligodendrocyte lineage cells and neutrophils in the CNS express CXCR2, and CXCR2-positive neutrophils promote inflammatory demyelination, which is the first chemokine receptor to show contributes to the control of remyelination and the inflammatory process that cause demyelination [69].
4.2. CXCL12/Cxcr4
CXCL12 is an effective chemoattractant for immune cells, interacts with the CXCR4 receptor, and also binds to atypical chemokine receptor 3. Migration of B cells, CD4 and CD8 cells can be blocked by using CXCR4 neutralizing antibodies in vitro. CXCL12 is elevated in both inactive and active MS lesions, as well as in astrocytes of MS patients. Some reports found that MS patients had higher CSF levels of CXCL12 than healthy subjects.
CXCR4 is the only chemokine receptor that has been proved to be vital to life, which is constitutively expressed in various tissues [56]. CXCR4 has been found in many kinds of cells such as microglia, astrocytes, neurons, naive T cells, effector T cells and vascular endothelial cells in the human brain [7], [56].
CXCL12 (P2G2) is a CXCL12 mutant that has been shown to be an antagonist of CXCL12, which is identical to human CXCL12(1 −6 7) except that the proline is replaced by glycine 7 in position two [56]. The data of Kohler RE et al. showed that CXCL12 (P2G2) can effectively inhibit the migration of activated mouse T cells by CXCL12. Interference with the action of CXCR4 and a synthetic receptor antagonist inhibits EAE(PLP induced in SJL/J mice) and reduces the accumulation of encephalitogenic CD4(+) T cells in the CNS, thus inhibiting the sensitization phase of EAE. CXCL11 is involved in the localization T cells in the CNS, CXCL11 inhibits the effector phase of the immune response, thus targeting CXCR4 and CXCR3 might be beneficial for the CNS autoimmune diseases treatment [56].
4.3. CXCL13/Cxcr5
CXCL13 is a B cell chemokine, also known as B lymphocyte chemoattractant (BLC) or B cell attracting chemokine(BCA-1). Infiltrating macrophages/DC myeloid cells and microglia may be one of the sources of CXCL13 in the central nervous system during inflammation. In addition, CXCL13-expressing cells were found only in the brainstem meninges with strong infiltrating leukocyte proliferation [70]. F. Sellebjerg et al. found that the CSF concentration of CXCL13 correlated with the CSF leukocyte count significantly in patients with RRMS, PPMS,
CIS, and SPMS and even stronger with the CSF B-cell count in patients with CIS and RRMS [105]. In addition to B cells, there is a direct correlation in the cerebrospinal fluid of MS patients between CXCL13 levels and the number of plasmablasts and T cells [59]. Khademi M et al. determined the concentration of CXCL13 in CSF in healthy control populations and patients with MS and other neurological diseases (bacterial and viral infections), and the results showed a lesser extent rise by MS patients. Taken together, we believe that early detection of CXCL13 predicts disease prognosis [53]. CXCR5 is expressed in most B cells in the CSF compartment and all B cells in the blood, and in 20% to 30% of CD4 + T cells in both CSF and blood [89]. The deficiency of CXCL13 attenuated the glial hyperplasia and inflammation of white matter in the acute and chronic phases of EAE, indicating an improvement in the condition [9]. The results reflect that CXCL13 may bind CXCR3. Thus, CXCL13 may be involved in recruiting mononuclear cells expressing CXCR5 or CXCR3 to the intrathecal compartment in MS [105]. CXCL13 levels are reduced by natalizumab treatment in MS [53], [82]. According to Quinn JL et al., the antibody against CXCL13 could block Tfh transport and significantly reduce disease expression in Th17 EAE (EAE that induced by the myelin-specific Th17 cells transfer in C57BL / 6 J mice) [91].
5. CX3C family and their receptors in MS
CX3C family has only one member, CX3CL1, an atypical chemokine in membrane-bound type and soluble form, which is able to act as an effective adhesion molecule and chemoattractant through a non-integrin-dependent mechanism [5].
5.1. CX3CL1/Cx3cr1
CX3CL1, also known as fractalkine, has 373 amino acids. CX3CL1 is the only neuronal chemokine in the body with pro-inflammatory, anti-inflammatory and neuroprotective effects [6]. In the brain, CX3CL1 is expressed in neuronal cells, and when stimulated with TNF-α and IFN-γ, microglia and astrocytes also produce CX3CL1. It can also be expressed by activated endothelial cells(ECs), which are located on the surface of the cavity, indicating that it arrests circulating cells in the EC membrane [14]. CX3CL1 promotes glial activation, ICAM-1 expression, pro-inflammatory cytokine secretion, and recruitment of CD4 + T cells into the CNS during neuroinflammatory processes [114]. CX3CL1 exists in two forms: shed glycoprotein and membrane anchoring. The former soluble form has chemotactic activity against monocytes, T cells and NK cells [78].
CX3CL1 only combines with CX3CR1, which is a GPCR (G-protein coupled receptor) that expressed by microglia, astrocytes, dendritic cells (DCs), NK cells, monocytes, B cells, and T cell subsets [14].
CX3CL1 levels are elevated in CSF from CIS patients, cerebrospinal fluid and serum samples from RRMS patients. In the early inflammatory response of multiple sclerosis, CX3CL1 induced accumulation of CX3CR1(+) ICAM-1(+)CD4(+) T cells in the CNS [14]. The results of Broux B et al. showed that CD4(+)CD28(-)T cells migrated to the inflammation sites that responsd to the chemotactic gradient of CX3CL1, which contributes to the inflammatory process in MS patients [17].
Choroid plexus, the barrier between the cerebrospinal fluid and blood in the brain, is the main entry site for immune cells to enter the central nervous system. CX3CL1 was induced on the choroid plexus during EAE. And the results showed that blocking CX3CL1 prevented the development of EAE and inhibited lymphocytes across into the CNS [77]. Blocking CX3CL1 can protect mice from EAE invasion. The data by Wenjun Zhu et al. showed that CX3CL1 signaling in dorsal horn SC neurons can activate the up-regulation pathway involved in nociceptive transmission during the early inflammatory phase of pre-myelinating MS [125]. MOG-induced EAE(in DA rats) is associated with cells that express CX3CR1 mRNA are clearly accumulated in inflammatory brain injury. The vast majority of these cells are microglia-macrophage lineage cells, and the accumulation of cells other than microglia indicates that CX3CL1 may participate in controlling the invasion of peripheral leukocytes into the brain [115].
NK cells were obviously reduced in the inflamed CNS of CX3CR1-deficient EAE mice(MOG or PLP induced in C57BL/6J mice), while monocytes/macrophages, T cells, and NKT cells are recruited to the CNS during EAE do not require CX3CR1, CX3CL1-mediated chemical attraction of NK cells is relatively specific for the CNS [42].
Arli B et al. believe that VI of the V249I CX3CL1 receptor haplotype may have a protective effect on conversion to SPMS, but V249I CX3CL1 receptor haplotype genotype II patients have a higher risk of disability [6].
6. Chemokine receptors as therapeutic targets
Studies of chemokine receptor antagonists offer potential lead compounds for the treatment of MS as well as a deep understanding of the pathogenesis of EAE. Their anti-inflammatory effects may be due to inhibition of chemotaxis, thereby preventing leukocytes from flowing into the affected tissues [11]. There are the following methods to modulate chemokine receptors: peptide antagonists, neutralizing antibodies and small non-peptidyl antagonists [72]. Immunoneutralization of specific chemokines (such as CCL3) does not provide information as to which receptors are important because they commonly interact with diverse chemokine receptors. In the case of CCL3, both CCR5 and CCR1 can combine with it. Both from the perspective of therapeutic and mechanistic, it is meaningful to administer a selective low molecular drug to inhibit the effective effect of chemokine receptors on acute EAE and MS in the induction phase of an autoimmune response [30]. Take the redundancy of chemokines expression in pathology in the account, it is preferred to select a compound that blocks multiple inflammatory chemokine receptors simultaneously.
6.1. CCR1 antagonists
CCR1 is one chemokine receptor that mediates monocytes trafficking to the site of inflammation when stimulated, particularly by CCL3 and CCL5 [76]. CCR1 is important for controlling the earliest pro-inflammatory events in the border zones of inflammatory brain injury in active MS and advanced EAE [30]. Interfering with CCL3-mediated CCR1 activation in vivo with a specific antibody or by genomic deletion of CCR1 gene results in a significant reduction in paralytic disease [30].
The first selective CCR1 antagonist, BX-471, was entered into clinical trials by Berlex Biosciences. There are no considerable effects of an orally administered CCR1 antagonist (BX-471) on ICAM-3 expression in RRMS patients [93]. It failed in the phase II trial because of the efficacy lack. But it lays the foundation for the clinical development of other chemokine receptor antagonists [103]. High-throughput screening of Merritt JR et al's combinatorial series identified a new, moderately effective CCR1 antagonist 3. Library Hit 3 was optimized in order to synthesis advanced lead compound, therefore compound 4 was obtained. The IC50 of Compound 4 that inhibits CCR1-mediated monocyte chemotaxis was 20 nM [76]. The results of the study showed that although the antagonist did not stop the peripheral anti-MOG response(in DA rats), it prevented T cell infiltration effectively, macrophage infiltration and activation, thereby preventing subsequent CNS inflammation in the model. A single CCR1 selective antagonist, 5-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-2,2-diphenylpentanenitrile, can suppress MOG-induced EAE, indicating that CCR1 plays a non-redundant role at this stage [30].
6.2. CCR2 antagonists
Many therapies currently target leukocyte trafficking and inflammation in the CNS. Some CCR2 targeted drugs have entered MS in the past, which generally shown a favorable safety profile in clinical trials but failed in efficacy and are not involved in clinical research currently [31].
BMS22 is a classic competitive antagonist CCR2 [8]. A great quantity of multi-QSAR modeling techniques (eg, pharmacodynamic mapping, 3D-QSAR CoMFA, etc.) were performed on a data set consisting of eighty-three CCR2 antagonists to design higher active molecules. Sk. Abdul Amin et al. designed seven new molecules that are expected to be more active than the best active antagonists in this series (cpd 34) [4].Extracellular loop 1 in verso (ECL1i), a native 7-d-amino acid peptide CCR2 inhibitor that selectively and potently inhibits CCL2 triggering chemotaxis and it has allosteric antagonism against human and mouse CCR2 [8].MLN-1202, a kind of humanized anti-CCR2 antibody did not show efficacy in patients with multiple sclerosis, although the administration of MLN-1202 reduced the circulating monocytes numbers in peripheral blood [119]. INCB3344 is a small molecule antagonist and the therapeutic dose significantly reduces the clinical symptoms of the disease in EAE mice(MOG induced in C57BL/6 mice) (disease incidence and severity) [16]. Buntinx M et al. identified JNJ-27141491 as a non-competitive, potent hCCR2 antagonist that exerts anti-inflammatory activity in mice expressing transgenic hCCR2. JNJ-27141491 (20 mg/kg q.d.) treatment delayed the onset and reduced signs of the nervous system in EAE temporarily but significantly. However, there are no pieces of evidence that the disease can be improved over the long term. Therefore the authors say that the use of JNJ-27141491 may not be successful in MS patients [19].
Merck Corporation has disclosed a number of CCR2 antagonists, containing compound MK-0812. The IC 50 of MK-0812 is 5.0 nM, indicating a potent CCR2 antagonistic activity, and has undergone clinical trials for MS. The primary endpoint of the trails was a new GD-enhanced lesion by MRI. MK-0812 entered phase II clinical trials of MS but did not improve significantly compared to placebo [31], [88] Incyte also exploited a variety of CCR2 antagonists, of which INCB3284 is one of the compounds in phase II clinical research of MS, but there are no subsequent reports of the trial results [88]. Lagumersindez-Denis, N et al. hypothesized that targeting CCR2 may be an effective treatment strategy against cortical demyelination. They developed a kind of novel mouse anti-human CCR2 monoclonal antibody aiming to deplete CCR2+ monocytes. Compared to isotype-treated controls, monocyte-depleted animals revealed less perivascular type 2 cortical demyelination and less subpial type 3 cortical demyelination significantly, which is in line with the ameliorated clinical disease course [61].
6.3. CCR4 & CCR6 antagonists
CCR4 is mainly expressed on Th2 / Treg lymphocytes [44].CCR4 antagonists are classified into two categories of aryl sulphonamides lipophilic and heteroarenes [108]. Small molecule antagonists, like endogenous ligands, have also shown different ability to induce receptor internalization, which may be relevant to different biological effects. Some CCR4 antagonists combine with the allosteric site of CCR4, not the binding site of CCR4 to the ligand [102]. It may be for this reason that CCR4 antagonist compound 22 inhibits the Th1 and Th17 cell polarization as well as ameliorate EAE, but the CCR4 antagonist AF399 / 420/18025 had no significant impact on the clinical score of EAE [80], [84]. It is not optimistic that most of the CCR4 antagonists tested have not been approved for MS treatment. For example, in Phase I clinical trials, there was only one kind of CCR4 antagonists, GSK 2239633, as a possible treatment for asthma but still had no further development due to low bioavailability. This condition might be caused by complex pharmacology that has not been resolved, such as the CCR4 regulation on surface expression and transport aspects [102]. CCR4-deficient mice(MOG induced in C57BL/6 mice) have been reported to exhibit low-grade EAE symptoms [33].
CCR6 is expressed on Th17 cells, immature dendritic cells, and Langerhans cells. CCR6 is a unique chemokine receptor because it combines with chemokine CCL20 exclusively [44]. CCR6 deficient mice have high resistance to EAE(MOG induced in C57BL/6 mice) that induced by MOG immunization [43]. In addition, double knockout CCR4 - / - and CCR6 - / - mice showed a low degree of EAE severity(MOG induced in C57BL/6 mice). The number of CNS infiltrating cells in knockout mice was obviously decreased compared with wild type mice [81]. Th17 cells highly express CCR6. In addition, dendritic cells, B cells, and other T cells subpopulations also express CCR6 [1].
6.4. CCR5 antagonists
CCL3, CCL4, and CCL5 are major chemokines that induce immune cells recruited to the CNS by interacting with their corresponding receptors CCR5 [37]. Initial studies have shown that gene targeting to disrupt CCR5 does not affect the severity of EAE [1].
Maraviroc (CelsentriTM) is the first CCR5 antagonist that approved for the treatment of HIV-1 infection by EMA (European Medicines Agency) [24]. Together with the approved use of AMD3100 (CXCR4 antagonist) and a large number of clinical trials of small molecules and biologics currently underway, reflecting the belief that chemokine systems serve as therapeutic targets for treatment [103]. During the development of EAE disease following influenza infection, CCL5 / CCR5 may mediate the type I T cells infiltration into the CNS. Administration of the CCR5 antagonist, TAK-779, significantly ameliorated the exacerbated EAE [24].
6.5. CX3CR1 antagonists
Ridderstad Wollberg A et al. demonstrated the expression of CX3CR1 mRNA on inflammatory cells in the active plaque region of MS brain autopsies. AZD8797, a CX3CR1 inhibitor, is effective outside the CNS. In order to verify the function of CX3CR1 in the pathogenesis of MS, AZD8797 was treated in Dark Agouti rats with MOG-induced EAE. Then it led to reduced CNS pathology, paralysis and recurrence and is effective after the acute phase [94]. Karlström S et al. exploited two parallel series, series A and series B of CX3CR1 antagonists for the MS treatment. By modifying the 7-Amino-5-thio-thiazolo[4,5-d]pyrimidines, they developed the first selective and potent oral antagonist of CX3CR1, 18a [50]. Blocking CX3CR1 on the leukocytes that outside the CNS could be another alternative approach to treat multiple sclerosis [94].
7. Conclusions
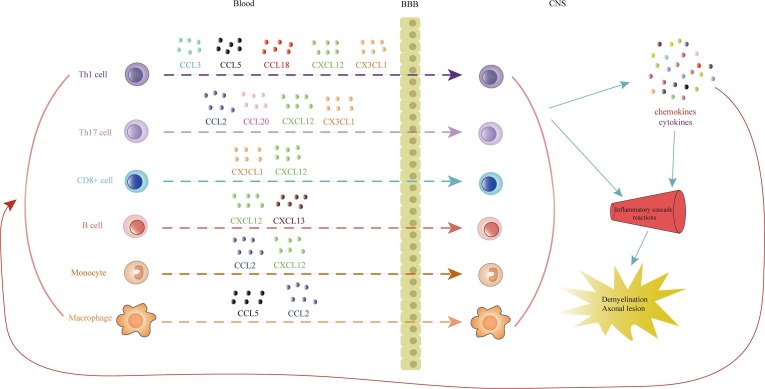
The onset of MS is closely related to immune dysfunction, which is demonstrated by inflammation, oligodendrocyte death, loss of myelin and various degrees of axonal damage. The formation of MS plaques begins with antigen-presenting cells activating myelin-reactive T lymphocytes, resulting in multifocal immune cells infiltrating into the CNS (see Fig. 1 ) [126]. In inflammatory infiltrating cells with active demyelinating lesions, 10% are CD8 + and CD4 + T cells and 90% are macrophages from resident microglia and infiltrating monocytes [23]. Activated immune cells activate the proinflammatory cytokine cascade in the CNS, ultimately leading to demyelination, glial scars, and axonal lesions. Regulating the immune response and cell infiltration in the CNS is a therapeutic strategy that will improve treatment efficacy [123]. The role of chemokines and their receptors in autoimmunity and inflammation is recognized, and they also have important impacts on normal homeostasis, including lymphoid tissue development and cell transport. The chemokine system is rich in ligands and receptors, and studies have shown that a lot of chemokines and their receptors can be effective therapeutic targets for MS disease. By specifically interfering with chemokine and chemokine receptor function, it can prevent the infiltration of inflammatory cells, control the cascade of inflammation, and may also prevent demyelinating damage and promote remyelination, bringing a new treatment to MS. hope. However, the development of a single chemokine receptor antagonist has not achieved imaginary success, overcoming the redundancy of chemokine action, the development of multi-target antagonists or the combination of multiple antagonists may be the future The direction of MS drug development.
Fig. 1.
Chemokines attract different immune cells infiltrating into the CNS during MS disease.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants (81873026, 81730096, 81973499), Beijing Natural Science Foundation Grants (7192135), CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-004), the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (BZ0150) and Shanxi Key R&D Program (201803D421006).
References
- 1.Abraham Michal, Karni Arnon, Mausner-Fainberg Karin, Weiss Ido D., Peled Amnon. Natural and induced immunization against CCL20 ameliorate experimental autoimmune encephalitis and may confer protection against multiple sclerosis. Clinical Immunol. 2017;183:316–324. doi: 10.1016/j.clim.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Allegretti Marcello, Cesta Maria Candida, Garin Alexandre, Proudfoot Amanda E.I. Current status of chemokine receptor inhibitors in development. Immunol. Lett. 2012;145(1-2):68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Allen Samantha J., Crown Susan E., Handel Tracy M. Chemokine: Receptor Structure, Interactions, and Antagonism. Annu. Rev. Immunol. 2007;25(1):787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 4.Amin Sk Abdul, Adhikari Nilanjan, Baidya Sandip Kumar, Gayen Shovanlal, Jha Tarun. Structural refinement and prediction of potential CCR2 antagonists through validated multi-QSAR modeling studies. J. Biomol. Struct. Dyn. 2019;37(1):75–94. doi: 10.1080/07391102.2017.1418679. [DOI] [PubMed] [Google Scholar]
- 5.Apostolakis Stavros, Spandidos Demetrios. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol. Sin. 2013;34(10):1251–1256. doi: 10.1038/aps.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arli Berna, Irkec Ceyla, Menevse Sevda, Yilmaz Akin, Alp Ebru. Fractalkine Gene Receptor Polymorphism in Patients With Multiple Sclerosis. Int. J. Neurosci. 2012;123(1):31–37. doi: 10.3109/00207454.2012.723079. [DOI] [PubMed] [Google Scholar]
- 7.Asashima T, Iizasa H, Terasaki T and Nakashima E.2003.Rat brain pericyte cell lines expressing beta2-adrenergic receptor, angiotensin II receptor type 1A, klotho, and CXCR4 mRNAs despite having endothelial cell markers. J. Cell Physiol. 197(1):69-76. [DOI] [PubMed]
- 8.Auvynet Constance, Baudesson de Chanville Camille, Hermand Patricia, Dorgham Karim, Piesse Christophe, Pouchy Charlotte, Carlier Ludovic, Poupel Lucie, Barthélémy Sandrine, Felouzis Virginie, Lacombe Claire, Sagan Sandrine, Chemtob Sylvain, Quiniou Christiane, Salomon Benoit, Deterre Philippe, Sennlaub Florian, Combadière Christophe. ECL1i, d(LGTFLKC), a novel, small peptide that specifically inhibits CCL2-dependent migration. FASEB J. 2016;30(6):2370–2381. doi: 10.1096/fj.201500116. [DOI] [PubMed] [Google Scholar]
- 9.Bagaeva Ludmila V., Rao Praveen, Powers James M., Segal Benjamin M. CXC Chemokine Ligand 13 Plays a Role in Experimental Autoimmune Encephalomyelitis. J. Immunol. 2006;176(12):7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 10.Baggiolini M.1998.Chemokines and leukocyte traffic. Nature 392(6676):565-8. [DOI] [PubMed]
- 11.Baggiolini M. Chemokines in pathology and medicine. J. Intern. Med. 2001;250(2):91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 12.Barlic Jana, Murphy Philip M. Chemokine regulation of atherosclerosis. J. Leukoc. Biol. 2007;82(2):226–236. doi: 10.1189/jlb.1206761. [DOI] [PubMed] [Google Scholar]
- 13.Bjelobaba Ivana, Begovic-Kupresanin Vesna, Pekovic Sanja, Lavrnja Irena. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J Neuro Res. 2018;96(6):1021–1042. doi: 10.1002/jnr.v96.610.1002/jnr.24224. [DOI] [PubMed] [Google Scholar]
- 14.Blauth Kevin, Zhang Xin, Chopra Manisha, Rogan Sarah, Markovic-Plese Silva. The role of fractalkine (CX3CL1) in regulation of CD4+ cell migration to the central nervous system in patients with relapsing–remitting multiple sclerosis. Clin. Immunol. 2015;157(2):121–132. doi: 10.1016/j.clim.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Bose Shambhunath, Cho Jungsook. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 2013;36(9):1039–1050. doi: 10.1007/s12272-013-0161-z. [DOI] [PubMed] [Google Scholar]
- 16.Brodmerkel Carrie M., Huber Reid, Covington Maryanne, Diamond Sharon, Hall Leslie, Collins Robert, Leffet Lynn, Gallagher Karen, Feldman Patricia, Collier Paul, Stow Mark, Gu Xiaomei, Baribaud Frederic, Shin Niu, Thomas Beth, Burn Tim, Hollis Greg, Yeleswaram Swamy, Solomon Kim, Friedman Steve, Wang Anlai, Xue Chu Biao, Newton Robert C., Scherle Peggy, Vaddi Kris. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J. Immunol. 2005;175(8):5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- 17.Broux Bieke, Pannemans Kim, Zhang Xin, Markovic-Plese Silva, Broekmans Tom, Eijnde Bert O., Van Wijmeersch Bart, Somers Veerle, Geusens Piet, van der Pol Susanne, van Horssen Jack, Stinissen Piet, Hellings Niels. CX3CR1 drives cytotoxic CD4+CD28− T cells into the brain of multiple sclerosis patients. J. Autoimmun. 2012;38(1):10–19. doi: 10.1016/j.jaut.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Browne RW, Jakimovski D, Ziliotto N, Kuhle J, Bernardi F, Weinstock-Guttman B, Zivadinov R and Ramanathan M.2019.High-density lipoprotein cholesterol is associated with multiple sclerosis fatigue: A fatigue-metabolism nexus? J Clin Lipidol 13(4):654-663 e1. [DOI] [PubMed]
- 19.Buntinx Mieke, Hermans Bart, Goossens Jan, Moechars Dieder, Gilissen Ron A.H.J., Doyon Julien, Boeckx Staf, Coesemans Erwin, Van Lommen Guy, Van Wauwe Jean P. Pharmacological Profile of JNJ-27141491 [(S)-3-[3,4-Difluorophenyl)-propyl]-5-isoxazol-5-yl-2-thioxo-2,3-dihydro-1-H-imidazole-4-carboxyl Acid Methyl Ester], as a Noncompetitive and Orally Active Antagonist of the Human Chemokine Receptor CCR2. J. Pharmacol. Exp. Ther. 2008;327(1):1–9. doi: 10.1124/jpet.108.140723. [DOI] [PubMed] [Google Scholar]
- 20.Burman Joachim, Svensson Emma, Fransson Moa, Loskog Angelica S.I., Zetterberg Henrik, Raininko Raili, Svenningsson Anders, Fagius Jan, Mangsbo Sara M. The cerebrospinal fluid cytokine signature of multiple sclerosis: A homogenous response that does not conform to the Th1/Th2/Th17 convention. J. Neuroimmunol. 2014;277(1-2):153–159. doi: 10.1016/j.jneuroim.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Burrows David John, McGown Alexander, Jain Saurabh A, De Felice Milena, Ramesh Tennore M, Sharrack Basil, Majid Arshad. Animal models of multiple sclerosis: From rodents to zebrafish. Mult Scler. 2019;25(3):306–324. doi: 10.1177/1352458518805246. [DOI] [PubMed] [Google Scholar]
- 22.Cardona SM, Garcia JA and Cardona AE.2013.The fine balance of chemokines during disease: trafficking, inflammation, and homeostasis. Methods Mol Biol 1013(1-16). [DOI] [PMC free article] [PubMed]
- 23.Charo Israel F., Ransohoff Richard M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 24.Chen Qingyun, Liu Yinping, Lu Aizhen, Ni Ke, Xiang Zheng, Wen Kun, Tu Wenwei. Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J. Autoimmun. 2017;77:1–10. doi: 10.1016/j.jaut.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu CS, Farooqi N, O'Brien K and Gran B.2011.Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164(4):1079-106. [DOI] [PMC free article] [PubMed]
- 26.De Angelis F, John NA and Brownlee WJ.2018.Disease-modifying therapies for multiple sclerosis. BMJ 363(k4674). [DOI] [PubMed]
- 27.DeVries Mark E., Kelvin Alyson A., Xu Luoling, Ran Longsi, Robinson John, Kelvin David J. Defining the Origins and Evolution of the Chemokine/Chemokine Receptor System. J. Immunol. 2006;176(1):401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 28.Dogan Rukiye-Nazan E., Long Nancy, Forde Eileen, Dennis Kristen, Kohm Adam P., Miller Stephen D., Karpus William J. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J. Leukoc. Biol. 2011;89(1):93–104. doi: 10.1189/jlb.0810442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Sharkawi Fathia Z., Ali Sahar A., Hegazy Mohamed I., Atya Hanaa B. The combined effect of IL-17F and CCL20 gene polymorphism in susceptibility to multiple sclerosis in Egypt. Gene. 2019;685:164–169. doi: 10.1016/j.gene.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Eltayeb Sana, Sunnemark Dan, Berg Anna-Lena, Nordvall Gunnar, Malmberg Åsa, Lassmann Hans, Wallström Erik, Olsson Tomas, Ericsson-Dahlstrand Anders. Effector stage CC chemokine receptor-1 selective antagonism reduces multiple sclerosis-like rat disease. J. Neuroimmunol. 2003;142(1-2):75–85. doi: 10.1016/S0165-5728(03)00264-9. [DOI] [PubMed] [Google Scholar]
- 31.Fantuzzi Laura, Tagliamonte Maria, Gauzzi Maria Cristina, Lopalco Lucia. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell. Mol. Life Sci. 2019;76(24):4869–4886. doi: 10.1007/s00018-019-03255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fife BT, Huffnagle GB, Kuziel WA and Karpus WJ.2000.CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192(6):899-905. [DOI] [PMC free article] [PubMed]
- 33.Forde Eileen A., Dogan Rukiye-Nazan E., Karpus William J. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J. Neuroimmunol. 2011;236(1-2):17–26. doi: 10.1016/j.jneuroim.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franciotta Diego, Martino Gianvito, Zardini Elisabetta, Furlan Roberto, Bergamaschi Roberto, Andreoni Laura, Cosi Vittorio. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J. Neuroimmunol. 2001;115(1-2):192–198. doi: 10.1016/S0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 35.Galimberti D., Fenoglio C., Comi C., Scalabrini D., De Riz M., Leone M., Venturelli E., Cortini F., Piola M., Monaco F., Bresolin N., Scarpini E. MDC/CCL22 intrathecal levels in patients with multiple sclerosis. Mult Scler. 2008;14(4):547–549. doi: 10.1177/1352458507084268. [DOI] [PubMed] [Google Scholar]
- 36.Galimberti Daniela, Scalabrini Diego, Fenoglio Chiara, De Riz Milena, Comi Cristoforo, Venturelli Eliana, Cortini Francesca, Piola Mirko, Leone Maurizio, Dianzani Umberto, D'Alfonso Sandra, Monaco Francesco, Bresolin Nereo, Scarpini Elio. Gender-specific influence of the chromosome 16 chemokine gene cluster on the susceptibility to Multiple Sclerosis. J. Neurol. Sci. 2008;267(1-2):86–90. doi: 10.1016/j.jns.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Ghorban K, Dadmanesh M, Hassanshahi G, Momeni M, Zare-Bidaki M, Arababadi MK and Kennedy D.2013.Is the CCR5 Delta 32 mutation associated with immune system-related diseases? Inflammation 36(3):633-42. [DOI] [PubMed]
- 38.Glass William G., Hickey Michelle J., Hardison Jenny L., Liu Michael T., Manning Jerry E., Lane Thomas E. Antibody Targeting of the CC Chemokine Ligand 5 Results in Diminished Leukocyte Infiltration into the Central Nervous System and Reduced Neurologic Disease in a Viral Model of Multiple Sclerosis. J. Immunol. 2004;172(7):4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- 39.Glatigny Simon, Bettelli Estelle. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS) Cold Spring Harb Perspect Med. 2018;8(11):a028977. doi: 10.1101/cshperspect.a028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haarmann A, Schuhmann MK, Silwedel C, Monoranu CM, Stoll G and Buttmann M.2019.Human Brain Endothelial CXCR2 is Inflammation-Inducible and Mediates CXCL5- and CXCL8-Triggered Paraendothelial Barrier Breakdown. Int. J. Mol. Sci. 20(3). [DOI] [PMC free article] [PubMed]
- 41.Hendrickx DAE, van Scheppingen J, van der Poel M, Bossers K, Schuurman KG, van Eden CG, Hol EM, Hamann J and Huitinga I.2017.Gene Expression Profiling of Multiple Sclerosis Pathology Identifies Early Patterns of Demyelination Surrounding Chronic Active Lesions. Front Immunol 8(1810). [DOI] [PMC free article] [PubMed]
- 42.Huang DeRen, Shi Fu-Dong, Jung Steffen, Pien Gary C., Wang Jintang, Salazar-Mather Thais P., He Toby T., Weaver Jennifer T., Ljunggren Hans-Gustaf, Biron Christine A., Littman Dan R., Ransohoff Richard M. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20(7):896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 43.Jafarzadeh A., Arabi Z., Ahangar-Parvin R., Mohammadi-Kordkhayli M., Nemati M. Extract Modulates the Expression of Chemokines CCL20 and CCL22 and Their Receptors (CCR6 and CCR4) in the Central Nervous System of Mice with Experimental Autoimmune Encephalomyelitis. Drug Res (Stuttg) 2017;67(11):632–639. doi: 10.1055/s-0043-113455. [DOI] [PubMed] [Google Scholar]
- 44.Jafarzadeh Abdollah, Azizi Sayyed Vahab, Arabi Zahra, Ahangar-Parvin Rayhaneh, Mohammadi-Kordkhayli Marziyeh, Larussa Tiziana, Khatami Fariba, Nemati Maryam. Vitamin D down-regulates the expression of some Th17 cell-related cytokines, key inflammatory chemokines, and chemokine receptors in experimental autoimmune encephalomyelitis. Nutritional Neurosci. 2019;22(10):725–737. doi: 10.1080/1028415X.2018.1436237. [DOI] [PubMed] [Google Scholar]
- 45.Jafarzadeh A., Bagherzadeh S., Ebrahimi H.A., Hajghani H., Bazrafshani M.R., Khosravimashizi A., Nemati M., Gadari F., Sabahi A., Iranmanesh F., Mohammadi M.M., Daneshvar H. Higher Circulating Levels of Chemokine CCL20 in Patients with Multiple Sclerosis: Evaluation of the Influences of Chemokine Gene Polymorphism, Gender, Treatment and Disease Pattern. J. Mol. Neurosci. 2014;53(3):500–505. doi: 10.1007/s12031-013-0214-2. [DOI] [PubMed] [Google Scholar]
- 46.Jafarzadeh A., Ebrahimi H.A., Bagherzadeh S., Zarkesh F., Iranmanesh F., Najafzadeh A., Khosravimashizi A., Nemati M., Sabahi A., Hajghani H., Daneshvar H., Mohammadi M.M. Lower Serum Levels of Th2-Related Chemokine CCL22 in Women Patients with Multiple Sclerosis: A Comparison Between Patients and Healthy Women. Inflammation. 2014;37(2):604–610. doi: 10.1007/s10753-013-9775-z. [DOI] [PubMed] [Google Scholar]
- 47.Janssen Katharina, Rickert Mira, Clarner Tim, Beyer Cordian, Kipp Markus. Absence of CCL2 and CCL3 Ameliorates Central Nervous System Grey Matter But Not White Matter Demyelination in the Presence of an Intact Blood–Brain Barrier. Mol. Neurobiol. 2016;53(3):1551–1564. doi: 10.1007/s12035-015-9113-6. [DOI] [PubMed] [Google Scholar]
- 48.Javor J., Parnicka Z., Michalik J., Copikova-Cudrakova D., Shawkatova I., Durmanova V., Gmitterova K., Klimova E., Bucova M., Buc M. +190 G/A (rs1799864) polymorphism in the C-C chemokine receptor 2 (CCR2) gene is associated with susceptibility to multiple sclerosis in HLA-DRB1* 15: 01-negative individuals. J. Neurol. Sci. 2015;349(1–2):138–142. doi: 10.1016/j.jns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Wei, St-Pierre Stéphanie, Roy Patrick, Morley Barbara J., Hao Junwei, Simard Alain R. Infiltration of CCR2+-Ly6C-high-Proinflammatory Monocytes and Neutrophils into the Central Nervous System Is Modulated by Nicotinic Acetylcholine Receptors in a Model of Multiple Sclerosis. J.I. 2016;196(5):2095–2108. doi: 10.4049/jimmunol.1501613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlstrom S., Nordvall G., Sohn D., Hettman A., Turek D., Ahlin K., Kers A., Claesson M., Slivo C., Lo-Alfredsson Y., Petersson C., Bessidskaia G., Svensson P.H., Rein T., Jerning E., Malmberg A., Ahlgen C., Ray C., Vares L., Ivanov V., Johansson R. Substituted 7-amino-5-thio-thiazolo[4,5-d]pyrimidines as potent and selective antagonists of the fractalkine receptor (CX3CR1) J. Med. Chem. 2013;56(8):3177–3190. doi: 10.1021/jm3012273. [DOI] [PubMed] [Google Scholar]
- 51.Karpus WJ and Ransohoff RM.1998.Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J Immunol 161(6):2667-71. [PubMed]
- 52.Kaskow BJ and Baecher-Allan C.2018.Effector T Cells in Multiple Sclerosis. Cold Spring Harb Perspect Med 8(4). [DOI] [PMC free article] [PubMed]
- 53.Khademi Mohsen, Kockum Ingrid, Andersson Magnus L, Iacobaeus Ellen, Brundin Lou, Sellebjerg Finn, Hillert Jan, Piehl Fredrik, Olsson Tomas. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17(3):335–343. doi: 10.1177/1352458510389102. [DOI] [PubMed] [Google Scholar]
- 54.Khaiboullina Svetlana F., Gumerova Aigul R., Khafizova Irina F., Martynova Ekaterina V., Lombardi Vincent C., Bellusci Saverio, Rizvanov Albert A. CCL27: Novel Cytokine with Potential Role in Pathogenesis of Multiple Sclerosis. Biomed Res. Int. 2015;2015:1–10. doi: 10.1155/2015/189638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kipp Markus, Nyamoya Stella, Hochstrasser Tanja, Amor Sandra. Multiple sclerosis animal models: a clinical and histopathological perspective: Multiple sclerosis animal models. Brain Pathol. 2017;27(2):123–137. doi: 10.1111/bpa.2017.27.issue-210.1111/bpa.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I and McColl SR.2008.Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol 18(4):504-16. [DOI] [PMC free article] [PubMed]
- 57.Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, van Het Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P and de Vries HE.2011.Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 134(Pt 2):555-70. [DOI] [PubMed]
- 58.Krohn SC, Bonvin P and Proudfoot AE.2013.CCL18 exhibits a regulatory role through inhibition of receptor and glycosaminoglycan binding. PLoS One 8(8):e72321. [DOI] [PMC free article] [PubMed]
- 59.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R and Meinl E.2006.Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129(Pt 1):200-11. [DOI] [PubMed]
- 60.Kurschus FlorianC. T cell mediated pathogenesis in EAE: Molecular mechanisms. Biomed J. 2015;38(3):183. doi: 10.4103/2319-4170.155590. [DOI] [PubMed] [Google Scholar]
- 61.Lagumersindez-Denis Nielsen, Wrzos Claudia, Mack Matthias, Winkler Anne, van der Meer Franziska, Reinert Marie C., Hollasch Heiko, Flach Anne, Brühl Hilke, Cullen Eilish, Schlumbohm Christina, Fuchs Eberhard, Linington Christopher, Barrantes-Freer Alonso, Metz Imke, Wegner Christiane, Liebetanz David, Prinz Marco, Brück Wolfgang, Stadelmann Christine, Nessler Stefan. Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis. Acta Neuropathol. 2017;134(1):15–34. doi: 10.1007/s00401-017-1706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lassmann Hans. Multiple Sclerosis Pathology. Cold Spring Harb Perspect Med. 2018;8(3):a028936. doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lassmann Hans, Bradl Monika. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133(2):223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laufer JM and Legler DF.2018.Beyond migration-Chemokines in lymphocyte priming, differentiation, and modulating effector functions. J Leukoc Biol 104(2):301-312. [DOI] [PubMed]
- 65.Li Rui, Sun Xiaobo, Shu Yaqing, Wang Yuge, Xiao Li, Wang Zhanhang, Hu Xueqiang, Kermode Allan G., Qiu Wei. Serum CCL20 and its association with SIRT1 activity in multiple sclerosis patients. J. Neuroimmunol. 2017;313:56–60. doi: 10.1016/j.jneuroim.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Li Xin, Lu Tingting, Xue Wenwen, Wang Yixuan, Luo Qiong, Ge Huiming, Tan Renxiang, Shen Yan, Xu Qiang. Small molecule-mediated upregulation of CCR7 ameliorates murine experimental autoimmune encephalomyelitis by accelerating T-cell homing. Int. Immunopharmacol. 2017;53:33–41. doi: 10.1016/j.intimp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Lintermans Lucas L., Rutgers Abraham, Stegeman Coen A., Heeringa Peter, Abdulahad Wayel H. Chemokine receptor co-expression reveals aberrantly distributed TH effector memory cells in GPA patients. Arthritis Res Ther. 2017;19(1) doi: 10.1186/s13075-017-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Cui G, Zhu M, Kang X and Guo H.2014.Neuroinflammation in Alzheimer's disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol 7(12):8342-55. [PMC free article] [PubMed]
- 69.Liu L., Darnall L., Hu T., Choi K., Lane T.E., Ransohoff R.M. Myelin Repair Is Accelerated by Inactivating CXCR2 on Nonhematopoietic Cells. J. Neurosci. 2010;30(27):9074–9083. doi: 10.1523/JNEUROSCI.1238-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Londoño Ana C., Mora Carlos A. Role of CXCL13 in the formation of the meningeal tertiary lymphoid organ in multiple sclerosis. F1000Res. 2018;7:514. doi: 10.12688/f1000research10.12688/f1000research.14556.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Jr., Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O'Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E and Polman CH.2014.Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83(3):278-86. [DOI] [PMC free article] [PubMed]
- 72.Matsui Masaru, Weaver Jennifer, Proudfoot Amanda E.I., Wujek Jerome R, Wei Tao, Richer Edward, Trapp Bruce D, Rao Ashwin, Ransohoff Richard M. Treatment of experimental autoimmune encephalomyelitis with the chemokine receptor antagonist Met-RANTES. J. Neuroimmunol. 2002;128(1-2):16–22. doi: 10.1016/S0165-5728(02)00121-2. [DOI] [PubMed] [Google Scholar]
- 73.Maurer M., von Stebut E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004;36(10):1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 74.Melief Jeroen, Schuurman Karianne G., van de Garde Martijn D.B., Smolders Joost, van Eijk Marco, Hamann Jörg, Huitinga Inge. Microglia in normal appearing white matter of multiple sclerosis are alerted but immunosuppressed: Microglia in NAWM of MS are alerted but immunosuppressed. Glia. 2013;61(11):1848–1861. doi: 10.1002/glia.22562. [DOI] [PubMed] [Google Scholar]
- 75.Mellergård J., Edström M., Vrethem M., Ernerudh J., Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16(2):208–217. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- 76.Merritt J. Robert, Liu Jinqi, Quadros Elizabeth, Morris Michelle L., Liu Ruiyan, Zhang Rui, Jacob Biji, Postelnek Jennifer, Hicks Catherine M., Chen Weiqing, Kimble Earl F., Rogers W. Lynn, O’Brien Linda, White Nicole, Desai Hema, Bansal Shalini, King George, Ohlmeyer Michael J., Appell Kenneth C., Webb Maria L. Novel Pyrrolidine Ureas as C−C Chemokine Receptor 1 (CCR1) Antagonists. J. Med. Chem. 2009;52(5):1295–1301. doi: 10.1021/jm801416q. [DOI] [PubMed] [Google Scholar]
- 77.Mills Jeffrey H, Alabanza Leah M, Mahamed Deeqa A, Bynoe Margaret S. Extracellular adenosine signaling induces CX3CL1 expression in the brain to promote experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9(1) doi: 10.1186/1742-2094-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizuno Tetsuya, Kawanokuchi Jun, Numata Kenji, Suzumura Akio. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1-2):65–70. doi: 10.1016/S0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 79.Mori Francesco, Nisticò Robert, Nicoletti Carolina G, Zagaglia Sara, Mandolesi Georgia, Piccinin Sonia, Martino Gianvito, Finardi Annamaria, Rossini Paolo M, Marfia Girolama A, Furlan Roberto, Centonze Diego. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Mult Scler. 2016;22(11):1405–1412. doi: 10.1177/1352458515621796. [DOI] [PubMed] [Google Scholar]
- 80.Moriguchi Kota, Miyamoto Katsuichi, Tanaka Noriko, Ueno Rino, Nakayama Takashi, Yoshie Osamu, Kusunoki Susumu. C-C chemokine receptor type 4 antagonist Compound 22 ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2016;291:54–58. doi: 10.1016/j.jneuroim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Moriguchi Kota, Miyamoto Katsuichi, Tanaka Noriko, Yoshie Osamu, Kusunoki Susumu. The importance of CCR4 and CCR6 in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013;257(1-2):53–58. doi: 10.1016/j.jneuroim.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Novakova Lenka, Axelsson Markus, Khademi Mohsen, Zetterberg Henrik, Blennow Kaj, Malmeström Clas, Piehl Fredrik, Olsson Tomas, Lycke Jan. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J. Neurochem. 2017;141(2):296–304. doi: 10.1111/jnc.2017.141.issue-210.1111/jnc.13881. [DOI] [PubMed] [Google Scholar]
- 83.Öckinger J., Stridh P., Beyeen A.D., Lundmark F., Seddighzadeh M., Oturai A., Sørensen P.S., Lorentzen Å.R., Celius E.G., Leppä V., Koivisto K., Tienari P.J., Alfredsson L., Padyukov L., Hillert J., Kockum I., Jagodic M., Olsson T. Genetic variants of CC chemokine genes in experimental autoimmune encephalomyelitis, multiple sclerosis and rheumatoid arthritis. Genes Immun. 2010;11(2):142–154. doi: 10.1038/gene.2009.82. [DOI] [PubMed] [Google Scholar]
- 84.Othy S., Topcu S., Kaveri S.V., Bayry J. Effect of CC chemokine receptor 4 antagonism on the evolution of experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. 2012;109(37):E2412–E2413. doi: 10.1073/pnas.1209124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Owens Trevor. The enigma of multiple sclerosis: inflammation and neurodegeneration cause heterogeneous dysfunction and damage. Curr. Opin. Neurol. 2003;16(3):259–265. doi: 10.1097/01.wco.0000073925.19076.f2. [DOI] [PubMed] [Google Scholar]
- 86.Palomino Diana Carolina Torres, Marti Luciana Cavalheiro. Chemokines and immunity. Einstein (São Paulo) 2015;13(3):469–473. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palumbo S and Pellegrini S (2017) Experimental In Vivo Models of Multiple Sclerosis: State of the Art. In: Zagon IS and McLaughlin PJ (eds) Multiple Sclerosis: Perspectives in Treatment and Pathogenesis, Brisbane (AU). [PubMed]
- 88.Pease James, Horuk Richard. Chemokine Receptor Antagonists. J. Med. Chem. 2012;55(22):9363–9392. doi: 10.1021/jm300682j. [DOI] [PubMed] [Google Scholar]
- 89.Piccio Laura, Naismith Robert T., Trinkaus Kathryn, Klein Robyn S., Parks Becky J., Lyons Jeri A., Cross Anne H. Changes in B- and T-Lymphocyte and Chemokine Levels With Rituximab Treatment in Multiple Sclerosis. Arch. Neurol. 2010;67(6) doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Procaccini Claudio, De Rosa Veronica, Pucino Valentina, Formisano Luigi, Matarese Giuseppe. Animal models of Multiple Sclerosis. Eur. J. Pharmacol. 2015;759:182–191. doi: 10.1016/j.ejphar.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quinn JL, Kumar G, Agasing A, Ko RM and Axtell RC.2018.Role of TFH Cells in Promoting T Helper 17-Induced Neuroinflammation. Front Immunol 9(382). [DOI] [PMC free article] [PubMed]
- 92.Raghu Harini, Lepus Christin M, Wang Qian, Wong Heidi H, Lingampalli Nithya, Oliviero Francesca, Punzi Leonardo, Giori Nicholas J, Goodman Stuart B, Chu Constance R, Sokolove Jeremy B, Robinson William H. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017;76(5):914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reuβ R., Schreiber V., Klein A., Infante-Duarte C., Filippi M., Pabst W., Pohl C., Oschmann P. No significant effect of orally administered chemokine receptor 1 antagonist on intercellular adhesion molecule-3 expression in relapsing—Remitting multiple sclerosis patients. Mult Scler. 2010;16(3):366–369. doi: 10.1177/1352458509358188. [DOI] [PubMed] [Google Scholar]
- 94.Ridderstad Wollberg A., Ericsson-Dahlstrand A., Jureus A., Ekerot P., Simon S., Nilsson M., Wiklund S.-J., Berg A.-L., Ferm M., Sunnemark D., Johansson R. Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc. Natl. Acad. Sci. 2014;111(14):5409–5414. doi: 10.1073/pnas.1316510111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridiandries Anisyah, Tan Joanne T.M., Ravindran Dhanya, Williams Helen, Medbury Heather J., Lindsay Laura, Hawkins Clare, Prosser Hamish C.G., Bursill Christina A. CC-chemokine class inhibition attenuates pathological angiogenesis while preserving physiological angiogenesis. FASEB J. 2017;31(3):1179–1192. doi: 10.1096/fj.201600540R. [DOI] [PubMed] [Google Scholar]
- 96.Rottman James B., Slavin Anthony J., Silva Robert, Weiner Howard L., Gerard Craig G., Hancock Wayne W. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur. J. Immunol. 2000;30(8):2372–2377. doi: 10.1002/(ISSN)1521-414110.1002/1521-4141(2000)30:8<>1.0.CO;2-010.1002/1521-4141(2000)30:8<2372::AID-IMMU2372>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 97.Roy Ishan, Evans Douglas B., Dwinell Michael B. Chemokines and chemokine receptors: Update on utility and challenges for the clinician. Surgery. 2014;155(6):961–973. doi: 10.1016/j.surg.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L and Segal BM.2015.Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med 212(1):23-35. [DOI] [PMC free article] [PubMed]
- 99.Rump Lisa, Mattey Derek L, Kehoe Oksana, Middleton Jim. An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium. Cytokine. 2017;97:133–140. doi: 10.1016/j.cyto.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katz Sand Ilana. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr. Opin. Neurol. 2015;28(3):193–205. doi: 10.1097/WCO.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 101.Schaller Teilo H., Batich Kristen A., Suryadevara Carter M., Desai Rupen, Sampson John H. Chemokines as adjuvants for immunotherapy: implications for immune activation with CCL3. Expert Review of Clinical Immunology. 2017;13(11):1049–1060. doi: 10.1080/1744666X.2017.1384313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheu S, Ali S, Ruland C, Arolt V and Alferink J.2017.The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int J Mol Sci 18(11). [DOI] [PMC free article] [PubMed]
- 103.Scholten DJ, Canals M, Maussang D, Roumen L, Smit MJ, Wijtmans M, de Graaf C, Vischer HF and Leurs R.2012.Pharmacological modulation of chemokine receptor function. Br. J. Pharmacol. 165(6):1617-1643. [DOI] [PMC free article] [PubMed]
- 104.Schraufstatter IU, Zhao M, Khaldoyanidi SK and Discipio RG.2012.The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 135(4):287-98. [DOI] [PMC free article] [PubMed]
- 105.Sellebjerg F., Bornsen L., Khademi M., Krakauer M., Olsson T., Frederiksen J.L., Sorensen P.S. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73(23):2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- 106.Sellebjerg F., Sørensen T.L. Chemokines and matrix metalloproteinase-9 in leukocyte recruitment to the central nervous system. Brain Res. Bull. 2003;61(3):347–355. doi: 10.1016/S0361-9230(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 107.Sindern E., Niederkinkhaus Y., Henschel M., Ossege L.M., Patzold T., Malin J.P. Differential release of beta-chemokines in serum and CSF of patients with relapsing-remitting multiple sclerosis. Acta Neurol. Scand. 2001;104(2):88–91. doi: 10.1034/j.1600-0404.2001.104002088.x. [DOI] [PubMed] [Google Scholar]
- 108.Solari Roberto, Pease James E. Targeting chemokine receptors in disease – a case study of CCR4. Eur. J. Pharmacol. 2015;763:169–177. doi: 10.1016/j.ejphar.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soleimani Mohammad, Soleymani Atiyeh, Seyyedirad Negarin. Elevated CSF concentration of CCL3 and CCL4 in relapsing remitting multiple sclerosis patients. J. Immunoassay Immunochem. 2019;40(4):378–385. doi: 10.1080/15321819.2019.1613242. [DOI] [PubMed] [Google Scholar]
- 110.Sørensen Torben L., Tani Marie, Jensen Jakob, Pierce Virginia, Lucchinetti Claudia, Folcik Virginia A., Qin Shixin, Rottman Jim, Sellebjerg Finn, Strieter Robert M., Frederiksen Jette L., Ransohoff Richard M. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 1999;103(6):807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sospedra Mireia, Martin Roland. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23(1):683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 112.Sternberg N., Hoess R. The molecular genetics of bacteriophage P1. Annu. Rev. Genet. 1983;17(1):123–154. doi: 10.1146/annurev.ge.17.120183.001011. [DOI] [PubMed] [Google Scholar]
- 113.Stone MJ, Hayward JA, Huang C, Z EH and Sanchez J.2017.Mechanisms of Regulation of the Chemokine-Receptor Network. Int J Mol Sci 18(2). [DOI] [PMC free article] [PubMed]
- 114.Stuart MJ, Singhal G and Baune BT.2015.Systematic Review of the Neurobiological Relevance of Chemokines to Psychiatric Disorders. Front Cell Neurosci 9(357. [DOI] [PMC free article] [PubMed]
- 115.Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, Berg AL and Ericsson-Dahlstrand A.2005.CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J. Neuroinflammation 2(17). [DOI] [PMC free article] [PubMed]
- 116.Tarique A.A., Logan J., Thomas E., Holt P.G., Sly P.D., Fantino E. functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015;53(5):676–688. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- 117.Van Kaer L., Postoak J.L., Wang C., Yang G., Wu L. innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell. Mol. Immunol. 2019;16(6):531–539. doi: 10.1038/s41423-019-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Langelaar J, van der Vuurst de Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, Dankers W, Verjans G, de Vries HE, Lubberts E, Hintzen RQ and van Luijn MM.2018.T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain 141(5):1334-1349. [DOI] [PubMed]
- 119.Vilums Maris, Zweemer Annelien J.M., Barmare Farhana, van der Gracht Anouk M.F., Bleeker Dave C.T., Yu Zhiyi, de Vries Henk, Gross Raymond, Clemens Jeremy, Krenitsky Paul, Brussee Johannes, Stamos Dean, Saunders John, Heitman Laura H., IJzerman Adriaan P. When structure–affinity relationships meet structure–kinetics relationships: 3-((Inden-1-yl)amino)-1-isopropyl-cyclopentane-1-carboxamides as CCR2 antagonists. Eur. J. Med. Chem. 2015;93:121–134. doi: 10.1016/j.ejmech.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 120.Vinader Victoria, Afarinkia Kamyar. A beginner’s guide to chemokines. Future Med. Chem. 2012;4(7):845–852. doi: 10.4155/fmc.12.49. [DOI] [PubMed] [Google Scholar]
- 121.Wang J., Knaut H. Chemokine signaling in development and disease. Development. 2014;141(22):4199–4205. doi: 10.1242/dev.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White Gemma E., Iqbal Asif J., Greaves David R., Garland Christopher J. CC Chemokine Receptors and Chronic Inflammation—Therapeutic Opportunities and Pharmacological Challenges. Pharmacol. Rev. 2013;65(1):47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y., Han J.J., Liang X.Y., Zhao L., Zhang F., Rasouli J., Wang Z.Z., Zhang G.X., Li X. Suppresses Leukocyte Migration and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Targeting CCL7. Mol. Ther. 2018;26(2):582–592. doi: 10.1016/j.ymthe.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng Yi, Qin Ling, Zacarías Natalia V. Ortiz, de Vries Henk, Han Gye Won, Gustavsson Martin, Dabros Marta, Zhao Chunxia, Cherney Robert J., Carter Percy, Stamos Dean, Abagyan Ruben, Cherezov Vadim, Stevens Raymond C., IJzerman Adriaan P., Heitman Laura H., Tebben Andrew, Kufareva Irina, Handel Tracy M. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature. 2016;540(7633):458–461. doi: 10.1038/nature20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Wenjun, Acosta Crystal, MacNeil Brian, Cortes Claudia, Intrater Howard, Gong Yuewen, Namaka Mike. Elevated Expression of Fractalkine (CX3CL1) and Fractalkine Receptor (CX3CR1) in the Dorsal Root Ganglia and Spinal Cord in Experimental Autoimmune Encephalomyelitis: Implications in Multiple Sclerosis-Induced Neuropathic Pain. Biomed Res. Int. 2013;2013:1–14. doi: 10.1155/2013/480702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ziliotto N, Bernardi F, Jakimovski D, Baroni M, Bergsland N, Ramasamy DP, Weinstock-Guttman B, Zamboni P, Marchetti G, Zivadinov R and Ramanathan M.2018.Increased CCL18 plasma levels are associated with neurodegenerative MRI outcomes in multiple sclerosis patients. Mult Scler Relat Disord 25(37-42). [DOI] [PubMed]
- 127.Zlotnik Albert, Yoshie Osamu. The Chemokine Superfamily Revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zweemer Annelien J.M., Toraskar Jimita, Heitman Laura H., IJzerman Adriaan P. Bias in chemokine receptor signalling. Trends Immunol. 2014;35(6):243–252. doi: 10.1016/j.it.2014.02.004. [DOI] [PubMed] [Google Scholar]