Abstract
Non-O1, non-O139 Vibrio cholerae (NOVC) does not agglutinate with O1 and O139 antisera and can cause intestinal and extraintestinal infections in immunocompromised individuals. NOVC bacteremia has the highest mortality among NOVC infections, and the number of reports has increased in recent years. Nevertheless, some clinicians are poorly informed about this disease. Herein, we describe a documented case of NOVC bacteremia in a male patient with impaired liver function. Blood cultures revealed the presence of V. cholerae, but this strain showed self-coagulation on the serum agglutination test. To our knowledge, this phenomenon is unreported among cases of NOVC infections. This pathogen was finally confirmed as NOVC via PCR. Because the patient worked as a garbage transporter, he was likely infected after contact with contaminated water through a foot wound. The patient developed septic shock shortly after admission and ultimately died from the illness. This paper reviews 23 cases of NOVC bacteremia from 2015 to 2019. To improve the accuracy of identifying NOVC and analyze its virulence factors, relevant detection methods were reviewed and analyzed.
Keywords: bacteremia, non-O1/non-O139 Vibrio cholerae, V. cholerae, virulence factors
Introduction
Vibrio cholerae (V. cholerae) is a halophilic, facultative, anaerobic, gram-negative, comma-shaped bacillus that is ubiquitous in aquatic and estuarine environments.1 The non-O1, non-O139 V. cholerae (NOVC) strains cannot cause cholera because they do not produce the cholera toxins; however, recent literature has reported that NOVC causes gastroenteritis and some extraintestinal infections.2 NOVC bacteremia has the highest mortality rates among NOVC infections and usually occurs in immunocompromised patients and those with underlying liver disease.3 However, the epidemiology, clinical manifestations and pathogenesis of NOVC bacteremia are unclear. Currently, no definitive guidelines exist for treating NOVC bacteremia.4 Therefore, clinicians should increase their knowledge of NOVC bacteremia to promptly diagnose and treat patients.
Here, we present a case of NOVC bacteremia in a patient with impaired liver function and summarize the available literature on NOVC bacteremia. Using the search terms: “non-O1”, “non-O139” and “Vibrio cholera” in PubMed from January 2015 to October 2019, we found 87 articles related to NOVC. Based on the title or abstract, 20 articles reported cases of NOVC-associated bacteremia; 18 of these articles were in English and were eventually included.
Case Report
A 47-year-old man was admitted to the emergency department for painful swelling of the face and right lower limb for 2 days. He did not significantly improve after anti-allergy treatment. Later, he appeared listless, and his family found large ecchymosis and blisters on his lower limbs. On the way to our hospital, the patient fell into a coma. The patient was mute and had a past medical history of impaired liver function. He worked as a garbage transporter.
On arrival, his body temperature was 38.1°C, heart rate was 78 beats per minute, blood pressure was 88/60 mmHg and oxygen saturation was 98% in room air. His physical examination revealed jaundice of the sclera and skin mucous membranes across his entire body except for the swelling and ecchymosis of both lower limbs. Additionally, a round black scab of ~0.5 cm was observed on the right foot. His blood glucose was only 0.9 mmol/L at admission.
No obvious abnormality was found on the computed tomography of the head or the arteriovenous ultrasonography of the lower extremities. Laboratory tests (Table 1) revealed a normal white blood cell count but with an increased neutrophil percentage (86.7%) and elevated procalcitonin (PCT) level (61.0 ng/mL). The alanine transaminase (ALT), aspartate transaminase (AST), urea, creatinine and pro-B-type natriuretic peptide (pro-BNP; 7248.5 pg/mL) were significantly increased. The prothrombin time and activated partial thromboplastin time (APTT) showed a prolonged coagulation function. Systemic infection was suspected based on the patient’s clinical manifestations and laboratory examinations. Blood cultures were immediately sent for microbiological examination (two sets each in aerobic and anaerobic bottles) using an automated blood culture system (BACTEC FX, BD Becton, Dickinson and Company). Empirical parenteral treatment was initiated with piperacillin tazobactam (4.5 g every 6 h) and vancomycin (1 g every 12 h).
Table 1.
Changes of Main Laboratory Results at Admission and After 6 Hours of Admission
| On Admission | After 6 Hours | Normal Range | ||
|---|---|---|---|---|
| Blood routine test | WBC | 6.35 | 0.83 | (3.50–9.50) ×109/L |
| RBC | 4.31 | 2.33 | (4.30–5.80) ×1012/L | |
| HGB | 158 | 82 | (130–175) g/L | |
| PLT | 29 | 5 | (125–350) ×109/L | |
| Liver and renal function | ALT | 243.4 | 196.6 | (13–69) U/L |
| AST | 688.1 | 1069.1 | (0–45) U/L | |
| Urea | 9.70 | 7.36 | (2.1–7.2) mmol/L | |
| CREA | 247.3 | 246.6 | (44–132) umol/L | |
| Blood coagulation function | PT | 26.5 | 52.0 | (8.0–14.0) s |
| PT-INR | 2.36 | 4.73 | ||
| APTT | 60.5 | >170 | (25.0–31.3) s | |
| TT | 21.5 | 23.7 | (15–21) s | |
| FIB | 1.94 | 0.86 | (2.0–4.0) g/L |
Abbreviations: WBC, white blood cell count; RBC, red blood cell count; HGB, hemoglobin; PLT, platelet count; ALT, alanine transaminase; AST, aspartate transaminase; CREA, creatinine; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; TT, thrombin time; FIB, fibrinogen.
After positive intravenous fluid and supportive therapy, the patient regained consciousness. However, 6 hours after admission, the patient’s clinical status again deteriorated. Arterial blood analysis showed severe metabolic acidosis. A retest of the blood (Table 1) revealed leukopenia, erythrocytopenia, thrombopenia and elevated liver enzymes. In addition, his APTT was significantly prolonged, and his fibrinogen showed a decreased coagulation function. During hospitalization, his urine volume was only 5 mL, and his blood potassium (6.47 mmol/L) was significantly increased. The patient ultimately died despite endotracheal intubation and cardiopulmonary resuscitation.
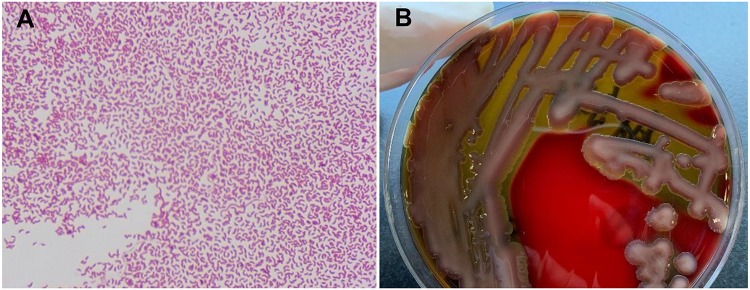
After 10 h of blood culture, gram-negative curved or straight rods were detected from all four blood culture bottles. Hemolytic, oxidase-positive colonies grew on the blood agar after 24 h of incubation (Figure 1). Blood culturing on thiosulfate-citrate-bile salt-sucrose agar revealed large yellow colonies. The strain was identified as V. cholerae (Vitek 2 Compact: 98%, biotype profile number: 0027601151502221) and subsequently confirmed by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS, BioMerieux) with a 99% confidence level.
Figure 1.
(A) Gram stain showing Gram-negative curved bacilli (×1000). (B) Blood agar showing β-hemolytic colonies.
A serum agglutination test was performed to classify the serotype of the pathogen. The pathogen showed self-coagulation in normal saline, which gradually weakened after several passages. The strain showed no agglutination with the O1 or O139 antisera; thus, NOVC was suspected and later confirmed via PCR by the Microbiology Laboratory of Jiangsu Provincial Center for Disease Control and Prevention. Antimicrobial susceptibility of the strain was analyzed using disk diffusion tests (Oxoid) on Muller-Hinton agar (BioMerieux) as per the Clinical and Laboratory Standards Institute guidelines. The test results showed that the strain was susceptible to penicillins, cephems, carbapenems, aminoglycosides, quinolones, and trimethoprim-sulfamethoxazole.
Discussion
Epidemiology
NOVC bacteria are usually nonpathogenic or asymptomatic colonizers in humans.3 However, cases of NOVC-associated infections continue to be reported worldwide.5 NOVC causes gastroenteritis6 and is related to extraintestinal infections, including skin and wound infections, otitis, bacteremia, biliary tract and urinary tract infections, pneumonia, peritonitis and meningitis.4,7,8 Statistical analysis of 83 NOVC-infected patients in Taiwan from 2009–2014 showed that gastroenteritis was the most common illness due to NOVC (accounting for 54.2%), followed by biliary tract infection (14.5%) and primary bacteremia (13.3%).9 Gastroenteritis can be mild to severe for both sporadic and break cases, but the prognosis of all cases is favorable. Although NOVC bacteremia is uncommon, its mortality is the highest among infections caused by this strain.1 Therefore, the case reports and research progress made in recent years must be systematically summarized.
Our review included 23 cases of NOVC bacteremia described in 18 reports from 2015–2019 (Table 2).1–5,7,10-21 The male-to-female ratio was 3.6:1, and the median age was 56 years. The youngest patient developed this disease at only 3 days of age, and the oldest patient was 83 years old. Of the patients with NOVC bacteremia, 39.1% (9/23) died.
Table 2.
Reports of Bacteremia Caused by NOVC in Recent Five Years
| Year | Country | Age (Years) | Gender | Risk Factors | Mode of Transmission | Clinical Presentation | Treatment | Clinical Outcome | Ref. no. |
|---|---|---|---|---|---|---|---|---|---|
| 2015 | USA | 54 | M | Multiple myeloma | A trip to Haiti | Diffuse abdominal pain, nausea, vomiting, and diarrhea | Piperacillin-tazobactam, levofloxacin | Death | [10] |
| 2015 | France | 70 | M | Hepatitis A | Seafood consumption | Fever, watery diarrhea, abdominal pain, vomiting and dizziness | Ceftriaxone, ciprofloxacin | Recover | [1] |
| 2015 | China | 11 days | F | None | Contaminated food and paraphernalia (most likely) |
Fever, lethargy, and a refusal to feed | Sulbenicillin, metronidazole | Recover | [11] |
| 2016 | India | 6.5 | F | Burkitt’s lymphoma | Not found | Abdominal pain, vomiting | Cefepime-tazobactam, teicoplanin, ciprofloxacin | Death | [12] |
| 2016 | India | 56 | F | Diabetes mellitus | Not found | Chills, swelling of the right lower leg | Piperacillin-tazobactam, imipenem | Death | |
| 2016 | India | 72 | M | Not mentioned | Not found | Fever, chills, nausea, dizziness | Not mentioned | Recover | |
| 2016 | Netherlands | 50 | M | Tuberculosis, COPD | Seafood consumption | Hypothermic, right ankle pain | Ciprofloxacin, cefotaxime | Death | [13] |
| 2016 | Netherlands | 60 | M | Heart disease, diabetes mellitus | Seafood consumption | Diarrhea, fever, mild jaundice | Ciprofloxacin | Recover | |
| 2016 | Netherlands | 70 | M | Heart failure, chronic cholangitis | Not found | Fever, coughing, dyspnea, dizziness, and decreased appetite | Ceftriaxone, gentamicin, amoxicillin-clavulanic acid | Recover | |
| 2016 | Austria | 80 | M | Ichthyosis cutis, several episodes of cellulitis | Contact with contaminated water through a wound | Swelling and pain in left lower leg, fever, dyspnea | Piperacillin-tazobactam, clindamycin, doxycycline | Death | [14] |
| 2016 | Greece | 43 | M | Liver cirrhosis, alcohol abuse | Not certain | Poor nutrition, icteric sclera, ascites, diffuse bleeding of the oral mucosa and bullous lesions of extremities. | Cefotaxime, metronidazole | Death | [15] |
| 2016 | Pakistan | 3 days | M | Very low birth weight | Goat’s milk | Fever and chills | Not mentioned | Death | [16] |
| 2017 | Canada | 66 | M | Biliary obstruction, benign pancreatic tumor | Seafood consumption | Epigastric pain, emesis and fever | Ciprofloxacin, doxycycline | Recover | [17] |
| 2017 | Saudi Arabia | 62 | M | Diabetes mellitus, cholecystectomy for chronic calculous cholecystitis | Not found | Fever, epigastric pain, intermittent vomiting | Piperacillin-tazobactam, ciprofloxacin | Recover | [7] |
| 2017 | Italy | 83 | M | COPD, cholecystectomy, and hypertension | Contact with contaminated water, and seafood consumption | Fever, cough, mild jaundice, and abdominal pain | Ceftriaxone, azithromycin | Recover | [3] |
| 2018 | Pakistan | 2 months | M | Neonatal jaundice, cataract | Not certain | Fever and abdominal pain | Cefotaxime, amikacin | Death | [18] |
| 2018 | Belgium | 45 | M | Klatskin tumor | Contact with contaminated water | Fever, epigastric pain, nausea, anorexia | Piperacillin-tazobactam, ciprofloxacin | Recover | [5] |
| 2018 | China | 47 | M | Hepatitis B cirrhosis | Not mentioned | Fever, chills and diarrhea | Ceftazidime | Recover | [19] |
| 2018 | China | 47 | M | Hepatitis B cirrhosis, hepatocellular carcinoma | Not mentioned | Fever, chills, abdominal distension, edema of both lower limbs | Moxifloxacin | Recover | |
| 2018 | Lebanon | 74 | F | Pancreatic adenocarcinoma with liver metastasis, diabetes mellitus | Not certain | Fever, nausea, vomiting, and abdominal pain | Ciprofloxacin | Recover | [2] |
| 2019 | USA | 62 | M | Chronic hepatitis C with liver cirrhosis | Not found | Right leg swelling, fatigue and chills | Ceftriaxone, doxycycline | Recover | [4] |
| 2019 | Saudi Arabia | 54 | M | Hypertension, diabetes mellitus, right lower limb cellulitis, ischemic heart disease, atrial fibrillation and old stroke | Not certain | Fever, confusion, right leg pain, bullae and pus discharge from the sole of the right foot. | Meropenem, vancomycin, tigecycline | Death | [20] |
| 2019 | USA | 63 | F | Chronic lower extremity lymphedema, hypertension, osteoarthritis, restless leg syndrome | Contact with contaminated water through a chronic wound | Left leg pain and discoloration, haemorrhagic blisters with foul-smelling discharge | Doxycycline | Recover | [21] |
Abbreviations: M, male; F: female.
Risk Factors
A literature review showed that most patients had an underlying disease (91.3%, 21/23), and a few had multiple predisposing conditions. The most frequently documented risk factors were liver cirrhosis or hepatitis, diabetes mellitus, malignancy, and biliary tract disease. Other risk factors included alcohol abuse, localized cellulitis, prolonged corticosteroid therapy, and pulmonary diseases such as chronic obstructive pulmonary disease. These risk factors indicated that immunocompromised individuals were more susceptible to NOVC.12 The patient in our study had a degree of hepatic impairment, which was in accordance with the susceptibility factors of NOVC infection. The review showed that middle-aged men were at high risk for NOVC infection. Abdelhafiz et al20 noted that NOVC should be included in the differential diagnoses of invasive infections.
Clinical Manifestations
The clinical manifestations of bacteremic patients varied. The disease may present as hypothermia or hyperthermia, chills, diarrhea, abdominal pain, vomiting, jaundice, and inappetence.18 Swelling or pain in the lower limbs were the first symptoms in some patients, as in the case we reported. A retrospective study found that patients who developed septic shock had a significantly increased risk of death.2 Therefore, early diagnosis and timely treatment can improve the prognosis of NOVC patients.
Mode of Transmission
The modes of infection in patients with bacteremia can be divided into endogenous and exogenous infections. NOVC bacteremia is strongly related to exposure to coastal or estuarine water or consumption of seafood, particularly oysters.22,23 Many NOVC stains have been isolated in the coastal waters of countries such as Germany, India, South Korea, and France.24–27 Our review found that 47.8% of patients (11/23) had a possible source of infection; 6 cases were attributed to consuming seafood and 4 to contaminated water. Two cases presented swelling or pain in the lower leg after contact with contaminated water through a wound. Given our patient’s workplace and the wound on his right foot, our patient was likely infected in the same manner. Some studies found that NOVC isolation varied seasonally, peaking during the summer (69%, especially June and July) and monsoons (46.5%) and decreasing in the winter (15.5%).9,25 An increasing abundance of NOVC may lead to an escalating incidence of NOVC-associated human infections as water surface temperatures rise.24,28 Therefore, people should be educated on the hazards of exposure to coastal and estuarine waters and consuming raw seafood to prevent small-scale outbreaks. In our review, 52.0% of patients denied consuming raw seafood or being exposed to coastal and estuarine waters; therefore, other possible sources of transmission must be determined.
Pathogenesis
To our knowledge, the NOVC pathogenesis remains unknown. Shanley et al hypothesized that NOVC caused bacteremia by spreading from the small intestine or skin wounds into the blood and lymphatic system.4 Patients with liver cirrhosis are susceptible to NOVC bacteremia because of increased intestinal permeability, altered iron metabolism, and impaired phagocytosis and complement.1 Previous studies revealed that several virulence factors were involved in the NOVC pathogenicity and invasiveness. NOVC strains typically did not carry the main virulence factors (cholera toxin and toxin-coregulated pilus), but clinical isolates expressed various synergistic factors, including hemolysin (hlyA), repeats in toxin (rtx), hemagglutinin protease (hapA), toxin regulatory gene (toxR), outer membrane proteins (omp), and the type III (T3SS) and type VI (T6SS) secretion systems.29,30 Furthermore, several new virulence gene variants were reported in some environmental NOVC strains.23 A study suggested that NOVC can release outer membrane vesicles that induce miR-146a to induce colonization.31 T3SS has also been demonstrated to facilitate colonization32 and enhance the virulence of NOVC isolates.6 In addition, hemolysin expression may contribute to the ability of NOVC isolates to invade the bloodstream in immunocompromised individuals.33
Treatment
Our review showed significant heterogeneity in antimicrobial therapies. Wong et al analyzed the antibiotic use and outcome patterns of 763 patients in the Cholera and Other Vibrio Illness Surveillance system and found that quinolone use may reduce the risk of death in patients with NOVC.34 In addition, dual-agent therapy (combining a third-generation cephalosporin with a tetracycline or fluoroquinolone) was recommended for sicker patients with NOVC septicemia or septic shock.35 However, recent studies discovered increased antimicrobial resistance among environmental and clinical NOVC isolates.36 Thus, antimicrobial treatment in patients with NOVC bacteremia must be based on antibiotic susceptibility testing.
NOVC Identification Methods
Detecting and accurately identifying pathogens from blood cultures is important for patients with bacteremia. Commercial identification systems based on biochemical reactions (eg, the Vitek 2 Compact and API 20E), mass spectrometry (eg, MALDI-TOF-MS) and gene sequencing are commonly used to identify NOVC. MALDI-TOF-MS analyzes the expression of intrinsic proteins and variations in the mass:charge ratio (m/z) of these proteins to identify pathogens. Gene sequencing is performed by sequencing the conserved bacterial 16S rRNA gene region and comparing the sequences with those in the NCBI database. Serum agglutination testing is performed to classify V. cholerae according to its serotype and outer membrane O-antigen composition. However, if the strains self-coagulate (as did our strain), serum agglutination testing is not applicable. PCR can then be used, which is based on the V. cholerae-specific outer membrane protein gene (ompW) and the O-antigen rfb genes specific for both O1 and O139.22 NOVC can be confirmed by ompW expression and the absence of O1-rfb and O139-rfb genes. Furthermore, the virulence genes and synergistic factors can be subjected to multiplex PCR,37 and pulsed-field gel electrophoresis and multilocus sequence typing can be used to determine the heterogeneity of the strains.19
Conclusion
NOVC should be included in the differential diagnoses of bacteremia patients, especially in immunocompromised individuals such as those with liver cirrhosis or hepatitis and diabetes mellitus. Exposure to coastal and estuarine waters and consuming raw seafood are sources of transmission for this pathogen. Given the different clinical manifestations and high mortality that NOVC bacteremia causes, timely examination of blood cultures and accurate identification of this strain are important for its diagnosis. Timely and adequate antimicrobial therapy can improve the outcomes of patients with NOVC bacteremia.
Acknowledgments
This study was supported by the Key Laboratory for Laboratory Medicine of Jiangsu Province of China, No. ZDXKB2016005.
Ethics and Consent Statement
Written informed consent was provided by the patient’s relative to allow the case details to be published, and our study was approved by the Ethics Committee at Jiangsu Province Hospital.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Deshayes S, Daurel C, Cattoir V, Parienti JJ, Quilici ML, de La Blanchardiere A. Non-O1, non-O139 Vibrio cholerae bacteraemia: case report and literature review. Springerplus. 2015;4(1):575. doi: 10.1186/s40064-015-1346-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zmeter C, Tabaja H, Sharara AI, Kanj SS. Non-O1, non-O139 Vibrio cholerae septicemia at a tertiary care center in Beirut, Lebanon; a case report and review. J Infect Public Health. 2018;11(5):601–604. doi: 10.1016/j.jiph.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 3.Marinello S, Marini G, Parisi G, et al. Vibrio cholerae non-O1, non-O139 bacteraemia associated with pneumonia, Italy 2016. Infection. 2017;45(2):237–240. doi: 10.1007/s15010-016-0961-4 [DOI] [PubMed] [Google Scholar]
- 4.Shanley J, Kanj A, El Zein S, et al. Non-O1, non-O139 Vibrio cholerae bacteremia in an urban academic medical center in the United States. IDCases. 2019;15:e00527. doi: 10.1016/j.idcr.2019.e00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Keukeleire S, Hoste P, Crivits M, Hammami N, Piette A. Atypical manifestation of Vibrio cholerae: fear the water! Acta Clin Belg. 2018;73(6):462–464. doi: 10.1080/17843286.2018.1483563 [DOI] [PubMed] [Google Scholar]
- 6.Zeb S, Shah MA, Yasir M, et al. Type III secretion system confers enhanced virulence in clinical non-O1/non-O139 Vibrio cholerae. Microb Pathog. 2019;135:103645. doi: 10.1016/j.micpath.2019.103645 [DOI] [PubMed] [Google Scholar]
- 7.Kaki R, El-Hossary D, Jiman-Fatani A, Al-Ghamdi R. Non-O1/non-O139 Vibrio cholerae septicaemia in a Saudi man: a case report. JMM Case Rep. 2017;4(2):e005077. doi: 10.1099/jmmcr.0.005077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan NP, Nga TV, Yen NT, et al. Two cases of bacteriemia caused by nontoxigenic, non-O1, non-O139 Vibrio cholerae isolates in Ho Chi Minh City, Vietnam. J Clin Microbiol. 2014;52(10):3819–3821. doi: 10.1128/JCM.01915-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YT, Tang HJ, Chao CM, Lai CC. Clinical manifestations of non-O1 Vibrio cholerae infections. PLoS One. 2015;10(1):e0116904. doi: 10.1371/journal.pone.0116904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel D, Kumar S. Rare strain of Vibrio cholerae septicemia in a patient with multiple myeloma. Case Rep Crit Care. 2015;2015:596906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y, Wang Y, Bi Z, et al. A case of non-O1/non-O139 Vibrio cholerae septicemia and meningitis in a neonate. Int J Infect Dis. 2015;35:117–119. doi: 10.1016/j.ijid.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury G, Joshi S, Bhattacharya S, et al. Extraintestinal infections caused by non-toxigenic Vibrio cholerae non-O1/non-O139. Front Microbiol. 2016;7:144. doi: 10.3389/fmicb.2016.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel MF, Muijsken MA, Mooi-Kokenberg E, Kuijper EJ, van Westerloo DJ. Vibrio cholerae non-O1 bacteraemia: description of three cases in the Netherlands and a literature review. Euro Surveill. 2016;21(15). doi: 10.2807/1560-7917.ES.2016.21.15.30197 [DOI] [PubMed] [Google Scholar]
- 14.Hirk S, Huhulescu S, Allerberger F, et al. Necrotizing fasciitis due to Vibrio cholerae non-O1/non-O139 after exposure to Austrian bathing sites. Wien Klin Wochenschr. 2016;128(3–4):141–145. doi: 10.1007/s00508-015-0944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maraki S, Christidou A, Anastasaki M, Scoulica E. Non-O1, non-O139 Vibrio cholerae bacteremic skin and soft tissue infections. Infect Dis. 2016;48(3):171–176. doi: 10.3109/23744235.2015.1104720 [DOI] [PubMed] [Google Scholar]
- 16.Sarwar S, Hannan A, Sultana Q, et al. Non-O1Vibrio cholerae bacteremia in an infant, first case report from Pakistan. J Infect Dev Ctries. 2016;10(2):188–189. doi: 10.3855/jidc.6554 [DOI] [PubMed] [Google Scholar]
- 17.Billick MJ, Lam PW, Bogoch II. Sinister seafood: bacteraemia secondary to non-O1/O139 Vibrio cholerae infection. JMM Case Rep. 2017;4(7):e005103. doi: 10.1099/jmmcr.0.005103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baig MZ, Abdullah UH, Shafquat Y, Humayun KN, Zafar A. Non O1, non O139 Vibrio cholerae bacteraemia in an infant; case report and literature review. J Pak Med Assoc. 2018;68(4):650–652. [PubMed] [Google Scholar]
- 19.Jiang F, Bi R, Deng L, Kang H, Gu B, Ma P. Virulence-associated genes and molecular characteristics of non-O1/non-O139 Vibrio cholerae isolated from hepatitis B cirrhosis patients in China. Int J Infect Dis. 2018;74:117–122. doi: 10.1016/j.ijid.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 20.Abdelhafiz TA, Alnimr AM, Alabduljabbar AM, et al. Non O1 Vibrio cholerae as a cause of bacteremic lower limb cellulitis: a case report. Int J Surg Case Rep. 2019;64:62–65. doi: 10.1016/j.ijscr.2019.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azeem A, Nogles T, Cherazard R. Septicemia secondary to Vibrio cholerae (non-O1/non-O139) in wound. BMJ Case Rep. 2019;12(10):e231901. doi: 10.1136/bcr-2019-231901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballal M, Shetty V, Bangera SR, et al. Vibrio cholerae O6 gastroenteritis in a patient with lupus nephritis - a report from coastal Karnataka, South India. JMM Case Rep. 2019;6(1):e005171. doi: 10.1099/jmmcr.0.005171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobrovic K, Rudman F, Ottaviani D, Sestan Crnek S, Leoni F, Skrlin J. A rare case of necrotizing fasciitis caused by Vibrio cholerae O8 in an immunocompetent patient. Wien Klin Wochenschr. 2016;128(19–20):728–730. doi: 10.1007/s00508-016-1060-3 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz K, Hammerl JA, Gollner C, Strauch E. Environmental and clinical strains of Vibrio cholerae non-o1, non-o139 from Germany possess similar virulence gene profiles. Front Microbiol. 2019;10:733. doi: 10.3389/fmicb.2019.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taneja N, Mishra A, Batra N, Gupta P, Mahindroo J, Mohan B. Inland cholera in freshwater environs of north India. Vaccine. 2019;38:A63–72. [DOI] [PubMed] [Google Scholar]
- 26.Di DYW, Lee A, Jang J, Han D, Hur HG. Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl Environ Microbiol. 2017;83(3). doi: 10.1128/AEM.02680-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron S, Larvor E, Chevalier S, et al. Antimicrobial susceptibility among urban wastewater and wild shellfish isolates of non-o1/non-o139 Vibrio cholerae from La Rance Estuary (Brittany, France). Front Microbiol. 2017;8:1637. doi: 10.3389/fmicb.2017.01637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezzulli L, Grande C, Reid PC, et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci U S A. 2016;113(34):E5062–5071. doi: 10.1073/pnas.1609157113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neogi SB, Chowdhury N, Awasthi SP, et al. Novel cholera toxin variant and ToxT regulon in environmental Vibrio mimicus isolates: potential resources for the evolution of Vibrio cholerae hybrid strains. Appl Environ Microbiol. 2019;85(3):e01977–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceccarelli D, Chen A, Hasan NA, Rashed SM, Huq A, Colwell RR. Non-O1/non-O139 Vibrio cholerae carrying multiple virulence factors and V. cholerae O1 in the Chesapeake Bay, Maryland. Appl Environ Microbiol. 2015;81(6):1909–1918. doi: 10.1128/AEM.03540-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bitar A, Aung KM, Wai SN, Hammarstrom ML. Vibrio cholerae derived outer membrane vesicles modulate the inflammatory response of human intestinal epithelial cells by inducing microRNA-146a. Sci Rep. 2019;9(1):7212. doi: 10.1038/s41598-019-43691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaand M, Miller KA, Sofia MK, et al. Type three secretion system island-encoded proteins required for colonization by non-o1/non-o139 serogroup Vibrio cholerae. Infect Immun. 2015;83(7):2862–2869. doi: 10.1128/IAI.03020-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Restrepo D, Huprikar SS, VanHorn K, Bottone EJ. O1 and non-O1 Vibrio cholerae bacteremia produced by hemolytic strains. Diagn Microbiol Infect Dis. 2006;54(2):145–148. doi: 10.1016/j.diagmicrobio.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 34.Wong KC, Brown AM, Luscombe GM, Wong SJ, Mendis K. Antibiotic use for Vibrio infections: important insights from surveillance data. BMC Infect Dis. 2015;15(1):226. doi: 10.1186/s12879-015-0959-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couzigou C, Lacombe K, Girard PM, Vittecoq D, Meynard JL. Non-O:1 and non-O:139 Vibrio cholerae septicemia and pyomyositis in an immunodeficient traveler returning from Tunisia. Travel Med Infect Dis. 2007;5(1):44–46. doi: 10.1016/j.tmaid.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 36.Laviad-Shitrit S, Sharaby Y, Izhaki I, Peretz A, Halpern M. Antimicrobial susceptibility of environmental Non-O1/Non-O139 Vibrio cholerae isolates. Front Microbiol. 2018;9:1726. doi: 10.3389/fmicb.2018.01726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awasthi SP, Chowdhury N, Neogi SB, et al. Development of a multiplex PCR assay for the detection of major virulence genes in Vibrio cholerae including non-O1 and non-O139 serogroups. J Microbiol Methods. 2019;157:54–58. doi: 10.1016/j.mimet.2018.12.012 [DOI] [PubMed] [Google Scholar]