Abstract
Endometrial carcinoma (EC), as described by Bokhman, has historically been classified as Type I (low-grade, hormone-dependant, young patients, good prognosis) or Type II (high-grade, hormone-independent, older patients, poor prognosis). This classification is no longer pragmatic, however, as EC is a much more heterogeneous disease. Four molecular subtypes of EC were identified by The Cancer Genome Atlas (TCGA), and subsequent studies have demonstrated its utility in predicting prognosis. While serous carcinoma (ESC), the prototypical Type II EC, largely occurs in older women, younger women with ESC were not accounted for in the Bokhman model and were underrepresented in the TCGA. We hypothesized that a subset of ESC in young patients do not represent bona fide serous carcinomas but rather high-grade endometrioid carcinomas mimicking a serous phenotype. We identified ESC and mixed endometrioid/serous carcinomas in women <60 years (n=37), and analyzed their clinical, morphologic, immunohistochemical, and molecular characteristics. Sixteen percent showed mismatch repair deficiency (MMR-D) and 11% were diagnosed with Lynch syndrome. Additionally, 16% of cases tested harbored a hotspot POLE exonuclease domain mutation (POLE-EDM). Morphologically, 47% of tumors showed confirmatory endometrioid features, including atypical hyperplasia, a low-grade endometrioid carcinoma component, or squamous differentiation. Clinically, the overall survival in patients with MMR-D and POLE-EDM was significantly better than that of patients without these abnormalities (p=0.0329). In conclusion, ESC in young patients comprise a heterogeneous group of tumors, demonstrating diverse clinical, immunohistochemical, morphologic, and molecular features which have implications for prognosis and adjuvant therapy.
INTRODUCTION
Historically, endometrial cancer (EC) has been classified into two broad subgroups, as described by Bokhman [1]. Type I cancers are usually low-grade, estrogen-dependent, occur in young women and show good outcomes, while Type II cancers are seen in older women, are high-grade and estrogen-independent and carry poor prognoses. The prototypical examples of Type I and Type II cancers are low-grade endometrioid and serous carcinomas, respectively. The accurate and reproducible sub-classification of high-grade EC is an ongoing challenge for pathologists, however.[2] Studies involving expert subspecialized gynecologic pathologists have shown a disappointingly low rate of inter-observer diagnostic agreement in these cases on morphologic assessment alone, with only moderate improvement in inter-pathologist agreement with the addition of immunohistochemistry [3–5]. These studies, coupled with new insights into the underlying molecular pathology of these tumors gained from studies such as The Cancer Genome Atlas (TCGA) [6] challenge existing beliefs about the utility and validity of the established morphology-based tumor classification in EC and have led to a richer understanding of the complexity of the molecular alterations present within these morphologically-defined carcinoma subtypes [7, 8]. It has become clear that the clinical and histological archetypes of Bokhman’s classification are of limited value in EC.
TCGA performed a comprehensive molecular characterization of endometrioid (EEC) and serous carcinomas (ESC) and identified four molecular subtypes based on the tumor somatic mutation rate, microsatellite instability status and of copy number alterations [6]. These four molecular groups include the copy number high (serous-like), copy number low (endometrioid-like), microsatellite-high (hypermutated) and polymerase epsilon (POLE) exonuclease domain mutated (ultramutated) [6]. This molecular classification showed significant associations with progression-free survival and subsequent studies have demonstrated its utility in predicting prognosis [9]. All of the morphologically-defined ESCs tested demonstrated high levels of copy number alterations (and almost all had a TP53 mutation), and were classified as of copy-number high (serous-like) subtype, as were 25% of tumors with a morphologic diagnosis of high-grade EEC. The remaining EECs fell into the other three groups. The POLE group represented a newly defined subtype of endometrial cancer and subsequent studies have confirmed the validity of this molecular subtype and have demonstrated that these tumors are often high grade and show morphological overlap with ESC and a subset may show p53 abnormalities by immunohistochemistry (IHC) [10, 11]. It should be noted, however, that ECs harboring a POLE exonuclease domain mutation (POLE-EDM) have a better prognosis than ESC, usually present at early stage, and often occur in younger women [12–15]. Morphologically ambiguous high grade ECs, including a subset with serous morphology have also been reported in DNA mismatch repair deficient (MMR-D) cancers and in patients with Lynch syndrome [16, 17]. In addition, rare p53 abnormalities by IHC in MMR-D ECs have also been described [11].
Since ESC is an exceptionally rare diagnosis in women under the age of 60, we hypothesized that many diagnosed as such may not actually be bona fide pure serous carcinomas but rather comprise tumors that show high grade features overlapping with ESC, as has been previously described in POLE-EDM and MMR-D ECs. In addition, we hypothesized that a subset could also have arisen from an endometrioid component given the young age of patients at diagnosis.
To this end, we analyzed a cohort of ECs diagnosed as ESC or mixed endometrioid/serous carcinomas in a group of patients less than 60 years. DNA MMR and p53 IHC and sequencing for exonuclease domain mutations in POLE was undertaken. In addition, the tumors were evaluated for the presence of endometrioid features including morphology and aberrant expression patterns for PTEN and ARID1A by IHC. Clinical data, including patient outcomes, were also recorded.
MATERIALS AND METHODS
Cases
This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center, and patient consent was obtained where appropriate.
Patients younger than 60 years, diagnosed with ESC (n=25) or mixed endometrioid/serous carcinoma (n=12) from 2006–2012 were identified from the clinical database. Details of patient epidemiologic and clinical features were recorded at electronic chart review. The archival tumor tissue was obtained from these cases, and all hematoxylin and eosin (H&E)-stained tumor sections of each case were reviewed by two specialized gynecologic pathologists (DFD, NC). Morphological review for features suggestive of serous or endometrioid morphology was undertaken. Discriminatory morphological “endometrioid” features were defined as squamous metaplasia/differentiation, background complex atypical hyperplasia, or a low-grade endometrioid carcinoma (LGEM) component. Serous morphology was defined as papillary, glandular or solid architecture and marked cytological atypia, cellular stratification and tufting, with no background evidence of discriminatory endometrioid features.
Immunohistochemistry
IHC for p53, ARID1A, PTEN and the DNA mismatch repair proteins MSH2, MSH6, MLH1 and PMS2 was performed on all cases. IHC for p53 was performed with a monoclonal p53 antibody (DO-7, Dako, pre-diluted) as previously described [18]. Cases were scored as aberrant if >75% of the cells were strongly p53 positive (overexpressed) or if 0% of cells were positive (null phenotype) [19]. If a distinct subset of cells showed aberrant p53 expression, this was classified as a geographic pattern. IHC for the mismatch repair proteins was performed using primary monoclonal antibodies against MLH1 (clone G168–728, diluted 1:250, PharMingen, San Diego, CA), MSH2 (clone FE11,diluted 1:50, Oncogene Research Products, Cambridge,MA), MSH6 (clone GRBP.P1/2.D4, diluted 1:200; Serotec Inc, Raleigh, NC), and PMS2 (clone A16–4, diluted 1:200, BD PharMingen, San Diego, CA) as previously described [20]. Expression was defined as abnormal if there was complete absence of one or more of the mismatch repair proteins from all tumor cell nuclei [20]. Immunohistochemistry for ARID1A was performed using a polyclonal antibody against ARID1A (HPA005456; Sigma-Aldrich, St. Louis, MO, USA) as previously described [21]. Abnormal ARID1A immunohistochemistry was defined as complete loss of nuclear staining in tumor cell nuclei. A monoclonal antibody for PTEN (clone 6H2.1, DAKO) was used as previously described [22]. PTEN expression was defined as lost when the tumor cells showed absence of immunoreactivity [22]. If a distinct subset of cells showed aberrant staining, this was classified as a geographic pattern.
With all of the antibodies, the presence of a positive internal control (blood vessels, stromal cells, lymphocytes) was required for interpretation.
Microdissection and DNA extraction
Eight-μm-thick representative tumor tissue sections from each case with adequate tumor (n=28) were stained with nuclear fast red and microdissected using a sterile needle under a stereomicroscope (Olympus SZ61) to ensure >80% of tumor cell content, as previously described [23]. Genomic DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen) and quantified using the Qubit Fluorometer assay (Life Technologies, Thermo Fisher Scientific).
Targeted amplicon sequencing
All DNA samples were subjected to targeted amplicon sequencing using a custom primer set encompassing the POLE codons D275, P286 and S297 (exon 9), V411, L424 and P436 (exon 13), and A456 and S459 (exon 14) (for primer sequences see Supplementary Table S1). DNA obtained from an EC harboring a previously validated POLE hotspot mutation (V411L) [24] was included as a positive control. For each sample, the PCR products were pooled, purified with Agencourt AMPure XP (Beckman Coulter, Indiana, USA), barcoded and sequenced on an Illumina Hiseq 4000 (Illumina, Inc., San Diego, CA, USA; 125 bp paired-end reads). Sequencing was performed to a median depth of 384× (range 216×−607×). Paired-end reads were aligned to the reference human genome GRCh37 using the Torrent Mapping Alignment Program (TMAP, v3.4.1) [25]. Local realignment was performed using GATK (v3.1.1)[26]. Somatic single nucleotide variants (SNVs) were identified using MuTect (v1.0)[27] and further curated by manual inspection using Integrative Genomics Viewer (IGV)[28].
Sanger sequencing
POLE hotspot mutations identified by targeted amplicon sequencing were independently validated using Sanger sequencing. PCR amplification and Sanger sequencing were performed as previously described [29]. Both the forward and reverse strands were analyzed using MacVector software.
Statistical Analysis
The association between morphologic features, and the presence of POLE-EDM and/or MMR-D and overall survival was analyzed, and survival curves were calculated by use of the Kaplan–Meier method with the log-rank test. P values of <0.05 were considered to be statistically significant.
RESULTS
Clinical Features
The median age at diagnosis of patients included in this study (n=37) was 56 years (range 27–59 years; Table 1). The menopausal status of two patients was not recorded; of the remainder, 74% (26/35) were post-menopausal; the median time since menopause was recorded as 9 years. Twenty-six percent of the patients (9/35) were described as pre- or peri-menopausal. The mean patient body-mass-index (BMI) at the time of diagnosis was 30.5 [median 28]; 35% (13/37) were clinically obese (BMI >30) while 43% (16/37) were overweight (BMI 25–30).
Table 1.
Clinico-pathologic factors of the endometrial serous carcinomas and mixed endometrioid/serous carcinomas in women less than 60 years of age included in this study.
| Case ID | Age (years) | Original diagnosis | Endometrioid features | Revised diagnosis | FIGO Stage | Survival (months) | p53 | PTEN | ARID1A | MMR | POLE EDM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||||
| 6 | 50 | ESC/EEC | LGEM | ESC/EEC | Ia | NED 132 | A/(OE) | R | R | MSH2,6, LS | NP |
| 11 | 27 | ESC/EEC | LEGM | ESC/EEC | Ib | NED 115 | A (OE/G), (LEGM WT) | R | R | PMS2, LS | NP |
| 24 | 49 | ESC/EEC | LGEM | ESC/EEC | Ia | AWD 87 | A (OE/G), (LGEM WT) | R | L | PMS2, LS | NP |
| 20 | 47 | ESC/EEC | LGEM | ESC/EEC | IIIc | NED 71 | WT | R | L | MSH2,6 LS | N |
| 10 | 59 | ESC | CAH | ESC/EEC | Ia | NED 123 | A (OE/G), (LEGM WT) | R | R | MSH2,6 | NP |
| 37 | 59 | ESC/EEC | LGEM | ESC/EEC | Ia | NED 21 | A (OE/G), (LGEM WT) | R | R | MSH6 | NP |
| 22 | 46 | ESC/EEC | LGEM | ESC/EEC | Ia | NED 68 | A (OE/G) | L | R | R | p.V411L |
| 30 | 57 | ESC/EEC | LGEM | ESC/EEC | IIIc | NED 94 | WT | L | R | R | p.V411L |
| 36 | 56 | ESC/EEC | LEGM | ESC/EEC | Ia | NED 88 | A (OE/G), (LEGM WT) | L | R | R | p.P286R |
| 12 | 52 | ESC/EEC | LGEM | ESC/EEC | Ia | NED 121 | WT | L | L | R | p.P286R |
| Group 2 | |||||||||||
| 3 | 55 | ESC | CAH | ESC/EEC | II | NED 140 | A (OE) | R | R | R | N |
| 5 | 59 | ESC/EEC | SQ | ESC/EEC | IIIc | DOD 10 | A (OE) | L | R | R | N |
| 9 | 55 | ESC/EEC | LGEM | ESC/EEC | IIIc | DOD 25 | A (OE) | L | R | R | N |
| 17 | 59 | ESC | SQ | ESC/EEC | Ib | AWD 94 | A (OE) | R | R | R | N |
| 18 | 53 | ESC | LGEM | ESC/EEC | Ia | NED 95 | A (OE) | R | R | R | NP |
| 19 | 59 | ESC | CAH | ESC/EEC | Ia | NED 90 | A (OE/G), (LEGM WT) | R | R | R | N |
| 25 | 50 | ESC/EEC | LGEM | ESC/EEC | Ia | NED 63 | A (OE/G), (LEGM WT) | R | R | R | N |
| 27 | 46 | ESC | SQ | ESC/EEC | IIIa | DOD 30 | A (OE) | L | R | R | N |
| 29 | 59 | ESC | SQ | ESC/EEC | Ia | NED 91 | null | L | R | R | N |
| 32 | 56 | ESC | SQ | ESC/EEC | IV | NED 99 | A (OE) | L | R | R | NP |
| 33 | 48 | ESC | LGEM | ESC/EEC | IIIa | NED 75 | null | L | R | R | NP |
| Group 3 | |||||||||||
| 1 | 59 | ESC | N | ESC | IIIc | DOD 36 | A (OE) | L (G) | R | R | N |
| 2 | 59 | ESC | N | ESC | IVb | DOD 15 | null | R | R | R | N |
| 4 | 59 | ESC | N | ESC | Ib | NED 128 | A (OE) | R | R | R | NP |
| 7 | 59 | ESC | N | ESC | Ib | NED 105 | A (OE) | R | R | R | N |
| 8 | 59 | ESC | N | ESC | IVb | DOD 13 | A (OE) | L | R | R | N |
| 13 | 58 | ESC | N | ESC | IVb | AWD 121 | A (OE) | L (G) | R | R | N |
| 14 | 56 | ESC | N | ESC | IIIa | DOD 47 | A (OE) | L | R | R | N |
| 15 | 53 | ESC | N | ESC | Ib | NED 44 | A (OE) | R | R | R | N |
| 16 | 54 | ESC | N | ESC | IIIc | NED 76 | A (OE) | L | R | R | N |
| 21 | 55 | ESC | N | ESC | IIIc | DOD 36 | A (OE) | R | R | R | N |
| 23 | 59 | ESC | N | ESC | Ia | NED 23 | A (OE) | L (G) | R | R | N |
| 26 | 54 | ESC | N | ESC | Ia | NED 31 | A (OE) | R | R | R | NP |
| 28 | 58 | ESC | N | ESC | IIIc | AWD 37 | null | R | R | R | NP |
| 31 | 59 | ESC | N | ESC | IVb | DOD 17 | A (OE) | L | R | R | N |
| 34 | 59 | ESC | N | ESC | Ia | NED 88 | WT | L | R | R | NP |
| 35 | 59 | ESC | N | ESC | IV | DOD 32 | A (OE) | L | R | R | N |
Abbreviations/Definitions: Group 1, POLE mutation or mismatch repair deficient; Group 2, serous and endometrioid morphology, MMR proficient, POLE wild type; Group 3, Prototypical serous morphology, MMR proficient, POLE wild-type; ESC, endometrial serous carcinoma; EEC, endometrial endometrioid carcinoma; MMR, mismatch repair; POLE EDM, POLE exonuclease domain mutation; CAH, complex atypical hyperplasia; LGEM, low grade endometrioid component; SQ, squamous differentiation; DOD, dead of disease; NED, no evidence of disease; AWD, alive with disease; A, aberrant; OE, overexpressed; G, geographic staining pattern; WT, wild-type staining pattern; null, null phenotype; L, lost; R, retained; NP not performed; N, no; Y, yes; LS patient has Lynch syndrome
At the time of diagnosis, 51% (19/37) of patients had stage I (14 stage Ia, 5 stage Ib), 3% (1/37) stage II, 27% (10/37) stage III (3 stage IIIa, 7 stage IIIc) and 19% (7/37) stage IV disease (Table 1). Of the 20 patients with low stage disease (stages I-II), 18 received chemotherapy and radiotherapy, 1 chemotherapy only and 1 received no adjuvant treatment. As expected, all patients with high stage disease (stages III-IV) at presentation received adjuvant chemotherapy with 6/17 (35%) also having had radiotherapy.
Histologic Review
Upon histologic re-review of the cases, tumors were subcategorized into those with exclusively serous morphology (n=16) and those with confirmatory endometrioid features in addition to serous morphology (n=21). Confirmatory endometrioid features consisted of evidence of endometrioid differentiation, including complex atypical hyperplasia, a low grade endometrioid component, and/or squamous differentiation. Of the 21 endometrial cancers with endometrioid features, 3 showed background complex atypical hyperplasia, 13 showed a low grade endometrioid component, and 5 showed squamous differentiation (Table 1). In summary, 21 of 37 (56.8%) endometrial cancers previously diagnosed as pure or mixed ESCs demonstrated at least one histologic finding considered a discriminatory endometrioid feature. Notably, 9 of 25 (36%) tumors originally diagnosed as pure ESCs displayed endometrioid features. As expected, all 12 ECs originally diagnosed as mixed endometrioid/serous had indeed evident discriminatory endometrioid features at the time of diagnosis.
POLE exonuclease domain mutations
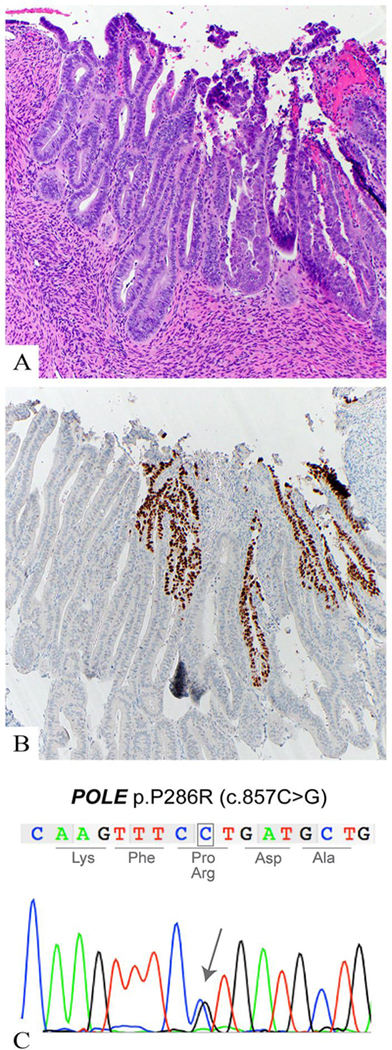
Given that ECs of POLE molecular subtype have been described as displaying high-grade/ambiguous morphologic features, [10] we sought to assess whether any of the tumors harbored hotspot POLE EDMs. A total of 25 of 37 ECs were successfully subjected to POLE targeted amplicon sequencing, and the mutations identified were validated by Sanger sequencing. Four out of the 25 (16%) ECs analyzed harbored a hotspot mutation in the exonuclease domain of POLE. The POLE mutations p.V411L and p.P286R were found in two cases respectively (Figure 1). The original diagnosis of all 4 POLE-EDM cases was mixed serous/endometrioid carcinoma. Two cases showed aberrant p53 overexpression but the staining pattern was geographic in both cases with the wild-type areas seen in the low-grade endometrioid component in one of the two cases (Figure 1).
Figure 1.
Case 36. a. Hematoxylin and eosin-stained section of a mixed endometrioid/serous carcinoma with a POLE exonuclease mutation. The left side of the image shows a low grade endometrioid component with smooth luminal borders and columnar shaped nuclei. To the right is the “serous” component with increased tufting and budding, nuclear stratification, and prominent nucleloi. b. Aberrant p53 over-expression by immunohistochemistry the in “serous” component. The adjacent low grade component shows a wild-type p53 expression pattern. C. Representative Sanger sequence electropherogram of POLE p.P286R
Mismatch Repair Protein Immunohistochemical Analysis
MMR-D was detected in 6 (16%) cases (Table 1). Three of these cases showed loss of MSH2 and MSH6 (Figure 2), 2 cases showed loss of PMS2 only, while one case showed loss MSH6 only. Four cases showed aberrant p53 in a geographic pattern while one was diffuse. Five of the six cases had an original diagnosis of mixed ESC/EEC and four of these patients have been diagnosed with Lynch Syndrome.
Figure 2.
Case 20 a. Hematoxylin and eosin-stained section of a case diagnosed as endometrial serous carcinoma in a patient with Lynch Syndrome. Papillary architecture and slit-like spaces suggest serous differentiation b. Normal/wild-type p53 expression by immunohistochemistry. c. Loss of MSH2 by immunohistochemistry (MSH6 was also lost, not shown).
Other Immunohistochemical Results
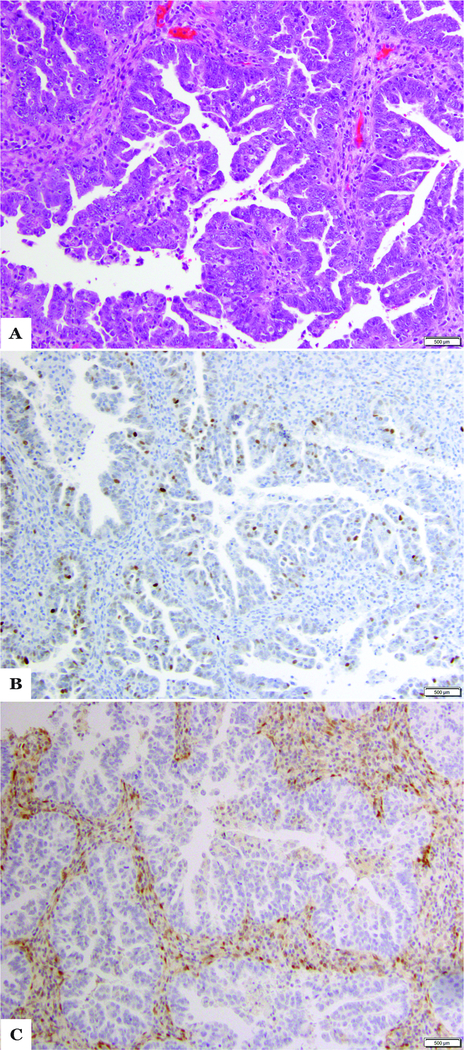
Overall, the majority (33/37; 89%) of the cases had aberrant p53 expression, with 8 of these showing a geographic pattern. In 7 of these cases the p53 wild-type pattern was present only in the low-grade endometrioid component. PTEN loss of expression was observed in 19/37 (51%) cases, with the pattern being geographic in 3 tumors while ARID1A loss was detected in only 3 (8%) of cases (2 MMR-D, 1 POLE-EDM). Aberrant expression of both p53 and PTEN was seen in 16 of 37 cases (43%) overall (Figure 3). Within the subgroup of ESCs lacking endometrioid features (n=16), only 7 (44%) demonstrated a classical serous-type immunohistochemical profile, including aberrant p53 expression and retained PTEN, ARID1A and MMR protein expression
Figure 3.
Case 5 a. Hematoxylin and eosin-stained section of case diagnosed as mixed endometrioid/serous carcinoma. Pictured here is the serous component showing focal papillary architecture, high nuclear grade, and prominent nucleoli. b. Loss of PTEN by immunohistochemistry. c. Aberrant p53 over-expression by immunohistochemistry.
Outcome Analysis
For the early stage patients, the median follow-up was 90 months (range 23–140); two of these patients developed a recurrence and are currently alive with disease (Table 1). In contrast, of the 17 high stage ECs, 10 had died of their disease, 2 patients are alive with disease and 5 patients have no evidence of disease at a median follow up of 36 months (range 10–121) (Table 1).
Based on the results of the histologic review, POLE-EDM testing, and MMR IHC, the cohort was stratified into three groups as outlined in Table 1. Group 1 represents those cases with POLE-EDM or MMR-D while Group 2 represents cases with at least focal endometrioid morphology but without POLE-EDM or MMR-D. Group 3 represents what can be described as apparently archetypal ESC, with no clear endometrioid morphology, no POLE-EDM and no MMR-D.
EC patients with tumors that showed MMR-D or a POLE-EDM (group 1) had better overall survival than those without these abnormalities (groups 2 and 3) in a univariate analysis (p=0.0329) (Fig. 4a). POLE-EDM/MMR-D showed the best overall survival followed by mixed EEC/ESC (POLE-EDM wild-type/DNA MMR proficient) while the prototypical serous group showed the worst overall survival. When comparing the outcome of all three groups, there was a trend with overall survival, but this did not reach statistical significance (p=0.0589, Fig 4b). No survival difference was found in patients with ESCs with PTEN loss versus those with retention of PTEN by IHC (Table 1; data not shown).
Figure 4.
a. Overall survival of patients with tumors that showed mismatch repair deficiency (MMR-D) or POLE exonuclease domain mutation versus patients with tumors that did not show these abnormalities. b. Overall survival of patients with tumors that show mismatch repair deficiency (MMR-D) or POLE exonuclease domain mutation versus tumors with pure prototypical serous morphology versus tumors with endometrioid and serous morphology which are mismatch repair proficient (MMR-P) or POLE exonuclease domain mutation wild-type (POLE-WT).
DISCUSSION
This study has demonstrated that in patients aged less than 60 at diagnosis, ECs that are diagnosed as showing a “serous” component are enriched for MMR-D, Lynch syndrome, and POLE-EDMs, abnormalities which have important clinical, prognostic, and therapeutic implications. Of all cases studied, 16% showed MMR-D and 11% of patients were diagnosed with Lynch Syndrome. Sixteen percent of all cases tested demonstrated a POLE-EDM.
One of the major findings of the TCGA study of endometrial carcinoma was the marked molecular heterogeneity in the category of tumors diagnosed histologically as high grade EEC. One quarter of such cases were characterized as “serous-like” in the TCGA study, having high levels of copy number aberrations (CNAs) and TP53 mutations, similar to ESCs. High grade EEC also comprised significant proportions of the POLE-EDM and MSI-H groups. It has been previously reported that intratumoral morphological heterogeneity is particularly prominent in POLE-EDM and MMR-D cancers (10, 31) and such cases have at times been categorized as having a serous component due to the level of cytological atypia present. The findings in our study that nine of twelve cases of mixed ESC/EEC showing either POLE-EDM or MMR-D are in keeping with these previous findings. It should be noted that our study cohort were diagnosed in 2006–2012, before the publication of the TCGA endometrial carcinoma study findings on POLE-EDM tumors, and prior to the introduction of universal MMR IHC in EC.
Of the cancers in our study cohort originally diagnosed as pure ESC (n=25), all but one had a p53 abnormality and intact DNA MMR protein expression and were negative for POLE-EDM. Thus, one could surmise that the vast majority of these tumors are best regarded as synonymous with the “copy number high (serous-like)” category as described in the TCGA study. However, it should be noted that our study population of younger patients with ESC were likely underrepresented in the TCGA study, as analysis of the clinical characteristics of that study cohort shows that only 3 of 41 patients with ESC were aged less than 60 years.
In this study, we hypothesised that a proportion of tumors diagnosed originally as ESC in younger patients would show an intermediate morphological and/or molecular phenotype between endometrioid and serous subtypes. This was based on the observation that these younger patients are not accounted for in the binary Bokhman Type I and II classification of EC. Our findings support this hypothesis, at least in a subset of tumors, as re-analysis of the original histologic slides in these cases revealed background endometrioid features present in 9 of the 25 (36%) cases originally diagnosed as pure ESC. However, the results of p53 and PTEN immunohistochemistry in this group are more striking, with approximately half of cases showing coincident loss of both p53 and PTEN expression. This p53/PTEN loss of expression phenotype was seen with equal frequency in the group with subtle endometrioid features (4/9) and those without (9/16) (p=0.69). Previous studies have suggested that while a PTEN mutation is present in up to 90% of high grade EEC, it is very rare in ESC (<3%) (7). In the TCGA study, 15 of 16 EECs of copy-number high (serous-like) subtype harbored a TP53 mutation and 5 of those had a concurrent mutation in PTEN. In contrast, only one of the ESCs in the TCGA cohort had a PTEN mutation [6, 30].
These findings raise the possibility that this p53/PTEN co-loss phenotype is uniquely common in younger patients with ESC and may reflect tumor progression from an underlying endometrioid component. One must also acknowledge that immunohistochemical loss of PTEN expression is not synonymous with mutation, copy number alterations may also lead to loss of expression and thus further studies are required.
The reproducibility of the histopathological diagnostic sub-categorization of high-grade endometrial carcinoma is poor, even between expert gynecologic pathologists [3–5]. Diagnostic reproducibility is undermined by the extensive overlap between the histologic features of the high-grade forms of endometrioid cancers and the other high-grade endometrial carcinoma subtypes, and also by variation in the incorporation of emerging molecular data into clinical practice. It is now recognized that ESCs may display glandular morphology with focally atypical, but monomorphic nuclei, while on the other hand, marked nuclear pleomorphism can occur in endometrioid adenocarcinomas. Such atypia is therefore not pathognomonic for ESC [31] and consequently many of the histopathological features traditionally emphasized in diagnosis of ESC are not specific. This study of younger women with a diagnosis of ESC illustrates the marked morphologic, molecular, and immunohistochemical heterogeneity within this group of patients.
It should be noted that ESC in a patient aged less than 60 years is a rare diagnosis, with only 37 cases identified in a 6-year period at our tertiary referral center. Thus, it is important that when dealing with a case of endometrial carcinoma, which is morphologically consistent with ESC (mixed-type or pure) in a woman aged 60 or less, one searches carefully for features suggestive of POLE-EDM mutation (marked cytological atypia, marked tumor infiltrating lymphocytes, and heterogeneity with areas of endometrioid morphology) [10], as this may suggest the patient has a better prognosis [6, 12, 14, 15] or could benefit from other treatment modalities. One should also search for morphology suggestive of DNA MMR-D, including involvement of the lower uterine segment, intratumoral heterogeneity and intratumoral lymphocytosis [32, 33]. Our data suggest that sequencing of the exonuclease domain of the POLE gene and IHC of the DNA MMR proteins should be performed in all ECs in young women in which a serous component is considered. In addition to the benefit of prognostic information and the detection of Lynch syndrome, the identification of POLE and DNA MMR abnormalities opens up alternative treatment regimens as recent data shows that these tumor types may respond well to immune checkpoint inhibitors [34]. Based on the young age of the patients we considered that some of the cases studied may represent drop metastases from the adnexa. Of tumors without endometrioid features and advanced stage at diagnosis (stages III or IV; n=10), the adnexa was involved in 6 cases, 2 of which were WT1 negative by IHC (performed at the time of diagnosis). The remaining four cases showed only focal adnexal surface involvement, arguing against an adnexal primary.
Due to limited material, mutation analysis to confirm the presence or absence of a TP53 mutation was not possible. Prior studies have shown, however, an excellent correlation between p53 IHC and TP53 mutation status. Singh et al recently reported an overall concordance rate of 92.3% between IHC and mutational status in a large series of endometrial cancers. [35] Subclonal or geographic aberrant p53 expression was found in a small subset of cases and were enriched for MMR-D and POLE-EDM, similar to the findings in the present study.
In summary, ESC at our center in women aged less than 60 years are a heterogeneous group of tumors spanning the clinical, morphological, immunohistochemical, and molecular spectrum between endometrioid and serous carcinomas. A subset with superior outcomes showed POLE EDMs and DNA MMR-D as well as other features usually restricted to endometrioid-type tumors. This study further emphasizes the importance of the identification of underlying molecular abnormalities in high-grade ECs in young patients as these findings may result in a diagnosis of Lynch syndrome as well as a distinct prognosis and adjuvant therapy.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this paper was supported in part by a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (Grant No P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BW is supported in part by a Cycle for Survival grant.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Bokhman JV, Two pathogenetic types of endometrial carcinoma. Gynecol Oncol, 1983. 15(1): p. 10–7. [DOI] [PubMed] [Google Scholar]
- 2.Murali R, et al. , High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int J Gynecol Pathol, 2019. 38 Suppl 1: p. S40–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han G, et al. , Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Hoang LN, et al. , Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol, 2013. 37(9): p. 1421–32. [DOI] [PubMed] [Google Scholar]
- 5.Gilks CB, Oliva E, and Soslow RA, Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol, 2013. 37(6): p. 874–81. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research, N., et al. , Integrated genomic characterization of endometrial carcinoma. Nature, 2013. 497(7447): p. 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConechy MK, et al. , Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol, 2012. 228(1): p. 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murali R, Soslow RA, and Weigelt B, Classification of endometrial carcinoma: more than two types. Lancet Oncol, 2014. 15(7): p. e268–78. [DOI] [PubMed] [Google Scholar]
- 9.Talhouk A, et al. , A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer, 2015. 113(2): p. 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussein YR, et al. , Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol, 2015. 28(4): p. 505–14. [DOI] [PubMed] [Google Scholar]
- 11.Talhouk A, et al. , Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: Earlier prognostic information to guide treatment. Gynecol Oncol, 2016. 143(1): p. 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billingsley CC, et al. , Prognostic Significance of POLE Exonuclease Domain Mutations in High-Grade Endometrioid Endometrial Cancer on Survival and Recurrence: A Subanalysis. Int J Gynecol Cancer, 2016. 26(5): p. 933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billingsley CC, et al. , Polymerase varepsilon (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer, 2015. 121(3): p. 386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church DN, et al. , Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst, 2015. 107(1): p. 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng B, et al. , POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol, 2014. 134(1): p. 15–9. [DOI] [PubMed] [Google Scholar]
- 16.Carcangiu ML, et al. , Lynch syndrome--related endometrial carcinomas show a high frequency of nonendometrioid types and of high FIGO grade endometrioid types. Int J Surg Pathol, 2010. 18(1): p. 21–6. [DOI] [PubMed] [Google Scholar]
- 17.Broaddus RR, et al. , Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer, 2006. 106(1): p. 87–94. [DOI] [PubMed] [Google Scholar]
- 18.Garg K, et al. , p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol, 2010. 23(1): p. 80–92. [DOI] [PubMed] [Google Scholar]
- 19.Yemelyanova A, et al. , Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol, 2011. 24(9): p. 1248–53. [DOI] [PubMed] [Google Scholar]
- 20.Modica I, et al. , Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol, 2007. 31(5): p. 744–51. [DOI] [PubMed] [Google Scholar]
- 21.Ye J, et al. , Immunohistochemical detection of ARID1A in colorectal carcinoma: loss of staining is associated with sporadic microsatellite unstable tumors with medullary histology and high TNM stage. Hum Pathol, 2014. 45(12): p. 2430–6. [DOI] [PubMed] [Google Scholar]
- 22.Garg K, et al. , Pathologic scoring of PTEN immunohistochemistry in endometrial carcinoma is highly reproducible. Int J Gynecol Pathol, 2012. 31(1): p. 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martelotto LG, et al. , Genomic landscape of adenoid cystic carcinoma of the breast. J Pathol, 2015. 237(2): p. 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLair DF, et al. , The genetic landscape of endometrial clear cell carcinomas. J Pathol, 2017. 243(2): p. 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Available from: https://github.com/iontorrent/TS/tree/master/Analysis/TMAP
- 26.McKenna A, et al. , The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res, 2010. 20(9): p. 1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cibulskis K, et al. , Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol, 2013. 31(3): p. 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JT, et al. , Integrative genomics viewer. Nat Biotechnol, 2011. 29(1): p. 24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piscuoglio S, et al. , Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and TERT promoter hotspot mutations and TERT gene amplification as likely drivers of progression. J Pathol, 2016. 238(4): p. 508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultheis AM, et al. , TP53 Mutational Spectrum in Endometrioid and Serous Endometrial Cancers. Int J Gynecol Pathol, 2016. 35(4): p. 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg K and Soslow RA, Strategies for distinguishing low-grade endometrioid and serous carcinomas of endometrium. Adv Anat Pathol, 2012. 19(1): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 32.Garg K and Soslow RA, Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma. J Clin Pathol, 2009. 62(8): p. 679–84. [DOI] [PubMed] [Google Scholar]
- 33.Garg K, et al. , Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol, 2009. 33(6): p. 925–33. [DOI] [PubMed] [Google Scholar]
- 34.Mittica G, et al. , Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget, 2017. 8(52): p. 90532–90544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh N, et al. , p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.