Abstract
TFIID is a large multiprotein assembly that serves as a general transcription factor for transcription initiation by eukaryotic RNA polymerase II (Pol II). TFIID is involved in the recognition of the core promoter sequences and neighboring chromatin marks, and can interact with gene-specific activators and repressors. In order to obtain a better molecular and mechanistic understanding of the function of TFIID, its structure has been pursued for many years. However, the scarcity of TFIID and its highly flexible nature have made this pursuit very challenging. Recent breakthroughs, largely due to methodological advances in cryo-electron microscopy, have finally described the structure of this complex, both alone and engaged with core promoter DNA, revealing the functional significance of its conformational complexity in the process of core promoter recognition and initiation of Pol II transcription. Here, we review these recent structural insights and discuss their implications for our understanding of eukaryotic transcription initiation.
Roles of TFIID in the early stages of transcription initiation
Eukaryotic transcription initiation involves a large number of protein factors that need to recognize the transcription start site, recruit the multi-subunit RNA polymerase complex, and, in the case of the RNA polymerase II (Pol II) system, open the DNA through the consumption of energy by ATP hydrolysis [1,2]. This system not only has to be robust and faithful, but also highly regulated to adjust transcriptional output to the cellular and organismal requirements in time and space [3]. Transcription of protein-coding genes starts with the recognition of core promoter regions on DNA by TFIID and the consequent assembly of a large pre-initiation complex (PIC) that includes Pol II, TFIIH, and Mediator complexes. TFIID is itself a very sizeable assembly (> 1 MDa) comprised of the TATA-binding protein (TBP) and 13 or 14 different TBP-associated factors (TAFs) [4], some of which are present in two copies [5,6]. TFIID binds to all protein gene promoters, where it loads TBP onto the DNA, irrespective of the presence or absence of a TATA box [7,8]. TFIID is not only essential for the recognition of core promoter sequences and the recruitment of the PIC via TBP, tasks that are critical for basal transcription, but is also involved in regulation of protein gene expression via its interactions with cofactors, gene-specific activators and repressors, and chromatin modifications associated with active regions of the genome [9–11]. Thus, TFIID must act as a molecular hub that integrates different regulatory cues into a certain level of transcriptional output, possibly through changes in its capacity to bind DNA and/or recruit Pol II.
Challenges and progress in the structural characterization of TFIID
While a large number of biochemical studies have uncovered important aspects of TFIID function [4,9], a structural framework that would provide a mechanistic understanding of how those functions can be integrated has been missing until recently. Structure determination of full TFIID has been challenging because, with a size of ~1.3 MDa, it is difficult to produce recombinantly and endogenous sources are very limiting. However, structures of several subunits or domains have been determined by X-ray crystallography or NMR, either alone or in small subcomplexes [10,11,12••,13–16]. Electron microscopy (EM), with its more modest sample requirements, has been the structural technique of choice to characterize full TFIID, but progress has been slow owing to the fragility and flexibility of this complex [17]. With the recent advent of new direct electron detector technology and improved cryo-EM image processing algorithms [18], significant progress has now been made, both toward defining the subunit arrangement within TFIID, and toward charting the conformational landscape of TFIID and its relationship to core promoter binding and TBP activation.
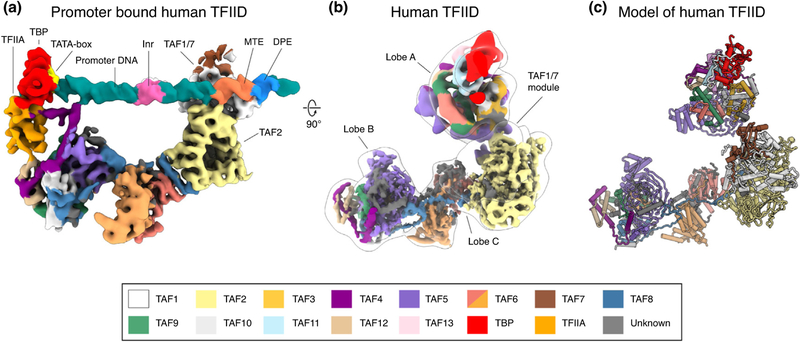
The earliest cryo-EM studies of human TFIID described its shape as resembling a horseshoe made of three main lobes, named A, B and C [19], and a similar structure was observed by cryo-EM for budding yeast TFIID [20,21]. Later studies showed that while Lobes B and C appear to form a relatively rigid core in human TFIID, Lobe A moves by about 150 Å, from being engaged with Lobe C (a state referred to as canonical), to crossing over to touch Lobe B (the rearranged state) [8]. Only the rearranged state was seen bound to DNA. The cryo-EM studies used super core promoter (SCP) DNA, optimized for high DNA template usage in transcriptional assays [22]. The DNA in these studies extends linearly from the edge of Lobe C, which binds downstream promoter regions, to the repositioned Lobe A near Lobe B, which binds the TATA box upstream of the transcription start site (TSS) [8,23•].
Exploiting new software and detector technology, cryo-EM studies led to an architectural model of human TFIID stably bound to DNA that defined all the TFIID subunits engaged in core promoter binding [22]. It revealed how TBP and TFIIA engage the TATA-end of the promoter after detaching from the rest of Lobe A and engaging with Lobe B (Figure 1a). It also showed regions in Lobe C engaging the motif ten element (MTE), downstream promoter element (DPE), and initiator (Inr) promoter sequences also present in the SCP [23•] (Figure 1a). Lobe C includes a dimer of TAF6 HEAT repeats, a large segment of TAF2 with homology to aminopeptidases [16], and a TAF1-TAF7 module. The latter had previously been described crystallographically and proposed to interact with DNA [14,15]. Consistent with this idea, the TAF1-TAF7 module interacts with the downstream core promoter elements in the cryo-EM structure [23•]. Furthermore, the structure suggested that a small helical segment in TAF1 that was disordered in the crystal structure of the human TAF1-TAF7 subcomplex [14,15] interacts with the Inr. Additionally, a zinc knuckle domain at the C-terminal end of TAF1 has been proposed to contribute also to promoter binding [24].
Figure 1.
Cryo-EM structures of TFIID and its binding to DNA. (a) Cryo-EM reconstruction of human TFIID bound to promoter DNA. The promoter elements in the SCP are highlighted. (b) Cryo-EM reconstructions of TFIID, with a transparent outline of TFIID in a canonical state and fitted cryo-EM maps from focused refinements of the BC core and Lobe A, colored by subunit following the code shown at the bottom of figure. (c) Atomic model of human TFIID using the same subunit color code.
Recent breakthroughs in TFIID structural studies
A complete architectural model for all three lobes of free human TFIID was finally obtained using a large cryo-EM dataset and a complex and iteratively optimized image processing strategy to generate a cryo-EM density map defining most of Lobes B and C — the comparably rigid BC core — at about 4.5 Å resolution [25••] (Figure 1b). Together with crystallographic structures, homology models of proteins or domains, and chemical crosslinking-mass spectrometry (CX-MS) data, it was possible to generate a model of this part of TFIID (Figure 1c). Three pairs of histone fold domains (HFDs) in TAF6-TAF9, TAF4-TAF12 and TAF8-TAF10 interact with the WD40 and helical N-terminal domains of TAF5 to form Lobe B. An extension from the HFD of TAF8 interacts with the homodimer of TAF6 HEAT repeats and staples it to the TAF2 subunit. The TAF1/TAF7 module, which is stably bound to DNA and TAF2 in the core promoter-bound form of TFIID [23•], is extremely flexible in the absence of DNA and thus could only be visualized at very low resolution. Classification and focused refinement of Lobe A led to a map of this structural region at about 10 Å resolution (Figure 1b), sufficient to generate an architectural model based on the knowledge of subunit stoichiometry and the modeling of Lobe B [25••] (Figure 1c). Lobe A includes a nucleosome core particle-like octamer of HFD-containing TAFs that interacts with TAF5. In addition to the TAF6-TAF9 and TAF4-TAF12 HFD pairs, TAF10 pairs with TAF3 in Lobe A, instead of TAF8, its partner in Lobe B. An additional TAF11-TAF13 pair completes the HFD octamer. Interacting with this Lobe A-specific HFD pair, and protruding from the lobe, is TBP, which is assumed to be bound to inhibitory N-terminal segments from TAF1 [26].
It is interesting that while the presence of nucleosome core particle-like structures had been predicted years before [27,28], and it seemed reasonable to assume that a DNA-binding complex that contained them would use them for DNA wrapping, these motifs in Lobes A and B do not interact with DNA in the DNA-bound cryo-EM structure [25••]. In fact, the structures, derived from the EM maps and homology models based on histones, show them to have completely lost the strong positively charged surfaces that histones use to engage the phosphate backbone of the DNA. Just like the aminopeptidase domain of TAF2 (which lacks the catalytic residues), the histone fold domains and the hexamer/octamer structures they form in TFIID have evolved to play scaffolding roles instead.
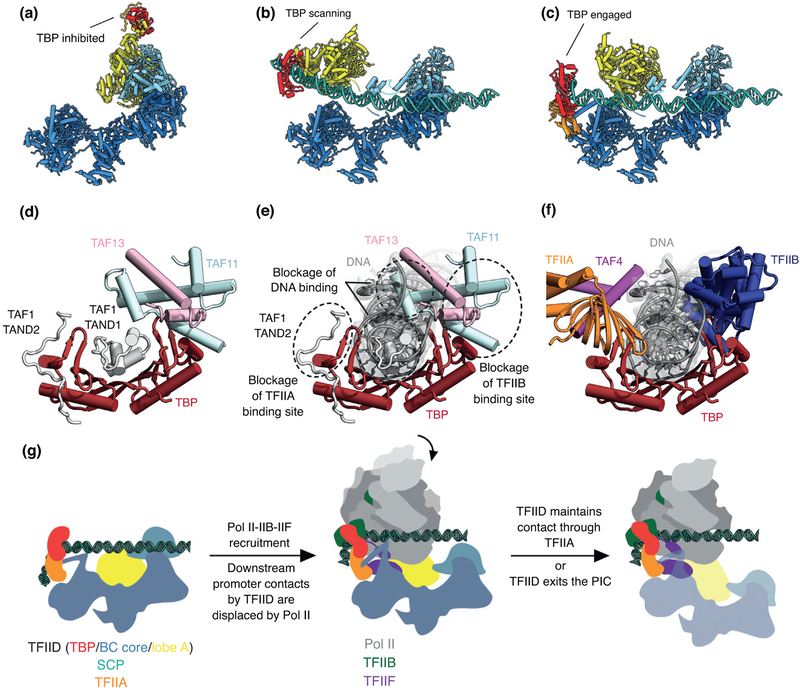
TFIID binding to the core promoter involves the release of TBP from Lobe A, likely in stages that overcome each of the inhibitory interactions blocking different functional surfaces of TBP (Figure 2). The interactions of the TAND1 and TAND2 regions of TAF1 with TBP [26] (Figure 2a,d) are likely to be released (i) as Lobe C engages downstream DNA and positions the upstream promoter region such that it can be ‘scanned’ by TBP (Figure 2b,e), and (ii) as TFIIA joins the complex via interaction with Lobe B and further stabilizes the location of TBP for DNA interaction (Figure 2c,f) [25••]. The final engagement of TBP with the promoter, with the concomitant bending of the DNA, is sterically incompatible with the TBP-TAF11 interaction, and therefore leads to TBP detachment from Lobe A and the opening of the binding site for TFIIB (Figure 2f), which in turn will engage Pol II, likely bound to TFIIF, thus promoting PIC assembly [25••] (Figure 2g).
Figure 2.
Changes in TBP binding partners through the process of promoter binding. On the top are the atomic structures of human TFIID in the process of promoter engagement. With (a) in the canonical state, (b) in the scanning state and (c) in the engaged state. On the bottom, (d) shows TBP bound by inhibitory TAFs, (e) how these TAFs act to inhibit DNA binding and (f) how TBP binds the general transcription factors TFIIA and TFIIB when in complex with the PIC. The engaged TFIID complex recruits Pol II (g) with the aid of TFIIB and TFIIF.
The complex architecture of TFIID, with the pseudo duplication of the core of Lobes A and B, poses questions about its evolution and biogenesis. Specifically, the importance of the stoichiometry of available subunits/subcomplexes in the nucleus, and the possible requirement of chaperone factors to promote the correct assembly of TFIID (Figure 1b) over theoretically feasible complexes containing a symmetrical arrangement of either two copies of Lobes A or Lobe B, dimerized via the HEAT repeats of TAF6, will require further study. Based on the available data today, we propose a plausible model for TFIID biogenesis below.
Models of TFIID assembly
Biochemical, cellular and structural studies have revealed many details concerning the early steps of TFIID assembly. In these early steps, several TAF subcomplexes are formed in the cytoplasm, including the well-characterized 5TAF (TAF4, 5, 6, 9, 12) and cTAF (TAF2, 8 and 10) subcomplexes [5,12••,29]. There is evidence that sTAF (TAF1, 7, 11, 13 and TBP) and TAF3/10 subcomplexes can also be formed, although no study has directly tested that they exist in the cytoplasm [30••,31,32•]. Each of these four subcomplexes contains a subunit (TAF4, 8, 1 and 3, respectively) with a nuclear localization sequence (NLS) that would allow them to enter the nucleus, where the assembly of TFIID would then continue [29,31,33] (Figure 3, center left).
Figure 3.
Possible model of human TFIID assembly. A number of TFIID subcomplexes initially assemble in the cytoplasm (5TAF: TAF4, 5, 6, 9, 12; cTAF: TAF2, 8, 10; sTAF: TAF1, 7, 11, 13 and TBP; TAF3/10) and can enter the nucleus due to the presence of nuclear localization signal sequences on TAF4, TAF8, TAF1 and TAF3. The final stages of TFIID assembly likely occur in the nucleus. We propose that the binding of either cTAF or TAF3/10 to the 5TAF leads to two different core subcomplexes, bcTAF and aTAF, respectively. Once the bcTAF and aTAF subcomplexes heterodimerize, they can then interact with the sTAF subcomplex to complete the assembly of TFIID.
It seems that the early steps in TFIID assembly occur in the cytoplasm due to the requirement of specific chaperones for folding of some TAFs. For example, the WD40 domain of TAF5 has been shown to require the TRiC/CCT chaperone to properly fold, while subsequent release from the chaperone requires binding to TAF6/9 [12••] (Figure 3, left). These 3 proteins form what has been referred to as the 3TAF subcomplex, a precursor of the larger 5TAF subcomplex [5]. Additionally, it is known that some TFIID subunits, such as TAF8 and TAF10 or TAF1 and TBP [32•], co-fold with each other co-translationally, which can occur only in the cytoplasm. TAF2 does not appear to associate with TAF8 while it is being translated, but it lacks an NLS and must bind TAF8 to get into the nucleus [29,32•]. In a similar way, TAF10 must associate with TAF3, while TAF7, 11, 13 and TBP will likely need to piggyback on TAF1 in order to enter the nucleus [30••,31,33].
One big question that remains with regards to the assembly of TFIID is how the asymmetry of the full complex is established. How does the cell make sure to prevent the integration of two copies of TAF3/10 or TAF8/10 into a single, ‘symmetric TFIID’ complex (i.e. with two Lobe As or two Lobe Bs, respectively)? Previous studies proposed a preexisting 2-fold symmetric 5TAF dimer. Based on this model, it was suggested that incorporation of a TAF8/10 pair would break the 5TAF symmetry and allow the incorporation of the rest of the TAF subunits, thus providing a possible mechanism for generating distinct Lobes A and B [5]. However, no such symmetric dimer exists in the final TFIID structure [23•]. While the structure of holo-TFIID does show the dimerization of the HEAT repeats in the two copies of TAF6 [23•], the rest of the two 5TAF subcomplexes are at opposite ends of the structure [25••]. This suggests that the incorporation of the TAF8/10 complex on one side is unlikely to influence the presence of the TAF 3/10 pair on the other side.
Additionally, it is unclear which interactions would mediate dimerization of the 5TAF complex. The TAF6 dimer generated by association of its HEAT repeats is not 2-fold symmetric and is also not seen in the crystal structure of the Antonospora locustae TAF6 HEAT repeat domain [34]. In fact, within TFIID the two copies of TAF6 appear to be held together by interaction with TAF8 [25••]. Thus, it is likely that on its own, the HEAT repeat domain of TAF6 either does not dimerize or does so only weakly. Another proposed dimerization involves TAF5. However, while SEC-LS data has shown that the isolated LisH/NTD2 domain of TAF5 can dimerize [10], within the structure of TFIID this region only transiently interacts when the complex is in the rearranged state [25••], leading us to speculate that TAF5 may also not dimerize on its own in vivo.
Given the structural considerations outlined above, it is possfible that the 5TAF subcomplex does not form a dimer on its own. A model that accounts for this possibility is that monomeric 5TAF complex in the nucleus can either interact with the cTAF (TAF2, 8 and 10) or TAF3/10 subcomplexes to give rise to distinct bcTAF and aTAF subcomplexes, respectively (Figure 3, center right). Once formed, these two distinct species would only be able to heterodimerize. In this scenario an aTAF complexes would contribute a TAF6 HEAT repeat for dimerization with the TAF6 HEAT repeat in the bcTAF subcomplex. The assembly would then be completed by the addition of the sTAF subcomplex [30••] (Figure 3, right). While the subunits in the proposed sTAF complex are all proximal in the final complex, whether they exist in vivo as a single subcomplex remains to be seen [25••]. Notably, while the model of TFIID assembly we are proposing requires that the 5TAF subcomplex does not dimerize, it does not require any undiscovered factors to assist in the assembly of TFIID. Alternatively, factors would be needed either to actively prevent 5TAF dimerization and/or to disrupt a preformed dimer to generate the final structure. Further studies will be needed to provide stronger evidence for either model.
Human versus yeast TFIID structures
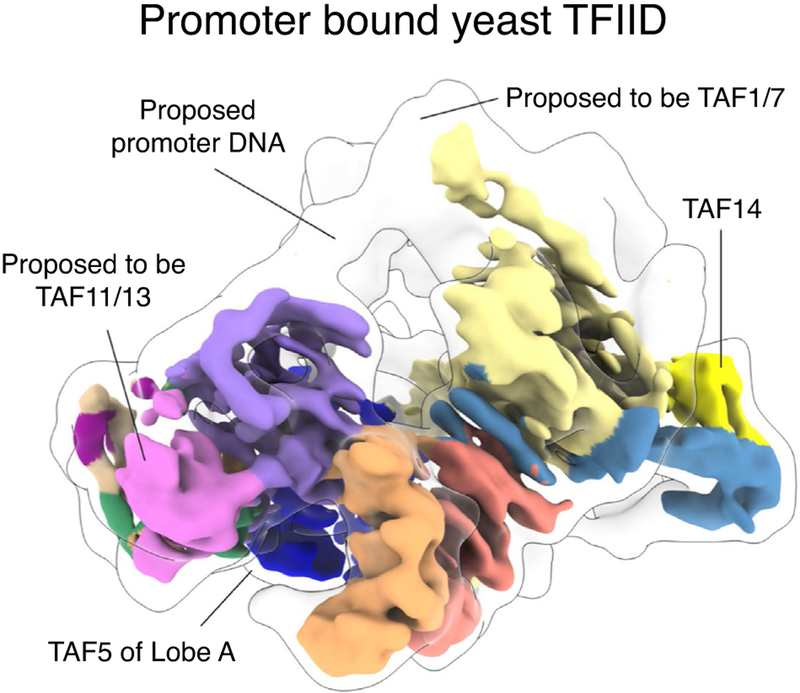
Recent cryo-EM studies of Komagataella phaffii TFIID [35••] have provided insight into the structure of a yeast TFIID. While the human and yeast structures share a similar architecture for the BC core (Figures 3b and 4), there are some clear differences. The BC core of yeast TFIID [35••] appears more compact than its human counterpart [23•,25••], possibly reflecting differences in the spacing or organization of human and yeast promoter elements. Also, the yeast complex contains an additional structured module that includes part of TAF8 and the yeast-specific TAF14. Other structural differences between the two models concern the content of Lobe B. The yeast study suggested that the histone-fold pair of TAF11-TAF13 occupies the position that corresponds to TAF8-TAF10 in the human structure [25••,35••]. A second difference within Lobe B is the lack of density for the TAF4 helix α3 within the yeast map [35••]. While these disparities in Lobe B architecture could reflect true differences between human and yeast TFIID, the apparent anisotropy of the cryo-EM map of yeast TFIID makes it difficult to unambiguously interpret it.
Figure 4.
Structure of the promoter bound yeast TFIID. Cryo-EM reconstruction of yeast (Komagataella phaffii) TFIID proposed to be bound to promoter DNA. Outlined is the 20 Å low-pass filtered map to help visualize potential DNA density. Two of the major differences from the human TFIID complex are indicated.
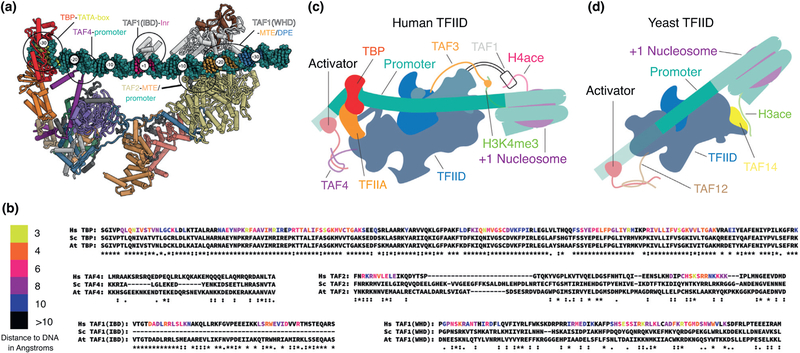
It still remains unclear whether the mechanism of TBP loading onto the TATA box is fully conserved between yeast and humans. While it has been shown that the dramatic flexibility of Lobe A plays an important role in TBP loading for the human complex [8,25••], and some poorly characterized flexibility is also apparent for yeast complexes [21,35••], its role in DNA binding has not been considered in the yeast case. On one hand, the downstream DNA binding residues identified in the human TAF1 and TAF2 are conserved in the yeast subunits (Figure 5a,b) but on the other, the downstream promoter elements proposed to be recognized by these TAFs have not been identified in yeast. Comparison of the DNA-bound structures of human and yeast TFIID is complicated by the fact that the yeast cryo-EM structure lacks clear density for DNA as well as TAF1, TAF2, TBP, and TFIIA [35••], which are all visible in the human promoter-bound TFIID complex and are important elements for DNA binding [23•].
Figure 5.
Evolutionary comparison of TFIID elements involved in interactions with DNA, activators and histone modifications. (a) Atomic model of human TFIID bound to promoter DNA, with subunits colored according to the code at the bottom of Figure 1. Major points of interaction of TFIID with promoter DNA are highlighted. (b) Sequence alignment of the regions that interact with promoter DNA as seen in human TFIID for human, yeast (Saccharomyces cerevisiae) and plant (Arabidopsis thaliana). Residues are colored based on the distance to the DNA in the model of human TFIID bound to promoter DNA, except for the TAF4 region proximal to the promoter for which the sequence register could not be determined. Alignment scores for the TAF1 Inr binding domain (IBD) was calculated using only the human and A. thaliana sequences, as S. cerevisiae lacks this domain entirely. (c) Model of interactions of human TFIID with chromatin marks on the +1 nucleosome and with upstream activators. (d) Model of interaction of yeast TFIID with chromatin marks on the +1 nucleosome and with upstream activators.
Interestingly, the best known differences between the yeast and human TFIID complexes do not involve the ordered regions observed in the EM structures, but small domains at the end of unstructured linkers that are too flexible to be visualized, and that include the activator and chromatin binding domains within TFIID. In humans, the best characterized activator binding motifs are in the N-terminal region of TAF4, while in yeast these are in the N-terminal region of TAF12. Interestingly, TAF4 and TAF12 dimerize, placing their N-terminal domains in roughly the same place with respect to the remainder of the complex (Figure 5c,d). The chromatin binding domains of TFIID, on the other hand, are significantly different between yeast and humans. In human TFIID there are two well characterized histone interacting domains: the TAF3 PHD domain, which binds to trimethyl marks on histone H3 lysine 4 (H3K4me3) [10], and the TAF1 double-bromo domain [11], which binds H4 acetyl-lysines (Figure 5c). Yeast TFIID lacks both of these domains and instead contains a YEATS domain in the yeast-specific TAF14 [13], which binds H3 acetyllysine (Figure 5d). This difference likely reflects major differences in how epigenetic marks are being used in humans and yeast, and more generally across eukaryotes. Extending the analysis of chromatin-binding domains within TAFs to plants (Figure 5b), we found that they much more closely resemble yeast, suggesting they represent a more general case for many eukaryotic systems, while metazoans may have evolved a more specialized form of TFIID, maybe to extend the possibilities for gene expression regulation. A more in-depth phylogenetic analysis will be required to better understand how TFIID has changed throughout eukaryotic evolution.
Future studies
The recent cryo-EM structures of TFIID represent a breakthrough in the structural and mechanistic understanding of the Pol II transcription initiation machinery, but also pose new questions regarding TFIID assembly and dynamics. The intriguing pseudo duplication of the core of Lobes A and B hints at a complex biogenesis pathway for the overall complex that has yet to be fully defined. The extreme dynamics of human TFIID appear to be linked to the release of TBP inhibition and its consequent deployment to DNA regions upstream of the transcription start site. Such dynamics are likely to be regulated by cofactors and gene specific activators and repressors that could shift the equilibrium of states to either promote or inhibit functional transitions, an idea that needs to be experimentally explored. How architecture and dynamics have been evolutionarily conserved or modified to fit the needs of different modes of gene regulation also needs further study. Transcription initiation is a critical process in the control of gene expression, and its deregulation is linked to human disease, including cancers. An improved structural description of the process is therefore critical for a fundamental understanding of this essential step in the regulation of gene expression, as well as to provide a structural framework to understand disease.
Acknowledgements
This work was supported by NIGMS grants R01-GM63072 and R35 GM127018 to E.N.; A.B.P. was supported by the NIGMS Molecular Biophysics Training Grant (GM008295). B.J.G. was supported by fellowships from the Swiss National Science Foundation (projects P300PA_160983, P300PA_174355). E.N. is a Howard Hughes Medical Institute Investigator.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Roeder RG: The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci 1996, 21:327–335. [PubMed] [Google Scholar]
- 2.Goodrich JA, Cutler G, Tjian R: Contacts in context: promoter specificity and macromolecular interactions in transcription. Cell 1996, 84:825–830. [DOI] [PubMed] [Google Scholar]
- 3.Levine M, Cattoglio C, Tjian R: Looping back to leap forward: transcription enters a new era. Cell 2014, 157:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burley SK, Roeder RG: Biochemistry and structural biology of transcription factor IID (TFIID). Annu Rev Biochem 1996, 65:769–799. [DOI] [PubMed] [Google Scholar]
- 5.Bieniossek C, Papai G, Schaffitzel C, Garzoni F, Chaillet M, Scheer E, Papadopoulos P, Tora L, Schultz P, Berger I: The architecture of human general transcription factor TFIID core complex. Nature 2013, 493:699–702. [DOI] [PubMed] [Google Scholar]
- 6.Sanders SL, Garbett KA, Weil PA: Molecular characterization of Saccharomyces cerevisiae TFIID. Mol Cell Biol 2002, 22:6000–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugh BF, Tjian R: Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev 1991, 5:1935–1945. [DOI] [PubMed] [Google Scholar]
- 8.Cianfrocco MA, Kassavetis GA, Grob P, Fang J, Juven-Gershon T, Kadonaga JT, Nogales E: Human TFIID binds to core promoter DNA in a reorganized structural state. Cell 2013, 152:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albright SR, Tjian R: TAFs revisited: more data reveal new twists and confirm old ideas. Gene 2000, 242:1–13. [DOI] [PubMed] [Google Scholar]
- 10.van Ingen H, van Schaik FMA, Wienk H, Ballering J, Rehmann H, Dechesne AC, Kruijzer JAW, Liskamp RMJ, Timmers HTM, Boelens R: Structural insight into the recognition of the H3K4me3 mark by the TFIID subunit TAF3. Structure 2008, 16:1245–1256. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson RH, Ladurner AG, King DS, Tjian R: Structure and function of a human TAF(II)250 double bromodomain module. Science (80-) 2000, 288:1422–1425. [DOI] [PubMed] [Google Scholar]
- 12. ••.Antonova SV, Haffke M, Corradini E, Mikuciunas M, Low TY, Signor L, van Es RM, Gupta K, Scheer E, Vos HR et al. : Chaperonin CCT checkpoint function in basal transcription factor TFIID assembly. Nat Struct Mol Biol 2018, 25:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes how the TAF5 WD40 requires the chaperonin CCT to fold before it can interact with the HFD of TAF6/9. The paper also provides the crystal structure of the TAF5,6 and 9 module that forms the common core of Lobe A and Lobe B.
- 13.Zhang W, Zhang J, Zhang X, Xu C, Tu X: Solution structure of the Taf14 YEATS domain and its roles in cell growth of Saccharomyces cerevisiae. Biochem J 2011, 436:83–90. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Curran EC, Hinds TR, Wang EH, Zheng N: Crystal structure of a TAF1-TAF7 complex in human transcription factor IID reveals a promoter binding module. Cell Res 2014, 24:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya S, Lou X, Hwang P, Rajashankar KR, Wang X, Gustafsson J-A, Fletterick RJ, Jacobson RH, Webb P: Structural and functional insight into TAF1-TAF7, a subcomplex of transcription factor II D. Proc Natl Acad Sci U S A 2014, 111:9103–9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkowska M, Kokoszynska K, Rychlewski L, Wyrwicz L: Structural bioinformatics of the general transcription factor TFIID. Biochimie 2013, 95:680–691. [DOI] [PubMed] [Google Scholar]
- 17.Nogales E, Louder RK, He Y: Cryo-EM in the study of challenging systems: the human transcription pre-initiation complex. Curr Opin Struct Biol 2016, 40:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogales E, Scheres SHW: Cryo-EM: a unique tool for the visualization of macromolecular complexity. Mol Cell 2015, 58:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grob P, Cruse MJ, Inouye C, Peris M, Penczek PA, Tjian R, Nogales E: Cryo-electron microscopy studies of human TFIID: conformational breathing in the integration of gene regulatory cues. Structure 2006, 14:511–520. [DOI] [PubMed] [Google Scholar]
- 20.Papai G, Tripathi MK, Ruhlmann C, Layer JH, Weil PA, Schultz P: TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation. Nature 2010, 465:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papai G, Tripathi MK, Ruhlmann C, Werten S, Crucifix C, Weil PA, Schultz P: Mapping the initiator binding Taf2 subunit in the structure of hydrated yeast TFIID. Structure 2009, 17:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juven-Gershon T, Cheng S, Kadonaga JT: Rational design of a super core promoter that enhances gene expression. Nat Methods 2006, 3:917–922. [DOI] [PubMed] [Google Scholar]
- 23. •.Louder RK, He Y, López-Blanco JR, Fang J, Chacón P, Nogales E: Structure of promoter-bound TFIID and model of human pre- initiation complex assembly. Nature 2016, 531:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]; The cryo-EM structure of promoter-bound human TFIID provides an architectural model for Lobe C, elucidates the DNA-protein interactions at the Initiator and downstream promotor elements, and visualizes the repositioning of TBP by Lobe B aided by TFIIA.
- 24.Curran EC, Wang H, Hinds TR, Zheng N, Wang EH: Zinc knuckle of TAF1 is a DNA binding module critical for TFIID promoter occupancy. Sci Rep 2018, 8:4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ••.Patel AB, Louder RK, Greber BJ, Grünberg S, Luo J, Fang J, Liu Y, Ranish J, Hahn S, Nogales E: Structure of human TFIID and mechanism of TBP loading onto promoter DNA. Science 2018, 362:eaau8872. [DOI] [PMC free article] [PubMed] [Google Scholar]; The cryo-EM structure of human TFIID defines the architecture of Lobes B and C at 4.5 Å resolution and provides an architectural model for Lobe A, resolved at about 10 Å resolution. This paper also defines the structural transitions of Lobe A during TBP loading onto the promoter and proposes a model of inhibition release for the different functional surfaces of TBP that relies on downstream promoter binding by Lobe C and TFIIA interactions with Lobe B.
- 26.Anandapadamanaban M, Andresen C, Helander S, Ohyama Y, Siponen MI, Lundström P, Kokubo T, Ikura M, Moche M, Sunnerhagen M: High-resolution structure of TBP with TAF1 reveals anchoring patterns in transcriptional regulation. Nat Struct Mol Biol 2013, 20:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann A, Chiang C-M, Oelgeschläger T, Xie X, Burley SK, Nakatani Y, Roeder RG: A histone octamer-like structure within TFIID. Nature 1996, 380:356–359. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Kokubo T, Cohen SL, Mirza UA, Hoffmann A, Chait BT, Roeder RG, Nakatani Y, Burley SK: Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature 1996, 380:316–322. [DOI] [PubMed] [Google Scholar]
- 29.Trowitzsch S, Viola C, Scheer E, Conic S, Chavant V, Fournier M, Papai G, Ebong IO, Schaffitzel C, Zou J et al. : Cytoplasmic TAF2-TAF8-TAF10 complex provides evidence for nuclear holo-TFIID assembly from preformed submodules. Nat Commun 2015, 6:6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.••.Fant C, Levandowski C, Gupta K, Maas Z, Moir J, Rubin J, Sawyer A, Esbin M, Rimel J, Marr M et al. : TFIID enables RNA polymerase II promoter-proximal pausing. SSRN Electron J 2019. 10.2139/ssrn.3429345 [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes the full reconstitution of human TFIID. The subcomplexes 8TAF (TAF2,4,5,6,8,9,10,12), STAF (TAF1,2,7,11,13 and TBP) and TAF3/10 were recombinantly expressed in insect cells, purified and reconstituted in vitro to form a complete human TFIID
- 31.Soutoglou E, Demény MA, Scheer E, Fienga G, Sassone-Corsi P, Tora L: The nuclear import of TAF10 is regulated by one of its three histone fold domain-containing interaction partners. Mol Cell Biol 2005, 25:4092–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. •.Kamenova I, Mukherjee P, Conic S, Mueller F, El-Saafin F, Bardot P, Garnier J-M, Dembele D, Capponi S, Timmers HTM et al. : Co-translational assembly of mammalian nuclear multisubunit complexes. Nat Commun 2019, 10:1740. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that TAF10 associcates/folds with cotranslating TAF8 and similarly how TBP associates/folds with cotranslating TAF1.
- 33.Singh MV, Bland CE, Weil PA: Molecular and genetic characterization of a Taf1p domain essential for yeast TFIID assembly. Mol Cell Biol 2004, 24:4929–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheer E, Delbac F, Tora L, Moras D, Romier C: TFIID TAF6-TAF9 complex formation involves the HEAT repeat-containing C-terminal domain of TAF6 and is modulated by TAF5 protein. J Biol Chem 2012, 287:27580–27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. ••.Kolesnikova O, Ben-Shem A, Luo J, Ranish J, Schultz P, Papai G: Molecular structure of promoter-bound yeast TFIID. Nat Commun 2018, 9:4666. [DOI] [PMC free article] [PubMed] [Google Scholar]; The cryo-EM reconstruction of yeast TFIID in free and promoter-bound states.