Abstract
Background and Aims:
Benzodiazepines are commonly prescribed to patients with opioid use disorder receiving buprenorphine treatment, yet may increase overdose risk. However, prescribed benzodiazepines may improve retention in care by reducing buprenorphine discontinuation and thus may prevent relapse to illicit opioid use. We aimed to test the association between benzodiazepine prescription and fatal opioid overdose, non-fatal opioid overdose, all-cause mortality, and buprenorphine discontinuation.
Design and Setting:
This was a retrospective cohort study using 5 individually linked data sets from Massachusetts, USA government agencies.
Participants:
We studied 63,389 Massachusetts residents aged 18 years or older who received buprenorphine treatment between January 2012 and December 2015.
Measurements:
Filled benzodiazepine prescription during buprenorphine treatment was the main independent variable. The primary outcome was time to fatal opioid overdose. Secondary outcomes were time to non-fatal opioid overdose, all-cause mortality, and buprenorphine discontinuation. We defined buprenorphine discontinuation as having a 30-day gap without another prescription following the end date of the previous prescription. We used Cox proportional hazards models to calculate hazards ratios that tested the association between receipt of benzodiazepines and all outcomes, restricted to periods during buprenorphine treatment.
Findings:
Of the 63,389 individuals who received buprenorphine, 24% filled at least one benzodiazepine prescription during buprenorphine treatment. Thirty-one percent of the 183 deaths from opioid overdose occurred when individuals received benzodiazepines during buprenorphine treatment. Benzodiazepine receipt during buprenorphine treatment was associated with an increased risk of fatal opioid overdose adjusted hazard ratio (HR) =3.02; (95% confidence interval [CI], 1.97–4.62), non-fatal opioid overdose, adjusted HR=1.98 (95% CI 1.58–2.48), all-cause mortality, adjusted HR=2.05 (95% CI 1.64–2.55), and a decreased risk of buprenorphine discontinuation, adjusted HR=0.78 (95% CI 0.75–0.80).
Conclusions:
Benzodiazepine receipt appears to be associated with both increased risk of opioid overdose and all-cause mortality and decreased risk of buprenorphine discontinuation among people receiving buprenorphine.
INTRODUCTION
Due to the epidemic of opioid-related harms in the United States, a growing number of people are being treated with medications for opioid use disorder, including buprenorphine, methadone, and injectable naltrexone. In particular, more people are receiving buprenorphine(1,2), the most widely prescribed medication for opioid use disorder in substance use treatment facilities(3). One concern among individuals receiving buprenorphine or methadone is the concurrent use of central nervous system depressants, particularly benzodiazepines. Pre-clinical studies indicate that benzodiazepines can cause a decrease in respiratory depression when combined with buprenorphine(4–6) and can eliminate buprenorphine’s protective respiratory “ceiling effect”, a result of buprenorphine’s partial agonism(7). Use of a benzodiazepine can cause decreased reaction time in participants also using buprenorphine(8) and prescription of benzodiazepines is associated with increased risk of emergency department visits for accidental injuries(9). In case series of fatalities involving buprenorphine, a large majority of deaths also involve benzodiazepines(10–13).
But deaths involving buprenorphine may often also involve benzodiazepines simply because benzodiazepine use is common among patients receiving buprenorphine. Approximately a third of buprenorphine patients are prescribed benzodiazepines(14–17) and up to another third regularly use benzodiazepines that are obtained illicitly(16,18). Benzodiazepines are efficacious treatments for anxiety and insomnia(19,20), two of the main reasons why patients with opioid use disorder take them(21,22). Additionally, untreated comorbidity can make achieving abstinence difficult and risk substance use relapse(23). Treatment with benzodiazepines may improve patient engagement in addiction treatment. In one study of opioid maintenance therapy patients who received daily, observed benzodiazepine treatment had better treatment retention and lower mortality than those who never used benzodiazepines(24). Benzodiazepine receipt prior to being hospitalized for opioid use disorder or overdose is associated with increased engagement post-hospitalization(25). In two recent cohort studies of patients receiving buprenorphine, benzodiazepine prescription was not associated with mortality(26,27). Due to concerns that benzodiazepine use might be a barrier to receiving opioid maintenance therapy, the U.S. Food and Drug Administration urged caution about withholding opioid agonist treatment of addiction in patients taking benzodiazepines(28).
The aim of this study was to assess whether: 1) benzodiazepine prescribing during buprenorphine treatment is associated with increased risks of fatal and non-fatal opioid overdose, and all-cause mortality, and 2) benzodiazepine prescribing during buprenorphine treatment is associated with reduced risk of buprenorphine discontinuation. We used a novel, population-based dataset of Massachusetts residents including linked records from 5 government agencies to identify a cohort of all individuals who filled a buprenorphine prescription over a 4-year period. We hypothesized that receipt of concurrent benzodiazepines would be associated with an increased risk of fatal opioid overdose, non-fatal opioid overdose, and all-cause mortality, but decreased risk of buprenorphine treatment discontinuation.
METHODS
Study design and population
We conducted a retrospective cohort study of 63,345 people aged 18 years or older who received buprenorphine treatment while residing in Massachusetts between January 2012 and December 2015. Observation time for all people started on the date of the first filled buprenorphine prescription during the study period and were only included if they did not have a buprenorphine fill in the year prior to start of observation. The potential minimum and maximum periods of observation were 1 day and 4 years respectively.
Data sources
We used data from the Massachusetts Public Health Data Warehouse, which combines multiple state government datasets in order to better understand the epidemic of opioid-related harms in the Commonwealth of Massachusetts. This dataset includes the Massachusetts All-Payer Claims Database (APCD), which contains health and pharmacy insurance claims data from private and public health care payers (including Medicare and MassHealth, the state’s Medicaid agency) and is estimated to represent more than 98% of Massachusetts residents. Using a deterministic linkage approach described elsewhere(29), APCD data were linked at the individual person level with multiple datasets. Datasets utilized in this study included: 1) the APCD, 2) the Registry of Vital Records and Statistics (RVRS) death records, 3) the Prescription Monitoring Program (PMP), 4) Acute Care Hospital Case Mix (Case Mix), and 5) Massachusetts Ambulance Trip Record Information System (MATRIS) (brief descriptions of datasets in Supplement). The combining of these datasets was authorized by Massachusetts law and conducted by a public health authority where institutional board review was not required. Additionally, the Boston University Medical Campus Institutional Review Board determined that this study was not human subjects research.
Cohort selection
People were included in the analysis if they filled a prescription for tablet or film buprenorphine or buprenorphine/naloxone, exclusive of transdermal and injectable formulations, between January 2012 and December 2015 as recorded in the PMP. People were included during times of buprenorphine treatment, defined by the date of buprenorphine fills and days supply as recorded in the PMP.
Benzodiazepine and buprenorphine treatment
We used the state PMP, which records filled outpatient prescriptions of controlled medications, the date of each fill, and the days supply, to define episodes of buprenorphine treatment. Episodes started on the day the buprenorphine prescription was filled and ended on the last day of the buprenorphine prescription according to the days supply. Only days during active buprenorphine use were included in this study. Individuals could have multiple episodes if they initiated and discontinued buprenorphine multiple times. When measuring buprenorphine use and daily dose, and benzodiazepine use, we assumed that patients took their medications according to the days supply recorded in the PMP. If there was an overlap between two prescriptions, it was assumed that the first prescription ended on the day the second prescription began. On days when buprenorphine prescriptions overlapped, the dose of the second prescription was used. Benzodiazepine use and buprenorphine dose were treated as time-varying and identified on each day.
We included the following benzodiazepine types: alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, prazepam, quazepam, temazepam, and triazolam. Buprenorphine was identified using the following Generic Cross Reference Codes: 929645, 92577, 923958, 927685, and 930981.
Outcomes
The primary outcome was fatal opioid overdose. Secondary outcomes were non-fatal opioid overdose, all-cause mortality, and buprenorphine treatment discontinuation. Classification of fatal opioid overdose was based on medical examiner determination or standardized assessment by the Massachusetts Department of Public Health (MDPH) (Supplement). Non-fatal opioid overdose was determined by one of two types of encounters. The first type was an ambulance encounter for opioid overdose identified in MATRIS and available in years 2013–2015 only, using an algorithm developed in collaboration between the Centers for Disease Control and Prevention and the MDPH and previously validated against internal emergency medical services data on opioid overdose events (Supplement). The second type was an emergency department, observation, or inpatient discharge in the Case Mix dataset with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) diagnosis code for opioid overdose (965.00, 965.01, 965.02, 965.09, E850.0, E850.1, and E850.2), codes validated to have high positive predictive value for opioid overdose or opioid-related adverse events(30). Those individuals who died within 7 days of discharge after having a nonfatal overdose event were excluded from having a nonfatal overdose and counted as having a fatal overdose. All-cause mortality was identified through the RVRS death records. For opioid overdose and mortality outcomes, only days with active buprenorphine treatment were analyzed. Short gaps between buprenorphine prescriptions occurred frequently and may not represent buprenorphine discontinuation. Thus, we defined buprenorphine discontinuation as having a 30-day gap without another prescription following the end date of their previous prescription based on fill date and days supply from the PMP. We censored buprenorphine treatment if they started methadone maintenance or were incarcerated.
Covariates
We obtained demographics and comorbidity data from the APCD and acute care utilization data from the Case Mix dataset during the year prior to start of observation for each person. Demographic data included age and sex. We also included Medicaid insurance status defined as any enrollment in Medicaid insurance coverage in the year prior to entering the cohort. Diagnoses were categorized into anxiety disorders, depressive disorders, and bipolar or psychotic disorders (Supplement). Any selective serotonin reuptake inhibitor prescription included the following types: citalopram, escitalopram, fluvoxamine, fluoxetine, paroxetine, and sertraline. We included whether the individual had any emergency department, observation, or inpatient encounters with a medical claim containing a mental health diagnosis code. Buprenorphine daily dose was calculated as described above and was included as a covariate.
Statistical analysis
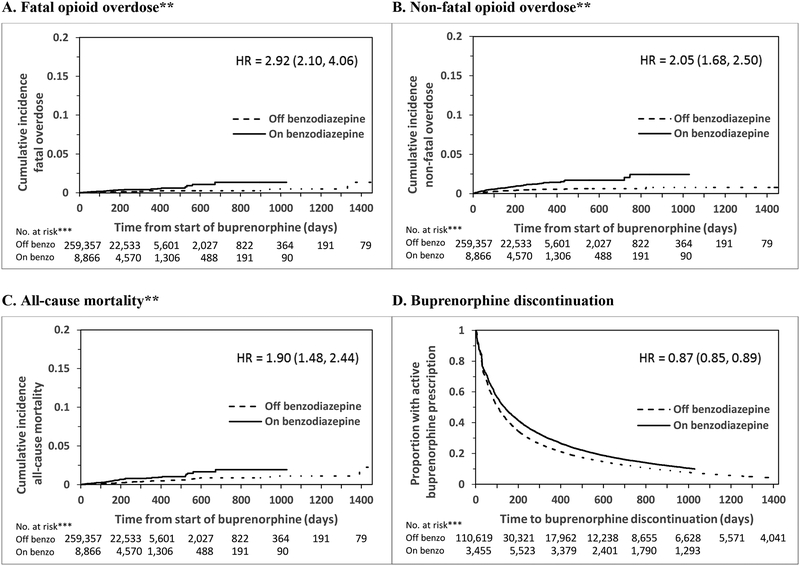
We compared baseline characteristics between individuals who received at least one benzodiazepine fill during the study period to those who did not receive a benzodiazepine fill with chi-square tests for categorical variables and t-tests for the continuous variable (age). We then calculated outcome event rates for fatal opioid overdose, non-fatal opioid overdose, and all-cause mortality by concurrent benzodiazepine and buprenorphine treatment and buprenorphine treatment alone. We calculated extended Kaplan-Meier curves (taking into account time-varying benzodiazepine receipt) to compare cumulative incidence of fatal opioid overdose, non-fatal opioid overdose, all-cause mortality, and the proportion of active buprenorphine receipt on and off benzodiazepines (Figure 1).
Figure 1: Extended Kaplan-Meier plots and adjusted hazard ratios* of fatal opioid overdoses, non-fatal opioid overdoses, all-cause mortality, and buprenorphine discontinuation by receipt of benzodiazepines.
*Adjusted for sex, age, race, Medicaid receipt, diagnosis of depressive disorder, anxiety disorder, bipolar/psychotic disorder, SSRI receipt, time-varying buprenorphine dose, and recent hospital-based mental health encounter (Supplement Table 2 shows full model results).
**Note truncated y-axis for fatal overdose, non-fatal opioid overdose, and all-cause mortality.
***Denotes number of buprenorphine treatment episodes
We used Cox proportional hazards models to calculate hazards ratios (HRs) for receipt of benzodiazepine treatment compared to no benzodiazepine receipt restricted to periods during buprenorphine treatment using robust sandwich variance estimators to account for possible clustering by individual (Figure 1 and Supplement). Our primary analysis studied the association between benzodiazepine treatment and fatal opioid overdose. We also studied the association between benzodiazepine treatment and non-fatal opioid overdose, all-cause mortality, and buprenorphine treatment discontinuation. All HRs were adjusted for all covariates. No violation of the proportional hazards assumption was detected.
We conducted one set of sensitivity analyses. We examined the association between benzodiazepine prescription history and fatal overdose, non-fatal overdose, all-cause mortality, and buprenorphine discontinuation. Benzodiazepine prescription history was defined as “current” during times when an individual was actively receiving a benzodiazepine, “former” during times when an individual was not actively receiving a benzodiazepine but had received one previously (including one year prior to start of observation), and “none” during times when an individual was not actively receiving a benzodiazepine and did not receive a benzodiazepine throughout the study period.
These analyses were not pre-registered and the results should be considered exploratory. Analyses were conducted using SAS Studio, version 3.5 (SAS Institute, Cary, North Carolina).
RESULTS
Sample
There were 63,345 people, aged 18 to 96, who received buprenorphine during the study period. Among the study sample, 15,283 people (24%) had filled at least one benzodiazepine prescription during buprenorphine treatment (Table 1). Of the 67,088 person-years of observation on buprenorphine, 57,825 person-years (86%) represented exposure to buprenorphine alone and 9,263 person-years (14%) represented exposure to buprenorphine and a benzodiazepine. Among the 114,971 buprenorphine treatment episodes, 102,918 (90%) ended in discontinuation. Those who received benzodiazepines were more likely to be female (49% of those who received a benzodiazepine were female vs. 34% of those who did not receive a benzodiazepine), have an anxiety disorder diagnosis (36% vs. 18%), depressive disorder (40% vs. 23%), bipolar or psychotic disorder (11% vs. 6%), have had a recent hospital-based mental health-related encounter (41% vs. 35%), and were older (mean age 40 vs. mean age 37).
Table 1:
Characteristics of the sample of individuals who received buprenorphine in Massachusetts between 2012–2015
| Receipt of benzodiazepine at any time while also prescribed buprenorphine* | Total | ||
|---|---|---|---|
| Characteristic | No (n=48,062) n (%) |
Yes (n=15,283) n (%) |
n=63,345 n (%) |
| Female | 16,343 (34) | 7,435 (49) | 23,778 (38) |
| Age, mean (SD) | 37 (11) | 40 (11) | 38 (11) |
| Medicaid enrollment | 21,172 (44) | 6,666 (44) | 27,838 (44) |
| Anxiety disorder | 8,479 (18) | 5,493 (36) | 13,972 (22) |
| Depressive disorder | 11,090 (23) | 6,045 (40) | 17,135 (27) |
| Bipolar/psychotic disorder | 2,849 (6) | 1,690 (11) | 4,539 (7) |
| Receipt of SSRI prescription | 3,178 (7) | 1,865 (12) | 5,043 (8) |
| Hospital-based mental health-related encounter** | 17,015 (35) | 6,238 (41) | 23,253 (37) |
p<0.001 for all comparisons between groups except for Medicaid enrollment which had p-value of 0.45.
Emergency department, observation, or inpatient discharge for mental health condition
Unadjusted rates of overdose and mortality
During the 4-year study period among people treated with buprenorphine, 183 people died of an opioid overdose, there were 693 non-fatal opioid overdoses, and 369 people died from any cause (Table 2). Approximately 31% of fatal opioid overdoses occurred during times when people received benzodiazepines during buprenorphine treatment. The total unadjusted incidence rates of fatal opioid overdoses, non-fatal opioid overdoses, and all-cause mortality were 27, 103, and 53 per 10,000 person-years respectively. During times when people received benzodiazepines in buprenorphine treatment, the rate of fatal opioid overdose (60 per 10,000 person-years) was almost three times the rate when they received buprenorphine alone (22 per 10,000 person-years). The rates of non-fatal opioid overdose and all-cause mortality during combined benzodiazepine and buprenorphine treatment alone were approximately twice the rates during buprenorphine treatment alone (172 vs. 92 per 10,000 person-years and 98 vs. 48 per 10,000 person-years, respectively).
Table 2:
Incidence rates of fatal opioid overdoses (FOD), non-fatal opioid overdoses (NFOD), and all-cause mortality during buprenorphine (BUP) treatment by receipt of benzodiazepine (BZD)
| Person-Years | FOD,No. | FOD rate per 10,000 Person-Years (95%CI) | NFOD, No. | NFOD rate per 10,000 Person-Years (95%CI) | All-cause Death, No. | All-cause Mortality rate per 10,000 Person-Years (95%CI) | |
|---|---|---|---|---|---|---|---|
| BUP only | 57,825 | 127 | 22 (18, 26) | 533 | 92 (85, 100) | 278 | 48 (43, 54) |
| BZD + BUP | 9,263 | 56 | 60 (47, 78) | 159 | 172 (147, 200) | 91 | 98 (80, 121) |
| Total | 67,088 | 183 | 27 (24, 32) | 693 | 103 (96, 111) | 369 | 55 (50, 61) |
Adjusted analyses
Figure 1 shows extended Kaplan-Meier estimates and hazard ratios describing the relationship between benzodiazepine receipt and fatal and non-fatal overdose, all-cause mortality, and buprenorphine discontinuation. Compared to periods during which people received buprenorphine alone, periods of concurrent benzodiazepine and buprenorphine receipt were associated with an increased risk of opioid-related overdose death (hazard ratio = 2.92; 95% confidence interval [CI], 2.10–4.06). Benzodiazepine treatment during buprenorphine treatment was also associated with an increased risk of non-fatal opioid overdose (HR = 2.05; 95%CI, 1.68–2.44) and all-cause mortality (HR = 1.90; 95%CI, 1.48–2.44). Benzodiazepine treatment during buprenorphine treatment was associated with a decreased risk of buprenorphine treatment discontinuation (HR = 0.87; 95%CI, 0.85–0.89).
Sensitivity analysis
We conducted a sensitivity analysis testing the association between benzodiazepine prescription history and fatal opioid overdose, non-fatal opioid overdose, all-cause mortality, and buprenorphine treatment discontinuation. We found only minor differences in the degree of association between current benzodiazepine receipt and all of the outcomes compared to our primary analyses. For fatal and non-fatal overdose and all-cause mortality, former benzodiazepine receipt was associated with a lower risk compared to current benzodiazepine receipt. In the case of non-fatal overdose, former benzodiazepine receipt was associated with a decreased risk compared to no benzodiazepine receipt. Lastly, former benzodiazepine receipt was associated with an increased risk of buprenorphine discontinuation.
DISCUSSION
In this large sample of people who received buprenorphine treatment in Massachusetts, receipt of benzodiazepines was associated with an increased risk of fatal opioid overdose, non-fatal opioid overdose, and all-cause mortality and a decreased risk of buprenorphine treatment discontinuation. The unadjusted rate of fatal opioid overdose while receiving benzodiazepines during buprenorphine treatment was almost three times that of receiving buprenorphine alone and rates of non-fatal overdose and all-cause mortality while receiving benzodiazepines during buprenorphine treatment were approximately two times that of receiving buprenorphine alone. Of note, fatal opioid overdose during buprenorphine treatment was relatively rare: there were only 183 fatal opioid overdoses during the 4-year study period representing less than 4% of the 4,754 estimated total opioid overdoses in the state of Massachusetts(31).
These findings differ from previous cohort studies that have suggested that benzodiazepines are not associated with an increased risk of overdose or mortality in patients receiving buprenorphine. Abrahamsson et al.(26) did not find a statistically significant association between concurrent benzodiazepine prescription and overdose death or all-cause mortality in a sample of 3,221 individuals receiving buprenorphine in Sweden. Likewise, Dupouy et al.(27), in a univariate analysis, did not find a statistically significant association between receipt of benzodiazepines and all-cause mortality in a sample of 713 individuals receiving buprenorphine in France, though this analysis also included times when individuals discontinued buprenorphine and were not receiving buprenorphine treatment. Those studies may have been underpowered to detect an association between benzodiazepines and mortality, in part due to the relatively small number of deaths in those studies (70 deaths in Abrahamsson and 29 deaths in Dupouy). Abrahamsson et al. adjusted for similar demographic characteristics and for previous inpatient treatment for non-fatal overdose. This differed from our approach of adjusting for all emergency or hospital mental health encounters and psychiatric diagnoses and may explain differences in our findings.
In this study, receipt of benzodiazepines during buprenorphine treatment was associated with an increased risk of overdose and mortality. Furthermore, former benzodiazepine receipt was associated with less risk of overdose compared to current benzodiazepine receipt in our sensitivity analysis. Because benzodiazepines can increase respiratory depression(32) and may remove the protective respiratory ceiling of buprenorphine(7), benzodiazepine use may increase the risk of overdose by inducing respiratory depression. Additionally, given that patients receiving methadone treatment for addiction often use benzodiazepines “to feel good” or “to get high”(21) or to “boost” the effect of methadone(33), benzodiazepine use in the setting of buprenorphine treatment may increase the risk of overdose by causing overuse of benzodiazepines or by inducing relapse to illicit opioid use. Alternatively, receipt of a benzodiazepine prescription may be a marker for a severe anxiety or insomnia disorder. Since insomnia and high levels of anxiety are predictors of substance use relapse(34–37), our findings may be a result of confounding by the indication for which benzodiazepines were prescribed.
We found that benzodiazepine receipt during buprenorphine treatment was associated with a decreased risk of buprenorphine treatment discontinuation, a novel finding in this population, and one that we suspected based on clinical experience. Since discontinuation of buprenorphine treatment is so common(38,39), clinicians should be aware of factors that are associated with an increased risk of discontinuation. Benzodiazepine treatment may lead to improved buprenorphine treatment retention because it can effectively treat anxiety symptoms. Given anxiety and sleep disturbance has been associated with an increased risk of medication nonadherence(40–42), relief of anxiety and insomnia symptoms might promote better buprenorphine treatment adherence. Additionally, we found that former benzodiazepine receipt was associated with an increased risk of buprenorphine discontinuation. This might suggest that people formerly but not actively prescribed benzodiazepines have under treatment of anxiety and insomnia leading to worse buprenorphine treatment adherence. Alternatively, those who received benzodiazepines may have been more likely to remain in treatment due to the indication for which benzodiazepines were prescribed, rather than the benzodiazepine itself. People with co-occurring mental disorders and drug use disorders are more likely to seek treatment than those with a single disorder alone(43,44). Furthermore, psychiatric diagnosis among those receiving buprenorphine has been associated with improved treatment retention(45,46). Thus, people who receive benzodiazepines may have a more severe underlying mental disorder and it may be the underlying mental disorder that leads them to seek and remain in buprenorphine treatment rather than receiving the benzodiazepine prescription.
Our findings suggest that there are both risks and benefits of prescribing benzodiazepines to individuals receiving buprenorphine. Though benzodiazepines were associated with an increased risk of overdose and mortality, they were also associated with a decreased risk of buprenorphine discontinuation. Since treatment with buprenorphine is associated with significant reductions in overdose and mortality(47,48), individuals who receive benzodiazepines during buprenorphine treatment may also have a lower risk of overdose and mortality in the long run by remaining in treatment. Because it is unclear which individuals who receive benzodiazepines during buprenorphine treatment are most likely to overdose or remain in treatment, clinicians should be cautious when prescribing benzodiazepines to patients who receive little clinical benefit from benzodiazepine treatment or do not have a diagnosis that warrants benzodiazepine treatment. Similarly, clinicians should be cautious when discontinuing benzodiazepines, even in patients who receive little clinical benefit from benzodiazepine treatment or do not have a diagnosis that warrants benzodiazepine treatment.
Limitations
Because we assumed individuals took their medications according to the prescriber’s instructions, we may not have accurately determined whether an individual took their prescribed medication on any given day which may have biased results. With regards to overlapping prescriptions, we assumed the first prescription ended the day the second prescription began which may have shortened the actual duration of medication exposure. We believe this is a relatively rare event given early refills for buprenorphine are often only granted for a limited number of emergencies by insurances in Massachusetts. Furthermore, the state PMP records prescription fill dates, not pick up dates. Thus, there may be a lag between the fill date and the date the patient picked up the medication. We believe this is a rare event given the withdrawal syndromes associated with buprenorphine and benzodiazepines. Because our research question was aimed at the safety of benzodiazepine prescribed during buprenorphine treatment, we restricted our analysis to times during receipt of buprenorphine. Thus, we do not know from this analysis whether the relationship between benzodiazepines and overdose or mortality changes when times outside of buprenorphine treatment are included. Dupouy et al.(27) included times outside of buprenorphine treatment and did not find an association between benzodiazepine receipt and mortality, though it is difficult to draw conclusions from that analysis given both benzodiazepine and buprenorphine exposure were not treated as time-varying. Because of the observational nature of this study’s design, we were unable to ascertain whether benzodiazepine use is a direct cause of overdose, mortality, or increased buprenorphine treatment retention, though we did adjust for several important confounders including psychiatric co-morbidities and service use. Furthermore, we did not analyze toxicology data at the time of death and therefore were not able to determine which opioids or benzodiazepines contributed or were actually present at the time of death.
CONCLUSIONS
In this large observational study of individuals receiving buprenorphine treatment, receipt of benzodiazepines was associated with an increased risk of fatal opioid overdose, non-fatal opioid overdose, and all-cause mortality. Receipt of benzodiazepines was also associated with a decreased risk of buprenorphine discontinuation. Further evaluation is needed in this population to determine the comparative risks and benefits of initiating, continuing, and discontinuing the co-prescription of buprenorphine and benzodiazepines.
Supplementary Material
DISCLOSURES AND ACKNOWLEDGMENTS:
Conflict of Interest Disclosures
RS is the principal investigator of a study funded by the National Institute on Alcohol Abuse and Alcoholism (R01 AA021335) for which Alkermes provides medication. No other disclosures were reported.
Funding/Support:
This work was supported by funding from a National Institute on Drug Abuse award (K23 DA044321). The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript of the decision to submit.
REFERENCES
- 1.Roberts AW, Saloner B, Dusetzina SB. Buprenorphine Use and Spending for Opioid Use Disorder Treatment: Trends From 2003 to 2015. PS. 2018. May 8;69(7):832–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piper BJ, Shah DT, Simoyan OM, McCall KL, Nichols SD. Trends in Medical Use of Opioids in the U.S., 2006–2016. American Journal of Preventive Medicine. 2018. May 1;54(5):652–60. [DOI] [PubMed] [Google Scholar]
- 3.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication Treatment For Opioid Use Disorders In Substance Use Treatment Facilities. Health Aff (Millwood). 2019;38(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gueye PN, Borron SW, Risède P, Monier C, Buneaux F, Debray M, et al. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol Sci. 2002. January;65(1):107–14. [DOI] [PubMed] [Google Scholar]
- 5.Borron SW, Monier C, Risède P, Baud FJ. Flunitrazepam variably alters morphine, buprenorphine, and methadone lethality in the rat. Hum Exp Toxicol. 2002. November;21(11):599–605. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder CA, Smith LJ. Respiratory Rates and Arterial Blood-Gas Tensions in Healthy Rabbits Given Buprenorphine, Butorphanol, Midazolam, or Their Combinations. J Am Assoc Lab Anim Sci. 2011. March;50(2):205–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen S, Taylor DA. The effect of buprenorphine and benzodiazepines on respiration in the rat. Drug Alcohol Depend. 2005. July;79(1):95–101. [DOI] [PubMed] [Google Scholar]
- 8.Lintzeris N, Mitchell TB, Bond A, Nestor L, Strang J. Interactions on mixing diazepam with methadone or buprenorphine in maintenance patients. J Clin Psychopharmacol. 2006. June;26(3):274–83. [DOI] [PubMed] [Google Scholar]
- 9.Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, Albanese MJ. Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend. 2013. October 1;132(3):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynaud M, Petit G, Potard D, Courty P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addiction. 1998. September 1;93(9):1385–92. [DOI] [PubMed] [Google Scholar]
- 11.Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int. 2001. September 15;121(1–2):65–9. [DOI] [PubMed] [Google Scholar]
- 12.Pirnay S, Borron SW, Giudicelli CP, Tourneau J, Baud FJ, Ricordel I. A critical review of the causes of death among post-mortem toxicological investigations: analysis of 34 buprenorphine-associated and 35 methadone-associated deaths. Addiction. 2004. August 1;99(8):978–88. [DOI] [PubMed] [Google Scholar]
- 13.Häkkinen M, Launiainen T, Vuori E, Ojanperä I. Benzodiazepines and alcohol are associated with cases of fatal buprenorphine poisoning. Eur J Clin Pharmacol. 2012. March 1;68(3):301–9. [DOI] [PubMed] [Google Scholar]
- 14.Bramness JG, Kornør H. Benzodiazepine prescription for patients in opioid maintenance treatment in Norway. Drug and Alcohol Dependence. 2007. October 8;90(2):203–9. [DOI] [PubMed] [Google Scholar]
- 15.Park TW, Bohnert AS, Austin K, Saitz R, Pizer SD. Regional Variation in Benzodiazepine Prescribing for Patients in Opioid Agonist Therapy. Psychiatr Serv. 2014. January 1;65(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lintzeris N, Nielsen S. Benzodiazepines, Methadone and Buprenorphine: Interactions and Clinical Management. The American Journal on Addictions. 2010. January 1;19(1):59–72. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AJ, Lo-Ciganic W-H, Cochran G, Gellad WF, Cathers T, Kelley D, et al. Patterns and Quality of Buprenorphine Opioid Agonist Treatment in a Large Medicaid Program. J Addict Med. 2015. December;9(6):470–7. [DOI] [PubMed] [Google Scholar]
- 18.Lavie E, Fatséas M, Denis C, Auriacombe M. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend. 2009. January 1;99(1–3):338–44. [DOI] [PubMed] [Google Scholar]
- 19.Offidani E, Guidi J, Tomba E, Fava GA. Efficacy and Tolerability of Benzodiazepines versus Antidepressants in Anxiety Disorders: A Systematic Review and Meta-Analysis. PPS. 2013;82(6):355–62. [DOI] [PubMed] [Google Scholar]
- 20.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000. January 25;162(2):225–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KW, Berger CC, Forde DP, D’Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011. May 19;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein MD, Kanabar M, Anderson BJ, Lembke A, Bailey GL. Reasons for Benzodiazepine Use Among Persons Seeking Opioid Detoxification. J Subst Abuse Treat. 2016;68:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: A review of major perspectives and findings. Clinical Psychology Review. 2000. March 1;20(2):149–71. [DOI] [PubMed] [Google Scholar]
- 24.Bakker A, Streel E. Benzodiazepine maintenance in opiate substitution treatment: Good or bad? A retrospective primary care case-note review. J Psychopharmacol (Oxford). 2017;31(1):62–6. [DOI] [PubMed] [Google Scholar]
- 25.Naeger S, Mutter R, Ali MM, Mark T, Hughey L. Post-Discharge Treatment Engagement Among Patients with an Opioid-Use Disorder. J Subst Abuse Treat. 2016. October;69:64–71. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsson T, Berge J, Öjehagen A, Håkansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—A nation-wide register-based open cohort study. Drug and Alcohol Dependence. 2017. May 1;174:58–64. [DOI] [PubMed] [Google Scholar]
- 27.Dupouy J, Palmaro A, Fatséas M, Auriacombe M, Micallef J, Oustric S, et al. Mortality Associated With Time in and Out of Buprenorphine Treatment in French Office-Based General Practice: A 7-Year Cohort Study. Ann Fam Med. 2017. July;15(4):355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration. Drug Safety and Availability - FDA Drug Safety Communication: FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks [Internet]. [cited 2018 Sep 4]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm575307.htm
- 29.The Commonwealth of Massachusetts. An assessment of fatal and nonfatal opioid overdoses in Massachusetts (2011–2015). Massachusetts Department of Public Health; [Internet] 2017. [cited 2018 Aug 12]. Available from: https://pilot.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf [Google Scholar]
- 30.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017. May;26(5):509–17. [DOI] [PubMed] [Google Scholar]
- 31.The Commonwealth of Massachusetts. Number of Opioid ‐Related Overdose Deaths, All Intents by City/Town, MA Residents: 2012–2016. Massachusetts Department of Public Health; [Internet] 2017. [cited 2018 Aug 12]. Available from: https://www.mass.gov/files/documents/2017/11/13/sec3-od%20deaths%20by%20city-town%20Nov-17.pdf [Google Scholar]
- 32.Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev. 2011. March;63(1):243–67. [DOI] [PubMed] [Google Scholar]
- 33.Stitzer ML, Griffiths RR, McLellan AT, Grabowski J, Hawthorne JW. Diazepam use among methadone maintenance patients: patterns and dosages. Drug Alcohol Depend. 1981. November;8(3):189–99. [DOI] [PubMed] [Google Scholar]
- 34.Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001. May 1;36(3):249–55. [DOI] [PubMed] [Google Scholar]
- 35.Willinger U, Lenzinger E, Hornik K, Fischer G, Schönbeck G, Aschauer HN, et al. ANXIETY AS A PREDICTOR OF RELAPSE IN DETOXIFIED ALCOHOL-DEPENDENT PATIENTS. Alcohol Alcohol. 2002. November 1;37(6):609–12. [DOI] [PubMed] [Google Scholar]
- 36.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003. December;7(6):523–39. [DOI] [PubMed] [Google Scholar]
- 37.Roehrs TA, Roth T. Sleep Disturbance in Substance Use Disorders. Psychiatr Clin North Am. 2015. December;38(4):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018. December;95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bet PM, Penninx BWJH, van Laer SD, Hoogendijk WJG, Hugtenburg JG. Current and remitted depression and anxiety disorders as risk factors for medication nonadherence. J Clin Psychiatry. 2015. September;76(9):e1114–1121. [DOI] [PubMed] [Google Scholar]
- 41.Sundbom LT, Bingefors K. The influence of symptoms of anxiety and depression on medication nonadherence and its causes: a population based survey of prescription drug users in Sweden. Patient Prefer Adherence. 2013. August 19;7:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips KD, Moneyham L, Murdaugh C, Boyd MR, Tavakoli A, Jackson K, et al. Sleep disturbance and depression as barriers to adherence. Clin Nurs Res. 2005. August;14(3):273–93. [DOI] [PubMed] [Google Scholar]
- 43.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990. November 21;264(19):2511–8. [PubMed] [Google Scholar]
- 44.Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry. 1996. January;66(1):17–31. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein ZM, Cheng DM, Quinn E, Hui D, Kim H, Gryczynski G, et al. Psychoactive medications and disengagement from office based opioid treatment (obot) with buprenorphine. Drug and Alcohol Dependence. 2017. January 1;170:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin ML, Dodd DR, Potter JS, Rice LS, Dickinson W, Sparenborg S, et al. Baseline characteristics and treatment outcomes in prescription opioid dependent patients with and without co-occurring psychiatric disorder. Am J Drug Alcohol Abuse. 2014. March;40(2):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med. 2018. August 7;169(3):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017. April 26;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.