Abstract
The Nucleosome Remodeling and Deacetylase (NuRD) complex uniquely combines both deacetylase and remodeling enzymatic activities in a single macromolecular complex. The Methyl-CpG Binding Domain 2 and 3 (MBD2 and MBD3) proteins provide a critical structural link between the deacetylase and remodeling components while MBD2 endows the complex with the ability to selectively recognize methylated DNA. Hence, NuRD combines three major arms of epigenetic gene regulation. Research over the past few decades has revealed much of the structural basis driving formation of this complex and started to uncover the functional roles of NuRD in epigenetic gene regulation. However, we have yet to fully understand the molecular and biophysical basis for methylation-dependent chromatin remodeling and transcription regulation by NuRD. In this review, we discuss the structural information currently available for the complex, the role MBD2 and MBD3 play in forming and recruiting the complex to methylated DNA, and the biological functions of NuRD.
Keywords: DNA methylation, nucleosome remodeling, histone deacetylase, epigenetics, chromatin
Introduction
The highly homologous Methyl-CpG Binding Domain proteins 2 and 3 (MBD2 and MBD3) are the ancestral orthologs to the larger MBD family found in all vertebrate species [1–3]. Members of this family share an approximately seventy amino acid domain that selectively recognizes methylated cytosine-guanosine dinucleotides (CpGs). Genetic duplication and diversification generated seven known family members (MBD1–6 and MeCP2), each of which independently associates with different co-regulatory complexes and have unique functional roles [2]. MBD2 and MBD3, which arose by duplication of the MBD2/3 protein found in invertebrates, provide critical structure to the Nucleosome Remodeling and Deacetylase (NuRD) complex [4–8]. NuRD comprises two separable functions consisting of histone deacetylase and chromatin remodeling sub-complexes which interact with MBD2 and MBD3 through distinct interfaces [7, 8].
Consequently, MBD2 and MBD3 physically bridge three major arms of epigenetic gene regulation to form NuRD: histone deacetylation, ATP-dependent nucleosome remodeling, and selective recognition of methylated DNA. Importantly, MBD2 and MBD3 form mutually exclusive NuRD complexes, each recognizing methylated DNA with markedly different affinity and selectivity [2, 9–12]. Therefore, MBD2-NuRD and MBD3-NuRD assemble functionally distinct complexes with high and low methylation selectivity, respectively. In this review, we discuss the formation of the NuRD complex focusing on the structures of the different components, the critical role provided by MBD2 or MBD3, how differences in methylation selectivity impact dynamic interaction with DNA, and we describe a model of NuRD function that attempts to reconcile these contrasting functional and biophysical data.
The NuRD complex
Early work in the Adrian Bird laboratory utilized methylated DNA probes to isolate two nuclear protein fractions that selectively bind methylated DNA, the methyl-CpG binding proteins 1 and 2 (MeCP1 and MeCP2) [13, 14]. MeCP1 was a large complex (400 – 800 kDa) that required multiple mCpGs for binding, while MeCP2 was a single polypeptide (52 kDa) that bound to individual methylated sites. Further characterization of MeCP2 led to the identification of the protein domain responsible for methylation selectivity and, by homology, the MBD family of proteins in mammals [2, 15]. A few years later, work by Paul Wade in the Alan Wolffe laboratory characterized a multi-protein nucleosome remodeling complex with methylation specificity [5, 6]. This complex became known as NuRD and likely represented the original MeCP1 protein complex identified in Bird’s laboratory [4]. At the same time the Danny Reinberg laboratory dissected the proteins comprising the histone deacetylase core complex of NuRD and co-expressed distinct combinations of these proteins to form stable sub-complexes [16, 17].
Together these and subsequent studies have shown that the core NuRD comprises at least seven proteins, each of which has multiple paralogs in humans (Figure 1) [5, 9, 17–20]. These proteins include Metastasis Tumor-Associated 1, 2, or 3 (MTA1, 2, or 3), Histone Deacetylase 1 or 2 (HDAC1 or 2), Retinoblastoma Binding Protein 4 or 7 (RBBP4 or 7), GATAD2A or B, Chromodomain Helicase DNA Binding Protein 3, 4, or 5 (CHD3, 4, or 5), Cyclin-Dependent Kinase 2 Associated Protein 1 (CDK2AP1), and MBD2 or 3 [5, 9, 16, 17].
Figure 1. Overview of the NuRD complex.
(a) Schematic diagrams of the seven major NuRD components highlight the domain architecture and relative size of each protein: CDKAP1, MBD2 or 3, RBBP4 or 7, HDAC 1 or 2, GATAD2 A or B, MTA 1, 2, or 3, and CHD 3, 4, or 5. (b) A model of NuRD depicts how these components interact.
HDAC1, HDAC2, RBBP4 and RBBP7 proteins each contain a single structured domain and function in a variety of co-regulatory histone-modifying complexes (e.g., Sin3a, NuRF, and CAF-1). HDACs provide histone deacetylase enzymatic activity while RBBPs present protein-protein interaction surfaces involved in the recruitment of the complex to chromatin.
MTA1, MTA2, and MTA3 proteins contain multiple domains separated by disordered stretches of the polypeptide. Different regions and domains of the MTAs make distinct protein-protein interactions with HDAC and RBBP paralogs [21–26]. Therefore, MTAs provide a critical structural role in forming the histone deacetylase core sub-complex (HDCC) of NuRD comprising the MTA, HDAC, and RBBP proteins.
GATAD2A and GATAD2B proteins contain two relatively small conserved regions implicated in protein-protein interactions separated by a long, likely unstructured, polypeptide [27]. These two conserved regions of the GATAD2s from critical interactions between the MBD and CHD proteins [8, 23].
The largest proteins in the complex, CHD3, CHD4, and CHD5 contain an Snf2-type helicase/ATPase domain that physically moves nucleosomes in an ATP dependent manner [5, 16, 28]. The CHDs have two chromodomains and two plant homeodomains (PHD) that interact with DNA and histones, respectively [29, 30]. CHDs also have an uncharacterized C-terminal domain and an N-terminal High Mobility Group (HMG) Box-like domain that binds poly-ADP ribose [31].
MBD2 and MBD3 proteins contain an N-terminal MBD and C-terminal coiled-coil domain separated by an intrinsically disordered region implicated in binding to the HDCC [4, 7, 8, 27]. The coiled-coil domain of MBD2 and MBD3 binds a GATAD2 protein, thereby linking the HDCC with the CHD proteins.
CDK2AP1 appears to be a core component of NuRD but often overlooked because of its small size (~12 kDa). Studies indicate that CDK2AP1 negatively regulates CDK2 activity, inhibits tumor growth, and acts as a tumor suppressor in oral cancer [9, 18, 32–34]. While its function within the NuRD complex has not been clearly defined, a recent study revealed that CDK2AP1 promotes NuRD recruitment to specific loci where it displaces SWI/SNF and represses transcription [35].
The presence of multiple paralogs for each core component leads to a sizeable combinatorial array of potentially distinct NuRD complexes. Experiments indicate that at least some of the paralogs interact with NuRD in a mutually exclusive manner and are expressed in a cell and tissue-specific manner with unique functional roles [9, 11, 36–39]. For example, the MTA3 paralog is expressed in a specific subset of B lymphocytes and promotes germinal center differentiation [40, 41]. The CHD3, CHD4, and CHD5 paralogs are expressed at different stages during cortical brain development in mouse and regulate distinct functions [42]. The GATAD2A paralog contains a Zinc Finger MYND-Type Containing 8 (ZMYND8) binding motif such that GATAD2A-NuRD localizes to sites of DNA damage [39].
In addition to the core components, other proteins bind to the complex at sub-stoichiometric ratios, such as spalt-like transcription factor 4 (SALL4), friend of GATA (FOG)- 1 and 2, B-cell lymphoma/leukemia 11a (BCL11a), ZMYND8, and several zinc finger proteins (ZNF-512B, −532, −592, and −687) [19, 20, 39, 43]. Several of these components interact with NuRD through a small (~12 amino acid) N-terminal peptide that binds to RBBP4/7 and was first identified in the FOG-1 protein [44–48]. This peptide can be used to isolate relatively pure NuRD complexes from cell extracts in a single step [45, 49], indicating that the interaction interface is uniquely accessible when the RBBPs are bound to NuRD. The sub-stoichiometric components can localize NuRD to different regions of the genome for distinct functions [39, 44, 47, 50–53]. Therefore, NuRD appears to act as a co-regulatory complex that can be targeted to different regions of the genome through either its intrinsic binding affinity for methylated DNA or co-recruitment by different transcription factors and chromatin-associated proteins.
Structural Analyses of NuRD
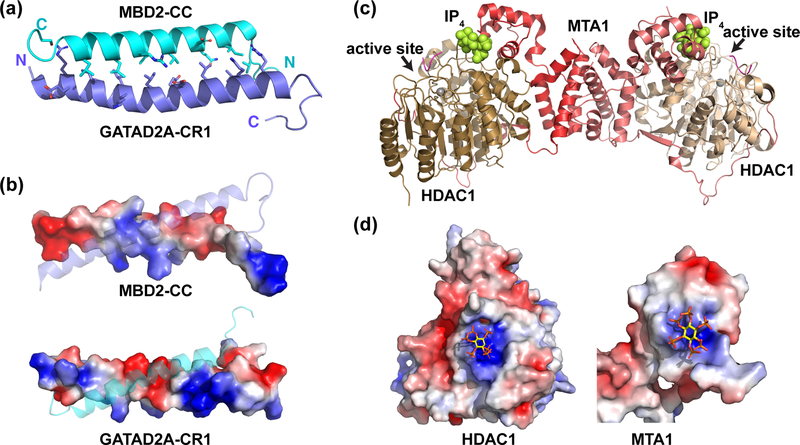
The variety of distinct NuRD complexes containing different isoforms with long disordered regions have challenged investigations of complex formation. As a result, structural studies have focused on well-defined sub-complexes between separate components. We determined one of the first structures of a NuRD sub-complex formed between the coiled-coil domains of GATAD2A (also known as p66α) and MBD2 [7, 54]. This complex involves two small helical peptides that form a very stable high-affinity interaction critical to NuRD function. Each of the coiled-coil domains behaves as monomeric helices in isolation, which is unusual for coiled-coil domains. The charge distribution of the domains inhibits homodimerization and stabilizes the heterodimeric interaction (Figure 2a,b) [54]. Importantly, we showed that enforced expression of the isolated coiled-coil domain from GATAD2A inhibits methylation-dependent gene silencing by NuRD. This same peptide can immuno-precipitate all native components of NuRD except CHD4 and GATAD2A. Hence, the coiled-coil interaction forms a necessary and sufficient link between the histone deacetylase and chromatin remodeling portions of NuRD. Of note, the coiled-coil domains of the MBD2/3 and GATAD2 proteins are conserved throughout Metazoan species, independent of the presence of DNA methylation. Therefore, this coiled-coil interaction and the NuRD complex emerged with the earliest multicellular organisms and has been maintained throughout animal evolution [1]. More recent work has shown that the second highly conserved region of the GATAD2 proteins interacts with the C-terminal portion of CHD4, thus delineating the minimal protein regions necessary for the structural link between NuRD and CHD3/4 [23, 55].
Figure 2. Structural analyses of the MBD2:GATAD2A and MTA1:HDAC1 complexes.
(a) A cartoon diagram depicts the solution structure of the coiled-coil complex between MBD2 (cyan) and GATAD2A (blue) (PBD ID: 2L2L). Amino acids at the protein-protein interface are shown as sticks. (b) A surface rendition of MBD2-CC and GATAD2A colored by electrostatic potential shows alternating patches of positive (blue) and negative (red) regions that promote heterodimerization and minimize homodimerization. (c) A cartoon diagram depicts the crystal structure of the complex between MTA1 (red and light red), HDAC1 (brown and light brown), and a peptide inhibitor (magenta). MTA1 forms a dimer and wraps around HDAC1. An inositol tetraphosphate molecule (space-filled, yellow) binds near the HDAC1 active site. (d) Surface rendering of HDAC1 and MTA1 colored by electrostatic potential shows that IP4 binds to positively charged regions (blue) of each, thereby bridging between surfaces that otherwise would not interact favorably (PDB ID: 5ICN). All structure figures were generated using PyMOL [130] and the electrostatic surface potential using the APBS plugin [131].
A breakthrough in structural analysis of NuRD came from studies of the histone deacetylase core by John Schwabe’s group. They determined high-resolution structures of a complex between the ELM2-SANT domains of MTA1 and HDAC1 [24, 25, 56]. This work built on prior research from the same group investigating the complex between homologous domains from the SMRT co-repressor and HDAC3 [57]. The MTA1-HDAC1 interaction involves an extensive protein-protein interface formed by ELM2 and SANT domains of MTA1 as well as an extended region between these two domains that wraps around the surface of HDAC1 (Figure 2c). Their structural analyses led to two interesting observations. First, the SANT domain of MTA1 binds to HDAC1 through inositol tetraphosphate (Ins(1,4,5,6)P4). The phosphate groups from Ins(1,4,5,6)P4 favorably interact with positively charged surfaces at the interface, bridging an otherwise repulsive interaction (Figure. 2d). The contact surface on HDAC1 is near the enzymatic cleft such that the presence of Ins(1,4,5,6)P4 augments deacetylase activity [56]. Also, previous work has suggested that Ins(1,4,5,6)P4 levels fluctuate during the cell cycle [58]. This observation raises the interesting possibility that Ins(1,4,5,6)P4 regulates histone deacetylation activity in a cell cycle-dependent manner. Second, the ELM2 domain forms a homodimer within the crystal lattice leading to a 2:2 molar ratio for the histone deacetylase core. Dimerization through the ELM2 domain helps clarify stoichiometric analyses of the full NuRD complex. While not wholly consistent, quantitative mass spectrometry and protein cross-linking experiments have found that a single CHD interacts with 1–2 copies of the MBD, GATAD2, and HDAC proteins, 2–3 copies of the MTA proteins, and at least four copies of the RBBP proteins [19, 20, 59]. These results, along with the structural studies, suggest an overall stoichiometry of 1:2:4 between the CHD/GATAD2/MBD/CDKAP1:MTA/HDAC:RBBP components.
Several studies have delineated the specific protein interactions involving the RBBP proteins. These proteins comprise a single WD-repeat domain, which forms a seven-bladed β propeller fold, with an extended N-terminal α-helix. This 3-dimensional fold generates a large surface area which allows RBBP4 and RBBP7 proteins to interact with different chromatin-associated proteins through independent interaction surfaces. Joel Mackay’s group mapped and solved the first structure of a complex between MTA1 and RBBP4 (also known as RbAp48) [21]. This interaction involves a conserved positively charged motif (KRAARR) from MTA1 (amino acids 678–683)* that binds in a groove formed between non-canonical structural elements from the N-terminal α-helix, an extended loop, and a C-terminal helical turn of RBBP4 (Figure 3). The MTA1 peptide occupies the same binding site as a homologous sequence from Histone H4 [60], so that interaction with MTA1 precludes binding to Histone H4. In subsequent work, the Mackay laboratory showed that a separate region in MTA1 (amino acids 440–550), contains a similar motif and binds RBBP4 [22]. At the same time, the Schwabe group determined the structure of this second interaction showing that it occupied the same binding site of RBBP4 as the more C-terminal peptide, but with a more extensive interface [26]. Unlike the more C-terminal peptide, this central region of MTA1 folds into three α-helices and a β-strand that wrap around RBBP4.
Figure 3. Binding of MTA1 and FOG1 to RBBP4.
A cartoon diagram depicts an overlay of crystal structures showing RBBP4 bound to four different peptides. Two different peptides from MTA1 (amino acids 462–546 in yellow and 670–695 in cyan; PDB ID: 5FXY and 4PBZ, respectively) bind RBBP4 (green) through a common interface. Likewise, the N-terminal helix from histone H4 (amino acids 24–41 in blue; PDB ID: 3CFV) binds to the same region of RBBP4. In contrast, the N-terminal FOG1 peptide (amino acids 1–15 in magenta; PDB ID: 2XU7) binds to a distinct region of RBBP4 that does not overlap with the binding surfaces for MTA1 or histone H4. Note that MTA1 residues not observed in the crystal structure (519–528) are indicated with a yellow dashed line.
To extend the crystallographic analyses of the RBBP-MTA peptide interaction, several groups have investigated larger complexes involving the MTA, HDAC, and RBBP proteins. First, the Schwabe group used a combination of small-angle x-ray scattering and single-particle negative-stain electron microscopy to study a complex formed between HDAC1, MTA1 (residues 162–546), and RBBP4 [26]. The MTA1 construct includes the ELM2-SANT region, adjacent zinc finger, and one of the two regions that bind RBBPs. This work showed that the ELM2-SANT region forms a dimer at the center of the complex with the associated HDAC1 proteins immediately adjacent to that center. The two RBBP proteins extend further away on opposite sides, while making contact with the HDAC1 proteins. In this model, the HDAC1 enzymatic cleft remains exposed to solvent and available for interaction with an acetylated histone tail. The MacKay group studied the complex between MTA1 (residues 449–715) and RBBP4 using chemical crosslinking analyses and single-particle negative-stain electron microscopy [22]. This MTA1 construct starts just after the ELM2-SANT domains and includes both of the RBPP binding motifs. The results confirmed that two copies of RBBP4 could bind MTA1 simultaneously. More recently, Brasen et al. [61] purified a complex between full-length MTA2 and RBBP7 for analysis by negative-stain electron microscopy. Two RBBP7 molecules extend from either side of the centrally located ELM2-SANT dimer to form an extended structure containing four RBBPs and two MTAs. The BAH domains protrude from the center of the complex adjacent to the ELM2-SANT domains. Together, these studies have shown how each MTA can homodimerize and recruit two independent copies of the RBBP proteins, for a total of four RBBP proteins per NuRD complex. This multiplicity also agrees with stoichiometric analyses [19, 20] and allows the RBBPs in NuRD to bridge multiple histones and simultaneously interact with transcription factors and other co-regulatory proteins.
As discussed previously, RBBPs bind to a small N-terminal peptide found in different transcription factors that recruit NuRD. Crystal structures have been determined for a number of these peptides bound to the RBBPs (BCL11A [46], SALL4 [48], AEBP2 [62], PHF6 [63] and others), all of which show a similar interaction as between FOG-1 and RBBP4 [45]. As shown in Figure 3, the FOG-1 peptide (amino acids 1–15) binds across one of the flat axial surfaces of the RBBP4 β-propeller, making multiple specific interactions. The conserved RRK motif from FOG-1 interacts with negatively charged glutamate residues at the surface, with the middle arginine protruding into the central cavity of RBBP4. Importantly, the peptide binding surface on RBBP4 does not overlap with that of MTA1, which should permit the RBBPs to bind both MTA and a transcription factor concurrently.
In addition, structural work by Schmitges et al. [64] showed that a similar peptide sequence from the N-terminal tail of histone H3 binds to the same region of Nurf55, the RBBP homolog from D. melanogaster. They studied this interaction in the context of the polycomb repressive complex 2 (PRC2) which contains an RBBP protein as a core component. Importantly, they found that lysine 4 of histone 3 (H3K4) makes a critical intermolecular contact, such that H3K4 methylation blocks interaction with Nurf55 and inhibits methylation of lysine 27 of histone 3 (H3K27) by PRC2. Together, these studies show that the RBBPs function as a handle for recruitment of NuRD by transcription factors and chromatin-interacting proteins and bind to nucleosomes that lack H3K4 methylation.
The CHD3, 4, and 5 proteins are the largest components of the NuRD complex (~110 kDa – Figure 1); however, only a few published studies have investigated the structure of these proteins. Solution structures of the PHD domains in isolation and bound to histone peptides reveal a canonical PHD fold in which the histone peptide binds to form a third β-strand on the central sheet of the domain [29, 65]. Affinity analyses show that the second PHD domain preferentially binds trimethylated lysine nine in the histone H3 peptide (H3K9me3) while the first PHD domain does not. However, both PHD domains favorably bind peptides with unmodified lysine four (H3K4). Work by Tatiana Kutateladze’s group showed that the separation between the dual PHD domains permits the CHDs to bridge neighboring nucleosomes in chromatin [30]. They found that gene silencing by NuRD requires this bivalent interaction with chromatin. This work provides tantalizing insight into how the CHD proteins from NuRD could establish an orderly array of nucleosomes, where bridging by the PHD domains could help establish compact spacing between nucleosomes. The Mackay group recently determined the structure of an N-terminal HMG-like domain from CHD4 [31]. They found that this domain binds poly-ADP ribose with high affinity, yet the role of this interaction in chromatin remodeling or DNA damage repair [39] remains unclear. At this point, the published data describing the structure of the CHDs and their interaction with nucleosomes has been limited to these few studies, which precludes a more detailed understanding of how the protein interacts with chromatin and an accurate model of CHD function.
Based on the above structural studies, a picture of the overall NuRD complex emerges. The MTAs form the structural backbone of the HDCC and provide a handle for recruitment to chromatin through binding of multiple RBBPs. The GATAD2 proteins bridge the CHDs and MBDs through two small conserved domains separated by a long intrinsically disordered region. The missing piece to this puzzle is how the MBD/GATAD2/CHD remodeling portion interacts with the MTA/HDAC/RBBP deacetylase portion of the complex. Based on our observation that the isolated coiled-coil domain from GATAD2A interacts with a sub-complex consisting of MBD, HDAC, MTA, and RBBP proteins, excluding native GATAD2A and CHD4 [7], we hypothesized that the MBD proteins would provide this missing connection. Importantly, NuRD appears to be present across all animal species; yet, in invertebrate species, the MBD2/3 proteins do not always contain an MBD domain. This latter observation implies that the MBD domain is not necessary for binding to other components to form NuRD.
Therefore, we anticipated that the region between the N-terminal MBD and the C-terminal coiled-coil likely binds to the HDCC comprising the MTA, HDAC, and RBBP proteins. Based on NMR and circular dichroism studies, we found that this central portion of MBD2 (amino acids 238–356) behaves as an intrinsically disordered region (IDR) in isolation (Figure 4a–c). Immune-precipitation studies showed that the IDR was necessary and sufficient to bind the HDCC [8]. Mutating highly conserved residues across the MBD2-IDR revealed that modifying two consecutive residues (R286E/L287A) disrupts binding to the HDCC and blocks methylation-dependent gene silencing by NuRD [66]. These observations lead to the current model depicted in Figure 1 which the MBDs play a critical structural role linking the GATAD2/CHD4 and HDCC subcomplexes.
Figure 4. The intrinsically disordered region and DNA binding domains of MBD2.
(a) A schematic diagram of MBD2b (amino acids 214–361) highlights the large intrinsically disordered region located between the N-terminal MBD and C-terminal coiled-coil domain. (b) The intrinsically disordered region (IDR) binds to the HDCC (brown), while mutating two consecutive residues (R286E/L287A, red) disrupts this interaction. (c) An alignment of a region from PWWP2A that binds to the HDCC shows that this peptide shares homology with the MBD2 IDR. This region includes the double-mutation (red) that disrupts MBD2 binding to to the HDCC. (d) A mixed diagram depicts the solution structure of MBD2 (cyan) bound to an mCpG dinucleotide (sticks) (PDB ID: 2KY8). MBD2 makes three key interactions with DNA through a conserved RRY motif. (e) A closeup view shows that arginine residues 24 and 46 each hydrogen bond to symmetrically related guanine bases and pack against methyl groups (orange spheres) of the adjacent methyl-cytosine bases. While tyrosine 36 positions its hydroxyl group towards methyl-cytosine and likely binds to structured water surrounding the methyl group.
More recent work by Zhang et al. [67] and Link et al. [68] found that the PWWP2A protein binds to the HDCC sub-complex and excludes the MBD, GATAD2, and CHD proteins of NuRD. The two groups mapped this interaction to slightly different but contiguous regions of PWWP2A. Interestingly, Zhang et al. [67] found that a small peptide from PWWP2A (amino acids 311–331) bound to a purified MTA1-HDAC1 complex. This peptide is homologous to the MBD2-IDR region containing R286-L287 (Figure 4c). Hence, PWWP2 and MBD2 competitively bind to the HDCC and appear to use a similar peptide motif in this interaction. Recently, we have shown that this same region of MBD2 shows intrinsic helical propensity and that the R286E/L287A mutation reduces this propensity [66]. These data suggest that the MBD2-IDR folds into α-helices upon binding to the HDCC. However, the structure of MBD2 or PWWP2 bound to the HDCC has yet to be determined to verify this model of interaction.
Ernest Laue’s group studied the formation of NuRD from D. melanogaster (dNuRD) using both pull-down assays of endogenous complexes and recombinant expression of sub-complexes in insect cells [69]. They demonstrated that the Drosophila homologs of the RBBP, MTA, HDAC, and MBD proteins formed a stable deacetylase sub-complex with a 5:2:2:1 stoichiometric ratio. This sub-complex could be purified with or without the MBD component. In contrast, the CHD4, GATAD2A, and DOC1 homologs were present in the endogenous dNuRD at sub-stoichiometric ratios and appeared to only weakly associate with histone deacetylase sub-complex. Using single-particle tracking, they showed that association of CHD4-like with dNuRD required the GATAD2A homolog. These observations further support that NuRD can be divided, both functionally and structurally, into chromatin remodeling and histone deacetylase sub-complexes. The instability of the full dNuRD may reflect the relatively low affinity of the coiled-coil interaction between MBD2 and GATAD2A homologs in Drosophila (KD ~ 7 μM) as compared to the human complex (KD ~ 50 nM) [1].
Methylation-specific targeting of NuRD
The NuRD complex was first described as selectively binding methylated DNA, and biochemical and cellular studies have supported that observation. However, methylation selectivity of the complex depends on which MBD it contains. Initially, MBD3 was considered a core component and MBD2 secondary. However, work by the Hendrik Stunnenberg laboratory showed that MBD2 and MBD3 form distinct and mutually exclusive complexes [9]. This finding has been well supported by subsequent quantitative mass spectrometry analyses [19, 20] and by genomic localization studies [70–72]. Also, mammalian cells express multiple isoforms of MBD2 and MBD3 [2]. MBD2a, the most abundant isoform of MBD2, contains a long (approximately 150 amino acid) N-terminal region only found in mammalian species. This region contains a positively charged glycine-arginine (GR) repeat that likely contributes to DNA binding and recruits additional cofactors (PRMT5 and MEP50) to NuRD [9]. However, the GR region is not required for methylation-dependent gene silencing by MBD2. The MBD2b isoform, which lacks the N-terminal extension, shares 76% identity with MBD3a isoform. Both isoforms contain an N-terminal MBD (77% identity) and C-terminal coiled-coil (85% identity) domains separated by the intrinsically disordered region (76% identity).
Embryonic stem cells express additional unique isoforms of MBD2 and MBD3. The MBD2c isoform is a splice variant that lacks the IDR and coiled-coil domains. MBD2c binds methylated DNA but does not recruit the NuRD complex, acting as a dominant-negative inhibitor of methylation-dependent gene silencing by NuRD [73]. The MBD3b isoform lacks a full MBD yet retains the IDR and coiled-coil domains [74]. Therefore, the MBD2c and MBD3b isoforms found in embryonic stem cells appear to disrupt the association of NuRD with methylated DNA. The precise role of the two proteins during embryonic development and pluripotency, though, remains an active area of investigation and hotly debated [74–78]. Early studies indicated that MBD3 promotes lineage commitment [74], while more recent research has indicated that MBD3 either promotes or blocks pluripotency and reprogramming [76, 77]. These confounding results likely reflect, at least in part, lack of a detailed molecular model for gene regulation by NuRD complexes.
After differentiation and development, most adult tissues express MBD2a, whereas only testis and brain tissues continue to express MBD3 [79]. This pattern of expression implies that methylation-independent functions of NuRD dominate in undifferentiated tissues while methylation-dependent functions play an essential role during cellular differentiation. However, until we understand the physical connection between NuRD recruitment and changes in gene expression, the biological phenotypes arising from manipulating NuRD expression are difficult to explain fully.
The MBD selectively recognizes symmetric mCpG dinucleotides
The structure of the DNA binding domain of MBDs bound to a variety of DNA substrates have been solved by x-ray crystallography and nuclear magnetic resonance spectroscopy [12, 80–88]. This domain comprises a 3–4 stranded β-sheet, a single α-helix, and a C-terminal loop. The sheet forms the base of the protein with a finger-like projection of two central β-strands that extends down the major groove of DNA (Figure 4d). The helix and C-terminal loop pack against this sheet to form the hydrophobic core of the domain. A conserved structural motif formed by two arginine and tyrosine residues protrudes from the base of the domain to interact with DNA. As depicted in Figure 4e, the two arginine sidechains form bidentate hydrogen bonds with symmetrically related guanosine bases of the CpG. This arrangement allows the arginine sidechain to form a stair motif cation-π interaction with the adjacent methyl-cytosine base [89]. The aliphatic portion of the arginine sidechains packs against the methyl groups of the symmetrically related methyl-cytosines (mC), which together with the cation-π interaction provides approximately 5-fold selectivity for mCpG [10, 12, 86]. The hydroxyl group of the conserved tyrosine sidechain points towards the methyl group of one mC and hydrogen bonds with structured water that surrounds this methyl group. The tyrosine hydroxyl provides the bulk of methylation selectivity, contributing an additional 10–20-fold binding preference for mCpG over CpG [10, 12, 85, 86]. Hence, the combination of these favorable interactions involving both the arginine and tyrosine residues leads to a net 100-fold selectivity for symmetrically methylated mCpGs.
The mC-arginine-guanosine interaction motif has been identified across a much larger group of DNA binding domains that recognize methyl-cytosines in one strand of DNA [90]. Notably, though, thymine has a methyl group in the major groove of DNA analogous to that of mC. Hence, the mC-arginine-guanosine triad can be replaced by thymine-arginine-guanosine, especially for those transcription factors that recognize the mC in only one strand of DNA [90]. In contrast, the symmetry of the two arginine residues in an MBD drives selectivity for symmetrically related guanosines, which, because of base-pairing, are found only in cytosine-guanosine dinucleotides. While other DNA binding domain can recognize one or more methyl-cytosines, the MBDs uniquely recognize the symmetrically related methyl-cytosines in mCpG dinucleotides.
Studies have shown that MBDs can adapt to alternative binding sequences that include a thymine-guanosine dinucleotide. For example, MeCP2 can bind non-CpG sequences such as mCAC which accumulate in neuronal tissues [91, 92]. These alternative binding sequences replace the second guanosine with adenosine, which cannot form a bidentate hydrogen bond with arginine. Therefore, one arginine residue must interact with the phosphate backbone or adjacent DNA bases to accommodate these alternative binding sequences. Nonetheless, MBDs generally show the highest binding affinity for mCpG dinucleotides and cellular studies have found that MBDs localize to methylated CpG islands [10, 71, 91]. Whether mCs in a non-CpG context are functional targets for MBDs, MeCP2 in particular, remains an exciting and active area of investigation.
MBD2 vs. MBD3
The mCpG DNA binding motif (RRY) is highly conserved across the MBD family of proteins, modifications of which predict a lack of methylation specificity. Unlike vertebrates which have both an MBD2 and MBD3, invertebrate species contain only a single MBD2/3 isoform. In species that show evidence of DNA methylation, the MBD2/3 retains the critical RRY motif and selectively binds mCpGs [1, 93–98]. In contrast, those species that lack DNA methylation, such as D. melanogaster, typically have MBD2/3 proteins with modifications that either eliminate or modify this motif [1, 99–101]. In either case, MBD2/3 retains both the IDR and coiled-coil domains critical to forming NuRD, which suggests that NuRD has a critical role in all multicellular organisms that is independent of DNA methylation.
The duplication event that generated MBD3 led to the replacement of the conserved tyrosine by phenylalanine in almost all vertebrate species studied to date (with the notable exception of X. laevis) [2, 3, 6]. This modification contributes to a reduction in overall DNA binding affinity and substantial loss of methylation specificity, which suggests sub-specialization of the complex in vertebrate biology. This sub-specialization correlates with a significant change in the pattern and extent of CpG methylation, and with the development CpG islands associated with gene promoters [93, 102–104].
In studying the MBDs bound to DNA by NMR, we have noted that several backbone and sidechain resonances show substantial chemical-shift differences between methylated and unmethylated DNA substrates. These chemical-shift differences are consistent across all MBDs we have studied to date, including MeCP2, MBD2, MBD4, and an invertebrate MBD2/3 from the freshwater sponge Ephydatia muelleri [1, 12, 85, 86, 92]. Importantly, we found that even MBD3 shows chemical-shift differences for these same resonances, moving in the same direction but not to the same extent when bound to methylated DNA [12]. We demonstrated that these shifts in MBD3 reflect rapid exchange between methylation-specific and non-specific binding modes. Consequently, peak positions can be used to calculate the fraction of MBD3 localized to the mCpG site. Using this information, we showed that the fraction bound to the methylated site depends on the density and relative affinity for mCpGs. Therefore, MBD3 shows structural recognition and preference for a methylated site even though it binds methylated DNA with significantly decreased overall affinity. In addition, we found that MBD3 did not preferentially bind hydroxymethylated DNA, showing identical chemical shifts for both hydroxymethylated and un-methylated substrates. This latter observation, along with binding affinity analyses by several groups [10, 12], provide strong structural evidence that MBD3 does not localize the NuRD complex to hydroxymethylated DNA through its MBD, although previous research has suggested otherwise [105].
These observations suggest to us that the behavior of an MBD when bound to DNA is more relevant to function than the absolute binding affinity and selectivity. Both MBD2 and MBD3 function to direct NuRD complexes to specific regions in the genome to modify chromatin compaction through nucleosome remodeling and deacetylation. If the MBD restricts the mobility of NuRD due to dense methylation of a CpG island, it should modify the ultimate positioning and density of the remodeled nucleosomes. To address this hypothesis, we have been studying the behavior of MBDs on methylated and unmethylated substrates using single-molecule approaches.
MBD2 sliding and bending DNA
In recent work, we developed an in vitro substrate to study MBD2 and MBD3 binding to DNA by atomic force microscopy and single-molecule fluorescence [106]. We inserted a large fragment of the DAPK1 CGI, a known target for MBD2 [107], into a vector completely devoid of CpGs. A surprising result came from atomic force microscopy analysis of MBD2 bound to this substrate. MBD2 induced an acute bend of DNA, especially for methylated CpG rich substrates. This bending has not been observed by NMR or x-ray crystallography studies of MBD2 bound to small DNA fragments, which suggests that it requires a more extended substrate. While the biological significance of this bending is not clear, the correlation between bending angle and methylation of DNA raises the possibility that it contributes to chromatin compaction and gene silencing.
Studies of MBD2 bound to DNA tightropes constructed from the same substrate show that MBD2 rapidly diffuses across unmethylated DAPK1 CGI, but when methylated MBD2 binds statically for a remarkably long time (1–2 minutes). Hence, methylation greatly restricts the dynamic mobility of MBD2 on a large scale, even though NMR studies indicate relatively rapid exchange between methylated sites separated by ~10 base pairs [86]. Ongoing studies with MBD3 show that it diffuses rapidly across CpG rich DNA even when fully methylated. The latter correlates with the rapid exchange between methylated and unmethylated binding modes we observed by NMR studies on small DNA substrates [12]. Together, these results fit with cellular studies which have shown that both MBD2 and MBD3 localize to unmethylated CpG rich regions within the genome while MBD2 more exclusively localizes to methylated CpG rich regions [37, 70, 71]. Knockdown of MBD2 leads to increased expression of associated genes while knockdown of MBD3 correlates with lower expression levels. Therefore, MBD2-NuRD is strongly associated with methylated DNA and silencing of gene expression, while MBD3-NuRD is associated with unmethylated CpG islands and can be associated with active transcription.
The biological function of NuRD
Despite the extensive structural and biophysical analyses over the past decade, the biological role of NuRD remains enigmatic. Early studies showing that MBD2-NuRD selectively binds methylated DNA, contains histone deacetylase activity and is associated with gene silencing lead to the conclusion that NuRD functions solely as a transcriptional repressor. However, more recent studies have begun to question that conclusion.
Several groups have determined the localization of MBD2- and MBD3-NuRD complexes and correlated their localization with gene expression. In studies by Rainer Renkawitz’s group, a green fluorescent protein (GFP) fused MBD2 localized to highly methylated sequences and was associated with repressed genes [37]. In contrast, GFP-MBD3 localized to unmethylated CpG rich regions and was associated with expressed genes, although the number of MBD3 binding sites was low (490 in total) as compared to MBD2 (8200 sites). Also, they found that LacI fused MBD2 dramatically compacted a LacO array in living cells, whereas LacI-MBD3 did not. Paul Wade’s group identified a much larger number of MBD3 binding sites in breast cancer cell lines (9310 sites in MCF-7 cells) using ChIP-seq of endogenous MBD3 [70]. Similarly, they found that MBD3 localized to active CpG rich promoters and was associated with a reduction in nucleosome occupancy near the transcriptional start site.
Finally, Stunnenberg’s group stably expressed a tagged MBD2 to study localization by CHIP-seq in the MCF-7 breast cancer cell line [71]. They found that MBD2 localized to heavily methylated and CpG rich loci, the majority of which were within promoters and exons. Interestingly, they identified a subset in which MBD2 localized downstream of active promoters.
More recently, Brian Hendrich’s laboratory developed a novel inducible MBD3 system in embryonic stem cells to follow MBD3 localization and chromatin remodeling over time [108]. They found that MBD3 rapidly induces nucleosome remodeling within a few hours of induction, which altered local transcription factor binding and gene expression. They could show by micrococcal nuclease digestion that MBD3 locally repositioned nucleosomes. Importantly, whether MBD3 induced remodeling increased or reduced transcription depended on the genetic context. The gene responses were relatively modest (2–3 fold), indicating that MBD3-NuRD functions to dampen or augment expression. Together, these studies show that MBD2-NuRD and MBD3-NuRD both localize to CpG rich regions of the genome, but that MBD2 dominates at methylated regions. The NuRD complex primarily functions by altering nucleosome positioning, while the biological effects depend on the local context and method of recruitment [53, 109–111].
NuRD complex and fetal hemoglobin regulation
The role of DNA methylation in the transcriptional switch from fetal to adult hemoglobin expression has been studied for more than 30 years [112–118]. Inhibition of DNA methylation increases the expression of fetal hemoglobin in adult erythroid cells, suggesting that methylation contributes to gene silencing. However, the γ-globin locus in humans does not contain a CpG island, indicating that silencing involves methylation of only a few CpGs or that methylation indirectly regulates γ-globin expression. In studying methylation-dependent gene silencing of globin, the laboratory of Gordon Ginder isolated an MBD2-NuRD complex that bound to the embryonic globin locus in primary chicken erythrocytes and contributed to methylation-dependent gene silencing [117, 119]. Importantly, disruption of MBD2-NuRD in mouse models of human β-globin gene regulation [117] as well as primary human cells and cell lines [66, 120] leads to a dramatic increase in the expression of fetal hemoglobin. This gene response exceeds the typical 2–3-fold changes associated with genetic disruption of NuRD, suggesting that the NuRD complex plays a particularly important role in globin regulation.
Furthermore, work by Stuart Orkin’s group has shown that Bcl11A binds directly to the γ-globin locus and recruits NuRD to silence gene expression [121–125]. Similarly, Takahiro Maeda’s group found that the ZBTB7A transcription factor (also known as LRF) binds to the γ-globin locus and recruits NuRD to silence gene expression [50]. Therefore, disrupting NuRD formation appears to be a potential strategy to restore fetal hemoglobin expression for therapy of sickle cell anemia and other β-hemoglobinopathies.
Recently, Daniel Bauer’s group used CRISPR screen to identify potential targets within the NuRD complex [55]. The results of those studies showed that the proteins and domains involved in forming NuRD are critical for fetal hemoglobin silencing. Both histone deacetylase and chromatin remodeling functions of the complex are necessary for gene silencing. Also, recent work by Ginder’s group showed that mutations of MBD2 that disrupt recruitment of either the HDCC or CHD4 portions inhibited silencing of fetal hemoglobin [66]. Therefore, fetal hemoglobin silencing requires a functional and complete NuRD complex.
A model of MBD2 and MBD3 function within NuRD
The structural and biological experiments described above raise the possibility that the functional differences between MBD2-NuRD and MBD3-NuRD reflect the behavior of the MBDs on densely methylated and unmethylated CpG islands [106]. As depicted in Figure 5, both complexes freely remodel nucleosomes over unmethylated CpGs. In contrast, MBD2-NuRD restricts mobility and hence drives accumulation of nucleosomes on methylated CpG islands, while MBD3-NuRD does not. Therefore, the two complexes have opposing actions on methylated promoters and enhancers. This model fits with genomic localization studies [37, 70, 71] as well as the expression patters of the two proteins [79]. Embryonic stem cells express MBD3 where it can remodel chromatin independent of DNA methylation status [79, 126, 127]. During the differentiation of embryonic stem cells, a subset of tissue-specific genes become methylated and silenced [128, 129]. Hence, MBD2 expression correlates with methylation and silencing of these genes. Of note, this model only addresses NuRD function when localized to DNA through the MBD components and does not necessarily apply when transcription factors recruit NuRD through the associated RBBP proteins [38]. In the latter case, the function likely depends on the location of the transcription factor binding site, the stability of the interaction with DNA, and whether the complex can interact with nearby methylated CpGs or other chromatin-associated factors.
Figure 5. A model of nucleosome remodeling by the NuRD complex.
A schematic diagram shows a simple model of NuRD function on CpG rich regions of the genome. Given the propensity for MBD2 to bind stably and with high affinity to methylated CpG rich DNA, MBD2-NuRD should reposition nucleosomes away from unmethylated (gray and open circles) and towards densely methylated (black and filled circles) CpG rich regions of the genome. In contrast, MBD3-NuRD would not show the same tendency.
Future Directions
Our understanding of NuRD structure and function has expanded considerably over the past twenty years. Crystal structures of the MTA/HDAC and RBBP/MTA have given us a firm understanding of the histone deacetylase sub-complex structure. However, the molecular details of how the deacetylase complex associates with chromatin and interacts with histone tails remains mostly unknown. The HDACs bind the MTA protein immediately adjacent to its N-terminal BAH domain. Homologous BAH domains have been shown to bind histone tails, and this arrangement suggests that the BAH domain of MTAs could bind and present a histone tail to HDACs for deacetylation. However, no direct evidence has shown whether the BAH domain directly binds to the histones or contributes to deacetylation. Alternatively, this same domain could be involved in protein-protein interactions with the MBDs. In support of the latter, crosslinking studies have indicated that the MBD and BAH domains are close [20, 23].
Furthermore, even though we have shown that the MBD2-IDR binds HDAC/MTA, we still do not know whether MBDs directly bind the MTAs, HDACs, or both. Therefore, structural analyses of the histone core complex, including one of the MBDs, bound to nucleosome core particles would address many of these open questions and provide mechanistic insight into histone deacetylation by NuRD. With the rapid development of cryo-electron microscopy over the past decade, we anticipate that an atomic resolution structure of this complex should be attainable soon.
We know much less about the interaction between the GATAD2 and CHD proteins. The structure of the C-terminal region of CHD4 remains unknown, in part because that region has been difficult to purify in isolation. Also, the GATA-type Zn-finger in the GATAD2 proteins (CR2) has not been studied in detail. In unpublished NMR studies, we found that this Zn-finger remains poorly structured in isolation. Therefore, we anticipate that detailed structural analysis of this region requires co-expression of the CHD and GATAD2 domains as a complex. Studies by Joel Mackay’s group have shown that the CHDs loosely associate with the rest of NuRD, which indicates that the CHD-GATAD2 interaction may be difficult to purify and study in isolation [59]. Nonetheless, recruitment of CHDs to NuRD is critical for function, and determining the structure of this complex should provide needed insight into the molecular mechanism of chromatin remodeling.
Ultimately, structural analyses of the NuRD complex strive to provide functional insight into its role in gene regulation. To accomplish this goal requires a better understanding of how the full complex remodels nucleosomes and how this remodeling depends on DNA methylation and co-recruitment by transcription factors. Dynamic flexibility of several of the core components likely contributes to this remodeling function but also impedes high-resolution structural analysis of the entire complex. Nonetheless, studies by both the Mackay group and Schwabe group have used a combination of electron microscopy and chemical crosslinking to build models of large portions of the complex [23, 26]. These studies suggest a pathway forward to solving the intact, full NuRD structure using a combination of techniques and careful modeling based on the extensive datasets available in the literature.
However, these types of models do not directly address how the NuRD complex functions to remodel chromatin. As discussed above, we do not understand how methylation impacts remodeling, nor do we understand how the remodeling and deacetylation activities function together to modify chromatin compaction and gene expression. Does methylation of specific CpG sites dictate the positioning of nucleosomes, or does it merely function as a barrier to remodeling leading to more condensed nucleosomes arrays? Also, NuRD can be recruited to chromatin through a variety of co-factors which may or may not impact how the complex repositions nucleosomes. Does the transcription factor binding site dictate where NuRD localizes a nearby nucleosome? Alternatively, does the inherent flexibility of NuRD allow it to remodel over a much larger distance even while tethered to specific transcription factor? Solving these questions will provide needed insight into the function of NuRD and lead to a better understanding of epigenetics and transcription regulation.
Abbreviations:
- MBD2(3)
methyl-CpG binding domain protein 2(3)
- CpGs
cytosine-guanosine dinucleotides
- NuRD
Nucleosome Remodeling and Deacetylase
- MeCP1(2)
methyl-CpG binding protein 1(2)
- MTA1(2,3)
metastasis tumor-associated protein 1(2,3)
- HDAC1(2)
histone deacetylase 1(2)
- RBBP4(7)
retinoblastoma binding protein 4(7)
- GATAD2A(B)
GATA zinc finger Domain 2A(B)
- CHD3(4,5)
Chromodomain Helicase DNA Binding Protein 3(4,5)
- CDK2AP1
cyclin-dependent kinase 2 associated protein 1
- HDCC
histone deacetylase core sub-complex
- PHD
plant homeodomain
- HMG
high mobility group
- SALL4
spalt-like transcription factor 4
- FOG1
friend of GATA protein 1
- BCL11a
B-cell lymphoma/leukemia 11a
- ZMYND8
zinc finger MYND-type containing 8
- Ins(1,4,5,6)P4
inositol tetraphosphate
- GR
glycine-arginine
Footnotes
Declaration of Interest: none
Amino acid numbers refer to human proteins throughout the text unless otherwise indicated
References
- [1].Cramer JM, Pohlmann D, Gomez F, Mark L, Kornegay B, Hall C, et al. Methylation specific targeting of a chromatin remodeling complex from sponges to humans. Sci Rep. 2017;7:40674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hendrich B, Tweedie S. The methyl-CpG binding domain and the evolving role of DNA methylation in animals. Trends Genet. 2003;19:269–77. [DOI] [PubMed] [Google Scholar]
- [4].Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, et al. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet. 1999;23:58–61. [DOI] [PubMed] [Google Scholar]
- [5].Wade PA, Jones PL, Vermaak D, Wolffe AP. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr Biol. 1998;8:843–6. [DOI] [PubMed] [Google Scholar]
- [6].Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, Wolffe AP. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet. 1999;23:62–6. [DOI] [PubMed] [Google Scholar]
- [7].Gnanapragasam MN, Scarsdale JN, Amaya ML, Webb HD, Desai MA, Walavalkar NM, et al. p66Alpha-MBD2 coiled-coil interaction and recruitment of Mi-2 are critical for globin gene silencing by the MBD2-NuRD complex. Proc Natl Acad Sci U S A. 2011;108:7487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Desai MA, Webb HD, Sinanan LM, Scarsdale JN, Walavalkar NM, Ginder GD, et al. An intrinsically disordered region of methyl-CpG binding domain protein 2 (MBD2) recruits the histone deacetylase core of the NuRD complex. Nucleic Acids Res. 2015;43:3100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Le Guezennec X, Vermeulen M, Brinkman AB, Hoeijmakers WA, Cohen A, Lasonder E, et al. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol Cell Biol. 2006;26:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hashimoto H, Liu Y, Upadhyay AK, Chang Y, Howerton SB, Vertino PM, et al. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 2012;40:4841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hendrich B, Guy J, Ramsahoye B, Wilson VA, Bird A. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 2001;15:710–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cramer JM, Scarsdale JN, Walavalkar NM, Buchwald WA, Ginder GD, Williams DC Jr. Probing the dynamic distribution of bound states for methylcytosine-binding domains on DNA. J Biol Chem. 2014;289:1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meehan RR, Lewis JD, McKay S, Kleiner EL, Bird AP. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989;58:499–507. [DOI] [PubMed] [Google Scholar]
- [14].Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–14. [DOI] [PubMed] [Google Scholar]
- [15].Nan X, Meehan RR, Bird A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993;21:4886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang Y, LeRoy G, Seelig H-P, Lane WS, Reinberg D. The Dermatomyositis-Specific Autoantigen Mi2 Is a Component of a Complex Containing Histone Deacetylase and Nucleosome Remodeling Activities. Cell. 1998;95:279–89. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spruijt CG, Bartels SJ, Brinkman AB, Tjeertes JV, Poser I, Stunnenberg HG, et al. CDK2AP1/DOC-1 is a bona fide subunit of the Mi-2/NuRD complex. Mol Biosyst. 2010;6:1700–6. [DOI] [PubMed] [Google Scholar]
- [19].Smits AH, Jansen PW, Poser I, Hyman AA, Vermeulen M. Stoichiometry of chromatin-associated protein complexes revealed by label-free quantitative mass spectrometry-based proteomics. Nucleic Acids Res. 2013;41:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kloet SL, Baymaz HI, Makowski M, Groenewold V, Jansen PW, Berendsen M, et al. Towards elucidating the stability, dynamics and architecture of the nucleosome remodeling and deacetylase complex by using quantitative interaction proteomics. FEBS J. 2015;282:1774–85. [DOI] [PubMed] [Google Scholar]
- [21].Alqarni SS, Murthy A, Zhang W, Przewloka MR, Silva AP, Watson AA, et al. Insight into the architecture of the NuRD complex: structure of the RbAp48-MTA1 subcomplex. J Biol Chem. 2014;289:21844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schmidberger JW, Sharifi Tabar M, Torrado M, Silva AP, Landsberg MJ, Brillault L, et al. The MTA1 subunit of the nucleosome remodeling and deacetylase complex can recruit two copies of RBBP4/7. Protein Sci. 2016;25:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Torrado M, Low JKK, Silva APG, Schmidberger JW, Sana M, Sharifi Tabar M, et al. Refinement of the subunit interaction network within the nucleosome remodelling and deacetylase (NuRD) complex. FEBS J. 2017;284:4216–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV, et al. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell. 2013;51:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Millard CJ, Fairall L, Schwabe JW. Towards an understanding of the structure and function of MTA1. Cancer Metastasis Rev. 2014;33:857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Millard CJ, Varma N, Saleh A, Morris K, Watson PJ, Bottrill AR, et al. The structure of the core NuRD repression complex provides insights into its interaction with chromatin. Elife. 2016;5:e13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brackertz M, Boeke J, Zhang R, Renkawitz R. Two highly related p66 proteins comprise a new family of potent transcriptional repressors interacting with MBD2 and MBD3. J Biol Chem. 2002;277:40958–66. [DOI] [PubMed] [Google Scholar]
- [28].Wang HB, Zhang Y. Mi2, an auto-antigen for dermatomyositis, is an ATP-dependent nucleosome remodeling factor. Nucleic Acids Res. 2001;29:2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mansfield RE, Musselman CA, Kwan AH, Oliver SS, Garske AL, Davrazou F, et al. Plant homeodomain (PHD) fingers of CHD4 are histone H3-binding modules with preference for unmodified H3K4 and methylated H3K9. J Biol Chem. 2011;286:11779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Musselman CA, Ramirez J, Sims JK, Mansfield RE, Oliver SS, Denu JM, et al. Bivalent recognition of nucleosomes by the tandem PHD fingers of the CHD4 ATPase is required for CHD4-mediated repression. Proc Natl Acad Sci U S A. 2012;109:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Silva AP, Ryan DP, Galanty Y, Low JK, Vandevenne M, Jackson SP, et al. The N-terminal Region of Chromodomain Helicase DNA-binding Protein 4 (CHD4) Is Essential for Activity and Contains a High Mobility Group (HMG) Box-like-domain That Can Bind Poly(ADP-ribose). J Biol Chem. 2016;291:924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuo K, Shintani S, Tsuji T, Nagata E, Lerman M, McBride J, et al. p12(DOC-1), a growth suppressor, associates with DNA polymerase alpha/primase. FASEB J. 2000;14:1318–24. [DOI] [PubMed] [Google Scholar]
- [33].Shintani S, Ohyama H, Zhang X, McBride J, Matsuo K, Tsuji T, et al. p12(DOC-1) is a novel cyclin-dependent kinase 2-associated protein. Mol Cell Biol. 2000;20:6300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ertekin A, Aramini JM, Rossi P, Leonard PG, Janjua H, Xiao R, et al. Human cyclin-dependent kinase 2-associated protein 1 (CDK2AP1) is dimeric in its disulfide-reduced state, with natively disordered N-terminal region. J Biol Chem. 2012;287:16541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mohd-Sarip A, Teeuwssen M, Bot AG, De Herdt MJ, Willems SM, Baatenburg de Jong RJ, et al. DOC1-Dependent Recruitment of NURD Reveals Antagonism with SWI/SNF during Epithelial-Mesenchymal Transition in Oral Cancer Cells. Cell Rep. 2017;20:61–75. [DOI] [PubMed] [Google Scholar]
- [36].Yao YL, Yang WM. The metastasis-associated proteins 1 and 2 form distinct protein complexes with histone deacetylase activity. J Biol Chem. 2003;278:42560–8. [DOI] [PubMed] [Google Scholar]
- [37].Gunther K, Rust M, Leers J, Boettger T, Scharfe M, Jarek M, et al. Differential roles for MBD2 and MBD3 at methylated CpG islands, active promoters and binding to exon sequences. Nucleic Acids Res. 2013;41:3010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Menafra R, Stunnenberg HG. MBD2 and MBD3: elusive functions and mechanisms. Front Genet. 2014;5:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spruijt CG, Luijsterburg MS, Menafra R, Lindeboom RG, Jansen PW, Edupuganti RR, et al. ZMYND8 Co-localizes with NuRD on Target Genes and Regulates Poly(ADP-Ribose)-Dependent Recruitment of GATAD2A/NuRD to Sites of DNA Damage. Cell Rep. 2016;17:783–98. [DOI] [PubMed] [Google Scholar]
- [40].Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, et al. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004;119:75–86. [DOI] [PubMed] [Google Scholar]
- [41].Jaye DL, Iqbal J, Fujita N, Geigerman CM, Li S, Karanam S, et al. The BCL6-associated transcriptional co-repressor, MTA3, is selectively expressed by germinal centre B cells and lymphomas of putative germinal centre derivation. J Pathol. 2007;213:106–15. [DOI] [PubMed] [Google Scholar]
- [42].Nitarska J, Smith JG, Sherlock WT, Hillege MM, Nott A, Barshop WD, et al. A Functional Switch of NuRD Chromatin Remodeling Complex Subunits Regulates Mouse Cortical Development. Cell Rep. 2016;17:1683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vermeulen M, Hubner NC, Mann M. High confidence determination of specific protein-protein interactions using quantitative mass spectrometry. Curr Opin Biotechnol. 2008;19:331–7. [DOI] [PubMed] [Google Scholar]
- [44].Hong W, Nakazawa M, Chen YY, Kori R, Vakoc CR, Rakowski C, et al. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 2005;24:2367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lejon S, Thong SY, Murthy A, AlQarni S, Murzina NV, Blobel GA, et al. Insights into association of the NuRD complex with FOG-1 from the crystal structure of an RbAp48.FOG-1 complex. J Biol Chem. 2011;286:1196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moody RR, Lo MC, Meagher JL, Lin CC, Stevers NO, Tinsley SL, et al. Probing the interaction between the histone methyltransferase/deacetylase subunit RBBP4/7 and the transcription factor BCL11A in epigenetic complexes. J Biol Chem. 2018;293:2125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lu J, Jeong HW, Kong N, Yang Y, Carroll J, Luo HR, et al. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS One. 2009;4:e5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gao C, Dimitrov T, Yong KJ, Tatetsu H, Jeong HW, Luo HR, et al. Targeting transcription factor SALL4 in acute myeloid leukemia by interrupting its interaction with an epigenetic complex. Blood. 2013;121:1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saathoff H, Brofelth M, Trinh A, Parker BL, Ryan DP, Low JK, et al. A peptide affinity reagent for isolating an intact and catalytically active multi-protein complex from mammalian cells. Bioorg Med Chem. 2015;23:960–5. [DOI] [PubMed] [Google Scholar]
- [50].Masuda T, Wang X, Maeda M, Canver MC, Sher F, Funnell AP, et al. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science. 2016;351:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaltenbrun E, Greco TM, Slagle CE, Kennedy LM, Li T, Cristea IM, et al. A Gro/TLE-NuRD corepressor complex facilitates Tbx20-dependent transcriptional repression. J Proteome Res. 2013;12:5395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gong F, Clouaire T, Aguirrebengoa M, Legube G, Miller KM. Histone demethylase KDM5A regulates the ZMYND8-NuRD chromatin remodeler to promote DNA repair. J Cell Biol. 2017;216:1959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Waldron L, Steimle JD, Greco TM, Gomez NC, Dorr KM, Kweon J, et al. The Cardiac TBX5 Interactome Reveals a Chromatin Remodeling Network Essential for Cardiac Septation. Dev Cell. 2016;36:262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Walavalkar NM, Gordon N, Williams DC Jr. Unique features of the anti-parallel, heterodimeric coiled-coil interaction between methyl-cytosine binding domain 2 (MBD2) homologues and GATA zinc finger domain containing 2A (GATAD2A/p66alpha). J Biol Chem. 2013;288:3419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sher F, Hossain M, Seruggia D, Schoonenberg VAC, Yao Q, Cifani P, et al. Rational targeting of a NuRD subcomplex guided by comprehensive in situ mutagenesis. Nat Genet. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Watson PJ, Millard CJ, Riley AM, Robertson NS, Wright LC, Godage HY, et al. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nat Commun. 2016;7:11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mattingly RR, Stephens LR, Irvine RF, Garrison JC. Effects of transformation with the v-src oncogene on inositol phosphate metabolism in rat-1 fibroblasts. D-myo-inositol 1,4,5,6-tetrakisphosphate is increased in v-src-transformed rat-1 fibroblasts and can be synthesized from D-myo-inositol 1,3,4-trisphosphate in cytosolic extracts. J Biol Chem. 1991;266:15144–53. [PubMed] [Google Scholar]
- [59].Low JK, Webb SR, Silva AP, Saathoff H, Ryan DP, Torrado M, et al. CHD4 Is a Peripheral Component of the Nucleosome Remodeling and Deacetylase Complex. J Biol Chem. 2016;291:15853–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Murzina NV, Pei XY, Zhang W, Sparkes M, Vicente-Garcia J, Pratap JV, et al. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure. 2008;16:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brasen C, Dorosz J, Wiuf A, Boesen T, Mirza O, Gajhede M. Expression, purification and characterization of the human MTA2-RBBP7 complex. Biochim Biophys Acta Proteins Proteom. 2017;1865:531–8. [DOI] [PubMed] [Google Scholar]
- [62].Kasinath V, Faini M, Poepsel S, Reif D, Feng XA, Stjepanovic G, et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Liu Z, Li F, Zhang B, Li S, Wu J, Shi Y. Structural basis of plant homeodomain finger 6 (PHF6) recognition by the retinoblastoma binding protein 4 (RBBP4) component of the nucleosome remodeling and deacetylase (NuRD) complex. J Biol Chem. 2015;290:6630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schmitges FW, Prusty AB, Faty M, Stutzer A, Lingaraju GM, Aiwazian J, et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011;42:330–41. [DOI] [PubMed] [Google Scholar]
- [65].Kwan AH, Gell DA, Verger A, Crossley M, Matthews JM, Mackay JP. Engineering a protein scaffold from a PHD finger. Structure. 2003;11:803–13. [DOI] [PubMed] [Google Scholar]
- [66].Yu X, Azzo A, Bilinovich SM, Li X, Dozmorov M, Kurita R, et al. Disruption of the MBD2-NuRD complex but not MBD3-NuRD induces high level HbF expression in human erythroid cells. Haematologica. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang T, Wei G, Millard CJ, Fischer R, Konietzny R, Kessler BM, et al. A variant NuRD complex containing PWWP2A/B excludes MBD2/3 to regulate transcription at active genes. Nature Communications. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Link S, Spitzer RMM, Sana M, Torrado M, Volker-Albert MC, Keilhauer EC, et al. PWWP2A binds distinct chromatin moieties and interacts with an MTA1-specific core NuRD complex. Nat Commun. 2018;9:4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang W, Aubert A, Gomez de Segura JM, Karuppasamy M, Basu S, Murthy AS, et al. The Nucleosome Remodeling and Deacetylase Complex NuRD Is Built from Preformed Catalytically Active Sub-modules. J Mol Biol. 2016;428:2931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shimbo T, Du Y, Grimm SA, Dhasarathy A, Mav D, Shah RR, et al. MBD3 localizes at promoters, gene bodies and enhancers of active genes. PLoS Genet. 2013;9:e1004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Menafra R, Brinkman AB, Matarese F, Franci G, Bartels SJ, Nguyen L, et al. Genome-wide binding of MBD2 reveals strong preference for highly methylated loci. PLoS One. 2014;9:e99603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Baubec T, Ivanek R, Lienert F, Schubeler D. Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell. 2013;153:480–92. [DOI] [PubMed] [Google Scholar]
- [73].Lu Y, Loh YH, Li H, Cesana M, Ficarro SB, Parikh JR, et al. Alternative splicing of MBD2 supports self-renewal in human pluripotent stem cells. Cell Stem Cell. 2014;15:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol. 2006;8:285–92. [DOI] [PubMed] [Google Scholar]
- [75].Kaji K, Nichols J, Hendrich B. Mbd3, a component of the NuRD co-repressor complex, is required for development of pluripotent cells. Development. 2007;134:1123–32. [DOI] [PubMed] [Google Scholar]
- [76].dos Santos RL, Tosti L, Radzisheuskaya A, Caballero IM, Kaji K, Hendrich B, et al. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell. 2014;15:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. [DOI] [PubMed] [Google Scholar]
- [78].Mor N, Rais Y, Sheban D, Peles S, Aguilera-Castrejon A, Zviran A, et al. Neutralizing Gatad2a-Chd4-Mbd3/NuRD Complex Facilitates Deterministic Induction of Naive Pluripotency. Cell Stem Cell. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wood KH, Johnson BS, Welsh SA, Lee JY, Cui Y, Krizman E, et al. Tagging methyl-CpG-binding domain proteins reveals different spatiotemporal expression and supports distinct functions. Epigenomics. 2016;8:455–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ho KL, McNae IW, Schmiedeberg L, Klose RJ, Bird AP, Walkinshaw MD. MeCP2 binding to DNA depends upon hydration at methyl-CpG. Mol Cell. 2008;29:525–31. [DOI] [PubMed] [Google Scholar]
- [81].Wakefield RI, Smith BO, Nan X, Free A, Soteriou A, Uhrin D, et al. The solution structure of the domain from MeCP2 that binds to methylated DNA. J Mol Biol. 1999;291:1055–65. [DOI] [PubMed] [Google Scholar]
- [82].Heitmann B, Maurer T, Weitzel JM, Stratling WH, Kalbitzer HR, Brunner E. Solution structure of the matrix attachment region-binding domain of chicken MeCP2. Eur J Biochem. 2003;270:3263–70. [DOI] [PubMed] [Google Scholar]
- [83].Ohki I, Shimotake N, Fujita N, Nakao M, Shirakawa M. Solution structure of the methyl-CpG-binding domain of the methylation-dependent transcriptional repressor MBD1. EMBO J. 1999;18:6653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ohki I, Shimotake N, Fujita N, Jee J, Ikegami T, Nakao M, et al. Solution structure of the methyl-CpG binding domain of human MBD1 in complex with methylated DNA. Cell. 2001;105:487–97. [DOI] [PubMed] [Google Scholar]
- [85].Scarsdale JN, Webb HD, Ginder GD, Williams DC Jr. Solution structure and dynamic analysis of chicken MBD2 methyl binding domain bound to a target-methylated DNA sequence. Nucleic Acids Res. 2011;39:6741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Walavalkar NM, Cramer JM, Buchwald WA, Scarsdale JN, Williams DC Jr. Solution structure and intramolecular exchange of methyl-cytosine binding domain protein 4 (MBD4) on DNA suggests a mechanism to scan for mCpG/TpG mismatches. Nucleic Acids Res. 2014;42:11218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Otani J, Arita K, Kato T, Kinoshita M, Kimura H, Suetake I, et al. Structural basis of the versatile DNA recognition ability of the methyl-CpG binding domain of methyl-CpG binding domain protein 4. J Biol Chem. 2013;288:6351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liu K, Lei M, Wu Z, Gan B, Cheng H, Li Y, et al. Structural analyses reveal that MBD3 is a methylated CG binder. FEBS J. 2019;286:3240–54. [DOI] [PubMed] [Google Scholar]
- [89].Zou X, Ma W, Solov’yov IA, Chipot C, Schulten K. Recognition of methylated DNA through methyl-CpG binding domain proteins. Nucleic Acids Res. 2012;40:2747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ren R, Horton JR, Zhang X, Blumenthal RM, Cheng X. Detecting and interpreting DNA methylation marks. Curr Opin Struct Biol. 2018;53:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lagger S, Connelly JC, Schweikert G, Webb S, Selfridge J, Ramsahoye BH, et al. MeCP2 recognizes cytosine methylated tri-nucleotide and di-nucleotide sequences to tune transcription in the mammalian brain. PLoS Genet. 2017;13:e1006793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sperlazza MJ, Bilinovich SM, Sinanan LM, Javier FR, Williams DC Jr. Structural Basis of MeCP2 Distribution on Non-CpG Methylated and Hydroxymethylated DNA. J Mol Biol. 2017;429:1581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tweedie S, Charlton J, Clark V, Bird A. Methylation of genomes and genes at the invertebrate-vertebrate boundary. Mol Cell Biol. 1997;17:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Y, Jorda M, Jones PL, Maleszka R, Ling X, Robertson HM, et al. Functional CpG methylation system in a social insect. Science. 2006;314:645–7. [DOI] [PubMed] [Google Scholar]
- [95].Albalat R. Evolution of DNA-methylation machinery: DNA methyltransferases and methyl-DNA binding proteins in the amphioxus Branchiostoma floridae. Dev Genes Evol. 2008;218:691–701. [DOI] [PubMed] [Google Scholar]
- [96].Albalat R, Marti-Solans J, Canestro C. DNA methylation in amphioxus: from ancestral functions to new roles in vertebrates. Brief Funct Genomics. 2012;11:142–55. [DOI] [PubMed] [Google Scholar]
- [97].Wang X, Li Q, Lian J, Li L, Jin L, Cai H, et al. Genome-wide and single-base resolution DNA methylomes of the Pacific oyster Crassostrea gigas provide insight into the evolution of invertebrate CpG methylation. BMC Genomics. 2014;15:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dabe EC, Sanford RS, Kohn AB, Bobkova Y, Moroz LL. DNA Methylation in Basal Metazoans: Insights from Ctenophores. Integr Comp Biol. 2015;55:1096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Uno T, Nomura Y, Nakamura M, Nakao A, Tajima S, Kanamaru K, et al. Expression, purification and characterization of methyl DNA binding protein from Bombyx mori. J Insect Sci. 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tweedie S, Ng HH, Barlow AL, Turner BM, Hendrich B, Bird A. Vestiges of a DNA methylation system in Drosophila melanogaster? Nat Genet. 1999;23:389–90. [DOI] [PubMed] [Google Scholar]
- [101].Ballestar E, Pile LA, Wassarman DA, Wolffe AP, Wade PA. A Drosophila MBD family member is a transcriptional corepressor associated with specific genes. Eur J Biochem. 2001;268:5397–406. [DOI] [PubMed] [Google Scholar]
- [102].Okamura K, Matsumoto KA, Nakai K. Gradual transition from mosaic to global DNA methylation patterns during deuterostome evolution. BMC Bioinformatics. 2010;11 Suppl 7:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sarda S, Zeng J, Hunt BG, Yi SV. The evolution of invertebrate gene body methylation. Mol Biol Evol. 2012;29:1907–16. [DOI] [PubMed] [Google Scholar]
- [104].Xu X, Li G, Li C, Zhang J, Wang Q, Simmons DK, et al. Evolutionary transition between invertebrates and vertebrates via methylation reprogramming in embryogenesis. National Science Review. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yildirim O, Li R, Hung JH, Chen PB, Dong X, Ee LS, et al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pan H, Bilinovich SM, Kaur P, Riehn R, Wang H, Williams DC Jr. CpG and methylation-dependent DNA binding and dynamics of the methylcytosine binding domain 2 protein at the single-molecule level. Nucleic Acids Res. 2017;45:9164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lopez-Serra L, Ballestar E, Fraga MF, Alaminos M, Setien F, Esteller M. A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer Res. 2006;66:8342–6. [DOI] [PubMed] [Google Scholar]
- [108].Bornelöv S, Reynolds N, Xenophontos M, Gharbi S, Johnstone E, Floyd R, et al. The Nucleosome Remodeling and Deacetylation Complex Modulates Chromatin Structure at Sites of Active Transcription to Fine-Tune Gene Expression. Molecular Cell. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hogstrom J, Heino S, Kallio P, Lahde M, Leppanen VM, Balboa D, et al. Transcription Factor PROX1 Suppresses Notch Pathway Activation via the Nucleosome Remodeling and Deacetylase Complex in Colorectal Cancer Stem-like Cells. Cancer Res. 2018;78:5820–32. [DOI] [PubMed] [Google Scholar]
- [110].Yoshida T, Hu Y, Zhang Z, Emmanuel AO, Galani K, Muhire B, et al. Chromatin restriction by the nucleosome remodeler Mi-2beta and functional interplay with lineage-specific transcription regulators control B-cell differentiation. Genes Dev. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Salamun SG, Sitz J, De La Cruz-Herrera CF, Yockteng-Melgar J, Marcon E, Greenblatt J, et al. The Epstein-Barr Virus BMRF1 Protein Activates Transcription and Inhibits the DNA Damage Response by Binding NuRD. J Virol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shen CK, Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980;77:6634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–58. [DOI] [PubMed] [Google Scholar]
- [114].Ley TJ, DeSimone J, Anagnou NP, Keller GH, Humphries RK, Turner PH, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–75. [DOI] [PubMed] [Google Scholar]
- [115].Busslinger M, Hurst J, Flavell RA. DNA methylation and the regulation of globin gene expression. Cell. 1983;34:197–206. [DOI] [PubMed] [Google Scholar]
- [116].Ginder GD, Whitters MJ, Pohlman JK. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci U S A. 1984;81:3954–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rupon JW, Wang SZ, Gaensler K, Lloyd J, Ginder GD. Methyl binding domain protein 2 mediates gamma-globin gene silencing in adult human betaYAC transgenic mice. Proc Natl Acad Sci U S A. 2006;103:6617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ginder GD, Gnanapragasam MN, Mian OY. The role of the epigenetic signal, DNA methylation, in gene regulation during erythroid development. Curr Top Dev Biol. 2008;82:85–116. [DOI] [PubMed] [Google Scholar]
- [119].Kransdorf EP, Wang SZ, Zhu SZ, Langston TB, Rupon JW, Ginder GD. MBD2 is a critical component of a methyl cytosine-binding protein complex isolated from primary erythroid cells. Blood. 2006;108:2836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Amaya M, Desai M, Gnanapragasam MN, Wang SZ, Zu Zhu S, Williams DC Jr., et al. Mi2beta-mediated silencing of the fetal gamma-globin gene in adult erythroid cells. Blood. 2013;121:3493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–42. [DOI] [PubMed] [Google Scholar]
- [122].Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A. 2008;105:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bauer DE, Orkin SH. Hemoglobin switching’s surprise: the versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr Opin Genet Dev. 2015;33:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Vinjamur DS, Bauer DE, Orkin SH. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br J Haematol. 2018;180:630–43. [DOI] [PubMed] [Google Scholar]
- [125].Liu N, Hargreaves VV, Zhu Q, Kurland JV, Hong J, Kim W, et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wood KH, Zhou Z. Emerging Molecular and Biological Functions of MBD2, a Reader of DNA Methylation. Front Genet. 2016;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Burgold T, Barber M, Kloet S, Cramard J, Gharbi S, Floyd R, et al. The Nucleosome Remodelling and Deacetylation complex suppresses transcriptional noise during lineage commitment. EMBO J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. [DOI] [PubMed] [Google Scholar]
- [130].Schrodinger L. The PyMOL Molecular Graphics System, Version 2.0. 2015.
- [131].Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]