Abstract
Background:
In patients with transthyretin amyloid cardiomyopathy (ATTR-CM), tafamidis reduces all-cause mortality and cardiovascular hospitalizations, and slows decline in quality-of-life compared with placebo. In May 2019, tafamidis received expedited approval from the US FDA as a breakthrough drug for a rare disease. However, at $225,000 per year, it is the most expensive cardiovascular drug ever launched in the US, and its long-term cost-effectiveness and budget impact are uncertain. We therefore sought to estimate the cost-effectiveness of tafamidis and its potential effect on US health care spending.
Methods:
We developed a Markov model of patients with wild-type or variant ATTR-CM and heart failure (mean age 74.5 years) using inputs from the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), published literature, US Food and Drug Administration review documents, healthcare claims, and national survey data. We compared no disease-specific treatment (“usual care”) with tafamidis therapy. The model reproduced 30-month survival, quality-of-life, and cardiovascular hospitalization rates observed in ATTR-ACT; future projections used a parametric survival model in the control arm, with constant hazards reduction in the tafamidis arm. We discounted future costs and quality-adjusted life years (QALYs) by 3% annually, and examined key parameter uncertainty using deterministic and probabilistic sensitivity analyses. The main outcomes were lifetime incremental cost-effectiveness ratio (ICER) and annual budget impact, assessed from the US healthcare sector perspective. This study was independent of the ATTR-ACT trial sponsor.
Results:
Compared with usual care, tafamidis was projected to add 1.29 (95% uncertainty interval, 0.47–1.75) QALYs at an incremental cost of $1,135,000 (872,000–1,377,000), resulting in an ICER of $880,000 (697,000–1,564,000) per QALY gained. Assuming a threshold of $100,000 per QALY gained and current drug price, tafamidis was cost-effective in 0% of 10,000 probabilistic simulations. A 92.6% price reduction from $225,000 to $16,563 would be necessary to make tafamidis cost-effective at $100,000/QALY. Results were sensitive to assumptions related to long-term effectiveness of tafamidis. Treating all eligible patients with ATTR-CM in the US with tafamidis (n = 120,000) was estimated to increase annual healthcare spending by $32.3 billion.
Conclusions:
Treatment with tafamidis is projected to produce substantial clinical benefit but would greatly exceed conventional cost-effectiveness thresholds at the current US list price. Based on recent US experience with high-cost cardiovascular medications, access to and uptake of this effective therapy may be limited unless there is a large reduction in drug costs.
Keywords: Tafamidis, Amyloidosis, Heart Failure, Cost-Effectiveness, Economics
Background
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous clinical syndrome that accounts for approximately 50% of all heart failure and lacks effective treatments. Transthyretin amyloid cardiomyopathy (ATTR-CM), caused by the accumulation in the myocardium of amyloid fibrils composed of misfolded transthyretin, is an underrecognized cause of HFpEF in older adults.1 In addition to causing heart failure, it increases the risk of conduction abnormalities, atrial fibrillation, embolic stroke, and cardiovascular death, and results in a median survival of 2.5 to 3.5 years from diagnosis if untreated.1, 2
Tafamidis is a drug that binds to and stabilizes transthyretin, thereby preventing transthyretin tetramer dissociation, the rate-limiting step in transthyretin amyloid deposition. In the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), patients with ATTR-CM and heart failure who received tafamidis experienced lower all-cause mortality, fewer cardiovascular hospitalizations, and a slower decline in quality-of-life and functional capacity compared with patients who received placebo.2 In May 2019, tafamidis received expedited approval from the US Food and Drug Administration (FDA) as a breakthrough drug, a designation reserved for therapies “intended to treat a serious or life-threatening disease” with preliminary evidence to suggest “a substantial improvement over existing therapies.”3 However, at a list price of $225,000 per year, it is the most expensive cardiovascular drug ever launched in the US, raising concerns about cost-effectiveness, affordability, and access. A timely and rigorous cost-effectiveness evaluation would help inform clinical and policy discussions regarding uptake of tafamidis and may influence drug pricing as the drug enters the market. We therefore sought to perform an independent cost-effectiveness analysis of tafamidis for ATTR-CM, with the goal of better understanding the potential effects of this high-priced therapy on US healthcare spending.
Methods
The simulation model and key inputs used to conduct this research are available to interested researchers who submit a 1- to 2-page research proposal and collaboration plan to Dr. Kazi (e-mail, dkazi@bidmc.harvard.edu) and sign a Creative Commons agreement, pending approval by the model team. Data used to generate the inputs for this study come from health care claims, surveys, and publications detailed in Table 1 and the Supplemental Material and may be requested directly from their primary source. Data on health surveys, vital statistics, and health care costs are publicly available from government sources as described. Because the study relied on publicly available de-identified data, this was deemed to not be human subjects research and institutional review board approval was therefore not required.
Table 1.
Input Parameters
| Parameter | Base-Case Value | Range in Sensitivity Analyses | Distribution for Probabilistic Analyses | Source |
|---|---|---|---|---|
| Transition Probabilities | ||||
| Rate of CV hospitalizations, per person per year | 0.70 | 0.62 – 0.80 | Log-normal | Maurer et al. 20182, CDER 20194 |
| Proportion of CV hospitalizations that are fatal | 0.0954 | 0.035 – 0.105 | Beta | Wadhera 20185, range assumed |
| Rate of death from any cause in the control arm, per person per year | Weibull distribution estimated from control arm of ATTR-ACT, with the following parameters: Sigma: 0.644 (SE 0.1081) k: 3.820 (SE 0.0887) | Weibull* | Maurer et al. 20182 | |
| Discount rate, per year | 0.03 | 0.01 – 0.08 | - | Sanders et al. 20166 |
| Effectiveness of Tafamidis | ||||
| Hazard Ratio for CV hospitalizations, compared with usual care | 0.68 | 0.56 – 0.81 | Log-normal | Maurer et al. 20182 |
| Rate of death from any cause in the tafamidis arm, per person per year | Months 0–18: Identical to the control arm | - | Maurer et al. 20182 | |
| Months 18–30: Weibull distribution estimated from pooled tafamidis arm of ATTR-ACT, with the following parameters: Sigma: 0.939 (SE 0.1628) k: 4.429 (SE 0.3116) | Weibull* | |||
| Months >30: Hazard ratio relative to usual care as observed in month 30 of the simulation | - | |||
| Costs | ||||
| Tafamidis therapy, USD per year | 225,000 | 2,250 – 500,000 | - | Truven Health Analytics7, Range assumed |
| Background healthcare costs, USD | Normal | MEPS8 | ||
| Age <75 years | 19,785 | 19,050 – 20,520 | ||
| Age 75–85 years | 18,462 | 17,967 – 18,958 | ||
| Age >85 years | 17,417 | 16,945 – 17,889 | ||
| CV hospitalization, USD | Normal | HCUP9, Peterson et al. 201510 | ||
| Age <75 years | 20,219 | 16,256 – 24,182 | ||
| Age 75–85 years | 20,219 | 16,256 – 24,182 | ||
| Age >85 years | 13,716§ | 11,028 – 16,404 | ||
| Clinic visit, USD | 148 | 120 – 175 | Normal | CMS11 |
| Quality of Life | ||||
| Baseline KCCQ-OS | 66.72 | 62.62 – 70.82 | Normal | Maurer et al. 20182 |
| Change in KCCQ-OS at 30 months in the control arm | −20.81 | −24.67 to 16.95 | Normal | CDER 20194 |
| Change in KCCQ-OS at 30 months in the tafamidis arm | −7.16 | −9.94 to −4.38 | Normal | CDER 20194 |
Abbreviations: CDER = Center for Drug Evaluation and Research, CMS = Centers for Medicare and Medicaid Services, CV = cardiovascular, HCUP = Healthcare Cost and Utilization Project, KCCQ-OS = Kansas City Cardiomyopathy Questionnaire – Overall Score, MLM = Medical Expenditure Panel Survey, SE = standard error, USD = United States dollar.
For the purpose of the probabilistic sensitivity analysis, we assumed that the underlying sigma and k parameter estimates of the Weibull survival curve had a bivariate normal distribution.
The small decline in mean hospitalization costs has been previously described, and may relate to lower rates of utilization, on average, of high-cost invasive procedures in adults over the age of 85 years.
Model Structure
We developed a state-transition Markov model of patients with wild-type or variant (i.e., hereditary) ATTR-CM and heart failure using inputs from the ATTR-ACT trial, published literature, FDA review documents, national claims data, and the Medical Expenditure Panel Survey (MEPS).2, 4, 5, 9, 10, 12–17 In monthly cycles, patients could continue to live with heart failure (with declining quality-of-life related to advancing disease), experience cardiovascular hospitalizations, or die from cardiovascular or non-cardiovascular causes (Figure 1). We adopted the US healthcare sector perspective, including all healthcare-related expenditures regardless of who incurs them, and a lifetime analytic horizon. Future costs and benefits were discounted at 3% per year. We adhered to the guidelines recommended by the Second Panel for Cost-Effectiveness in Health and Medicine.6
Figure 1. Schematic of Model and Model Calibration.
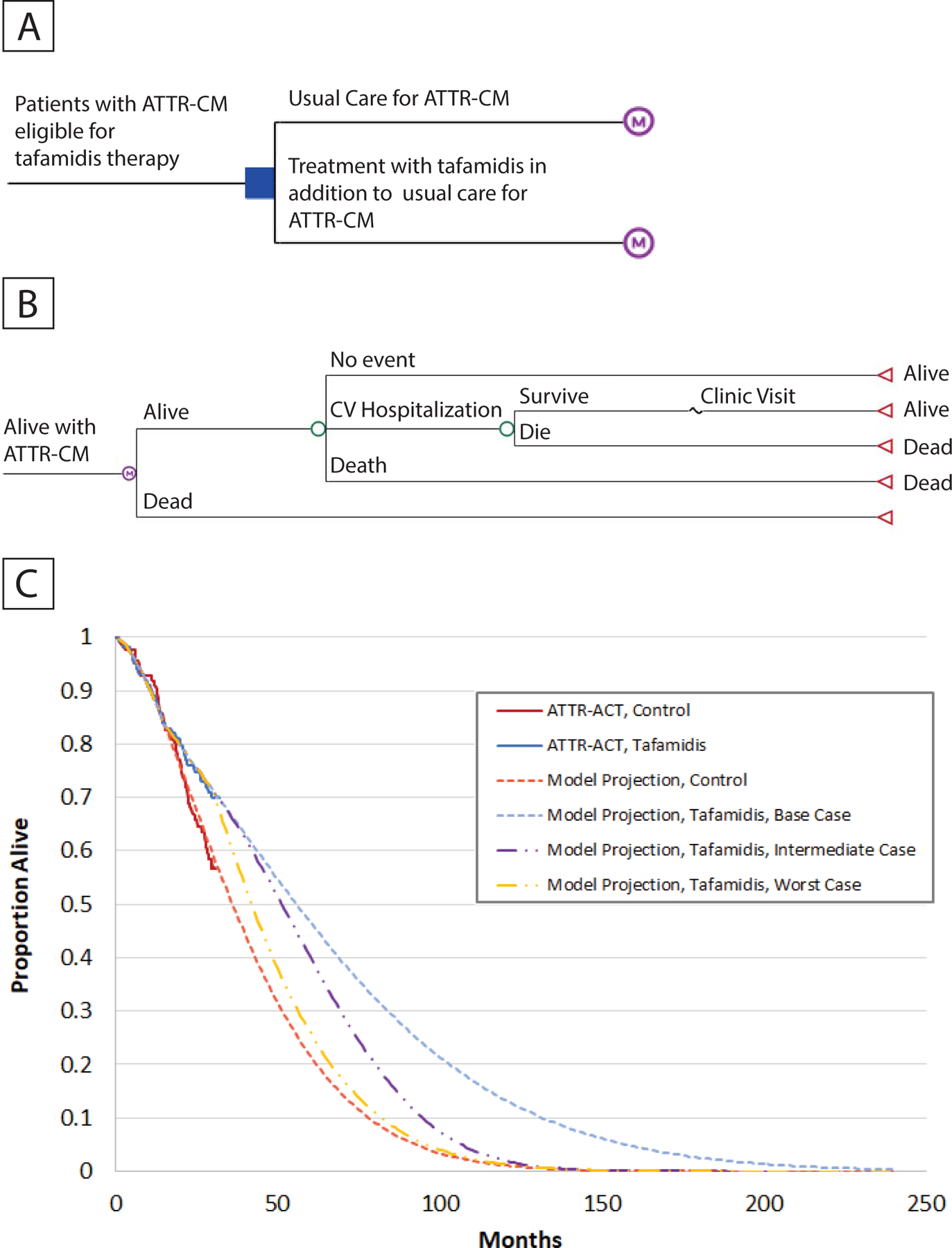
We developed a state-transition Markov model to evaluate the cost-effectiveness of tafamidis therapy compared with usual care among patients with symptomatic heart failure due to transthyretin amyloid cardiomyopathy (ATTR-CM, panel A). In monthly cycles, patients could experience cardiovascular hospitalizations (some proportion of which were fatal), or die from other causes (Panel B). In the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), treatment with tafamidis compared with usual care reduced the risk of cardiovascular hospitalizations throughout the course of therapy, and the risk of death after the first 18 months of treatment. Over the initial 30 months, the model reproduced survival rates seen in the tafamidis and control arms in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT, Panel C). The model used a parametric (Weibull) model to project long-term survival in the control arm beyond 30 months. The base case assumed that the effectiveness of tafamidis would be preserved beyond 30 months (best-case scenario). In sensitivity analyses, we modeled an intermediate-case scenario that assumed that the effectiveness of tafamidis gradually would wane beyond 30 months so that there would be no meaningful differences between the control and intervention groups beyond 90 months, and a worst-case scenario that assumed a complete loss of effectiveness of tafamidis beyond 30 months (Panel C). See text for additional modeling details. Abbreviations: ATTR-ACT = Transthyretin Amyloidosis Cardiomyopathy Clinical Trial, ATTR-CM = transthyretin amyloid cardiomyopathy.
Target Population
The simulated population reflected the characteristics of patients in ATTR-ACT, a phase 3, multicenter, placebo-controlled, double-blind, randomized trial that enrolled patients between 18 and 90 years of age who had wild-type or variant ATTR-CM, a history of heart failure, and at least one prior hospitalization for heart failure or clinical evidence of heart failure.2 Patients with New York Heart Association Class IV symptoms, an implanted left ventricular assist device, or severe renal insufficiency (estimated glomerular filtration rate <25 ml/min/m2) were excluded. The mean age at study entry was 74.5 years and 11% were women.
Treatment Strategies
We evaluated the effect of tafamidis compared with no disease-specific ATTR-CM treatment (“usual care”).
Transition Probabilities for the first 30 months
The model was calibrated to reproduce 30-month survival, quality-of-life, and cardiovascular hospitalization rates observed in ATTR-ACT, based on published data and publicly available FDA review documents.2, 4 In ATTR-ACT, compared with patients receiving placebo, patients receiving tafamidis had lower all-cause mortality at 30 months (hazard ratio 0.70, 95% confidence interval [CI] 0.56–0.81) and a lower rate of cardiovascular hospitalizations (relative risk 0.68, 95% CI 0.56–0.80). In order to estimate survival in both arms, we fit separate parametric Weibull models to the observed survival in the control and intervention arms of the trial (Figure 1). The Weibull was chosen because it is a flexible class of distributions that allows for increasing or decreasing hazard over time (e.g., increasing cardiovascular mortality with age), and also covers simpler distributions like the exponential distribution.18 We used parametric bootstrapping to estimate the uncertainty of the Weibull curve, assuming a bivariate normal joint distribution of the estimates of its sigma and k parameters (Table 1). As observed in ATTR-ACT, we assumed that survival in the intervention arm was identical to that in the control arm for the first 18 months. We then used the Weibull models described above – based on the published survival curves from the trial – to model the reduction in all-cause mortality in the intervention arm relative to the control arm between months 18 and 30.
We assumed that patients in the control arm would have an average of 0.70 cardiovascular hospitalizations a year (95% CI 0.62–0.80) as observed in ATTR-ACT, and applied a 32% hazard reduction in the tafamidis arm (Table 1).2
Extrapolation Beyond Trial Duration
Since patients enrolled in ATTR-ACT were only followed for 30 months, we used the Weibull model described above to project survival in the control arm beyond 30 months. In the base case, we made the optimistic assumption that the benefits of tafamidis would be sustained beyond 30 months, so that the hazard ratio for survival observed in month 30 would continue throughout the duration of therapy. We also assumed that the monthly rate of decline in mean quality-of-life in each arm would continue unchanged beyond 30 months. These assumptions favored tafamidis and represented a “best-case scenario” for the cost-effectiveness of tafamidis compared with usual care (Supplemental Table 1). In sensitivity analyses, we modeled: 1) an “intermediate-case scenario” that assumed that the effectiveness of tafamidis waned over the 60 months after trial completion so that there was no difference between the intervention and control arms beyond month 90; and 2) a “worst-case scenario” that assumed that tafamidis becomes completely ineffective after month 30.
Adverse Events
In ATTR-ACT, the safety profile of tafamidis appeared to be similar to placebo at 30 months. We therefore did not include any costs and quality-of-life penalties related to medication-related adverse events in the model.
Costs
We assumed that one year of tafamidis therapy would cost $225,000, its wholesale acquisition cost in September 2019 (Supplement, Tafamidis Dose and Pricing).7 We varied this assumption in sensitivity analyses. The cost of cardiovascular hospitalizations was estimated from the 2014 Healthcare Cost and Utilization Project data using Ninth Revision of International Classification of Diseases (ICD-9) codes, adjusted to include physician fees (Supplemental Table 2, Estimating Costs of Cardiovascular and Non-Cardiovascular Care).9, 10 Because ATTR-CM has substantial prognostic implications for patients and, in the case of hereditary ATTR-CM, for family members, we assumed that patients with heart failure with preserved ejection fraction would be tested for ATTR-CM regardless of the decision to initiate tafamidis therapy. We therefore did not incorporate diagnostic costs in this analysis as their inclusion would not be expected to alter the incremental cost-effectiveness of tafamidis therapy. Background healthcare costs (defined as all direct medical costs, excluding cardiovascular hospitalizations and tafamidis drug costs) were estimated as the adjusted, survey-weighted mean total expenditures for individuals with history of heart failure and without any cardiovascular hospitalizations in the year prior or during the survey year from the 2006–2015 MEPS, stratified by age.8 We included long-term care costs by multiplying the proportion of all US adults using each type of long-term care service by published annual long-term care cost estimates from the US Department of Health and Human Services (Supplemental Tables 3 and 4).14, 15 All costs were inflated to 2019 US dollars using the Personal Consumption Expenditure index.16 Additional modeling details are available in the online Supplemental Material.
Quality-of-Life Estimates
ATTR-ACT used the overall score of the Kansas City Cardiomyopathy Questionnaire (KCCQ-OS) to measure study participants’ heart failure-specific health status at baseline and throughout follow-up.17 We used observed changes in the KCCQ-OS from ATTR-ACT (placebo 20.81±1.97 vs tafamidis 7.16±1.42) in the first 30 months of the model and linearly extrapolated these afterward. To map KCCQ-OS scores to quality-of-life weights, we developed a mapping algorithm using individual-level data from a prospective, 14-center cohort of 476 outpatients with heart failure (Supplement, Estimating Quality-of-Life Parameters, Supplemental Table 5, and Supplemental Table 6).19 To capture the uncertainty in this mapping process, we used parametric bootstrapping to generate 1000 paired values for the mapping parameters, which we incorporated into probabilistic sensitivity analyses.20 Since patients with HFpEF typically receive other oral medications like diuretics, we did not model any additional pill-related disutility related to tafamidis therapy.
Main Outcome Measures
The primary outcome was the incremental cost-effectiveness ratio (ICER) of tafamidis compared with usual care, assessed in terms of both cost per life year and cost per quality-adjusted life year (QALY). We assumed a cost-effectiveness threshold of $100,000 per QALY, and examined alternative thresholds in sensitivity analyses (high value, <$50,000; intermediate value, ≥$50,000 to <$150,000, and low value, ≥$150,000 per QALY gained).21 We also evaluated the impact on annual healthcare spending if all US patients eligible for tafamidis were to receive the drug. For this budget impact analysis, we estimated a target population of 120,000 US adults, based on a conservative estimate that 4% of adults older than 60 years who have HFpEF have ATTR-CM, but varied this number between 100,000 to 200,000 in sensitivity analyses (Supplemental Material, Approach to the Budget Impact Analysis). We first estimated total change in healthcare spending over 5 years and then generated annualized estimates of budget impact.
Sensitivity Analyses
We performed deterministic and probabilistic sensitivity analyses to reflect uncertainty in the key parameters. In deterministic analyses, we varied input parameters one-at-a-time across the range shown in Table 1 while holding all other parameters at their base-case value. Probabilistic sensitivity analyses were performed by drawing (with replacement) 10,000 sets of input parameters from pre-specified statistical distributions in order to generate 95% uncertainty intervals (UI) for key clinical and economic outcomes as well as acceptability curves.
Software
Modeling was performed using TreeAge Pro 2019 (TreeAge Software Inc, Williamstown, Massachusetts) and Microsoft Excel version 16 (Microsoft Corporation, Redmond, Washington), and statistical analyses were performed using R version 3.6.1 (Vienna, Austria).
Funding and Role of the Sponsor
This study was independent of the commercial sponsor of the ATTR-ACT trial, and was funded by the Richard A. and Susan F. Smith Center for Cardiovascular Outcomes Research and a Northwestern University Multidisciplinary Amyloidosis Program pilot grant. Dr. Bellows is supported by K01-HL140170 from the National Heart, Lung, and Blood Institute. The funders had no role in study design, data collection and analysis, or preparation of the manuscript, or the decision to submit it for publication.
Results
Model Calibration
The mean age of the simulated cohort was 74.5 years and 11% were women. The model accurately replicated the all-cause mortality and cardiovascular hospitalization rates observed in ATTR-ACT over 30 months of follow-up (Supplemental Table 7). For instance, all-cause mortality at 30 months among patients receiving tafamidis was 29.6% in the model and 29.5% in ATTR-ACT. At 30 months, the hazard ratio for all-cause mortality (tafamidis vs. usual care) was 0.68 (95% UI 0.51 to 0.86) in the model compared with 0.70 (95% CI 0.51 to 0.96), and the relative risk ratio for cardiovascular hospitalizations was 0.70 [95% UI 0.59 to 0.83) in the model vs 0.68 (95% CI 0.56 to 0.81) in ATTR-ACT (Supplemental Table 7).
Base-Case Analysis
Mean survival in the usual care arm was 3.46 (95% UI, 2.88–4.25) years; this was prolonged to 5.43 (95% UI, 4.17–6.76) years in the tafamidis arm (Table 2). Patients receiving tafamidis were projected to have fewer cardiovascular hospitalizations over the first 30 months compared with patients receiving usual care, but this was offset by additional hospitalizations over the long-term due to increased survival, resulting resulted in a higher total number of lifetime cardiovascular hospitalizations in patients receiving tafamidis (Table 2 and Supplemental Table 8). Compared with usual care, treatment with tafamidis over the lifetime horizon was projected to generate 1.29 (95% UI, 0.60–1.89) additional QALYs at an incremental cost of $1,135,000 (95% UI, 872,000–1,377,000), resulting in an ICER of $880,000 (95% UI, 697,000–1,553,000) per QALY gained (Table 2). The incremental cost in the intervention arm was almost entirely comprised of the cost of tafamidis ($1,086,000 [95% UI, 861,000–1,303,000]), with a small contribution from increased background costs related to prolonged survival (Table 2). There was no meaningful reduction in lifetime costs of cardiovascular care because savings from fewer cardiovascular hospitalizations per year were offset by increased cardiovascular costs in the added years of life.
Table 2.
Base Case Results
| Usual Care | Tafamidis | |
|---|---|---|
| Healthcare Outcomes | ||
| Survival, life years (undiscounted) | 3.46 (2.88 – 4.25) | 5.43 (4.17 – 6.76) |
| Survival, life years (discounted) | 3.23 (2.73 – 3.84) | 4.83 (3.82 – 5.79) |
| Incremental life years (discounted) | Comparator | 1.60 (0.48 – 2.47) |
| Quality-adjusted survival, QALYs (discounted) | 2.19 (1.94 – 2.56) | 3.48 (2.85 – 4.15) |
| Incremental QALYs (discounted) | Comparator | 1.29 (0.47 – 1.75) |
| Cardiovascular Hospitalizations, number | 2.36 (1.87 – 3.02) | 2.53 (1.78 – 3.43) |
| Direct Healthcare Costs | ||
| Lifetime Healthcare Costs, 2019 USD (discounted) | 126,000 (105,000 – 157,000) | 1,262,000 (996,000 – 1,515,000) |
| Spending on Tafamidis | - | 1,086,000 (861,000 – 1,303,000) |
| Spending on CV Hospitalizations | 34,000 (26,000 – 46,000) | 34,000 (23,000 – 47,000) |
| Background Healthcare Costs | 92,000 (77,000 – 113,000) | 142,000 (110,000 – 174,000) |
| Incremental healthcare costs, 2019 USD (discounted) | Comparator | $1,135,000 (872,000–1,377,000) |
| ICER, USD per life-year gained | Comparator | $709,000 (547,000 – 1,943,000) |
| ICER, USD per QALY gained | Comparator | $880,000 (697,000–1,564,000) |
Abbreviations: ICER = incremental cost-effectiveness ratio; USD = United States Dollar; QALY = quality-adjusted life year.
Sensitivity Analyses
In deterministic analyses, our findings were sensitive to three key parameters. First, an increase in the discount rate to 8% increased the ICER to $977,000 per QALY gained. Second, assuming a lower effectiveness of tafamidis with regard to reduction in all-cause mortality would increase the ICER to $1,321,000 per QALY gained. Third, the ICER was very sensitive to the annual cost of tafamidis. A 92.6% price reduction from $225,000 to $16,563 would make tafamidis cost-effective at a threshold of $100,000 per QALY gained, while an 87% price reduction to $29,925 would make it cost-effective at $150,000 per QALY gained (Figure 2). Additionally, shortening the time horizon to 30 months, the duration of ATTR-ACT, increased the ICER to $3,903,000 per QALY gained (Supplemental Table 8). The model was relatively insensitive to other parameters (altering the ICER by less than 20%).
Figure 2. One-Way Sensitivity Analysis by Price of Tafamidis.
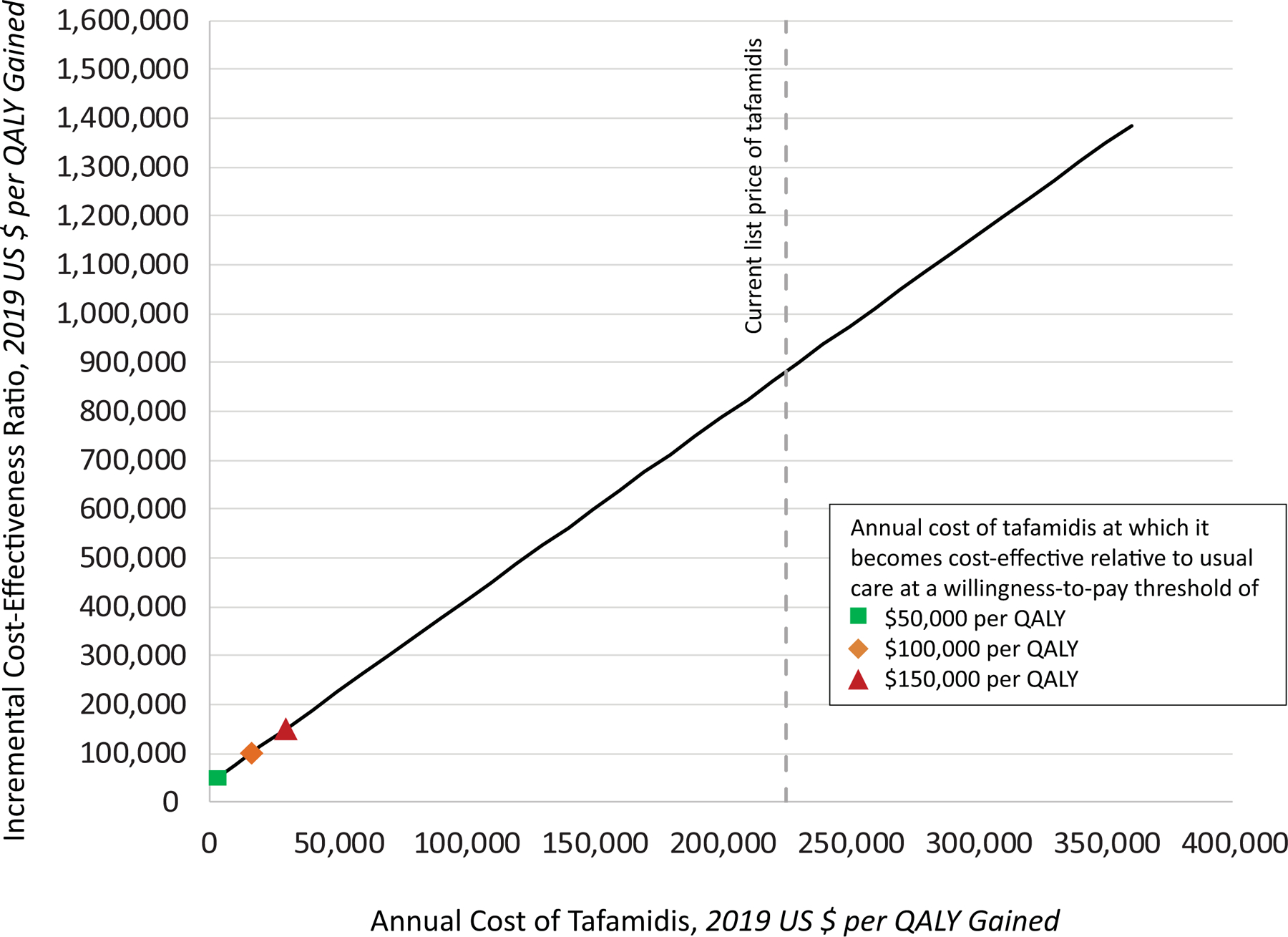
We evaluated the effect of varying the annual cost of tafamidis on the incremental cost-effectiveness ratio (ICER) of tafamidis compared with usual care, holding all other input parameters at their base-case value. At the 2019 annual price of $225,000, tafamidis does not meet conventional cost-effectiveness thresholds. An 86.7% reduction in price to $29,925 would be needed to achieve a threshold of $150, 000 per quality-adjusted life year (QALY) gained, a 92.6% reduction in price to $16,563 would be needed to meet a cost-effectiveness threshold of $100,000 per QALY gained, and a 98.6% reduction to $3,200 would be needed to achieve a cost-effectiveness threshold of $50,000 per QALY gained.
In probabilistic sensitivity analyses, tafamidis was cost-effective in 0% of the 10,000 probabilistic simulations at a cost-effectiveness threshold of $100,000 per QALY gained (Figure 3 and Supplemental Figure 1).
Figure 3. Cost-Effectiveness Acceptability Curves.
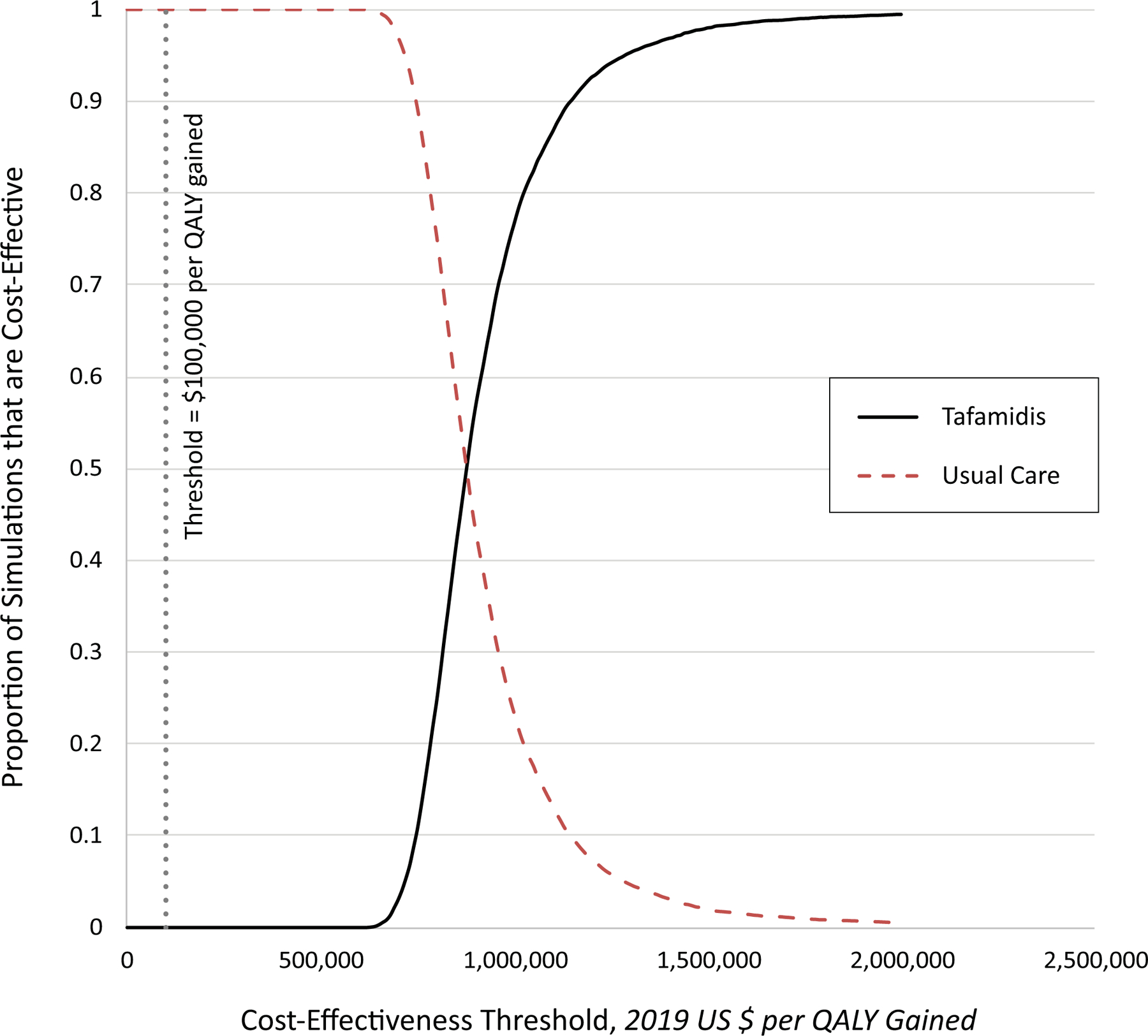
In probabilistic sensitivity analyses, we ran 10,000 iterations of the model after sampling key input parameters from pre-specified statistical distributions (with replacement). The results are shown below as acceptability curves, which indicate the proportion of simulations (y-axis) in which a given strategy is the optimal strategy at various cost-effectiveness thresholds (x-axis). Under our base case assumptions and assuming a societal cost-effectiveness threshold of $100,000 per quality-adjusted life year (QALY), treatment with tafamidis was cost-effective compared with usual care in 0% of the simulations.
Scenario Analyses
The results were sensitive to the long-term durability of effectiveness of tafamidis. Assuming that the effectiveness of tafamidis wanes over 5 years after trial completion (intermediate-case scenario) reduced the incremental benefit of tafamidis to 0.63 QALYs and increased the ICER to $1,517,000 per QALY gained (Supplemental Table 9). Assuming complete loss of tafamidis effectiveness beyond 30 months (worst-case scenario) increased the ICER to $3,122,000 per QALY gained. In the intermediate-case and worst-case scenarios, the price of tafamadis would need to be 95.7% and 97.4% lower, respectively, for the therapy to be cost-effective at a threshold of $100,000 per QALY gained (Supplemental Table 10).
Budget Impact Analysis
Treating all eligible US patients with ATTR-CM with tafamidis (n = 120,000) was projected to increase annual healthcare spending by $32.3 billion. Nearly all of the budget impact ($31.9 billion) was due to the cost of tafamidis. The change in healthcare spending varied with estimated prevalence of ATTR-CM, ranging from $26.9 billion if the prevalence was assumed to be 100,000 to $53.8 billion if the prevalence was assumed to be 200,000.
Discussion
In a simulation model calibrated to the results of the ATTR-ACT trial, we found that tafamidis therapy for patients with ATTR-CM would increase quality-adjusted life expectancy by an average of 1.29 QALYs, but the ICER of $880,000 per QALY gained would be substantially higher than conventional cost-effectiveness thresholds. A 92.6% reduction in the annual price of tafamidis – from $225,000 to $16,563 – would be needed for the drug to be cost-effective at a commonly accepted threshold of $100,000 per QALY gained. Savings from fewer cardiovascular hospitalizations per patient per year would be offset by increases in healthcare costs related to prolonged survival, such that U.S. healthcare spending would increase by $32.3 billion a year if all eligible patients were to receive tafamidis therapy. This includes a $31.9 billion increase in annual prescription drug expenditures, which would increase the total US spending for all prescription drugs by 9.3% (from $344 billion in 2018 to $375.9 billion).22 As diagnosis rates increase – as a result of greater awareness about ATTR-CM, increased use of nuclear scintigraphy for accurate diagnosis, and more widespread uptake of genetic tests to screen family members of individuals with variant ATTR-CM – the budget impact of tafamidis is expected to increase as well.23 The challenge for health systems and payers is therefore likely to grow over time. Our findings are concordant with those of two prior analyses that found that tafamidis was not cost-effective for ATTR familial amyloid polyneuropathy in Europe.24, 25 However, our analysis is the to examine tafamidis from a US healthcare sector perspective and include the survival benefits observed in ATTR-ACT.
Over the past 3 decades, numerous cost-effective and potentially life-saving therapies have been approved and implemented for heart failure with reduced ejection fraction, but pharmaceutical innovation for HFpEF has largely been disappointing. One potential explanation for the difficulty in developing effective therapies for HFpEF is that it represents a heterogeneous group of conditions that are unlikely to all respond to a single therapy, arguing against a “one-size-fits-all” approach. Given this heterogeneity, HFpEF is an ideal syndrome for the application of precision medicine, and recent efforts have focused on identifying subgroups of HFpEF to tailor therapy accordingly. The recognition of ATTR-CM as a distinct phenotype of HFpEF, the use of nuclear scintigraphy to noninvasively and accurately diagnose ATTR-CM, and the development of tafamidis to inhibit a key step in the pathogenesis of ATTR-CM are therefore major advances in the targeted treatment of the HFpEF syndrome.2 Our study suggests that for patients similar to those in the ATTR-ACT trial, tafamidis therapy for ATTR-CM will prolong quality-adjusted survival by 1.29 QALYs, a relatively large health gain compared with other contemporary cardiovascular therapies, justifying its designation as a “breakthrough therapy” that addresses an important unmet clinical need.26, 27 For comparison, the use of sacubitril-valsartan in heart failure with reduced ejection fraction is projected to yield 0.62 QALYs over a patient’s lifetime.26
But these substantial health benefits of tafamidis are projected to come at a high cost. Our study demonstrates that, at current drug prices, widespread uptake of tafamidis would produce a large increase in healthcare spending, with an ICER that is greatly exceeds US thresholds for high or even intermediate economic value. Although manufacturers typically offer discounts and rebates amounting to 25–30% of the wholesale acquisition cost, our findings suggest that massive discounts or price reductions would be needed in order to make tafamidis economically attractive at conventional thresholds. It should be noted, however, that manufacturers of rare drugs have substantial pricing power, so that these drugs are often discounted less than 5% if at all. Moreover, manufacturers’ discounts do little to alleviate the financial burden for Medicare Part D beneficiaries (who, given the natural history of the disease, are likely to represent the majority of US patients eligible for this novel therapy) because their copayments are calculated from the list price of the drug before the application of any rebates or discounts. As a result, Medicare Part D beneficiaries without secondary insurance may be responsible for tens of thousands of dollars per year in out-of-pocket costs. This would put the drug out of reach for many fixed-income seniors.
In many ways, tafamidis is emblematic of contemporary challenges in rewarding pharmaceutical innovation – it is a highly personalized therapy that is effective, safe, and unaffordable. Its expedited FDA approval in May 2019 relied on several regulatory and financial incentives available to manufacturers of “orphan drugs” that target rare diseases without pre-existing treatments.28,29 These incentives, embedded in the Orphan Drug Act and designed to help patients gain expedited access to transformative therapies, have been spectacularly successful in spurring pharmaceutical innovation: 58% of all new drugs approved in 2018 were for a rare-disease indication.30 But once approved, drugs that receive “orphan drug status” typically enter the market at a very high price: the 100 best-selling rare disease drugs in 2017 cost an average of $116,000 more than 100 best-selling drugs for other indications.31 Innovators have argued that these higher prices are necessary to recoup large investments in research and development when only a small number of patients are eligible for therapy. But the high price tag defeats the very purpose of the expedited approval process, as it makes the drug unaffordable for the target population. The case of tafamidis lends further credence to recent calls to reform the Orphan Drug Act to ensure access to the rare-disease drugs that utilize its incentives. For instance, regulatory and financial incentives could be conditioned on the manufacturer setting a value-based price, based on an appropriate cost-effectiveness threshold for rare-disease drugs.30 In return, payers would cover these drugs without onerous pre-authorization requirements or large co-payments, eliminating financial barriers to access and adherence. While the details would need to be worked out, it is clear that a framework for the responsible pricing of novel therapies guided by rigorous and timely value-based assessments is urgently needed to ensure broad access.
Recent experience with other high-price cardiovascular medications such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors may foretell the adoption patterns for tafamidis. Launched in 2016 at an average price of $14,350 per year and with a potential target population of 10 million US adults, PCSK9 inhibitors saw their uptake stymied due to a combination of onerous pre-authorization requirements (related to high drug costs for payers) and frequent prescription abandonment (related to high out-of-pocket costs for patients).32, 33 Subsequently, a series of independent cost-effectiveness analyses argued for a 70–80% price-reduction to meet conventional cost-effectiveness thresholds; other studies showed that average annual out-of-pocket costs for a Medicare Part D beneficiary receiving a PCSK9 inhibitor and a generic statin was $5000.33, 34 Finally, in 2019, both manufacturers announced an unprecedented 60% price reduction in an effort to increase uptake. We suspect that the widespread adoption of tafamidis is likely to face similar hurdles. Unless the price of the drug falls substantially, onerous pre-authorization requirements and high out-of-pocket costs are likely to present substantial hurdles to widespread adoption. Consequently, the projected population health gains with tafamidis are unlikely to be achieved at 2019 prices.
Limitations
Our study has a few limitations. Our estimates of the efficacy and safety of tafamadis were based on a single randomized clinical trial with a mean follow-up of 30 months and should be updated when longer follow-up data and information regarding high-risk subgroups become available. We have previously argued that the “life-cycle approach” advocated by the National Academy of Medicine to evaluate the safety and effectiveness of drugs based on new data should be extended to cost-effectiveness evaluations.34 Our base-case analysis assumed an annual drug price equivalent to the wholesale acquisition cost of tafamidis before any discounts or rebates offered by the manufacturer. However, our findings suggest that the typical discounts are not likely to make the drug cost-effective. We did not incorporate the cost of provider time spent on meeting onerous pre-authorization requirements often introduced by by payers to limit uptake of expensive medications, the clinican time spent appealing initial denials, or the effect of delays in initiating treatment. We did not model the costs or consequences of cardiac transplantation and cardiac-assist devices, which are uncommon in patients with ATTR-CM because the average age at diagnosis in the US is 70–75 years. If future diagnostic advances facilitate the diagnosis of amyloid cardiomyopathy at younger ages, and should tafamidis therapy in younger patients alter the rates of transplantation or implantation of cardiac-assist devices during follow-up, this cost-effectiveness analysis would need to be updated to include the high cost of these procedures. Health-related quality-of-life data were not directly available for trial participants in ATTR-ACT; we therefore estimated quality-of-life data from published KCCQ-OS estimates. Our budget impact evaluation was based on current estimates of the prevalence of ATTR-CM, which several experts believe to be an underestimate of its true prevalence. If rates of diagnosis of ATTR-CM substantially increase due to more widespread application of nuclear scintigraphy and genetic testing, as well as from greater awareness of the condition, the impact of tafamidis therapy on total health spending would exceed our projections.
Conclusions
In a disease-simulation model calibrated to the results of the ATTR-ACT trial, treatment with tafamidis is projected to produce substantial clinical benefit but would greatly exceed conventional cost-effectiveness thresholds at the current list price. Based on recent US experience with high-cost cardiovascular medications, access to and uptake of this effective therapy may be limited unless there is a large reduction in drug costs.
Supplementary Material
Clinical Perspective.
What is new?
In patients with transthyretin amyloid cardiomyopathy (ATTR-CM), tafamidis reduces all-cause mortality and slows decline in quality-of-life compared with placebo, but it is the most expensive cardiovascular drug ever launched in the US.
In this simulation model of US adults, tafamidis therapy for ATTR-CM was estimated to cost $880000 per quality-adjusted life-year gained compared with usual care and increased annual health care costs by $32.3 billion (including a 9.3% increase in total spending on all prescription drugs over 2018 levels).
A 92.6% reduction in drug price from $225000 annually to $16,563 would be necessary to meet a $100000 per QALY threshold.
What are the clinical implications?
Assuming 2019 prices, tafamidis use does not meet generally accepted cost-effectiveness thresholds and is estimated to increase US health care costs substantially.
Based on recent US experience with high-cost cardiovascular medications, access to and uptake of this potentially life-saving therapy may be limited unless there is a large reduction in drug costs.
Acknowledgments and Author Contributions:
Dr. Kazi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kazi, Shah.
Acquisition, analysis, or interpretation of data: Kazi, Bellows, Shen, Spertus, Shah.
Drafting of the manuscript: Kazi, Bellows, Shah.
Critical revision of the manuscript for important intellectual content: Kazi, Bellows, Baron, Shen, Cohen, Spertus, Yeh, Arnold, Sperry, Maurer, Shah.
Statistical analysis: Kazi, Bellows, Shen.
Obtaining funding: Kazi, Shah.
Administrative, technical, or material support: Kazi.
Study supervision: Kazi.
The authors acknowledge Linda Valsdottir for editorial assistance.
Funding/Support: This study was independent of the commercial sponsor of the ATTR-ACT trial, and was funded by the Richard A. and Susan F. Smith Center for Cardiovascular Outcomes Research and a Northwestern University Multidisciplinary Amyloidosis Program pilot grant. Dr. Bellows is supported by K01-HL140170 from the National Heart, Lung, and Blood Institute.
Role of the Funder/Sponsor: Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Non-standard Abbreviations and Acronyms
- ATTR-ACT
Transthyretin Amyloidosis Cardiomyopathy Clinical Trial
- ATTR-CM
transthyretin amyloid cardiomyopathy
- FDA
Food and Drug Administration
- HFpEF
heart failure with preserved ejection fraction
- ICER
incremental cost-effectiveness ratio
- KCCQ-OS
Kansas City Cardiomyopathy Questionnaire Overall Score
- MEPS
Medical Expenditure Panel Survey
- PCSK9
proprotein convertase subtilisin/kexin type 9
- UI
uncertainty interval
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Cohen reports research grant support from Edwards Lifesciences, Abbott Vascular, Boston Scientific, Medtronic, and Corvia, and consulting income from Edwards Lifesciences, Abbott Vascular, and Medtronic. Dr. Spertus owns copyright for the Kansas City Cardiomyopathy Questionnaire, and has an equity interest in Health Outcomes Sciences. He also reports consulting income from Novartis, AstraZeneca, Bayer, Merck, Amgen, Cytokinetics, United Healthcare, and Janssen, and serves on the Board of Blue Cross Blue Shield of Kansas City. Dr. Yeh has research grants and scientific advisory board and/or consulting income from Abbott Vascular, AstraZeneca, Boston Scientific, and Medtronic. Dr. Maurer receives grant support from NIH R01HL139671–01, R21AG058348 and K24AG036778. He has had consulting income from Pfizer, GSK, Eidos, Prothena, Akcea and Alnylam, and his institution received clinical trial funding from Pfizer, Prothena, Eidos, and Alnylam. Dr. Shah is supported by grants from the National Institutes of Health (NIH; R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423); the American Heart Association (AHA; #16SFRN28780016); and Actelion, AstraZeneca, Corvia, and Novartis; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer-Ingelheim, Cardiora, CVRx, Eisai, Ionis, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics. Other authors report no conflicts of interest. No other disclosures are reported.
REFERENCES
- 1.Ruberg FL, Grogan M, Hanna M, Kelly JW and Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 3.Food US and Administration Drug. Frequently Asked Questions: Breakthrough Therapies. June 28, 2019 Available from: https://www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/frequently-asked-questions-breakthrough-therapies. Accessed October 14, 2019.
- 4.Center for Drug Evaluation and Research. Application Number 211996Orig1s000 212161Orig1s000: Summary Review. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211996Orig1s000,%20212161Orig1s000SumR.pdf. Accessed August 1, 2019.
- 5.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C and Yeh RW. Association of the Hospital Readmissions Reduction Program With Mortality Among Medicare Beneficiaries Hospitalized for Heart Failure, Acute Myocardial Infarction, and Pneumonia. JAMA. 2018;320:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 7.Micromedex Red Book Online. Accessed through Micromedex 2.0. Truven Health Analytics Inc. Accessed October 14, 2019. [Google Scholar]
- 8.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey. Available from: https://meps.ahrq.gov/mepsweb/. Accessed August 12, 2019. [PubMed]
- 9.Cost Healthcare and Project Utilization. Statistics on Hospital Stays. Available from: https://hcupnet.ahrq.gov/#setup. Accessed October 14, 2019.
- 10.Peterson C, Xu L, Florence C, Grosse SD and Annest JL. Professional Fee Ratios for US Hospital Discharge Data. Med Care. 2015;53:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services. Physician Fee Schedule. Available from: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed July 19, 2019.
- 12.Howden LM and Meyer JA. Age and Sex Composition: 2010 US Census Brief. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed October 14, 2019.
- 13.Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R, Caffrey C, Rome V and Lendon J. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013–2014. Vital Health Stat 3 2016:x–xii; 1–105. [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Costs of Care. Available from: https://longtermcare.acl.gov/costs-how-to-pay/costs-of-care.html Accessed May 7, 2019.
- 15.Genworth Financial Inc. Cost of Care Survey 2018. Available from: https://www.genworth.com/aging-and-you/finances/cost-of-care.html. Accessed May 7, 2019.
- 16.US Bureau of Economic Analysis. Table 2.4.4.U Price Indexes for Personal Consumption Expenditures by Type of Product. Available from: https://apps.bea.gov/iTable/iTable.cfm?reqid=19&step=2#reqid=19&step=2&isuri=1&1921=underlying. Accessed May 1, 2019.
- 17.Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW Jr., Lemeshow S and May S Applied Survival Analysis: Regression Modeling of Time-to-Event Data. 2nd ed: John Wiley and Sons Inc.; 2008. [Google Scholar]
- 19.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 20.Efron B and Tibshirani RJ. An introduction to the bootstrap: CRC press; 1994.
- 21.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 22.Institute IQVIA. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Accessed November 26, 2019.
- 23.Gilstrap LG, Dominici F, Wang Y, El-Sady MS, Singh A, Di Carli MF, Falk RH and Dorbala S. Epidemiology of Cardiac Amyloidosis-Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ Heart Fail. 2019;12:e005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faria R, Walker S, Palmer S, Corbett M, Stirk L and McDaid C. Tafamidis for Transthyretin Familial Polyneuropathy (TTR-FAP) Evidence Review Group assessment of manufacturer submission. Available from: https://www.york.ac.uk/media/crd/Tafamidis%20ERG%20Report_CRDCHE%20September%204%202013.pdf. Accessed November 26, 2019.
- 25.Borowiack E, Marzec M, Jarosz J, Snarska K and Konopka-Pliszka M. Cost-utility analysis of tafamidis for the treatment of adult patients with transthyretin familial amyloid polyneuropathy in Poland. Journal of Health Policy and Outcomes Research 2019;1 Available from: https://www.jhpor.com/article/2212-cost-utility-analysis-of-tafamidis-for-the-treatment-of-adult-patients-with-transthyretin-familial-amyloid-polyneuropathy-in-poland. Accessed January 28, 2020. [Google Scholar]
- 26.Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD and Heidenreich PA. Cost-Effectiveness of Sacubitril–Valsartan in Patients With Heart Failure With Reduced Ejection Fraction. Ann Intern Med. 2016;165:681–689. [DOI] [PubMed] [Google Scholar]
- 27.Kazi DS, Garber AM, Shah RU, Dudley RA, Mell MW, Rhee C, Moshkevich S, Boothroyd DB, Owens DK and Hlatky MA. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann Intern Med. 2014;160:221–232. [DOI] [PubMed] [Google Scholar]
- 28.Food US and Administration Drug. Fast Track. Available from: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track. Accessed October 14, 2019.
- 29.Food US and Administration Drug. Kempf L CDER’s Office of New Drugs Rare Diseases Program. Available from: https://www.fda.gov/media/112176/download. Accessed October 14, 2019.
- 30.Sarpatwari A and Kesselheim AS. Reforming the Orphan Drug Act for the 21st Century. N Engl J Med. 2019;381:106–108. [DOI] [PubMed] [Google Scholar]
- 31.EvaluatePharma. Orphan Drug Report 2018. Available from: https://info.evaluategroup.com/OD2018-SOC.html. Accessed October 14, 2019.
- 32.Navar AM, Taylor B, Mulder H, Fievitz E, Monda KL, Fievitz A, Maya JF, López JAG and Peterson ED. Association of Prior Authorization and Out-of-pocket Costs With Patient Access to PCSK9 Inhibitor Therapy. JAMA Cardiol. 2017;2:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazi DS, Lu CY, Lin GA, DeJong C, Dudley RA, Chen R and Tseng C-W. Nationwide Coverage and Cost-Sharing for PCSK9 Inhibitors Among Medicare Part D Plans. JAMA Cardiol. 2017;2:1164–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazi DS, Moran AE, Coxson PG and et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


